Abstract
Early threat exposure is a transdiagnostic risk factor for psychopathology, and evidence suggests that genetic variation in the oxytocin receptor (OXTR) moderates this association. However, it is unclear if this gene-by-environment (G×E) interaction is tied to unique risk for disorder-specific outcomes or instead increases shared risk for general psychopathology. Moreover, little is known about how this G×E interaction increases risk. The current study utilized a prospective, longitudinal sample of females (n = 2,020) to examine: (a) whether the interaction between early threat exposure and OXTR variation (rs53576, rs2254298) confers risk for disorder-specific outcomes (depression, anxiety, borderline and antisocial personality disorders) and/or general psychopathology in early adulthood; and (b) whether social–emotional deficits (emotion dysregulation, callousness, attachment quality) during adolescence constitute mediating mechanisms. Consistent with hypotheses, the interactive effects of early threat exposure and OXTR variation (rs53576) predicted general psychopathology, with threat-exposed women carrying at least one copy of the rs53576 A-allele at greatest risk. This interaction was mediated via emotional dysregulation in adolescence, with threat-exposed A-allele carriers demonstrating greater emotion dysregulation, and greater emotion dysregulation predicting general psychopathology in early adulthood. Findings suggest that this G×E places women at risk for a broad range of psychopathology via effects on emotion dysregulation.
Keywords: callousness, emotion dysregulation, maltreatment, oxytocin, psychopathology
Overview
Childhood maltreatment, specifically early threat exposure (e.g., experiencing violence or abuse), is a transdiagnostic risk factor for later psychopathology (Cicchetti & Toth, 2005; Kessler et al., 2010), and emerging evidence suggests that genetic variation in the oxytocin receptor (OXTR) may moderate this association (Cicchetti, Rogosch, Hecht, Crick, & Hetzel, 2014). Given the well-documented impact of early threat exposure and oxytocin on neurobiological systems supporting social–emotional development (Donadon, Martin-Santos, & Osório, 2018), it is not surprising that this gene-by-environment (G×E) interaction has been implicated in a wide range of disorders characterized by social-emotional deficits (i.e., depression, anxiety, borderline personality disorder (BPD) and antisocial personality disorder (ASPD); McQuaid, McInnis, Abizaid, & Anisman, 2014; Myers et al., 2014; Steele & Siever, 2010). However, it is unclear whether this G×E interaction is tied to unique risk for disorder-specific outcomes, or whether it increases shared risk for general psychopathology. Moreover, very little is known about how this G×E interaction functions to increase risk. Research suggests that social-emotional deficits like emotion dysregulation, callousness, and poor attachment quality may function as mediating mechanisms linking the interaction between early threat exposure and OXTR variation with psychopathology in adulthood (e.g., Bradley et al., 2011; Dadds et al., 2014; McQuaid, McInnis, Stead, Matheson, & Anisman, 2013). However, these putative intermediate behavioral phenotypes have yet to be examined simultaneously, and never as mediators of the association between this G×E interaction and disorder-specific versus general risk for psychopathology.
To extend the current literature and improve the characterization of this developmental risk pathway, the current study seeks to: (a) examine whether the interaction between early threat exposure and OXTR variation (rs53576, rs2254298) confers risk for disorder-specific outcomes and/or for general psychopathology, and (b) assess whether individual variability in social-emotional deficits (emotion dysregulation, callousness, and quality of attachment) during adolescence constitutes potential mediating mechanisms connecting this G×E interaction with disorder-specific and/or general risk for psychopathology in early adulthood. In light of research suggesting that early threat exposure affects the oxytocin system in a sex-dependent way (Cicchetti et al., 2014; Israel et al., 2009; Seltzer, Ziegler, Connolly, Prososki, & Pollak, 2014), we examined these questions in a large, at-risk sample of females assessed prospectively from childhood through early adulthood.
Early threat exposure and risk for psychopathology
Recent research highlights the importance of differentiating between distinct dimensions of maltreatment (e.g., threat exposure vs. deprivation) when examining risk for psychopathology, as the specific mechanisms through which these experiences influence development vary (Cicchetti & Manly, 2001; McLaughlin, Sheridan, & Lambert, 2014b; Sheridan & McLaughlin, 2014). Along these lines, early threat exposure, defined as any experience involving harm or threat of harm (i.e., witnessing and/or experiencing violence or abuse), has been linked to profound disruptions in the stress-response system (Tarullo & Gunnar, 2006; Teicher et al., 2003). Specifically, research suggests that threat exposure in childhood influences the development of cortico–limbic circuits that underlie social and emotional learning processes (McCrory et al., 2011), and disrupts physiological and subjective responses to environmental stressors (Cicchetti, 2016; Cicchetti & Toth, 2005; Perry, Pollard, Blakley, Baker, & Vigilante, 1995). Not surprisingly, decades of research have consistently linked early threat exposure to the emergence of psychiatric disorders characterized by marked deficits in social and emotional processing, namely depression, anxiety, and personality disorders such as BPD and ASPD (Heim & Nemeroff, 2001; Jaffee, Caspi, Moffitt, & Taylor, 2004; Johnson, Cohen, Brown, Smailes, & Bernstein, 1999; Rogosch & Cicchetti, 2005; Stepp, Lazarus, & Byrd, 2016). Moreover, risk for these disorders substantially increases as threat exposures become more chronic (Anda et al., 2006; Hughes et al., 2017; Manly, Kim, Rogosch, & Cicchetti, 2001; Mersky, Topitzes, & Reynolds, 2013), emphasizing the importance of prospective assessments that account for chronicity when examining experiences of threat exposure over time (e.g., Cowell, Cicchetti, Rogosch, & Toth, 2015; Jaffee & Maikovich-Fong, 2011).
Robust associations between threat exposure and disorder-specific outcomes have emerged primarily from studies examining these disorders in isolation (Heim & Nemeroff, 2001; Jaffee et al., 2004; Johnson et al., 1999; Rogosch & Cicchetti, 2005; Stepp et al., 2016). This leaves unanswered questions about the extent to which threat exposure is tied to unique risk for disorder-specific outcomes or instead linked to risk for general psychopathology. A growing body of work suggests that the impact of early threat exposure may be largely nonspecific, and therefore better understood as a shared liability for the development of a wide range of psychopathologies (Eaton et al., 2011; Green et al., 2010; Kessler et al., 2010; Keyes et al., 2012; McLaughlin et al., 2012). Along these lines, recent theoretical and empirical work supports a general factor of psychopathology (Caspi et al., 2014; Laceulle, Vollebergh, & Ormel, 2015; Lahey et al., 2012, 2015)—termed the “p factor”—that likely reflects a shared etiology. Thus, there is growing interest in better understanding how early risk factors, like threat exposure, may contribute to an overall latent vulnerability to psychopathology (Kotov et al., 2017; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011; Lahey et al., 2012; Tackett et al., 2013), as such an approach has the potential to further clarify shared risk mechanisms (McLaughlin et al., 2014b; Sheridan & McLaughlin, 2014).
Along these lines, research suggests that moderating influences of polymorphic variation in the gene encoding the OXTR may play a role in this developmental risk pathway. Importantly, OXTR variation may help to explain why only some youth who experience early threat exposure go on to develop psychopathology (Cicchetti, 2013; Jaffee et al., 2005), particularly with regard to the development of disorders characterized by social–emotional deficits (McQuaid et al., 2014; Myers et al., 2014; Steele & Siever, 2010). OXTR variation plays a central role in the development and functioning of social and emotional processing (see below). Thus, it is possible that the synergistic effects of both early threat exposure and OXTR variation increase one’s vulnerability for developing later psychopathology via shared risk mechanisms.
OXTR variation as a moderator of risk for psychopathology
There is growing evidence that OXTR variation moderates the association between early threat exposure and the emergence of mood, anxiety, and personality disorders (e.g., Cicchetti et al., 2014; Hammen, Bower, & Cole, 2015; Hostinar, Cicchetti, & Rogosch, 2014; Thompson, Parker, Hallmayer, Waugh, & Gotlib, 2011). Like early threat exposure, oxytocin significantly impacts the development of the stress-response system, and this regulatory neuropeptide continues to influence our physiological, emotional, and behavioral responses throughout the lifespan (Carter, 2014, 2003). Oxytocin is synthesized in the hypothalamus and, in addition to subsequent pituitary release into the peripheral circulation, oxytocin-releasing neurons project widely throughout the brain. The OXTR is highly concentrated in limbic regions (e.g., amygdala, hippocampus) and autonomic centers in the brainstem, which is not surprising given their influence on the functioning of physiological systems underlying the regulation of emotions and complex social behaviors (Neumann, 2008).
Molecular variation in the gene encoding the OXTR, located on chromosome 3p25.3, has been shown to interact with early threat exposure to predict a range of psychopathologies characterized by emotional and social deficits (Kumsta & Heinrichs, 2013). Two single nucleotide polymorphisms (SNPs) have emerged as particularly promising candidates and represent two of the most well-researched OXTR genotypes: rs53576 (A/G), located within intron 3, and rs2254298 (A/G), located within the 3I untranslated region (Bakermans-Kranenburg & van IJzendoorn, 2014; Kumsta & Heinrichs, 2013; Toepfer et al., 2017). Variation in these SNPs has been linked to significantly higher levels of depression and anxiety in the context of early adversity (Hostinar et al., 2014; Myers et al., 2014; Thompson et al., 2011), as well as higher BPD symptoms (Cicchetti et al., 2014; Hammen et al., 2015) and higher ASPD symptoms (Malik, Zai, Abu, Nowrouzi, & Beitchman, 2012; Smearman, Winiarski, Brennan, Najman, & Johnson, 2015). While these studies provide support for the role of the OXTR in this developmental risk pathway, it is unclear whether genetic variation in the OXTR moderates risk for disorder-specific outcomes (as outlined above) or instead functions to increase risk for a shared liability in the development of a range of psychopathology.
Notably, findings are inconsistent regarding which OXTR genotypes confer greater vulnerability for psychopathology in the presence of early threat exposure. Studies examining rs2254298 have consistently documented A-allele carriers as more susceptible to threat exposure, though the notably larger body of empirical work examining the moderating effects of rs53576 has been more mixed (i.e., higher susceptibility in GG homozygotes vs. A-allele carriers; for review see Toepfer et al., 2017). There is some suggestion that sex-dependent differences in OTXR allelic risk may contribute to these discrepancies. Though the majority of research in this area has focused on male or undifferentiated samples, studies stratified by gender have shown sex-dependent associations for rs53576, with male GG homozygotes and female A-allele carriers showing higher levels of psychopathology in the presence of early threat exposure (see Cicchetti et al., 2014 for an example). Moreover, studies with predominantly female samples have shown rs53576 A-allele carriers to be most at risk for psychopathology (Hammen et al., 2015; McInnis, McQuaid, Matheson, & Anisman, 2015; Thompson, Hammen, Starr, & Najman, 2014), suggesting that, like rs2254298, female rs53576 A-allele carriers may be more vulnerable to the negative effects of early threat exposure. While the exact mechanisms underlying sex-dependent oxytocinergic functioning continue to be investigated, this research echoes a larger body of literature suggesting that the interaction between early threat exposure and OXTR may function differently in males versus females (see Seltzer et al., 2014). Taken together, these findings highlight the importance of accounting for sex when examining this developmental risk pathway and expanding the literature to include female samples.
