Abstract
Chest imaging is often used as a complementary tool in the evaluation of coronavirus disease 2019 (COVID-19) patients, helping physicians to augment their clinical suspicion. Despite not being diagnostic for COVID-19, chest CT may help clinicians to isolate high suspicion patients with suggestive imaging findings. However, COVID-19 findings on CT are also common to other pulmonary infections and non-infectious diseases, and radiologists and point-of-care physicians should be aware of possible mimickers. This state-of-the-art review goal is to summarize and illustrate possible etiologies that may have a similar pattern on chest CT as COVID-19. The review encompasses both infectious etiologies, such as non-COVID viral pneumonia, Mycoplasma pneumoniae, Pneumocystis jiroveci, and pulmonary granulomatous infectious, and non-infectious disorders, such as pulmonary embolism, fat embolism, cryptogenic organizing pneumonia, non-specific interstitial pneumonia, desquamative interstitial pneumonia, and acute and chronic eosinophilic pneumonia.
Introduction
The coronavirus disease 2019 (COVID-19) is a new type of coronavirus disease caused by SARS-CoV-2, which first emerged in Wuhan-China in December 2019. There was a significant spread worldwide, resulting in an increasing number of cases and deaths in the first half of 2020. By early June 2020, there were more than 6 million confirmed cases around the world and almost 400,000 deaths caused by COVID-19, according to the World Health Organization (WHO). Just in the United States in the same timeline, approximately 1,8 million individuals were infected, with more than 100,000 deaths. 1
Most COVID-19 cases are asymptomatic or demonstrate mild symptoms, mostly presenting with upper respiratory tract symptoms such as sore throat, dry cough, headache, fatigue, and fever. 2 The disease may also begin with gastrointestinal symptoms, including diarrhea, nausea, and vomiting. In a smaller number of cases, patients can present with dyspnea, ventilatory disfunction, and respiratory failure. 3,4 Older adults and patients with multiple comorbidities have a worse outcome, as they have a higher risk of developing diffuse alveolar damage requiring mechanical ventilation. 4 Laboratory tests commonly show normal white blood cell or decreased lymphocyte count, as would be expected in viral infections.
Since COVID-19 has a high community transmission rate, it is essential to define the diagnosis in order to isolate infected patients and to monitor their outcome. Real-time reverse transcription polymerase chain reaction (rRT-PCR), a nucleic acid amplification test, is considered the gold-standard test for COVID-19 diagnosis. 5 However, this test presents some limitations, such as unavailability in some countries and significant false-negative rates, mainly in the first days after the infection. 3,6
Chest imaging has been used as a complementary tool in the evaluation of COVID-19 patients, including chest radiography, lung ultrasound, and chest CT scans. Despite not being definitively diagnostic for COVID-19, CT imaging can establish the presence of lung pathology and demonstrate findings deemed typical of COVID-19. 6 In this way, patients with suggestive symptoms and imaging of COVID-19 infection can be allocated to a holding room for contagious disease until RT-PCR test results become available. However, COVID-19 findings on CT can also overlap other viral infections and noninfectious etiologies, and radiologists and point-of-care physicians should be aware of possible mimickers. 7
This review aims to discuss the potential differential diagnoses of COVID-19 on chest CT and clarify the distinguishing clinical and radiological features.
