Abstract
Background
Seasonal malaria chemoprevention (SMC) is a strategy for malaria control recommended by the World Health Organization (WHO) since 2012 for Sahelian countries. The Mali National Malaria Control Programme adopted a plan for pilot implementation and nationwide scale-up by 2016. Given that SMC is a relatively new approach, there is an urgent need to assess the costs and cost effectiveness of SMC when implemented through the routine health system to inform decisions on resource allocation.
Methods
Cost data were collected from pilot implementation of SMC in Kita district, which targeted 77,497 children aged 3–59 months. Starting in August 2014, SMC was delivered by fixed point distribution in villages with the first dose observed each month. Treatment consisted of sulfadoxine-pyrimethamine and amodiaquine once a month for four consecutive months, or rounds. Economic and financial costs were collected from the provider perspective using an ingredients approach. Effectiveness estimates were based upon a published mathematical transmission model calibrated to local epidemiology, rainfall patterns and scale-up of interventions. Incremental cost effectiveness ratios were calculated for the cost per malaria episode averted, cost per disability adjusted life years (DALYs) averted, and cost per death averted.
Results
The total economic cost of the intervention in the district of Kita was US $357,494. Drug costs and personnel costs accounted for 34% and 31%, respectively. Incentives (payment other than salary for efforts beyond routine activities) accounted for 25% of total implementation costs. Average financial and economic unit costs per child per round were US $0.73 and US $0.86, respectively; total annual financial and economic costs per child receiving SMC were US $2.92 and US $3.43, respectively. Accounting for coverage, the economic cost per child fully adherent (receiving all four rounds) was US $6.38 and US $4.69, if weighted highly adherent, (receiving 3 or 4 rounds of SMC). When costs were combined with modelled effects, the economic cost per malaria episode averted in children was US $4.26 (uncertainty bound 2.83–7.17), US $144 (135–153) per DALY averted and US $ 14,503 (13,604–15,402) per death averted.
Conclusions
When implemented at fixed point distribution through the routine health system in Mali, SMC was highly cost-effective. As in previous SMC implementation studies, financial incentives were a large cost component.
Keywords: Malaria, Mali, Seasonal malaria chemoprevention, Cost, Cost-effectiveness, Economic, Financial, Disability-adjusted life year (DALY)
Background
After an unprecedented period of success, with substantial investment in malaria control and reductions in malaria episodes, the progress has stalled [1]. In 2018, there were an estimated 228 million cases and 405,000 related deaths worldwide, with a high proportion of these deaths occurring in sub-Saharan Africa (93%) mostly in children under 5 years [1].
In addition to the human morbidity and mortality impact, malaria places economic burdens on individuals, health systems and national governments. In Mali, malaria is the main cause of outpatient visits, hospitalizations and mortality in health facilities; children under five and pregnant women are the most affected [2]. According to a national survey in 2013, the prevalence of malaria parasitaemia by microscopy was 52% in children under 5 years overall in Mali and 37% in the southern region of Kayes, the setting of this study [3]. In 2014, health facilities across Mali registered more than two and half million cases of suspected malaria, of which 1.7 million were clinical cases, 800,000 severe cases and 2309 deaths [4].
Seasonal malaria chemoprevention (SMC) was recommended by the World Health Organization (WHO) in areas of highly seasonal malaria transmission across the Sahel sub-region in 2012. It consists of the administration of a complete treatment course of sulfadoxine-pyrimethamine and amodiaquine (SP + AQ) at monthly intervals to children aged 3 to 59 months, beginning before the start of the transmission season, with up to a maximum of four doses during transmission season [5]. The National Malaria Control Programme (NMCP) in Mali was one of the early adopters and introduced SMC in 2012 with pilot implementation in one district and progressive expansion to other districts.
Few studies have assessed the cost and cost-effectiveness of SMC introduction and distribution at scale through the routine health system. In addition, little is known about the detailed cost breakdown of SMC when implemented using fixed point distribution method [6, 7]. Peer-reviewed cost and cost-effectiveness studies using data collected alongside clinical trials and modelling efforts have suggested that SMC is a low cost and highly effective intervention (Table 1). The lowest cost per child per dose reported was in Senegal at $1.96 for 3 doses (inflated to 2016 USD) [7] and the highest cost per dose at $ 22.81 in the upper west region in Ghana (inflated to USD 2016) [6]. Cost drivers included the cost of the drugs, scale of the study, the delivery mode (fixed point vs. door-to-door), personnel costs (salaries) and incentives. Incentives reflect a payment (either in kind or in monetary terms) to an individual independent of any salary they may receive, for efforts carried out in addition to their routine activities.
Table 1.
