Abstract
Objective:
To investigate the association of mural parameters of MR-enterography (MRE) with one-year therapeutic management of Crohn’s disease (CD) patients.
Methods:
CD patients, undergone MRE with diffusion-weighted imaging (DWI) and apparent diffusion coefficient (ADC) maps between January 2017 and June 2018, were retrospectively enrolled. Extramural complications represented an exclusion criterion because of their potential influence on the intrinsic characteristic of the bowel wall. Two groups of patients were defined on the base of the therapeutic management adopted at 1-year follow-up: Medical-group and surgical-group. The following MRE parameters were evaluated: wall-thickening, longitudinal-extension, T2-fat-suppression-mural-signal, ulcers, mural-oedema, wall-enhancement-rate/pattern, DWI-scores, ADC-values, strictures.
Results:
70 CD patients were enrolled. 57/70 (81.4%) were included in Medical-group and 13/70 (18.6%) in Surgical-group. ADCmean and strictures resulted to be significantly (p < 0.01) different between the two groups. The ADCmean showed to be significantly associated to conservative management [p < 0.01; OR: 0.0003; 95% CI (0.00–0.13)], while the strictures to surgical management [p < 0.01; OR: 29.7; 95% CI (4.9–179.7)]. ROC curves for ADCmean showed that AUC was 0.717 [95% CI (0.607–0.810), p < 0.01] with an optimal cut-off value of 1.081 × 10−3 mm2 s−1. A negative predictive value of 90.2% was observed associating ADCmean values > 1.081 × 10−3 mm2 s−1 to conservative therapy. 13/17 (76%) strictures with an ADCmean > 1.081 × 10−3 mm2 s−1 benefited of conservative therapy.
Conclusion:
ADCmean values calculated on DWI-MRE may be associated to 1-year conservative medical therapy in patients with CD without extramural complications.
Advances in knowledge:
ADC maps may be proposed to select CD patients with a lower burden of mural active inflammatory cells and/or fibrosis benefiting of 1-year conservative treatment.
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease with a relapsing and remitting course. The most common disease behavior at diagnosis is the inflammatory phenotype, however stricturing or penetrating phenotypes can represent the first manifestation of the disease in up to 20% of patients or they can develop in up to 50% of patients within 20 years after the diagnosis. 1–3
Patients with CD may benefit of medical or surgical treatment depending on current severity, location and phenotype of the disease. 1,2 Medical therapy is based on steroids, 5-aminosalycilates, immunosuppressants and biologic agents with the aim to induce and maintain steroids-free clinical and endoscopic remission and the hopes to prevent or delay complications. Surgery may be required once stricturing or penetrating complications occur.
The choice of the optimal treatment strategy should also consider the features of the disease predictive of its evolution in the course of the time with the aim of selecting an early and/or intensive and/or aggressive therapy in high-risk patients or avoiding unnecessary or potentially harmful treatments in patients with less severe disease. 1–3 By the clinical, endoscopic and cross-sectional imaging point of view, initial requirement for steroids, age below 40 years and perianal disease at diagnosis, 4 terminal ileum or ileocolonic or upper gastrointestinal location, 5–7 smoking, 8,9 endoscopic Rutgeerts score 10 and Simple Endoscopic Score for CD (SES-CD), 11 stricturing and penetrating disease 6,11–15 may accurately predict the risk of surgery. Conversely, immunosuppressants and/or biologics agents reduce such risk. 12,13 As a result, stricturing disease has a significant impact on surgical risk, while active inflammatory disease usually benefit of medical therapy. On the other hand, Jauregui-Amezaga et al 12 reported that the 53% of stenosis disclosed on MR-enterography (MRE) were not operated, while severe inflammatory activity refractory to medical therapy can represent a surgical indication. The management of stenosis in CD disease represents a real dilemma for the clinicians, because they are unsure whether to continue anti-inflammatory therapy in the hope that it will resolve the obstruction or to send the patient for surgery. Ideally, we need tools to differentiate the group of patients with active inflammatory strictures responding to medical therapy from the group requiring surgery that includes active inflammatory strictures not responding to medical therapy and irreversibly fibrostenotic strictures. As a result, CD offers a grey zone of unpredictable therapeutic management outcome, which should be investigated when the disease is still confined to bowel wall focusing on mural intrinsic characteristics of involved intestinal segments.
