Abstract
Background
Genetic testing in hypertrophic cardiomyopathy (HCM) is a published guideline-based recommendation. The diagnostic yield of genetic testing and corresponding HCM-associated genes have been largely documented by single center studies and carefully selected patient cohorts. Our goal was to evaluate the diagnostic yield of genetic testing in a heterogeneous cohort of patients with a clinical suspicion of HCM, referred for genetic testing from multiple centers around the world.
Methods
A retrospective review of patients with a suspected clinical diagnosis of HCM referred for genetic testing at Blueprint Genetics was undertaken. The analysis included syndromic, myopathic and metabolic etiologies. Genetic test results and variant classifications were extracted from the database. Variants classified as pathogenic (P) or likely pathogenic (LP) were considered diagnostic.
Results
A total of 1376 samples were analyzed. Three hundred and sixty-nine tests were diagnostic (26.8%); 373 P or LP variants were identified. Only one copy number variant was identified. The majority of diagnostic variants involved genes encoding the sarcomere (85.0%) followed by 4.3% of diagnostic variants identified in the RASopathy genes. Two percent of diagnostic variants were in genes associated with a cardiomyopathy other than HCM or an inherited arrhythmia. Clinical variables that increased the likelihood of identifying a diagnostic variant included: an earlier age at diagnosis (p < 0.0001), a higher maximum wall thickness (MWT) (p < 0.0001), a positive family history (p < 0.0001), the absence of hypertension (p = 0.0002), and the presence of an implantable cardioverter-defibrillator (ICD) (p = 0.0004).
Conclusion
The diagnostic yield of genetic testing in this heterogeneous cohort of patients with a clinical suspicion of HCM is lower than what has been reported in well-characterized patient cohorts. We report the highest yield of diagnostic variants in the RASopathy genes identified in a laboratory cohort of HCM patients to date. The spectrum of genes implicated in this unselected cohort highlights the importance of pre-and post-test counseling when offering genetic testing to the broad HCM population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-021-01927-5.
Keywords: Hypertrophic cardiomyopathy, Genetic testing, Next generation sequencing, Diagnosis, Counseling
Background
Hypertrophic cardiomyopathy (HCM) is an inherited cardiac disorder that is defined by the presence of increased left ventricular (LV) wall thickness that is not solely explained by loading conditions [1]. In terms of genetic conditions, it is relatively common; historical studies have estimated a prevalence of 1 in 500 [2], but recent work demonstrates this to be closer to 1 in 200 [3]. HCM is clinically heterogenous as individuals with severe hypertrophy may remain asymptomatic but those with mild hypertrophy may develop significant arrhythmias, heart failure and/or sudden death. Classic HCM is primarily caused by variants in the genes encoding proteins of the cardiac sarcomere [4] and follows an autosomal dominant pattern of inheritance. Although rarer, a number of multisystem diseases are genocopies of HCM, which require additional surveillance, treatment options, and may follow a different inheritance pattern. Some examples include Fabry disease, PRKAG2-related disease, Danon disease, neuromuscular diseases, mitochondrial myopathies and the RASopathies such as Noonan syndrome. Obtaining a correct diagnosis is therefore of utmost importance for medical management purposes, but also for the identification of at-risk family members who require ongoing screening.
Genetic testing provides one way in which a diagnosis can be confirmed in this patient population. Although clinical genetic testing for HCM has been available since 2003 [4], its diagnostic yield in patients with HCM has remained relatively constant, despite advances in testing technology, broader gene inclusion, established reference databases, and guidelines to improve variant interpretation.
Clinical and laboratory cohorts from the pre-next generation sequencing (NGS) era report a yield in the range of 30–40% [5–8] similar to what has been reported in studies utilizing NGS [9–13]. Variant interpretation in most of the older published studies (prior to 2015) did not follow a systematic variant classification scheme such as the ACMG (American College of Medical Genetics and Genomics)—AMP (Association for Molecular Pathology) 2015 guidelines [14]. Integration of copy number variant (CNV) analysis has not significantly increased the yield [11, 15]. The sensitivity of CNV analysis using NGS is constantly improving; it is highly dependent on bioinformatics pipeline and data interpretation. Smaller variants such as exon-level deletions or duplications may not always have been detected [16].
Although NGS has enabled high throughput testing, studies with clinical cohorts have repeatedly shown that increasing the number of analyzed genes does not significantly increase the yield [12, 17]. Recent work by the HCM ClinGen Gene Curation Expert Panel determined that only 46% of evaluated HCM/intrinsic cardiomyopathy and syndromic genes associated with isolated left ventricular hypertrophy (LVH) routinely included on testing panels were categorized as having a definite, strong or moderate association with the disease [18]. Among these are three RASopathy genes (RAF1, RIT1 and PTPN11), which have been shown to cause isolated LVH in some patients, but have only been comprehensively evaluated in a small number of studies [19, 20]. An absence of syndromic features in some patients could be explained by mild syndromic gestalt and/or low to intermediate level mosaicism, which may not be detected by certain NGS platforms without sufficient read depth.
Studies of well-defined clinical cohorts have reported similar diagnostic yields as presumably more heterogeneous referral laboratory cohorts. However, one of the largest HCM laboratory cohorts published to date [9] excluded patients with left ventricular hypertrophy explained by a clinical syndrome. Due to their more stringent inclusion criteria, the reported yield (32%) may not be completely representative of a broad HCM population.
Clinical variables that increase the likelihood of a positive genetic test result include a positive family history [5–7, 9, 21], an earlier age of onset/diagnosis [5–7, 9, 21], maximal left ventricular wall thickness (MLVWT) [6], and specific types of septal morphology [8]. While these factors have been compiled into genotype-predictor scores by two different groups [8, 22], they have been primarily used and validated in well-defined cohorts.
Genetic testing for any patient with HCM has become a guideline-based recommendation [1, 23], suggesting that it may be increasingly performed in patients with a lower index of suspicion and also identify some who fall within the nonfamilial HCM group, which has important implications for the follow up care of family members [24]. When compared with a cohort of patients explicitly meeting diagnostic criteria for HCM, a heterogeneous HCM population specifically ascertained through referral for genetic testing may have a lower diagnostic yield, and a higher rate of clinically significant variants in genes outside those encoding the proteins of the sarcomere.
