Abstract
Objective:
To investigate the feasibility of using deep learning image reconstruction (DLIR) to significantly reduce radiation dose and improve image quality in contrast-enhanced abdominal CT.
Methods:
This was a prospective study. 40 patients with hepatic lesions underwent abdominal CT using routine dose (120kV, noise index (NI) setting of 11 with automatic tube current modulation) in the arterial-phase (AP) and portal-phase (PP), and low dose (NI = 24) in the delayed-phase (DP). All images were reconstructed at 1.25 mm thickness using ASIR-V at 50% strength. In addition, images in DP were reconstructed using DLIR in high setting (DLIR-H). The CT value and standard deviation (SD) of hepatic parenchyma, spleen, paraspinal muscle and lesion were measured. The overall image quality includes subjective noise, sharpness, artifacts and diagnostic confidence were assessed by two radiologists blindly using a 5-point scale (1, unacceptable and 5, excellent). Dose between AP and DP was compared, and image quality among different reconstructions were compared using SPSS20.0.
Results:
Compared to AP, DP significantly reduced radiation dose by 76% (0.76 ± 0.09 mSv vs 3.18 ± 0.48 mSv), DLIR-H DP images had lower image noise (14.08 ± 2.89 HU vs 16.67 ± 3.74 HU, p < 0.001) but similar overall image quality score as the ASIR-V50% AP images (3.88 ± 0.34 vs 4.05 ± 0.44, p > 0.05). For the DP images, DLIR-H significantly reduced image noise in hepatic parenchyma, spleen, muscle and lesion to (14.77 ± 2.61 HU, 14.26 ± 2.67 HU, 14.08 ± 2.89 HU and 16.25 ± 4.42 HU) from (24.95 ± 4.32 HU, 25.42 ± 4.99 HU, 23.99 ± 5.26 HU and 27.01 ± 7.11) with ASIR-V50%, respectively (all p < 0.001) and improved image quality score (3.88 ± 0.34 vs 2.87 ± 0.53; p < 0.05).
Conclusion:
DLIR-H significantly reduces image noise and generates images with clinically acceptable quality and diagnostic confidence with 76% dose reduction.
Advances in knowledge:
(1) DLIR-H yielded a significantly lower image noise, higher CNR and higher overall image quality score and diagnostic confidence than the ASIR-V50% under low signal conditions. (2) Our study demonstrated that at 76% lower radiation dose, the DLIR-H DP images had similar overall image quality to the routine-dose ASIR-V50% AP images.
Introduction
CT has advantages of fast scan, large axial coverage, good image quality, high spatial and temporal resolution and is widely used clinically to evaluate patients with liver diseases. Contrast-enhanced CT is better than non-enhanced CT in detecting and differentiating between benign and malignant hepatic lesions. However, the radiation dose also inevitably increases. Many techniques have been developed and used to decrease the radiation dose to patients. These techniques include automatic tube current modulation (ATCM), hybrid/partial model-based iterative reconstruction (IR) algorithms, such as the adaptive statistical iterative reconstruction (ASIR-V, GE Healthcare) and full model-based iterative reconstruction algorithms. 1 IR algorithms can provide different levels of image noise reduction while attempting to preserve image quality. Many clinical researches have shown that the noise reduction ability of these IR algorithms can be converted to reduce radiation dose. 2–6 However, some studies 7,8 reported that the use of IR algorithms, especially at high strengths, may cause images to appear “smooth,” “blotchy” or simply “unnatural”, because many IR algorithms change the magnitude of the noise as well as the texture details of the images, which may have adverse impact on the detection of low-contrast lesions.
Recently, a deep learning image reconstruction (DLIR) algorithm was developed by an imaging manufacture (TrueFidelity, GE Healthcare) and has been tested by some researchers in phantom and clinical studies. The DLIR algorithm is based on the deep convolution neural network to simulate the texture of standard dose filtered back projection (FBP) image, and is capable of providing powerful noise reduction and while maintaining high spatial resolution for detailed structures. The purpose of our study was to investigate the feasibility of using DLIR to significantly reduce radiation dose and improve image quality in contrast-enhanced abdominal CT compared with using the most advanced ASIR-V algorithm.