Therefore, the aim of our study was to investigate the association of mural MRE parameters with therapeutic management outcome during a period of 1-year in patients with CD without extramural complications.
Methods and materials
Population selection
This retrospective study included all patients with CD who underwent MRE with DWI sequences at our institution between January 2017 and June 2018. All patients were included irrespective of medical treatment (failed or ongoing or successful or no medical treatment) as well as of previous surgical history (no or one or multiple surgeries) for CD.
The inclusion criteria were as follows:
Diagnosis of CD according to the current European Crohn’s and Colitis Organisation (ECCO) guidelines; 16,17
MRE performed using basal and contrast-enhanced sequences and supplemented by DWI sequences with ADC maps calculation;
Clinical, laboratory and instrumental follow-up for at least one year or until abdominal resection surgery.
The exclusion criteria were as follows:
The presence of artifacts on MRI examination preventing an accurate imaging evaluation.
The presence of extramural complications. This point was dictated by the necessity to prevent that the presence of extramural complications could influence the intrinsic characteristic of the bowel wall.
Two groups were defined on the base of the therapeutic management adopted in 1-year follow up: Medical Group, including patients undergoing conservative treatment; Surgical Group, including patients requiring surgery.
As standard protocol of our institution, (1) patients are requested to give the permission for the use of their anonymised data for research purposes at the moment of hospitalisation; (2) patients are requested to give their informed consent before performing any examination; (3) the institutional review board (IRB) is exempt if the study is retrospective and the examinations have been performed for the routine clinical management of the patients. Before MRE, all patients of our population gave the permission for the use of their anonymised data for research purposes as well as their informed consent to perform examination. IRB was exempt.
MRE imaging protocol
On the day of MRE, patients had to have been fasting for at least 6 h before the examination.
Images were acquired with the patients in the prone position using a 3 T MR scanner (Magnetom Trio Siemens, Erlangen, Germany) equipped with two paired “multichannel phased array body coil”, which allowed the coverage of the whole abdomen. To achieve an adequate distension of the small bowel, the patients assumed 1.500 ml of PEG solution (34.8 g/500 ml) 50 min before MRE. A dose of 1.000 ml of PEG solution was administered 40 min before MRE to those patients with previous small bowel resection. Small bowel motility was reduced with intravenous administration of 20 mg of N-butyl-scopolamine (Buscopan; Boehringer, Ingelheim, Germany) immediately prior to imaging.
MRE protocol with HASTE T2, True FISP and VIBE T1 sequences is reported in Table 1. Prior to contrast-enhanced imaging, free-breathing axial diffusion-weighted imaging (DWI) sequences were obtained using “echoplanar imaging single shot” (SS-EPI) sequences with fat-suppression and integrated parallel imaging (GRAPPA-2). The following parameters were used: TR 5700 ms, TE 69 ms, slice thickness 4 mm; matrix size128 × 128; averages 5; b-value 50, 500 and 1000 s mm−2, acquisition time 3.07 min. ADC map was computed by using monoexponential model on the imaging console (Syngo VE 36 A, Siemens, Erlangen, Germany).
Table 1.
MRE parameters
| HASTE T2 Sequences with and without FS a | True FISP Sequences b | VIBE T1 Sequences c | ||||
|---|---|---|---|---|---|---|
| Coronal | Axial | Coronal | Axial | Coronal | Axial | |
| Field of view | Variable d | Variable | Variable | Variable | Variable | Variable |
| No of sections | Variable | Variable | Variable | Variable | Variable | Variable |
| Repetition time (ms) | 800 | 800 | 4.18 | 4.59 | 2.79 | 2.91 |
| Echo time (ms) | 90 | 90 | 2.09 | 2.3 | 1.02 | 1.04 |
| Image matrix | 384 × 307 | 320 × 246 | 256 × 179 | 256 × 230 | 256 × 243 | 256 × 256 |
| Section thickness (mm) | 4 | 3 | 3 | 3 | 1.7 | 2.5 |
| Section gap (mm) | 0.4 | 0.9 | 0.6 | 0.6 | 0 | 0 |
| Turbo factor | 269 | 216 | Not applicable | Not applicable | Not applicable | Not applicable |
| Integrated parallel acquisition technique | GRAPPA e | GRAPPA | Not applicable | Not applicable | GRAPPA | GRAPPA |
| Flip angle (degrees) | 150 | 150 | 60 | 60 | 11 | 11 |
HASTE = half-Fourier acquisition single-shot turbo spin-echo. The HASTE sequences were performed with and without fat suppression (FS).