The aim of this study is to report on the diagnostic yield of genetic testing, outline the genes in which diagnostic variants were identified, by applying a systematic ACMG/AMP-compatible variant classification scheme and determine which clinical variables influence the likelihood of a diagnostic test result in a heterogeneous HCM cohort evaluated over a 5-year period.
Methods
Study patients
The cohort in this study included 1376 patients having been identified as having a suspected diagnosis of HCM by their ordering provider in their laboratory requisiton. HCM diagnostic criteria was not utilized to include or exclude patients. Complete clinical data was not available to verify the diagnoses. Only patients for whom panel testing was ordered were included. The patients were presumed to be affected and unrelated. Demographic, clinical and diagnosis information including age, sex, family history, documented arrhythmias, type of medical device and patient outcomes was obtained from requisitions completed by ordering providers. This work was reviewed by the Western Institutional Review Board (IRB) and received an exemption determination.
Genetic testing
Patients underwent testing as ordered by their healthcare provider for either an HCM panel (16, 19 or 38 genes), a broad cardiomyopathy panel (72, 101, 103, 134 or 155 genes) or a broad cardiology panel (133, 165 or 184 genes associated with arrhythmias/cardiomyopathies) (Additional file 1). All of the genes on the HCM panel are included in the broad cardiomyopathy and broad cardiology panels. A total of 1133 (82.3%) cases were analyzed using the oligonucleotide-selective sequencing (OS-Seq™) (25) NGS method on the NextSeq sequencing system (Illumina). The remaining patients (17.7%) were analyzed using an in-house tailored Integrated DNA Technologies (IDT) based whole exome sequencing platform run on the NovaSeq sequencing system (Illumina). In the analysis, mean sequencing depth was > 150× and > 99% of target nucleotides were covered with > 20× sequencing depth for all assays. The target nucleotides include all protein coding exons of the genes on the panels, as well as 20 base pairs (bp) inside each intron/exon boundary. Later versions of the panels were customized by adding oligonucleotides targeting deep intronic variants (≥ 20 bp from the intron/exon boundary) and non-coding variants [promoter region, 5′ or 3′ untranslated regions (UTR)] that have been reported as disease causing in association with cardiomyopathy and arrhythmia (Additional file 1). The sequence variant analysis pipeline has been validated in a CLIA (Clinical Laboratory Improvement Amendments) and CAP (College of American Pathologists) accredited Blueprint Genetics diagnostic laboratory. Bi-directional Sanger sequencing was used to confirm likely pathogenic (LP) and pathogenic (P) sequencing variants whenever stringent quality criteria for a true positive call were not met. The series of quality criteria utilized include a variant call quality score, variant genomic location, sequence content, and integrative genomics viewer visual analysis. This algorithm was established based on the outcome of an internal validation performed in the CLIA and CAP accredited Blueprint Genetics diagnostic laboratory.
Copy number variant analysis
CNV analysis was performed bioinformatically from the NGS data using a bioinformatic pipeline; one component used for calling is CNVkit and another is an in-house developed proprietary technology. CNVs were confirmed using quantitative polymerase chain reaction (qPCR) technology. The CNV analysis pipeline has been validated in the CLIA and CAP accredited Blueprint Genetics diagnostic laboratory.
Interpretation of test results
Variants were classified according to an adaptation of the ACMG/AMP guidelines [14] as outlined in the Blueprint Genetics website (https://blueprintgenetics.com/variant-classification/). Tests were considered diagnostic if the variants were classified as likely pathogenic (LP) or pathogenic (P). The Blueprint Genetics classification scheme is similar to the ACMG/AMP guidelines [14] in that multiple independent lines of evidence must be met (e.g. rare in population databases, predicted deleterious by in silico software, segregation with disease, de novo in a patient with no family history, damaging impact shown in well-established functional studies, etc.) to achieve a likely pathogenic or pathogenic classification, and several of these criteria are equivalent in both schemes. For example, LP missense variants are most often rare in population databases, predicted deleterious by in silico software tools, are reported in affected individuals/segregate in families and demonstrate a clear gene-phenotype association.
Statistical analysis
Comparisons between groups were performed with either Fisher’s exact or Chi-Square test for categorical variables, as appropriate, and unpaired T-test for normally distributed continuous variables. P-values less than 0.05 were considered statistically significant.
Results
Genetic testing was performed on 1376 cases of patients with a clinical suspicion of HCM. LP/P variants were identified in 369 cases, resulting in a diagnostic yield of 26.8%. Demographic and clinical variables are outlined in Table 1. LP/P variants were identified in 30.8% (151/491) of women and 24.7% (218/884) of men (p = 0.015). In one case (n = 1), the patient’s gender was not specified. A broad cardiomyopathy panel was ordered for 51.1% of cases (n = 703); an HCM specific panel was ordered for 45.9% (n = 632), and the remainder underwent a broad cardiology (cardiomyopathy and arrhythmia genes) panel (n = 41). The diagnostic yield was highest for the broad cardiology panel (31.7%, n = 13), but the overall yield was not significantly higher when compared to the HCM-specific panel (24.4%, n = 154, p = 0.29) or the broad cardiomyopathy panel (28.7%, n = 202, p = 0.68) (Table 2). Both sequencing and deletion/duplication analysis were completed in 40.3% of cases (n = 554); the remainder had sequence variant analysis alone.
Table 1.