Methods and materials
This was a prospective study approved by the local institutional review committee. All patients were informed of the potential risks of contrast-enhanced CT and filled out an informed consent. This study was not funded by any grants. Patients with suspected hepatic lesions scheduled for abdominal CT angiology per the standard protocol of our institution were enrolled in the study. The patient inclusion criteria were: (1) patients with hepatic lesions requiring abdominal enhancement imaging and (2) normal renal function based on blood biochemical analysis. The patient exclusion criteria were: (1) impaired renal function; (2) contraindication for iodinated contrast medium and (3) children and pregnant females.
Imaging technique and post-processing
All patients were scanned on a 256-slice wide-detector CT scanner (Revolution CT, GE Healthcare, Milwaukee, USA). The routine dose scanning protocol (tube voltage:120kV, automatic tube current modulation for a noise index (NI) of 11) was used in the arterial-phase (AP) and portal-phase (PP), and reduced radiation dose scanning with NI of 24 was used in the delayed-phase (DP). The other parameters included the following: gantry speed, 0.5 sec; pitch, 0.992:1; detector coverage: 80 mm; tube current modulation range, 200–500 mA (AP and PP) and 50-500mA (DP); scan slice thickness: 5 mm, and a pre-set ASIR-V for scanning (pre-ASIR-V) at 40% was used for reducing X-ray dose requirement. The Iohexol (Omnipaque 300, Yangtze river pharmaceutical group) was used as the IV contrast agent. Weight-based IV contrast dose protocol of 1.2 ml /kg was used allowing a range of 50–95 ml contrast dose and injection speed of 2.7 ml s−1. Bolus tracking technique was used to monitor the area of interest and 120 HU at the abdominal aorta level was used as the trigger threshold. The AP scan started with a scan delay of 12 s after triggering, PP scan started 30 s after AP scan and DP scan started 50 s after the finish of PP scan. Images of 1.25 mm slice thickness were reconstructed using ASIR-V at 50% intensity in all scanning phases. In addition, images in DP were reconstructed using DLIR in high setting (DLIR-H).
Objective image assessment
All images were transferred to an advantage workstation (AW4.7, GE Healthcare, Waukesha, Wisconsin) for clinical diagnosis and the AP and DP images were selected for further analysis. The ROIs were drawn on the ASIR-V50% reconstruction images with 1.25 mm slice thickness (in AP and DP) and DLIR-H reconstruction images with 1.25 mm slice thickness (in DP). The images were linked in order to make sure identical ROIs were used for the same anatomic structure of different reconstructions. The CT value and standard deviation (SD) of hepatic parenchyma (excluding visible hepatic vessels), spleen, left side-of the paraspinal muscle, lesion (the largest was selected) were measured. For the two reconstruction algorithms, the contrast-to-noise ratio (CNR) for the hepatic parenchyma, spleen and lesion was calculated using the muscle as the background: (ROIorgan – ROImuscle) / SDmuscle, where ROIorgan and ROImuscle represents the mean attenuation of organ-of-interest and paraspinal muscle, respectively and SDmuscle represents image noise. 9
Subjective image assessment
Two experienced radiologists with at least 5 years of abdominal CT imaging experience independently and blindly evaluated the qualitative image quality. Images are randomly displayed in a soft tissue window: window width of 400HU and window level of 60HU. The overall subjective image noise, image sharpness and diagnostic confidence was evaluated using a 5-point Likert scale; and the image artifact with a 4-point scale (Table 1). 10 Consensus reading was performed if disagreements occurred between the two readers.
Table 1.
Grading Scales for the Qualitative Image Analysis
| Grading Score |
Subjective image noise | Image sharpness | Artifacts | Diagnostic confidence |
|---|---|---|---|---|
| 1 | Unacceptable noise | Blurry | Major | Unacceptable |
| 2 | Above average | Worse than average | Minor | Poor |
| 3 | Average | Average | Very little | Average |
| 4 | Less than average | Better than average | None | High |
| 5 | Minimal | Sharpest | Excellent |
Radiation dose evaluation
The system recorded and presented the volume CT dose index (CTDIvol) and dose length product (DLP) at the end of CT scan. The effective dose (ED) was calculated using the product of DLP and a conversion coefficient k for the abdomen of 0.015 millisieverts/(mGy-cm).