COVID-19 typical CT findings
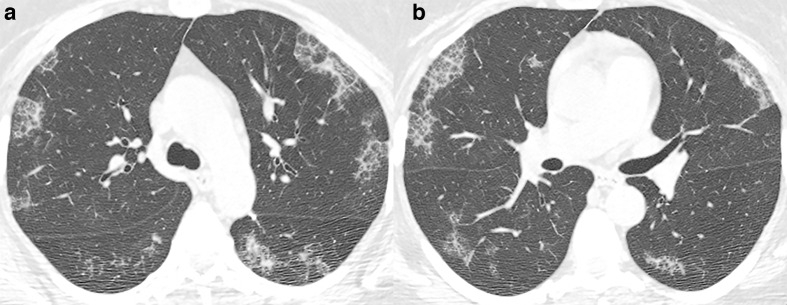
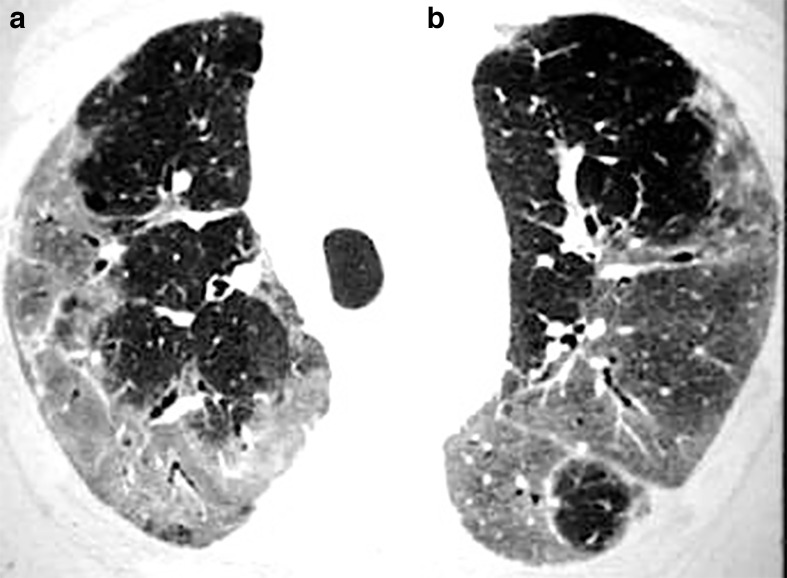
COVID-19 presentation on CT imaging will change according to the stage of the disease, which can be divided into early, intermediate, and late phases. 7 Not all patients will go through all these imaging stages. Although some CT scans during the early phase of the disease (0–5 days after the onset of symptoms) may be normal, there is a predominance of small areas of ground-glass opacities (GGOs) with subpleural distribution in one or, most commonly, both lungs (Figure 1). Less frequently, the chest CT may show a consolidation pattern. 7,8
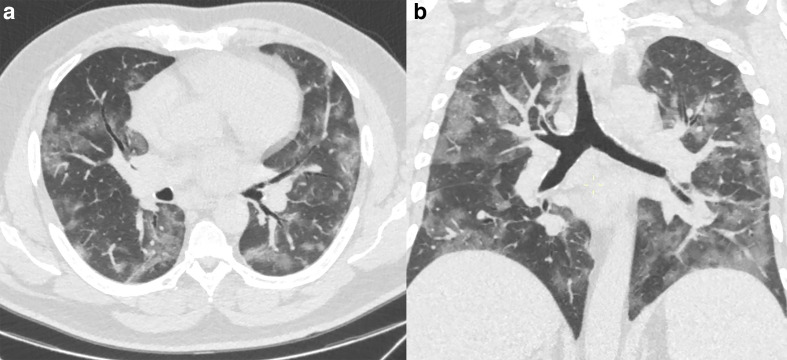
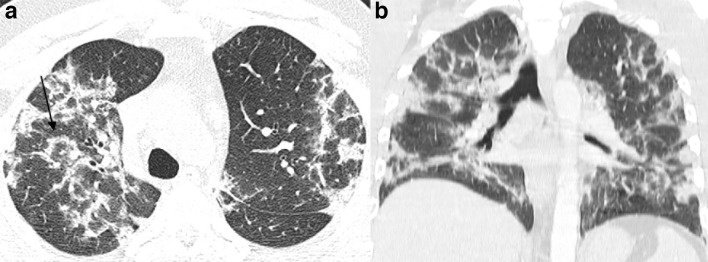
Figure 1.
51-year-old patient presenting with CT findings of COVID-19 confirmed by RT-PCR. (a) Axial chest CT in lung windows demonstrates bilateral predominantly peripheral ground-glass attenuation without consolidation. (b) Coronal reformatted CT shows bilateral ground-glass attenuation without a significant gradient. RT-PCR, reverse transcription polymerase chain reaction.
In the intermediate phase (about 6–11 days after the onset of symptoms), there is still a predominance of ground-glass pattern in most patients. However, there may be an expansion of CT findings in the pulmonary parenchyma with a higher number of GGO and bilateral involvement, as well as denser areas (foci of consolidation). 7,8
In the late phase (about 12–17 days after the onset of symptoms), most patients have confluent lesions with mixed consolidation and GGO in a bilateral distribution. In association, there may also be inter- and intralobular septal thickening that manifests as a “crazy-paving” pattern. 7,8 Less frequently, the “reversed halo sign” can also be seen in COVID-19, mainly in the intermediate to the late phase of the disease. 7,8
Some findings, such as atypical GGO distribution (sparing subpleural areas or unilateral), “tree-in-bud” pattern, moderate to severe pleural effusion, segmental or lobar consolidation without ground-glass opacities, diffuse nodules, mediastinal lymphadenopathy, pneumothorax, pneumomediastinum, and lung cavitation are rare findings in COVID-19. Those findings should raise a suspicion of other etiologies.
Mimickers of typical CT findings in COVID-19
Infectious etiologies that can mimic COVID-19 on CT
Other viral pneumonia
Viral pathogens are one of most frequent etiology of pneumonia, accounting for around 25 and 70% of cases of community-acquired pneumonia in adults and children, respectively. 9 There are some characteristics which may help radiologists and clinicians to raise their suspicion of a specific virus. The disease pathogenesis, age group, and immune status can provide some clues on the diagnosis. 10–12 However, there are several unspecific findings on CT scans, that may overlap with other lung conditions.
In this context, some viral infections tend to have some particular characteristics and show a predominant pattern on chest CT. In general, viral pneumonia presents as multifocal (random or segmental) findings with GGO and consolidations 10,12,13 (Figures 2 and 3). A recent meta-analysis with 2263 patients reported overlapping CT findings between COVID-19 and other viral pneumonia, including high prevalences of a mixed pattern of GGO and consolidation, bilateral distribution, and involvement of lower lobes. 13 On the other hand, COVID-19 pneumonia presented a higher prevalence of peripheral distribution, and involvement of upper and middle lobes when compared to other viral pneumonia. 13
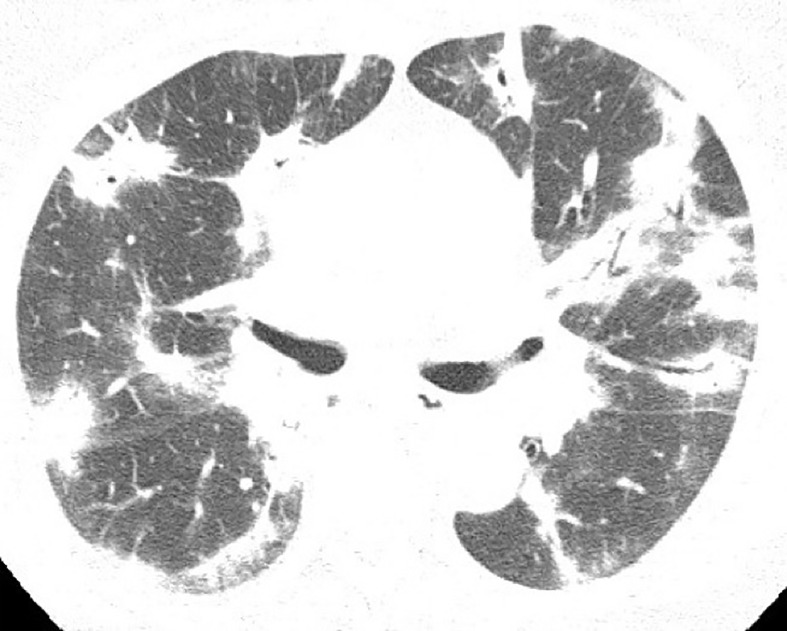
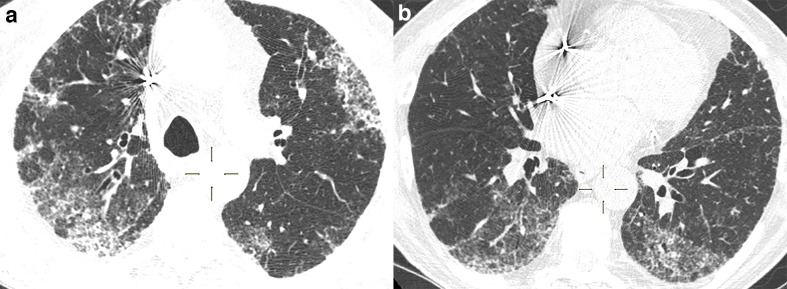
Figure 2.