Patient demographic and clinical variables
| Number of cases | % | |
|---|---|---|
| Age at diagnosis (n = 769) | ||
| Infant (< 1 year) | 35 | 4.6 |
| Child (1–17 years) | 72 | 9.4 |
| Adult (≥ 18 years) | 662 | 86.1 |
| Average | 769 | 44.7 |
| Gender (n = 1376) | ||
| Female | 491 | 35.7 |
| Male | 884 | 64.2 |
| Unknown/not provided | 1 | 0.1 |
| Positive family history (n = 1376) | 421 | 30.6 |
| Clinical findings | ||
| Maximum wall thickness > 16 years (n = 849) | ||
| ≤ 12 mm | 39 | 4.6 |
| 13–14 mm | 45 | 5.3 |
| 15–20 mm | 457 | 53.8 |
| 21–25 mm | 228 | 26.9 |
| ≥ 26 mm | 80 | 9.4 |
| Hypertension (n = 1376) | 158 | 11.5 |
| Aortic stenosis (n = 1376) | 8 | 0.6 |
| AV block (n = 1376) | 15 | 1.1 |
| Ventricular tachycardia (n = 1376) | 115 | 8.4 |
| Resuscitated cardiac arrest (n = 1376) | 42 | 3.1 |
| ICD (n = 1376) | 108 | 7.8 |
| Heart transplantation (n = 1376) | 18 | 1.3 |
AV Block for atrioventricular block, ICD for implantable cardioverter-defibrillator
Table 2.
Overall diagnostic yield by panel type
| Panel type | Number of genes/panel | Number of panels ordered (% of total) | Number of diagnostic tests | Number of diagnostic variants | Diagnostic yield (%) |
|---|---|---|---|---|---|
| Broad cardiomyopathy | 72–155 | 703 (51%) | 202 | 205 | 28.7 |
| Broad cardiology | 133–184 | 41 (3%) | 13 | 13 | 31.7 |
| HCM | 16–38 | 632 (46%) | 154 | 155 | 24.4 |
| Total | 1376 | 369 | 373 | 26.8 |
Overall diagnostic yield presented by panel type. Percentages in column “number of panels ordered” represent the proportion of all panels ordered (n = 1376)
HCM for hypertrophic cardiomyopathy
Variant profile
Altogether, 373 P/LP variants were identified in 31 unique genes (Additional file 2). Most (85.0%, n = 317) of the diagnostic variants were in genes encoding sarcomere proteins, followed by the RASopathy genes (4.3%, n = 16), non-sarcomere HCM genes (4.3%, n = 16), metabolic/storage disease genes (3.5%, n = 13) and other cardiomyopathy genes (1.3%, n = 5). The remainder were in genes associated with myopathy, arrhythmias/channelopathies and neurofibromatosis (n = 6).
The HCM-specific panel yielded the highest proportion of sarcomere variants (92.3%, n = 143) compared to the broad cardiomyopathy (80.5%, n = 165) and broad cardiology (69.2%, n = 9) panels. The broad cardiology panel yielded the highest proportion of diagnostic variants in non-sarcomere HCM genes (15.4%, n = 2) compared to the broad cardiomyopathy (5.9% n = 12) and HCM-specific panel (1.3% n = 2).
According to the ClinGen gene validity evaluation [18], 95.4% (n = 356) of LP/P variants were identified in genes that are definitively or moderately associated with HCM and LVH, an overall yield of 25.8% for the cohort. The remaining variants (n = 17) were in genes that have not been evaluated or have limited evidence of an association with HCM/LVH (Fig. 1).
Fig. 1.
Distribution of LP/P gene by ClinGen HCM Gene Disease Association. Bar chart demonstrates the distribution of LP/P gene by ClinGen HCM Gene Disease Association. The Y axis indicates percentage of all diagnostic variants (n = 373) and the X axis indicates the ClinGen disease association. LP for likely pathogenic, P for pathogenic and HCM for hypertrophic cardiomyopathy. Genes included in sections: Definitive: ACTC1, MYH7, MYL2, MYL3, TNNI3, TNNT2, TPM1, MYBPC3, PLN; Definitive syndromic: DES, FHL1, FLNC, GLA, LAMP2, PRKAG2, RAF1, TTR; Moderate: JPH2; Limited: TTN, RYR2; Not evaluated: CPT2, DSG2, HRAS, JUP, LMNA, MAP2K1, NF1, SCN5A, SHOC2, TRPM4
LP/P variants were seen most often in MYBPC3 (39.7%, n = 148) MYH7 (29.0%, n = 108) followed by TPM1 (8.0%, n = 30), TNNI3 (2.9%, n = 11), JPH2 (2.7%, n = 10), TNNT2 (2.4%, n = 9), RAF1 (2.1%, n = 8) and GLA (1.6%, n = 6). The remaining 23 genes each had five variants or less (Fig. 2).
Fig. 2.
Distribution of Diagnostic Variants by Gene. Bar chart demonstrates the distribution of diagnostic variants by gene as a percentage of total variants (n = 373). The Y axis indicates the percentage of all diagnostic variants (rounded to the nearest whole percent) and the X axis indicates the gene. Section titled “Other” includes genes that had five variants or less detected. Genes with ≤ 5 variants detected: MYL2 (n = 5), PTPN11 (n = 4), DES (n = 3), TTN (n = 3), LMNA (n = 2), FHL1 (n = 2), FLNC (n = 2), HRAS (n = 2), LAMP2 (n = 2), MYL3 (n = 2), PRKAG2 (n = 2), TTR (n = 2), DSG2 (n = 2), CPT2 (n = 1), MAP2K1 (n = 1), SHOC2 (n = 1), ACTC1 (n = 1), PLN (n = 1), TRPM4 (n = 1), JUP (n = 1), NF1 (n = 1), RYR2 (n = 1), SCN5A (n = 1)
Loss of function variants in MYBPC3 and missense variants in MYH7 were the most common LP/P variants seen. A single CNV was identified in exon 3 of the RYR2 gene.
Table 3 illustrates the breakdown of the most commonly implicated genes and variant types.
Table 3.