Summary of peer reviewed SMC cost and cost-effectiveness estimates
| Setting | Delivery strategy | Unit Cost/US $ (2016)a | Cost–effectiveness ratiosb | |||||
|---|---|---|---|---|---|---|---|---|
| Cost per round | Cost per fully adherent childc | |||||||
| Financial | Economic | Financial | Economic | |||||
|
Hohoe, Ghana (Conteh et al. 2010) |
DTD, 6 rounds, AS + AQ monthly – trial conditions DTD, 6 rounds, AS + AQ monthly – modelled to district level |
1.84 | 2.54 |
13.17 4.02 |
16.70 4.83 |
80.46 (66.18–94.81) per case averted 24.87 (22.63–27.36) per case averted |
||
|
Basse, Gambia (Bojang et al. 2011) |
FPD, 3 rounds, SP + AQ monthly DTD, 3 rounds, SP + AQ monthly |
1.04 0.74 |
1.20 0.97 |
3.31 1.37 |
3.87 1.82 |
Not applicable | ||
|
Jasikan, Ghana (Patoulllard et al. 2012) |
FPD, 3 rounds SP + AQ monthly DTD, 3 rounds, SP + AQ monthly |
2.93–3.09 2.65 |
3.65–3.79 3.46 |
– – |
9.37 8.32 |
Not undertaken | ||
|
Upper West Region, Ghana (Nonvignon et al. 2016) |
DTD, 4 rounds, SP + AQ at monthly | 22.81 |
108.41 (101.01–123.01) per additional case avertedd 3339.97 (3112.03–3789.85) per additional death averted |
|||||
|
Bambey, Mbour, Fatick & Niakhar, Senegal (Pitt et al., 2017) |
DTD, 3 rounds SP + AQ monthly | 0.55 | 0.65 | 1.65 | 1.96 | Not undertaken | ||
| Burkina Faso, Chad, The Gambia, Guinea, Mali, Niger, and Nigeria (ACCESS-SMC Partnership, 2020) |
DTD and FPD (country dependent) 4 rounds SP + AQ monthly |
– | 0.91e | – | – |
2.91–30.73 per case averted 119.63- 506.00 per severe case averted 533.56- 2256.92 per death averted |
||
DTD: Door to Door distribution, FPD: Fixed Point Distribution (Health facility, outreach or focal point in village)
aCosts from Provider perspective and inflated from original source base year to 2016 using Inflation Calculator from U.S. Labor Department's Bureau of Labor Statistics on September 12, 2019
bBased on intervention costs only
cThe unit cost per round weighted by adherence levels
dNonvignon et al. (2016) used a similar approach to this study (combined primary cost data with a transmission model to estimate cost-effectiveness)
eAccording to the manuscript, ‘The weighted average cost of four treatments per child was obtained by dividing the total recurrent cost by the total number of doses administered divided by 4′ (p1834). This amounted to US $3.63, a calculation based on a multiple of treatment rounds that appears to exclude adherence. To obtain the cost per round, US $3.63 was divided by 4. Mali specific costs were not presented
There have been two implementation studies of SMC in Mali [8, 9]. In 2012, an evaluation of SMC was undertaken by “Médecins Sans Frontières” (MSF) in Koutiala, the first Malian health district to implement SMC. Using budgetary estimates, the average cost of providing SMC to a child was estimated at $1.30 per child per round [8]. In addition, Mali was part of a large multi-county rapid expansion of seasonal malaria chemoprevention in the Sahel (ACCESS SMC) implementation study [10]. Preliminary ACCESS SMC cost estimates suggested that the recurrent costs of delivering 4 doses of SMC to a child in Mali was $4.05 (2015 USD) [11]. In a later publication, an average economic cost for four treatments per child, across all seven ACCESS countries, of US $ 3.63 (US $ 2.71 to US $ 8.20) was obtained [12]. To date, this is the first study to use primary cost data to estimate the cost per disability-adjusted life year (DALY) averted for SMC.
SMC is a relatively new approach compared to other malaria control tools. Therefore, rigorous evaluations of the strategy when implemented through a routine health system, outside the context of clinical trials, are needed to inform resource allocation decisions. Often trials are well funded and able to incentivize those involved in ensuring the delivery of SMC, both in terms of financial payments and non-financial incentives such as additional training and improved access to drugs and other resources. The aim of this evaluation was three-fold: firstly, to assess the district-wide primary cost data associated with the implementation of SMC in Kita as part of routine care; secondly to combine these costs with coverage levels identified in the project to estimate the effectiveness of SMC in Kita based upon an established dynamic malaria transmission model. Thirdly, to use the costs and the effectiveness estimates to calculate the incremental cost-effectiveness of the intervention compared to no intervention.
Methods
Study site and population
Kita district, where SMC was implemented and evaluated in 2014, is located in the western region of Kayes, about 180 kms north of Bamako, the capital city of Mali. Kita was one of the 21 districts in Mali to roll out SMC in 2014 as part of the progressive national scale up of SMC. Kita has one district hospital, 40 community health centres, and 72 community health workers (CHWs). The population was estimated to be 516,649 in 2014 with approximately 77,497 children between 3 and 59 months of age and parasitaemia prevalence for the region in 2013 was 37% [13, 14]. SMC was implemented in all 336 villages/quartiers and while approximately 77,497 children were forecast to receive SMC, an estimated 104,255 children actually received the intervention. This higher number of children than expected is explained by three factors. Firstly, some children older than 5 years are likely to have received the intervention; secondly, Bafoulabe, the district next to Kita did not receive SMC and it is likely that children came from bordering villages to receive SMC; and finally, the study site covered a large agricultural area that, at the time of SMC administration, might have experienced a population swell given it was growing season so there was a need for increased labour.
SMC delivery and administration
SMC delivery refers to the process of obtaining the resources (mainly drugs and incentives) from the central stores to the end user (in this case the child). In the context of this study, incentives capture the financial compensation for supporting for SMC implementation in Kita. SMC administration (sometimes referred to in previous SMC costing papers as distribution) refers to the activities associated with giving the drugs to the child on the day(s) of SMC.
In Kita, the SMC campaign was organized in 2014 using a fixed point distribution method. Specifically, drug distribution was channelled through the district hospital and community health centres, and SMC was delivered at village focal points. Distribution was the responsibility of government employed health staff, primarily nurses and community health workers (CHWs).
Children aged 3–59 months received sulfadoxine-pyrimethamine and amodiaquine (SP + AQ) at monthly intervals over the four months of August, September, October, and November 2014. Before drug distribution started, several meetings were held from May to August 2014 at the national, regional, district and sub-districts levels in Kita in preparation for SMC implementation. Two technical groups composed of 8 staff members from NMCP and implementing partners were created: one to establish, review and update the SMC distribution modules, and develop data collection tools; and the second group to develop a communication plan for SMC implementation. SMC dispensers, who were CHWs and nurses, received a two-day training course on SMC administration using the training modules developed. Normally, the Central Pharmacy (Pharmacie Populaire du Mali, PPM) provides drugs and medical supplies to the Regional Health Directorate in Kayes. The Regional Directorate then provides supplies and drugs to health districts who dispatch them to the district and Community Health Centres. However, because of a delay in preparing for implementation, on this occasion SMC drugs were paid for by the Ministry of Health (MoH) through the NMCP and delivered directly to the district heath centre of Kita by the PPM without passing through the Regional Health Directorate. It then took five days for the district health centre to deliver the drugs and supplies to community health centres.
The SP + AQ used for SMC in Kita were not co-blistered or pre-packed conditional on age group. The drugs, therefore, had to be cut and repacked by the district health team. For this, 10 persons were recruited and worked on the drugs packaging for 25 days. After the drugs were packaged, an allocation plan was developed to distribute the SMC drugs and necessary supplies for implementation to the community health centres and villages heath workers. Information, sensitization and communication messages on SMC were developed and delivered through the two most popular local radio channels for six months (July to December 2014). Additional specific messages were developed and added during the distribution period. In addition, at the community level, mobilization and sensitization activities were carried out.
