Abstract
Background and aims
Patients infected with the SARS-CoV-2 usually report fever and respiratory symptoms. However, multiple gastrointestinal (GI) manifestations such as diarrhoea and abdominal pain have been described. The aim of this study was to evaluate the prevalence of GI symptoms, elevated liver enzymes and mortality of patients with COVID-19.
Methods
A systematic review and meta-analysis of published studies that included a cohort of patients infected with SARS-CoV-2 were performed from 1 December 2019 to 15 December 2020. Data were collected by conducting a literature search using PubMed, Embase, Scopus, and Cochrane according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We analysed pooled data on the prevalence of individual GI symptoms and elevated liver enzymes and performed subanalyses to investigate the relationship between GI symptoms/elevated liver enzymes, geographical location, mortality, and intensive care unit (ICU) admission.
Results
The available data of 78 798 patients positive for SARS-CoV-2 from 158 studies were included in our analysis. The most frequent manifestations were diarrhoea (16.5%, 95% CI 14.2% to 18.4%), nausea (9.7%, 95% CI 9.0% to 13.2%) and elevated liver enzymes (5.6%, 95% CI 4.2% to 9.1%). The overall mortality and GI mortality were 23.5% (95% CI 21.2% to 26.1%) and 3.5% (95% CI 3.1% to 6.2%), respectively. Subgroup analysis showed non-statistically significant associations between GI symptoms/elevated liver enzymes and ICU admissions (OR=1.01, 95% CI 0.55 to 1.83). The GI mortality was 0.9% (95% CI 0.5% to 2.2%) in China and 10.8% (95% CI 7.8% to 11.3%) in the USA.
Conclusion
GI symptoms/elevated liver enzymes are common in patients with COVID-19. Our subanalyses showed that the presence of GI symptoms/elevated liver enzymes does not appear to affect mortality or ICU admission rate. Furthermore, the proportion of GI mortality among patients infected with SARS-CoV-2 varied based on geographical location.
Keywords: COVID-19, gastrointestinal tract, liver
Introduction
In December 2019, China was faced with a new strain of coronavirus, novel coronavirus (2019 nCov). Within a short period of time, it manifested into a full pandemic.1 It was first noticed by the innumerable cases of pneumonia that suddenly surged among local inhabitants in the province of Wuhan.2 Soon, the virus was detected through sequencing, leading to it officially being renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the International Committee on Taxonomy of Viruses.3 The disease caused by SARS-CoV-2 was allocated the title of COVID-19 or ‘coronavirus disease’.3 Coronaviruses in general are single-stranded RNA viruses falling under the family of Coronaviridae, which also include Middle East respiratory syndrome coronavirus (MERS Cov) and SARS (SARS-CoV).4 By the end of December 2020, more than 81 million cases of COVID-19 have officially been confirmed worldwide, and mortality from COVID-19 was more than 1 798 050 deaths worldwide.5 In addition, new variants of SARS-CoV-2 have been discovered in the UK, South Africa, and other regions around the world.6
It has been established that the transmission of SARS-CoV-2 occurs from person to person through the upper airway tract (droplet infection) or through direct contact.7 The virus can also be detected in saliva, urine, gastrointestinal (GI) tract and possibly through airborne spread.8 9 The spectrum of symptoms attributable to SARS-CoV-2 includes fever, cough, myalgia, fatigue, and, to a lesser extent, headache. Patients may also be asymptomatic.10–12 Diarrhoea, nausea and vomiting, as well as liver involvement have all been reported in the literature.13 14 In fact, GI involvement is plausible, given that ACE2, the major human cellular receptor for the SARS-CoV-2, is expressed in the GI tract, as well as in liver cells.15 We thus conducted a systematic review of published GI symptoms and elevated liver enzymes associated with COVID-19 on the basis of disease severity, mortality, intensive care unit (ICU) admission, and geographical region. This will aid in understanding the magnitude of involvement of the GI tract and liver in patients with COVID-19.
Methods
Search strategy
A systematic review was conducted using PubMed, Scopus, Cochrane, and Embase databases. Medical literature searches for human studies were performed from 1 December 2019 up to 15 December 2020. The key terms used for the literature search were ((“COVID-19” OR “COVID 2019” OR “severe acute respiratory syndrome coronavirus 2” OR “severe acute respiratory syndrome coronavirus 2” OR “2019 nCoV” OR “SARS-COV2” OR “2019nCoV” OR (“severe acute respiratory syndrome coronavirus 2” OR “SARS-COV2” AND “gastrointestinal” AND (“manifestations” OR “clinical characteristics”) OR (“gastrointestinal tract” OR (“gastrointestinal’ AND “tract”) OR “gastrointestinal tract” OR (“gi” AND “tract”) OR (“fatality” or “Mortality”). In addition, a manual search of all review articles, editorials and retrieved original studies was also performed. All procedures used in this meta-analysis were consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and prespecified protocol, which described our method and analysis before data collection was initiated (see online supplemental material PRISMA checklist).
bmjgast-2020-000571supp001.pdf (2.1MB, pdf)
Selection criteria and data extraction
Data were independently extracted by two investigators (MS and FA) and any discrepancies between the two authors were resolved through discussion. Inclusion and exclusion criteria were defined prior to the literature search. The inclusion criteria were (1) study type: case reports/case series (including chart reviews), prospective/retrospective cohort studies, case–control studies, cross-sectional studies and randomised controlled trials; (2) patient population: adult patients with COVID-19, inpatient or outpatient setting; and (3) outcome measured: at least one reported GI symptom or elevated liver enzyme, number of patients admitted to ICU, and number of deaths reported. In addition, systematic reviews and meta-analyses were also reviewed for any relevant studies.
For the purpose of this study, elevated liver enzyme defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) value above the upper limit of normal of each study laboratory reference range. Furthermore, overall mortality was defined as the proportion of deaths among identified confirmed COVID-19 cases in all studies that reported it. The number of deaths among patients experiencing GI symptoms/elevated liver enzymes was extracted and referred to as GI mortality.
Exclusion criteria were (1) review, opinion, abstracts from conferences, editorials, commentary articles, and review articles; (2) studies without data for retrieval; (3) duplicate studies; (4) asymptomatic patients with COVID-19; and (5) studies that did not report GI symptoms.
Data extraction was performed using Microsoft Excel. The following data were extracted:
Study: author, journal, date, country, number of patients, and study type.
Patients characteristics: mean age, ethnicity, gender, and comorbidities.