Variant type and classification in genes (> 5 LP/P variants)
| Gene | Pathogenic | Likely Pathogenic | LoF | Splice | Missense | Inframe deletion |
|---|---|---|---|---|---|---|
| MYBPC3 | 101 (27.1%) | 47 (12.6%) | 81 (21.7%) | 39 (10.5%) | 23 (6.2%) | 5 (1.3%) |
| MYH7 | 75 (20.1%) | 33 (8.8%) | 0 | 0 | 106 (28.4%) | 2 (0.5%) |
| TPM1 | 21 (5.6%) | 9 (2.4%) | 0 | 0 | 30 (8.0%) | 0 |
| TNNI3 | 7 (1.9%) | 4 (1.1%) | 0 | 0 | 11 (2.9%) | 0 |
| JPH2 | 10 (2.7%) | 0 | 0 | 0 | 10 (2.7%) | 0 |
| TNNT2 | 7 (1.9%) | 2 (0.5%) | 0 | 0 | 7 (1.9%) | 2 (0.5%) |
| RAF1 | 3 (0.8%) | 5 (1.3%) | 0 | 0 | 8 (2.1%) | 0 |
| GLA | 3 (0.8%) | 3 (0.8%) | 2 (0.5%) | 0 | 4 (1.1%) | 0 |
Variant types and classification of likely pathogenic and pathogenic variants in genes in which more than five variants were detected. Percentages represent the proportion of total variants identified (n = 373)
LP for likely pathogenic, P for pathogenic, LoF for Loss of function, Splice for consensus splice site variant or other variant with known or suspected effect on splicing. A total of five splice variants were at a position greater than ± 10 bp from the intron/exon boundary
RASopathy findings
A total of 959 tests included at least two RASopathy genes. A LP/P variant was found in 1.7% (n = 16) of these, representing 4.3% of all LP/P variants. The majority (81.3%, n = 13) of all diagnostic variants in RASopathy variants were identified on the broad cardiomyopathy panel; the remainder were from the HCM-specific panel. In 15/16 cases, the RASopathy gene variant was the only LP/P variant identified, in one case two LP variants were seen; (RAF1 and TNNT2). Variants were seen most commonly in the RAF1 gene (n = 8), followed by PTPN11 (n = 4), HRAS (n = 2), MAP2K1 (n = 1) and SHOC2 (n = 1). The mean age at diagnosis in the RASopathy group was 19.1 years, which is significantly lower than seen in patients without a RASopathy variant (33.8 years, p = 0.002). Syndromic features were reported by the clinician for 31.3% of patients (n = 5), all but one of whom had a RASopathy variant as their only genetic finding. All patients were heterozygous for their identified variant; no patients showed evidence of mosaicism within the limits of what is detectable by the assay.
Other findings
A total of 0.9% (n = 13) of all patients had LP/P variants in genes associated with a metabolic or storage disease. Just over half of these were identified from the HCM-specific panel (53.8%, n = 7) and the remainder from the broad cardiomyopathy panel (n = 6). Most were in the GLA gene (n = 6), followed by 2 patients with variants in TTR, PRKAG2 and LAMP2 respectively and finally one with CPT2. A total of 0.6% of cases (n = 8) had LP/P variants in genes associated with non-HCM cardiomyopathy (two in LMNA, and DSG2, one in JUP) or arrhythmias (one in SCN5A, RYR2, TRPM4 respectively). One third of channelopathy/arrhythmia genes were identified on the broad cardiomyopathy panel. The remainder of the channelopathy/arrhythmia variants were identified on the broad cardiology panel. Diagnostic variants in syndromic (n = 1), other cardiomyopathy (n = 5) and myopathy genes (n = 1) were only identified from the broad cardiomyopathy panel. Of all cases, 0.3% (n = 4) had two or more LP/P variants.
Clinical variables and diagnostic yield
Age at diagnosis had a significant effect on the likelihood of identifying a LP or P variant. The average age at diagnosis was 37.2 years in those with a diagnostic finding (reported in 216/369) and 47.6 years for those with no diagnostic findings (reported in 553/1007), p = 6.4 × 10–10. The diagnostic yield was highest for patients who were 11–20 years of age at the time of diagnosis, (45.8%, reported in 33/72) and lowest for patients who were 61–70 years of age at the time of diagnosis (17.5%, reported in 21/120) (Fig. 3). In considering only genes in which 5 or more LP/P variants were found, the age at diagnosis was the lowest (26.5 years) in patients who had a missense variant in TNNT2 and the highest (46.3 years) in those with a missense variant in GLA.
Fig. 3.
Diagnostic Yield (LP/P variants) by Age at Diagnosis (Where Reported). The Y axis indicates percentage of diagnostic yield, and the X axis indicates age at diagnosis
A positive family history was significantly associated with the likelihood of identifying a LP/P variant. LP/P variants were significantly more common in patients with a family history (37.1%; 156/421) than in those without a family history (22.3%; 213/955) (p < 0.0001).
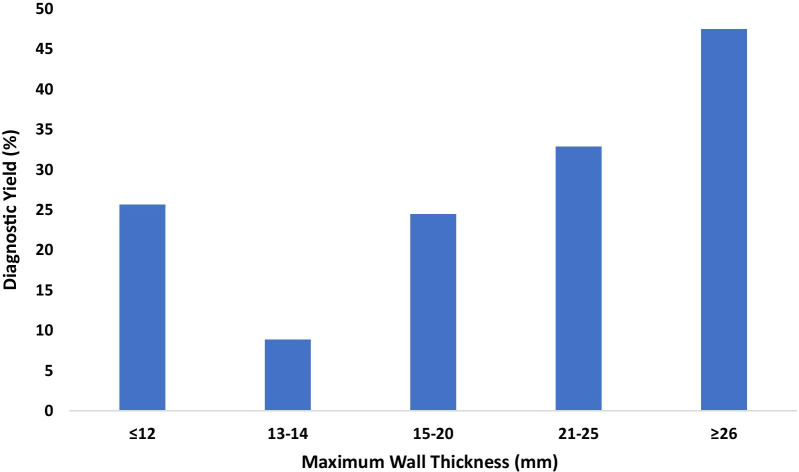
Higher maximum wall thickness (MWT) increased the likelihood of identifying a LP or P variant. MWT was higher (20.7 ± 4.8 mm and 19.0 ± 4.2 mm, p = 2.1 × 10–7) in patients over 16 years old with a diagnostic finding (reported in 239/849) compared to those patients with no molecular diagnosis (reported in 610/849). Patients older than 16 years of age with a LP/P variant in a ClinGen definitive HCM gene had a MWT that was similar to patients older than 16 years of age with LP/P variants in ClinGen moderate, limited evidence/not evaluated HCM genes (20.9 ± 4.8 and 19.6 ± 5.0, p = 0.22). Where MWT information was available, the diagnostic yield was the highest in patients with a wall thickness of ≥ 26 mm (47.5%, 38/80) and the lowest in patients with a maximum wall thickness of 13–14 mm (8.9%, 4/45) (Fig. 4). In considering only genes in which 5 or more variants were identified, the greatest MWT was seen in patients with a splice site LP/P variant in MYBPC3 (22.8 mm) and smallest in those with a LP/P variant in TNNT2 (14.5 mm).