SMC drug administration was performed by Kita district health staff and comprised 588 drug dispensers (nurses and CHWs) organized into 133 teams of 2–6 health workers each at health centres (n = 37 teams) and village fixed points (n = 96 teams). The first dose of AQ and the single dose of SP was given the first day by health workers. Children were observed for 30 min, and if the child vomited within this time, another dose was administered. The second and third doses of AQ were given to parents to be administrated at home. Further details are provided elsewhere [3].
All labour used in SMC distribution was employed and paid by the MoH, except implementing partner staff who were paid by the non-government organization Save the Children [12]. Supervision and monitoring were performed during the distribution period by teams composed of staff at national level ((NMCP, Maternal and Child Survival Programme, and National Federation for Community Health Centres Association)), regional level and district level. As with all SMC programmes, incentives were given to all of those involved: NMCP personnel, Fédération Nationale des Associations Communautaires, staff from the district hospital and community health centres, CHWs and drivers. Incentives (per-diems and accommodation where required) varied according to categories of the staff and location (local, district level, regional). In Kita incentives were set at daily rates comparable to financial incentives paid for delivering other interventions and research studies conducted in the area previously. The daily incentive for a rural doctor was comparable to their daily salary, and a little less than a CHW could expect to receive for their daily duties. On average those involved in the implementation were paid approximately 36 days of incentives for helping with SMC implementation in 2014.
Costing
Costs are presented from the provider perspective using an ingredients approach whereby the relevant resources were identified and measured at the time of the SMC implementation to estimate the total cost of the intervention [5]. The choice of provider perspective was based on data availability and the intention of the study to inform decision makers how much the intervention would cost for funding proposes. Both financial and economic costs (recurrent and capital) are estimated for the 4 rounds of distribution. Financial costs reflect the actual expenditure required to deliver the intervention such as the cost of drugs and incentives. Economic costs capture the opportunity cost of all resources used to provide SMC, whether or not they incur a financial cost. For example, the time of health personnel involved in SMC delivery represents an economic cost as the staff already received a salary so there was no additional financial commitment, however they could have spent their time on other activities, so we need to capture the opportunity cost [6, 15]. Only 2014 cost data were used in this study and are presented in 2016 Communauté Financière en Afrique (CFA) and US dollars (USD). A conversion rate of CFA 494.17: USD 1 was assumed based on the average of August 2014 inflated to 2016 using US Inflation Calculator [16].
Table 2 presents the cost categories used in the analysis: planning, communication, training, drugs, personnel, equipment and transport. In addition to the total implementation cost and the total cost per round, unit costs are estimated in terms of cost per child fully adherent (i.e. a child that had the first of each of the SMC drugs under observation in all 4 rounds) and cost per child partially covered (i.e. a child that had the first SMC drugs under observation in 1, 2 or 3 rounds). Fully adherent is defined as child who received all 4 rounds and highly adherent as child who received at least 3 rounds.
Table 2.
Cost categories
| Cost category Input or Activity |
Description | Sample size | Data source |
|---|---|---|---|
| Planning meetings: | In total six meetings were held, including five field visits | 6 meetings | MCSP expenses record |
| Training | A group of trainers was trained on SMC distribution. In turn, community health center staff, community and voluntary health workers (n = 100) were trained by trainers (n = 9). Total training cost was estimated using the number of participants and daily per diems; accommodation, refreshments and transportation costs were also included | 6 trainings | MCSP expenses record |
| Drugs |
Quantities were estimated by age group from NMCP 2014 drug accountability plan Number of drugs received is from the drug accountability plan |
AQ: 179,627 packs of 6 tablets SP: 359 boxes of 1000 tablets |
Costs were collected from Pharmacie Populaire du Mali drug stock inventory state, ACT, RDT, and anti- malarial store in November 2014 |
| Personnel | Personnel costs were collected and calculated in planning, training and distribution categories and captured both those directly responsible for delivering SMC and those supervising. Staff costs were classified by specialty; salary (plus associated benefits such as pensions, housing allowance etc.) Per diems and incentives were calculated according to activity | 588 | Ministry of Health personnel pay slips for 2014 |
| Equipment | The costs of materials and equipment such as drinking glasses, buckets, chairs and tables used during dispensing SMC | MCSP expenses record | |
| Transport | For transport cost during meetings, trainings and drugs distribution, MCSP/SC vehicles were used as well as rented vehicles. Vehicles maintenance costs were included in renting costs |
The total number of vehicles used and the costs were estimated from vehicle renting cost per day from the Ministry of Health vehicle renting documents Fuel costs were extracted from MCSP/SC monthly activities accounts |
NMCP National Malaria Control Program, MCSP/SC Maternal and Child Survival Program
Effectiveness
An aim of this district-wide SMC delivery was to see how feasible the intervention was at scale outside of trial conditions. The project focused on coverage and collected limited data on health outcomes (specifically prevalence of malaria and anaemia) [13]. Instead, modelling was used to provide an indication of the likely effectiveness of the intervention in Kita. An established dynamical transmission model was used [17]; this model has previously been used for a range of applications including to establish global investment targets for the WHO Global Technical Strategy [18]. Full details, as well as source code and instructions for compilation and running, of the model are available elsewhere [19, 20]. In brief, the model is individual-based and non-spatial, with rainfall driving seasonal patterns of mosquito emergence and subsequent patterns of the entomological inoculation rate, incidence of malaria and prevalence of infection, which then determine the level of onwards transmissibility to the mosquito. The human component of the model incorporates heterogeneity in exposure and both acquired and age-dependent immunity, which affects (i) the probability of developing severe and clinical disease, (ii) the duration of detectable blood-stage infection and (iii) onwards infectivity to the mosquito. This allows the model to capture the relationship between transmission and age-dependent patterns of prevalence and disease across a wide range of settings and to simulate the onwards impact of multiple interventions upon transmission (though the magnitude of this impact within the model is limited when SMC is only provided to under 5 s [21]. The model has also been shown to replicate seasonal patterns of malaria burden, driven by rainfall [22] and the effectiveness of SMC within trials (Cairns et al., pers. commun.), to a high degree of accuracy. Here the model was calibrated to patterns of seasonality, based upon rainfall gauge data between 2002 and 2008 in the region, smoothed by Fourier transformation, to provide typical patterns of rainfall throughout the year. Long-lasting insecticidal nets (LLIN) use within the area was also captured by the model, assuming a linear increase from 0% in 2000 to 56.9% observed in Kayes region in 2013 [3] which had risen to 65.2% in 2015 [2] following the universal coverage campaign in 2014. Usage immediately following the 2014 campaign (which was not measured) was calibrated to achieve 65% usage in the area at the end of 2015 (when the above survey was done), with no further assumed distribution in 2015–2017. The model assumes a constant per-capita rate of attrition in usage in the absence of receiving a new net of 0.2 per year [23]. Simulations were then conducted using 1000 parameter sets representing separate draws from the joint posterior distribution of the underlying transmission model. Baseline vectorial capacity in each simulation was calibrated to 2014 dry-season prevalence according to a triangle distribution with mode of 24.1% and a 95% interval between 20.7% and 27.8%, based upon the mean and binomially-distributed 95% confidence intervals (CIs) observed in this survey, and further accounting for temporal trends in seasonality, LLIN usage and insecticidal coverage.