True FISP = true fast imaging with steady-state precession.
VIBE = volume interpolated breath-hold.
Variable = the field of the view and the number of sections were adapted to the body habitus.
GRAPPA = examination generalised autocalibrating partially parallel acquisition.
Coronal T 1 weighted fat-suppressed 3D spoiled gradient-recalled echo (VIBE, volume interpolated breath-hold examination) sequences were obtained before, 30 s and 80 s after the intravenous bolus administration into an arm vein of 0.2 mmol kg−1 body weight of gadopentetate dimeglumine (Magnevist; Berlex Laboratories, Wayne, NJ) at the rate of 3 ml s−1, followed by 20 ml saline. A single late T1-VIBE acquisition in the axial plane was obtained 3 min after contrast agent injection.
Image interpretation
All MRE were evaluated independently by two subspecialist gastro-intestinal radiologists respectively with 15 years (Reader 1) and 6 years (Reader 2) of experience. The qualitative imaging evaluation was performed by both readers, blinded to clinical, laboratory, endoscopic and surgical information; they reviewed independently the studies and then compared their interpretations. A third subspecialist gastrointestinal radiologist with 20 years (Reader 3) of experience resolved the cases of disagreement. The quantitative analysis was performed by the Reader 1. To test intra- and interobserver variability, the measurements of one or more quantitative parameters appearing significantly different between the two Medical and Surgical Groups were repeated by the Reader 1 after a wash out period of 1 month and independently by the Reader 2.
Qualitative analysis
The localisation of the disease was defined according to the following regions: jejunum, ileum, neoterminal ileum, ileocolonic and colorectal. In patients who had previous intestinal resection, the small-bowel loop segment (up to 10 cm) involved in the anastomosis was regarded as the “neoterminal ileum”.
The following pathological mural qualitative parameters were evaluated: submucosal wall oedema, mucosal ulcers, qualitative analysis of venous wall enhancement, qualitative DWI analysis, presence of strictures. 18–21 All these features were described on per-segment basis. Submucosal wall oedema was defined as an increased signal of the intestinal wall compared with normal bowel wall evaluated on FS-T2 sequences. Pathological bowel wall enhancement was defined by an increased intensity compared to normal bowel wall after intravenous gadolinium. The following three patterns were described at qualitative analysis of venous phase: mucosal, layered (mucosal–serosal) and transmural enhancement. Regarding standard DW images, each involved segment was graded on a 4-point scale on the basis of wall signal as follows: 0 = definitely absent (imperceptible signal); 1 = probably absent (signal intensity similar to the surrounding bowel segments); 2 = probably present (mild increased signal intensity respect to the surrounding bowel segments); 3 = definitely present (severe increased signal intensity respect to the surrounding bowel segments). Strictures were defined as intestinal wall thickening with constant luminal narrowing and prestenotic dilatation larger than 2.5 cm in transversal diameter.
Quantitative analysis
The following pathological mural quantitative parameters were evaluated: increased wall thickness, longitudinal extension of the disease, fat-suppression-T2 (FS-T2) mural signal intensity, rate of arterial and venous wall enhancement, ADC maps analysis. 18–22 All these features were described on per-segment basis. Bowel wall thickness was determined on T2 sequences and was defined as increased if it measured more than 3 mm. The maximum mural thickness of each involved intestinal segment had to be reported.
FS-T2 mural signal intensity, rate of arterial and venous wall enhancement, ADC maps analysis had to be performed at the level of the maximum mural thickness of each involved intestinal segment. As a result, the regions of interest (ROIs) had to be placed on FS-T2, 3D VIBE and DW images according to the slice of the T2 images where the maximum mural thickness was measured. The ROIs had to be placed on the largest possible area covering the bowel wall. The choice of the present method was dictated by the following reasons: (1) the ROIs could be enough large to obtain a representative mural sample; (2) the different measurements were obtained at the level of the same mural section; (3) the thickness as dictating reference could benefit the reproducibility of each measurements. Mural signal intensity was measured in a ROI (mean area, 0.35 cm2; range, 0.2–0.6 cm2) placed on axial FS-T2 weighted images and it was expressed as ratio with a similar ROI placed on ileopsoas muscles. The quantitative analysis of wall enhancement was expressed as ratio of enhancement by placing a ROI (mean area, 0.35 cm2; range, 0.2–0.6 cm2) on coronal 3D VIBE images and applying the following equation [(SI-post – SI-pre)/SI-pre) × 100] for both the arterial and venous phase, where SI-pre is signal intensity on precontrast images and SI-post is signal intensity on post-contrast images. Regarding the ADC maps, each involved segment was analysed placing a ROI (mean area, 0.35 cm2; range, 0.2–0.6 cm2) on the highest b-value DW images at level of the maximum mural thickness. Successively, the ROI was propagated to the corresponding ADC maps. The ADCmin (minimum apparent diffusion coefficient), ADCmax (maximum apparent diffusion coefficient), ADCmean (mean apparent diffusion coefficient) and ADCratio (ADCmin/ADCmax) values were calculated.