Fig. 4.
Diagnostic Yield (LP and P variants) by Maximum Wall Thickness (mm). The Y axis indicates percentage of diagnostic yield, and the X axis indicates the maximum wall thickness in millimeters. mm millimeter
The absence of hypertension significantly affected the likelihood of identifying a LP/P variant. Of all patients without hypertension, 28.4% (346/1218) had a LP/P variant identified. Of all patients with hypertension, 13.9% (22/158) had a LP/P variant identified (p = 0.0002).
Patients with an implantable cardioverter-defibrillator (ICD) were more likely to have a LP/P variant identified (41.7%, 45/108) compared to those without an ICD (25.6%, 324/1268) (p = 0.0004). Clinical variables not significantly associated with the likelihood of identifying a LP/P variant were atrioventricular (AV) block, aortic stenosis, heart transplantation, ventricular tachycardia, cardiac arrest, pacemaker or whether previous genetic testing had been performed.
Discussion
Genetic testing for HCM is more accessible than ever and is supported by multiple international societies [1, 23]. Describing the genetic findings and diagnostic yield in a large, heterogeneous, cohort of patients referred for genetic testing further informs clinicians about the likelihood of obtaining a diagnostic result and the breadth of genes in which these variants may be found as to further tailor counseling, surveillance and management strategies.
A retrospective review of 1376 probands with a suspected diagnosis of HCM as indicated by the ordering provider and referred for genetic testing via NGS technology over a 5-year period identified a diagnostic finding in 26.8% (n = 369/1376) of cases. This yield is lower than previously reported in other studies [31.5%; 917/2912 (9), 32.3%; 1998/6179 (26), 33.3%; 129/387 (11), 33.2%; 127/382 (12), 46.7%; 491/1198 (13), 54.3%; 38/70 (10)]. Our cohort of HCM patients is one of the most heterogeneous reported to date, as this study only included cases where the clinician specifically indicated a suspicion of HCM; no supporting diagnostic criteria were required for inclusion and no clinical data excluded patients. Although the inclusion criteria utilized here aren’t as stringent as those used for some clinical cohorts, they allow for the reporting of the diagnostic yield in all patients with suspected HCM referred for genetic testing. The lower yield reported in this study could be explained by the inclusion of patients with a low suspicion of HCM, including those with an alternate explanation for LVH such as systemic findings and/or a syndrome. Other comparable cohorts such as the cohort of 2,912 HCM patients reported by Alfares et al. excluded individuals with LVH explained by a clinical syndrome and unaffected individuals with only a family history [9]. In our study, nearly 10% of patients who were > 16 years of age at the time of testing were reported to have a MWT of 14 mm or less, raising the possibility that these patients may have an alternative diagnosis. It should also be noted that the high diagnostic yield reported in some of these earlier studies may be explained by the inclusion of variants in genes that have now been shown to have limited or no evidence of being associated with HCM [18], or by including all suspicious variants in the total yield. For example, Rubattu et al. [10] reported a high diagnostic yield (54.3%; 38/70) but these included some debatable findings; for example, three ‘causative’ variants in MYH6 and CAV3. According to ClinGen or the Genomic England PanelApp, neither of these two genes are considered to be associated with HCM at gene level or without overt syndromic features [18, 27]. The study by Mazzarotto et al. [13] includes P, LP and variants of uncertain significance (VUS) in their total diagnostic yield. Finally, earlier versions of the Blueprint Genetics HCM panel (see Additional file 1: Table 1) did not include the now well-established HCM/left ventricular hypertrophy genes PLN and PTPN11, which might also have an impact on overall yield.
Notably, 4.6% of all LP/P variants were in genes deemed to have limited evidence, or not evaluated in association with HCM [18]. Also, 2.4% of all LP/P variants, representing 0.7% of all cases, were in genes that are not typically included on an HCM panel (LMNA, JUP, DSG2, SCN5A, RYR2, TRPM4, NF1). These genes are associated with other cardiomyopathies, arrhythmias or syndromes, which have different treatment or management strategies and so are of the utmost importance to identify. Sufficient clinical data was not available to verify the accuracy of the HCM diagnoses in patients with a LP/P variant in LMNA, JUP, DSG2, SCN5A, RYR2, TRPM4 or NF1. Diagnostic variants in the broad cardiomyopathy panel were the most varied and were distributed across all gene categories (sarcomere, RASopathy, non-sarcomere HCM, metabolic/storage, other cardiomyopathy, channelopathy/arrhythmia and syndromic/myopathy). This would be expected as the broad cardiomyopathy and broad cardiology panels include a number of other cardiomyopathy, channelopathy, channelopathy/arrhythmia, syndromic/myopathy genes that are not included on the HCM-specific panel. Further, patients offered a broad cardiomyopathy panel may have a lower index of suspicion for HCM or an unclear cardiomyopathy presentation. Unfortunately, there is limited clinical information available on individual patients, and so it is difficult to draw conclusions about specific features that prompted choosing a broader cardiomyopathy/arrhythmia panel over an HCM-specific one. Finally, the MWT in patients over 16 years of age at the time of testing was similar in patients with LP/P variants in definitive, strong, moderate, limited or not evaluated ClinGen HCM genes [18]. Higher MWT in patients with variants in non-HCM related genes could be explained by other factors that were not described in the requisition or due to possible discrepancies in imaging measurements.