Four rounds of SMC with SP + AQ were simulated, with a duration of prophylaxis following a Weibull distribution with scale parameter 38.1 and shape parameter 4.3 fitted to published point estimates of the protective efficacy over time from a trial in Burkina Faso [20] (see Fig. 1). This profile provides strong protection for the first 3–4 weeks after a round, declining to 50% protection around 5 weeks, and then subsequently declining rapidly. The model was calibrated to match the per-capita number of doses delivered during the intervention by setting the coverage of the intervention within the model to the overall probability of receiving a dose in any given round according to interviews with the caregivers of children who reported receiving the intervention in 3396 of 4505 (75.3%) potential per-protocol rounds within the survey [13]. Uncertainty in the timing of these doses was then captured by varying the timing of the intervention relative to the seasonal peak by summarising simulations using 1000 draws from a triangle distribution with mode centred around the seasonal peak in transmission, with extremes between a month before and after this optimal scheduling.
Fig. 1.
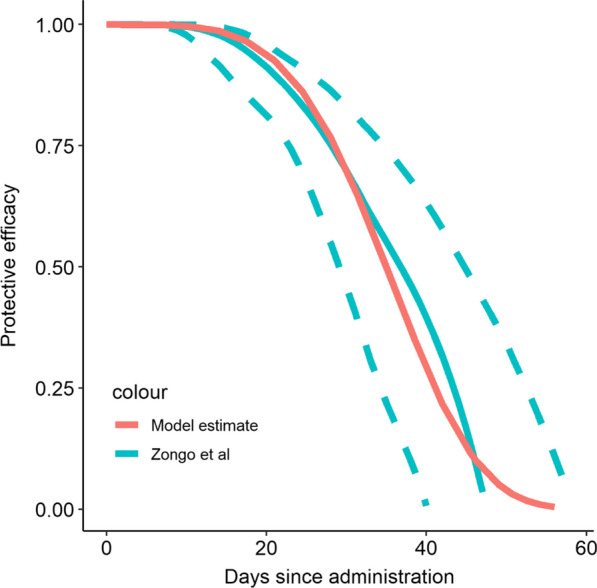
Figure shows the estimated protective efficacy from time since receiving a round of SMC as estimated in [reference 24] compared to the profile used within the model
Goodness-of-fit was assessed by comparing the model prevalence to prevalence survey collected at the end of 2012 [3] and a month after the final round of SMC [13]. Burden outputs considered were uncomplicated clinical malaria and severe malaria in children under five years and in the wider population, with estimates of lives saved based upon a case-fatality rate of 0.3% [24] per malaria case. The modelling incorporated the universal LLIN campaign within the area in 2014; as a result, in addition to our estimates of 2014 impact, were present both the estimates of the impact of SMC in 2014 specifically, and estimates if SMC at the same coverage as in 2014 had been extended across the 3-year LLIN distribution cycle, in order to provide a measure of the likely effectiveness of SMC if applied continuously in the area.
Incremental cost effectiveness analysis
An incremental cost effectiveness ratio (ICER) was calculated by dividing the annual total economic cost of SMC by the annual modelled estimates of (1) the number of uncomplicated and severe malaria cases averted, (2) the DALYs averted, and (3) the deaths averted. The annual number of episodes averted was an average of a three-year cycle where the effects of LLINs were also included. DALYs are a measure of burden of disease and can be used as a summary measure to determine and compare the cost-effectiveness of different types of interventions across different diseases and settings [25, 26]. They allow for a more informed assessment of cost effectiveness in relation to thresholds and acceptability. There is no gold-standard for when an intervention is deemed cost-effective. Indeed, the use and mis-use of thresholds is much debated [27, 28]. In helping interpret whether SMC was cost effective we present our findings using two very conservative thresholds: (i) US $780.51, Mali’s 2016 Gross Domestic Product (GDP) per capita and (ii) an even more stringent World Bank threshold of US $250 per DALY averted [29, 30].
Ethical considerations
The study protocol was reviewed and approved by the Ethical Committee of the Faculty of Medicine, Pharmacy and Dentistry of the University of Bamako prior to the study. The clinical trial was registered at Clinicaltrials.gov as number NCT02894294.
Results
SMC cost
The total financial cost to dispense SMC to the 104,255 children in Kita was US $316,111 and the total economic cost was US $357,494. The main economic costs by input were drugs (34%) and personnel (31%). The bulk of personnel costs were due to $81,455 spent on incentives (23% of the total economic costs) (Table 3). The most costly activity was the end stage, dispensing the drugs to the children (Table 4). Financial and economic cost were similar, the main difference being the already covered salary costs of facility-based health staff that were included in economic costs.
Table 3.