Number of reported deaths in all studies.
Number of patients admitted to the ICU.
Number of patients who experienced the following GI symptoms/elevated liver enzymes: abdominal pain, diarrhoea, nausea, anorexia, loss of taste, AST or ALT above the upper limit of normal of each study laboratory reference range.
Risk of bias and certainty of evidence
The Methodical Index for Non-randomized Studies (MINORS)16 was used to assess bias risk. In addition, risk of bias was assessed based on four domains: selection, ascertainment, causality, and reporting. An overall judgement of risk of bias was made based on factors deemed to be most critical for the systematic review (selection criteria, ascertainment of outcome, and follow-up duration).
Statistical analysis
Our primary analysis focused on assessing the weighted pooled prevalence of GI symptoms/elevated liver enzymes in patients with COVID-19 infection, occurring any time during the course of illness. We also conducted subanalyses that looked at the association between GI symptoms/elevated liver enzymes and mortality as well as ICU admission. Categorical variables were described as count (%). Continuous variables were described using mean (SD) if they are normally distributed, median (IQR) if they are not. We pooled the single-arm event rates using a random effects method, and we measured heterogeneity within our studies using the I2 statistic. Subanalyses were described and tested using ORs and 95% CIs to determine statistical significance. STATA V.16 was used to calculate ORs and their respective 95% CI and to create Forest and box plots.
Sensitivity analysis
To examine the effect of the quality of studies on our results, we performed a sensitivity analysis on the prevalence of GI symptoms/elevated liver enzymes by excluding low-quality studies. To do so, we used the modified Newcastle-Ottawa Quality Assessment Scale for non-randomised studies.17 A study with a score of 0–3 was classified as a low-quality study. On the other hand, studies that scored 4 or above were included in the analysis.
Results
Research selection and quality assessment
Overall, 158 studies (online supplemental table 1) from 3175 potentially relevant citations were included in the analysis (figure 1). Most of the included studies were single arm only; very few studies included comparator groups. Furthermore, outcome assessors in all 158 studies were not blinded. Both inpatient and outpatient studies were included. The risk of evidence imprecision was rated as very serious, given that the included studies were all observational studies. Overall, all included studies were rated as having very serious risk of bias because they lacked a control group and had a high risk of confounding and selection bias (online supplemental table 2).
Figure 1.
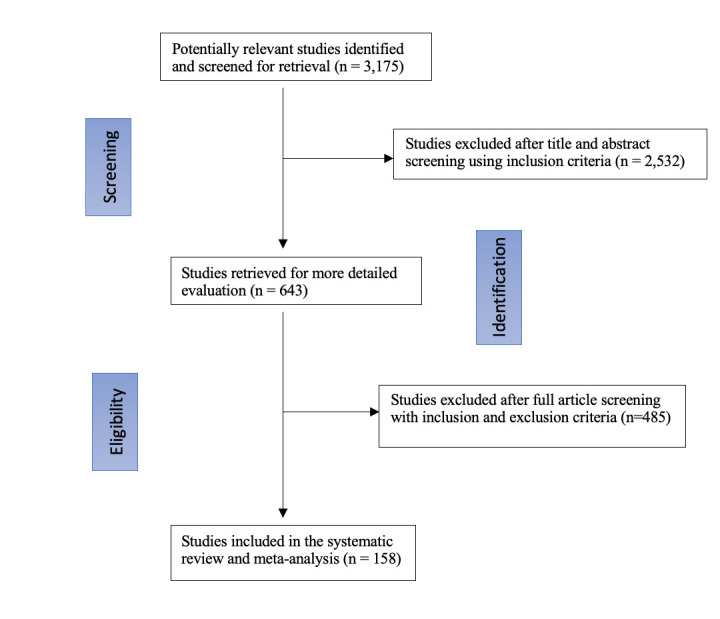
Flow diagram for study selection.
Clinical data
This systematic review included 158 studies2–4 8 12–14 16 18–147 with a total of 78 798 patients that tested positive for SARS-CoV-2 and were included in the analysis. The mean patient age was 55.6 years (±14, 95% CI 48 to 57.3) and 45.2% of the patients were men. Most patients had several comorbidities, the most common being hypertension (28.7%, 95% CI 21.3% to 29.1%), diabetes mellitus (17.4%, 95% CI 13.0% to 19.2%), and cardiovascular diseases (15.7%, 95% CI 13.3% to 17.1%). GI symptoms included nausea, vomiting, abdominal pain, loss of taste, anorexia and diarrhoea (figure 2). Heterogeneity statistic I2 is 95%, which signifies a significant heterogeneity among our studies. The most common reported manifestation among GI symptoms/elevated liver enzymes was diarrhoea (online supplemental figure 1). Specifically, GI symptoms/elevated liver enzymes of patients infected with SARS-CoV-2 are diarrhoea (16.5%, 95% CI 14.2% to 18.4%), nausea (9.7%, 95% CI 9.0% to 13.2%), anorexia or loss of appetite (1.6%, 95% CI 1.2% to 5.1%), vomiting (1.5%, 95% CI 5.1% to 8.0%), abdominal pain (4.5%, 95% CI 3.1% to 7.3%), loss of taste (1.3%, 95% CI 1.1% to 4.1%), and elevated liver enzymes (5.6%, 95% CI 4.2% to 9.1%) (online supplemental table 3).
Figure 2.
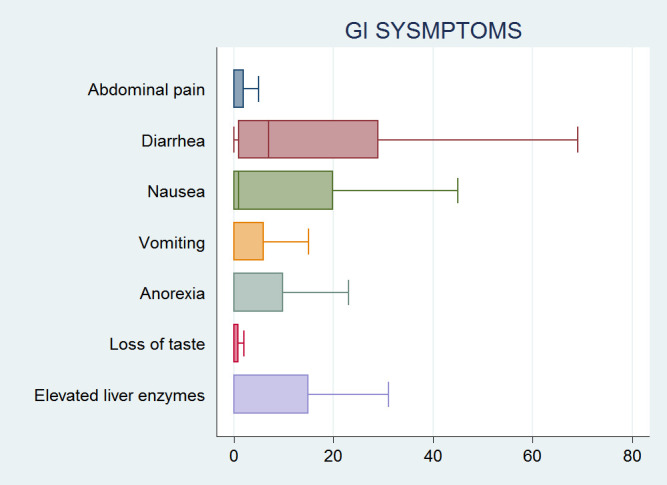
Box plots showing the distribution and proportion of GI symptoms/elevated liver enzymes in patients with COVID-19. GI, gastrointestinal.