The prevalence of RASopathy LP/P variants in patients whose panel included at least two or more RASopathy genes was more than double what has previously been published in a similar HCM cohort (1.7% vs. 0.7%) [20]. Our reported proportion may be an underestimate of the true prevalence of RASopathy findings, as earlier versions of the HCM and cardiomyopathy panels utilized in this study did not include the most common of the RASopathy genes associated with left ventricular hypertrophy (PTPN11, RAF1, RIT1). Despite small numbers, it is worth noting that patients harboring RASopathy LP/P variants were significantly younger than those with non-RASopathy LP/P variants. A systematic evaluation of syndromic features in these patients was not possible as limited clinical data was available. Further studies are needed to better understand the phenotypic spectrum of these patients presenting with apparently isolated LVH. Our work supports the inclusion of RASopathy genes on HCM specific panels.
This study reports a higher proportion of JPH2 diagnostic variants (2.7%) than has been previously reported, all of which were the c.482C > A; p. (Thr161Lys) variant. A recent publication by our group describes nine Finnish HCM families with this variant and no other LP/P variant which would cause HCM [28], suggesting that this could be a founder variant in Finland. Segregation with disease was seen in 6 of these families [28]. This JPH2 missense variant is also absent in population databases (gnomAD) and predicted to be deleterious by the majority of in silico tools used. The laboratory where testing was performed is located in Finland and is used by Finnish providers, thus explaining the higher proportion of patients with this one specific variant, in a gene that is not commonly associated with HCM.
We identified a small proportion (0.3%)of patients in this cohort with multiple LP/P variants, which is comparable to what has recently been reported (0%) in HCM using strict ACMG/AMP classification criteria [12]. This further supports the notion that HCM patients rarely have more than one LP/P variant.
None of the deep intronic (≥ 20bps from the intron/exon boundary) or non-coding (located in the promoter or 5′ and 3′ UTR) variants included in later versions of the panels (Additional file 1) were identified in this patient cohort. Only 1.3% of all LP/P variants identified (5/373) were splice variants at positions greater than ± 10 bp, which is what is covered by most NGS panels. These two variants were MYBPC3 c.1224-19G > A (n = 1) and MYBPC3 c.1227-13G > A (n = 4). Including ± 20 bp from the intron/exon boundary in the target region of this analysis increased the diagnostic yield from 26.5% (364/1373) to 26.8% (369/1373). The deep intronic variants recently identified by Bagnall et al. [29] such as MYBPC3 c.1090 + 453C > T, MYBPC3 c.1091-575A > C, MYBPC3 c.1224-52G > A which increased their diagnostic yield by 8.7%, were not included in the analysis of any of our patients. The inclusion of non-coding regions brings a challenge to the interpretation of variants when accompanying RNA studies are not available. Therefore, this study included only deep intronic variants (≥ 20 bps from the intron/exon boundary) or non-coding variants (such as those in the promoter, 5′ or 3′ UTRs) listed in Additional file 1 that have a previously established disease association or known splice defect in their target region at the time the analysis was performed. More extensive analysis of deep intronic and non-coding variants in this cohort is needed to determine whether they may have a greater impact on diagnostic yield.
Despite the above differences, several aspects of our findings are comparable to, and support what has previously been published in HCM cohorts from all genetic testing eras. The use of a broader, larger genetic testing panel did not lead to a significant increase in diagnostic yield, which has been demonstrated by others [12, 17] even when compared to whole genome sequencing [30]. In this study, LP/P variants were most commonly found in genes encoding the proteins of the sarcomere, the majority being in MYBPC3 and MYH7, as has been previously demonstrated [5, 7, 9, 31]. We identified a single CNV in this study (0.1% of all cases) in a gene not associated with HCM (RYR2). This represents a lower proportion than what has previously been reported using NGS technologies (1.3% (11) and 0.6% (15)). Our data further demonstrates that CNVs are rare in HCM patients. However, it is worth noting that less than half of the cases (40.3%) in this study underwent both sequencing and deletion/duplication analysis.
Finally, a younger age of onset, a greater MWT, the absence of hypertension and a family history of HCM were all individually, significantly associated with a greater likelihood of identifying a LP/P variant. These are well known clinical variables that have been integrated into both the Mayo [8] and Toronto [22] scores for predicting the yield of genetic testing in HCM patients. One important limitation to our family history analysis is that if the family history section in the requisition was left incomplete, it was assumed that the family history was negative. The diagnostic yield in patients with a MWT of 13–14 mm was lower than that of those with a MWT ≤ 12 mm. Without additional clinical details, this finding is difficult to explain. We found that the presence of an ICD was associated with a higher likelihood of identifying a LP/P variant, which has been shown previously [6]. Because of the limited information provided in the test requisitions, we were not specifically able to evaluate whether patients with negative genetic test results fit into a non-familial HCM group, which is defined by Ingles et al. by HCM index patients with a low Toronto score [22], a negative family history including at least two adult children, and no sarcomere variants identified [24]. Although we were not able to establish a proportion of true non-familial HCM patients in our cohort, it is of value for providers to appreciate the clinical variables associated with a negative result, particularly in a heterogeneous cohort that likely includes patients with a lower index of suspicion for HCM.
Conclusions
The diagnostic yield in this heterogeneous cohort of patients is lower than what has previously been reported in other published cohorts, likely due to differences in patient cohorts, study inclusion criteria, improvements in variant classifications and HCM gene curation efforts. The majority of LP/P variants were in genes that are definitively associated with HCM [18]. An important proportion of patients referred for HCM genetic testing have a LP/P variant in a RASopathy gene. An evaluation for syndromic features is warranted, as the phenotypic spectrum of the RASopathy syndromes continues to expand. A comprehensive inclusion of RASopathy genes on HCM panels may increase the diagnostic yield, and the findings in these genes have important implications for patients given the need for extra-cardiac management. Offering a broader panel may be of value in some patients as, despite a clinical suspicion of HCM, their presentation may actually be explained by a different diagnosis. Further work is needed to understand how to increase the diagnostic yield of genetic testing in patients with HCM. Thus far, the analysis of deep intronic/non-coding variants has been shown to provide the most substantial increase in diagnostic yield [29], more so than new gene discovery at this time.