58-year-old male with Influenza A H1N1 pneumonia. Axial CT image demonstrates a predominant pattern of consolidation with diffuse distribution, air bronchograms, and sparse ground-glass opacities.
Figure 3.
67-year-old male with adenovirus pneumonia. (a, b) Axial CT images demonstrate areas of consolidation with peripheral distribution, lower lobe predominance, and interlobular septal thickening.
Although rare in COVID-19, bronchial wall thickening, and pleural effusion are common findings in infections by adenovirus, parvovirus, paramyxovirus (measles), herpes (HSV), hantavirus, and phenuivirus. 12 Hantaviruses infections and COVID-19 may have a shared CT finding as interlobular septal thickening. Pulmonary micronodules and larger nodules are predominantly seen in adenoviruses, herpes (Herpes virus and varicella-zoster), paramyxovirus (measles), pneumovirus, and orthomyxovirus, and are uncommon in COVID-19 patients. 12
Mycoplasma pneumoniae infection
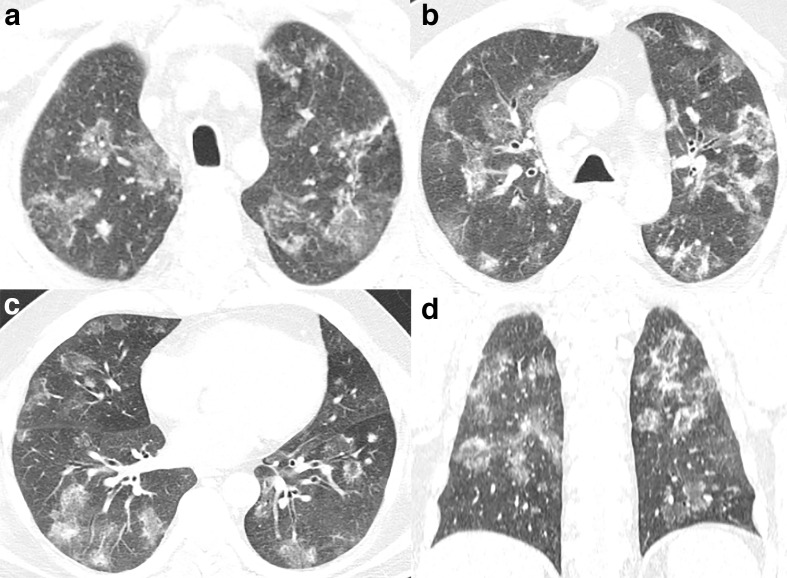
Mycoplasma pneumoniae is a frequent cause of pneumonia, especially in children and young adults. However, it may affect all ages, regardless of the patient’s immune status. Pneumonia is the most common clinical manifestation in school-aged children, and common symptoms include fever, non-productive cough, fatigue, dyspnea, headache, and sore throat. 14 In a study with more than 2200 children hospitalized with radiographically confirmed community-acquired pneumonia in the United States, M. pneumoniae was the most prevalent bacterial pathogen, affecting around 8% of cases. 9 In adults, upper respiratory tract symptoms and acute bronchitis are the most common syndromes, while M. pneumoniae pneumonia is less common, affecting from 2 to 12% of adults with community-acquired pneumonia. 9
Typical CT findings include bronchial wall thickening and centrilobular nodules, as well as GGO and consolidations, commonly affecting more than one lobe (Figure 4). Less frequently, it demonstrates reticular or linear opacities, lymphadenopathy, and pleural effusion. 15 Centrilobular nodules and bronchial wall thickening are commonly seen in Mycoplasma pneumoniae infections but are uncommon in COVID-19. The presence of such findings could help to differentiate M. pneumoniae and COVID-19, as the clinical presentation of both diseases might overlap.