Sensitivity analysis
The sensitivity analysis included 52 studies (online supplemental tables 4 and 5). The results did not differ from our main analysis. Among the GI manifestations experienced by patients with COVID-19, diarrhoea (16.6%, 95% CI 12.1% to 17.3%) was still the most common symptom, followed by nausea (9.9%, 95% CI 8.2% to 11.7%). The proportion of patients experiencing loss of taste was 4.7% (95% CI 3.8% to 5.9%). The percentage of patients experiencing elevated liver enzymes was 1.9% (95% CI% 1.3% to 3.4%).
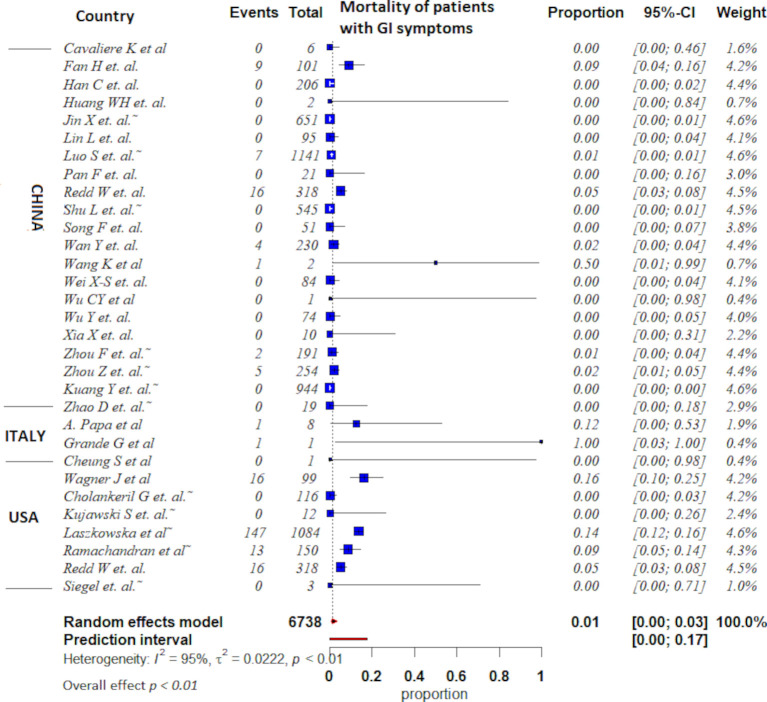
Mortality and geographical variation
A total of 83 studies reported mortality. Of those, 82 studies reported mortality as the number of deaths at the time of the study. Only one study reported mortality as death over 30 days.143 The overall prevalence of overall mortality and GI mortality were 23.5% (95% CI 21.2% to 26.1%) and 3.5% (95% CI 3.1% to 6.2%), respectively (online supplemental tables 6 and 7). The subgroup analysis included eight studies19 20 57 110 136 139 141 143 that directly compared the number of deaths in patients with and without GI symptoms/elevated liver enzymes. In this analysis, the number of patients who experienced GI symptoms/elevated liver enzymes and those who did not were 1593 and 3321, respectively. The results showed that patients with GI symptoms/elevated liver enzymes were not more likely to die compared with those who did not, with a statistically insignificant pooled odds of patients of 1.01 (95% CI 0.46 to 2.25) (figure 3).
Figure 3.
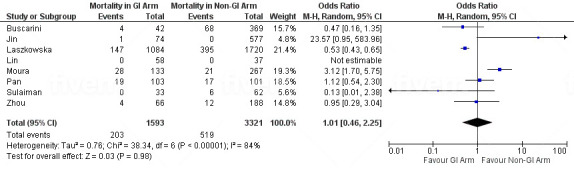
Forest plot of GI mortality in patients with COVID-19, showing no significant difference in the pooled odds of patients with GI symptoms/elevated liver enzymes and those without. GI, gastrointestinal.
Moreover, out of the 158 studies, a total of 42 studies reported mortality in patients with GI symptoms/elevated liver enzymes based on their geographical location (figure 4). This analysis showed that 44 out 4946 patients (0.9%) in China died (95% CI 0.5 to 2.2), whereas 192 out 1783 patients (10.8%) in the USA died (95% CI 7.8 to 11.3). In addition, 2 out of 9 patients (22.2%) in Italy died, while 28 out of 400 patients (7%) in Brazil died. Furthermore, three studies from Taiwan, Korea, and Japan reported zero GI mortality (table 1).
Figure 4.
Forest plot of GI mortality in patients with COVID-19 who are experiencing GI symptoms/elevated liver enzymes in three different countries. GI, gastrointestinal.
Table 1.
GI mortality by geographical location
| Study | Patients (total n) | Mortality in patients with GI symptoms | Country |
| Fan et al48 | 101 | 9 | China |
| Han et al | 206 | 0 | China |
| Huang et al | 2 | 0 | China |
| Kuang et al | 944 | 0 | China |
| Shu et al | 545 | 0 | China |
| Jin et al | 651 | 0 | China |
| Lin et al | 95 | 0 | China |
| Pan et al | 21 | 0 | China |
| Zhao et al | 19 | 0 | China |
| Redd et al | 318 | 16 | China |
| Luo et al | 1141 | 7 | China |
| Song et al | 51 | 0 | China |
| Wan et al | 230 | 4 | China |
| Wei et al | 84 | 0 | China |
| Wu et al | 74 | 0 | China |
| Zhou et al | 191 | 2 | China |
| Zhou et al | 254 | 5 | China |
| Xia et al | 10 | 0 | China |
| Cavaliere et al | 6 | 0 | China |
| Wu et al | 1 | 0 | China |
| Wang et al | 2 | 1 | China |
| Total China | 4946 | 44 | 0.9% |
| Ramachandran et al | 150 | 13 | USA |
| Wagner et al | 99 | 16 | USA |
| Cheung et al | 1 | 0 | USA |
| Cholankeril et al | 116 | 0 | USA |
| Kujawski et al | 12 | 0 | USA |
| Redd et al | 318 | 16 | USA |
| Siegel et al | 3 | 0 | USA |
| Laszkowska et al | 1084 | 147 | USA |
| Total USA | 1783 | 192 | 10.8% |
| Grande et al | 1 | 1 | Italy |
| Papa et al | 8 | 1 | Italy |
| Total Italy | 9 | 2 | 22.2% |
| Hsih et al | 2 | 0 | Taiwan |
| Tabata et al | 104 | 0 | Japan |
| Moura et al | 400 | 28 | Brazil (7%) |
| Wahab et al | 1 | 0 | Denmark |
| Dietrich et al | 1 | 0 | Germany |
| Kandasamy et al | 1 | 0 | India |
| Sulaiman et al | 140 | 0 | Iraq |
| Hassani et al | 2 | 1 | Iran |
| Khader et al | 1 | 0 | Qatar |
| Gulen et al | 1 | 0 | Turkey |
| Kim et al | 28 | 0 | South Korea |
ICU admission rate
Five studies22 85 139 141 148 reported differences in ICU admissions among patients manifesting GI symptoms/elevated liver enzymes and patients who did not. The total number of patients with GI symptoms/elevated liver enzymes who were admitted to the ICU was 1282, and the number of patients who did not experience GI symptoms/elevated liver enzymes and were admitted to the ICU was 2512. No statistically significant difference in ICU admission rate was noted between those who experienced GI symptoms/elevated liver enzymes and those who did not. The pooled proportion was 1.01 (95% CI 0.55 to 1.83) (figure 5).
