Abstract
Status epilepticus (SE) or prolonged epileptic seizure activity is a common neurological emergency with a high mortality rate and, if left untreated, can lead to irreversible cerebral damage and systemic complications. Fast and effective first-line management is of paramount importance, particularly in the at-home management of seizures where drug administration routes are limited. Benzodiazepines (BZDs) have been exclusively used in veterinary medicine for decades as first-line drugs based on their high potency and rapid onset of action. Various administration routes exist in dogs, such as oral, intravenous, intramuscular, rectal, and intranasal, all with different advantages and limitations. Recently, intranasal drug delivery has become more popular due to its unique and favourable characteristics, providing potential advantages over other routes of drug administration in the management of canine SE. This narrative review provides an outline of the management of SE at home and in a hospital setting, discusses considerations and challenges of the various routes of BZD administration, and evaluates the impact of intranasal drug administration (nose-brain pathway) for controlling canine SE at home and within hospital settings.
Background
Although most epileptic seizures are self-limiting and last for a few seconds or minutes (usually < 2–3 min), in some cases seizures can be prolonged leading to the development of status epilepticus (SE) [1]. SE is broadly defined clinically as seizures lasting > 5 min or multiple seizures with incomplete inter-seizure recovery and remains a common neurological emergency [1–3]. In self-limiting seizures, an array of processes lead to seizure termination including i) excitatory neurotransmitter (glutamate) and ATP depletion, ii) enhanced γ-aminobutyric acid (GABA)-induced inhibition, iii) adenosine release, iv) ionic (calcium, potassium) level alterations, and v) acidification of intra- and extracellular space [4]. Insufficiency of the seizure termination mechanisms and imbalance between excitatory and inhibitory activity within the forebrain’s neuronal network may lead to SE in both humans and animals [5, 6]. In addition, other mechanisms that promote seizure activity during SE include inflammatory processes (e.g. interleukins), enhanced pro-epileptogenic peptide expression (e.g. substance P), and blood-brain barrier (BBB) dysfunction [7–11].
SE can occur in dogs with idiopathic epilepsy (IE), structural epilepsy or reactive seizures [12–14]. In general, 0.5–2.6% of dogs are admitted to the emergency hospital for SE [15, 16]. Among the population of dogs presenting to hospital for seizures, 16.5% manifest SE [12] and the latter has been identified as the first clinical manifestation of an epileptic seizure disorder in 58% of dogs [16]. Studies showed that 32–40%, 27–59%, and 7–23% of dogs with structural epilepsy, IE, and reactive seizures, respectively, can present with SE [12–14, 16]. SE can lead to permanent brain damage (e.g. neuronal cell necrosis, network reorganization, gliosis) and severe systemic complications (e.g. cardiorespiratory collapse, shock, acidosis, electrolyte imbalances) [5]. The occurrence and severity of SE-induced complications are proportionally related to the duration of seizure activity [17–21]. With regard to the survival rates, an overall mortality rate of 25.3–38.5% among all dogs presented with SE has been reported [12, 16]. Therefore, clinicians or owners should quickly intervene to cease the continuous seizure activity either in hospital or at home, respectively [22–25]. Owners, in particular, have a substantial role in seizure control because appropriate administration of antiseizure drugs at home could prevent seizure progression to SE or reduce the risk of progression to more refractory stages. First-line management is of paramount importance and should include drugs with high potency and rapid onset of action. The aims of first-line management are i) cessation of seizures, ii) prevention of SE refractory phases, iii) prevention of complications, and iv) avoidance of adjunction of anaesthetic and non-anaesthetic antiseizure medications that could increase the risk of adverse effects [24]. Benzodiazepines (BZDs) have been exclusively used for decades in humans and animals as first-line antiseizure treatment due to their high potency and rapid onset of action [22–25].
The primary goals of this review are to i) provide an outline about the management of SE at home and in the hospital, with particular focus on first-line pharmacological intervention, ii) discuss the considerations and challenges of the various routes of BZD administration, iii) analyse and evaluate the recently introduced intranasal (IN) drug delivery method for controlling SE in dogs, and iv) provide guidance for primary and specialist clinicians regarding SE management within home and hospital settings.
Therapeutic considerations in status epilepticus
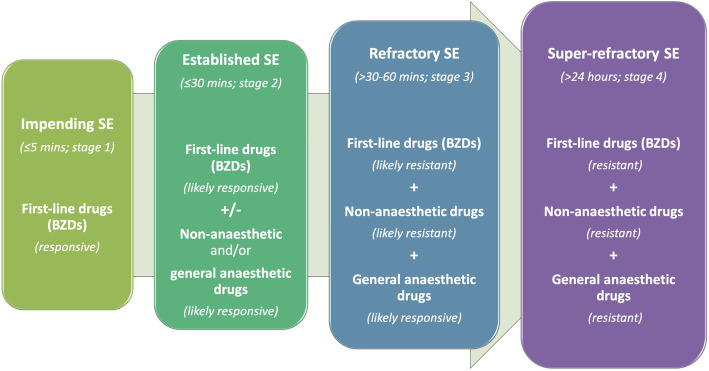
SE therapy should incorporate a combination of medical interventions including antiseizure medication and measurements for treating seizure-related complications (e.g. intravenous fluid therapy for addressing in case of electrolyte imbalances) and underlying aetiological conditions (e.g. radiotherapy or surgical therapy in case of brain neoplasia, immunosuppressive therapy in case of immune-mediated meningoencephalitis) [5, 26]. Regarding seizure activity termination, SE may be subdivided into four different stages (Fig. 1), which differ in terms of sensitivity to the drugs used, treatment options as well as morbidity and mortality rates [17, 19, 20, 27–31]:
- Impending SE
- Less than 5 min of continuous seizure activity.
- Seizures are most likely responsive solely to first-line antiseizure therapy.
- Established SE
- Less than 30 min of continuous seizure activity.
- Seizures are still, but likely less, responsive to first-line antiseizure therapy.
- Adjunctive non-anaesthetic (e.g. phenobarbital, levetiracetam) or general anaesthetic (e.g. propofol, ketamine, pentobarbital, etomidate, inhalation anaesthetics) antiseizure therapy might be needed.
- Refractory SE
- Less than 30–60 min of continuous seizure activity.
- Seizures are resistant to first-line and non-anaesthetic antiseizure therapy.
- Adjunctive general anaesthetic antiseizure therapy is needed.
- Super-refractory
- More than 24 h of continuous seizure activity or seizure recurrence after initiation of treatment with general anaesthetic antiseizure therapy.
- Seizures are likely resistant to any antiseizure therapy.
Fig. 1.
Stages of SE according to time and responsiveness to antiseizure medication. The more advanced the stage of SE is, the less responsive to antiseizure medication, and in particular first-line drugs (benzodiazepines), will be. Thus, in more advanced stages of SE, further antiseizure medication might be gradually added-on in order to control the epileptic seizures
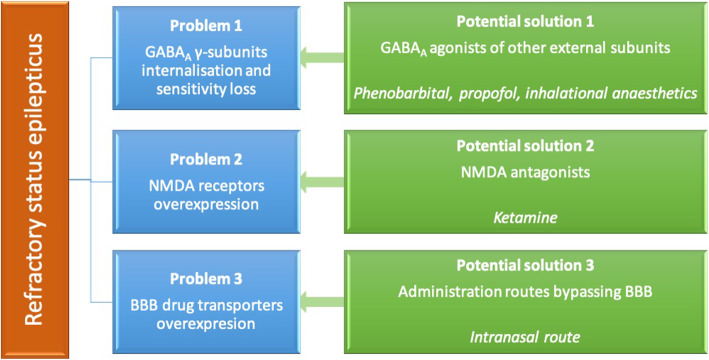
The reasons SE progresses towards more refractory stages over time are related to multiple processes that inhibit cessation of activity including mainly i) loss of GABA-induced inhibition, ii) upregulation of excitation induced by N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors for glutamate, and iii) BBB transporters overexpression [6, 32]. GABAergic drugs (e.g. benzodiazepines (BDZs)) are particularly used in the management of SE [24, 25]. BZD’s effects derive from their action on pre- and postsynaptic GABA-ergic transmission; specifically, they bind on the γ-subunit of GABAA receptors, enhancing the inhibitory effect of GABA, and result in an opening of chloride channels and influx of chloride within the neurons. This effect leads to hyperpolarisation of the cell membrane and inhibition of the transmission of nerve impulses [30, 33]. BZDs’ effectiveness, though, may gradually decrease with prolonged SE due to reduced synaptic targets (e.g. internalization of GABAA receptors γ-subunits, alterations in GABAA receptor trafficking and conversion of receptors subunits to less BZD-responsive) and changes in chloride homeostasis [34, 35]. Medications that act also on other external subunits (e.g. α, β) of GABAA receptors (e.g. phenobarbital, propofol, inhalation anaesthetics) should be more efficient in cases of BZD-resistant SE [36–38]. In (super) refractory SE, resistance to most of GABAA-acting drugs may occur due to several factors including phosphorylation and internalization of the potassium-chloride transporter and increased concentration of intracellular chloride [39]. In addition, loss of AMPA receptors GluA2 subunit and overexpression of NMDA receptors occur, which promote glutamate-induced excitation [40, 41]; these changes lead to calcium accumulation within the cells and trigger apoptosis [41]. Glutamate receptor (NMDA) antagonists (e.g ketamine) may be beneficial particularly in refractory stages of SE and they may even help preventing resistance, if administered at early stages [6]. Overexpression and activation of NMDA receptors may also contribute towards calcineurin-induced internalization of the GABAA receptors γ-subunits, leading further to BZD resistance [42, 43]. Therefore, NMDA receptor inhibitors may also have another benefit by means of enhancing BZDs potency as it was observed in animal models for SE [44–46]. Another process that occurs during SE is the overexpression of BBB drug transporters, which results in pharmaco-resistance [47]. A significant upregulation by 87–166% of endothelial P-glycoprotein (PGP; BBB drug transporter) was demonstrated in the canine brain following SE that leads to enhanced BBB efflux of antiseizure drugs and limited concentrations of drugs into the brain [48]. Lastly, after prolonged seizure activity, alterations in gene expression and associated protein production responsible for drug transporters as well as reorganization of synapses occur; all these processes contribute further to the drug resistance as it was shown in animal and human studies [17, 49, 50]. Therefore, the early application of drugs with different mechanisms of action (e.g. GABAA agonists and NMDA antagonists) and through different routes (e.g. administration routes that might avoid BBB) with the aim to circumvent the mechanisms that sustain continuous seizure activity is fundamental for the management of SE (Fig. 2).
Fig. 2.
Summary of the main causes of pharmaco-resistance in refractory stages of SE and potential solutions to them
Benzodiazepines
Diazepam (DZP), midazolam (MDZ), and lorazepam (LZP) are the main representatives of BZDs used as first-line treatment of SE [22–25]. In veterinary medicine, MDZ and DZP have been mainly used, although only DZP is currently licenced for small animals. In dogs, both DZP and MDZ can be effective for ceasing seizure activity, but this can depend on the administration route and dose [5, 22, 23, 25]; the recommended dose ranges for MDZ or DZP remain the same regardless the route of administration. Although not well-defined, it has been estimated that DZP should reach serum concentration of 0.15–0.5 μg/mL [33, 51–53] within 10–15 min in order to provide a clinically acceptable seizure control in canine SE and prevent progression to more refractory stages. For MDZ, these values have not been established for dogs, but it is speculated from human medicine that serum concentration of < 0.04 μg/mL might be adequate for seizure cessation [54]. In addition, MDZ might be potentially more potent than DZP because, in one canine study, MDZ manifested a higher suppressive effect on lidocaine-induced epileptic seizures compared to DZP [55]. In a human pharmacodynamic and encephalographic study, MDZ was found to be 5–6 times more potent than DZP [56]. In addition, MDZ has gained more popularity in the management of SE due to its safer drug profile, i.e. MDZ-induced brain and respiratory depression are less severe compared to DZP and LZP [30].
MDZ is a hydrophilic drug but converts to lipophilic at physiologic pH (e.g. tissues), which facilitates penetration of BBB [30, 56]. MDZ can be administered at a dose of 0.2–0.5 mg/kg IV, IN or intramuscular (IM) but has a short half-life (approximately 1 h in the dog), and thus, frequent administration or constant rate infusion (CRI) might be necessary for adequate seizure control [5, 25]. An IV CRI at the dose of 0.2–0.5 mg/kg/h might be advised to sustain good seizure control after the delivery of the initial MDZ bolus dose [5, 25]. The CRI dose is administered via an infusion pump and usually diluted in 0.9% saline or 5% dextrose solution, with the volume used being equal to the dog’s hourly maintenance fluid requirements [5, 25]. The dosage rate should be reduced by 50% every 6 h for at least two times before discontinuation of the drug [5, 25]. In humans, it has been shown that MDZ’s half-life increases after CRI [57].