Social–emotional deficits as potential mediators
Although recent research has identified allelic variation in the OXTR as a moderator of the association between early threat exposure and later psychopathology, very little is known about how this G×E interaction functions to increase psychopathology risk. The examination of intermediate behavioral phenotypes as mediating mechanisms has the potential to further elucidate this developmental risk pathway (Dodge, 2009; Kumsta & Heinrichs, 2013). Given the synergistic influence of threat exposure and oxytocin on social–emotional development (Donadon et al., 2018; Kumsta & Heinrichs, 2013), deficits within this domain have received growing attention. As described above, early threat exposure and oxytocin significantly impact the stress-response system, and disruption within this system has been linked to specific social–emotional deficits such as difficulties with social–emotional processing and regulation, as well as attachment quality (Carter, 2014, 2003; Tarullo & Gunnar, 2006; Teicher et al., 2003). Importantly, these deficits are robust indicators of risk for later psychopathology, underscoring the need for continued research in this area (Tabak, 2013).
Indeed, research examining early threat exposure and OXTR variation in isolation has shown associations with social–emotional deficits strongly implicated in psychopathology (Dadds et al., 2014; Kim & Cicchetti, 2010; Kumsta & Heinrichs, 2013; Maughan & Cicchetti, 2002). Three specific social-emotional deficits—emotion dysregulation, callousness, and poor attachment quality—have received growing attention in the literature as putative endophenotypes. Threat exposure and OXTR variation have been independently linked to increased sensitivity to stress as evidenced by higher physiological reactivity and emotion dysregulation, as well as to social skills deficits like callousness, and to measures of attachment quality, such as reduced trust (Krueger et al., 2012; Kumsta & Heinrichs, 2013; Rodrigues, Saslow, Garcia, John, & Keltner, 2009). Further, emerging research shows that the combined effects of these risk factors (i.e., G×E interaction) increases emotion dysregulation (Bradley et al., 2011) and callousness (Verona, Murphy, & Bresin, 2018) as well as poor quality of attachment (McQuaid et al., 2013). These deficits often peak during adolescence (Blakemore & Choudhury, 2006; Casey, Jones, & Hare, 2008), a critical period for social–emotional development (Steinberg, 2005; Steinberg & Morris, 2001), and increased levels during this sensitive window have been linked to risk for mood, anxiety, and personality disorders (Buist, Deković, Meeus, & van Aken, 2004; Cole, Michel, & Teti, 1994; Frick & White, 2008). However, research has yet to examine these social–emotional deficits simultaneously as potential intermediate behavioral phenotypes linking the interaction between early threat exposure and OXTR variation with disorder-specific and/or general risk for psychopathology.
Current study
The current study sought to replicate and extend prior work by examining whether the interaction between threat exposure in childhood and OXTR variation (rs53576, rs2254298) confers risk for disorder-specific outcomes and/or general psychopathology in young adulthood. Additionally, the current study examined whether social–emotional factors (emotion dysregulation, callousness, and attachment quality) in adolescence constitute potential mediating mechanisms connecting the interaction between early threat exposure and OXTR variation to psychopathology. Importantly, the present study addresses these questions in a large, urban female sample that has been comprehensively assessed from childhood into early adulthood. Assessments include annual comprehensive multi-informant reports of multiple early threat exposures throughout childhood, social–emotional deficits during adolescence, and mood, anxiety and personality disorder symptoms in early adulthood.
We hypothesized that the interactive effects of early threat exposure and OXTR variation would be predictive of a shared liability for “general” psychopathology. Specifically, we predicted that threat-exposed women with at least one copy of the A allele (AA/AG) of either rs53576 or rs2254298 would show the highest levels of the “general” psychopathology factor in early adulthood. We also predicted that the interaction between early threat exposure and OXTR variation would increase risk for general psychopathology (vs. disorder-specific outcomes) via the impact on social–emotional development during adolescence. That is, threat-exposed, A-allele carriers would show higher levels of social–emotional deficits in adolescence, putting them at increased risk for “general” psychopathology in early adulthood.
Finally, because this study extends from one of two strands of contemporary G×E research, some justification for the approach employed here is warranted, beyond its obvious provenance in a corresponding literature. The first strand of G×E research, or the candidate gene G×E approach, proceeds from fundamentally biological considerations, hence drawing “candidate” gene polymorphisms from plausible pathways of developmental influence. In this sense, it is hypothesis driven, but necessarily constrained in selection of genetic variants for study. Thus, one frequently cited limitation of the candidate G×E approach is its limited coverage of potentially relevant genetic variation, often coupled with reliance on small study samples and attendant failures of replication (Dick et al., 2015; Duncan & Keller, 2011). Still, it is not the case that candidate or single-gene G×E studies never replicate or fail on meta-analytic review (Bakermans-Kranenburg & Van Ijzendoorn, 2011, 2015; Byrd & Manuck, 2014; Hartman, Widaman, & Belsky, 2015; Kilpeläinen et al., 2011; Wang, Shelton, & Dwivedi, 2018; Zhao et al., 2018). The second strand of G×E research draws on the power of genome-wide association (GWA) to identify variants of phenotypic association at high levels of statistical significance, which may then be aggregated into polygenic scores (Bogdan, Baranger, & Agrawal, 2018). The latter may be constructed as well from all genetic variants typed on a GWA microarray, thus also incorporating predictive variants undetectable at individual loci. Such polygenic scores will account for a larger portion of phenotypic variation, both as main effects and, in principle (as there are yet few examples), when moderated by candidate environmental factors (G×E). A limitation of this approach is that because few GWA-identified variants have known functionality, their use in G×E research lacks mechanistic rationale and rests on the uncertain assumption that all phenotype-associated polymorphisms (whatever their biological actions) interact in unison and uniformly with the same environmental moderator. At present, then, molecular G×E research is split between two approaches, neither without limitations. In that context, we believe continued research in the first tradition remains warranted, with the understanding that the present study, like all such work, ultimately demands replication and gains credence by consilience with the literatures giving rise to it.
Method
Sample
Participants were drawn from the Pittsburgh Girls Study (PGS), an ongoing longitudinal study of 2,450 women that began when they were 5- to 8-years old. Girls were identified by a stratified sampling strategy that included a total of 103,238 households in Pittsburgh, where households in low-income neighborhoods were over-sampled. Of those girls initially identified, 2,876 were asked to take part in the longitudinal study, and a total of 2,450 (85.2%) girls agreed to participate. At the time of the first interview, the sample comprised 588 5-year olds, 630 6-year olds, 611 7-year olds, and 622 8-year olds, of whom 41.2% were Caucasian and over half were racial minorities (52.9% African American; 4.9% multi-racial, <1% Asian).1 Girls and their caregivers (92.9% biological mothers) were interviewed annually, and at the time of analyses girls had been interviewed through age 22. Participant retention was over 85% at each assessment. Further demographic information can be found in Hipwell et al. (2002).
Procedure
Separate in-home interviews for both the girl and the caregiver were conducted annually by trained interviewers using a laptop computer. Analyses for the current study utilize data collected during childhood (ages 5 to 11 years), adolescence (ages 13 to 17 years) and early adulthood (ages 18 to 22 years). When participants were between the ages of 15 and 20, DNA was isolated from saliva samples using the Oragene DNA self-collection kit following manufacturer instructions (DNA Genotek Inc., Ottawa, Ontario, Canada). Procedures were reviewed and approved by the Institutional Review Board at the University of Pittsburgh. Written informed consent was obtained from caregivers and/or girls prior to each assessment. Families were compensated for their participation.
DNA extraction and genotyping
Genotyping of the OXTR SNPs rs53576 (A/G) and rs2254298 (A/G) was performed using the Agena iPlex platform (Agena Bioscience, San Diego, California). Following multiplex polymerase chain reaction (PCR), allele adjacent Extend primers are subjected to single base extension. Extension products are analyzed on the Agena MassArray®. Agena Typer® software compares the known mass of the extension primers to identify each locus and the increase caused by each distinct nucleotide to identify the alleles present in each sample. Genotypes are assigned by peak analysis in TyperAnalyzer 4.0.
Overall, 84.6% and 85.2% of the sample provided genetic information for rs53576 and rs2254298, respectively, and 96.7% of this sample were successfully genotyped. This yielded a final sample of 794 Caucasians and 1,210 African Americans for rs53576, and 805 Caucasians and 1,215 African Americans for rs2254298. The distribution of OXTR genotypes for both SNPs conformed to Hardy-Weinberg equilibrium in the Caucasian (ps > 0.30) and African American samples (ps > 0.30). Consistent with prior reports (e.g., Lerer et al., 2008), alleles were not found to be in linkage disequilibrium (LD; D1 = 0.18; r2 = .002). In line with previous research (e.g., Cicchetti et al., 2014) and given the low frequency of AA genotype in both SNPs (<10%), rs53576 and rs2254298 genotypes were grouped by presence of any A allele (rs53576: n = 925, 46%; rs2254298: n = 625, 31%) versus GG (rs53576: n = 1082, 54%; rs2254298: n = 1398, 69%; see Supplemental Table S1). Based on evidence suggesting racial–ethnic variation in the OXTR allele frequencies (Creswell et al., 2014; Butovskaya et al., 2016), all analyses included race as a covariate, and secondary analyses were conducted separately by race.
Genetic data were unavailable for 443 participants, including 376 participants whose genetic data were not collected due to inability to recontact or refusal to provide genetic data, 56 participants who provided saliva samples after genotyping had concluded, and 13 participants whose genetic data were unusable due to laboratory error. Girls without genetic data (n = 443) were compared to participants included in primary analyses for all study variables. Girls without genetic data were more likely to be Caucasian (χ2 = 12.16, p < .05) and less likely to experience early threat exposure (χ2 = 16.31, p < .05). Additionally, girls without genetic data reported greater BPD symptom severity at ages 18 (t = 2.71, p < .05) and 19 (t = 2.80, p < .05), greater depression symptom severity at age 19 (t = 2.18, p < .05), and greater anxiety symptom severity at ages 18 (t = 2.80, p < .05) and 19 (t = 3.37, p < .05). Finally, girls without genetic data reported higher levels of emotion dysregulation during adolescence (t = 2.55, p < .05).
Measures
Early threat exposure
Consistent with previous research (McLaughlin et al., 2014b; Sheridan & McLaughlin, 2014), the current study defined early threat exposure as any experience involving harm or threat of harm, including observing community violence, witnessing domestic violence, and being the victim of physical, emotional or sexual abuse, that occurred prior to age 12. To assess this construct comprehensively, we utilized all available data reflecting threat exposure, including three dichotomous indicators (whether or not participants experienced community violence, domestic violence, and/or sexual abuse), and one continuous indicator (harsh physical and emotional punishment). A chronicity index of early threat exposure was derived based on the number of years participants reported experiencing any of the threat experiences (0 = none; 1 = 1 year; 2 = 2 years; 3 = 3+years) in line with research suggesting that risk for psychopathology substantially increases as threat exposure becomes more chronic (Anda et al., 2006; Hughes et al., 2017; Manly et al., 2001; Mersky et al., 2013). In order to count the number of years during which any experience of threat exposure occurred, we dichotomized our sole continuous variable (harsh physical and emotional punishment). Although we realize the inherent limitations of this approach (e.g., loss of information; see MacCallum, Zhang, Preacher, & Rucker, 2002), this method allowed us to retain the rich information provided by all four indicators of threat exposure. Details on each of these indicators and their prevalence within the sample (n = 2,020) are described below. Prior to the age of 12, approximately 20% of the sample had been exposed to threat during 1 year, 10% was exposed during 2 years, and 12% was exposed during 3 or more years.