SMC implementation in Kita: Total annual financial and economic costs by input of 2016 USD
| Financial cost | Economic cost | |||
|---|---|---|---|---|
| $ | % | $ | % | |
| Personnel | 88,429 | 28 | 110,729 | 31 |
| Drugs | 122,060 | 39 | 122,060 | 34 |
| Transport | 36,190 | 11 | 36,190 | 10 |
| IECb | 6584 | 2 | 6584 | 2 |
| Equipment | 35,393 | 11 | 35,392 | 10 |
| Other | 27,454 | 9 | 46,538 | 13 |
| Total | 316,111 | 100 | 357,494 | 100 |
aPPM Central Pharmacy/Pharmacie Populaire du Mali
bInformation Education & Communication
Table 4.
SMC implementation in Kita: Total annual financial and economic costs by activity of (2016 USD)
| Activity | Financial cost | Economic cost | ||||
|---|---|---|---|---|---|---|
| CFA | $ | Percentage of total | CFA | $ | Percentage of total | |
| Planning meetings | 2,305,217 | 3006 | 1.39% | 2,887,971 | 5,925 | 1.66% |
| SP-AQ Tablets | 100,325 | 100,325 | ||||
| Drug Packing | 5,823 | 5,823 | ||||
| PPM Feesa | 17,632 | 17,632 | ||||
| Purchasing and preparing SMC drugs | 59,495,789 | 122,060 | 35.91% | 59,495,789 | 122,060 | 34.14% |
| Training | 6,674,267 | 13,693 | 4.03% | 8,093,772 | 16,605 | 4.64% |
| Equipment | 17,251,396 | 35,393 | 10.41% | 17,251,396 | 35,392 | 9.90% |
| Delivering to distribution point | 374,863 | 626 | 0.23% | 382,512 | 785 | 0.22% |
| Dispensing | 74,073,986 | 134,752 | 44.71% | 82,933,503 | 170,144 | 47.59% |
| IECa | 3,209,310 | 6,584 | 1.94% | 3,209,310 | 6,584 | 1.84% |
| Total | 165,690,044 | 316,111 | 100% | 174,254,252 | 357,494 | 100% |
aInformation Education & Communication
The average financial unit costs per child per round was US $0.73. The economic costs by round of SMC and per child are shown in Table 5. The economic costs by round were similar for all the rounds except round 1, where the number of children reached was lower compared to the other rounds. On average, SMC cost $0.86 (424.986 FCFA) per child/per round. Adding the averages across the four rounds gives a cost, on average, of $3.43 per child for the annual economic cost per child receiving SMC. However, this does not take into account adherence. In terms of how the costs changed relative to adherence, the cost of a fully adherent child having received all four rounds was US $6.38. (i.e. total economic costs divided by the 53.8% of 104,255 children estimated to have received at least the first day treatment of all four SMC rounds in the trial [13]) There is evidence to suggest that receiving even three rounds of SMC provides substantial protection [31, 32] which would make the cost per child highly adherent US $4.69.
Table 5.
Economic cost of SMC per rounds and accounting coverage in Kita (US $ 2016)
| 1st round | 2nd round | 3rd round | 4th round | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Cost (US $) | 90,526 | 88,345 | 89,466 | 89,157 | 357,494 | |||
| Total Number of Individual Children reached | 98,790 | 104,477 | 108,659 | 105,093 | ||||
| Cost per child per round | 0.92 | 0.85 | 0.82 | 0.85 | 3.43 | |||
| Adherence descriptiona | Coverage rate | Cost per child accounting for adherence rates | ||||||
|---|---|---|---|---|---|---|---|---|
| Received at least the first day treatment of SMC in all 4 rounds | 53.8% | US $ 6.38 | ||||||
| Received at least the first day treatment of SMC in at least 3 rounds | 73.1% | US $ 4.69 | ||||||
| Received at least the first day treatment of SMC in at least 2 rounds | 84.8% | US $4.05 | ||||||
aBased on information from maternal interview only Taken from Diawara et al. [13]. Table 1 Coverage of SMC defined as proportion of children who received SMC drugs at day 1 and days 1–3 according to the source of the information (interview or SMC card) during the coverage survey and Prof Alassane Dicko personal communication
Effectiveness
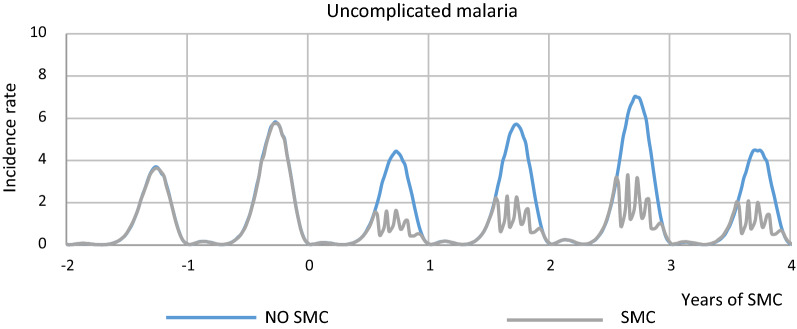
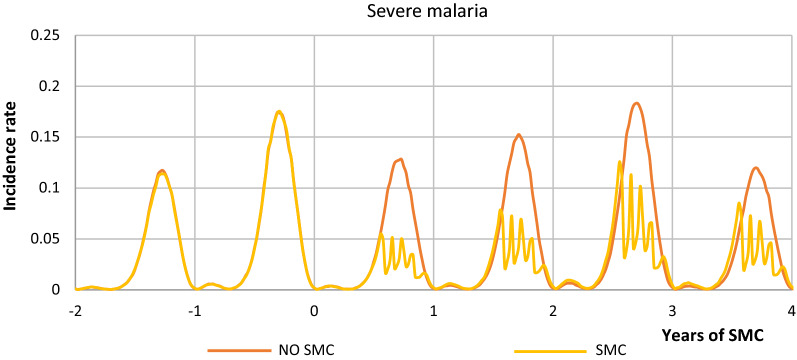
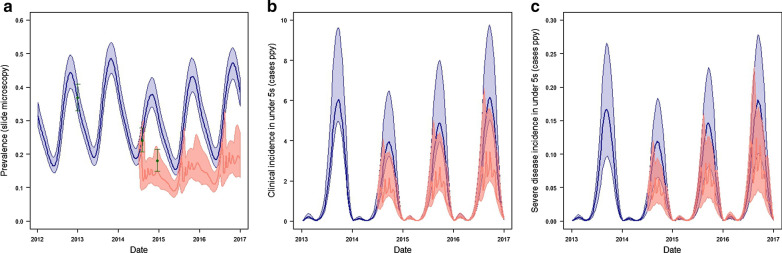
Figures 2, 3 shows the range of model simulations for transmission in Kita prior to SMC and during SMC implementation, as well as counter-factual simulations for the trajectory of transmission and patterns of burden that would have occurred in the absence of SMC. Simulations were calibrated to the pre-intervention survey, LLIN scale-up and seasonal rainfall; show a good fit to prevalence measured soon after the end of the 2012 transmission season; and are within the range of the prevalence observed soon after the end of the intervention (Fig. 4). Counter-factual simulations capture the likely reductions in burden that will have occurred following the LLIN campaign in 2014, with transmission increasing towards the end of the 3-year LLIN distribution cycle. In the year of SMC implementation our simulations are consistent with SMC preventing 660 (435–922 95% uncertainty interval (UI)) uncomplicated cases and 15.4 (8.42–24.3 95% UI) cases of severe malaria per 1000 children reached among children below five years of age. In the all-age population, which also captures the impact of SMC on transmission, this represents 118 (78.2–163 95% UI) cases and 2.61 (1.43–4.14 UI) severe cases per 1000 person-years. Averaged over the 3-year LLIN distribution cycle this gives 804 (478–1213 95% UI) uncomplicated cases and 16.5 (8.89–27.1 95% UI) cases of severe malaria per 1000 children reached and 141 (84.3–209 95% UI) cases and 2.73 (1.46–4.43) severe cases per 1000 person-years. Table 6 provides the number of cases averted annually incorporating population size and these figures inform the ICER.