Statistical analysis
Because of the non-Gaussian distribution at normality test, the data of the continuous variables are presented as median and range. The Mann–Whitney U test was used to compare the continuous variables between the two Medical and Surgical Groups. Χ2 test was used to compare the categorical variables between the two Medical and Surgical Groups. A pvalue < 0.05 was considered to be statistically significant.
Stepwise logistic regression was used to examine the relationship between conservative treatment and surgery (dependent variables) and possible predictors (independent variables). The coefficients obtained from the logistic regression were also expressed in terms of odds of event occurrence (odds ratios = ORs). A p-value < 0.05 was considered to be statistically significant.
Receiver operating curve (ROC) curve analysis was used to evaluate the ability of continuous variables, statistically significant at logistic regression, to discriminate between Medical and Surgical Group, and to individuate the optimal cut-off value. Sensitivity, specificity, positive (PPV) and negative (PPN) predictive values and the area under the ROC-curve (AUC) were calculated. The 95% CI for the area was used to test the null hypothesis that the theoretical area is 0.5. If the confidence interval does not include the 0.5 value, then there is evidence that the classification variable does have the ability to distinguish between the two Groups. The p-value for comparison of observed AUC versus null hypothesis AUC (=0.5) was also reported, and this was considered significant if lower than 0.05.
A Spearman’s coefficient rank correlation was finally performed to test if one or more MRE independent variables were influenced by age, duration of the disease, location of the disease, previous surgery, therapy and CDAI.
For continuous variables appearing significantly different at Mann–Whitney U test, intra- and interobserver variability was analysed according to the method of Bland and Altman and by calculating intraclass correlation coefficient (ICC) (0.00–0.2 poor, 0.21–04 fair, 0.41–0.6 moderate, 0.61–0.80 good and 0.81–1.00 excellent correlation).
Statistical analysis was performed exclusively on per-segment basis. MedCalc Statistical Software v. 13.1.2 was used for statistical analysis (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014
Results
During the study period, a total of 90 patients with CD underwent MRE in our department. Of these, 70 showed a disease limited to mural involvement at MRE and were therefore eligible to participate in the study, while 20 patients were excluded because of the presence of extramural complications (fistulas: 12; abscesses: 1; mesenteric inflammatory masses: 7). The presence of artifacts on MRE examination preventing an accurate imaging evaluation was not observed in any patient. As a result, the study included 70 patients (age: median 35 years; range 16–72 years) with CD who performed MRE in our department. Their baseline features are shown in Table 2.
Table 2.
Demographic, clinical, laboratory features of the study population reported on per-patient basis (n = 70)
| Gender | Males/Females | 38/32 |
| Age at inclusion | Years, median (range) | 35 (16–72) |
| Disease duration | Months, median (range) | 65 (1–384) |
| Previous intestinal resection | Yes/no | 32/38 |
| Age at diagnosis | A2: 16–40 years | 43 |
| A3:>40 years | 27 | |
| CDAI | <150/>150 | 47/23 |
| Location | L1: ileal | 58 |
| L2: colonic | 5 | |
| L3: ileo-colonic | 7 | |
| Behaviour | B1: inflammatory | 45 |
| B2: stricturing | 25 | |
| Treatment before enrollment | No treatment | 11 |
| Antibiotics | 4 | |
| Mesalamine | 12 | |
| Steroids | 13 | |
| Azathioprine | 14 | |
| Infliximab | 9 | |
| Adalimumab | 7 | |
| Surgery into 1 year | Yes/no | 13/57 |
| Indication for surgery into 1 year of follow-up | Chronic bowel obstruction | 11 |
| Refractory disease | 2 | |
| Days to surgery | Days, median (range) | 68 (10–242) |
During the 1-year follow up after MRE, a conservative therapy was adopted in 57/70 (81.4%) patients (Medical Group), while 13/70 (18.6%) underwent intestinal resection (Surgical Group). A median interval of 68 days (range 10–242 days) between MRE and surgery was observed. Indications for surgery were the presence of stenosis in 11 patients (84.6%) and severe inflammatory disease resistant to medical therapy in 2 (15.4%).