First, exposure to community violence was assessed annually by asking parents if their child (a) witnessed any violent crime and/or (b) was a victim of any violent crime. Second, exposure to domestic violence was assessed via prospective caregiver reports of domestic violence in the home using four items from the Conflict Tactics Scale-Revised (CTS2; Straus, Hamby, Boney-McCoy, & Sugarman, 1996) and two items from the Difficult Life Circumstances Scale (DLC; Barnard, Johnson, Booth, & Bee, 1994). Nineteen percent of girls were characterized as having been exposed to violence. Third, harsh physical and emotional punishment was assessed annually via caregiver reports on six items of the harsh punishment subscale (e.g., “I spank or hit her,” “I swear or curse at her,” “I say I will send her away”) from the Conflict Tactics Scale-Parent/Child Version (CTSPC; Straus, Hamby, Finkelhor, Moore, & Runyan, 1998), and girls scoring in the top decile (10%) were classified as experiencing harsh punishment. Finally, sexual abuse was assessed prospectively at ages 10 and 11 via child report on six items from the Abuse Questionnaire (Hipwell, Murray, Xiong, Stepp, & Keenan, 2016) and the Child Post-Traumatic Stress Disorder symptom scale (CPSS; Foa, Johnson, Feeny, & Treadwell, 2001). Less than 1% of girls reported experiencing sexual abuse.
Early adult outcomes
All early adult outcomes were assessed annually from ages 18 through 22 using the Adult Self-Report Index (ASRI; Gadow, Sprafkin, & Weiss, 2004). All symptoms were rated on a 4-point Likert scale (0 = never, 1= sometimes, 2 = a lot and 3 = all the time), and items specific to each disorder (described below) were summed to create a total severity score for each age (18–22). Summed scores for each age (18–22) were then utilized to create latent factors representing both general and specific factors in early adulthood (see analysis section for more detail).
Depression.
Participants reported annually on the past-year severity of the ten depression symptoms (e.g., “felt depressed,” “thought about death,” “felt worthless”; range = 0 to 29). The internal consistency for this scale was good at each time point (α range = .87 to .89).
Anxiety.
Participants reported annually on the past-year severity of the eight generalized anxiety disorder symptoms (e.g., “worried about bad thing,” “trouble stopping worry,” “felt nervous”; range = 0 to 24). The internal consistency for this scale was good at each time point (α range = .86 to .89).
Borderline personality disorder.
Participants reported annually on the past-year severity of the nine BPD symptoms (e.g., affective instability, inappropriate or intense anger, recurrent suicide behavior, frantic efforts to avoid abandonment; range = 0 to 23). The internal consistency for this scale was good at each time point (α range = .79 to .84).
Antisocial personality disorder.
Participants reported annually on the past-year severity of the seven ASPD symptoms (e.g., repeatedly performing acts that are grounds for arrest, aggression, impulsivity; range = 0 to 15). The internal consistency for this scale this was acceptable at each time point (α range = .75 to .78).
Social–emotional mediators
Emotion dysregulation.
Emotion dysregulation was assessed annually from ages 14–17 using the affective instability subscale of the seven-item Personality Assessment Inventory-Borderline Features (PAI-BOR; Morey, 2007). Participants rated items (e.g., “mood can shift quite suddenly,” “moods can get quite intense,” “has little control over anger,” “times when so mad, could not do enough to express all anger”) on a 4-point Likert scale (1 = false, 2 = slightly true, 3 = mainly true, or 4 = very true). Total scores were created by summing all items each year, and these scores were averaged across adolescence. The internal consistency for this scale was acceptable at each time point (α range = .73 to .79).
Callousness.
Callousness was assessed annually from ages 13–17 using six items from the parent-reported Antisocial Processes Screening Device (APSD; Frick & Hare, 2001). Caregivers rated items (i.e., “feels bad or guilty when she does something wrong,” “does not show her emotions to others,” “concerned about the feelings of others,” “concerned about doing well in school,” “is good at keeping promises,” “keeps the same friends”) on a 3-point Likert scale (0 = not at all true to 2 = definitely true). Total scores were created by summing all items for each year, and these scores were averaged across adolescence. The internal consistency for this scale ranged from low to adequate at each time point (α range = .57 to .61), consistent with previous studies that have used this scale (Hipwell et al., 2007; Pardini, Lochman, & Frick, 2003).
Quality of attachment.
Perceived quality of attachment was assessed annually from ages 13–17 using the ten item Trust subscale from the Revised Inventory of Parent and Peer Attachment (IPPA-R; Gullone & Robinson, 2005). Participants reported on their trust in the availability and sensitivity of their primary caregiver and their perception of mutual understanding and respect in the relationship (i.e., “My parents respect my feelings,” “My parents accept me as I am,” “When I talk about things with my parents they listen to what I think,” “My parents listen to my opinions,” “My parents understand me,” “I trust my parents,” “When I am angry about something, my parents try to understand,” “My parents are good parents,” “I wish I had different parents”) using a 3-point Likert scale (1 = never true, 2 = sometimes true, 3 = always true). Items were worded to reflect the adolescent’s perception of the primary caregiver and their relationship. One item, “My parents expect too much from me,” was not administered because it had the lowest factor loading on the Trust factor and the lowest item-total correlation in previous studies (Armsden & Greenberg, 1987). This scale has been utilized in previous work to assess perceived quality of attachment (Scott et al., 2013) and is highly correlated with the overall attachment to parents’ scale (r’s = .86–.90; Gullone & Robinson, 2005). Thus, it represents a good estimate of the adolescent’s perceived quality of attachment to primary caregivers. The internal consistency for this scale was good at each time point (alpha range = .89 to .92).
Control variables
Race.
Caregivers reported on their child’s race at the initiation of data collection. As described above, race (0 = Caucasian, 1 = African American) was controlled for in all analyses in light of evidence suggesting racial–ethnic variation in oxytocin allele frequencies (Butovskaya et al., 2016).
Public assistance.
Caregivers reported on receipt of public assistance at the first assessment. Those receiving public assistance were coded as 1, and those not receiving public assistance were coded as 0. Receipt of public assistance was also controlled for in all analyses, given the evidence linking poverty to psychopathology (Costello, Compton, Keeler, & Angold, 2003).
Data analytic strategy
The following analyses included only those participants with available genetic data (n = 2,020). Testing the main study hypotheses required preliminary analyses including obtaining descriptive statistics and examining bivariate correlations between all study variables, all of which were conducted using SPSS version 25 (SPSS, Inc., Chicago, IL). To assess genetically moderated associations between early threat exposure and general and specific factors of psychopathology, we utilized a general-specific bifactor model (see Figure 1 for conceptual model). The bifactor requires that all indicators load onto orthogonal factors: a general factor that represents common variance shared by all disorders and specific factors that represent unique, disorder-specific variance (Chen, Hayes, Carver, Laurenceau, & Zhang, 2012). To achieve this, age-based (18–22) indicators of depression, anxiety, BPD, and ASPD symptom severity (20 indicators total) were each loaded on a “general” psychopathology factor as well as their respective “specific” psychopathology factor. All factors (general and specific) were orthogonal (correlation set to 0), and all age-based residuals were correlated to account for time-varying effects. Verification of good model fit and configural invariance were established across genotype and race based on the fit indices described below.
Figure 1.
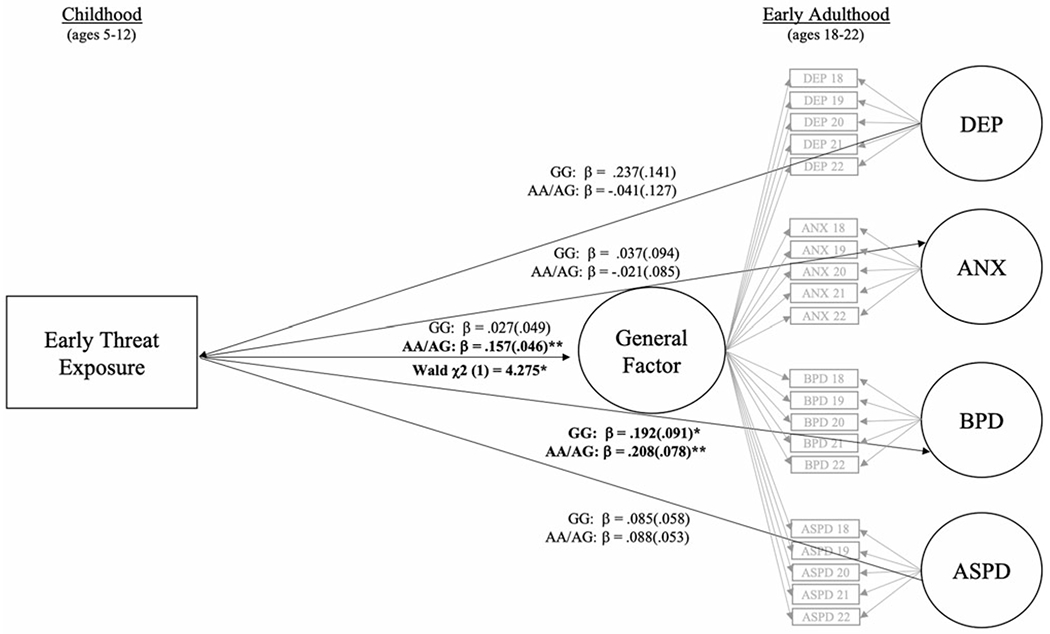
Multiple-group (GG vs. AA/AG) general-specific (bifactor) model testing oxytocin receptor (OXTR) (rs53576) genotype differences in the association between early threat exposure and disorder-specific versus general factors of psychopathology in early adulthood. Note: Standardized (β) coefficients were estimated separately for each group. Overall model fit was good (χ2 (470) = 1049.99. p < .05; RMSEA = .04; CFI = .97). All significant associations are bolded and represent effects after accounting for race, receipt of public assistance, cohort, and number of years with available threat exposure data. Significant Wald tests indicate differences between women with the GG versus AA/AG OXTR (rs53576) genotype. ANX = anxiety symptom severity; ASPD = antisocial personality disorder symptom severity; BPD = borderline personality disorder symptom severity; DEP = depression symptom severity. *p < .05. **p < .01.
Primary study hypotheses were tested using multiple-group models in MPlus version 8 (Muthén & Muthén, 2012), and the effects of race, public assistance, cohort, and number of years with threat exposure data were controlled for in all analyses. First, using full-information maximum likelihood estimation (ML estimator), multiple-group models (GG vs. AA/AG) assessing potential differences in the strength of the association between early threat exposure and general and specific psychopathology factors in early adulthood outcomes were examined. This yielded estimates of direct associations between variables. A series of Wald tests of parameter constraints (Wald, 1943) were used to examine whether differences in the strength of these associations for those with GG versus AA/AG alleles reached statistical significance. This involved the standard practice of fixing and freeing cross-group equality constraints on path coefficients. Second, using the ML estimator with bootstrapped standard errors, multiple group mediation models were estimated examining the indirect effects of early threat exposure on general and specific factors in early adulthood outcomes via (a) emotion dysregulation, (b) callousness, and (c) quality of attachment during adolescence. Again, a series of Wald tests examined the statistical significance of differences in these direct and indirect paths across groups. Estimates of indirect effects between these variables were calculated using the product of indirect pathway coefficients, and Wald tests were used to assess significant differences between indirect effects across groups.
For all models, chi-square statistic (Δχ2), comparative fit index (CFI), root mean square error of approximation (RMSEA), and standardized root mean square residual (SRMR) values are reported. For the CFI, conventional cutoff values of .90 or greater indicate acceptable fit and .95 or greater indicate good fit (McDonald & Ho, 2002). RMSEA values less than .05 indicate a good fit (Kline, 2005; McDonald & Ho, 2002). Within the text and tables, we report effect sizes as standardized βs and confidence intervals for all estimates.
Results
Descriptive statistics and bivariate correlations
Supplemental Table S1 shows frequencies of the OXTR alleles and genotypes, including the final categorization of genotypes for each SNP for the total sample and across racial groups. The distribution of genotypes varied significantly across racial groups for both SNPs [for rs53576, χ2 (2, n = 2004) = 19.59, p < .01; for rs2254298, χ2 (2, n = 2020) = 39.68, p < .01]. Because none of the hypothesized associations were significant for rs2254298 (see Supplemental Tables S2 and S3), only rs53576 genotype (referred to as OXTR hereafter) was included in subsequent analyses.