Fig. 2.
Uncomplicated malaria incidence in children under 5 with SMC and no SMC in Kita district
Fig. 3.
Severe malaria incidence in children under 5 with SMC and no SMC in Kita district
Fig. 4.
Simulations of the effectiveness of SMC in Kita shows a simulations of the impact of SMC upon prevalence in ages 3 -59 months, with estimates from prevalence surveys, including the 2014 pre-intervention to which the simulations were calibrated are shown as green dots with bars showing binomial 95% confidence intervals, b simulated incidence of uncomplicated clinical malaria in children under 5 and c incidence of severe malaria in children under 5. In all plot simulations using model parameters from the median of the joint posterior distribution and modal values from the triangle-distributed uncertainty distributions are shown in dark lines (blue for simulations in the absence of SMC, salmon in the presence of SMC), shaded areas show 95% uncertainty intervals based upon the 1000 simulated parameter draws. * Note these are 1 year averages based on a 3 year cycle that takes into account the waning protective efficacy of LLINs
Table 6.
Effectiveness outcomes: malaria episodes averted
| Total over 1 year time horizona Kita pop | Children | All population | ||||
|---|---|---|---|---|---|---|
| Cases | Uncomplicated | Severe | Cases | Uncomplicated | Severe | |
| Mean | 62,313 | 61,031 | 1282 | 72,676 | 71,265 | 1411 |
| Lower 95% limita (based on 1000 simulations) | 37,047 | 36,358 | 689 | 43,535 | 42,781 | 754 |
| Higher 95% limit (based on 1000 simulations) | 94,004 | 91,904 | 2099 | 108,144 | 105,853 | 2291 |
aUncertainty bound
ICER
The incremental cost effectiveness ratios (ICER) per malaria episode averted were estimated for children and for the entire population (Table 7). Based on predicted coverage, the financial cost per childhood episode averted was estimated at US $5.07 (uncertainty bounds 3.36–8.53) and the economic cost was US $5.74 (3.80–9.65), respectively. In the all-ages population in Kita, the economic cost per malaria episode averted was US $4.92 (range 3.31–8.21). Given that coverage was higher than predicted, it could be argued that the more accurate ICERs are a financial cost per childhood episode averted of US $3.77 (uncertainty bounds 2.50–6.34) and an economic cost of US $4.26 (range 2.83—7.17) per childhood episode averted. As shown in Table 7, the ICER is US $144 (135–153) per DALY averted and US $14,503 (13,604–15,402) per death averted based on actual children covered. See Appendix 1 for DALY calculations.
Table 7.
Cost effectiveness analysis of SMC (US $ 2016)
| Mean | Lower 95% limita | Higher 95% limit | |
|---|---|---|---|
| Costs Per Episode Averted | |||
| Financial cost per childhood episode averted (actual coverage) | 3.77 | 2.50 | 6.34 |
| Economic cost per childhood episode averted (actual coverage) | 4.26 | 2.83 | 7.17 |
| Financial cost per childhood episode averted (predicted coverage) | 5.07 | 3.36 | 8.53 |
| Economic cost per childhood episode averted (predicted coverage) | 5.74 | 3.80 | 9.65 |
| Financial cost per all ages episode averted (predicted coverage) | 4.35 | 2.92 | 7.26 |
| Economic cost per all ages episode averted (predicted coverage) | 4.92 | 3.31 | 8.21 |
| Economic Cost per DALY averted (actual coverage) | 144 | 135 | 153 |
| Economic Cost per Death averted (actual coverage) | 14,503 | 13,604 | 15,402 |
aUncertainty bounds – for cost per episode these were taken from the ranges of the model effectiveness estimates. For the DALYs and Deaths a ± 20% was used in the absence of any cost uncertainty, a gamma distribution was assumed for all parameters and 10,000 iterations were run using monte carlo simulations
Discussion
The economic cost of SMC was US $0.86 per child per SMC round and the cost per child fully adherent was $6.38. The cost effectiveness ratios were estimated at an economic cost of US $4.26 per childhood episode averted and US $144 per DALY averted. Thus, SMC was cost effective as US $144 per DALY averted was well below two very conservative thresholds: (i) USD $779.94 per DALY averted, based on Mali’s 2016 GDP per capita [33] and (ii) an even more stringent World Bank threshold of $250 per DALY averted [29, 30].