DZP is a lipophilic drug, which can also penetrate the BBB [30, 56]. Because of its lipophilicity, it is prepared in propylene glycol, which can induce phlebitis and hypotension, especially when rapidly administered [5]. Therefore, central IV access may be needed for preventing phlebitis, although establishing such an access might be quite challenging and time-consuming during SE [5]. DZP can also adsorb to plastic and, therefore, should not be stored in plastic syringes or infusion lines for any length of time [58]. DZP may be administered as a bolus at the dose of 0.5–2.0 mg/kg IV, IN or rectally (R) [5, 25]. Repeat DZP bolus leads to accumulation and high concentrations of the drug in the central nervous system (CNS), cerebrospinal fluid (CSF), and serum; although this may result in prolonged antiseizure activity, it can also cause severe CNS depression and cardiorespiratory collapse [59]. Therefore, only 2–3 DZP boluses should be considered and, if unsuccessful, an IV CRI (at the dose of 0.1–0.5 mg/kg/h) or another antiseizure drug should be considered [5, 25]. Co-administration of levetiracetam enhances DZP’s antiseizure effect and, thus, DZP’s dose adjustment might be needed [60]; this phenomenon might also occur with the combination of levetiracetam and other BDZs [61].
Administration routes
Various administration routes have been studied in human and veterinary medicine for managing SE either at home or in hospital. One of the main challenges is the at-home management of emergency seizures as therapeutic options and routes of administration are quite limited and restricted to non-IV routes of administration. Given the fact that SE management should be commenced at the earliest possible, there is an undoubted need for quickly effective routes of administration that can be applied in the out-of-hospital environment by owners or even clinicians within hospital settings (especially when IV line has not been yet established) [62]. Advantages and challenges of the various administration routes are discussed below and summarised in Table 1. Information regarding each BZD’s recommended dose and target serum concentration as well as reported serum concentrations, time to peak serum concentrations and time to seizure control achieved with each administration route in dogs is provided in Table 2.
Table 1.
Advantages and limitations of benzodiazepines delivery routes in dogs
| Administration route | Advantages | Limitations |
|---|---|---|
| Intravenous |
Likely effective (clinical evidence) Likely rapid onset of action (clinical evidence) Precise control of the administered dose Avoidance of first-pass hepatic metabolism |
Subject to blood-brain barrier Requirement for hospitalisation Requirement for medically-trained staff Hard to establish during seizures Not for at-home use |
| Intramuscular |
Likely favourable pharmacokinetics Avoidance of first-pass hepatic metabolism |
Subject to blood-brain barrier Requirement for training or medical staff Needle/syringe misuse by non-trained caregivers Less suitable for at-home use Soft tissue or nerve damage risk Infection risk Painful |
| Transdermal |
Painless Easy to use Suitable for home No requirement for medical training Avoidance of first-pass hepatic metabolism |
Subject to blood-brain barrier Slow release not suitable for emergency |
| Buccal |
Painless Ease to administer Suitable for home No requirement for medical training Avoidance of first-pass hepatic metabolism |
Subject to blood-brain barrier Potentially unfavourable pharmacokinetics Delivery of limited drug amount If swallowed, functions as oral Dog’s compliance is needed Incorrect administration during seizures |
| Sublingual | Similar to buccal | Similar to buccal |
| Oral |
Painless Easy to use No requirement for medical training Suitable for home |
Subject to blood-brain barrier Potentially unfavourable pharmacokinetics Slow absorption not suitable for emergency Potential for gastrointestinal degradation Subject to first-pass hepatic metabolism Dog’s compliance is needed |
| Rectal |
Minimal pain/discomfort Relatively easy to use No requirement for medical training Suitable for home |
Subject to blood-brain barrier Variability in effectiveness (clinical evidence) Variability in pharmacokinetics Partially subject to first-pass hepatic metabolism Likely slow onset of action Socially unacceptable |
| Intranasal |
Likely effective (clinical evidence) Likely rapid onset of action (clinical evidence) Likely favourable pharmacokinetics Avoidance of first-pass hepatic metabolism Avoidance of blood-brain barrier No requirement for medical training Relatively easy to use Painless Suitable for home |
Need for high concentration drug Potentially affected by mucosal factors Potentially affected by drug formulation Need for a veterinary nasal device |
Table 2.
Information regarding each benzodiazepine’s recommended dose and target serum concentration as well as reported serum concentrations, time to peak serum concentration and time to seizure control achieved with each administration route in dogs
| Midazolam | Diazepam | |
|---|---|---|
| Recommended dose | 0.2–0.5 mg/kg intravenous, intranasal or intramuscular | 0.5–2.0 mg/kg intravenous, intranasal or rectal |
| Recommended target serum concentration for seizure control (pharmakokinetic studies) | < 0.04 μg/mL (value derived from humans) | 0.15–0.5 μg/mL |
| Serum concentration achieved with each administration route (pharmakokinetic studies) | Intravenous | |
| NA | NA | |
| Intranasal | ||
| 0.21 ± 0.02 μg/mL (solution) or 0.45 ± 0.09 μg/mL (gel) | 0.44 ± 0.04 μg/mL (solution) or 0.31 +/− 0.17 (solution/atomised formulation) | |
| Intramuscular | ||
| 0.20 ± 0.06 μg/mL or 0.55 ± 0.12 μg/mL (solution) | NA | |
| Rectal | ||
| NA | 0.5 μg/mL (solution) and or 0.01–0.1 μg/mL (suppository) | |
| Buccal | ||
| 0.1–0.2 μg/mL (gel) | NA | |
| Sublingual | ||
| NA | NA | |
| Time to peak serum concentration achieved with each administration route (pharmakokinetic studies) | Intravenous | |
| NA | NA | |
| Intranasal | ||
| 12 min (gel) or 17 min (solution) | 4.5–8.0 min (solution/atomised formulation) | |
| Intramuscular | ||
| 10–15 min (solution) | NA | |
| Rectal | ||
| NA | 15 min (solution) or 80 min (suppository) | |
| Buccal | ||
| 15 min (gel) | NA | |
| Sublingual | ||
| NA | NA | |
| Time to seizure control achieved with each administration route (clinical studies) | Intravenous | |
| 1.0–4.5 min | NA | |
| Intranasal | ||
| 0.5–1.6 min | NA | |
| Intramuscular | ||
| NA | NA | |
| Buccal | ||
| NA | NA | |
| Sublingual | ||
| NA | NA | |
| Rectal | ||
| NA | 2.5 min | |
Intravenous
IV administration of BZDs has an onset of action approximately within < 2–7 min, circumvents first-pass hepatic metabolism (i.e. i.e. liver-induced drug metabolism whereby the concentration of a drug might be substantially reduced) [30, 56], and is likely effective for ceasing SE in humans [63–71] and dogs [23, 25, 33, 72–74]. In humans, IV administration of BDZs has been considered the “gold standard” route as it can result in the highest drug efficacy and shorter seizure cessation time [75–77]; similar recommendations have been made in dogs [25, 78]. In clinical practice, however, delays in establishing IV access in a seizuring human [79–81] or dog [23, 72] can be significant and negatively affect IV drugs’ onset of action. Based on a systematic review/meta-analysis in humans, non-IV BDZs could be administered faster to patients and demonstrated superior efficacy and onset of action in terminating seizures compared to IV-BDZs [79]. In veterinary medicine, there are not enough clinical trials to allow the conduction of a systematic review/meta-analysis evaluating and comparing different BDZ or IV versus non-IV routes of administration, but it is likely that the experience and evidence derived from human medicine could be translated to veterinary medicine. Existing clinical data, though, already indicates that IV route, despite being effective, might not be the “gold standard” delivery method as it was widely speculated up to date. Specifically, based on a recent multicenter clinical trial in canine SE, IV administration of MDZ was inferior to IN with regard to median seizure cessation time (1 min for IV versus 0.5 min for IN), especially when the time needed to establish an IV catheter was considered (4.5 min for IV versus 1.6 min for IN) [23]. The main factors in clinical practice that pose significant difficulties in establishing or maintaining functional IV access and delays in IV drug administration and seizure cessation include i) convulsive seizure activity, ii) requirement of experienced medically-trained staff, iii) patient’s cardiovascular collapse, and iv) small or toy canine breeds [23, 68, 72, 79, 82, 83].
Intramuscular
IM BZDs provide onset of action within 15 min after administration and have been suggested for at-home and in-hospital SE management in humans [64, 66, 84–88]. IM administration is not subject to first-pass hepatic metabolism and has been also shown that IM administration of MDZ can be as effective as IV-DZP for the management of human SE [86, 87]. In two double-blinded randomised controlled clinical studies in humans, IM-MDZ could be administered by trained medical staff quicker and easier than and was as effective as IV-LZP [64, 66]. Based on meta-analysis in humans, both IM and IN administration of BDZs have been shown to be two of the most effective and fastest methods for ceasing SE, especially in out-of-hospital settings [69, 89]. Clinical trials to evaluate IM-BZDs’ efficacy and safety have not been conducted in dogs, apart from pharmacokinetic studies [72, 73]. Specifically, after IM administration of MDZ solution (at the lowest clinically recommended dose of 0.2 mg/kg [72] or 0.5 mg/kg [73]), mean bioavailability was 50% [72] and > 90% [73]. The mean serum concentration was 0.20 ± 0.06 μg/mL [72] or 0.55 ± 0.12 μg/mL [73]. The maximum serum concentrations were achieved within 10–15 min [72, 73]. DZP is not advised to be given IM because of its erratic absorption [30].
In comparison to other non-IV routes, IM drug administration can be quite painful and pose risks [90], such as syringe/needle misuse, soft tissue or nerve injury, and administration in wrong sites or tissues, especially when administered by non-experienced, non-medically-trained individuals, such as dog owners.
Transdermal
The transdermal drug administration is easily performed (no requirement for syringes or injections), not subject to first-pass hepatic metabolism, and could be a reasonable method for gradual and long-term delivery of drugs (lipophilic drugs with < 500 Da molecular weight, such as BZDs, can penetrate through the skin layers and reach the systemic circulation) [91–93]. However, before therapeutic levels of any drug appear to the systemic circulation, drug crossing and accumulation through the dermal layers is necessary [93, 94]; the latter depends on several factors such as pharmacological characteristics and delivery systems, skin thickness and barrier, and enzymes present in skin that degrade drugs [91–94]. Therefore, a rapid effect that is vital in emergency situations is unlikely in SE, even if permeation enhancers to increase drugs’ absorption are co-administered [91, 92]. The transdermal route for administering long-term antiseizure drugs, i.e. levetiracetam or phenobarbital, has been reported in epileptic cats [95–97] but there is a lack of evidence regarding transdermal BZDs for treating emergency seizures in dogs, likely due to the limitations discussed above.
Buccal
Buccal-BZD might provide an alternative administration option in humans due to its relatively easy use (no requirement for syringes or injections) and the fact that it is socially acceptable (avoidance of rectal drug administration especially in public) [98]. Buccal MDZ has an onset of action within 5–10 min, avoids first-pass hepatic metabolism and has showed good efficacy and safety profile [98–104]. Based on a randomised controlled study, both buccal-MDZ and IV-DZP were successful in ceasing SE but IV-DZP had significantly better mean seizure cessation time (1.1 min) than buccal-MDZ (1.7 min); however, when the time to establish IV access was considered, buccal-MDZ demonstrated significantly shorter mean seizure cessation time (2.4 min) compared to IV (3 min), indicating that preparing the IV medication and introducing an IV line can delay the treatment [103]. According to a systematic review/meta-analysis, buccal-MDZ was more effective than R-DZP in ceasing seizures [69]. Buccal-MDZ, though, was not as effective and fast as IN-MDZ or IM-MDZ for terminating seizures, based on the conclusion of another systematic review/meta-analysis [89]. In dogs, only pharmacokinetic studies have been performed. One study showed that after buccal administration of various MDZ gel formulations (at the dose of 0.3 mg/kg), bioavailability ranged from 25 to 41% [105], mean serum concentrations ranged from 0.1–0.2 μg/mL and time to peak concentration was achieved within 15 min [105]. Another study showed a pH-dependent absorption of buccal-BZDs, with bioavailability ranging from 6.2–22.6% [106]. No clinical trials to support its efficacy in canine SE exist up to date.
Administering the correct dose via the buccal route poses limitations in humans (e.g. hypersalivation and risks of incomplete absorption and aspiration as well as need for patient’s cooperation that might not be realistic in cases of SE) [107]; these limitations might be higher in dogs, adding the risk of the owners being bitten or injured. Additionally, buccal route is beneficial only for small drug doses and volumes as some amount of the buccally administered drug can be swallowed; the latter can lead to decreased bioavailability and delayed time to peak concentration mainly due to the first-pass hepatic metabolism and gastrointestinal tract absorption time, respectively [108, 109].
Sublingual
The sublingual route is another administration method within the oral cavity similar to buccal. The sublingual route provides a thinner and more permeable layer of absorption compared to buccal and, thus, could potentially provide a faster onset of action [110]. To benefit from this, it is essential that the drug should be administered in specific areas of the oral cavity, i.e. sublingual drugs are administered under the tongue, while buccal drugs at the caudal aspect of the oral cavity between the upper or lower molars and the cheek in humans. One of the main limitations in both routes is the necessity for cooperation of the patient for correct administration, which is quite challenging during SE and even more difficult or nearly impossible in dogs. The limitations mentioned in the buccal administration apply also in sublingual route. Absorption can also be very slow [111]. Therefore, sublingual and buccal drug delivery might not be ideal for humans and particularly dogs during seizures. This was also supported by one randomised controlled trial in 436 children showing that sublingual-LZP was less effective than R-DZP in managing seizures [112]. In dogs, no studies evaluating the sublingual BZDs administration have been performed.