On MRE, 83 bowel segments appeared to be involved by the disease, because 13 patients showed two intestinal localisations. CD localisations were distributed as follows: 3 (3.6%) jejunal, 43 (51.8%) ileum, 24 (28.9%) neoterminal ileum, 13 (15.7%) colorectal. A MRE pattern of strictures was associated to 32 intestinal segments in 25 patients.
Tables 3 and 4 show the descriptive statistics of MRE mural parameters, analysed respectively as continuous and dichotomous variables. ADCmean and strictures resulted to be significantly different between the two Medical and Surgical Groups. Univariate logistic regression showed a significant relationship between conservative management and ADCmean (p < 0.01) and between surgical management and strictures (p < 0.01). At the multivariate analysis, the ADCmean [p < 0.01; OR: 0.0003; 95% CI (0.00–0.13)] showed to be an independent variable significantly associated to conservative management, as well as, the strictures [p < 0.01; OR: 29.7; 95% CI (4.9–179.7)] showed to be independent variables significantly associated to surgical management.
Table 3.
MRE parameters measured as continuous in Medical and Surgical Group. 83 intestinal CD segments in 70 patients were evaluated
| Total intestinal CD segments (n 83) | Medical Group (n 66) | Surgical Group (n 17) | |
|---|---|---|---|
| Wall thickening (mm) | 9 (4–13) | 9 (5–20) | p = 0.8 |
| Length of involved segment (cm) | 29 (1,5–92) | 12,5 (2–70) | p = 0.44 |
| T2 FS Wall signal | 3.9 (1.7–6.1) | 4.1 (2.5–6.7) | p = 0.41 |
| Arterial wall enhancement (%) | 160 (145–240) | 194 (77–365) | p = 0.42 |
| Venous wall enhancement (%) | 232 (115–517) | 225 (87–402) | p = 0.62 |
| ADCmax (×10–3 mm2 s–1) | 1.39 (0.98–2.7) | 1.34 (1.05–2.27) | p = 0.07 |
| ADCmean (×10–3 mm2 s–1) | 1.18 (0.89–1.93) | 1.08 (0.78–1.22) | p = 0.002 |
| ADCmin (×10–3 mm2 s–1) | 0.95 (0.36–1,67) | 0.91 (0.75–1.64) | p = 0.43 |
| ADCratio | 0.7 (0.25–0.94) | 0.72 (0.46–0.83) | p = 0.56 |
ADC, apparent diffusion coefficient; CD, Crohn's disease; FS, fat-suppressed.
The continuous variables are expressed as median and range (parenthesis) because of the non-Gaussian distribution.
Table 4.
MRE parameters measured as dichotomous variables in Medical and Surgical Group
| Total intestinal CD segments (n 83) | Medical Group (n 66) | Surgical Group (n 17) | |
|---|---|---|---|
| Wall oedema | |||
| Absent | 39 (59%) | 11 (65%) | |
| Present | 27 (41%) | 6 (35%) | p = 0.6 |
| Ulcers | |||
| Absent | 22 (33%) | 6 (35%) | |
| Present | 44 (67%) | 11 (65%) | p = 0.88 |
| DWI visual analysis | |||
| 0 | 0 (0%) | 0 (0%) | |
| 1 | 1 (1.5%) | 0 (0%) | |
| 2 | 12 (18,2%) | 5 (29,4%) | |
| 3 | 53 (80.3%) | 12 (70.6%) | p = 0.58 |
| Enhancement patterns | |||
| Mucosal | 38 (57.6%) | 9 (53%) | |
| Mucosal–serosal | 25 (37.8%) | 7 (41.2%) | |
| Transmural | 3 (4.6%) | 1 (5.8%) | p = 0.28 |
| Strictures | |||
| Absent | 49 (74.2%) | 2 (11.8%) | |
| Present | 17 (25.8%) | 15 (88.2%) | p < 0.0001 |
CD, Crohn's disease; DWI, diffusion-weighted imaging; MRE, MR-enterography.