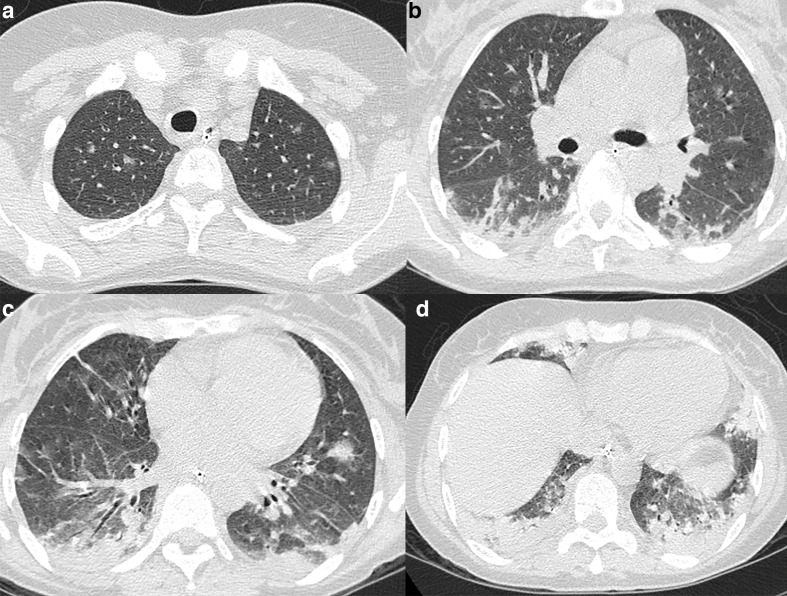
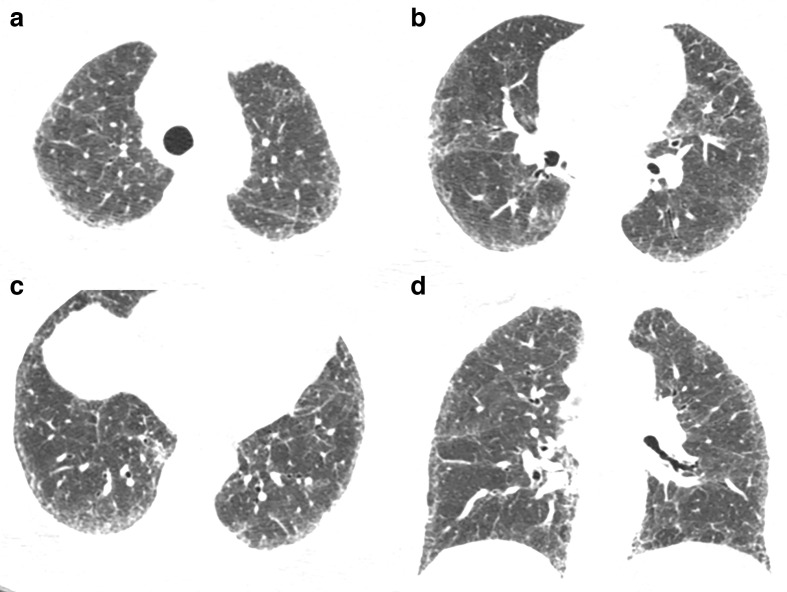
Figure 4.
48-year-old female with non-Hodgkin lymphoma and Mycoplasma pneumoniae infection. (a–d) Axial CT images demonstrate random centrilobular nodules with ground-glass and solid attenuation, bronchial wall thickening and consolidations predominantly in the lower lobes.
Pulmonary Pneumocystis jiroveci infection
Pneumocystis jiroveci pneumonia (PJP) is an opportunistic disease usually affecting immunocompromised patients with CD4 lymphocyte counts lower than 200 cells mm−³ and especially under 100 cells mm−³. There are few patients (around 10–15%) with CD4 lymphocyte count above 200 cells mm−³ presenting PJP. 16 Despite being historically associated with HIV-infected patients, PJP can also occur in HIV-uninfected patients with immunosuppression due to malignancies, organ transplant, or autoimmune diseases. Incidence rates of PJP between HIV-infected patients are around 3.9 to 7.7 per 100,000 person-years. 17
When immunosuppression is known, there is a higher chance of distinguishing PJP from COVID-19, although CT scans may be similar. Sometimes immunocompromised patients may be first aware of their diagnosis due to respiratory symptoms onset as a result of pulmonary pneumocystis infection. While COVID-19 has a CT predominance of multifocal and peripheral GGOs, the main pattern of PJP is represented as a central and diffuse distribution with relative subpleural sparing (Figure 5). 16 Both conditions can present with some degree of consolidations, interlobular septal thickening, and “crazy paving pattern”. Around one-third of PJP cases present pulmonary cysts with variable shape, wall thickness, and size, 16 findings that have not been reported in COVID-19. Nodules and tree-in-bud opacities are uncommon in both PJP and COVID-19. 6
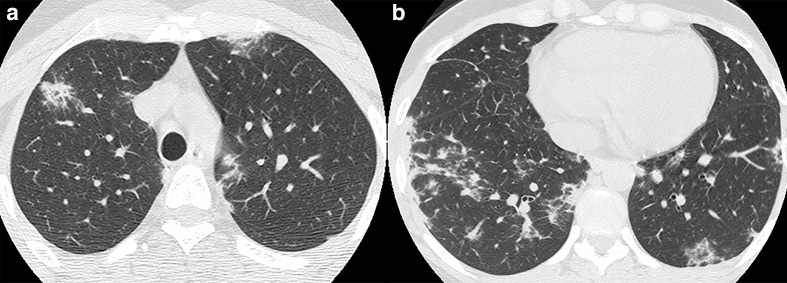
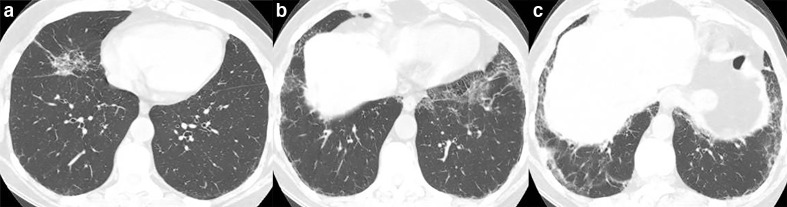
Figure 5.