Table 1 provides means and standard deviations for all study variables as well as bivariate correlations. Relative to their Caucasian peers, African American girls were more likely to carry the GG OXTR genotype, to receive public assistance, and to experience early threat exposure. Additionally, African American girls reported higher levels of emotion dysregulation and callousness during adolescence, as well as higher levels of BPD symptom severity and lower levels of anxiety symptom severity across ages 18–22. Girls receiving public assistance were more likely to carry the GG OXTR genotype, to experience early threat exposure, and to report higher levels of emotion dysregulation and callousness, as well as lower perceived quality of attachment. Girls receiving public assistance also reported higher BPD symptom severity in early adulthood. While chronicity of threat exposure did not differ by genotype, girls carrying the GG OXTR genotype reported higher levels of emotion dysregulation during adolescence. Girls experiencing early threat exposure reported higher levels of emotion dysregulation, higher levels of callousness, and lower perceived quality of attachment during adolescence. They also reported higher ASPD, BPD, depression, and anxiety symptom severity in early adulthood. Higher levels of emotion dysregulation and lower perceived quality of attachment during adolescence were associated with greater depression, anxiety, BPD, and ASPD symptom severity in early adulthood. Higher levels of callousness in adolescence were associated with greater BPD and ASPD symptom severity. Similar patterns of associations were seen when separated by race (see Supplemental Table S4).
Table 1.
Means and correlations for all study variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| %/Mean (SD) | 60% | 40% | 46% | 0.77 (1.05) | 6.28 (3.61) | 3.32 (1.67) | 2.73 (0.36) | 6.39 (3.94) | 6.86 (3.88) | 2.55 (2.22) | 1.57 (1.82) |
| 1. Race | |||||||||||
|
| |||||||||||
| 2. Public Assistance | .34 ** | ||||||||||
|
| |||||||||||
| 3. OXTR (rs53576) | −.10 ** | −.06 ** | |||||||||
|
| |||||||||||
| 4. Threat Exposure | .19 ** | .18 ** | −.03 | ||||||||
|
| |||||||||||
| 5. Emotion Dysregulation | .10 ** | .06 * | −.03 | .19 ** | |||||||
|
| |||||||||||
| 6. Callousness | .32 ** | .28 ** | −.04 | .28 ** | .28 ** | ||||||
|
| |||||||||||
| 7. Quality of Attachment | .02 | −.06 * | .00 | −.10 ** | −.35 ** | −.23 ** | |||||
|
| |||||||||||
| 8. DEP | −.03 | .01 | .02 | .09 ** | .43 ** | .00 | −.24 ** | ||||
|
| |||||||||||
| 9. ANX | −.07 ** | −.03 | .01 | .05 * | .43 ** | −.04 | −.21 ** | .83 ** | |||
|
| |||||||||||
| 10. BPD | .07 ** | .07 ** | .02 | .15 ** | .51 ** | .11 ** | −.22 ** | .71 ** | .67 ** | ||
|
| |||||||||||
| 11. ASPD | −.02 | −.02 | .02 | .09 * | .49 ** | .10 ** | −.26 ** | .51 ** | .46 ** | .64 ** | |
Note: ANX = anxiety; ASPD = antisocial personality disorder; BPD = borderline personality disorder; DEP = depression; OXTR = oxytocin receptor.
Significant correlations are bolded.
p < .05.
p < .01.
General-specific bifactor model
Prior to testing our primary hypotheses, we examined the general-specific bifactor model in the total sample. The model demonstrated good fit (χ2 (120) = 437.201, p < .05; RMSEA = 0.034, 95% CI: .031–.038; CFI = 0.983), with significant factor loadings for all indicators on both the general and specific factors. All indicators loaded more strongly on the general factor (β range = .45–.78, ps < .05) than on specific factors. ASPD indicators demonstrated the highest specific factor loadings (β range = .42–.53, ps < .05), followed by BPD (β range = .26–.34, ps < .05), anxiety (β range = .25–.33, ps < .05), and depression (β range = .10–.32, ps < .05). Next, configural invariance was examined in a series of multi-group models. All models demonstrated good fit to the data, and a consistent pattern of factor loadings was observed across both genotype and race, supporting configural invariance in this measurement model across subgroups. Supplemental Table S5 shows factor loadings by both genotype and race.
Multiple group models: Direct associations between general and specific factors by OXTR genotype
Table 2 depicts findings from the multiple group (GG vs. AA/AG) bifactor model assessing potential differences in the association between early threat exposure and general and specific psychopathology factors in early adulthood. All results account for race, receipt of public assistance, cohort, and number of years with available threat exposure data.
Table 2.
Multiple-group (GG vs. AA/AG) general-specific (bifactor) model testing oxytocin receptor (OXTR) (rs53576) genotype differences in the association between early threat exposure and disorder-specific versus general factors of psychopathology in early adulthood
| GG |
AA/AG |
Wald Difference Test |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | 95% CI | β | SE | 95% CI | Wald | df | p | |
| Threat Exposure → General Factor | 0.027 | 0.049 | −0.070–0.123 | 0.157 | 0.046 | 0.066–0.247 | 4.275 | 1 | 0.039 |
|
| |||||||||
| Threat Exposure → DEP | 0.237 | 0.141 | −0.040–0.513 | −0.041 | 0.127 | −0.289–0.208 | 1.291 | 1 | 0.256 |
|
| |||||||||
| Threat Exposure → ANX | 0.037 | 0.094 | −0.147–0.222 | −0.021 | 0.085 | −0.187–0.146 | 0.211 | 1 | 0.646 |
|
| |||||||||
| Threat Exposure → BPD | 0.192 | 0.091 | 0.014–0.370 | 0.208 | 0.078 | 0.056–0.361 | 0.182 | 1 | 0.700 |
|
| |||||||||
| Threat Exposure → ASPD | 0.085 | 0.058 | −0.029–0.198 | 0.088 | 0.053 | −0.015–0.194 | 0.101 | 1 | 0.750 |
Note: Standardized (β) coefficients were estimated separately for each OXTR (rs53576) group. Overall model fit was good (χ2 (470) = 1049.99, p < .05; RMSEA = .04; CFI = .97). ANX = anxiety symptom severity; ASPD = antisocial personality disorder symptom severity; BPD = borderline personality disorder symptom severity; DEP = depression symptom severity; OXTR = oxytocin receptor.
Significant associations are bolded and represent effects after accounting for race, receipt of public assistance, cohort, and number of years with available threat exposure data. Significant Wald tests indicate differences between women with the GG and AA/AG OXTR (rs53576) genotype.
Only those with the AA/AG genotype demonstrated a significant association between early threat exposure and the general psychopathology factor in early adulthood, and the strength of this association was significantly stronger than for those with GG genotype (Figure 2). When examining associations with specific factors, only associations with the BPD specific factor reached significance. Both groups demonstrated a significant association between early threat exposure and BPD in early adulthood, though the strength of these associations did not differ by genotype.
Figure 2.

General psychopathology scores as a function of early threat exposure and the oxytocin receptor (OXTR) (rs53576). Note. General psychopathology scores are standardized (β) coefficients extracted from the multiple-group (GG vs. AA/AG) general-specific (bifactor) model.
Multiple group mediation models: Indirect associations
Table 3 shows findings from multiple-group (GG vs. AA/AG) bifactor mediation models examining the indirect effects of early threat exposure on adulthood outcomes via social–emotional deficits during adolescence (see Figure 3).2
Table 3.
Multiple-group (GG vs. AA/AG) general-specific (bifactor) mediation models testing oxytocin receptor (OXTR) (rs53576) genotype differences in the association between early threat exposure and general factor of psychopathology in early adulthood via social-emotional deficits in adolescence
| GG |
AA/AG |
Wald Difference Test |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | 95% CI | β | SE | 95% CI | Wald | df | p | |
| Direct Path | |||||||||
| Threat Exposure → General Factor | −0.304 | 0.251 | −0.815–0.492 | −0.445 | 0.255 | −0.963–0.219 | 0.353 | 1 | 0.553 |
|
| |||||||||
| Indirect Paths via Emotion Dysregulation | |||||||||
| Threat Exposure → Emotion Dysregulation | 0.110 | 0.031 | 0.050–0.165 | 0.211 | 0.032 | 0.140–0.266 | 5.150 | 1 | 0.023 |
|
| |||||||||
| Emotion Dysregulation → General Factor | 0.537 | 0.054 | 0.070–0.591 | 0.500 | 0.072 | 0.069–0.588 | 1.097 | 1 | 0.295 |
|
| |||||||||
| Indirect Effect via Emotion Dysregulation | 0.059 | 0.018 | 0.008–0.087 | 0.106 | 0.023 | 0.016–0.140 | 5.896 | 1 | 0.015 |
|
| |||||||||
| Indirect Paths via Callousness | |||||||||
| Threat Exposure → Callousness | 0.220 | 0.028 | 0.162–0.277 | 0.197 | 0.031 | 0.133–0.266 | 0.072 | 1 | 0.788 |
|
| |||||||||
| Callousness → General Factor | −0.123 | 0.034 | −0.172–−0.017 | −0.141 | 0.037 | −0.211–−0.019 | 0.441 | 1 | 0.507 |
|
| |||||||||
| Indirect Effect via Callousness | −0.028 | 0.008 | −0.039–−0.006 | −0.028 | 0.009 | −0.047–−0.006 | 0.188 | 1 | 0.664 |
|
| |||||||||
| Indirect Paths via Quality of Attachment | |||||||||
| Threat Exposure → Quality of Attachment | −0.105 | 0.031 | −0.174–−0.040 | −0.090 | 0.034 | −0.166–−0.014 | 0.082 | 1 | 0.774 |
|
| |||||||||
| Quality of attachment → General Factor | −0.143 | 0.033 | −0.206–−0.020 | −0.149 | 0.036 | −0.216–−0.023 | 0.425 | 1 | 0.515 |
|
| |||||||||
| Indirect Effect via Quality of Attachment | 0.015 | 0.006 | 0.001–0.024 | 0.013 | 0.006 | 0.001–0.026 | 0.010 | 1 | 0.919 |
Note: Standardized (β) coefficients were estimated separately for each OXTR (rs53576) group. Overall model fit was good (χ2 (470) = 1470.851, p < .05; RMSEA = .04; CFI = .95). OXTR = oxytocin receptor. Significant associations are bolded and represent effects after accounting for the effects of early threat exposure on all specific factors as well as the effect of race, receipt of public assistance, cohort, and number of years with available threat exposure data. Significant Wald tests indicate differences between women with the GG and AA/AG OXTR (rs53576) genotype.
Figure 3.