In addition to the multi-country ACCESS SMC study (which included Mali) (12), other studies of routine SMC implementation comparable to Kita in terms of scale and their attempt to deliver through the routine health system have been conducted in Senegal [7], Upper West Region of Ghana [6], Koutiala in Mali [8], and elsewhere (see Table 1). At US $0.86 per child per round, Kita SMC unit costs are amongst the lowest reported to date. Only Senegal, where the economic cost per child per round was reported to be $0.55, shows a lower economic cost per round. A major difference in the Kita study and the Senegalese study is that costs incurred at national level were not included in the total Senegalese unit cost. Kita SMC delivery unit costs were lower than the two Malian SMC studies conducted to date. In Koutiala, an estimate based on the budget expenses found a unit cost per child per round at 1.12 euros or $1.30 (inflated to 2016 $US) [8], compared to an average economic cost of US $ 0.86 per round in Kita. The other Malian study, ACCESS-SMC, suggests a preliminary average estimates of $4.05 (USD 2015) for the delivery of four doses using both door-to-door and fixed point delivery strategies [10, 11] compared to US $3.43 delivery of four rounds in Kita. The average cost of four treatments per child, weighted across the seven ACCESS countries was US $3.63 (12).
This study shows drug costs and personnel costs (largely made up of incentives) were the main cost drivers. This is in line with most previous SMC cost studies, for example in the Senegal study incentives to CHWs and drugs costs were the highest costs, representing 44% and 27% of the total cost, respectively. The economic cost estimate of US $4.26 per malaria case averted (2.83–7.17), is lower than that found in Hohoe Ghana US $24.87 (22.63–27.36) per case averted and Upper West Region Ghana US $108.41 (101.01–123.01). However, the per death were higher than Upper West Region Ghana. At US $144 per DALY averted it is well below both conservative thresholds we enlisted to identify highly cost effective interventions. This is the first SMC cost-effectiveness analysis to use primary cost data to estimate the cost per DALY averted for SMC and also one of very few large-scale implementation studies to estimate the cost- effectiveness of SMC using a fixed point distribution.
The study has limitations. While the costs were taken directly from the intervention site, the effectiveness of the intervention was modelled on data drawn from surveys as data on malaria episodes and deaths averted were not available. However, the model was calibrated to local data and has been widely published. In terms of generalising beyond this study setting, SMC drugs were not in co- blister packs, so had to be packed manually and this resulted in additional costs. Using co-blister drugs, depending on their price, might lead to an increase the cost of the intervention.
Conclusion
SMC was highly cost effective when delivered at large scale, fixed-points through the routine health system in Mali. The main costs were drugs and personnel, with financial incentives as the largest component of the personnel cost.
Acknowledgements
Not applicable.
Disclaimer
The opinions expressed in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention.
Appendix 1: DALY and deaths averted calculations
DALYs for a disease or health condition are calculated as the sum of the Years of Life Lost (YLL) due to premature mortality in the population and the Years Lost due to Disability (YLD) for people living with the health condition or its consequences,1,2:
These calculations incorporate weights for life expectancy, age, future time and disability. See the table below for the weightings assigned to our analysis.3
where K: age weighting modulation factor, C: constant, r: discount rate expressed as decimal, a: age of onset disability, β: parameter from the age weighting function, L: duration of disability, D: disability weight.
Inputs
| Formula | Malaria DALY Calculations and weightings | Assumption and source | |
|---|---|---|---|
| Uncomplicated | Severe | ||
| DALYs | DALYs | ||
| C | 0.1658 | 0.1658 | |
| K | 0 | 0 | No age weighting applied |
| r | 0.03 | 0.03 | A discount rate of 3% applied |
| a | 2.5 | 2.5 | Mid point between sample cohort of 0–5 |
| B | 0 | 0 | No age weighting applied |
| LD (7 or 30 days) | 0.019178082 | 0.082191781 | The assumed duration of an uncomplicated malaria episodes was 7 days, expressed in years (7/365) and a severe episode, 30 days, expressed in years (30/365) |
| D | 0.006 | 0.133 | IHME, GBD 2016–2017 (based on 2015 estimates at the most recent weightings available) |
| Life expectancy | 72 | 72 | According to the Global Health Observatory 72.0 years was the average life expectancy at birth of the global population in 2016 https://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends/en/ |
| CFR malaria | 0.3% | 0.3% | WHO Health Information 2015—Indicators https://www.who.int/healthinfo/indicators/2015/chi_2015_33_mortality_malaria.pdf |
| DALY per episode using the above parameters | |||
| YLD | 0.019 | 0.082 | |
| YLL | 30.31 | ||
The total number of DALYs was calculated by multiplying the DALYs weights with the annual sum of clinical and severe episodes predicted in the epidemiological model
Authors’ contributions
HD: Collect data, draft the design and contribute to the analysis and interpretation of data. Write the manuscript. PW and MC: Contribute to the study design, analysis and the work revision. FD: Contribute to data collection and substantively revised the manuscript. BK: Contribute to data collection, study design and substantively revised the manuscript. LD: Contribute to the study design and the manuscript review. LCS, ES, IS, AS, MJ, EE: Contribute to substantively revised the manuscript. AD: contribute to the study design, data analysis and interpretation of data. LC: led the study design, contributed substantively to data analysis and interpretation and the manuscript writing and multiple revisions. All authors read and approved the final manuscript.
Funding
This work was funded by the U.S. President’s Malaria Initiative (PMI).
Availability of data and materials
The datasets analysed for this study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
References
- 1.WHO. World Malaria Report 2019. Geneva: World Health Organization; 2019. https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019
- 2.PNLP/EIPM. République du Mali - Enquête sur les Indicateurs du Paludisme (EIPM) 2015, 2016.
- 3.CPS/SSDSPF. Enquête Démographique et de Santé au Mali 2012–2013 / Mali DHS 2012–2013, 2014.
- 4.Mali Gd. Système National d’Information Sanitaire et Social. Annuaire. 2015;2015:206. [Google Scholar]
- 5.Programme WGM. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum malaria control in highly seasonal transmission areas of the Sahel sub-region in Africa. Geneva: World Health Organization; 2012. https://www.whoint/malaria/publications/atoz/smc_policy_recommendation_en_032012pdf?ua=1.