Supplementary Information
Additional file 1. Genetic Testing Panels Ordered for Study Patients.
Additional file 2. Likely Pathogenic and Pathogenic Variants Identified in the Study Patients.
Acknowledgements
We express our gratitude to providers who ordered the testing through our laboratory which enabled the collection of valuable, publishable data.
Abbreviations
- ACMG
American College of Medical Genetics and Genomics
- AMP
Association for Molecular Pathology
- AV block
Atrioventricular block
- Bp
Base pairs
- CAP
College of American Pathologists
- CLIA
Clinical Laboratory Improvement Amendments
- CNV
Copy Number Variant
- HCM
Hypertrophic cardiomyopathy
- ICD
Implantable cardioverter-defibrillator
- IDT
Integrated DNA Technologies
- LoF
Loss of function
- LP
Likely pathogenic
- LV
Left ventricle
- LVH
Left ventricular hypertrophy
- MLVWT
Maximum left ventricular wall thickness
- Mm
Millimeter
- MWT
Maximum wall thickness
- NGS
Next generation sequencing
- OS-Seq™
Oligonucleotide-selective sequencing
- P
Pathogenic
- qPCR
Quantitative polymerase chain reaction
- UTR
Untranslated region
- VUS
Variants of Uncertain Significance
Authors’ contributions
JH, KH, IS, JP, TPA, TH and JK contributed to the conception and design of the study. JH, KH, IS and JK compiled the clinical data and performed all of the statistical analysis. JH, KH, IS and JK analyzed the data. JH, KH, IS, JT, EHS, ST, HT, TKK, JS, JTo, VK, MV, MM, JSi, MG, PS, TPA, and JK analyzed individual patient data and performed variant interpretation. VK, MV, MM, JSi, MG, PS, SM, JP, TPA, and JK developed and implemented the methodology used for DNA analysis, and variant interpretation. JH, KH, IS, TPA, TH and JK contributed in the making of tables and figures. JH, KH, TH and JK drafted and wrote the manuscript. JH, KH, IS, JT, EHS, ST, HT, TKK, JS, JTo, VK, MV, MM, JSi, MG, PS, SM, JP, TPA, TH and JK revised the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available as Additional files 1, 2: Tables 1 and 2. Additional data is available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Our study has been reviewed by the Western Institutional Review Board (IRB) and received an exemption determination.
Consent for publication
Not applicable.
Competing interests
Minor conflict of interest: SM, TPA, JK are co-founders of Blueprint Genetics and JH, IS, JT, EHS, ST, TKK, JS, JTo, VK, MV, MM, JSi, MG, PS, SM, JP, TPA, and JK are full-time employees of Blueprint Genetics, which offers genetic diagnostic services.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Julie Hathaway and Krista Heliö have contributed equally to this work
References
- 1.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathythe task force for the diagnosis and management of hypertrophic cardiomyopathy of the european society of cardiology (ESC) Eur Heart J. 2014;35(39):2733–2779. doi: 10.1093/eurheartj/ehu284. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Circulation. 1995;92(4):785–789. doi: 10.1161/01.CIR.92.4.785. [DOI] [PubMed] [Google Scholar]
- 3.Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1249–1254. doi: 10.1016/j.jacc.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ, Maron MS, Semsarian C. Genetics of hypertrophic cardiomyopathy after 20 years clinical perspectives. J Am Coll Cardiol. 2012;60(8):705–715. doi: 10.1016/j.jacc.2012.02.068. [DOI] [PubMed] [Google Scholar]
- 5.Erdmann J, Daehmlow S, Wischke S, Senyuva M, Werner U, Raible J, et al. Mutation spectrum in a large cohort of unrelated consecutive patients with hypertrophic cardiomyopathy. Clin Genet. 2003;64(4):339–349. doi: 10.1034/j.1399-0004.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 6.Driest SLV, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Yield of genetic testing in hypertrophic cardiomyopathy. Mayo Clin Proc. 2005;80(6):739–744. doi: 10.1016/S0025-6196(11)61527-9. [DOI] [PubMed] [Google Scholar]
- 7.Andersen PS, Havndrup O, Hougs L, Sørensen KM, Jensen M, Larsen LA, et al. Diagnostic yield, interpretation, and clinical utility of mutation screening of sarcomere encoding genes in Danish hypertrophic cardiomyopathy patients and relatives. Hum Mutat. 2009;30(3):363–370. doi: 10.1002/humu.20862. [DOI] [PubMed] [Google Scholar]
- 8.Bos JM, Will ML, Gersh BJ, Kruisselbrink TM, Ommen SR, Ackerman MJ. Characterization of a phenotype-based genetic test prediction score for unrelated patients with hypertrophic cardiomyopathy. Mayo Clin Proc. 2014;89(6):727–737. doi: 10.1016/j.mayocp.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfares AA, Kelly MA, McDermott G, Funke BH, Lebo MS, Baxter SB, et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: expanded panels offer limited additional sensitivity. Genet Med. 2015;17(11):880–888. doi: 10.1038/gim.2014.205. [DOI] [PubMed] [Google Scholar]
- 10.Rubattu S, Bozzao C, Pennacchini E, Pagannone E, Musumeci BM, Piane M, et al. A next-generation sequencing approach to identify gene mutations in early- and late-onset hypertrophic cardiomyopathy patients of an italian cohort. Int J Mol Sci. 2016;17(8):1239. doi: 10.3390/ijms17081239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mademont-Soler I, Mates J, Yotti R, Espinosa MA, Pérez-Serra A, Fernandez-Avila AI, et al. Additional value of screening for minor genes and copy number variants in hypertrophic cardiomyopathy. PLoS ONE. 2017;12(8):e0181465. doi: 10.1371/journal.pone.0181465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burns C, Bagnall RD, Lam L, Semsarian C, Ingles J. Multiple gene variants in hypertrophic cardiomyopathy in the era of next-generation sequencing. Circ Cardiovasc Genet. 2018;10(4):e001666. doi: 10.1161/CIRCGENETICS.116.001666. [DOI] [PubMed] [Google Scholar]