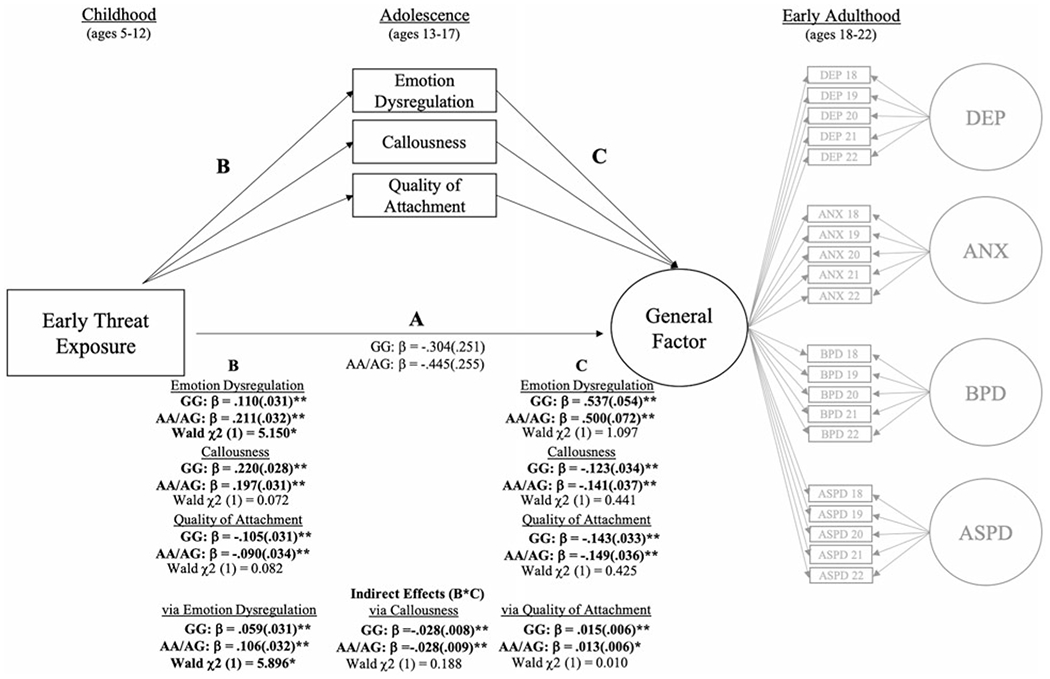
Multiple-group (GG vs. AA/AG) mediation model testing oxytocin receptor (OXTR) (rs53576) genotype differences in the association between early threat exposure and the general factor of psychopathology in early adulthood. Note: Standardized (β) coefficients were estimated separately for each group. Overall model fit was good (χ2 (470) = 1470.851, p < .05; RMSEA = .04; CFI = .95). All significant associations are bolded and represent effects after accounting for race, receipt of public assistance, cohort, and number of years with available threat exposure data. Significant Wald tests indicate differences between women with the GG versus AA/AG OXTR (rs53576) genotype. ANX = anxiety symptom severity; ASPD = antisocial personality disorder symptom severity; BPD = borderline personality disorder symptom severity; DEP = depression symptom severity. *p < .05. **p < .01.
The direct association between early threat exposure and the general psychopathology factor was reduced to nonsignificance for both genotype groups. In both groups, early threat exposure predicted higher emotion dysregulation and callousness, and lower perceived quality of attachment during adolescence. Additionally, in both groups, higher emotion dysregulation and lower perceived quality of attachment predicted the greater general psychopathology factor in early adulthood, while higher callousness predicted lower general psychopathology factor in early adulthood. There was also a significant indirect effect for all mediators in both groups. However, only the association between early threat exposure and emotion dysregulation was moderated by OXTR, with A carriers showing a significantly stronger association. The indirect pathway through emotion dysregulation (early threat exposure → emotion dysregulation → general psychopathology factor) was also stronger for those women who were A carriers, compared to GG homozygous women.
Multiple group models stratified by race
The inclusion of race did not moderate findings in the total sample. Secondary analyses were re-run for Caucasian and African American samples separately (see Supplemental Table S6). Though results were similar to those seen in the total sample, findings were reduced to trend-level or nonsignificance in both samples.
Discussion
Previous research has demonstrated that OXTR variation moderates the influence of early threat exposure on various psychiatric disorders characterized by social–emotional deficits (i.e., depression, anxiety, BPD, and ASPD; McQuaid et al., 2014; Myers et al., 2014; Steele & Siever, 2010). The current study sought to clarify whether this G×E interaction is better understood as conferring risk for disorder-specific outcomes and/or general psychopathology, and whether individual differences in social–emotional deficits (i.e., emotion dysregulation, callousness, and perceived quality of attachment) during adolescence constitute potential mediating mechanisms. These questions were examined in a large, at-risk sample of females assessed prospectively from childhood through early adulthood. Consistent with hypotheses, the interactive effects of early threat exposure and OXTR variation predicted a shared liability for “general” psychopathology, with threat-exposed women carrying at least one copy of the rs53576 A allele (AA/AG) being at greatest risk. Additionally, the interaction between early threat exposure and OXTR variation (rs53576) increased risk for general psychopathology via higher levels of emotional dysregulation during adolescence.
Disorder-specific versus general risk for psychopathology
This is the first study to test the hypothesis that the interaction between early threat exposure and OXTR variation contributes to an overall latent vulnerability for psychopathology, echoing prior theory and research that suggests this developmental risk pathway may be nonspecific (McLaughlin et al., 2014b; Sheridan & McLaughlin, 2014). Specifically, the current study found that the interaction between early threat exposure and OXTR (rs53576) variation was related to higher levels of “general” psychopathology in early adulthood; however, this G×E failed to predict any unique, disorder-specific outcome. In the current study, this “general” liability reflected common variance across four psychiatric disorders—depression, anxiety, BPD, and ASPD—all hallmarked by social–emotional deficits (American Psychiatric Association, 2013). These disorders had previously been examined in isolation, with research suggesting that the interaction between early threat exposure and OXTR variation increased risk for each of these disorders independently (Jaffee et al., 2004; Johnson et al., 1999; Heim & Nemeroff, 2001; Rogosch & Cicchetti, 2005; Stepp et al., 2016). Findings also demonstrated a significant association between early threat exposure and unique risk for BPD (see also Stepp et al., 2016); however, this risk was not moderated by OXTR variation, and early threat exposure was unrelated to unique risk for depression, anxiety or ASPD. Consistent with high rates of comorbidity (Kessler, Chiu, Demler, Merikangas, & Walters, 2005; Krueger & Markon, 2006) and the shift toward understanding transdiagnostic risk as predictors of general risk or multifinality (Barlow, Allen, & Choate, 2004; Cicchetti & Rogosch, 1996; Nolen-Hoeksema & Watkins, 2011), results provide support for the notion that the synergistic influence of these risk factors contributes to a shared liability for the development of a wide range of psychopathologies (Eaton et al., 2011; Green et al., 2010; Kessler et al., 2010; Keyes et al., 2012; McLaughlin et al., 2012). Moreover, findings highlight the need to further elucidate factors that help to explain divergent trajectories of symptom expression, leading to disorder-specific outcomes (Nolen-Hoeksema & Watkins, 2011).
It is noteworthy that the impact of the interaction between early threat exposure and OXTR variation was specific to variation in the rs53576 SNP and was not found in rs2254298. Specifically, threat-exposed women carrying at least one copy of the rs53576 A allele were at greatest risk for “general” psychopathology in early adulthood. Indeed, rs53576 has been more extensively investigated as a moderator of early threat exposure, and while results from the broader literature are notably mixed, our findings echo those from other predominately female samples, which show threat-exposed rs53576 A-allele carriers to be most at risk for various forms of psychopathology (Cicchetti et al., 2014; Hammen et al., 2015; McInnis et al., 2015; Thompson et al., 2014). This reflects a similar pattern of sex-dependent findings seen when examining the interaction between early maltreatment and genetic variation in monoamine oxidase-A (MAOA), where when seen in women, this G×E is likely to reflect a reverse allelic association compared to men (Byrd & Manuck, 2014).
Along these lines, there is some suggestion that discrepancies in research examining the interaction between early threat exposure and OXTR variation may be a function of sex-dependent differences in the development and functioning of the oxytocin system (Carter, 2003, 2014). For example, sex differences in the action of oxytocin early in life are thought to directly impact the development of the stress-response system, and it is hypothesized that these differences are fundamental to sex differences in behavior (Carter, 2014). Moreover, the impact of early experiences has been shown to have sex-dependent effects on behavior (Carter, Boone, Pournajafi-Nazarloo, & Bales, 2009), and this differential functioning impacts physiological, emotional, and behavioral responses throughout the lifespan (Carter, 2014, 2003). While the biological function of polymorphic variation in rs53576 remains unknown, these findings highlight the importance of considering sex-dependent effects when examining this developmental risk pathway.
Emotion dysregulation as a mediating mechanism
The OXTR-moderated association between early threat exposure and general psychopathology was fully mediated by emotion dysregulation in adolescence, and the strength of this effect was driven by allele-dependent differences in the association between early threat exposure and emotion dysregulation. In other words, women who experienced early threat exposure and carried at least one copy of the rs53576 A allele were characterized by higher levels of emotion dysregulation during adolescence, and higher emotion dysregulation in adolescence predicted general psychopathology for all women. This is consistent with previous research documenting associations between threat exposure, OXTR, and emotion dysregulation (Bradley et al., 2011; Rodrigues et al., 2009), as well as a broader literature demonstrating that disruptions to emotion regulation constitute an important transdiagnostic marker of risk (Byrd et al., 2019; Cole, Hall, & Hajal, 2008; McLaughlin, Hatzenbuehler, Mennin, & Nolen-Hoeksema, 2011).
Notably, early threat exposure has been linked specifically to alterations in emotional processing, like attention to threat and heightened emotional reactivity (Heleniak, Jenness, Vander Stoep, McCauley, & McLaughlin, 2016; McLaughlin, Sheridan, Alves, & Mendes, 2014a; McLaughlin et al., 2014b; Sheridan & McLaughlin, 2014). This is in line with deficits proposed in the social salience hypothesis of oxytocin (see Shamay-Tsoory & Abu-Akel, 2016), which suggests that oxytocin plays a major role in orienting attention to environmental social cues. Specifically, this model posits that in the context of a positive and supportive environment, oxytocin functions to increase the salience of safety signals, while in the context of unpredictable, threatening circumstances, oxytocin enhances the salience of environmental threat. Consistent with theories of social information processing (Crick & Dodge, 1996), this heightened acuity to environmental threat may generalize to potentially nonthreatening stimuli, contributing to greater emotion dysregulation over time and increased risk for maladaptive behavioral responses. Our measure of emotion dysregulation in adolescence, while only a summary construct, may serve as a proxy of these concrete and fundamental emotional processes. Future replications could extend our work by using multimodal assessments of emotion dysregulation (e.g., psychophysiology, neuroimaging) or by focusing on more precise indices of emotional processing using task-based assessments (e.g., of attention to threat) as potential mediators in longitudinal investigations of the early threat-by-OXTR interaction.
Although callousness and perceived quality of attachment did not mediate the impact of this G×E interaction on psychopathology in early adulthood, these social–emotional deficits were higher among women who experienced early threat exposure (regardless of genotype), and they were significantly associated with general psychopathology (again, regardless of genotype). Specifically, women with early threat exposure reported higher levels of callousness and lower perceived quality of attachment during adolescence, consistent with prior research linking threat exposure to increased risk for both callousness (Byrd, Hawes, Loeber, & Pardini, 2018; Kimonis, Centifanti, Allen, & Frick, 2014; Kimonis, Fanti, Isoma, & Donoghue, 2013) and attachment quality (Cicchetti, Toth, & Maughan, 2000; Egeland & Sroufe, 1981; Reyome, 2010). Moreover, while lower perceived quality of attachment was associated with greater general psychopathology, higher callousness was related to lower levels of general psychopathology in early adulthood. This is consistent with theory and research demonstrating that callousness in youth shows negative associations with depression and anxiety symptoms, and predicts the “unique” portion of antisocial behavior (Byrd, Kahn, & Pardini, 2013; Byrd, Loeber, & Pardini, 2012; Frick, Lilienfeld, Ellis, Loney, & Silverthorn, 1999). Given that the current study was limited to examining mediators of genetically moderated associations between threat exposure and general psychopathology, future work may seek to further elucidate factors that contribute to divergent trajectories of symptomatology (Nolen-Hoeksema & Watkins, 2011).