Oral
Oral is considered a practical and easy (no requirement for syringes or injections) route of drug administration [113], although it might not be feasible during SE. Certain oral drugs including BZDs and in particular MDZ display low or variable bioavailability in humans (approximately 53–97% and 15–40% for DZP and MDZ, respectively) as well as reduced efficacy and quite prolonged onset of action (approximately 15–60 and 10–45 min for DZP and MDZ, respectively) due to their slow absorption and enzymatic degradation in the gastrointestinal system (small intestine and stomach), and extensive first-pass hepatic metabolism [113–121]. In addition, oral BZDs cannot be administered in people with difficulty in swallowing or have severe CNS suppression [122], as it occurs in SE, and may lead to aspiration pneumonia, especially after administering oily solutions such as DZP. Similar limitations exist in dogs, including the risk of caregiver’s injury due to accidental dog bites, which impair the effect and use of oral BDZs in canine SE. BZDs’ mean availability after oral administration in dogs is 69% for MDZ [73] and > 70% for DZP [123]. Overall, oral BZDs are deemed inconvenient, risky as well as inadequate or ineffective in both human and canine SE.
Rectal
Rectal administration of BZDs and in particular DZP has been well recommended and widely used as a relatively cheap and potentially effective managing option in human SE, with an onset of action within 10–15 min [124, 125]. Rectal drugs can be administered by non-medically trained individuals in contrast to IM and IV drug delivery routes [117]. Empty rectum provides a stable environment with low activity of degrading enzymes that favours absorption of drugs into the systemic circulation [117], but faecal material may impair drug absorption. R-DZP has been suggested as a non-IV method of treating SE in humans, especially within out-of-hospital settings [102, 126–128]. Based on a systematic review/meta-analysis the time periods from arrival in the hospital to drug administration and seizure cessation were shorter with IN, IM and buccal routes of MDZ administration compared to R-DZP [129]. Based on another meta-analysis, R-DZP was not considered as effective as other non-IV methods of MDZ administration and in particular IN- and IM-MDZ [89]. In a third systematic review, non-R BZDs routes of administration were suggested as better or preferred SE treatment options compared to R-DZP [130].
R-DZP in dogs has been widely recommended as a management option for SE in the absence of IV access. This recommendation has been mainly based on pharmacokinetic studies and one small-scale uncontrolled clinical trial [51–53, 131] with conflicting evidence. Specifically, after R administration of DZP (at the dose of 1 mg/kg as solution [52] or 2 mg/kg as solution [53] or 2 mg/kg as gel formulation/suppository [131]), mean bioavailability was reported to be 52% [52] or 7.4% [53] for the solution but was not detected for suppositories [131]. There was a notable variability in DZP serum concentrations among dogs but, in general, the mean serum concentration was approximately 0.5 μg/mL [52, 53] for the solution and ranged between 0.01–0.1 μg/mL for the suppository [131]. The maximum serum concentrations were achieved within 15 min [52, 53] for the solution and 80 min [131] for the suppository. In addition, one recent multicenter open-labelled controlled clinical study compared R-DZP to IN-MDZ and showed that R-DZP was successful in terminating SE in only 20% of the dogs (versus 70% in the IN-MDZ group) and was significantly inferior to IN-MDZ [22]. Hence, R-DZP, in particular suppositories, might provide variable results and potentially inadequate seizure control within the time frame needed for successful control of SE. Regarding R administration of MDZ, studies report erratic bioavailability and serum concentration ranging from undetectable to low [72, 73]. Therefore, MDZ is unlikely to be successful, but there are no clinical studies evaluating drug’s effect in SE.
R route of administration is generally not preferred by people due to cultural and social issues and the potential for discomfort and faecal or drug leakage out of the rectum [117]. Leakage of drugs together with other organic fluids can be an issue in dogs too, while application of rectal tubes might be difficult and performed incorrectly by the owners, especially during SE [22]. In addition, drugs can partially be subject to first-pass hepatic metabolism, which reduces their availability and increases their onset of action time [22, 117]. Given the fact that R-DZP in dogs with SE is relatively inconvenient and likely less successful compared to other routes [22, 131], the promising value of alternative delivery methods (i.e. IN) was highlighted in the recent years [22, 23].
Intranasal
IN drug administration is a noninvasive method for delivering molecules and drugs aiming to act on local, systemic, and CNS level. IN delivery of BZDs offers multiple advantages because it i) requires minimum training and can be performed by non-medically trained individuals, ii) is easily executed, iii) carries minimal or no risk of injury for the owner, clinician or the dog (there were no reports of injury such as accidental bites of the personnel by the seizuring dog or trauma of the dog’s nostrils during IN device application and drug administration), and iv) is generally well accepted for use at home compared to other non-IV routes [22, 23, 122].
The IN route provides fast and efficient drug delivery to the brain. Specifically, human studies reported that IN-MDZ (at the minimum clinically recommended dose of 0.2 mg/kg) can reach the human brain and cease seizure activity within 2–5 min, as shown on electroencephalography [132]. In addition, IN-MDZ at the same dose can reach serum concetration of 0.1–0.18 μg/mL to achive sedation within 12 min after administration (minimum therapeutic concentration for sedation in adult humans is 0.04 μg/mL) [133–135]. It was suggested that the MDZ serum concentration needed to cease activity is even less compared to sedation in humans (< 0.04 μg/mL) [54]. IN-MDZ is also considered a good and successful alternative to other non-IV and IV routes of administration because its efficacy, safety and feasibility has been shown in multiple different species [22, 23, 122, 136–151]. Two human meta-analyses also strongly supported the effectiveness of IN-MDZ in SE [69, 89]. In one meta-analysis, IN-MDZ was found to terminate > 90% of seizures within 5–10 min and sustain seizure freedom for minimum an hour in 80% of people with SE [89].
In humans, both MDZ and DZP can be effective and potent via IN delivery [80, 152–154]. When compared, DZP is more lipophilic than MDZ, which can result in DZP’s better absorption by the nasal mucosa and potentially higher brain concentration [80, 152, 154]. However, DZP’s high lipophilicity also causes the drug to be rapidly redistributed into peripheral tissues which eventually results in DZP’s decreased concentration in the brain [80, 152]. MDZ demonstrates quicker rate of absorption by the nasal mucosa, but lower and more variable degree of absorption as well as shorter duration of action than DZP [80, 152–154]. However, MDZ’s higher potency and better safety profile compared to DZP [30, 55, 56] might make the drug a preferable choice in SE.
In veterinary medicine, pharmacokinetic studies showed that IN-MDZ [155, 156], IN-DZP [33, 157] and IN-flurazepam [156] are rapidly and efficiently absorbed by the nasal mucosa and can reach adequate therapeutic serum concentrations. Specifically, after IN administration of MDZ (lowest clinically recommended dose of 0.2 mg/kg) and DZP (lowest clinically recommended dose of 0.5 mg/kg), mean bioavailability was 52% (solution) [155] or 70.4% (gel formulation) [155] for MDZ and 80% (solution) [33] or 42% (solution/atomised formulation) [157] for DZP. The mean serum concentration was 0.21 ± 0.02 μg/mL (solution) [155] or 0.45 ± 0.09 μg/mL (gel formulation) [155] for MDZ and 0.44 ± 0.04 μg/mL (solution) [33] or 0.31 +/− 0.17 (solution/atomised formulation) [157] for DZP. The maximum serum concentrations were achieved within 17 min (solution) [155] or 12 min (gel formulation) [155] for MDZ and 4.5 min (solution) [33] or 8 min (solution/atomised formulation) [157] for DZP. Regarding results from veterinary clinical studies, two recent open-labelled randomised controlled clinical trials demonstrated that IN-MDZ was not only safe and superior to R-DZP but also superior to the “gold standard” IV route of MDZ administration, especially when the time to place an IV catheter was considered [22, 23].
An important consideration regarding IN administration of BZD is that drugs’ penetration into the brain and antiseizure effect can occur earlier than the time needed to reach maximum serum concentration [54, 155]. This can be supported by the fact that nasally administered drugs can follow both the blood systemic circulation and direct nerve pathways to reach the brain (as it will be thoroughly discussed later in the text); this may result in decreased drug concentration into the bloodstream, but successful penetration into the brain [90, 108, 158]. Therefore, estimating BZDs’ therapeutic serum concentration and bioavailability after IN administration might not be an accurate tool for estimating drugs’ efficacy, as it occurs with other administration routes.
Nasal drug administration considerations
Anatomical considerations
The nasal cavity consists of two equal chambers (left and right), separated by the nasal septum, each of which has a vestibule (entrance of the nasal cavity) and main cavity. The nasal vestibule carries no cilia and is covered by stratified squamous epithelia [159]. The nasal vestibules’ blood perfusion is reduced compared to the main cavity, which results in insignificant drug absorption. The nasal sinuses can pose another potential area for drug absorption, but they are considered hard to reach due to their anatomical features (located into deeper and upper parts of nasal cavity with narrow passages and complex geometry) in both humans and dogs [90, 159–163]. The main nasal cavity consists of the respiratory and olfactory areas and is covered by highly vascularised mucus membranes, a fact that favours absorption into the systemic circulation. The respiratory region, in particular, consists of highly convoluted turbinates (conchae) and microvilli [164] that provide large surface-to-volume ratio and, hence, can benefit rapid drug absorption into the blood vessels.
Physiological considerations
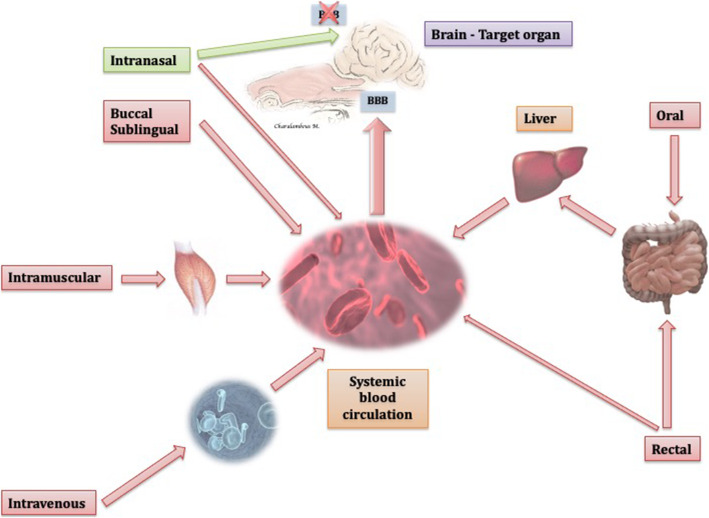
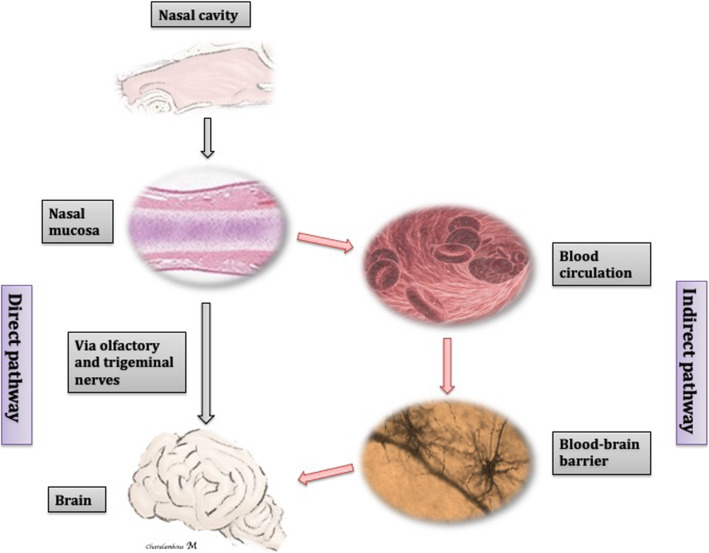
When compared to other administration routes, IN is the only route that can enter the brain via both the blood circulation (indirect pathway) and specific nerves (direct or nose-brain pathway), circumventing the BBB [90, 108, 158], as illustrated in Figs. 3 and 4.
Fig. 3.
Schematic illustration of the different routes of drug administration’ pathways to the brain. The intranasal route is the only route that provides a direct pathway to the brain avoing the BBB (green arrow), along with an indirect pathway (red arrow). The remaining routes reach the brain indirectly (red arrows) via the systemic blood circulation passing via the BBB. Oral, in particular, and rectal route undergo first-pass hepatic metabolism, although rectally administered drugs could potentially avoid the first-pass metabolism, if they do not reach more cranial parts of the colon. Figures from authors’ personal record modified with microsoft power point
Fig. 4.
Schematic illustration of the indirect and direct (nose-brain) nasal drug delivery pathways. Drugs administered IN can penetrate directly into the brain through the olfactory and/or trigeminal nerve pathways or indirectly following absorption into the systemic blood circulation. Figures from authors’ personal record modified with microsoft power point
Indirect nasal-brain drug delivery
The indirect pathway involves, firstly, a rapid drug absorption by the relatively large and highly-vascularised nasal epithelium and, secondly, delivery of the drug to the brain via the systemic circulation [90]. The less lipophilicity and higher molecular weight a drug exhibits, the less is absorbed by the nasal mucosa [109, 165, 166]. Lipophilic drugs with molecular weight < 1000 Da can be absorbed, but drugs with < 200 Da manifest the highest absorption [109, 165, 166]. Drugs are not subject to first-pass (presystemic) hepatic metabolism after absorption [90, 108, 158]. However, following absorption to the systemic circulation, IN drugs, similar to drugs administered via other routes, are subject to the systemic hepatic metabolism, renal function and plasma proteases, and they have to transverse the BBB for reaching the brain [90, 109, 167]. The BBB functions as a barrier, i.e., physical (intercellular tight junctions between endothelial cells and astrocyte end-feet), transport (multidrug transporters such as PGP), metabolic or enzymatic as well as immunological (perivascular mast cells, microglia and macrophages) [168, 169]. The physical barrier prevents molecules that are hydrophilic and/or have a high molecular weight (> 400–600 Da) to enter the brain [170]. Interestingly, more than 98% of the drugs cannot cross the BBB freely [171–174]. Modern drugs that target the brain are chemically modified to increase their stability and degree of BBB penetration [175]. BZDs are lipophilic drugs with molecular weight < 400 Da; therefore, not only can they be absorbed by the nasal mucosa to the systemic blood circulation, but the drugs can also penetrate the BBB and reach the brain [90, 166, 176].