Statistical analysis
The SPSS statistical software (Windows v.22.0; SPSS, Chicago, Illinois) was used for statistical analyses. A p < 0.05 in the analysis would indicate that the difference is statistically significant. The measured values are expressed as mean ± standard deviation. The Kolmogorov-Smirnov test was used to test the normality of continuous data (CT value and noise). The paired sample t-test was used for normal-distributed continuous data and Mann-Whitney U-test was used to compare image quality scores. For qualitative analysis, the consistency between two readers was tested using κ statistics. A κ value between 0.81 and 1.00 was interpreted as excellent, 0.61–0.80 as substantial, 0.41–0.60 as moderate, 0.21–0.40 as fair, and 0.00–0.20 as poor.
Results
There were no complications in all CT scans. The study included 40 patients (24 males, 16 females; mean age, 57 ± 12 years; range, 30–85 years), with a mean weight of 66 ± 10 kg (range, 45–85 kg). The average volume of intravenous contrast media based on body weight was 78 ± 13 ml (range, 50–99 ml). CT scans diagnosed the following diseases among the 40 patients: hepatic metastases (n = 5), hepatic cysts (n = 4), hepatic carcinoma (n = 17, included hepatectomy and radiofrequency patients), cirrhosis (n = 5), hepatic hemangiomas (n = 2), and others (n = 7). Compared to AP, the radiation dose for DP was significantly reduced by 76% (0.76 ± 0.09 mSv vs 3.18 ± 0.48 mSv).
Quantitative analysis of the image noise and CNR
Table 2 summarizes the results of the objective image quality parameters (CT value, image noise, and CNR). For DP images, The image noise (in HU) for the hepatic parenchyma, spleen, muscle and lesion were significantly reduced from (24.95, 25.42, 23.99, 27.01) on the ASIR-V50% images to (14.77, 14.26, 14.08, 16.25) on the DLIR-H images, respectively, and the CNR of liver, spleen and lesion were also significantly higher with DLIR-H (all p < 0.001). Compared to the routine-dose AP ASIR-V50% images, the image noises of DLIR-H DP images in hepatic parenchyma, spleen, muscle and lesion were also reduced (14.77, 14.26, 14.08, 16.25 vs 17.09, 17.92, 16.67, 17.92), all p < 0.05), despite the significantly reduced radiation dose in DP than in AP.
Table 2.
Mean Attenuation, Noise, and Contrast-to-Noise Ratio in the Abdomen
| DLIR-H DP | ASIR-V50% DP | ASIR-V 50% AP | P1 | P2 | |
|---|---|---|---|---|---|
| Attenuation | |||||
| Liver | 93.08 ± 7.39 | 92.41 ± 9.33 | 69.20 ± 7.39 | 0.84 | —— |
| Spleen | 93.62 ± 7.17 | 92.63 ± 12.60 | 104.56 ± 18.51 | 0.65 | —— |
| Muscle | 59.81 ± 6.38 | 59.76 ± 9.35 | 54.85 ± 8.99 | 0.94 | —— |
| lesion | 44.55 ± 26.67 | 43.39 ± 26.84 | 40.73 ± 26.97 | 0.26 | —— |
| Noise | |||||
| Liver | 14.77 ± 2.61 | 24.95 ± 4.32 | 17.09 ± 3.09 | <0.001 | <0.001 |
| Spleen | 14.26 ± 2.67 | 25.42 ± 4.99 | 17.92 ± 4.18 | <0.001 | <0.001 |
| Muscle | 14.08 ± 2.89 | 23.99 ± 5.26 | 16.67 ± 3.74 | <0.001 | <0.001 |
| lesion | 16.25 ± 4.42 | 27.01 ± 7.11 | 17.92 ± 5.19 | <0.001 | 0.034 |
| Contrast-to-Noise Ratio | |||||
| Liver | 2.48 ± 1.05 | 1.41 ± 0.62 | 0.88 ± 0.67 | <0.001 | —— |
| Spleen | 2.50 ± 0.83 | 1.33 ± 0.99 | 3.09 ± 1.86 | <0.001 | —— |
| lesion | 1.94 ± 1.39 | 1.12 ± 0.78 | 1.85 ± 1.47 | <0.001 | —— |
Measurements are mean ± SD. ASIR-V50% = 50% adaptive statistical iterative reconstruction V, DLIR-H = deep learning image reconstruction at high strengths. AP: arterial-phase; DP: delayed-phase. ASIR-V50% DP = ASIR-V50% reconstructions in the delayed phase. DLIR-H DP: DLIR-H reconstructions in the delayed phase. ASIR-V50% AP = ASIR-V50% reconstructions in the arterial phase. P1 = P value value between ASIR-V50% images in DP and DLIR-H images in DP. P2 = P value value between DLIR-H images in DP and ASIR-V50% images in AP (only image noise was compared due to the different scan phases).