Limitations
One limitation of the current study is its focus on a single gene, and hence, a candidate gene approach to G×E investigation. This is necessitated by the study’s mechanistic rationale, which derives from the role of oxytocin in social and emotional development and from prior literature implicating variation in the OXTR. Unfortunately, this archival data set did not allow an analysis based on broader coverage of polymorphic variants (haplotypes) within the OXTR. In the larger perspective of G×E research, increasing use of polygenic scores, particularly as constructed from large sample GWA studies, may ultimately capture a greater portion of the variance to be explained by environmentally moderated genetic associations (Bogdan et al., 2018). Nonetheless, this is a fundamentally different approach, one that capitalizes on largely anonymous genetic variation and, therefore, proceeds in the absence of mechanistic hypotheses. Hence, we believe our findings merit consideration by contributing to the growing body of work implicating the OXTR in risk for psychopathology and underscore the utility of biologically informed approaches to understanding nature-nurture effects. Specifically, our findings provide impetus for continuing to investigate the mechanistic role of the OXTR, particularly in interaction with early threat exposure, as predictors of a general liability for psychopathology and highlight the potential importance examining intermediate behavioral phenotypes, like emotion dysregulation.
Additional limitations include our focus on an urban sample of females, meaning findings cannot be generalized to males or clinical populations. Moreover, primary results were reported after collapsing across race due to concerns about reducing power in our multiple group mediation models. While supplementary analyses were stratified by race and did show a similar pattern of results, significance levels were reduced to trend level. Furthermore, we did not have access to ancestry informative markers, and could not detect moderating effects that might stem from differences in extent of genetic admixture among African American participants. Additionally, we had to balance the psychometric limitations of dichotomizing harsh physical and emotional punishment for each year of the seven annual childhood assessments with our overarching goal of evaluating an index of threat exposure that was (a) comprehensive (McLaughlin et al., 2014b; Sheridan & McLaughlin, 2014) and (b) reflected the critical dimension of chronicity (Cowell et al., 2015; Jaffee & Maikovich-Fong, 2011; Manly et al., 2001). Given the noted limitations of dichotomizing continuous variables (e.g., loss of information; see MacCallum et al., 2002), replication is needed. Lastly, our early adult outcomes focused on four common psychiatric disorders characterized by social–emotional deficits (depression, anxiety, BPD, and ASPD) and, in turn, our “general” psychopathology factor represented liability shared only by these specific disorders. Notably, our general-specific bifactor model does not include the full range of disorders used in previous nosologically-focused work seeking to characterize the structure of psychopathology (Caspi et al., 2014; Kotov et al., 2017; Laceulle et al., 2015; Lahey et al., 2012; Lahey et al., 2015). Future research may seek to extend findings from the current study by incorporating additional diagnoses/symptomatology.
Finally, the present study focused on three potential social–emotional mediators: emotional dysregulation, callousness, and perceived quality of attachment. This focus was based on research suggesting that (a) the synergistic effects of early threat exposure and OXTR variation increases risk for these specific deficits, and (b) these specific deficits increase risk for the mood, anxiety, and personality disorders examined in the current study. However, our examination of these social–emotional mediators was limited by the timing of our measurements. Specifically, the measurement of these mediators was initiated in adolescence, precluding our ability to control for earlier manifestations of these constructs and their impact on adolescent development. It is possible that other risk factors serve as intermediate behavioral phenotypes, and as such, it may be important to consider alternative mediating pathways and/or how these risk factors interact to contribute to complex developmental cascades of risk (Masten & Cicchetti, 2010). Along these lines, future work may also seek to examine how parental warmth and/or sensitivity may function to buffer risk, especially considering research highlighting the role of oxytocin in child–caregiver bonding (Feldman, 2012; Feldman, Gordon, Influs, Gutbir, & Ebstein, 2013) and the potential impact of gene–environment correlations (Jaffee & Price, 2007; Rutter, 2007). Lastly, social–emotional mediators in the current study were assessed prospectively across adolescence using only questionnaire data. Future research may seek to utilize more comprehensive measures that include multimodal assessments (e.g., multiple informants, psychophysiology, and neuroimaging).
Summary and clinical implications
The current study extends our understanding of the impact of early threat exposure and OXTR variation on risk for psychopathology. Specifically, it suggests that this G×E interaction confers risk for an overall latent vulnerability for general psychopathology versus disorder-specific risk in a large, prospectively assessed, longitudinal sample of urban women. Moreover, results bridge a gap in the extant literature and suggest that this developmental risk pathway is mediated by emotion dysregulation during the sensitive adolescent period. Specifically, findings indicate that women who experienced early threat exposure and carry at least one copy of the rs53576 A allele (AA/AG) showed significantly higher emotion dysregulation during adolescence, which placed them at risk for general psychopathology in early adulthood. These findings have significant implications for prevention and intervention programs designed to target youth at risk for the development of disorders characterized by social–emotional deficits (i.e., depression, anxiety, BPD, and ASPD). Namely, prevention efforts may focus on ways to reduce emotion dysregulation and/or promote adaptive strategies for managing high levels of emotion dysregulation. Moreover, because these youth are particularly vulnerable, prolonged caregiver scaffolding and targeted emotion socialization during the critical adolescent period may be crucial for risk reduction (Klimes-Dougan et al., 2007; Morris, Silk, Steinberg, Myers, & Robinson, 2007).
Supplementary Material
Acknowledgments.
We are grateful to all the families who took part in this study, and to the PGS team, which includes interviewers and their supervisors, data managers, student workers, and volunteers. This project used the University of Pittsburgh HSCRF Genomics Research Core SNP genotyping service/s. This research was specifically funded by grants from the Office of Juvenile Justice and Delinquency Prevention, Office of Justice Programs, US Department of Justice (Grant #2013-JF-FX-0058); the National Institute of Mental Health (R01 MH056630); the National Institute on Drug Abuse (R01 DA012237); and by funding from the FISA Foundation and the Falk Fund. Additional funding from the National Institute of Mental Health (K01 MH119216; T32 MH018951) and the National Institute on Alcohol Abuse and Alcoholism (T32 AA00745) also supported this work. The opinions, findings, and conclusions or recommendations expressed in this report are those of the authors and do not necessarily reflect those of the Department of Justice, National Institutes of Health, or the FISA Foundation and Falk Fund.
Footnotes
Participants who described their race as “multiracial” and identified as African American were coded as African American for all genetic analyses.
To be as comprehensive as possible, we tested a full mediation model which examined the indirect effects of all potential mediators on all outcomes (general and specific factors). However, this model did not converge. Thus, we focused only on the potential mediation of genetically moderated paths (i.e., the general factor).
Supplemental material. The supplementary material for this article can be found at https://doi.org/10.1017/S0954579420000462.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, … Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry Clinical Neuroscience, 256, 174–186. doi: 10.1007/s00406-005-0624-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armsden GC, & Greenberg MT (1987). The inventory of parent and peer attachment: Individual differences and their relationship to psychological well-being in adolescence. Journal of Youth and Adolescence, 16(5), 427–454. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2011). Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology, 23, 39–52. doi: 10.1017/S0954579410000635 [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & van IJzendoorn MH (2014). A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatric Genetics, 24, 45–51. doi: 10.1097/YPG.0b013e3283643684 [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, & Van Ijzendoorn MH (2015). The hidden efficacy of interventions: Gene×environment experiments from a differential susceptibility perspective. Annual Review of Psychology, 66, 381–409. doi: 10.1146/annurev-psych-010814-015407 [DOI] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, & Choate ML (2004). Toward a unified treatment for emotional disorders. Behavior Therapy, 35, 205–230. doi: 10.1016/S0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- Barnard K, Johnson S, Booth C, & Bee H (1994). Difficult life circumstances scale. Seattle, WA: University of Washington. [Google Scholar]
- Blakemore SJ, & Choudhury S (2006). Development of the adolescent brain: Implications for executive function and social cognition. Journal of Child Psychology and Psychiatry, 47, 296–312. doi: 10.1111/j.1469-7610.2006.01611.x [DOI] [PubMed] [Google Scholar]
- Bogdan R, Baranger DA, & Agrawal A (2018). Polygenic risk scores in clinical psychology: Bridging genomic risk to individual differences. Annual Review of Clinical Psychology, 14, 119–157. doi: 10.1146/annurev-clinpsy-050817-084847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, Binder EB, Jovanovic T, Crain D, … Heim C (2011). Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: Moderation by oxytocin receptor gene. Development Psychopathology, 23, 439–452. doi: 10.1017/S0954579411000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buist KL, Deković M, Meeus W, & van Aken MA (2004). The reciprocal relationship between early adolescent attachment and internalizing and externalizing problem behaviour. Journal of Adolescence, 27, 251–266. doi: 10.1016/j.adolescence.2003.11.012 [DOI] [PubMed] [Google Scholar]
- Butovskaya PR, Lazebny OE, Sukhodolskaya EM, Vasiliev VA, Dronova DA, Fedenok JN, … Butovskaya ML (2016). Polymorphisms of two loci at the oxytocin receptor gene in populations of Africa, Asia and South Europe. BMC Genetics, 17, 17. doi: 10.1186/s12863-015-0323-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Hawes SW, Loeber R, & Pardini DA (2018). Interpersonal callousness from childhood to adolescence: Developmental trajectories and early risk factors. Journal of Clinical Child Adolescent Psychology, 47, 467–482. doi: 10.1080/15374416.2016.1144190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Kahn RE, & Pardini DA (2013). A validation of the inventory of callous-unemotional traits in a community sample of young adult males. Journal of Psychopathology and Behavioral Assessment, 35, 20–34. doi: 10.1007/s10862-012-9315-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Loeber R, & Pardini DA (2012). Understanding desisting and persisting forms ofd elinquency: The unique contributions of disruptive behavior disorders and interpersonal callousness. Journal of Child Psychology and Psychiatry, 53, 371–380. doi: 10.1111/j.1469-7610.2011.02504.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, & Manuck SB (2014). MAOA, childhood maltreatment, and antisocial behavior: Meta-analysis of a gene-environment interaction. Biological Psychiatry, 75, 9–17. doi: 10.1016/j.biopsych.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd AL, Manuck SB, Hawes SW, Vebares TJ, Nimgaonkar V, Chowdari KV, … Stepp SD (2019). The interaction between monoamine oxidase A (MAOA) and childhood maltreatment as a predictor of personality pathology in females: Emotional reactivity as a potential mediating mechanism. Development and Psychopathology, 31, 361–377. doi: 10.1017/S0954579417001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS (2003). Developmental consequences of oxytocin. Physiology & Behavior, 79, 383–397. doi: 10.1016/S0031-9384(03)00151-3 [DOI] [PubMed] [Google Scholar]