Direct nasal-brain drug delivery
The direct pathway has gained attention in recent years as it offers a direct nose-brain axis for drug delivery via specific cranial nerves [164, 177–183]. Specifically, drugs gain access to the brain via the olfactory (nerve travels via the cribriform plate to provide special visceral afferent innervation to the olfactory mucosal epithelium) and trigeminal nerve (ophthalmic and maxillary branches travel via the cribriform plate to provide somatic afferent innervation to the respiratory mucosal epithelium) [178, 181, 184–186]. This nose-brain pathway is likely more advantageous for molecules that cannot enter the brain via other routes, as a result of their low systemic bioavailability (e.g. due to inadequate absorption into the systemic circulation or extensive metabolism and elimination) or inability to penetrate the BBB (e.g. hydrophilic or drugs with molecular weight > 400–600 Da) [90, 170]. There is accumulating evidence of IN administration of various hydrophilic and/or high molecular-weight molecules that reached rapid concentrations in the brain exceeding those that obtained after IV administration [158, 187, 188]; a fact that supports further the direct nose-to-brain pathway. In a study, it has been demonstrated that IN administration of drugs might lead to a drug CSF concentration that exceeds the blood plasma concentration [189–191] and can be identical to the direct intracerebroventricular administration [174]. In the olfactory region, in particular, there might be a possibility of another direct pathway, which could also contribute to the nose-brain pathway [173, 192, 193]. Specifically, the submucosal zone of the olfactory region is adjacent to the CSF flow paths of the olfactory bulb. Therefore, the IN drug could reach the CSF through the nasal epithelium and meninges that separate the submucosal space from the CSF [192].
Drugs following the direct pathway are transported via the nerves by various mechanisms including a) extracellular diffusion of the drug along the axonal myelin sheath and endoneurium of the nerves, b) extracellular convection of the drug following the fluid bulk flow through the perivascular zones of vessels that travel across the distal parts of the nerves and c) intracellular transport through the neuronal axons [185, 194, 195]. The extracellular convection of the drug (bulk flow) was suggested as the main mechanism of these nerve pathways, in particular for the olfactory nerve, that can be quick enough to cause the desired effect [182, 185, 195]. Final distribution of the drug from the point of entry into the brain, i.e. the olfactory bulb (drug entering through the nasal epithelium and olfactory nerve) and the brainstem (drugs entering through the trigeminal nerve), to other brain regions is likely performed via various transport mechanisms; these include intracellular (drug uptake and transfer via further connective neurons) and extracellular (drug distribution and transfer by convective bulk flow transport through the brain perivascular spaces or drug diffusion from the perivascular spaces into the brain parenchyma) [196–199].
Direct versus indirect pathway predominance in each nasal region
In humans, the respiratory and olfactory regions account for > 80–90% [164] and approximately 3–8% [164, 200] of the total nasal surface, respectively. The respiratory epithelium is considered more vascularised than the olfactory epithelium because one of its roles is to warm and humidify the inhaled air [201]. Therefore, the indirect pathway is likely favoured at the respiratory area, causing less amount of drug to become available for the direct (trigeminal nerve) pathway. In contrast, the olfactory area does not offer adequate highly vascularised surface [164, 200, 201] for the indirect pathway to occur and, thus, the direct (olfactory nerve) pathway is favoured. It might be possible that, due to the above anatomical reasons, trigeminal nerve might not be as significant as the olfactory nerve for transporting drugs into the human brain [90, 202]. On contrary, in dogs [162, 201] and rats [203], the respiratory and olfactory regions have almost equal distribution on the overall nasal cavity. Based on the fact that animals have remarkable larger olfactory area compared to humans [109, 162, 164, 203], it might be likely that there is similar drug distribution between direct and indirect methods of drug transport in each nasal area, although this assumption has not been proven yet.
Intranasal drug administration and pharmaco-resistance
A vital future consideration is a potential connection between IN route and pharmaco-resistance. As described earlier in the text, IN delivery of drugs may follow the direct or nose-brain pathway to enter the brain avoiding BBB vascular transporters, such as PGP. This can be quite advantageous for dogs with pharmaco-resistance, where there is impaired transfer of antiseizure drugs through the BBB due to overexpression of these transporters [48, 204, 205]. Therefore, it would be quite interesting to conduct future studies to assess the effect of IN delivery of various antiseizure drugs specifically in dogs with pharmaco-resistant epilepsy or refractory stages of SE.
Intranasal drug administration potential challenges
Anatomical and physiological challenges of the nasal administration route
Even though IN route is promising for drug delivery into the brain, it can pose important challenges regarding drug absorption. First, hydrolytic enzymes (e.g. cytochrome P450 isoenzymes and aminopeptidases) are excreted by the nasal mucosa and can metabolise nasally administered drugs reducing their local or systemic bioavailability [200, 206]. Second, the mucociliary clearance (i.e. elimination of nasally entering substances by nasal mucosa) regulates the contact time of drugs with the nasal mucosa affecting the degree of their absorption [207]. Co-administration of mucoadhesion-enhancing agents could improve drugs’ contact time and absorption [90]. Third, there are epithelial transporters in nasal epithelium that can cause efflux of drugs from cells and reduce their absorption [200, 208–212]. Fourth, constriction or dilation of the nasal mucosa vessels can influence blood flow and, hence, drug absorption. Co-administering vasoconstriction agents (e.g. ephedrine or phenylephrine) can decrease drug nasal absorption [200, 213], while vasodilator agents (e.g. hydralazine) can enhance absorption [214, 215]. Fifth, nasal blood flow and drug absorption can be influenced by environmental factors such as nasal pathology, humidity, temperature, fear and stress [159, 216]. Lastly, the distribution of the IN drug can be potentially affected by anatomical features of specific canine breeds [217]. Precisely, in brachycephalic dogs, the conchae are hypertrophic, and the overall nasal cavity surface is decreased compared to dolichocephalic breeds [218, 219]; facts that could potentially limit the absorption and volume of nasally administered drugs. However, in two canine clinical studies [22, 23], various small, medium, and large breed as well as brachycephalic and dolichocephalic dogs were included, but no difference in the efficacy of IN-MDZ was detected among the dogs. Difficulties in applying the IN mucosal atomization device (MAD; nasal drug delivery device for MDZ) during SE were reported in < 24% of dogs [22, 23] and these were related to the initial unfamiliarity of personnel with the IN drug administration. Establishing IV access by placing an IV catheter [23] or using a syringe for R administration was perceived more difficult [22] during SE in dogs than applying the MAD at the entrance of the nasal cavity.
An ideal drug for IN administration should be characterised by adequate mucus solubility, ability for fast absorption, and rapid onset of action; highly concentrated solutions with small administration volume are also important because excess drug volume can flow out of the nasal cavity or drain into the oesophagus [59]. Combined with the above drug characteristics, attention should be given to the delivery device and the head position during administration as these factors can also affect drug’s distribution within the nasal cavity [220, 221]. Pump sprays are widely used in human medicine nowadays to deliver between 25 and 200 μL of drug volume per spray and they are relatively convenient and easy to use while allowing accurate dosage [222, 223]. Nasal drops [222] might be distributed over a larger area, although they might be cleared faster in comparison to sprays [224]. An essential limitation of both spray and, in particular, nasal drop systems is that they might require specific head positioning for correct administration, which can influence the drug’s distribution within the nasal cavity, absorption and therefore efficacy [222, 223]. In veterinary medicine, the IN drug delivery method has not yet well implemented or widely explored and there are no nasal devices specifically designed for dogs. In the two veterinary clinical trials evaluating the effect of IN-MDZ in dogs with SE [22, 23], a human device (i.e. MAD) was used for IN delivery and the majority of the dogs (70–76%) successfully responded. MAD functions like a syringe with a soft conical plug attached on it that converts the drug solution into an atomised mist. However, this device does not provide MDZ formulation already included in the device, requires time for preparation and drug administration and is not specifically adapted for the anatomical features of dogs. This can be problematic for small or brachycephalic breeds in which the correct application of the current human nasal devices might be challenging or even impossible. Dogs with SE may benefit from the design of a specific nasal device which would be adapted for small animals (e.g. thinner and more elongated device tip to adequately enter the nasal cavity and administer the drug into the whole nasal cavity) and contain drug solution ready for dosing and administration. Such a device might provide rapid and convenient delivery as well as enhanced efficacy of MDZ in dogs with SE.
Conclusions
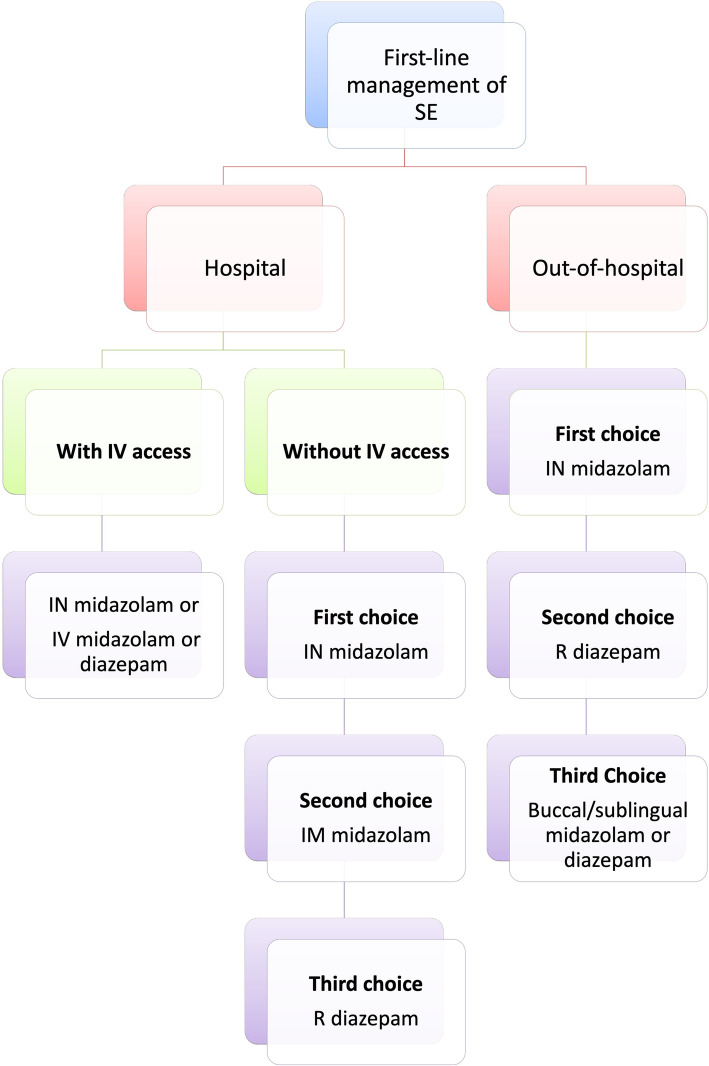
BDZs represent the first-line and widely-used treatment choice and still remain crucial for management of canine SE, despite their potential decline in effectiveness with more advanced SE stages. Multi-drug therapy, including drugs with different mechanisms of action, are also essential for a successful treatment. Evidence in dogs shows that efficacy and safety of non-IV routes of administration may be equal or even more effective to IV routes. This is more profound when the time to establish an IV catheter is considered. For at-home SE management, IN-MDZ is likely an effective and safe first-choice for terminating SE and well supported by clinical studies compared to other non-IV routes of administration. R-DZP is unlikely to be as effective as IN-MDZ to terminate SE, based on the current evidence. IM and buccal/sublingual administration routes might also be effective but there is currently insufficient to no clinical evidence supporting their efficacy and safety in canine SE, while their application at home by owners might be problematic. Regarding the in-hospital SE management, both IV- and IN-MDZ can be successful first choices but IN-MDZ can be advantageous particularly when IV access has not yet been established. Overall, based on the current evidence, IN-MDZ is recommended as a first-choice treatment in dogs with SE at home or in hospital and a proposed cascade is provided by the authors (Fig. 5). The IN pathway of drug delivery for SE provides several advantages as an administration method because it i) likely circumvents practical and efficacy-related issues associated with other IV and non-IV routes, ii) provides non-invasive and ease of administration, iv) offers capability for direct drug delivery into the brain, and v) provides enhanced drug bioavailability due to the high vascularisation of the nasal tissue, large surface available for drug absorption and avoidance of first-pass hepatic metabolism. Olfactory and trigeminal pathways might provide further advantages such as i) increased drug efficacy at lower dosages, ii) decreased risk for systemic adverse effects and iii) circumvention of BBB; the latter can be quite beneficial in pharmaco-resistant cases. These pathways are the only channels through which the brain is somewhat directly bridged to the external environment making the nose an effective “window to the brain”. A better understanding of the nasal anatomy and its limitations as well as formulation strategies can result in improved characteristics and efficacy of IN drugs.