83 intestinal CD segments in 70 patients were evaluated.
Analysis of ROC curves for ADCmean showed that AUC was 0.717 [95% CI (0.607–0.810), p < 0.01] with an optimal cut-off value of 1.081 × 103 mm2 s−1. Thus, associating high surgical risk to ADCmean < 1.081 × 10−3 mm2 s−1 and conservative therapy to ADCmean > 1.081 × 10−3 mm2 s−1, sensitivity, specificity, PPV and NPV were 70.6, 69.7, 37.5 and 90.2% respectively. As a result, 46 out of 51 intestinal segments (90.2%) with ADCmean value >1.081 × 10−3 mm2 s−1 benefited of conservative management.
Of the 32 strictures disclosed on MRE, 17/32 (53.1%) were not resected, while 15/32 (46.9%) underwent surgery. 13 out of 17 (76%) strictures benefiting of conservative therapy showed an ADCmean > 1.081 × 10−3 mm2 s−1 (Figure 1), while 10 out of 15 (66.6%) strictures requiring surgery an ADCmean < 1.081 × 10−3 mm2 s−1 (Figure 2). Both the two intestinal segments resected for a severe inflammatory disease resistant to medical therapy showed an ADCmean < 1.081 × 10−3 mm2 s−1.
Figure 1.
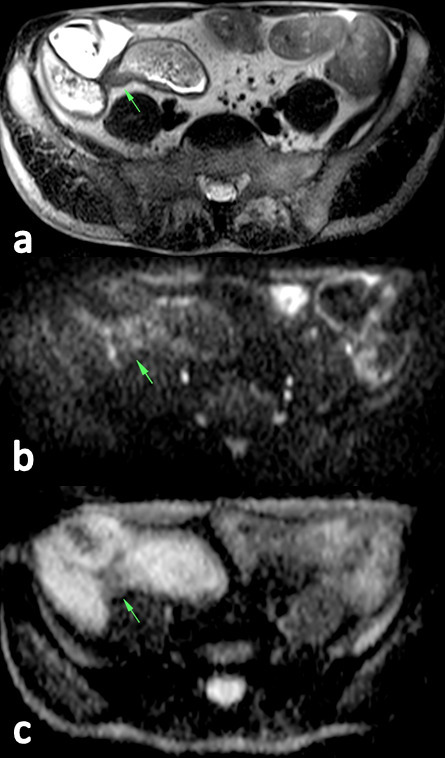
HASTE-T2 image (a) show a stenosis of the ileum with mural thickening of 7 mm and proximal lumen dilatation of 32 mm. On the DWI (b), the stenosis shows a signal intensity similar to the surrounding bowel segments. On ADC maps (c), an ADCmean of 1.265 × 10−3 mm2 s−1 was calculated. The patient underwent conservative treatment during a 1-year follow-up. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; HASTE, half-Fourier acquisition single-shot turbo spin-echo.
Figure 2.
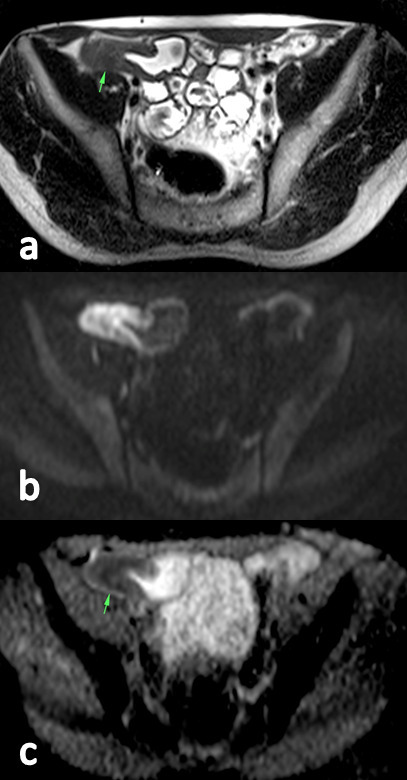
HASTE-T2 image (a) show a stenosis of the ileum with mural thickening of 10 mm and proximal lumen dilatation of 26 mm. On the DWI (b), the stenosis shows a severe increased signal intensity respect to the surrounding bowel segments. On ADC maps (c), an ADCmean of 1.047 × 10−3 mm2 s−1 was calculated. The patient underwent intestinal resection during a 1-year follow-up. ADC, apparent diffusion coefficient; DWI, diffusion-weighted imaging; HASTE, half-Fourier acquisition single-shot turbo spin-echo.