- Carter CS (2014). Oxytocin pathways and the evolution of human behavior. Annual Review of Psychology, 65, 17–39. doi: 10.1146/annurev-psych-010213-115110 [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, & Bales KL (2009). Consequences of early experiences and exposure to oxytocin and vasopressin are sexually dimorphic. Developmental Neuroscience, 31, 332–341. doi: 10.1159/000216544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, & Hare TA (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–126. doi: 10.1196/annals.1440.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE (2014). The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2, 119–137. doi: 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FF, Hayes A, Carver CS, Laurenceau JP, & Zhang Z (2012). Modeling general and specific variance in multifaceted constructs: A comparison of the bifactor model to other approaches. Journal of Personality, 80, 219–251. doi: 10.1111/j.1467-6494.2011.00739.x [DOI] [PubMed] [Google Scholar]
- Cicchetti D (2013). Annual research review: Resilient functioning in maltreated children-past, present, and future perspectives. Journal of Child Psychology Psychiatry, 54, 402–422. doi: 10.1111/j.1469-7610.2012.02608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D (2016). Socioemotional, personality, and biological development: Illustrations from a multilevel developmental psychopathology perspective on child maltreatment. Annual Review of Psychology, 67, 187–211. doi: 10.1146/annurev-psych-122414-033259 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Manly JT (2001). Operationalizing child maltreatment: Developmental processes and outcomes. Development Psychopathology, 13, 755–757. doi: 10.1017/S0954579401004011 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, & Rogosch FA (1996). Equifinality and multifinality in developmental psychopathology. Development and Psychopathology, 8, 597–600. doi: 10.1017/S0954579400007318 [DOI] [Google Scholar]
- Cicchetti D, Rogosch FA, Hecht KF, Crick NR, & Hetzel S (2014). Moderation of maltreatment effects on childhood borderline personality symptoms by gender and oxytocin receptor and FK506 binding protein 5 genes. Development Psychopathology, 26, 831–849. doi: 10.1017/S095457941400042X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, & Toth SL (2005). Child maltreatment. Annual Review of Clinical Psycholology, 1, 409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029 [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Toth S, & Maughan A. (2000). An ecological-transactional model of child maltreatment. In Sameroff A, Lewis M, & Miller S (Eds.), Handbook of developmental psychopathology (pp. 689–722). Springer [Google Scholar]
- Cole PM, Hall SE, & Hajal NJ (2008). Emotion dysregulation as a risk factor for psychopathology. Child and Adolescent Psychopathology, 341–373. [Google Scholar]
- Cole PM, Michel MK, & Teti LOD (1994). The development of emotion regulation and dysregulation: A clinical perspective. Monographs of the Society for Research in Child Development, 59, 73–102. doi: 10.1111/j.1540-5834.1994.tb01278.x [DOI] [PubMed] [Google Scholar]
- Costello EJ, Compton SN, Keeler G, & Angold A (2003). Relationships between poverty and psychopathology: A natural experiment. JAMA, 290, 2023–2029. doi: 10.1001/jama.290.15.2023 [DOI] [PubMed] [Google Scholar]
- Cowell RA, Cicchetti D, Rogosch FA, & Toth SL (2015). Childhood maltreatment and its effect on neurocognitive functioning: Timing and chronicity matter. Development and Psychopathology, 27, 521–533. doi: 10.1017/S0954579415000139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell KG, Wright AG, Troxel WM, Ferrell RE, Flory JD, & Manuck SB (2014). OXTR polymorphism predicts social relationships through its effects on social temperament. Social Cognitive Affective Neuroscience, 10, 869–876. doi: 10.1093/scan/nsu132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick NR, & Dodge KA (1996). Social information-processing mechanisms in reactive and proactive aggression. Child Development, 67, 993–1002. doi: 10.2307/1131875 [DOI] [PubMed] [Google Scholar]
- Dadds MR, Moul C, Cauchi A, Dobson-Stone C, Hawes DJ, Brennan J, … Ebstein RE (2014). Polymorphisms in the oxytocin receptor gene are associated with the development of psychopathy. Development Psychopathology, 26, 21–31. doi: 10.1017/S0954579413000485 [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, … Sher KJ (2015). Candidate gene–environment interaction research: Reflections and recommendations. Perspectives on Psychological Science, 10, 37–59. doi: 10.1177/1745691614556682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge KA (2009). Mechanisms of gene-environment interaction effects in the development of conduct disorder. Perspectives on Psychological Science, 4, 408–414. doi: 10.1111/j.1745-6924.2009.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donadon MF, Martin-Santos R, & Osório F d. L. (2018). The associations between oxytocin and trauma in humans: A systematic review. Frontiers in Pharmacology, 9, 154. doi: 10.3389/fphar.2018.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, & Keller MC (2011). A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry, 168, 1041–1049. doi: 10.1176/appi.ajp.2011.11020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Keyes KM, Skodol AE, Markon KE, Grant BF, & Hasin DS (2011). Borderline personality disorder co-morbidity: Relationship to the internalizing-externalizing structure of common mental disorders. Psychological Medicine, 41, 1041–1050. doi: 10.1017/S0033291710001662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland B, & Sroufe LA (1981). Attachment and early maltreatment. Child Development, 44–52. doi: 10.2307/1129213 [DOI] [PubMed] [Google Scholar]
- Feldman R (2012). Oxytocin and social affiliation in humans. Hormones and Behavior, 61, 380–391. doi: 10.1016/j.yhbeh.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Influs M, Gutbir T, & Ebstein RP (2013). Parental oxytocin and early caregiving jointly shape children’s oxytocin response and social reciprocity. Neuropsychopharmacology, 38, 1154. doi: 10.1038/npp.2013.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Johnson KM, Feeny NC, & Treadwell KR (2001). The Child PTSD Symptom Scale: A preliminary examination of its psychometric properties. Journal of Clinical Child Psychology, 30, 376–384. doi: 10.1207/S15374424JCCP3003_9 [DOI] [PubMed] [Google Scholar]
- Frick PJ, & Hare RD (2001). Antisocial process screening device: APSD. Toronto: Multi-Health Systems. [Google Scholar]
- Frick PJ, Lilienfeld SO, Ellis M, Loney B, & Silverthorn P (1999). The association between anxiety and psychopathy dimensions in children. Journal of Abnormal Child Psychology, 27, 383–392. doi: 10.1023/A:1021928018403 [DOI] [PubMed] [Google Scholar]
- Frick PJ, & White SF (2008). Research Review: The importance of callous-unemotional traits for developmental models of aggressive and antisocial behavior. Journal of Child Psychology and Psychiatry, 49, 359–375. doi: 10.1111/j.1469-7610.2007.01862.x [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, & Weiss MD (2004). Adult Self-Report Inventory 4 manual. Stony Brook, NY: Checkmate Plus. [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2010). Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry, 67, 113–123. doi: 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullone E, & Robinson K (2005). The inventory of parent and peer attachment—Revised (IPPA-R) for children: A psychometric investigation. Clinical Psychology Psychotherapy: An International Journal of Theory Practice, 12, 67–79. doi: 10.1002/cpp.433 [DOI] [Google Scholar]
- Hammen C, Bower JE, & Cole SW (2015). Oxytocin receptor gene variation and differential susceptibility to family environment in predicting youth borderline symptoms. Journal of Personality Disorders, 29, 177–192. doi: 10.1521/pedi_2014_28_152 [DOI] [PubMed] [Google Scholar]
- Hartman S, Widaman KF, & Belsky J (2015). Genetic moderation of effects of maternal sensitivity on girl’s age of menarche: Replication of the Manuck et al. study. Development and Psychopathology, 27, 747–756. doi: 10.1017/S0954579414000856 [DOI] [PubMed] [Google Scholar]
- Heim C, & Nemeroff CB (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry, 49, 1023–1039. doi: 10.1016/S0006-3223(01)01157-X [DOI] [PubMed] [Google Scholar]
- Heleniak C, Jenness JL, Vander Stoep A, McCauley E, & McLaughlin KA (2016). Childhood maltreatment exposure and disruptions in emotion regulation: A transdiagnostic pathway to adolescent internalizing and externalizing psychopathology. Cognitive Therapy and Research, 40, 394–415. doi: 10.1007/s10608-015-9735-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Loeber R, Stouthamer-Loeber M, Keenan K, White HR, & Kroneman L (2002). Characteristics of girls with early onset disruptive and antisocial behaviour. Criminal Behaviour and Mental Health, 12, 99–118. doi: 10.1002/cbm.489 [DOI] [PubMed] [Google Scholar]
- Hipwell AE, Murray J, Xiong S, Stepp SD, & Keenan KE (2016). Effects of adolescent childbearing on maternal depression and problem behaviors: A prospective, population-based study using risk-set propensity scores. PLoS One, 11, e0155641. doi: 10.1371/journal.pone.0155641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Pardini DA, Loeber R, Sembower M, Keenan K, & Stouthamer-Loeber M (2007). Callous-unemotional behaviors in young girls: Shared and unique effects relative to conduct problems. Journal of Clinical Child and Adolescent Psychology, 36, 293–304. doi: 10.1080/15374410701444165 [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Cicchetti D, & Rogosch FA (2014). Oxytocin receptor gene polymorphism, perceived social support, and psychological symptoms in maltreated adolescents. Development and Psychopathology, 26, 465–477. doi: 10.1017/S0954579414000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, … Dunne MP (2017). The effect of multiple adverse childhood experiences on health: A systematic review and meta-analysis. The Lancet Public Health, 2, e356–e366. doi: 10.1016/S2468-2667(17)30118-4 [DOI] [PubMed] [Google Scholar]
- Israel S, Lerer E, Shalev I, Uzefovsky F, Riebold M, Laiba E, … Knafo A (2009). The oxytocin receptor (OXTR) contributes to prosocial fund allocations in the dictator game and the social value orientations task. PLoS One, 4, e5535. doi: 10.1371/journal.pone.0005535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, Dodge KA, Rutter M, Taylor A, & Tully LA (2005). Nature × nurture: Genetic vulnerabilities interact with physical maltreatment to promote conduct problems. Development and Psychopathology, 17, 67–84. doi: 10.1017/S0954579405050042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Caspi A, Moffitt TE, & Taylor A (2004). Physical maltreatment victim to antisocial child: Evidence of an environmentally mediated process. Journal of Abnormal Psychology, 113, 44–55. doi: 10.1037/0021-843X.113.1.44 [DOI] [PubMed] [Google Scholar]
- Jaffee SR, & Maikovich-Fong AK (2011). Effects of chronic maltreatment and maltreatment timing on children’s behavior and cognitive abilities. Journal of Child Psychology and Psychiatry, 52, 184–194. doi: 10.1111/j.1469-7610.2010.02304.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, & Price TS (2007). Gene–environment correlations: A review of the evidence and implications for prevention of mental illness. Molecular Psychiatry, 12, 432. doi: 10.1038/sj.mp.4001950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JG, Cohen P, Brown J, Smailes EM, & Bernstein DP (1999). Childhood maltreatment increases risk for personality disorders during early adulthood. Archive General Psychiatry, 56, 600–606. doi: 10.1001/archpsyc.56.7.600 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, & Walters EE (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archive of General Psychiatry, 62, 617–627. doi: 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Angermeyer M (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. The British Journal of Psychiatry, 197, 378–385. doi: 10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, & Hasin DS (2012). Childhood maltreatment and the structure of common psychiatric disorders. The British Journal of Psychiatry, 200, 107–115. doi: 10.1192/bjp.bp.111.093062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpeläinen TO, Qi L, Brage S, Sharp SJ, Sonestedt E, Demerath E, … Sandholt CH (2011). Physical activity attenuates the influence of FTO variants on obesity risk: A meta-analysis of 218,166 adults and 19,268 children. PLoS Medicine, 8, e1001116. doi: 10.1371/journal.pmed.1001116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Cicchetti D (2010). Longitudinal pathways linking child maltreatment, emotion regulation, peer relations, and psychopathology. Journal of Child Psychology Psychiatry, 51, 706–716. doi: 10.1111/j.1469-7610.2009.02202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimonis ER, Centifanti LC, Allen JL, & Frick PJ (2014). Reciprocal influences between negative life events and callous-unemotional traits. Journal of Abnormal Child Psychology, 42, 1287–1298. doi: 10.1007/s10802-014-9882-9 [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Fanti KA, Isoma Z, & Donoghue K (2013). Maltreatment profiles among incarcerated boys with callous-unemotional traits. Child Maltreatment, 18, 108–121. doi: 10.1177/1077559513483002 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Brand AE, Zahn-Waxler C, Usher B, Hastings PD, Kendziora K, & Garside RB (2007). Parental emotion socialization in adolescence: Differences in sex, age and problem status. Social Development, 16(2), 326–342. [Google Scholar]