32-year-old male with acquired immunodeficiency syndrome and Pneumocystis jiroveci pneumonia. (a, b) Axial CT images demonstrate ground-glass opacities and intralobular septal thickening in the periphery of the upper lobes and the superior segments of the lower lobes.
Infectious pulmonary granulomatous disease
There are a considerable number of etiologies for granulomatous pulmonary disease, both infectious or noninfectious, and therefore chest CT scans can show different patterns of presentation. 18 Some of these findings are commonly seen in COVID-19 CT scans and may confound radiologists and clinicians in determining the correct diagnosis. As demonstrated in COVID-19, CT finding in granulomatous diseases include GGOs, especially in paracoccidioidomycosis (Figure 6). A reversed halo sign may also be present in tuberculosis, among others. 18 Clinical, laboratory and immune status details are indispensable for investigating the etiology in order to differentiate from COVID-19.
Figure 6.
58-year-old male with paracoccidioidomycosis. (a–c) Axial and (d) coronal CT images demonstrate diffuse ground-glass lesions in a random distribution, some with a peripheral rim of consolidation yielding the reversed halo sign and some solid nodules.
Non-infectious etiologies that can mimic COVID-19 on CT
Pulmonary embolism
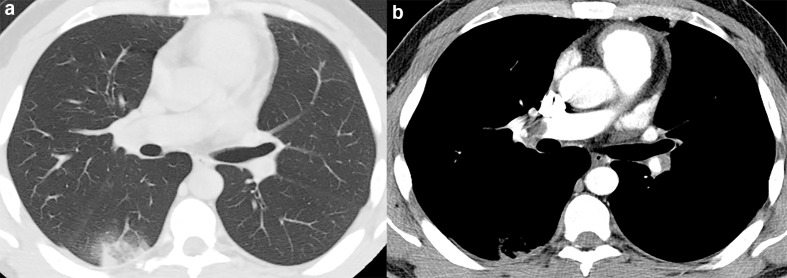
CT pulmonary angiography (CTPA) is one of the most accurate methods for the diagnosis of pulmonary embolism (PE). 19 The diagnostic criteria for acute PE include the direct visualization of the emboli causing arterial occlusion with failure to enhance the entire lumen due to a filling defect. 19,20 Pulmonary infarct due to acute PE can present on CTPA as wedge-shaped peripheral lung opacities, often with central ground glass and a rim of consolidation: the reversed halo sign 18,19,21 (Figure 7). In COVID-19, the reversed halo sign is uncommon, found in only 4% of patients in the late phase in one study. 8 Therefore, when this sign is present, pulmonary infarct should be included in the differential diagnosis.
Figure 7.
48-year-old male with pulmonary embolism. (a) Axial CT image demonstrate a wedge-shaped peripheral lung opacity in the right lower lobe with a ground-glass core and a peripheral rim of consolidation yielding the reversed halo sign. (b) CT angiography evidencing the thrombus in the right interlobar artery.
Nevertheless, the association of PE and COVID-19 appears to be common, with an incidence in CT angiography scans of COVID-19 patients ranging from 22 to 30%. 22–24 Before the COVID-19 pandemics, respiratory viruses had already been associated with procoagulant activity in human endothelial cells. 25 Likewise, the SARS-CoV-2 has also been associated with thrombotic events in macro- and micro-circulation. Severe stages of COVID-19 may induce a proinflammatory and hypercoagulable state, which could increase the risk of PE development. 26
In patients with a clinical suspicion of COVID-19 infection, presence of additional findings in non-enhanced CT scans such as dilatation of pulmonary trunk, enlargement of the right cardiac chamber, and presence of pleural effusion could indicate the concomitant presence of PE. 27 Likewise, some clinical parameters, such as high D-dimer levels, hemoptysis, and sudden worsening of respiratory function or chest pain, should also be considered. 27 When these other findings are present, complementing the investigation with a CTPA scan may be helpful.
Fat embolism
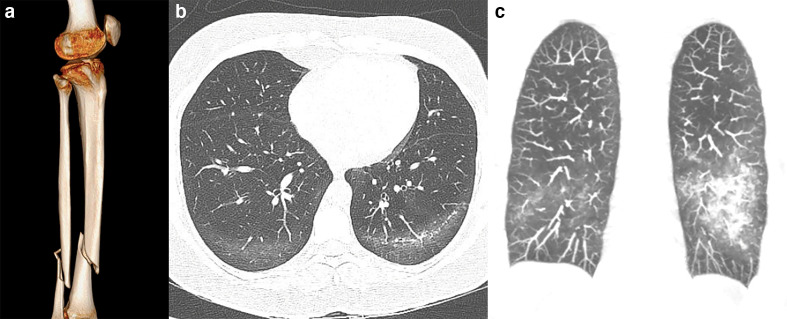
Fat embolism usually occurs as a rare complication of long bone fracture with a prevalence of 1–3% of patients with tibial or femoral fractures and 20% in more severe osseous polytrauma. 28 The classical clinical triad consists of respiratory distress, cerebral abnormalities, and petechial hemorrhages. Pulmonary fat embolism can present at CT as areas of GGO with interlobular septal thickening, ground-glass changes with patchy distribution resulting in a geographic appearance, or as nodular opacities without any zone predominance 29 (Figure 8). Therefore, some CT findings may overlap with COVID-19 presentation. However, the clinical history of recent long bone fracture, trauma, or surgical fixation can assist the differentiation, and the use of Gurd’s and Wilson’s criteria for fat embolism syndrome might be helpful. 30
Figure 8.