Figure 5.
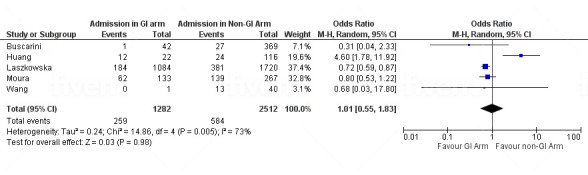
Forest plot showing odd ratio (OR) of intensive care unit admissions in patients with COVID-19 with and without GI symptoms/elevated liver enzymes. GI, gastrointestinal.
Discussion
This meta-analysis of 78 798 patients with COVID-19 found that GI symptoms/elevated liver enzymes are common in patients infected with SARS-CoV-2. Subgroup analysis found that no association between the presence of GI symptoms/elevated liver enzymes and mortality or ICU admission, which is similar to the finding of other meta-analyses.149 150
GI symptoms including abdominal pain, diarrhoea, nausea, vomiting, loss of appetite, loss of taste and elevated liver enzyme are among the presenting symptoms or laboratory abnormalities of SARS-CoV-2 infection found in this study. Diarrhoea was the most common GI symptom; this is particularly important because previous studies have shown that patients with diarrhoea on presentation have a higher stool RNA positivity and viral load than those without.22 36 151 One study showed that 44 of 153 patients with COVID-19 tested positive for the virus in the stools.148 In addition, a report of a patient with COVID-19 with positive faecal but negative pharyngeal and sputum viral tests has been described.33 Moreover, a meta-analysis concluded that SARS‐CoV‐2 is commonly present in stool samples or anal swabs in which the virus can persist for a long period after respiratory samples become negative and that the virus may be viable.152 This may imply that faecal oral route is a possible route of SARS-CoV-2 transmission.
The possibility of faecal oral transmission of SARS-CoV-2 emphasises the importance of frequent and proper hand hygiene. This is important in every clinical setting, but especially in low-resource areas with poor sanitation.38 Intuitively, proper handling of the excreta of patients with COVID-19 should still be strongly enforced, and sewage from hospitals should also be properly disinfected. The presence of the virus in the digestive tract also raises the concerns of COVID-19 infection in patients with established GI conditions, as well as potential faecal microbiota transplant donors.148 Nevertheless, the unknown effect of COVID-19 on patients with pre-existing GI diseases and its influence on treatment and outcome is a cause for concern. These implications warrant further investigation. The American Gastroenterological Association and joint society recommend the use of enhanced personal protective equipment, including the use of N95 (or N99) masks instead of surgical masks, for healthcare workers performing upper or lower GI procedures regardless of COVID-19 status.35
It is believed that the prevalence of GI symptoms is underestimated because the majority of studies included in our study reported GI symptoms only on the day of admission but not throughout the disease course. Furthermore, many earlier studies did not report on other GI symptoms except for diarrhoea.22 Based on these findings, clinicians must be aware that digestive symptoms, such as diarrhoea, may be a presenting feature of COVID-19 that can arise before respiratory symptoms and, on rare occasions, may be the only presenting manifestation of COVID-19.33
The pooled analysis showed that the overall mortality and GI mortality were 23.5% and 3.5%, respectively. However, it is important to emphasise that reporting of COVID-19 mortality in each country varies.153 Some countries do not depend on the availability of confirmed laboratory tests; instead, both probable and confirmed cases are used when calculating COVID-19 mortality.154
In this meta-analysis, a subanalysis of mortality in patients with GI symptoms/elevated liver enzymes varied between countries. This difference in GI mortality can be attributed to several reasons. Differences in reporting cases, case definition, and the mortality measure used might have a great role in this geographical variation. The available mortality data mostly reported as case fatality rate, which measures the number of deaths out of all confirmed cases.155 Furthermore, using case fatality rate is influenced by reporting and testing strategies in each country, where countries that do not have good reporting or intensive testing might miss a lot of confirmed cases and eventually overestimate mortality.153 In addition, it is well known that comorbidities increase the risk of death from COVID-19,154 and countries with the highest burden of chronic diseases had the highest COVID-19 mortality. Small sample size of the published GI mortality reports of some countries is another factor that can lead to inaccurate presentation of the actual GI mortality.
Our study did not show higher GI mortality among patients manifesting GI symptoms/elevated liver enzymes. However, any possible true difference in mortality may be worth further investigation among better defined patients with COVID-19 subgroups with GI symptoms/elevated liver enzymes because one study showed that prevalence of severe disease was more common in patients who had GI symptoms than those who did not.156 Our meta-analysis did not find a statistically significant association between patients with GI symptoms/elevated liver enzymes and ICU admission. However, to investigate such an association, it is important to consider other causes of elevated liver enzymes in patients admitted to ICU such as sepsis, hypoperfusion, hepatotoxic drugs, and parenteral nutrition.157
Strengths and limitations
Our study has several strengths. This is one of the more recent meta-analyses that summarises the literature on COVID-19 and the prevalence of overall and individual GI manifestations.149 158–161 The large patient population and comprehensive inclusion of 158 studies allow a more precise estimation of the prevalence of GI symptoms/elevated liver enzymes associated with COVID-19. Moreover, our search included studies over 1-year period, from 1 December 2019 up to 15 December 2020, which makes it more up-to-date and more inclusive of the recent evidence. Furthermore, our meta-analysis included studies from different countries and regions.
