Abstract
Objectives:
To assess the efficacy of the second measured glomerular filtration rate (GFR) during the course of weekly cisplatin-based chemoradiotherapy in head and neck cancer.
Methods:
Data was collected on consecutive 221 head and neck cancer patients who underwent cisplatin-based chemoradiotherapy.
Results:
68% patients managed to complete at least five out six proposed cycles of cisplatin, with a cumulative dose of ≥200 mg/m 2 . 181 patients underwent second measured GFR and it showed a mean fall in measured GFR by 12.0 ml/min/1.73 m 2 (p < 0.0001). Out of these 181 patients, in 16 patients (9%), the decision to discontinue cisplatin was purely based on a low second measured GFR (below 50 ml/min/1.73 m 2 ).
Conclusion:
Our study has shown that obtaining a second measured GFR is valuable in 9% of these patients. We propose that this should be considered as a standard procedure in these settings and also should be considered incorporating this additional safety measure, into future clinical trials as a mandatory procedure.
Advances in knowledge:
To the best of author’s knowledge, this is first study of its kind. The results of our study suggest that it should be a standard procedure of obtaining a second GFR in these settings.
Introduction
Head and neck cancer (HNC) is a heterogeneous group of diseases, originating from multiple sites in the head and neck and represent approximately 5% of all malignancies. 1 A large number of patients present at an advanced locoregional stage and management is complex and often involves a combination of surgery, radiotherapy and chemotherapy. For locoregionally advanced disease, either surgery followed by post*operative radiotherapy or definitive radiotherapy by itself is commonly used. There is established evidence that concomitant cisplatin chemotherapy with radiotherapy improves survival 2 and therefore cisplatin-based chemoradiotherapy is the standard of care in fit patients with optimal kidney function. Cisplatin (CDDP) is an alkylating agent which covalently binds to DNA and disrupts DNA function. 3 Cisplatin has a range of side-effects, some severe. In particular, cisplatin-induced nephrotoxicity (CIN) is a major concern and is a dose-limiting side-effect of cisplatin. 4 In approximately 25–35% of patients treated with cisplatin chemotherapy, reduced renal function has been shown to be a clinical problem. 5
In HNC, 3-weekly cisplatin is most commonly used and is considered the standard of care, however, use of weekly cisplatin at lower doses is gaining popularity and is being increasingly used worldwide. 6 Although there is no randomised controlled trial between these two cisplatin regimens, a comparative meta-analysis and systematic review showed that 3-weekly high-dose cisplatin chemotherapy was more toxic, including CIN, when compared to weekly cisplatin chemotherapy, with similar efficacy. 6 We previously published our results of the use of weekly cisplatin 7 and it is our standard practice to use weekly cisplatin 40 mg/m2 with radiotherapy 60–65 Gy in 30 daily fractions over a 6-week period. As cisplatin is considered highly nephrotoxic, two radionuclide ethylenediamine tetraacetic acid (EDTA)-based glomerular filtration rate (GFR) measurements (referred as ‘measured GFR’ in this paper) are conducted during this concomitant cisplatin chemotherapy. The first measured GFR is carried out before the first cycle of cisplatin and the second measured GFR is performed prior to third cycle of cisplatin chemotherapy to monitor closely for CIN.
The primary objective of this study was to assess the efficacy of the second measured GFR, and the potential clinical outcomes of withdrawing this second measured GFR.