ADCmean did not show any correlation with age of the patients, duration of the disease, location of the disease, previous surgery, the type of medical therapy and CDAI.
Because of the ADCmean was the only continuous variable significantly different between the two Medical and Surgical Groups at Mann–Whitney U test, the intra and interobserver variability of ADCmean measurements was calculated resulting excellent (ICC >0.81) (Figure 3a and b).
Figure 3.
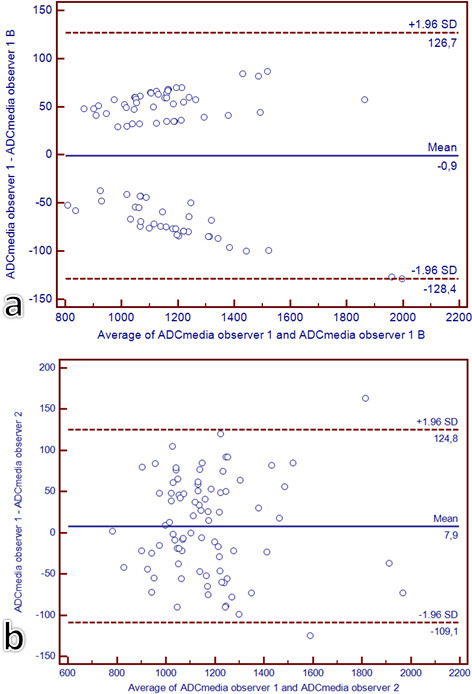
Intra- (a) and inter- (b) observers reproducibility for ADCmean measurements. For the intraobserver reproducibility, the same operator calculated twice the ADCmean. For the interobservers reproducibility, a second operator calculated the ADCmean. Bland–Altman plots of the average of the ADCmean (x axis) against the difference between ADCmean (y axis) of the two measurements performed by the same operator (a) and by the two operators (b); the dashed lines represent the 95% confidence intervals of the mean differences. ADC, apparent diffusion coefficient.
Discussion
Our study suggest that, the ADCmean values, as mural intrinsic parameter, may be associated to 1-year conservative therapeutic management in patients with CD without extramural complications. The well-known prognostic surgical impact of the strictures was also confirmed in our series.
DWI associated to a MRE protocol has been demonstrated to be a reliable tool for detecting and localising CD 21–28 and differentiating actively inflamed intestinal segments from inactive involved ones. 21,22,27 Particularly, Hordonneau et al 22 Oto et al 26 and Buisson et al 27 identified an ADC value cut-off below which the disease may be classified in active phase respectively of 1.9 × 10−3 mm2 s−1 (sensitivity 93.7%, specificity 96%), of 2.0 × 10−3 mm2 s−1 (sensitivity 84%, specificity 91%) and 1.6 × 10−3 mm2 s−1 (sensitivity 82.4%, specificity 100%), respectively. Li et al 23 reported that the active inflamed segments showed ADC value (0.92 × 10−3 mm2 s−1) significantly lower than that of inactive involved segments (1.68 × 10−1 mm2 s−1). Catalano et al 29 observed an ADC value of 1.24 × 10−3 mm2 s−1 in active inflammatory disease, of 1.18 × 10−3 mm2 s−1 in fibrosis disease and 1.30 × 10−3 mm2 s−1 in fibrosis plus active inflammatory disease. Tielbeek et al 30 found significantly lower ADC in fibrotic (1.714 × 10−3 mm2 s−1) compared to non-fibrotic lesions (2.282 × 10−3 mm2 s−1). A large difference of ADC values are reported in the above studies. 22,23,26,29,30 As a result the thresholds of ADC values for differentiating active inflammatory, non-active and fibrotic lesions are not established, yet. The following technical factors could explain in part this point: (1) different scanners with varying magnetic filed strengths and lack of reproducibility between MRI vendors; (2) non-standardised sequence parameters (using of different b values). On the basis of these considerations, also the clinical use of ADC values in the therapeutic decisions of patients with CD can be hampered by the variability introduced by the scanners and the acquisition protocols. 31
Independently from the ADCmean cut-off value used, it is reasonable to assume that ADC values depend on intramural accumulation of active inflammatory cells and/or fibrotic tissue which determines restriction of the Brownian motion of the water in the extracellular space. Referred to our series, the higher ADC values observed on pathological segments benefiting of conservative therapy can be supposed to be related to a lower intramural burden of acute inflammatory cells and/or fibrotic tissue. Considering that the strictures in CD are never pure but usually represent a mixture of fibrosis and inflammation and the non-invasive evaluation of the amount of each component of the disease represents a real diagnostic challenge, 32 the availability of a quantitative MRE parameter indirectly representative of the intramural total burden of inflammatory cells and fibrotic tissue might represent a new way to solve the dilemma about the therapeutic management of stenosis in CD.