Fig. 5.
Cascade of choices for the first-line management of SE at home and in-hospital, with or without IV access
Acknowledgements
Not applicable.
Abbreviations
- BBB
Blood-brain barrier
- BZD(s)
Benzodiazepines
- CNS
Cental nervous system
- CRI
Constant rate infusion
- CSF
Cerebrospinal fluid
- DZP
Diazepam
- GABA
Gabapentine
- IE
Idioapthic epilepsy
- IM
Intramuscular
- IN
Intranasal
- IV
Intravenous
- LZP
Lorazepam
- MDZ
Midazolam
- NMDA
N-methyl-d-aspartate
- PGP
P-glycoprotein
- R
Rectal
- SE
Status epilepticus
Authors’ contributions
MC contributed to the conception of the review and drafting of the manuscript. MC, HV, LVH and SB contributed to the manuscript revision. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
HV is a member of the editorial board of the journal.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marios Charalambous, Email: marios.charalambous@ugent.be.
Holger A. Volk, Email: holger.volk@tiho-hannover.de
Luc Van Ham, Email: luc.vanham@ugent.be.
Sofie F. M. Bhatti, Email: sofie.bhatti@ugent.be
References
- 1.Berendt M, Farquhar RG, Mandigers PJ, Pakozdy A, Bhatti SF, De Risio L, et al. International veterinary epilepsy task force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. doi: 10.1186/s12917-015-0461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinka E, Brigo F, Shorvon S. Recent advances in status epilepticus. Curr Opin Neurol. 2016;29(2):189–198. doi: 10.1097/WCO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 3.Walker M. Status epilepticus: an evidence based guide. BMJ. 2005;331(7518):673–677. doi: 10.1136/bmj.331.7518.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lado FA, Moshe SL. How do seizures stop? Epilepsia. 2008;49(10):1651–1664. doi: 10.1111/j.1528-1167.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blades Golubovic S, Rossmeisl JH., Jr Status epilepticus in dogs and cats, part 1: etiopathogenesis, epidemiology, and diagnosis. J Vet Emerg Crit Care (San Antonio) 2017;27(3):278–287. doi: 10.1111/vec.12605. [DOI] [PubMed] [Google Scholar]
- 6.Walker MC. Pathophysiology of status epilepticus. Neurosci Lett. 2018;667:84–91. doi: 10.1016/j.neulet.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 7.Janigro D, Iffland PH, 2nd, Marchi N, Granata T. A role for inflammation in status epilepticus is revealed by a review of current therapeutic approaches. Epilepsia. 2013;54(Suppl 6):30–32. doi: 10.1111/epi.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu HT, Mazarati AM, Katsumori H, Sankar R, Waterlain CG. Substance P is expressed in hippocampal principal neurons during status epilepticus and plays a critical role in the maintenance of status epilepticus. P Natl Acad Sci USA. 1999;96(9):5286–5291. doi: 10.1073/pnas.96.9.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kostic D, Carlson R, Henke D, Rohn K, Tipold A. Evaluation of IL-1beta levels in epilepsy and traumatic brain injury in dogs. BMC Neurosci. 2019;20(1):29. doi: 10.1186/s12868-019-0509-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segers E, Martle V, Piepers S, Van Ham L, Bhatti SFM. Serum C-reactive protein concentrations in dogs with idiopathic epilepsy. Vlaams Diergen Tijds. 2017;86(2):79–83. doi: 10.21825/vdt.v86i2.16292. [DOI] [Google Scholar]
- 11.Hanael E, Veksler R, Friedman A, Bar-Klein G, Senatorov VV, Jr, Kaufer D, et al. Blood-brain barrier dysfunction in canine epileptic seizures detected by dynamic contrast-enhanced magnetic resonance imaging. Epilepsia. 2019;60(5):1005–1016. doi: 10.1111/epi.14739. [DOI] [PubMed] [Google Scholar]
- 12.Bateman SW, Parent JM. Clinical findings, treatment, and outcome of dogs with status epilepticus or cluster seizures: 156 cases (1990-1995) J Am Vet Med Assoc. 1999;215(10):1463–1468. [PubMed] [Google Scholar]
- 13.Platt SR, Haag M. Canine status epilepticus: a retrospective study of 50 cases. J Small Anim Pract. 2002;43(4):151–153. doi: 10.1111/j.1748-5827.2002.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 14.Saito M, Munana KR, Sharp NJ, Olby NJ. Risk factors for development of status epilepticus in dogs with idiopathic epilepsy and effects of status epilepticus on outcome and survival time: 32 cases (1990-1996) J Am Vet Med Assoc. 2001;219(5):618–623. doi: 10.2460/javma.2001.219.618. [DOI] [PubMed] [Google Scholar]
- 15.Knowles K. Idiopathic epilepsy. Clin Tech Small Anim Pract. 1998;13(3):144–151. doi: 10.1016/S1096-2867(98)80035-2. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann R, Hulsmeyer V, Sauter-Louis C, Fischer A. Status epilepticus and epileptic seizures in dogs. J Vet Intern Med. 2009;23(5):970–976. doi: 10.1111/j.1939-1676.2009.0368.x. [DOI] [PubMed] [Google Scholar]
- 17.Costello DJ, Cole AJ. Treatment of acute seizures and status epilepticus. J Intensive Care Med. 2007;22(6):319–347. doi: 10.1177/0885066607307506. [DOI] [PubMed] [Google Scholar]
- 18.Koestner A. Neuropathology of canine epilepsy. Probl Vet Med. 1989;1(4):516–534. [PubMed] [Google Scholar]
- 19.Lothman E. The biochemical basis and pathophysiology of status epilepticus. Neurology. 1990;40(5 Suppl 2):13–23. [PubMed] [Google Scholar]
- 20.Shorvon S, Ferlisi M. The treatment of super-refractory status epilepticus: a critical review of available therapies and a clinical treatment protocol. Brain. 2011;134(Pt 10):2802–2818. doi: 10.1093/brain/awr215. [DOI] [PubMed] [Google Scholar]
- 21.Watson C. Status epilepticus. Clinical features, pathophysiology, and treatment. West J Med. 1991;155(6):626–631. [PMC free article] [PubMed] [Google Scholar]
- 22.Charalambous M, Bhatti SFM, Van Ham L, Platt S, Jeffery ND, Tipold A, et al. Intranasal midazolam versus rectal diazepam for the Management of Canine Status Epilepticus: a multicenter randomized parallel-group clinical trial. J Vet Intern Med. 2017;31(4):1149–1158. doi: 10.1111/jvim.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charalambous M, Volk HA, Tipold A, Erath J, Huenerfauth E, Gallucci A, et al. Comparison of intranasal versus intravenous midazolam for management of status epilepticus in dogs: a multi-center randomized parallel group clinical study. J Vet Intern Med. 2019;33(6):2709–2717. doi: 10.1111/jvim.15627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Waele L, Boon P, Ceulemans B, Dan B, Jansen A, Legros B, et al. First line management of prolonged convulsive seizures in children and adults: good practice points. Acta Neurol Belg. 2013;113(4):375–380. doi: 10.1007/s13760-013-0247-x. [DOI] [PubMed] [Google Scholar]
- 25.Patterson EN. Status epilepticus and cluster seizures. Vet Clin North Am Small Anim Pract. 2014;44(6):1103–1112. doi: 10.1016/j.cvsm.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Nair PP, Kalita J, Misra UK. Status epilepticus: why, what, and how. J Postgrad Med. 2011;57(3):242–252. doi: 10.4103/0022-3859.81807. [DOI] [PubMed] [Google Scholar]
- 27.Falco-Walter JJ, Bleck T. Treatment of Established Status Epilepticus. J Clin Med. 2016:5. [DOI] [PMC free article] [PubMed]
- 28.Gollwitzer S, Hamer HM. The treatment and prognosis of refractory and super-refractory status Epilepticus. Aktuel Neurol. 2017;44(9):662–668. doi: 10.1055/s-0043-110223. [DOI] [Google Scholar]
- 29.Podell M, Fenner WR, Powers JD. Seizure classification in dogs from a nonreferral-based population. J Am Vet Med Assoc. 1995;206(11):1721–1728. [PubMed] [Google Scholar]
- 30.Tesoro EP, Brophy GM. Pharmacological management of seizures and status epilepticus in critically ill patients. J Pharm Pract. 2010;23(5):441–454. doi: 10.1177/0897190010372321. [DOI] [PubMed] [Google Scholar]
- 31.Zaccara G, Giannasi G, Oggioni R, Rosati E, Tramacere L, Palumbo P, et al. Challenges in the treatment of convulsive status epilepticus. Seizure. 2017;47:17–24. doi: 10.1016/j.seizure.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(a) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25(34):7724–7733. doi: 10.1523/JNEUROSCI.4944-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platt SR, Randell SC, Scott KC, Chrisman CL, Hill RC, Gronwall RR. Comparison of plasma benzodiazepine concentrations following intranasal and intravenous administration of diazepam to dogs. Am J Vet Res. 2000;61(6):651–654. doi: 10.2460/ajvr.2000.61.651. [DOI] [PubMed] [Google Scholar]
- 34.Niquet J, Baldwin R, Suchomelova L, Lumley L, Naylor D, Eavey R, et al. Benzodiazepine-refractory status epilepticus: pathophysiology and principles of treatment. Ann N Y Acad Sci. 2016;1378(1):166–173. doi: 10.1111/nyas.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deeb TZ, Maguire J, Moss SJ. Possible alterations in GABAA receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia. 2012;53(Suppl 9):79–88. doi: 10.1111/epi.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtkamp M, Tong X, Walker MC. Propofol in subanesthetic doses terminates status epilepticus in a rodent model. Ann Neurol. 2001;49(2):260–263. doi: 10.1002/1531-8249(20010201)49:2<260::AID-ANA49>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 37.Orser BA, Canning KJ, Macdonald JF. Mechanisms of general anesthesia. Curr Opin Anaesthesiol. 2002;15(4):427–433. doi: 10.1097/00001503-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Rogawski MA, Loya CM, Reddy K, Zolkowska D, Lossin C. Neuroactive steroids for the treatment of status epilepticus. Epilepsia. 2013;54(Suppl 6):93–98. doi: 10.1111/epi.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silayeva L, Deeb TZ, Hines RM, Kelley MR, Munoz MB, Lee HH, et al. KCC2 activity is critical in limiting the onset and severity of status epilepticus. Proc Natl Acad Sci U S A. 2015;112(11):3523–3528. doi: 10.1073/pnas.1415126112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naylor DE, Liu H, Niquet J, Wasterlain CG. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobiol Dis. 2013;54:225–238. doi: 10.1016/j.nbd.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajasekaran K, Todorovic M, Kapur J. Calcium-permeable AMPA receptors are expressed in a rodent model of status epilepticus. Ann Neurol. 2012;72(1):91–102. doi: 10.1002/ana.23570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckel R, Szulc B, Walker MC, Kittler JT. Activation of calcineurin underlies altered trafficking of alpha2 subunit containing GABAA receptors during prolonged epileptiform activity. Neuropharmacology. 2015;88:82–90. doi: 10.1016/j.neuropharm.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi S, Rajasekaran K, Hawk KM, Brar J, Ross BM, Tran CA, et al. Phosphatase inhibition prevents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia. 2015;56(9):1355–1365. doi: 10.1111/epi.13098. [DOI] [PubMed] [Google Scholar]
- 44.Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49(2):248–255. doi: 10.1111/j.1528-1167.2007.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice AC, DeLorenzo RJ. N-methyl-D-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience. 1999;93(1):117–123. doi: 10.1016/S0306-4522(99)00132-3. [DOI] [PubMed] [Google Scholar]
- 46.Walton NY, Treiman DM. Motor and electroencephalographic response of refractory experimental status epilepticus in rats to treatment with MK-801, diazepam, or MK-801 plus diazepam. Brain Res. 1991;553(1):97–104. doi: 10.1016/0006-8993(91)90235-N. [DOI] [PubMed] [Google Scholar]
- 47.Volk HA, Loscher W. Multidrug resistance in epilepsy: rats with drug-resistant seizures exhibit enhanced brain expression of P-glycoprotein compared with rats with drug-responsive seizures. Brain. 2005;128(Pt 6):1358–1368. doi: 10.1093/brain/awh437. [DOI] [PubMed] [Google Scholar]
- 48.Pekcec A, Unkruer B, Stein V, Bankstahl JP, Soerensen J, Tipold A, et al. Over-expression of P-glycoprotein in the canine brain following spontaneous status epilepticus. Epilepsy Res. 2009;83(2–3):144–151. doi: 10.1016/j.eplepsyres.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Sisodiya SM, Thom M. Widespread upregulation of drug-resistance proteins in fatal human status epilepticus. Epilepsia. 2003;44(2):261–264. doi: 10.1046/j.1528-1157.2003.42802.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Ong WY, Lee T. Induction of P-glycoprotein expression in astrocytes following intracerebroventricular kainate injections. Exp Brain Res. 1999;126(4):509–516. doi: 10.1007/s002210050759. [DOI] [PubMed] [Google Scholar]