Qualitative analysis
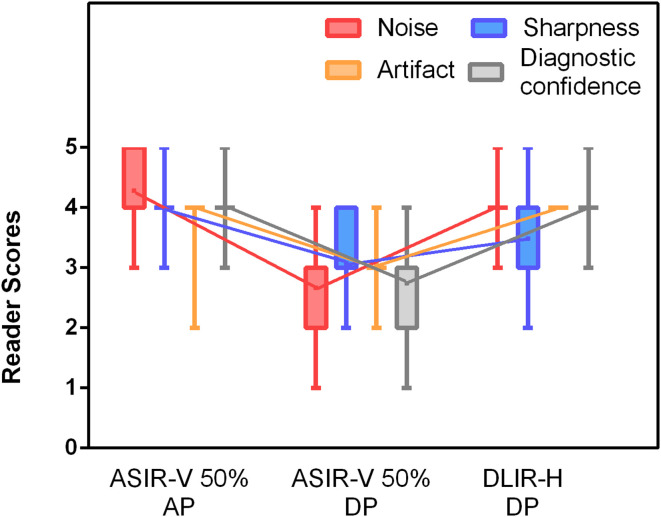
Image quality for the low-dose DLIR-H DP images was significantly improved over the ASIR-V50% DP images ((3.88 ± 0.34 vs 2.87 ± 0.53; p < 0.05) (Figure 1), but was similar to the routine-dose ASIR-V50% AP images (3.88 ± 0.34 vs 4.05 ± 0.44; p > 0.0.05) (Figure 2 and Figure 3). In the delayed-phase that used extremely low radiation dose, 30% of the ASIR-V50% images had scores of poor or Unacceptable (score one or 2) in diagnostic confidence, but the proportion dropped to 0% after using DLIR-H. In the subjective image noise score, 35% of ASIR-V50% DP images were rated to have unacceptable noise or above average noise (score one or 2), but all DLIR-H DP images had scores higher than 3 with 92.5% scored four or above points (Figure 4). There was a good agreement between the two readers (κ value was between 0.81 and 0.90).
Figure 1.
Box-and-whisker plots of reader scores from qualitative image evaluation. DLIR-H DP images were scored as significantly better than ASIR-V50% DP images in image noise, image sharpness, artifacts and lesion diagnostic confidence, but were similar to the routine-dose ASIR-V50% AP images.
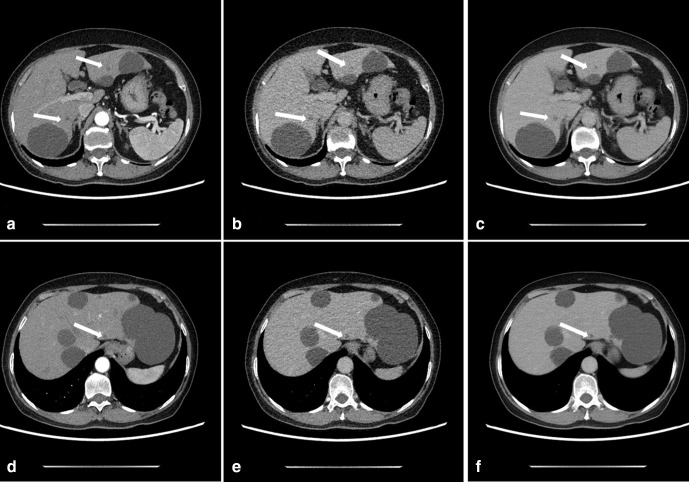
Figure 2.