Methods and materials
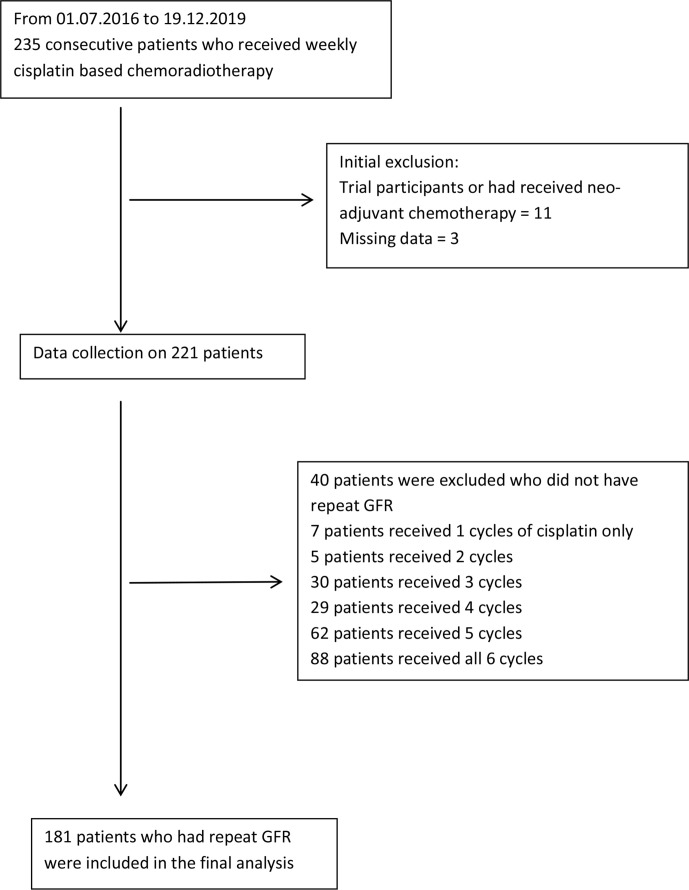
From 1 July 2016 to 19 December 2019, 235 consecutive HNC patients who received concomitant, weekly cisplatin-based chemoradiotherapy were identified. 14 patients were excluded from the study, including 11 patients who were either participants in trials or had received neoadjuvant chemotherapy; data were missing in the remaining 3 patients. Data were collected retrospectively for the remaining patients (n = 221). 181 patients had 2 radionuclide measured GFR tests during their concomitant chemoradiotherapy treatment, as not all 221 patients received the 6 cycles of proposed cisplatin chemotherapy (Figure 1).
Figure 1.
Consort diagram of patient selection.
Standard criteria for selection for chemoradiotherapy in HNC was applied including disease stage, performance status and pre-treatment optimal kidney function.
All patients underwent measured GFR prior to Cycle 1 and a repeat GFR prior to Cycle 3 of weekly cisplatin chemotherapy. Until 3 March 2019, GFR was calculated with an EDTA method. From 4 March 2019 onwards, it was calculated using a technetium radiolabled diethylene tiamine pentaacetic acid (Tc-99m DTPA) test.
Statistical analysis was performed using SPSS (v. 24). A two-tailed paired Student’s t-test was used to compare the difference between matched samples, e.g EDTA GFR before and after two cycles of chemotherapy. ANOVA or the Student‘s t-test was used to compare differences in the mean change in GFR following two cycles of chemotherapy between different groups. The χ2 test was applied to compare relative proportions of nephrotoxicity or completion of chemotherapy between groups. A p-value of <0.05 was considered statistically significant.
This project was registered with local hospital’s clinical effective register as a service review project. The registration number was 1,0157.
Results
Median age of the patient cohort was 59 years (range: 32–73). Out of the total 221 patients, 154 (70%) patients had oropharynx as the primary tumour site, 20 patients with oral cavity cancer (9%), 21 patients with the laryngeal cancer (10%), 14 patients with hypopharynx tumours (6%) and in remaining 12 patients (5%) the primary site was ‘other’ in head and neck region. 162 patients (73%) received definitive chemoradiotherapy (CRT) and remaining 59 patients (27%) were treated with adjuvant CRT, post-operatively.
The median number of weekly cisplatin chemotherapy cycles received was 5 (range: 1–6). 150 patients (68%) managed to complete at least 5 out 6 proposed cycles of cisplatin, with a cumulative dose of ≥200 mg/m 2 . 179 patients (81%) managed to complete at least 4 cycles of weekly cisplatin chemotherapy giving a cumulative dose of ≥160 mg/m 2 .