- 51.Wagner SO, Sams RA, Podell M. Chronic phenobarbital therapy reduces plasma benzodiazepine concentrations after intravenous and rectal administration of diazepam in the dog. J Vet Pharmacol Ther. 1998;21(5):335–341. doi: 10.1046/j.1365-2885.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 52.Mealey KL, Boothe DM. Bioavailability of benzodiazepines following rectal administration of diazepam in dogs. J Vet Pharmacol Ther. 1995;18(1):72–74. doi: 10.1111/j.1365-2885.1995.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 53.Papich MG, Alcorn J. Absorption of diazepam after its rectal administration in dogs. Am J Vet Res. 1995;56(12):1629–1636. [PubMed] [Google Scholar]
- 54.Wermeling DP, Record KA, Archer SM, Rudy AC. A pharmacokinetic and pharmacodynamic study, in healthy volunteers, of a rapidly absorbed intranasal midazolam formulation. Epilepsy Res. 2009;83(2–3):124–132. doi: 10.1016/j.eplepsyres.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 55.Horikawa H, Tada T, Sakai M, Karube T, Ichiyanagi K. Effects of midazolam on the threshold of lidocaine-induced seizures in the dog--comparison with diazepam. J Anesth. 1990;4(3):265–269. doi: 10.1007/s0054000040265. [DOI] [PubMed] [Google Scholar]
- 56.Buhrer M, Maitre PO, Crevoisier C, Stanski DR. Electroencephalographic effects of benzodiazepines. II. Pharmacodynamic modeling of the electroencephalographic effects of midazolam and diazepam. Clin Pharmacol Ther. 1990;48(5):555–567. doi: 10.1038/clpt.1990.192. [DOI] [PubMed] [Google Scholar]
- 57.Rossetti AO. Treatment options in the management of status epilepticus. Curr Treat Options Neurol. 2010;12(2):100–112. doi: 10.1007/s11940-010-0060-2. [DOI] [PubMed] [Google Scholar]
- 58.Winsnes M, Jeppsson R, Sjoberg B. Diazepam adsorption to infusion sets and plastic syringes. Acta Anaesthesiol Scand. 1981;25(2):93–96. doi: 10.1111/j.1399-6576.1981.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 59.Walker MC, Tong X, Brown S, Shorvon SD, Patsalos PN. Comparison of single- and repeated-dose pharmacokinetics of diazepam. Epilepsia. 1998;39(3):283–289. doi: 10.1111/j.1528-1157.1998.tb01374.x. [DOI] [PubMed] [Google Scholar]
- 60.Mazarati AM, Baldwin R, Klitgaard H, Matagne A, Wasterlain CG. Anticonvulsant effects of levetiracetam and levetiracetam-diazepam combinations in experimental status epilepticus. Epilepsy Res. 2004;58(2–3):167–174. doi: 10.1016/j.eplepsyres.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 61.Uges JW, van Huizen MD, Engelsman J, Wilms EB, Touw DJ, Peeters E, et al. Safety and pharmacokinetics of intravenous levetiracetam infusion as add-on in status epilepticus. Epilepsia. 2009;50(3):415–421. doi: 10.1111/j.1528-1167.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- 62.Lowenstein DH, Cloyd J. Out-of-hospital treatment of status epilepticus and prolonged seizures. Epilepsia. 2007;48(Suppl 8):96–98. doi: 10.1111/j.1528-1167.2007.01363.x. [DOI] [PubMed] [Google Scholar]
- 63.Leppik IE, Derivan AT, Homan RW, Walker J, Ramsay RE, Patrick B. Double-blind study of lorazepam and diazepam in status epilepticus. JAMA. 1983;249(11):1452–1454. doi: 10.1001/jama.1983.03330350028021. [DOI] [PubMed] [Google Scholar]
- 64.Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Treiman DM, Meyers PD, Walton NY, Collins JF, Colling C, Rowan AJ, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans affairs status Epilepticus cooperative study group. N Engl J Med. 1998;339(12):792–798. doi: 10.1056/NEJM199809173391202. [DOI] [PubMed] [Google Scholar]
- 66.Welch RD, Nicholas K, Durkalski-Mauldin VL, Lowenstein DH, Conwit R, Mahajan PV, et al. Intramuscular midazolam versus intravenous lorazepam for the prehospital treatment of status epilepticus in the pediatric population. Epilepsia. 2015;56(2):254–262. doi: 10.1111/epi.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- 68.Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001;345(9):631–637. doi: 10.1056/NEJMoa002141. [DOI] [PubMed] [Google Scholar]
- 69.Brigo F, Bragazzi NL, Bacigaluppi S, Nardone R, Trinka E. Is intravenous lorazepam really more effective and safe than intravenous diazepam as first-line treatment for convulsive status epilepticus? A systematic review with meta-analysis of randomized controlled trials. Epilepsy Behav. 2016;64(Pt A):29–36. [DOI] [PubMed]
- 70.McTague A, Martland T, Appleton R. Drug management for acute tonic-clonic convulsions including convulsive status epilepticus in children. Cochrane Database Syst Rev. 2018;1:CD001905. doi: 10.1002/14651858.CD001905.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu W, Zhang L, Xue R. Lorazepam or diazepam for convulsive status epilepticus: a meta-analysis. J Clin Neurosci. 2016;29:133–138. doi: 10.1016/j.jocn.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz M, Munana KR, Nettifee-Osborne JA, Messenger KM, Papich MG. The pharmacokinetics of midazolam after intravenous, intramuscular, and rectal administration in healthy dogs. J Vet Pharmacol Ther. 2013;36(5):471–477. doi: 10.1111/jvp.12032. [DOI] [PubMed] [Google Scholar]
- 73.Court MH, Greenblatt DJ. Pharmacokinetics and preliminary observations of behavioral changes following administration of midazolam to dogs. J Vet Pharmacol Ther. 1992;15(4):343–350. doi: 10.1111/j.1365-2885.1992.tb01026.x. [DOI] [PubMed] [Google Scholar]
- 74.Brown SA, Jacobson JD, Hartsfield SM. Pharmacokinetics of midazolam administered concurrently with ketamine after intravenous bolus or infusion in dogs. J Vet Pharmacol Ther. 1993;16(4):419–425. doi: 10.1111/j.1365-2885.1993.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 75.Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus. Eur J Neurol. 2006;13(5):445–450. doi: 10.1111/j.1468-1331.2006.01397.x. [DOI] [PubMed] [Google Scholar]
- 76.Meierkord H, Boon P, Engelsen B, Gocke K, Shorvon S, Tinuper P, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17(3):348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 77.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status Epilepticus in children and adults: report of the guideline Committee of the American Epilepsy Society. Epilepsy Cur. 2016;16(1):48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas WB. Idiopathic epilepsy in dogs and cats. Vet Clin North Am Small Anim Pract. 2010;40(1):161–179. doi: 10.1016/j.cvsm.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 79.Alshehri A, Abulaban A, Bokhari R, Kojan S, Alsalamah M, Ferwana M, et al. Intravenous versus nonintravenous benzodiazepines for the cessation of seizures: a systematic review and meta-analysis of randomized controlled trials. Acad Emerg Med. 2017;24:875–883. doi: 10.1111/acem.13190. [DOI] [PubMed] [Google Scholar]
- 80.Manno EM. Status epilepticus: current treatment strategies. Neurohospitalist. 2011;1(1):23–31. doi: 10.1177/1941875210383176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Marawar R, Basha M, Mahulikar A, Desai A, Suchdev K, Shah A. Updates in refractory status Epilepticus. Crit Care Res Pract. 2018;2018:9768949. doi: 10.1155/2018/9768949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang T, Hirsch LJ. Treatment of convulsive and nonconvulsive status Epilepticus. Curr Treat Options Neurol. 2005;7(4):247–259. doi: 10.1007/s11940-005-0035-x. [DOI] [PubMed] [Google Scholar]
- 83.Sirven JI, Waterhouse E. Management of status epilepticus. Am Fam Physician. 2003;68(3):469–476. [PubMed] [Google Scholar]
- 84.Abou-Khalil B, Wheless J, Rogin J, Wolter KD, Pixton GC, Shukla RB, et al. A double-blind, randomized, placebo-controlled trial of a diazepam auto-injector administered by caregivers to patients with epilepsy who require intermittent intervention for acute repetitive seizures. Epilepsia. 2013;54(11):1968–1976. doi: 10.1111/epi.12373. [DOI] [PubMed] [Google Scholar]
- 85.Silbergleit R, Lowenstein D, Durkalski V, Conwit R. Neurological Emergency Treatment Trials I. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011;52(Suppl 8):45–47. doi: 10.1111/j.1528-1167.2011.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chamberlain JM, Altieri MA, Futterman C, Young GM, Ochsenschlager DW, Waisman Y. A prospective, randomized study comparing intramuscular midazolam with intravenous diazepam for the treatment of seizures in children. Pediatr Emerg Care. 1997;13(2):92–94. doi: 10.1097/00006565-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Shah I, Deshmukh CT. Intramuscular midazolam vs intravenous diazepam for acute seizures. Indian J Pediatr. 2005;72(8):667–670. doi: 10.1007/BF02724074. [DOI] [PubMed] [Google Scholar]
- 88.Riva A, Iapadre G, Grasso EA, Balagura G, Striano P, Verrotti A. Intramuscular midazolam for treatment of status Epilepticus. Expert Opin Pharmacother. 2020:1–8. [DOI] [PubMed]
- 89.Arya R, Kothari H, Zhang Z, Han B, Horn PS, Glauser TA. Efficacy of nonvenous medications for acute convulsive seizures: a network meta-analysis. Neurology. 2015;85(21):1859–1868. doi: 10.1212/WNL.0000000000002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapoor M, Cloyd JC, Siegel RA. A review of intranasal formulations for the treatment of seizure emergencies. J Control Release. 2016;237:147–159. doi: 10.1016/j.jconrel.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Godin B, Touitou E. Transdermal skin delivery: predictions for humans from in vivo, ex vivo and animal models. Adv Drug Deliv Rev. 2007;59(11):1152–1161. doi: 10.1016/j.addr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 92.Nokhodchi A, Shokri J, Dashbolaghi A, Hassan-Zadeh D, Ghafourian T, Barzegar-Jalali M. The enhancement effect of surfactants on the penetration of lorazepam through rat skin. Int J Pharm. 2003;250(2):359–369. doi: 10.1016/S0378-5173(02)00554-9. [DOI] [PubMed] [Google Scholar]
- 93.Akhtar N, Singh V, Yusuf M, Khan RA. Non-invasive drug delivery technology: development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed Tech (Berl) 2020;65(3):243–272. doi: 10.1515/bmt-2019-0019. [DOI] [PubMed] [Google Scholar]
- 94.Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13(3):175–187. doi: 10.1080/10717540500455975. [DOI] [PubMed] [Google Scholar]
- 95.Barnes Heller HL, Trepanier LA, Robertson M, Mei C. Prospective crossover clinical trial comparing transdermal with oral phenobarbital administration in epileptic cats. J Feline Med Surg. 2019;21(12):1181–1187. doi: 10.1177/1098612X18823577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krull DP, Thomovsky SA, Chen AV, Mealey KL, Papich MG. Evaluation of transdermal Administration of Phenobarbital in healthy cats. J Am Anim Hosp Assoc. 2019;55(1):1–7. doi: 10.5326/JAAHA-MS-6670. [DOI] [PubMed] [Google Scholar]
- 97.Smith C, Barnes Heller HL, Reif N, Van Hesteren M, Reinhart JM. Serum levetiracetam concentrations after transdermal levetiracetam administration, 3 times daily, to healthy cats. J Vet Intern Med. 2019;33(2):827–830. doi: 10.1111/jvim.15412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McIntyre J, Robertson S, Norris E, Appleton R, Whitehouse WP, Phillips B, et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet. 2005;366(9481):205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 99.Ashrafi MR, Khosroshahi N, Karimi P, Malamiri RA, Bavarian B, Zarch AV, et al. Efficacy and usability of buccal midazolam in controlling acute prolonged convulsive seizures in children. Eur J Paediatr Neurol. 2010;14(5):434–438. doi: 10.1016/j.ejpn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Nakken KO, Lossius MI. Buccal midazolam or rectal diazepam for treatment of residential adult patients with serial seizures or status epilepticus. Acta Neurol Scand. 2011;124(2):99–103. doi: 10.1111/j.1600-0404.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 101.Scheepers M, Comish S, Cordes L, Clough P, Scheepers B. Buccal midazolam and rectal diazepam for epilepsy. Lancet. 1999;353(9166):1797–1798. doi: 10.1016/S0140-6736(05)75897-9. [DOI] [PubMed] [Google Scholar]
- 102.Scott RC, Besag FM, Neville BG. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomised trial. Lancet. 1999;353(9153):623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 103.Talukdar B, Chakrabarty B. Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: a randomized controlled trial. Brain and Development. 2009;31(10):744–749. doi: 10.1016/j.braindev.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Kutlu NO, Dogrul M, Yakinci C, Soylu H. Buccal midazolam for treatment of prolonged seizures in children. Brain and Development. 2003;25(4):275–278. doi: 10.1016/s0387-7604(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 105.Aldawsari MF, Lau VW, Babu RJ, Arnold RD, Platt SR. Pharmacokinetic evaluation of novel midazolam gel formulations following buccal administration to healthy dogs. Am J Vet Res. 2018;79(1):73–82. doi: 10.2460/ajvr.79.1.73. [DOI] [PubMed] [Google Scholar]