A 67-year-old female with small hepatic cysts in the arterial-phase (AP) and delayed-phase (DP). CTDIvol value was 6.8 mGy and 1.7 mGy in AP and DP, respectively. A and D: Axial AP images reconstructed with ASIR-V50% shows small hepatic cysts (arrows) with high diagnostic confidence. B and E: Axial DP images reconstructed with ASIR-V50% shows small hepatic cysts (arrows) with low diagnostic confidence due to unclear cyst boundary and high image noise. C and F: Axial DLIR-H DP images for confidence diagnosis of cysts, image noise was significantly reduced, and cyst boundary was clear.
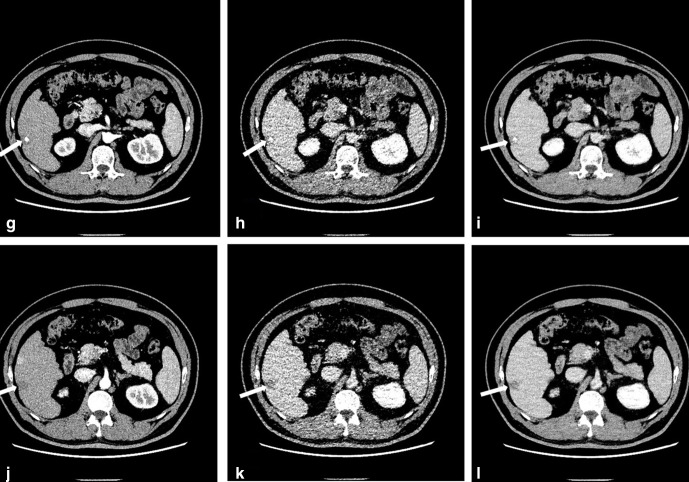
Figure 3.
Images of a 54-year-old male after radio frequency ablation (RFA). The CTDIvol value in AP and DP was 9.8 mGy and 1.7 mGy, respectively. G and J: Axial AP images reconstructed with ASIR-V50%. H and K: Axial DP images reconstructed with ASIR-V50% showing lesions (arrows). But lesion boundaries were not very clear with high image noise. I and L: Images with DLIR-H. Image noise was significantly reduced, and contrast resolution was significantly improved.
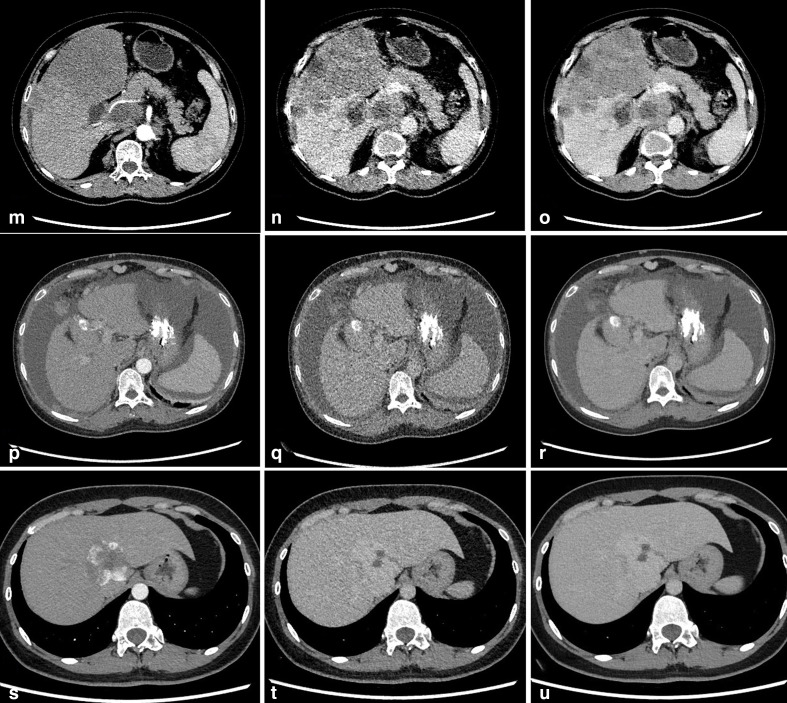
Figure 4.