- 13.Mazzarotto F, Girolami F, Boschi B, Barlocco F, Tomberli A, Baldini K, et al. Defining the diagnostic effectiveness of genes for inclusion in panels: the experience of two decades of genetic testing for hypertrophic cardiomyopathy at a single center. Genet Med. 2019;21(2):284–292. doi: 10.1038/s41436-018-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceyhan-Birsoy O, Pugh TJ, Bowser MJ, Hynes E, Frisella AL, Mahanta LM, et al. Next generation sequencing-based copy number analysis reveals low prevalence of deletions and duplications in 46 genes associated with genetic cardiomyopathies. Mol Genet Genom Med. 2016;4(2):143–151. doi: 10.1002/mgg3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Russo CD, Giacomo GD, Cignini P, Padula F, Mangiafico L, Mesoraca A, et al. Comparative study of aCGH and Next Generation Sequencing (NGS) for chromosomal microdeletion and microduplication screening. J Prenat Med. 2014;8(3–4):57–69. [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson KL, Ormondroyd E, Harper AR, Dent T, McGuire K, Baksi J, et al. Analysis of 51 proposed hypertrophic cardiomyopathy genes from genome sequencing data in sarcomere negative cases has negligible diagnostic yield. Genet Med. 2019;21(7):1576–1584. doi: 10.1038/s41436-018-0375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingles J, Goldstein J, Thaxton C, Caleshu C, Corty EW, Crowley SB, et al. Evaluating the clinical validity of hypertrophic cardiomyopathy genes. Circ Genom Precis Med. 2019;12(2):e002460. doi: 10.1161/CIRCGEN.119.002460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aljeaid D, Sanchez AI, Wakefield E, Chadwell SE, Moore N, Prada CE, et al. Prevalence of pathogenic and likely pathogenic variants in the RASopathy genes in patients who have had panel testing for cardiomyopathy. Am J Med Genet A. 2019;179(4):608–614. doi: 10.1002/ajmg.a.61072. [DOI] [PubMed] [Google Scholar]
- 20.Ceyhan-Birsoy O, Miatkowski MM, Hynes E, Funke BH, Mason-Suares H. NGS testing for cardiomyopathy: utility of adding RASopathy-associated genes. Hum Mutat. 2018;39(7):954–958. doi: 10.1002/humu.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Millat G, Bouvagnet P, Chevalier P, Dauphin C, Jouk PS, Costa AD, et al. Prevalence and spectrum of mutations in a cohort of 192 unrelated patients with hypertrophic cardiomyopathy. Eur J Med Genet. 2010;53(5):261–267. doi: 10.1016/j.ejmg.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Gruner C, Ivanov J, Care M, Williams L, Moravsky G, Yang H, et al. Toronto hypertrophic cardiomyopathy genotype score for prediction of a positive genotype in hypertrophic cardiomyopathy. Circ Cardiovasc Genet. 2013;6(1):19–26. doi: 10.1161/CIRCGENETICS.112.963363. [DOI] [PubMed] [Google Scholar]
- 23.Hershberger RE, Givertz M, Ho CY, Judge DP, Kantor P, McBride KL, et al. Genetic evaluation of cardiomyopathy—a heart failure society of america practice guideline. J Card Fail. 2018;24(5):281–302. doi: 10.1016/j.cardfail.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingles J, Burns C, Bagnall RD, Lam L, Yeates L, Sarina T, et al. Nonfamilial hypertrophic cardiomyopathy. Circulation cardiovasc. Genetics. 2017;10(2):e001620. doi: 10.1161/CIRCGENETICS.116.001620. [DOI] [PubMed] [Google Scholar]
- 25.Myllykangas S, Buenrostro JD, Natsoulis G, Bell JM, Ji HP. Efficient targeted resequencing of human germline and cancer genomes by oligonucleotide-selective sequencing. Nat Biotechnol. 2011;29(11):1024–1027. doi: 10.1038/nbt.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh R, Thomson KL, Ware JS, Funke BH, Woodley J, McGuire KJ, et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet Med. 2017;19(2):192–203. doi: 10.1038/gim.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin AR, Williams E, Foulger RE, Leigh S, Daugherty LC, Niblock O, et al. PanelApp crowdsources expert knowledge to establish consensus diagnostic gene panels. Nat Genet. 2019;51(11):1560–1565. doi: 10.1038/s41588-019-0528-2. [DOI] [PubMed] [Google Scholar]
- 28.Vanninen SUM, Leivo K, Seppälä EH, Aalto-Setälä K, Pitkänen O, Suursalmi P, et al. Heterozygous junctophilin-2 (JPH2) p.(Thr161Lys) is a monogenic cause for HCM with heart failure. PLoS ONE. 2018;13(9):e0203422. doi: 10.1371/journal.pone.0203422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagnall RD, Ingles J, Dinger ME, Cowley MJ, Ross SB, Minoche AE, et al. Whole genome sequencing improves outcomes of genetic testing in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2018;72(4):419–429. doi: 10.1016/j.jacc.2018.04.078. [DOI] [PubMed] [Google Scholar]
- 30.Cirino AL, Lakdawala NK, McDonough B, Conner L, Adler D, Weinfeld M, et al. A comparison of whole genome sequencing to multigene panel testing in hypertrophic cardiomyopathy patients. Circ Cardiovasc Genet. 2018;10(5):e001768. doi: 10.1161/CIRCGENETICS.117.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapplinger JD, Landstrom AP, Bos JM, Salisbury BA, Callis TE, Ackerman MJ. Distinguishing hypertrophic cardiomyopathy-associated mutations from background genetic noise. J Cardiovasc Transl. 2014;7(3):347–361. doi: 10.1007/s12265-014-9542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Genetic Testing Panels Ordered for Study Patients.
Additional file 2. Likely Pathogenic and Pathogenic Variants Identified in the Study Patients.
Data Availability Statement
The datasets used and/or analysed during the current study are available as Additional files 1, 2: Tables 1 and 2. Additional data is available from the corresponding author on reasonable request.