- 106.Zhang J, Niu S, Zhang H, Streisand JB. Oral mucosal absorption of midazolam in dogs is strongly pH dependent. J Pharm Sci. 2002;91(4):980–982. doi: 10.1002/jps.10079. [DOI] [PubMed] [Google Scholar]
- 107.Spencer D. Hope for new treatments for acute repetitive seizures. Epilepsy Curr. 2014;14(3):147–149. doi: 10.5698/1535-7597-14.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anderson GD, Saneto RP. Current oral and non-oral routes of antiepileptic drug delivery. Adv Drug Deliv Rev. 2012;64(10):911–918. doi: 10.1016/j.addr.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 109.Musumeci T, Bonaccorso A, Puglisi G. Epilepsy Disease and Nose-to-Brain Delivery of Polymeric Nanoparticles: An Overview. Pharmaceutics. 2019;11:3. doi: 10.3390/pharmaceutics11030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10. doi: 10.1002/jps.2600810102. [DOI] [PubMed] [Google Scholar]
- 111.Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Pharmacokinetic comparison of sublingual lorazepam with intravenous, intramuscular, and oral lorazepam. J Pharm Sci. 1982;71(2):248–252. doi: 10.1002/jps.2600710227. [DOI] [PubMed] [Google Scholar]
- 112.Malu CK, Kahamba DM, Walker TD, Mukampunga C, Musalu EM, Kokolomani J, et al. Efficacy of sublingual lorazepam versus intrarectal diazepam for prolonged convulsions in sub-Saharan Africa. J Child Neurol. 2014;29(7):895–902. doi: 10.1177/0883073813493501. [DOI] [PubMed] [Google Scholar]
- 113.Gavhane YN, Yadav AV. Loss of orally administered drugs in GI tract. Saudi Pharm J. 2012;20(4):331–344. doi: 10.1016/j.jsps.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolars JC, Lown KS, Schmiedlin-Ren P, Ghosh M, Fang C, Wrighton SA, et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics. 1994;4(5):247–259. doi: 10.1097/00008571-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 115.Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, et al. First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther. 1996;60(1):14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 116.Thummel KE, OShea D, Paine MF, Shen DD, Kunze KL, Perkins JD, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59(5):491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 117.Jannin V, Lemagnen G, Gueroult P, Larrouture D, Tuleu C. Rectal route in the 21st century to treat children. Adv Drug Deliv Rev. 2014;73:34–49. doi: 10.1016/j.addr.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 118.Dhaliwal JS, Rosani A, Saadabadi A. Diazepam. Treasure Island: StatPearls; 2020. [Google Scholar]
- 119.Betcher DL, Burnham N. Midazolam for outpatient sedation. J Pediatr Oncol Nurs. 1992;9(3):136–138. doi: 10.1177/104345429200900307. [DOI] [PubMed] [Google Scholar]
- 120.Guittet C, Manso M, Burton I, Granier LA, Marcon F. A two-way randomized cross-over pharmacokinetic and Pharmacodynamic study of an innovative Oral solution of midazolam (ADV6209) Pharm Res. 2017;34(9):1840–1848. doi: 10.1007/s11095-017-2193-4. [DOI] [PubMed] [Google Scholar]
- 121.Dhillon S, Oxley J, Richens A. Bioavailability of diazepam after intravenous, oral and rectal administration in adult epileptic patients. Br J Clin Pharmacol. 1982;13(3):427–432. doi: 10.1111/j.1365-2125.1982.tb01397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Costa C, Moreira JN, Amaral MH, Sousa Lobo JM, Silva AC. Nose-to-brain delivery of lipid-based nanosystems for epileptic seizures and anxiety crisis. J Control Release. 2019;295:187–200. doi: 10.1016/j.jconrel.2018.12.049. [DOI] [PubMed] [Google Scholar]
- 123.Loscher W, Frey HH. Pharmacokinetics of diazepam in the dog. Arch Int Pharmacodyn Ther. 1981;254(2):180–195. [PubMed] [Google Scholar]
- 124.Crawshaw AA, Cock HR. Medical management of status epilepticus: emergency room to intensive care unit. Seizure. 2020;75:145–152. doi: 10.1016/j.seizure.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 125.Seigler RS. The administration of rectal diazepam for acute management of seizures. J Emerg Med. 1990;8(2):155–159. doi: 10.1016/0736-4679(90)90225-K. [DOI] [PubMed] [Google Scholar]
- 126.Dieckmann RA. Rectal diazepam for prehospital pediatric status epilepticus. Ann Emerg Med. 1994;23(2):216–224. doi: 10.1016/S0196-0644(94)70034-6. [DOI] [PubMed] [Google Scholar]
- 127.Alldredge BK, Wall DB, Ferriero DM. Effect of prehospital treatment on the outcome of status epilepticus in children. Pediatr Neurol. 1995;12(3):213–216. doi: 10.1016/0887-8994(95)00044-G. [DOI] [PubMed] [Google Scholar]
- 128.Dreifuss FE, Rosman NP, Cloyd JC, Pellock JM, Kuzniecky RI, Lo WD, et al. A comparison of rectal diazepam gel and placebo for acute repetitive seizures. N Engl J Med. 1998;338(26):1869–1875. doi: 10.1056/NEJM199806253382602. [DOI] [PubMed] [Google Scholar]
- 129.Brigo F, Nardone R, Tezzon F, Trinka E. Nonintravenous midazolam versus intravenous or rectal diazepam for the treatment of early status epilepticus: a systematic review with meta-analysis. Epilepsy Behav. 2015;49:325–336. doi: 10.1016/j.yebeh.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 130.Haut SR, Seinfeld S, Pellock J. Benzodiazepine use in seizure emergencies: a systematic review. Epilepsy Behav. 2016;63:109–117. doi: 10.1016/j.yebeh.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 131.Probst CW, Thomas WB, Moyers TD, Martin T, Cox S. Evaluation of plasma diazepam and nordiazepam concentrations following administration of diazepam intravenously or via suppository per rectum in dogs. Am J Vet Res. 2013;74(4):611–615. doi: 10.2460/ajvr.74.4.611. [DOI] [PubMed] [Google Scholar]
- 132.ORegan ME, Brown JK, Clark M. Nasal rather than rectal benzodiazepines in the management of acute childhood seizures? Dev Med Child Neurol. 1996;38(11):1037–1045. doi: 10.1111/j.1469-8749.1996.tb15064.x. [DOI] [PubMed] [Google Scholar]
- 133.Allonen H, Ziegler G, Klotz U. Midazolam kinetics. Clin Pharmacol Ther. 1981;30(5):653–661. doi: 10.1038/clpt.1981.217. [DOI] [PubMed] [Google Scholar]
- 134.Malinovsky JM, Lejus C, Servin F, Lepage JY, Le Normand Y, Testa S, et al. Plasma concentrations of midazolam after i.v., nasal or rectal administration in children. Br J Anaesth. 1993;70(6):617–620. doi: 10.1093/bja/70.6.617. [DOI] [PubMed] [Google Scholar]
- 135.Rey E, Delaunay L, Pons G, Murat I, Richard MO, Saint-Maurice C, et al. Pharmacokinetics of midazolam in children: comparative study of intranasal and intravenous administration. Eur J Clin Pharmacol. 1991;41(4):355–357. doi: 10.1007/BF00314967. [DOI] [PubMed] [Google Scholar]
- 136.Lahat E, Goldman M, Barr J, Bistritzer T, Berkovitch M. Comparison of intranasal midazolam with intravenous diazepam for treating febrile seizures in children: prospective randomised study. BMJ. 2000;321(7253):83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mahmoudian T, Zadeh MM. Comparison of intranasal midazolam with intravenous diazepam for treating acute seizures in children. Epilepsy Behav. 2004;5(2):253–255. doi: 10.1016/j.yebeh.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Javadzadeh M, Sheibani K, Hashemieh M, Saneifard H. Intranasal midazolam compared with intravenous diazepam in patients suffering from acute seizure: a randomized clinical trial. Iran J Pediatr. 2012;22(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 139.Ugwoke MI, Exaud S, Van Den Mooter G, Verbeke N, Kinget R. Bioavailability of apomorphine following intranasal administration of mucoadhesive drug delivery systems in rabbits. Eur J Pharm Sci. 1999;9(2):213–219. doi: 10.1016/S0928-0987(99)00061-5. [DOI] [PubMed] [Google Scholar]
- 140.Varshosaz J, Sadrai H, Alinagari R. Nasal delivery of insulin using chitosan microspheres. J Microencapsul. 2004;21(7):761–774. doi: 10.1080/02652040400015403. [DOI] [PubMed] [Google Scholar]
- 141.Varshosaz J, Sadrai H, Heidari A. Nasal delivery of insulin using bioadhesive chitosan gels. Drug Deliv. 2006;13(1):31–38. doi: 10.1080/10717540500309040. [DOI] [PubMed] [Google Scholar]
- 142.Furubayashi T, Kamaguchi A, Kawaharada K, Masaoka Y, Kataoka M, Yamashita S, et al. Evaluation of the contribution of the nasal cavity and gastrointestinal tract to drug absorption following nasal application to rats. Biol Pharm Bull. 2007;30(3):608–611. doi: 10.1248/bpb.30.608. [DOI] [PubMed] [Google Scholar]
- 143.Leonard AK, Sileno AP, Brandt GC, Foerder CA, Quay SC, Costantino HR. In vitro formulation optimization of intranasal galantamine leading to enhanced bioavailability and reduced emetic response in vivo. Int J Pharm. 2007;335(1–2):138–146. doi: 10.1016/j.ijpharm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 144.Cattet MR, Caulkett NA, Wilson C, Vandenbrink T, Brook RK. Intranasal administration of xylazine to reduce stress in elk captured by net gun. J Wildl Dis. 2004;40(3):562–565. doi: 10.7589/0090-3558-40.3.562. [DOI] [PubMed] [Google Scholar]
- 145.Duque JC, Oleskovicz N, Guirro EC, Valadao CA, Soares VE. Relative potency of ketamine and S(+)-ketamine in dogs. J Vet Pharmacol Ther. 2008;31(4):344–348. doi: 10.1111/j.1365-2885.2008.00965.x. [DOI] [PubMed] [Google Scholar]
- 146.Gerritsmann H, Stalder GL, Seilern-Moy K, Knauer F, Walzer C. Comparison of S(+)-ketamine and ketamine, with medetomidine, for field anaesthesia in the European brown hare (Lepus europaeus) Vet Anaesth Analg. 2012;39(5):511–519. doi: 10.1111/j.1467-2995.2012.00754.x. [DOI] [PubMed] [Google Scholar]
- 147.Micieli F, Santangelo B, Napoleone G, Di Dona F, Mennonna G, Vesce G. Intranasal fentanyl for acute severe pain episodes control in a dog. Vet Anaesth Analg. 2017;44(6):1400–1401. doi: 10.1016/j.vaa.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 148.Shury TK, Caulkett NA, Woodbury MR. Intranasal naltrexone and atipamezole for reversal of white-tailed deer immobilized with carfentanil and medetomidine. Can Vet J. 2010;51(5):501–505. [PMC free article] [PubMed] [Google Scholar]
- 149.Weiland LC, Kluge K, Kutter APN, Kronen PW. Clinical evaluation of intranasal medetomidine-ketamine and medetomidine-S(+)-ketamine for induction of anaesthesia in rabbits in two centres with two different administration techniques. Vet Anaesth Analg. 2017;44(1):98–105. doi: 10.1111/vaa.12408. [DOI] [PubMed] [Google Scholar]
- 150.Vlerick L, Devreese M, Peremans K, Dockx R, Croubels S, Duchateau L, et al. Pharmacokinetics, absolute bioavailability and tolerability of ketamine after intranasal administration to dexmedetomidine sedated dogs. PLoS One. 2020;15(1):e0227762. doi: 10.1371/journal.pone.0227762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Humphries LK, Eiland LS. Treatment of acute seizures: is intranasal midazolam a viable option? J Pediatr Pharmacol Ther. 2013;18(2):79–87. doi: 10.5863/1551-6776-18.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Boddu SHS, Kumari S. A Short Review on the Intranasal Delivery of Diazepam for Treating Acute Repetitive Seizures. Pharmaceutics. 2020:12. [DOI] [PMC free article] [PubMed]
- 153.Ivaturi VD, Riss JR, Kriel RL, Cloyd JC. Pharmacokinetics and tolerability of intranasal diazepam and midazolam in healthy adult volunteers. Acta Neurol Scand. 2009;120(5):353–357. doi: 10.1111/j.1600-0404.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- 154.Schrier L, Zuiker R, Merkus FW, Klaassen ES, Guan Z, Tuk B, et al. Pharmacokinetics and pharmacodynamics of a new highly concentrated intranasal midazolam formulation for conscious sedation. Br J Clin Pharmacol. 2017;83(4):721–731. doi: 10.1111/bcp.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eagleson JS, Platt SR, Strong DL, Kent M, Freeman AC, Nghiem PP, et al. Bioavailability of a novel midazolam gel after intranasal administration in dogs. Am J Vet Res. 2012;73(4):539–545. doi: 10.2460/ajvr.73.4.539. [DOI] [PubMed] [Google Scholar]
- 156.Lui CY, Amidon GL, Goldberg A. Intranasal absorption of flurazepam, midazolam, and triazolam in dogs. J Pharm Sci. 1991;80(12):1125–1129. doi: 10.1002/jps.2600801207. [DOI] [PubMed] [Google Scholar]
- 157.Musulin SE, Mariani CL, Papich MG. Diazepam pharmacokinetics after nasal drop and atomized nasal administration in dogs. J Vet Pharmacol Ther. 2011;34(1):17–24. doi: 10.1111/j.1365-2885.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- 158.Hong SS, Oh KT, Choi HG, Lim SJ. Liposomal Formulations for Nose-to-Brain Delivery: Recent Advances and Future Perspectives. Pharmaceutics. 2019;11:10. doi: 10.3390/pharmaceutics11100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):13–38. doi: 10.1016/s0169-409x(97)00059-8. [DOI] [PubMed] [Google Scholar]
- 160.Agu RU. Challenges in nasal drug absorption: how far have we come? Ther Deliv. 2016;7(7):495–510. doi: 10.4155/tde-2016-0022. [DOI] [PubMed] [Google Scholar]