M-O: Images of a 62-year-old female with hepatic metastases: ASIR-V50% AP image (M), ASIR-V50% DP image (N), DLIR-H DP image (O). The qualitative image quality scores were 4.25, 2.5 and 3.25, respectively.P-R: Images of a 48-year-old female with hepatic cirrhosis: ASIR-V50% AP image (P), ASIR-V50% DP image (Q), DLIR-H DP image (R). The qualitative image quality scores were 3.75, 2.75 and 3.75, respectively. S-U: Images of a 30-year-old female with hepatic cirrhosis: ASIR-V50% AP image (S), ASIR-V50% DP image (T), DLIR-H DP image (U). The qualitative image quality scores were 4.25, 3.25 and 4.25, respectively.
Discussion
In this study, we evaluated the clinical value of DLIR in contrast-enhanced abdominal CT with extremely low radiation dose. DLIR-H yielded a significantly lower image noise, higher CNR and higher overall image quality score and diagnostic confidence than the ASIR-V50% under low signal conditions. The use of DLIR-H resulted in turning non-diagnostic image (scores less than 3) into diagnostic images (scores greater or equals 3). In addition, our study demonstrated that at 76% lower radiation dose, the DLIR-H DP images had similar overall image quality to the routine-dose ASIR-V50% AP images, indicating a potential 76% dose reduction with the use of DLIR-H algorithm for achieving the current diagnostic image quality.
The growing number of contrast-enhanced CT in clinical use increases the cumulative burden of radiation exposure, which increases the need for radiation dose reduction. However, for malignant tumor patients, radiation dose reduction may cause a reduction in diagnostic accuracy. 11 So, how to balance the radiation dose reduction and image quality and diagnostic accuracy is a big challenge. At present, efforts are being made to reduce radiation dose while maintaining image quality, often through technical advances in the use of reconstruction algorithms. 12–14 Although the current iterative reconstruction (IR) algorithms improve the image quality significantly, especially in low-dose CT studies, the image texture, spatial resolution, and object detectability remain unsatisfactory, especially when IR algorithms with high strength are used. IR algorithm images with high strengths sometime appear “overly smooth,” “blotchy,” or simply “unnatural” which makes low-contrast detection tasks challenging 7,8 and limits the dose reduction potential.
Recently, the potential of using artificial intelligence through deep convolution neural network (DNN) to improve CT image reconstruction has been reported. In the process of training, these networks are optimized by minimizing the difference between their outputs and the ideal training samples. A new deep-learning-based image reconstruction (DLIR) technique (TrueFidelityTM, GE Healthcare) has been developed and has recently entered into clinical application. DLIR is a CT image reconstruction method applying DNN to improve image quality and overcome the limitations of existing iterative reconstruction algorithms. In DLIR model, DNN is trained using the low-dose CT projections as input to generate images as close as possible to the high-dose images reconstructed by filtered back projection (FBP). The “ground truth” images used to train DNN are high-dose FBP CT images under ideal data acquisition conditions, either from high-dose phantom scans or clinical data. Considering this design, it is expected that the image generated by DLIR has similar spatial resolution and noise texture attributes as FBP images, but with the magnitude of noise significantly reduced. 15 The technical details describing DLIR algorithm (TrueFidelityTM) can be found on a white paper from the manufacturer, 16 but the details about the network architecture and training/testing/validation regimes are not publicly available.