Prior to commencing chemotherapy, the mean value of measured GFR was 96 ml/min/1.73 m 2 (n = 221), (SD 23.5). 181 patients had 2 radionuclide measured GFR tests during their concomitant chemoradiotherapy treatment, as not all 221 patients received the 6 cycles of proposed cisplatin chemotherapy. There was a significant deterioration in measured GFR in the 181 patients who underwent a repeat GFR measurement, with a mean fall in measured GFR by 12.0 ml/min/1.73 m 2 (p < 0.0001). The mean value of measured GFR was 84 ml/min/1.73 m 2 , (SD 26.1) following two cycles of chemotherapy. The mean reduction of GFR was 10.1 in patients with measured GFR value of <70 and 13.5 in patients with measured GFR value of ≥70 and this small difference was not significant (p 0.202).
Statistical analyses showed that treatment intention (radical vs adjuvant) was not a significant factor in developing CIN (p 0.215). However, there was a trend in difference in mean reduction of the second measured GFR value, between primary sites (p = 0.053). Multiple analyses showed that patients with oral cavity cancers experienced a significantly smaller fall in measured GFR than other primary sites (p = 0.032). The mean fall in measured GFR in patients where further chemotherapy cycles were stopped due to nephrotoxicity was 30.7 ml/min/1.73 m 2 (SD 18.9), which was significantly higher than the fall in measured GFR in patients who stopped chemotherapy for other causes (mean 9.8, SD 15.9; p < 0.001). There was no difference in incidence of CIN in radical vs adjuvant patients (p = 0.491). While comparing patients who completed chemotherapy vs those who did not, there was no difference in radical vs adjuvant groups (p = 0.376) or primary sites of disease (p = 0.253). The results are summarised in Table 1.
Table 1.
A summary of the study findings
| Characteristic | Number | Percentage | p-value | |
|---|---|---|---|---|
| Total number | 221 | 100% | ||
| Treatment intention | ||||
| Radical | 162 | 73% | 0.256 | |
| Adjuvant | 59 | 27% | ||
| Number of cycles of chemotherapy | ||||
| 6 | 88 | 40% | ||
| 5 | 62 | 28% | ||
| 4 | 29 | 13% | ||
| 3 | 30 | 14% | ||
| 2 | 5 | 2% | ||
| 1 | 7 | 3% | ||
| GFR (mean value with standard deviation) | ||||
| Pre-chemotherapy (n = 221) | 96.20 (23.482) | <0.001 | ||
| second GFR (n = 181) | 84.24 (26.051) | |||
| Mean difference in second GFR (mean value with SD) | ||||
| Radical | −12.976 (17.221) | 0.215 | ||
| Adjuvant | −9.442 (17.471) | |||
| Mean difference in second GFR according to primary sitea | ||||
| Oropharynx (n = 127) | −12.189 (16.841) | 0.053 | ||
| Oral cavity (n = 16) | −4.125 (18.786) | |||
| Larynx (n = 15) | −8.866 (9.620) | |||
| Hypopharynx (n = 13) | −13.153 (14.926) | |||
| Other (n = 10) | −24.700 (26.276) | |||
| Mean difference in second GFR according to reasons of stopping chemotherapy | ||||
| Nephrotoxicity (n = 27) | −30.666 (18.945) | <0.001 | ||
| All other reasons (n = 74) | −9.770 (15.887) |
GFR, glomerular filtration rate.
Multiple analyses showed that patients with oral cavity cancers experienced a significantly smaller fall in GFR than other primary sites (p = 0.032).
We also analysed the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) using a 20% change in creatinine or measured GFR as an indicator of nephrotoxicity. The analyses showed that sensitivity and PPV of creatinine was poor, meaning that a change in creatinine alone failed to detect 42% (22 out of 52 patients) of the true positive rates of nephrotoxicity (Table 2). Further cycles of cisplatin were discontinued due to CIN in 30 patients. Within this 30 patient group, 16 patients (9% of the total patients who had a second measured GFR, 7% of the overall patient cohort of 221 patients), decision to discontinue cisplatin was based on a low second measured GFR (below 50 ml/min/1.73 m 2 ), despite having an adequate estimated GFR using measurement of creatinine. The remaining 14 patients, included 11 patients whose renal function became impaired even after a second acceptable value of measured GFR.
Table 2.