- 161.Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3(1):42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Craven BA, Neuberger T, Paterson EG, Webb AG, Josephson EM, Morrison EE, et al. Reconstruction and morphometric analysis of the nasal airway of the dog (Canis familiaris) and implications regarding olfactory airflow. Anat Rec (Hoboken) 2007;290(11):1325–1340. doi: 10.1002/ar.20592. [DOI] [PubMed] [Google Scholar]
- 163.Craven BA, Paterson EG, Settles GS. The fluid dynamics of canine olfaction: unique nasal airflow patterns as an explanation of macrosmia. J R Soc Interface. 2010;7(47):933–943. doi: 10.1098/rsif.2009.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Illum L. Nasal clearance in health and disease. J Aerosol Med. 2006;19(1):92–99. doi: 10.1089/jam.2006.19.92. [DOI] [PubMed] [Google Scholar]
- 165.McMartin C, Hutchinson LE, Hyde R, Peters GE. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci. 1987;76(7):535–540. doi: 10.1002/jps.2600760709. [DOI] [PubMed] [Google Scholar]
- 166.Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14(9):3754–3779. doi: 10.3390/molecules14093754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kozlovskaya L, Abou-Kaoud M, Stepensky D. Quantitative analysis of drug delivery to the brain via nasal route. J Control Release. 2014;189:133–140. doi: 10.1016/j.jconrel.2014.06.053. [DOI] [PubMed] [Google Scholar]
- 168.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 169.Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol. 2005;30(1):57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 170.Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9(Suppl 1):S3. doi: 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37(1):13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 172.Cardoso FL, Brites D, Brito MA. Looking at the blood-brain barrier: molecular anatomy and possible investigation approaches. Brain Res Rev. 2010;64(2):328–363. doi: 10.1016/j.brainresrev.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 173.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–1972. doi: 10.1038/jcbfm.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Merkus P, Guchelaar HJ, Bosch DA, Merkus FW. Direct access of drugs to the human brain after intranasal drug administration? Neurology. 2003;60(10):1669–1671. doi: 10.1212/01.WNL.0000067993.60735.77. [DOI] [PubMed] [Google Scholar]
- 175.Marianecci C, Rinaldi F, Hanieh PN, Di Marzio L, Paolino D, Carafa M. Drug delivery in overcoming the blood-brain barrier: role of nasal mucosal grafting. Drug Des Devel Ther. 2017;11:325–335. doi: 10.2147/DDDT.S100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Griffin CE, 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 177.Talegaonkar S, Mishra PR. Intranasal delivery: an approach to bypass the blood brain barrier. Ind J Pharmacol. 2004;36:140–147. [Google Scholar]
- 178.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 179.Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 180.Illum L. Nasal drug delivery - recent developments and future prospects. J Control Release. 2012;161(2):254–263. doi: 10.1016/j.jconrel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 181.Johnson NJ, Hanson LR, Frey WH. Trigeminal pathways deliver a low molecular weight drug from the nose to the brain and orofacial structures. Mol Pharm. 2010;7(3):884–893. doi: 10.1021/mp100029t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64(7):614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 183.Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7(23):1184–1189. doi: 10.1016/S1359-6446(02)02529-1. [DOI] [PubMed] [Google Scholar]
- 184.Bourganis V, Kammona O, Alexopoulos A, Kiparissides C. Recent advances in carrier mediated nose-to-brain delivery of pharmaceutics. Eur J Pharm Biopharm. 2018;128:337–362. doi: 10.1016/j.ejpb.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 185.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 186.Chapman CD, Frey WH, 2nd, Craft S, Danielyan L, Hallschmid M, Schioth HB, et al. Intranasal treatment of central nervous system dysfunction in humans. Pharm Res. 2013;30(10):2475–2484. doi: 10.1007/s11095-012-0915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Frey WH, Liu J, Chen X, Thorne RG, Fawcett JR, Ala TA, et al. Delivery of 125I-NGF to the brain via the olfactory route. Drug Deliv. 1997;4:87–92. doi: 10.3109/10717549709051878. [DOI] [Google Scholar]
- 188.Sakane T, Akizuki M, Yoshida M, Yamashita S, Nadai T, Hashida M, et al. Transport of cephalexin to the cerebrospinal fluid directly from the nasal cavity. J Pharm Pharmacol. 1991;43(6):449–451. doi: 10.1111/j.2042-7158.1991.tb03510.x. [DOI] [PubMed] [Google Scholar]
- 189.Anand Kumar TC, David GF, Sankaranarayanan A, Puri V, Sundram KR. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc Natl Acad Sci U S A. 1982;79(13):4185–4189. doi: 10.1073/pnas.79.13.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang X, He H, Leng W, Tang X. Evaluation of brain-targeting for the nasal delivery of estradiol by the microdialysis method. Int J Pharm. 2006;317(1):40–46. doi: 10.1016/j.ijpharm.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 191.Striepens N, Kendrick KM, Hanking V, Landgraf R, Wullner U, Maier W, et al. Elevated cerebrospinal fluid and blood concentrations of oxytocin following its intranasal administration in humans. Sci Rep. 2013;3:3440. doi: 10.1038/srep03440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kristensson K, Olsson Y. Uptake of exogenous proteins in mouse olfactory cells. Acta Neuropathol. 1971;19(2):145–154. doi: 10.1007/BF00688493. [DOI] [PubMed] [Google Scholar]
- 193.Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci. 2002;5(6):514–516. doi: 10.1038/nn0602-849. [DOI] [PubMed] [Google Scholar]
- 194.Anton F, Peppel P. Central projections of trigeminal primary afferents innervating the nasal mucosa: a horseradish peroxidase study in the rat. Neuroscience. 1991;41(2–3):617–628. doi: 10.1016/0306-4522(91)90354-Q. [DOI] [PubMed] [Google Scholar]
- 195.Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH., 2nd Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152(3):785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 196.Bilston LE, Fletcher DF, Brodbelt AR, Stoodley MA. Arterial pulsation-driven cerebrospinal fluid flow in the perivascular space: a computational model. Comput Methods Biomech Biomed Eng. 2003;6(4):235–241. doi: 10.1080/10255840310001606116. [DOI] [PubMed] [Google Scholar]
- 197.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The "perivascular pump" driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO. Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol. 2006;238(4):962–974. doi: 10.1016/j.jtbi.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 199.Lochhead JJ, Wolak DJ, Pizzo ME, Thorne RG. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 2015;35(3):371–381. doi: 10.1038/jcbfm.2014.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pires A, Fortuna A, Alves G, Falcao A. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci. 2009;12(3):288–311. doi: 10.18433/J3NC79. [DOI] [PubMed] [Google Scholar]
- 201.Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol. 2006;34(3):252–269. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 202.Jansson B, Bjork E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J Drug Target. 2002;10(5):379–386. doi: 10.1080/1061186021000001823. [DOI] [PubMed] [Google Scholar]
- 203.Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982;135(Pt 1):83–88. [PMC free article] [PubMed] [Google Scholar]
- 204.Loscher W, Luna-Tortos C, Romermann K, Fedrowitz M. Do ATP-binding cassette transporters cause pharmacoresistance in epilepsy? Problems and approaches in determining which antiepileptic drugs are affected. Curr Pharm Des. 2011;17(26):2808–2828. doi: 10.2174/138161211797440212. [DOI] [PubMed] [Google Scholar]
- 205.Grewal GK, Kukal S, Kanojia N, Saso L, Kukreti S, Kukreti R. Effect of Oxidative Stress on ABC Transporters: Contribution to Epilepsy Pharmacoresistance. Molecules. 2017;22:3. doi: 10.3390/molecules22030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Romeo VD, de Meireles J, Sileno AP, Pimplaskar HK, Behl CR. Effects of physicochemical properties and other factors on systemic nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1–2):89–116. doi: 10.1016/s0169-409x(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 207.Schipper NG, Verhoef JC, Merkus FW. The nasal mucociliary clearance: relevance to nasal drug delivery. Pharm Res. 1991;8(7):807–814. doi: 10.1023/A:1015830907632. [DOI] [PubMed] [Google Scholar]
- 208.Graff CL, Pollack GM. P-glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20(8):1225–1230. doi: 10.1023/A:1025053115583. [DOI] [PubMed] [Google Scholar]
- 209.Graff CL, Pollack GM. Functional evidence for P-glycoprotein at the nose-brain barrier. Pharm Res. 2005;22(1):86–93. doi: 10.1007/s11095-004-9013-3. [DOI] [PubMed] [Google Scholar]
- 210.Graff CL, Pollack GM. Nasal drug administration: potential for targeted central nervous system delivery. J Pharm Sci. 2005;94(6):1187–1195. doi: 10.1002/jps.20318. [DOI] [PubMed] [Google Scholar]
- 211.Graff CL, Zhao R, Pollack GM. Pharmacokinetics of substrate uptake and distribution in murine brain after nasal instillation. Pharm Res. 2005;22(2):235–244. doi: 10.1007/s11095-004-1191-5. [DOI] [PubMed] [Google Scholar]
- 212.Westin U, Piras E, Jansson B, Bergstrom U, Dahlin M, Brittebo E, et al. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci. 2005;24(5):565–573. doi: 10.1016/j.ejps.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 213.Dhuria SV, Hanson LR, Frey WH., 2nd Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther. 2009;328(1):312–320. doi: 10.1124/jpet.108.145565. [DOI] [PubMed] [Google Scholar]
- 214.Bennett DA, Lal H. Discriminative stimulus properties of the vasodilator, hydralazine: differential generalization with alpha 1 and alpha 2 adrenoreceptor drugs. Prog Neuro-Psychopharmacol Biol Psychiatry. 1982;6(1):17–26. doi: 10.1016/S0364-7722(82)80103-3. [DOI] [PubMed] [Google Scholar]
- 215.Kamata K, Numazawa T, Kasuya Y. Vasodilator effects of clonidine on the mesenteric arterial beds in normotensive and spontaneously hypertensive rats. Res Commun Chem Pathol Pharmacol. 1994;84(3):371–374. [PubMed] [Google Scholar]
- 216.Holsti M, Sill BL, Firth SD, Filloux FM, Joyce SM, Furnival RA. Prehospital intranasal midazolam for the treatment of pediatric seizures. Pediatr Emerg Care. 2007;23(3):148–153. doi: 10.1097/PEC.0b013e3180328c92. [DOI] [PubMed] [Google Scholar]
- 217.Jenkins EK, DeChant MT, Perry EB. When the nose Doesn't know: canine olfactory function associated with health, management, and potential links to microbiota. Front Vet Sci. 2018;5:56. doi: 10.3389/fvets.2018.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Fernandez-Parra R, Pey P, Zilberstein L, Malve M. Use of computational fluid dynamics to compare upper airway pressures and airflow resistance in brachycephalic, mesocephalic, and dolichocephalic dogs. Vet J. 2019;253:105392. doi: 10.1016/j.tvjl.2019.105392. [DOI] [PubMed] [Google Scholar]
- 219.Schuenemann R, Oechtering G. Inside the brachycephalic nose: Conchal regrowth and mucosal contact points after laser-assisted turbinectomy. J Am Anim Hosp Assoc. 2014;50(4):237–246. doi: 10.5326/JAAHA-MS-6086. [DOI] [PubMed] [Google Scholar]
- 220.Kumar A, Pandey AN, Jain SK. Nasal-nanotechnology: revolution for efficient therapeutics delivery. Drug Deliv. 2016;23(3):681–693. doi: 10.3109/10717544.2014.920431. [DOI] [PubMed] [Google Scholar]
- 221.Mittal D, Ali A, Md S, Baboota S, Sahni JK, Ali J. Insights into direct nose to brain delivery: current status and future perspective. Drug Delivery. 2014;21(2):75–86. doi: 10.3109/10717544.2013.838713. [DOI] [PubMed] [Google Scholar]
- 222.Vidgren MT, Kublik H. Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev. 1998;29(1–2):157–177. doi: 10.1016/s0169-409x(97)00067-7. [DOI] [PubMed] [Google Scholar]
- 223.Warnken ZN, Smyth HDC, Davis DA, Weitman S, Kuhn JG, Williams RO., 3rd Personalized medicine in nasal delivery: the use of patient-specific administration parameters to improve nasal drug targeting using 3D-printed nasal replica casts. Mol Pharm. 2018;15(4):1392–1402. doi: 10.1021/acs.molpharmaceut.7b00702. [DOI] [PubMed] [Google Scholar]
- 224.Hardy JG, Lee SW, Wilson CG. Intranasal drug delivery by spray and drops. J Pharm Pharmacol. 1985;37(5):294–297. doi: 10.1111/j.2042-7158.1985.tb05069.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.