This study, however, is subject to some limitations. Most of the studies we base our analyses on are observational, single-arm cohorts. The lack of control groups and comparison arms can lead to bias due to confounding. Additionally, regarding mortality among patients with COVID-19, most studies reported mortality at the time of the study. In other words, studies did not report mortality over a specific period of time. Furthermore, most studies reported patients with COVID-19 who have been admitted to hospital, who are more likely to have severe disease, resulting in under-representation of patients with milder disease.
Conclusion
In this meta-analysis, we summarise the recent reports of GI symptoms/elevated liver enzymes among patients infected with SARS-CoV-2. GI symptoms/elevated liver enzymes are commonly observed in patients with COVID-19; therefore, clinicians should be aware that diarrhoea and nausea can be the only manifestations of patients with COVID-19. Our subanalysis showed that GI mortality among patients infected with SARS-CoV-2 varied based on geographical location. We also could not find a statistically significant association between ICU admission in patients with GI symptoms/elevated liver enzymes compared with those without GI symptoms/elevated liver enzymes. However, further investigation is warranted to better assess this possible association.
Acknowledgments
We acknowledge and thank all health care workers and front liners around the globe for their hard work and sacrifices made during this pandemic.
Footnotes
Twitter: @drmohamadshehab
Contributors: MS: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, and submission of the manuscript. FA: acquisition of data, analysis and interpretation of data, drafting of the manuscript.SS: acquisition of data and drafting of the manuscript. DA: acquisition of data and drafting of the manuscript. AB: critical revision of the manuscript for important intellectual content, statistical analysis, study supervision; he is also responsible for the overall work as a guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data generated or analysed during this study are included in this published article (and its supplementary information files).
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Centers for Disease Control and Prevention . Coronavirus disease 2019 (COVID-19), 2020. Available: https://www.cdc.gov/coronavirus/2019-ncov/cdcresponse/about-COVID-19.html#:~:text=On%20February%2011%2C%202020%2C%20the,and%20'D'%20for%20disease [Accessed 25 Dec 2020].
- 2.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Peng W, Zhu Y, et al. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: detection, mechanisms and treatment. Int J Antimicrob Agents 2020;55:105950. 10.1016/j.ijantimicag.2020.105950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Coronavirus Disease (COVID-19) – World HealthOrganization [online]. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQiAuJb_BRDJARIsAKkycUl7HGvUu5_W-YzjcWARo-V5Uy3YreqHt7_FFvJpd1hcYCzeWxeH20kaAswjEALw_wcB [Accessed 31 Dec 2020].
- 6.World Health Organization . SARS-CoV-2 variants, 2020. Available: https://www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ [Accessed 05 Jan 2021].
- 7.World Health Organization . Who coronavirus disease (COVID-19) Dashboard, 2020. Available: https://covid19.who.int/?gclid=CjwKCAjwiMj2BRBFEiwAYfTbCs-zYYng4rwUJqKD946CSFHwJgC2YP9erGKVDphwwdudJsa3uWTxaBoCdLUQAvD_BwE [Accessed 05 Jun 2020].
- 8.Luo C, Yao L, Zhang L, et al. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public Bath center in Huai'an, Jiangsu Province, China. JAMA Netw Open 2020;3:e204583. 10.1001/jamanetworkopen.2020.4583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morawska L, Milton DK. It is time to address airborne transmission of COVID-19. Clinical Infectious Diseases 2020:ciaa939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323:1406–7. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145–51. 10.3760/cma.j.issn.0254-6450.2020.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Lai C-C, Liu YH, Wang C-Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect 2020;53:404–12. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 2020;296:E15–25. 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W-jie, Ni Z-yi, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv 2020. [Google Scholar]
- 16.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712–6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O'Connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomised studies in meta-analyses, 2014. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 18.Hung IFN, Cheng VCC, Wu AKL, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis 2004;10:1550–7. 10.3201/eid1009.040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997–1001. 10.1136/gutjnl-2020-321013 [DOI] [PubMed] [Google Scholar]
- 20.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020;115:766–73. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Z, Zhao N, Shu Y, et al. Effect of gastrointestinal symptoms in patients with COVID-19. Gastroenterology 2020;158:2294–7. 10.1053/j.gastro.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus–Infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benvenuto D, Giovanetti M, Salemi M, et al. The global spread of 2019-nCoV: a molecular evolutionary analysis. Pathog Glob Health 2020;114:64–7. 10.1080/20477724.2020.1725339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Who coronavirus disease (COVID-19) Dashboard, 2020. Available: covid19.who.int/?gclid=CjwKCAjwiMj2BRBFEiwAYfTbCs-zYYng4rwUJqKD946CSFHwJgC2YP9erGKVDphwwdudJsa3uWTxaBoCdLUQAvD_BwE
- 26.Morawska L, Milton DK. It is time to address airborne transmission of coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020;71:2311–3. 10.1093/cid/ciaa939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323:1406–7. 10.1001/jama.2020.2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W-jie, Ni Z-yi, Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv 2020. [Google Scholar]
- 29.Qi F, Qian S, Zhang S, et al. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun 2020;526:135–40. 10.1016/j.bbrc.2020.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung IFN, Cheng VCC, Wu AKL, et al. Viral loads in clinical specimens and SARS manifestations. Emerg Infect Dis 2004;10:1550–7. 10.3201/eid1009.040058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831–3. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen L, Lou J, Bai Y, et al. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol 2020;115:790. 10.14309/ajg.0000000000000610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut 2020;69:1141–3. 10.1136/gutjnl-2020-320832 [DOI] [PubMed] [Google Scholar]
- 35.American college of gastroenterology . Joint Gi Society statement: use of personal protective equipment in Gi endoscopy, 2020. Available: https://gi.org/2020/04/01/joint-gi-society-message-on-ppe-during-COVID-19/
- 36.Zhou J, Li C, Zhao G, et al. Human intestinal tract serves as an alternative infection route for middle East respiratory syndrome coronavirus. Sci Adv 2017;3:eaao4966. 10.1126/sciadv.aao4966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic Society and infectious diseases Society of America. Am J Respir Crit Care Med 2019;200:e45–67. 10.1164/rccm.201908-1581ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020;159:81–95. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.PaC A, Chen H. Clinical features of 2019 novel coronavirus pneumonia presented gastrointestinal symptoms but without fever onset. Lancet 2020. https://ssrn.com/abstract=3532530 [Google Scholar]