- 6.Novignon J, Aryeetey GA, Issah S, Ansah O, Malm KL, Ofosu W, et al. Cost-effectiveness of seasonal malaria chemoprevention in upper west region of Ghana. Malar J. 2016;15:367. doi: 10.1186/s12936-016-1418-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitt C, Ndiaye M, Conteh L, Sy O, Hadj Ba E, Cissé B, et al. Large-scale delivery of seasonal malaria chemoprevention to children under 10 in Senegal: an economic analysis. Health Policy Plan. 2017;32:1256–1266. doi: 10.1093/heapol/czx084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Médecins sans Frontières. Chimio-Prevention du Paludisme Saisonnier, 2013. p. 1–2.
- 9.Malaria Consortium. Learning from experience: Seasonal chemoprevention as an effective malaria preventive strategy for children in the Sahel. https://www.malariaconsortiumorg/media-downloads/1202/Seasonal%20chemoprevention%20as%20an%20effective%20malaria%20preventive%20strategy%20for%20children%20in%20the%20Sahel, 2019.
- 10.ACCESS SMC. https://www.access-smc.org/. 2019 (ACCESS SMC Project Website). Accessed 14 Aug 2019.
- 11.Collins D, Gilmartin C. SMC Cost Analyses. MSH 2017. https://www.malariaconsortium.org/media-downloads/959/SMC%20Cost%20Analyses. Accessed 15 Aug 2019.
- 12.ACCESS-SMC Partnership. Effectiveness of seasonal malaria chemoprevention at scale in west and central Africa: an observational study. Lancet. 2020;396:1829–40. [DOI] [PMC free article] [PubMed]
- 13.Diawara F, Steinhardt LC, Mahamar A, Traore T, Kone DT, Diawara H, et al. Measuring the impact of seasonal malaria chemoprevention as part of routine malaria control in Kita. Mali Malar J. 2017;16:325. doi: 10.1186/s12936-017-1974-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mali Demographic and Health Survey. http://ghdx.healthdata.org/record/mali-demographic-and-health-survey-2012-2013 2012–2013. Accessed June 2014.
- 15.Mogyorosy Z, Smith P. The main methodological issues in costing health care services: A literature review. Centre for Health Economics Research Paper 7. University of York, 2005.
- 16.Patouillard E, Conteh L, Webster J, Kweku M, Chandramohan D, Greenwood B. Coverage, adherence and costs of intermittent preventive treatment of malaria in children employing different delivery strategies in Jasikan. Ghana PLoS One. 2011;6:e24871. doi: 10.1371/journal.pone.0024871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calculator UI. https://www.usinflationcalculator.com/. 2019. Accessed 7 Feb 2019.
- 18.Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun. 2014;5:3136. doi: 10.1038/ncomms4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin JT, Bhatt S, Sinka ME, Gething PW, Lynch M, Patouillard E, et al. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis. 2016;16:465–472. doi: 10.1016/S1473-3099(15)00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Source code, https://github.com/jamiegriffin/Malaria_simulation
- 21.Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4:e474–e484. doi: 10.1016/S2214-109X(16)30073-0. [DOI] [PubMed] [Google Scholar]
- 22.Cairns ME, Walker PG, Okell LC, Griffin JT, Garske T, Asante KP, et al. Seasonality in malaria transmission: implications for case-management with long-acting artemisinin combination therapy in sub-Saharan Africa. Malar J. 2015;14:321. doi: 10.1186/s12936-015-0839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zongo I, Milligan P, Compaore YD, Some AF, Greenwood B, Tarning J, et al. Randomized noninferiority trial of dihydroartemisinin-piperaquine compared with sulfadoxine-pyrimethamine plus amodiaquine for seasonal malaria chemoprevention in Burkina Faso. Antimicrob Agents Chemother. 2015;59:4387–4396. doi: 10.1128/AAC.04923-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Health Information 2015 - Indicators https://www.who.int/healthinfo/indicators/2015/chi_2015_33_mortality_malaria.pdf
- 25.Gunda R, Chimbari MJ. Cost-effectiveness analysis of malaria interventions using disability adjusted life years: a systematic review. Cost Eff Resour Alloc. 2017;15:10. doi: 10.1186/s12962-017-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva, World Health Organization; 2003.
- 27.Woods B, Revill P, Sculpher M, Claxton K. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Health. 2016;19:929–935. doi: 10.1016/j.jval.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertram MY, Lauer JA, De Joncheere K, Edejer T, Hutubessy R, Kieny MP, et al. Cost-effectiveness thresholds: pros and cons. Bull World Health Organ. 2016;94:925–930. doi: 10.2471/BLT.15.164418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marseille E, Larson B, Kazi DS, Kahn JG, Rosen S. Thresholds for the cost-effectiveness of interventions: alternative approaches. Bull World Health Organ. 2015;93:118–124. doi: 10.2471/BLT.14.138206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shillcutt SD, Walker DG, Goodman CA, Mills AJ. Cost effectiveness in low- and middle-income countries: a review of the debates surrounding decision rules. Pharmacoeconomics. 2009;27:903–917. doi: 10.2165/10899580-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dicko A, Diallo AI, Tembine I, Dicko Y, Dara N, Sidibe Y, et al. Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide-treated bednet in Mali: a randomised, double-blind, placebo-controlled trial. PLoS Med. 2011;8:e1000407. doi: 10.1371/journal.pmed.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson AL. A systematic review and meta-analysis of the efficacy and safety of intermittent preventive treatment of malaria in children (IPTc) PLoS ONE. 2011;6:e16976. doi: 10.1371/journal.pone.0016976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Bank. Mali GDP per capita (current US $) 2019. Accessed 7 Feb 2019.
- 34.Devleesschauwer B, Havelaar AH, Maertens de Noordhout C, Haagsma JA, Praet N, Dorny P, Duchateau L, Torgerson PR, Van Oyen H, Speybroeck N. Calculating disability-adjusted life years to quantify burden of disease. Int J Public Health. 2014;59(3):565–9. 10.1007/s00038-014-0552-z. [DOI] [PubMed]
- 35.Larson BA. Calculating disability-adjusted-life-years lost (DALYs) in discrete-time. Cost Eff Resour Alloc. 2013;11(1):18. 10.1186/1478-7547-11-18. [DOI] [PMC free article] [PubMed]
- 36.Fox-Rushby JA, Hanson K. Calculating and presenting disability adjusted life years (DALYs) in cost-effectiveness analysis. Health Policy Plan. 2001; 16(3):326–31. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed for this study are available from the corresponding author on reasonable request.