- Kline RB (2005). Principles and practice of structural equation modeling. New York: Guilford. [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby RM, … Clark LA (2017). The Hierarchical Taxonomy of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of Abnormal Psychology, 126, 454. doi: 10.1037/abn0000258 [DOI] [PubMed] [Google Scholar]
- Krueger RF, & Markon KE (2006). Reinterpreting comorbidity: A model-based approach to understanding and classifying psychopathology. Annual Review of Clinical Psycholology, 2, 111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Parasuraman R, Iyengar V, Thornburg M, Weel J, Lin M, … Lipsky R (2012). Oxytocin receptor genetic variation promotes human trust behavior. Frontiers in Human Neuroscience, 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumsta R, & Heinrichs M (2013). Oxytocin, stress and social behavior: Neurogenetics of the human oxytocin system. Current Opinion in Neurobiology, 23, 11–16. doi: 10.1016/j.conb.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Laceulle OM, Vollebergh WA, & Ormel J (2015). The structure of psychopathology in adolescence: Replication of a general psychopathology factor in the TRAILS study. Clinical Psychological Science, 3, 850–860. doi: 10.1177/2167702614560750 [DOI] [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, & Rathouz PJ (2012). Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology, 121, 971. doi: 10.1037/a0028355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Rathouz PJ, Keenan K, Stepp SD, Loeber R, & Hipwell AE (2015). Criterion validity of the general factor of psychopathology in a prospective study of girls. Journal of Child Psychology Psychiatry, 56, 415–422. doi: 10.1111/jcpp.12300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, & Rathouz P (2011). Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of General Psychiatry, 68, 181–189. doi: 10.1001/archgenpsychiatry.2010.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, & Ebstein R (2008). Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Molecular Psychiatry, 13, 980. doi: 10.1038/sj.mp.4002087 [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, & Rucker DD (2002). On the practice of dichotomization of quantitative variables. Psychological Methods, 7, 19. doi: 10.1037/1082-989X.7.1.19 [DOI] [PubMed] [Google Scholar]
- Malik AI, Zai CC, Abu Z, Nowrouzi B, & Beitchman JH (2012). The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes, Brain and Behavior, 11, 545–551. doi: 10.1111/j.1601-183X.2012.00776.x [DOI] [PubMed] [Google Scholar]
- Manly JT, Kim JE, Rogosch FA, & Cicchetti D (2001). Dimensions of child maltreatment and children’s adjustment: Contributions of developmental timing and subtype. Development and Psychopathology, 13, 759–782. doi: 10.1017/S0954579401004023 [DOI] [PubMed] [Google Scholar]
- Masten AS, & Cicchetti D (2010). Developmental cascades. Development and Psychopathology, 22(3), 491–495. [DOI] [PubMed] [Google Scholar]
- Maughan A, & Cicchetti D (2002). Impact of child maltreatment and inter-adult violence on children’s emotion regulation abilities and socioemotional adjustment. Child Development, 73, 1525–1542. doi: 10.1111/1467-8624.00488 [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, & Viding E (2011). Heightened neural reactivity to threat in child victims of family violence. Current Biology, 21, 947–948. doi: 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- McDonald RP, & Ho MR (2002). Principles and practice in reporting structural equation analyses. Psychological Methods, 7, 64–82. doi: 10.1037/1082-989X.7.1.64 [DOI] [PubMed] [Google Scholar]
- McInnis OA, McQuaid RJ, Matheson K, & Anisman H (2015). The moderating role of an oxytocin receptor gene polymorphism in the relation between unsupportive social interactions and coping profiles: Implications for depression. Frontiers in Psychology, 6, 1133. doi: 10.3389/fpsyg.2015.01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Mennin DS, & Nolen-Hoeksema S (2011). Emotion dysregulation and adolescent psychopathology: A prospective study. Behaviour Research and Therapy, 49, 544–554. doi: 10.1016/j.brat.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Alves S, & Mendes WB (2014a). Child maltreatment and autonomic nervous system reactivity: Identifying dysregulated stress reactivity patterns using the biopsychosocial model of challenge and threat. Psychosomatic Medicine, 76, 538. doi: 10.1097/PSY.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014b). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience ans Biobehavioral Reviews, 47, 578–591. doi: 10.1016/j.neubiorev.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, & Anisman H (2014). Making room for oxytocin in understanding depression. Journal of Neuroscience and Biobehavioral Reviews, 45, 305–322. doi: 10.1016/j.neubiorev.2014.07.005 [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matheson K, & Anisman H (2013). A paradoxical association of an oxytocin receptor gene polymerphism: Early-life adversity and vulnerability to depression. Frontiers in Neuroscience, 7, 128. doi: 10.3389/fnins.2013.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersky J, Topitzes J, & Reynolds A (2013). Impacts of adverse childhood experiences on health, mental health, and substance use in early adulthood: A cohort study of an urban, minority sample in the US. Child Abuse and Neglect, 37, 917–925. doi: 10.1016/j.chiabu.2013.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey LC. (2007). Personality assessment inventory (PAI): Professional manual. PAR (Psychological Assessment Resources). [Google Scholar]
- Muthén BO, & Muthén LK (2012). Mplus user’s guide (7th ed.). Los Angeles, CA. [Google Scholar]
- Myers AJ, Williams L, Gatt JM, McAuley-Clark EZ, Dobson-Stone C, Schofield PR, & Nemeroff CB (2014). Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. Journal of Psychiatric Research, 59, 93–100. doi: 10.1016/j.jpsychires.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID (2008). Brain oxytocin: A key regulator of emotional and social behaviours in both females and males. Journal of Neuroendocrinology, 20, 858–865. doi: 10.1111/j.1365-2826.2008.01726.x [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, & Watkins ER (2011). A heuristic for developing transdiagnostic models of psychopathology: Explaining multifinality and divergent trajectories. Perspectives on Psychological Science, 6, 589–609. doi: 10.1177/1745691611419672 [DOI] [PubMed] [Google Scholar]
- Pardini DA, Lochman JE, & Frick PJ (2003). Callous-unemotional traits and social-cognitive processes in adjudicated youths. Journal of the American Academy of Child and Adolescent Psychiatry, 42, 364–371. doi: 10.1097/00004583-200303000-00018 [DOI] [PubMed] [Google Scholar]
- Perry BD, Pollard RA, Blakley TL, Baker WL, & Vigilante D (1995). Childhood trauma, the neurobiology of adaptation, and “use-dependent” development of the brain: How “states” become “traits”. Infant Mental Health Journal, 16, 271–291. doi: [DOI] [Google Scholar]
- Reyome ND (2010). Childhood emotional maltreatment and later intimate relationships: Themes from the empirical literature. Journal of Aggression, Maltreatment, and Trauma, 19, 224–242. doi: 10.1080/10926770903539664 [DOI] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, & Keltner D (2009). Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proceedings of the National Academy of Sciences, 106, 21437–21441. doi: 10.1073/pnas.0909579106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch FA, & Cicchetti D (2005). Child maltreatment, attention networks, and potential precursors to borderline personality disorder. Development and Psychopathology, 17, 1071–1089. doi: 10.1017/S0954579405050509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M (2007). Gene–environment interdependence. Developmental Science, 10, 12–18. doi: 10.1111/j.1467-7687.2007.00557.x [DOI] [PubMed] [Google Scholar]
- Scott LN, Whalen DJ, Zalewski M, Beeney JE, Pilkonis PA, Hipwell AE, & Stepp SD (2013). Predictors and consequences of developmental changes in adolescent girls’ self-reported quality of attachment to their primary caregiver. Journal of Adolescence, 36, 797–806. doi: 10.1016/j.adolescence.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler T, Connolly MJ, Prososki AR, & Pollak SD (2014). Stress-induced elevation of oxytocin in maltreated children: Evolution, neurodevelopment, and social behavior. Child Development, 85, 501–512. doi: 10.1111/cdev.12136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, & Abu-Akel A (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79, 194–202. doi: 10.1016/j.biopsych.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18, 580–585. doi: 10.1016/j.tics.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smearman EL, Winiarski DA, Brennan PA, Najman J, & Johnson KC (2015). Social stress and the oxytocin receptor gene interact to predict antisocial behavior in an at-risk cohort. Development and Psychopathology, 27, 309–318. doi: 10.1017/S0954579414000649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele H, & Siever L (2010). An attachment perspective on borderline personality disorder: Advances in gene–environment considerations. Current Psychiatry Reports, 12, 61–67. doi: 10.1007/s11920-009-0091-0 [DOI] [PubMed] [Google Scholar]
- Steinberg L (2005). Cognitive and affective development in adolescence. Trends in Cognitive Sciences, 9, 69–74. doi: 10.1016/j.tics.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Steinberg L, & Morris AS (2001). Adolescent development. Journal of Cognitive Education and Psychology, 2, 55–87. doi: 10.1891/1945-8959.2.1.55 [DOI] [Google Scholar]
- Stepp SD, Lazarus SA & Byrd AL (2016). A systematic review of risk factors prospectively associated with borderline personality disorder: Taking stock and moving forward. Personality Disorders: Theory, Research, and Treatment, 7, 316–323. doi: 10.1037/per0000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Boney-McCoy S, & Sugarman DB (1996). The revised conflict tactics scales (CTS2) development and preliminary psychometric data. Journal of Family Issues, 17, 283–316. doi: 10.1177/019251396017003001 [DOI] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, & Runyan D (1998). Identification of child maltreatment with the parent-child conflict tactics scales: Development and psychometric data for a national sample of American parents. Child Abuse and Neglect, 22, 249–270. doi: 10.1016/S0145-2134(97)00174-9 [DOI] [PubMed] [Google Scholar]
- Tabak BA (2013). Oxytocin and social salience: A call for gene-environment interaction research. Frontiers in Neuroscience, 7, 199. doi: 10.3389/fnins.2013.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Lahey BB, Van Hulle C, Waldman I, Krueger RF, & Rathouz PJ (2013). Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. Journal of Abnormal Psychology, 122(4), 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, & Gunnar MR (2006). Child maltreatment and the developing HPA axis. Hormones and Behavior, 50, 632–639. doi: 10.1016/j.yhbeh.2006.06.010 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, & Kim DM (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioral Reviews, 27, 33–44. doi: 10.1016/S0149-7634(03)00007-1 [DOI] [PubMed] [Google Scholar]
- Thompson SM, Hammen C, Starr LR, & Najman JM (2014). Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology, 43, 11–19. doi: 10.1016/j.psyneuen.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, & Gotlib IH (2011). Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology, 36, 144–147. doi: 10.1016/j.psyneuen.2010.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepfer P, Heim C, Entringer S, Binder E, Wadhwa P, & Buss C (2017). Oxytocin pathways in the intergenerational transmission of maternal early life stress. Neuroscience & Biobehavioral Reviews, 73, 293–308. doi: 10.1016/j.neubiorev.2016.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verona E, Murphy B, & Bresin K (2018). Oxytocin-related single-nucleotide polymorphisms, family environment, and psychopathic traits. Personality Disorders: Theory, Research, and Treatment, 9, 584. doi: 10.1037/per0000290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A (1943). Tests of statistical hypotheses concerning several parameters when the number of observations is large. Transactions of the American Mathematical society, 54, 426–482. doi: 10.1090/S0002-9947-1943-0012401-3 [DOI] [Google Scholar]
- Wang Q, Shelton RC, & Dwivedi Y (2018). Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: A systematic review and meta-analysis. Journal of Affective Disorders, 225, 422–428. doi: 10.1016/j.jad.2017.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Chen L, Yang J, Han D, Fang D, Qiu X, … Wang L (2018). BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. Journal of Affective Disorders, 227, 226–235. doi: 10.1016/j.jad.2017.10.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