- 40.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang D, Lin M, Wei L, et al. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 2020;323:1092. 10.1001/jama.2020.1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The Lancet 2020;395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Quan B, Li X, et al. A report of clinical diagnosis and treatment of nine cases of coronavirus disease 2019. J Med Virol 2020;92:683–7. 10.1002/jmv.25755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 2020;48:543–51. 10.1007/s15010-020-01432-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cholankeril G, Podboy A, Aivaliotis VI, et al. High prevalence of concurrent gastrointestinal manifestations in patients with severe acute respiratory syndrome coronavirus 2: early experience from California. Gastroenterology 2020;159:775–7. 10.1053/j.gastro.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention . Early epidemiological and clinical characteristics of 28 cases of coronavirus disease in South Korea. Osong Public Health Res Perspect 2020;11:8–14. 10.24171/j.phrp.2020.11.1.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.COVID-19 National Incident Room Surveillance Team . COVID-19, Australia: epidemiology report 7 (reporting week ending 19:00 AEDT 14 March 2020). Commun Dis Intell 2020:44. 10.33321/cdi.2020.44.23 [DOI] [PubMed] [Google Scholar]
- 48.Fan H, Zhang L, Huang B. Retrospective analysis of clinical features in 101 death cases with COVID-19 2020. [DOI] [PMC free article] [PubMed]
- 49.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 2020;20:1849–58. 10.1111/ajt.15929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gritti G, Raimondi F, Ripamonti D. Use of siltuximab in patients with COVID-19 pneumonia requiring ventilatory support. MedRxiv 2020. [Google Scholar]
- 51.Hajifathalian K, Krisko T, Mehta A, et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology 2020;159:1137–40. 10.1053/j.gastro.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol 2020;115:916–23. 10.14309/ajg.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsih W-H, Cheng M-Y, Ho M-W, et al. Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan. J Microbiol Immunol Infect 2020;53:459–66. 10.1016/j.jmii.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang R, Xia J, Chen Y, et al. A family cluster of SARS-CoV-2 infection involving 11 patients in Nanjing, China. Lancet Infect Dis 2020;20:534–5. 10.1016/S1473-3099(20)30147-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang W-H, Teng L-C, Yeh T-K, et al. 2019 novel coronavirus disease (COVID-19) in Taiwan: reports of two cases from Wuhan, China. J Microbiol Immunol Infect 2020;53:481–4. 10.1016/j.jmii.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang Y, Tu M, Wang S, et al. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis 2020;36:101606. 10.1016/j.tmaid.2020.101606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin X, Lian J-S, Hu J-H, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020;69:1002–9. 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci 2020;35:e142. 10.3346/jkms.2020.35.e142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klopfenstein T, Kadiane-Oussou N'dri Juliette, Royer P-Y, et al. Diarrhea: an underestimated symptom in coronavirus disease 2019. Clin Res Hepatol Gastroenterol 2020;44:282–3. 10.1016/j.clinre.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kluytmans M, Buiting A, Pas S. SARS-COV2 infection in 86 healthcare workers in two Dutch hospitals in March 2020. medRxiv 2020. [Google Scholar]
- 61.Kuang Y, Zhang H, Zhou R. Epidemiological and clinical characteristics of 944 cases of 2019 novel coronavirus infection of Non-COVID-19 exporting City, Zhejiang, China. Zhejiang, China (February 20, 2020) 2020.
- 62.Liu K, Fang Y-Y, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J 2020;133:1025–31. 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kujawski SA, Wong KK, Collins JP. First 12 patients with coronavirus disease 2019 (COVID-19) in the United States. MedRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–61. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020;55:327–31. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997–1001. 10.1136/gutjnl-2020-321013 [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–74. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo S, Zhang X, Xu H. Don't overlook digestive symptoms in patients with 2019 novel coronavirus disease (COVID-19). Clin Gastroenterol Hepatol 2020;18:1636–7. 10.1016/j.cgh.2020.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nobel YR, Phipps M, Zucker J, et al. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology 2020;159:373–5. 10.1053/j.gastro.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020;295:715–21. 10.1148/radiol.2020200370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China. Am J Gastroenterol 2020;115:766–73. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pung R, Chiew CJ, Young BE, et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet 2020;395:1039–46. 10.1016/S0140-6736(20)30528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 2020;159:765–7. 10.1053/j.gastro.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren L-L, Wang Y-M, Wu Z-Q, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J 2020;133:1015–24. 10.1097/CM9.0000000000000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shu L, Wang X, Li M. Clinical characteristics of 545 cases confirmed COVID-19 in Wuhan stadium cabin Hospital. available at SSRN 3552844, 2020. Available: https://ssrn.com/abstract=3552844
- 78.Siegel A, Chang PJ, Jarou ZJ, et al. Lung base findings of coronavirus disease (COVID-19) on abdominal CT in patients with predominant gastrointestinal symptoms. AJR Am J Roentgenol 2020;215:607–9. 10.2214/AJR.20.23232 [DOI] [PubMed] [Google Scholar]
- 79.Song F, Shi N, Shan F, et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020;295:210–7. 10.1148/radiol.2020200274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID-19) in the who European region, 24 January to 21 February 2020. Euro Surveill 2020;25. 10.2807/1560-7917.ES.2020.25.9.2000178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabata S, Imai K, Kawano S. The clinical characteristics of COVID-19: a retrospective analysis of 104 patients from the outbreak on board the diamond Princess cruise ship in Japan. medRxiv 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wan Y, Li J, Shen L, et al. Enteric involvement in hospitalised patients with COVID-19 outside Wuhan. Lancet Gastroenterol Hepatol 2020;5:534–5. 10.1016/S2468-1253(20)30118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Gao Y-H, Lou L-L, et al. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur Respir J 2020;55:2000398. 10.1183/13993003.00398-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang L, Duan Y, Zhang W, et al. Epidemiologic and clinical characteristics of 26 cases of COVID-19 arising from Patient-to-Patient transmission in Liaocheng, China. Clin Epidemiol 2020;12:387–91. 10.2147/CLEP.S249903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020;80:639–45. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang Hospital. Clin Microbiol Infect 2020;26:1063–8. 10.1016/j.cmi.2020.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang Z, Chen X, Lu Y, et al. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends 2020;14:64–8. 10.5582/bst.2020.01030 [DOI] [PubMed] [Google Scholar]
- 88.Wei X-S, Wang X, Niu Y-R. Clinical characteristics of SARS-COV2 infected pneumonia with diarrhea 2020. Available at SSRN 3546120.