27-year-old male victim of a motorcycle accident with multiples injuries and developed respiratory distress a couple of days after the trauma due to fat embolism. (a) Three-dimensional rendering reconstruction of CT images demonstrates tibial and fibular fracture. (b) Axial CT image and (c) coronal maximum intensity projection reconstruction demonstrate patchy ground-glass opacities in the lower lobes with subpleural sparing.
Non-infectious organizing pneumonia
The organizing pneumonia pattern is the imaging representation of a healing process. Pathology shows loose plugs of connective tissue in airspaces and distal airways, sometimes progressing with filled bronchial endoluminal spaces by granulation material, known as Masson bodies. 31,32 There may also be interstitial inflammatory involvement.
Cryptogenic organizing pneumonia is an interstitial lung disease that represents the idiopathic form of this CT pattern. The reported annual incidence varies from 1.1 to 7 cases per 100,000 hospital admissions. 21,33 Patients are usually between 50 and 60 years old, equally affecting males and females. They may present with subacute symptoms, such as cough, mild dyspnea, fever, malaise, and sometimes weight loss. 34,35 Therefore, the assessment of clinical and epidemiological presentation and progression is essential for differential diagnoses.
CT imaging is mostly polymorphic, characterized by a mixed pattern of consolidation and GGO, that may be single or multiple, with a focal, bronchocentric, or subpleural distribution (Figure 9). 31 Moreover, there may be a nodular or crazy paving pattern and reversed halo sign. Due to its several forms of presentation, organizing pneumonia may have a considerable number of differential diagnoses other than infections, such as drug toxicity, immune diseases, actinic lesions, among others (Figure 10).
Figure 9.
57-year-old female with cryptogenic organizing pneumonia. (a) Axial and (b) coronal CT images shows bilateral perilobular opacities, interlobular septal thickening, and the reversed halo sign (arrow).
Figure 10.
61-year-old male with amiodarone-induced organizing pneumonia. (a, b) Axial CT images with peripheral and bilateral areas of ground-glass lesions in random distribution and with some reticular opacities.
Non-specific interstitial pneumonia
Non-specific interstitial pneumonia (NSIP) is one of the most common interstitial lung disease patterns in chest CT. NSIP is usually associated with connective tissue diseases (CTD), such as systemic sclerosis, Sjögren syndrome, polymyositis, and dermatomyositis. NSIP can also be due to HIV infection or be idiopathic. 34,36 Clinical history commonly includes dyspnea and cough that develop subacutely over weeks to months. 36 About one-third of cases present fever or flu-like symptoms. NSIP associated with CTD has a similar distribution between males and females, while idiopathic NSIP affects more middle-aged females who are never smokers. 37
Chest CT often demonstrates a subpleural, bilateral, and lower lobe predominance of ground glass with or without reticular abnormalities and mild subpleural fibrosis (Figure 11). Therefore, the ground-glass component can be considered an alternative diagnosis for COVID-19. 38 However, the ground glass areas are less geographic, and there are nearly always some component of subpleural fibrotic findings, such as subpleural reticulation, with fibrotic subtype also having traction bronchiectasis and volume loss of the affected lobe. Likewise, clinical symptoms, the time course of the disease symptoms, and other associated underlying conditions can guide the diagnosis. Identifying this non-acute lung differential diagnosis is essential as these patients do need outpatient follow-up at an appropriate time to have full workup to confirm the diagnosis and medication management to reduce lung involvement and symptoms.
Figure 11.
49-year-old female with non-specific interstitial pneumonia. (a–c) Axial and (d) coronal CT images demonstrate reticulations in the subpleural zones, predominantly in the lower lobes, with some ground-glass opacities and bronchiectasis.
Desquamative interstitial pneumonia
Desquamative interstitial pneumonia (DIP) is seen predominantly in smokers, with few additional conditions associated with its presentation such as autoimmune diseases and HIV. In pathology analysis, there is an interstitial reaction caused by the tobacco, showed by pigmented macrophages conglomerates in the alveoli and various degrees of interstitial fibrosis. 34 DIP is rare, occurring in less than 1% of ILD. More than 90% of patients are smokers, in their fourth and fifth decades, in a 2:1 male to female ratio with insidious onset symptoms, such as dry cough and dyspnea. 39,40
Chest CT shows subpleural and bilateral ground-glass opacities frequently associated with irregular linear opacities predominantly involving the lower lobes 34 (Figure 12). Due to the peripheral, diffuse, and bilateral involvement of pulmonary parenchyma in chest CT, generally with GGO, desquamative interstitial pneumonia may also be considered a COVID-19 differential diagnosis in early stages, even though, as other pathologies, the disease’s course and tobacco use can help in the diagnosis.
Figure 12.
44-year-old male, smoker, with desquamative interstitial pneumonia. (a–c) Axial CT images demonstrate ground-glass and reticular opacities in the periphery of the lower lobes and the middle lobe.
Acute and chronic eosinophilic pneumonia
Eosinophilic lung diseases are a heterogeneous group of disorders marked by lung opacities with tissue or peripheral eosinophilia. The diagnosis of eosinophilic lung disease can be made when the following features are encountered: (1) pulmonary opacities with peripheral eosinophilia, (2) tissue eosinophilia confirmed at lung biopsy, or (3) increased eosinophils in bronchoalveolar lavage fluid. 41 Regarding the disease onset, they can be divided into acute and chronic eosinophilic pneumonia.