In the study, we used the state-of-the-art ASIR-V50% as the comparing algorithm. ASIR-V is a hybrid technique, with models include the system noise (photon statistics and electronic noise), X-ray physics and objects model. The more complex optical model that requires much longer computational time is omitted in ASIR-V. It has shorter imaging processing time than the more advanced full model-based iterative reconstruction (MBIR). ASIR-V has higher dose reduction capability with better image quality than the previous generation ASIR, it is described as “Augmented ASIR” or “Simplified MBIR”. 17,18 Cho’s study showed images generated using 50% ASIR-V were significantly better than other series. 10 We have been using 50% as the routine reconstruction strength in our institution for abdominal CT imaging. However, as indicated in our study, when we used extremely low dose (less than 1 mSv) in DP, the use of ASIR-V50% for some of the patients did not produce satisfactory images, and the image noise was too high to provide confident diagnosis. For the same patients, image noise in hepatic parenchyma, spleen and musculature with the use of DLIR-H was significantly reduced and the DLIR-H image quality was significantly improved over the ASIR-V50%, resulted in all images being acceptable for confident diagnosis. Due to the scan phase difference, we did not compare the quantitative lesion enhancement and CNR values between the ASIR-V50% AP images and DLIR-H DP images. We did compare the image noise and overall image quality between the two scans to estimate the dose reduction potential with DLIR-H algorithm. Compared to the routine dose (AP) ASIR-V50% images, the image noises of DLIR-H DP images in the hepatic parenchyma, spleen, muscle were actually further reduced, and the overall image quality was similar, but the radiation dose was reduced by 76%.
This study had some limitations. First, this was a single institution investigation and the study population was small, the results require further verification. Second, we only measured the magnitude of image noise, the detailed noise power spectrum and modulation transfer function were not evaluated. Finally, the lesions in our study were diagnosed using the images from multi-phase scans (arterial, portal and delayed phases) and some of the lesions (especially small lesions) lacked gold standard. Since the study was focused on evaluating the performance of DLIR in a specific phase (delayed-phase) with extremely low-dose, we only reported the diagnostic confidence using DLIR images on the lesions that were already detected with the multi-phase scans. The sensitivity, specificity and accuracy for lesion detection need further validation.
In summary, DLIR-H reduces image noise and generates images with clinically acceptable quality and diagnostic confidence with 76% dose reduction, but without disruptive image texture alteration, in comparison with routine-dose CT with the state-of-the-art ASIR-V50% algorithm.
Footnotes
The authors Le Cao and Xiang Liu contributed equally to the work.
Contributor Information
Le Cao, Email: 13072985707@163.com.
Xiang Liu, Email: lx329705298@126.com.
Jianying Li, Email: jianying.li@med.ge.com.
Tingting Qu, Email: 1036354669@qq.com.
Lihong Chen, Email: 316676511@qq.com.
Yannan Cheng, Email: 1291068604@qq.com.
Jieliang Hu, Email: 2452108713@qq.com.
Jingtao Sun, Email: 253705770@qq.com.
Jianxin Guo, Email: gjx1665@xjtufh.edu.cn.
REFERENCES
- 1. Greffier J, Pereira F, Macri F, Beregi J-P, Larbi A. Ct dose reduction using automatic exposure control and iterative reconstruction: a chest paediatric phantoms study. Phys Med 2016; 32: 582–9. doi: 10.1016/j.ejmp.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 2. Katsura M, Matsuda I, Akahane M, Sato J, Akai H, Yasaka K, et al. Model-Based iterative reconstruction technique for radiation dose reduction in chest CT: comparison with the adaptive statistical iterative reconstruction technique. Eur Radiol 2012; 22: 1613–23. doi: 10.1007/s00330-012-2452-z [DOI] [PubMed] [Google Scholar]