- 89.Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020;581:465–9. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 90.Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of coronavirus disease 2019 (COVID-19) in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis 2020;71:706–12. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu J, Wu X, Zeng W, et al. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol 2020;55:257–61. 10.1097/RLI.0000000000000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020;5:434–5. 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xia X-Y, Wu J, Liu H-L, et al. Epidemiological and initial clinical characteristics of patients with family aggregation of COVID-19. J Clin Virol 2020;127:104360. 10.1016/j.jcv.2020.104360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831–3. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie H, Zhao J, Lian N, et al. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int 2020;40:1321–6. 10.1111/liv.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong Y, Sun D, Liu Y, et al. Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol 2020;55:332–9. 10.1097/RLI.0000000000000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 2020;47:1275–80. 10.1007/s00259-020-04735-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xu X-W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang F, Shi S, Zhu J, et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol 2020;92:2067–73. 10.1002/jmv.25972 [DOI] [PubMed] [Google Scholar]
- 100.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323:1488. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis 2020;221:1757–61. 10.1093/infdis/jiaa077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020;127:104364. 10.1016/j.jcv.2020.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–41. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol 2020;92:680–2. 10.1002/jmv.25742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao D, Yao F, Wang L, et al. A comparative study on the clinical features of coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin Infect Dis 2020;71:756–61. 10.1093/cid/ciaa247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao W, Zhong Z, Xie X, et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol 2020;214:1072–7. 10.2214/AJR.20.22976 [DOI] [PubMed] [Google Scholar]
- 108.Zhao X-Y, Xu X-X, Yin H-S, et al. Clinical characteristics of patients with 2019 coronavirus disease in a non-Wuhan area of Hubei Province, China: a retrospective study. BMC Infect Dis 2020;20:311. 10.1186/s12879-020-05010-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou S, Wang Y, Zhu T, et al. Ct features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR Am J Roentgenol 2020;214:1287–94. 10.2214/AJR.20.22975 [DOI] [PubMed] [Google Scholar]
- 111.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177–9. 10.1056/NEJMc2001737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med Overseas Ed 2020;382:929–36. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khader M, Al Bishawi A, Kambal A, et al. SARS-CoV-2 infection presenting as colitis with chest and abdomen CT findings. Radiol Case Rep 2020;15:2427–32. 10.1016/j.radcr.2020.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Grande G, Cocca S, Russo S, et al. COVID-19 and the gastrointestinal system: lesions beyond the symptoms? ACG Case Rep J 2020;7:e00464. 10.14309/crj.0000000000000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gulen M, Satar S. Uncommon presentation of COVID-19: gastrointestinal bleeding. Clin Res Hepatol Gastroenterol 2020;44:e72–6. 10.1016/j.clinre.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cholankeril G, Podboy A, Aivaliotis VI, et al. Association of digestive symptoms and hospitalization in patients with SARS-CoV-2 infection. Am J Gastroenterol 2020;115:1129–32. 10.14309/ajg.0000000000000712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cavaliere K, Levine C, Wander P, et al. Management of upper Gi bleeding in patients with COVID-19 pneumonia. Gastrointest Endosc 2020;92:454–5. 10.1016/j.gie.2020.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hassani AH, Beheshti A, Almasi F, et al. Unusual gastrointestinal manifestations of COVID-19: two case reports. Gastroenterol Hepatol Bed Bench 2020;13:410–4. [PMC free article] [PubMed] [Google Scholar]
- 119.Wu C-Y, Yu X-P, Ma AHY, et al. Coronavirus disease 19 with gastrointestinal symptoms as initial manifestations: a case report. J Int Med Res 2020;48:300060520952256. 10.1177/0300060520952256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang K, Luo J, Tan F, et al. Acute pancreatitis as the initial manifestation in 2 cases of COVID-19 in Wuhan, China. Open Forum Infect Dis 2020;7:ofaa324. 10.1093/ofid/ofaa324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dietrich CG, Hübner D, Marx G, et al. Primary presentation of COVID-19 solely with gastrointestinal symptoms: a problem for the containment of the disease. Eur J Gastroenterol Hepatol 2020;32:1475–8. 10.1097/MEG.0000000000001922 [DOI] [PubMed] [Google Scholar]
- 122.Kandasamy S. An unusual presentation of COVID-19: acute pancreatitis. Ann Hepatobiliary Pancreat Surg 2020;24:539–41. 10.14701/ahbps.2020.24.4.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wagner J, Garcia-Rodriguez V, Yu A, et al. The model for end-stage liver Disease-Sodium score at admission is prognostic of Covid-19 disease severity. SN Compr Clin Med 2020:1–5. 10.1007/s42399-020-00534-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wahab SF, Løgstrup BB. Atypical manifestations of COVID-19 in general practice: a case of gastrointestinal symptoms. BMJ Case Rep 2020;13:e237520. 10.1136/bcr-2020-237520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent acute pancreatitis in a patient with COVID-19 infection. Am J Case Rep 2020;21:e927076. 10.12659/AJCR.927076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Docherty AB, Green CA. Featuresof 16, 749 hospitalised UK patients with COVID-19 using the ISARIC who clinical characterization protocol. MedRvix 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.CDC COVID-19 Response Team . Coronavirus Disease 2019 in Children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep 2020;69:422–6. 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.CDC COVID-19 Response Team . Characteristics of Health Care Personnel with COVID-19 - United States, February 12-April 9, 2020. MMWR Morb Mortal Wkly Rep 2020;69:477–81. 10.15585/mmwr.mm6915e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Borobia A, Carcas A, Arnalich F, et al. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J Clin Med 2020;9:1733. 10.3390/jcm9061733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Elmunzer BJ, Spitzer RL, Foster LD. DigestiveManifestations in patients hospitalized with COVID-19. MedRxiv 2020. [Google Scholar]
- 131.Gil-Rodrigo A, Miró Òscar, Piñera P, et al. Analysis of clinical characteristics and outcomes in patients with COVID-19 based on a series of 1000 patients treated in Spanish emergency departments. Emergencias 2020;32:233–41. [PubMed] [Google Scholar]