In the acute form, patients present with acute/subacute onset of fever and hypoxemia. The exact prevalence is unknown, and some series reported an incidence of 9.1 per 100,000 person-years. 42 All age groups can be affected, with an average of 30 years. Tobacco smoking is the most frequently implicated trigger. Males and females equally affected. On chest CT imaging, acute EP present patchy areas of GGO accompanied by consolidation opacities and smooth interlobular septal thickening (Figure 13), findings that overlap with COVID-19 pneumonia. 41 Thickening of bronchovascular bundles, lymph node enlargement, and pleural effusions are also common findings.
Figure 13.
40-year-old male with acute eosinophilic pneumonia. Axial CT demonstrates bilateral and peripheral ground-glass opacites with interlobular septal thickening.
On the other hand, patients with chronic eosinophilic pneumonia present with an insidious onset of symptoms and show not only bronchoalveolar lavage fluid eosinophilia but also peripheral eosinophilia and frequently increased IgE serum levels. 41 Chest CT in chronic eosinophilic pneumonia mostly demonstrates nonsegmental areas of airspace consolidation with peripheral predominance. GGO, nodules, reticulation, and pleural effusions are rare findings in the chronic phase. 41
Conclusion
Due to COVID-19 high infectivity rates and the significant spread around the world in early 2020, it is essential not only to prepare health-care systems in order to manage all infected patients but also to conclude the right diagnosis. The latter will reduce acute care burden when diseases are identified as chronic or guide alternative management in other acute infections. Despite not being diagnostic for COVID-19, CT may help clinicians to isolate high suspicion patients with suggestive image findings. There is a large number of differential diagnoses for COVID-19 on chest CT scans, and clinicians and radiologists must be aware of these conditions. The careful consideration of acuity of symptoms and patient history with the knowledge of chest CT patterns in COVID-19 and its primary clinical confounders is indispensable for making the right diagnosis.
Contributor Information
Bruno Hochhegger, Email: brunoho@ufcspa.edu.br.
Matheus Zanon, Email: mhgzanon@hotmail.com.
Stephan Altmayer, Email: stephanaltmayer@gmail.com.
Nicole S Mandelli, Email: nicolesmandelli@gmail.com.
Guilherme Stüker, Email: gstuker.pucrs@gmail.com.
Tan-Lucien Mohammed, Email: mohtan@radiology.ufl.edu.
Nupur Verma, Email: vermnu@radiology.ufl.edu.
Gustavo Souza Portes Meirelles, Email: gmeirelles@gmail.com.
Edson Marchiori, Email: edmarchiori@gmail.com.
REFERENCES
- 1. World Health Organization . WHO Coronavirus Disease (COVID-19) Dashboard 2020. 2020. Available from: https://covid19.who.int/.
- 2. Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the Fleischner Society. Chest 2020; 158: 106–16. doi: 10.1016/j.chest.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 2020; 296: E15–25. doi: 10.1148/radiol.2020200490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding X, Xu J, Zhou J, Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur J Radiol 2020; 127: 109009. doi: 10.1016/j.ejrad.2020.109009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization. Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases: interim guidance, 17 January 2020. 2020. Available from: https://apps.who.int/iris/handle/10665/330676.
- 6. Dai W-C, Zhang H-W, Yu J, Xu H-J, Chen H, Luo S-P, et al. Ct imaging and differential diagnosis of COVID-19. Can Assoc Radiol J 2020; 71: 195–200. doi: 10.1177/0846537120913033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, et al. Coronavirus disease (COVID-19): spectrum of CT findings and temporal progression of the disease. Acad Radiol 2020; 27: 603–8. doi: 10.1016/j.acra.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, et al. Radiological society of north America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of Thoracic Radiology, the American college of Radiology, and RSNA - secondary publication. J Thorac Imaging 2020; 35: 219–27. doi: 10.1097/RTI.0000000000000524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med 2015; 372: 835–45. doi: 10.1056/NEJMoa1405870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koo HJ, Lim S, Choe J, Choi S-H, Sung H, Do K-H. Radiographic and CT features of viral pneumonia. Radiographics 2018; 38: 719–39. doi: 10.1148/rg.2018170048 [DOI] [PubMed] [Google Scholar]
- 11. Kim EA, Lee KS, Primack SL, Yoon HK, Byun HS, Kim TS, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002; 22 Spec No(suppl_1): S137–49. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137 [DOI] [PubMed] [Google Scholar]
- 12. Franquet T. Imaging of pulmonary viral pneumonia. Radiology 2011; 260: 18–39. doi: 10.1148/radiol.11092149 [DOI] [PubMed] [Google Scholar]
- 13. Altmayer S, Zanon M, Pacini GS, Watte G, Barros MC, Mohammed T-L, et al. Comparison of the computed tomography findings in COVID-19 and other viral pneumonia in immunocompetent adults: a systematic review and meta-analysis. Eur Radiol 2020; 0: 1–12. doi: 10.1007/s00330-020-07018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, et al. The clinical presentation of pediatric Mycoplasma pneumoniae Infections-A single center cohort. Pediatr Infect Dis J 2019; 38: 698–705. doi: 10.1097/INF.0000000000002291 [DOI] [PubMed] [Google Scholar]