- 3. Larbi A, Orliac C, Frandon J, Pereira F, Ruyer A, Goupil J, et al. Detection and characterization of focal liver lesions with ultra-low dose computed tomography in neoplastic patients. Diagn Interv Imaging 2018; 99: 311–20. doi: 10.1016/j.diii.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 4. Tang H, Liu Z, Hu Z, He T, Li D, Yu N, et al. Clinical value of a new generation adaptive statistical iterative reconstruction (ASIR-V) in the diagnosis of pulmonary nodule in low-dose chest CT. Br J Radiol 2019; 92: 20180909. doi: 10.1259/bjr.20180909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han WK, Na JC, Park SY. Low-Dose CT angiography using ASiR-V for potential living renal donors: a prospective analysis of image quality and diagnostic accuracy. Eur Radiol 2020; 30: 798–805. doi: 10.1007/s00330-019-06423-1 [DOI] [PubMed] [Google Scholar]
- 6. Chen L-H, Jin C, Li J-Y, Wang G-L, Jia Y-J, Duan H-F, et al. Image quality comparison of two adaptive statistical iterative reconstruction (ASiR, ASiR-V) algorithms and filtered back projection in routine liver CT. Br J Radiol 2018; 91: 20170655. doi: 10.1259/bjr.20170655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verdun FR, Racine D, Ott JG, Tapiovaara MJ, Toroi P, Bochud FO, et al. Image quality in CT: from physical measurements to model observers. Phys Med 2015; 31: 823–43. doi: 10.1016/j.ejmp.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 8. Samei E, Richard S. Assessment of the dose reduction potential of a model-based iterative reconstruction algorithm using a task-based performance metrology. Med Phys 2015; 42: 314–23. doi: 10.1118/1.4903899 [DOI] [PubMed] [Google Scholar]
- 9. Marin D, Nelson RC, Schindera ST, Richard S, Youngblood RS, Yoshizumi TT, et al. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm--initial clinical experience. Radiology 2010; 254: 145–53. doi: 10.1148/radiol.09090094 [DOI] [PubMed] [Google Scholar]
- 10. Lee S, Kwon H, Cho J. The detection of focal liver lesions using abdominal CT: a comparison of image quality between adaptive statistical iterative reconstruction V and adaptive statistical iterative reconstruction. Acad Radiol 2016; 23: 1532–8. doi: 10.1016/j.acra.2016.08.013 [DOI] [PubMed] [Google Scholar]
- 11. Jensen CT, Wagner-Bartak NA, Vu LN, Liu X, Raval B, Martinez D, et al. Detection of colorectal hepatic metastases is superior at standard radiation dose CT versus reduced dose CT. Radiology 2019; 290: 400–9. doi: 10.1148/radiol.2018181657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodenberger MH, Wagner-Bartak NA, Gupta S, Liu X, Yap RQ, Sun J, et al. Computed tomography image quality evaluation of a new iterative reconstruction algorithm in the abdomen (adaptive statistical iterative Reconstruction-V) a comparison with model-based iterative reconstruction, adaptive statistical iterative reconstruction, and filtered back projection reconstructions. J Comput Assist Tomogr 2018; 42: 184–90. doi: 10.1097/RCT.0000000000000666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee NK, Kim S, Hong SB, Kim TU, Ryu H, Lee JW, et al. Low-Dose CT with the adaptive statistical iterative reconstruction V technique in abdominal organ injury: comparison with Routine-Dose CT with filtered back projection. AJR Am J Roentgenol 2019; 213: 659–66. doi: 10.2214/AJR.18.20827 [DOI] [PubMed] [Google Scholar]
- 14. Xu Y, He W, Chen H, Hu Z, Li J, Zhang T. Impact of the adaptive statistical iterative reconstruction technique on image quality in ultra-low-dose CT. Clin Radiol 2013; 68: 902–8. doi: 10.1016/j.crad.2013.03.024 [DOI] [PubMed] [Google Scholar]
- 15. Solomon J, Lyu P, Marin D, Samei E. Noise and spatial resolution properties of a commercially available deep learning-based CT reconstruction algorithm. Med Phys 2020;: 3961–71 07 Jun 2020. doi: 10.1002/mp.14319 [DOI] [PubMed] [Google Scholar]
- 16. Healthcare GE. A new era of image reconstruction: TrueFidelity™ technical white paper on deep learning image reconstruction. 2019 [1/1/ 2010;Available from. [Google Scholar]
- 17. Deák Z, Grimm JM, Treitl M, Geyer LL, Linsenmaier U, Körner M, et al. Filtered back projection, adaptive statistical iterative reconstruction, and a model-based iterative reconstruction in abdominal CT: an experimental clinical study. Radiology 2013; 266: 197–206. doi: 10.1148/radiol.12112707 [DOI] [PubMed] [Google Scholar]
- 18. Lim K, Kwon H, Cho J, Oh J, Yoon S, Kang M, et al. Initial phantom study comparing image quality in computed tomography using adaptive statistical iterative reconstruction and new adaptive statistical iterative reconstruction V. J Comput Assist Tomogr 2015; 39: 1–8. doi: 10.1097/RCT.0000000000000216 [DOI] [PubMed] [Google Scholar]