Significant change in renal function (20%)
| Creatinine | Measured | GFR | |
| Positive | Negative | ||
| Positive | 30 | 22 | |
| Negative | 22 | 99 |
GFR, glomerular filtration rate.
Sensitivity 0.576923
Specificity 0.818182
Positive predictive value 0.576923
Negative predictive value 0.818182
Discussion
A systematic review and meta-analysis of aggregate data of weekly low–dose versus three–weekly high–dose cisplatin for concurrent chemoradiotherapy in locoregionally advanced non-nasopharyngeal HNC included 52 studies involving 4209 patients. 6 Although the authors of this meta-analysis concluded, that due to a lack of prospective randomised studies, low-dose weekly cisplatin should be first prospectively studied in a Phase III trial, their results showed that there was no difference in treatment efficacy in terms of overall survival or response rate between patients treated with either low-dose weekly or high-dose 3-weekly cisplatin regimens. The treatment compliance was significantly better with the weekly cisplatin chemotherapy in definitive chemoradiotherapy settings and it was also associated with less toxicity in terms of Grade 3–4 myelosuppression (leukopenia p = 0.0083, neutropenia p = 0.0024), severe nausea/vomiting (p < 0.0001) and severe CIN (p = 0.0099). 6 There are many published retrospective series and individual institutional experiences where cisplatin has been used on a weekly basis and many centres in the UK prefer this due to its simpler logistics and improved cost-effectiveness.
Currently, two large UK–based head and neck prospective Phase III trials allow use of weekly cisplatin in their protocols. PATHOS is an ongoing Phase III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for Human Papillomavirus (HPV)-positive oropharyngeal cancer. 8 Patients with high-risk pathology (positive margins < 1 mm with negative marginal biopsies, and/or extracapsular spread) are being randomised to either post-operative radiotherapy (RT) alone or RT with concurrent cisplatin. Cisplatin can be given 100 mg/m 2 , using two cycles on a 3-weekly basis, or as 40 mg/m2 weekly for a maximum of 6 weeks. The trial protocol requires a measured or estimated GFR >60 ml/min to proceed with full dose of cisplatin. The protocol does not require a second measured GFR, but it specifies a dose reduction for cisplatin by 25% if the estimated GFR is between 50 and 60 ml/min. 9 Similarly, another ongoing Phase III randomised control trial comparing alternative regimens for escalating treatment of intermediate and high risk oropharyngeal cancer (CompARE trial) allows the use of either three weekly high-dose cisplatin or weekly cisplatin at 40 mg/m2 for a maximum of 7 weeks. Again, repeating a measured GFR was also not mandatory. 10
There is evidence that patients with cancer commonly present with underlying impaired kidney function. Studies have shown that over 50% of patients with solid tumours have an estimated GFR of less than 90 ml/min/1.73 m2 and up to 20% of cancer patients have an estimated GFR below 60. 11,12 There is also evidence that the value of an estimated GFR based on creatinine measurement alone, could be higher than the actual GFR in cancer patients undergoing cisplatin chemotherapy. In a study of 112 oncology patients, the median value of the estimated GFR based on creatinine 1.460 ml s−1/1.73 m 2 (interquartile range: 1.210–1.660) was significantly higher than the median value of GFR (by DTPA was 1.335 ml s−1/1.73 m 2 (interquartile range: 1.070–1.725) (p < 0.05). 13
Our analysis showed that 9% of HNC patients undergoing weekly cisplatin chemotherapy were found to have suboptimal kidney function (<50 ml/min/1.73 m 2 ) for further cisplatin chemotherapy, based on second measured GFR despite having an acceptable estimated GFR (>50 ml/min). Creatinine alone failed to detect 42% of the true-positive rates of nephrotoxicity. This is a significant finding and had these patients received further cisplatin chemotherapy, they would be at risk of developing severe and possibly irreversible CIN.
The authors do not think that the increased marginal cost of introducing a second mandatory measured GFR test will be prohibitive in the context of the overall costs of a radical or adjuvant 6 week course of chemoradiotherapy using cisplatin, as currently, the total NHS cost for a measured GFR is £239/€268/$292.