- 15. Miyashita N, Sugiu T, Kawai Y, Oda K, Yamaguchi T, Ouchi K, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging 2009; 9: 7. doi: 10.1186/1471-2342-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kanne JP, Yandow DR, Meyer CA. Pneumocystis jiroveci pneumonia: high-resolution CT findings in patients with and without HIV infection. AJR Am J Roentgenol 2012; 198: W555–61. doi: 10.2214/AJR.11.7329 [DOI] [PubMed] [Google Scholar]
- 17. Solano L MF, Alvarez Lerma F, Grau S, Segura C, Aguilar A. Pneumocystis jiroveci pneumonia: clinical characteristics and mortality risk factors in an intensive care unit. Med Intensiva 2015; 39: 13–19. doi: 10.1016/j.medin.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 18. Marchiori E, Zanetti G, Meirelles GSP, Escuissato DL, Souza AS, Hochhegger B. The reversed halo sign on high-resolution CT in infectious and noninfectious pulmonary diseases. AJR Am J Roentgenol 2011; 197: W69–75. doi: 10.2214/AJR.10.5762 [DOI] [PubMed] [Google Scholar]
- 19. Stein PD, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, et al. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med 2006; 354: 2317–27. doi: 10.1056/NEJMoa052367 [DOI] [PubMed] [Google Scholar]
- 20. Souza LVS, Zanon M, Souza AS, Irion K, Penha D, Alves GRT, et al. “Pulmonary vein sign” for pulmonary embolism diagnosis in computed tomography angiography. Lung 2017; 195: 769–74. doi: 10.1007/s00408-017-0057-7 [DOI] [PubMed] [Google Scholar]
- 21. Gudmundsson G, Sveinsson O, Isaksson HJ, Jonsson S, Frodadottir H, Aspelund T. Epidemiology of organising pneumonia in Iceland. Thorax 2006; 61: 805–8. doi: 10.1136/thx.2006.059469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology 2020; 296: E186–8. doi: 10.1148/radiol.2020201544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, et al. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-dimer levels. Radiology 2020; 201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020; 41: 1858. doi: 10.1093/eurheartj/ehaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost 2000; 84: 319–24. [PubMed] [Google Scholar]
- 26. Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020; 18: 1559–61. doi: 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moreira BL, Santana PRP, Zanetti G, Marchiori E. COVID-19 and acute pulmonary embolism: what should be considered to indicate a computed tomography pulmonary angiography scan? Rev Soc Bras Med Trop 2020; 53: e20200267. doi: 10.1590/0037-8682-0267-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am 2018; 53: 335–47. doi: 10.1016/j.cnur.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 29. Malagari K, Economopoulos N, Stoupis C, Daniil Z, Papiris S, Müller NL, et al. High-Resolution CT findings in mild pulmonary fat embolism. Chest 2003; 123: 1196–201. doi: 10.1378/chest.123.4.1196 [DOI] [PubMed] [Google Scholar]
- 30. Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J Crit Illn Inj Sci 2013; 3: 64. doi: 10.4103/2229-5151.109426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246: 697–722. doi: 10.1148/radiol.2462070712 [DOI] [PubMed] [Google Scholar]
- 32. Baque-Juston M, Pellegrin A, Leroy S, Marquette CH, Padovani B. Organizing pneumonia: what is it? A conceptual approach and pictorial review. Diagn Interv Imaging 2014; 95: 771–7. doi: 10.1016/j.diii.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 33. Alasaly K, Müller N, Ostrow DN, Champion P, FitzGerald JM. Cryptogenic organizing pneumonia. A report of 25 cases and a review of the literature. Medicine 1995; 74: 201–11. doi: 10.1097/00005792-199507000-00004 [DOI] [PubMed] [Google Scholar]
- 34. Mueller-Mang C, Grosse C, Schmid K, Stiebellehner L, Bankier AA. What every radiologist should know about idiopathic interstitial pneumonias. Radiographics 2007; 27: 595–615. doi: 10.1148/rg.273065130 [DOI] [PubMed] [Google Scholar]
- 35. Lee JW, Lee KS, Lee HY, Chung MP, Yi CA, Kim TS, et al. Cryptogenic organizing pneumonia: serial high-resolution CT findings in 22 patients. AJR Am J Roentgenol 2010; 195: 916–22. doi: 10.2214/AJR.09.3940 [DOI] [PubMed] [Google Scholar]
- 36. Kligerman SJ, Groshong S, Brown KK, Lynch DA. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics 2009; 29: 73–87. doi: 10.1148/rg.291085096 [DOI] [PubMed] [Google Scholar]
- 37. Travis WD, Hunninghake G, King TE, Lynch DA, Colby TV, Galvin JR, et al. Idiopathic nonspecific interstitial pneumonia: report of an American thoracic Society project. Am J Respir Crit Care Med 2008; 177: 1338–47. doi: 10.1164/rccm.200611-1685OC [DOI] [PubMed] [Google Scholar]
- 38. Hani C, Trieu NH, Saab I, Dangeard S, Bennani S, Chassagnon G, et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv Imaging 2020; 101: 263–8. doi: 10.1016/j.diii.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Godbert B, Wissler M-P, Vignaud J-M. Desquamative interstitial pneumonia: an analytic review with an emphasis on aetiology. Eur Respir Rev 2013; 22: 117–23. doi: 10.1183/09059180.00005812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Attili AK, Kazerooni EA, Gross BH, Flaherty KR, Myers JL, Martinez FJ. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation. Radiographics 2008; 28: 1383–96. doi: 10.1148/rg.285075223 [DOI] [PubMed] [Google Scholar]
- 41. Jeong YJ, Kim K-I, Seo IJ, Lee CH, Lee KN, Kim KN, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics 2007; 27: 617–37. doi: 10.1148/rg.273065051 [DOI] [PubMed] [Google Scholar]
- 42. Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA 2004; 292: 2997–3005. doi: 10.1001/jama.292.24.2997 [DOI] [PubMed] [Google Scholar]