- 132.Livanos AE, Jha D, Cossarini F, et al. Gastrointestinal involvement attenuates COVID-19 severity and mortality. medRxiv 2020. 10.1101/2020.09.07.20187666. [Epub ahead of print: 09 Sep 2020]. [DOI] [Google Scholar]
- 133.Tsibouris P, Ekmektzoglou K, Agorogianni A, et al. Gastrointestinal involvement in COVID-19 patients: a retrospective study from a Greek COVID-19 referral hospital. Ann Gastroenterol 2020;33:465–72. 10.20524/aog.2020.0514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aghemo A, Piovani D, Parigi TL, et al. COVID-19 digestive system involvement and clinical outcomes in a large academic hospital in Milan, Italy. Clin Gastroenterol Hepatol 2020;18:2366–8. 10.1016/j.cgh.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020. 10.1093/cid/ciaa270. [Epub ahead of print: 16 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fanelli V, Fiorentino M, Cantaluppi V, et al. Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 2020;24:155. 10.1186/s13054-020-02872-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Colaneri M, Sacchi P, Zuccaro V, et al. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill 2020;25:2000460. 10.2807/1560-7917.ES.2020.25.16.2000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buscarini E, Manfredi G, Brambilla G, et al. Gi symptoms as early signs of COVID-19 in hospitalised Italian patients. Gut 2020;69:1547–8. 10.1136/gutjnl-2020-321434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hundt MA, Deng Y, Ciarleglio MM, et al. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. Hospital network. Hepatology 2020;72:1169–76. 10.1002/hep.31487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Laszkowska M, Faye AS, et al. Disease course and outcomes of COVID-19 among hospitalized patients with gastrointestinal manifestations. Clin Gastroenterol Hepatol 2020:31367–7. 10.1016/j.cgh.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ferm S, Fisher C, Pakala T, et al. Analysis of gastrointestinal and hepatic manifestations of SARS-CoV-2 infection in 892 patients in queens, NY. Clin Gastroenterol Hepatol 2020;18:2378–9. 10.1016/j.cgh.2020.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sulaiman T, Algharawi AA, Idrees M, et al. The prevalence of gastrointestinal symptoms among patients with COVID-19 and the effect on the severity of the disease. JGH Open 2020;4:1162–6. 10.1002/jgh3.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suleyman G, Fadel RA, Malette KM, et al. Clinical characteristics and morbidity associated with coronavirus disease 2019 in a series of patients in metropolitan Detroit. JAMA Netw Open 2020;3:e2012270. 10.1001/jamanetworkopen.2020.12270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhan T, Liu M, Tang Y, et al. Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J Int Med Res 2020;48:300060520949039. 10.1177/0300060520949039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Moura DTHde, Proença IM, McCarty TR, et al. Gastrointestinal manifestations and associated health outcomes of COVID-19: a Brazilian experience from the largest South American public hospital. Clinics 2020;75:e2271. 10.6061/clinics/2020/e2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lei P, Zhang L, Han P, et al. Liver injury in patients with COVID-19: clinical profiles, CT findings, the correlation of the severity with liver injury. Hepatol Int 2020;14:733–42. 10.1007/s12072-020-10087-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Papa A, Covino M, Pizzolante F, et al. Gastrointestinal symptoms and digestive comorbidities in an Italian cohort of patients with COVID-19. Eur Rev Med Pharmacol Sci 2020;24:7506–11. 10.26355/eurrev_202007_21923 [DOI] [PubMed] [Google Scholar]
- 148.Hindson J. COVID-19: faecal-oral transmission? Nat Rev Gastroenterol Hepatol 2020;17:259. 10.1038/s41575-020-0295-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tariq R, Saha S, Furqan F, et al. Prevalence and mortality of COVID-19 patients with gastrointestinal symptoms: a systematic review and meta-analysis. Mayo Clin Proc 2020;95:1632–48. 10.1016/j.mayocp.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mao R, Qiu Y, He J-S, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020;5:667–78. 10.1016/S2468-1253(20)30126-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sultan S, Altayar O, Siddique SM, et al. AGA Institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology 2020;159:320–34. 10.1053/j.gastro.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van Doorn AS, Meijer B, Frampton CMA, et al. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment Pharmacol Ther 2020;52:1276–88. 10.1111/apt.16036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.World Health Organization . Estimating mortality from COVID-19. Available: https://www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19 [Accessed 27 Dec 2020].
- 154.Sorci G, Faivre B, Morand S. Explaining among-country variation in COVID-19 case fatality rate. Sci Rep 2020;10:18909. 10.1038/s41598-020-75848-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.World Health Organization . Health systems response monitor (HSRM), 2020Published April. Available: https://analysis.covid19healthsystem.org/index.php/2020/04/16/how-do-covid-19-testing-criteria-differ-across-countries/ [Accessed December 29, 2020].
- 156.Zhang Y, Ma P, Zhang X, et al. Association of digestive symptoms with severity and mortality of COVID-19: a protocol for systematic review and meta-analysis. Medicine 2020;99:e22736. 10.1097/MD.0000000000022736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Koch A, Streetz K, Tischendorf J, et al. [Abnormal liver function tests in the intensive care unit]. Med Klin Intensivmed Notfmed 2013;108:599–610. 10.1007/s00063-013-0287-2 [DOI] [PubMed] [Google Scholar]
- 158.Akin H, Kurt R, Tufan F, et al. Newly reported studies on the increase in gastrointestinal symptom prevalence withCOVID-19 infection: a comprehensive systematic review and meta-analysis. Diseases 2020;8:41. 10.3390/diseases8040041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Aziz M, Haghbin H, Lee-Smith W, et al. Gastrointestinal predictors of severe COVID-19: systematic review and meta-analysis. Ann Gastroenterol 2020;33:615–30. 10.20524/aog.2020.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dong Z-Y, Xiang B-J, Jiang M, et al. The prevalence of gastrointestinal symptoms, abnormal liver function, digestive system disease and liver disease in COVID-19 infection: a systematic review and meta-analysis. J Clin Gastroenterol 2021;55:67–76. 10.1097/MCG.0000000000001424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zarifian A, Zamiri Bidary M, Arekhi S, et al. Gastrointestinal and hepatic abnormalities in patients with confirmed COVID-19: a systematic review and meta-analysis. J Med Virol 2020 10.1002/jmv.26314. [Epub ahead of print: 18 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgast-2020-000571supp001.pdf (2.1MB, pdf)