Conclusion
To the best of the authors` knowledge, this is the first study looking at the clinical value in performing a second measured GFR during the course of concomitant weekly cisplatin-based chemoradiotherapy in HNC. Our study has shown that obtaining a second measured GFR is valuable in 9% of these patients, where a suboptimal GFR of less than 50 ml/min/1.73 m2, prevented further use of cisplatin with its attendant risk of worsening CIN which is significant. We propose that this should be considered as a standard procedure in these settings and also should be considered incorporating this additional safety measure, into future clinical trials as a mandatory procedure.
Contributor Information
Lily Akmar, Email: Lily.Akmar@nhs.net.
Michelle Cunnell, Email: Michelle.Cunnell@nhs.net.
Charles Kelly, Email: Charles.Kelly2@nhs.net.
Josef Kovarik, Email: Josef.Kovarik@nhs.net.
Muhammad Shahid Iqbal, Email: shahid.iqbal@nhs.net.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Pignon J-P, le Maître A, Maillard E, Bourhis J, .MACH-NC Collaborative Group . Meta-Analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009; 92: 4–14. doi: 10.1016/j.radonc.2009.04.014 [DOI] [PubMed] [Google Scholar]
- 3. Chabner BA, Longo DL. ed. Cancer chemotherapy and biotherapy. 3rd . Philadelphia PA: Lippincott Williams & Wilkins; 2001. pp. 453–9. [Google Scholar]
- 4. Sato K, Watanabe S, Ohtsubo A, Shoji S, Ishikawa D, Tanaka T, et al. Nephrotoxicity of cisplatin combination chemotherapy in thoracic malignancy patients with CKD risk factors. BMC Cancer 2016; 16: 222. doi: 10.1186/s12885-016-2271-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. dos Santos NAG, Carvalho Rodrigues MA, Martins NM, dos Santos AC. Cisplatin-Induced nephrotoxicity and targets of nephroprotection: an update. Arch Toxicol 2012; 86: 1233–50. doi: 10.1007/s00204-012-0821-7 [DOI] [PubMed] [Google Scholar]
- 6. Szturz P, Wouters K, Kiyota N, Tahara M, Prabhash K, Noronha V, et al. Weekly low-dose versus Three-Weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced Non-Nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist 2017; 22: 1056–66. doi: 10.1634/theoncologist.2017-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iqbal MS, Chaw C, Kovarik J, Aslam S, Jackson A, Kelly J, et al. Primary concurrent chemoradiation in head and neck cancers with Weekly cisplatin chemotherapy: analysis of compliance, toxicity and survival. Int Arch Otorhinolaryngol 2017; 21: 171–7. doi: 10.1055/s-0036-1594020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. https.. Available from: ://clinicaltrials.gov/ct2/show/NCT02215265.
- 9. Owadally W, Hurt C, Timmins H, Parsons E, Townsend S, Patterson J, et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for human papillomavirus (HPV) positive oropharyngeal cancer. BMC Cancer 2015; 15: 602. doi: 10.1186/s12885-015-1598-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehanna H, Sen M, Chester J, Sanghera P, Paleri V, Gaunt P, et al. Phase III randomised controlled trial (RCT) comparing alternative regimens for escalating treatment of intermediate and high-risk oropharyngeal cancer (compare). JCO volume 15 Suppl.. [DOI] [PMC free article] [PubMed]
- 11. Launay-Vacher V, Oudard S, Janus N, Gligorov J, Pourrat X, Rixe O, et al. Prevalence of renal insufficiency in cancer patients and implications for anticancer drug management: the renal insufficiency and anticancer medications (IRMA) study. Cancer 2007; 110: 1376–84. doi: 10.1002/cncr.22904 [DOI] [PubMed] [Google Scholar]
- 12. Janus N, Launay-Vacher V, Byloos E, Machiels J-P, Duck L, Kerger J, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer 2010; 103: 1815–21. doi: 10.1038/sj.bjc.6605979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salek T, Vesely P, Bernatek J. Estimated glomerular filtration rate in oncology patients before cisplatin chemotherapy. Klin Onkol 2015; 28: 273–7. doi: 10.14735/amko2015273 [DOI] [PubMed] [Google Scholar]