Neither the magnetic resonance index of activity (MaRIA) nor its individual components (wall thickness, relative contrast enhancement, mural oedema and mucosal ulceration) significantly predict the surgical risk of patients with CD. 12,33 Similar findings were observed in our population, suggesting that the conventional mural parameters of CD are not enough representative of the severity of intramural architectural tissue change.
The quantitative analysis of ADC maps performed better than qualitative analysis of DWI, as well as, an excellent intra-/interobserver variability of ADCmean measurements was observed in our population. These observations are in contrast with previous findings. 34,35 The above mismatch may be related to the method of measurement adopted. Indeed, the ROI placement on the ADC maps was dictated by the maximum mural thickness observed on T2 images rather than the brightest signal observed on DW images. This approach might have offered a better representation of the intramural architectural tissue change respect to the qualitative analysis, as well as, a reproducible ADC measurement.
In our series, strictures showed a significant surgical impact, as just largely reported in previous study; 11,12,14,15 however, the ADC values could have aided to identify the 76% of stenosis benefiting of conservative therapy.
A few observations have to be offered.
The impact of clinical and laboratory data on therapeutic management outcome was not investigated; however, we were exclusively interested to explore the role of imaging information to this scope.
The retrospective design of the study does not allow to define how every single imaging mural parameter including ADCmean truly impacted on treatment strategy. At the same time, the physicians were not blinded to MRE assessment in establishing the therapeutic management, and some indications for surgery may have been directly related to the information of the MRE.
The choice to exclude penetrating disease may represent a limitation for evaluating the impact of ADC values on the therapeutic management of all CD manifestations; however, we aimed to prevent that extramural complications could influence the intrinsic characteristic of the bowel wall. At the same time, the exclusion of penetrating disease can have impacted on the relative small number of surgical patients (13) vs conservative managed patients (57).
Although the excellent intra-/interobserver variability of ADCmean measurements observed in our population, the significant difference of ADCmean values between Medical and Surgical Groups (median 1.18 × 10–3 mm2/s vs 1.08 × 10–3 mm2/s) might not be sufficient to balance the variability induced by different scanners and/or acquisition protocols previously reported. 17,23,24,29,30 A more comprehensive studies in a multi-institutional setting with DW-MRI data acquired at different field strengths and/or with different vendor systems and/or with different acquisition protocols should be conducted. In the meantime, these studies are performed, it is probably best to refer to institution’s own cut-off ADCmean values obtained from the same magnet, MRE protocol and b values.
The patients were enrolled independently from the type of medical therapy performed before MRE examination. However, the analysis stratified on the type of medical treatment showed no correlation between ADCmean values and the type of medical agents used.
The ROC curves for ADCmean showed an AUC of 0.717, which represents a not excellent value in terms of ability to differentiate conservative therapy vs need for surgery despite the significant difference on average. As a consequence, the derived cut-off value of 1.081 × 10−3 mm2 s−1 may be not effective when applied to a single patient. For this reason, we have exclusively emphasised the NPV of 90.2% observed applying the above cut-off value.
In conclusion, ADCmean values calculated on DWI-MRE may be associated to 1-year conservative medical therapy of patients with CD without extramural complications, indicating patients who have a low intramural total burden of both acute inflammatory cells and/or fibrotic tissue. Further prospective studies are needed to validate the prognostic role of ADCmean in the routine clinical practice, focalising primarily on the standardisation of ADC values measurement.
Contributor Information
Pier Paolo Mainenti, Email: pierpamainenti@hotmail.com.
Fabiana Castiglione, Email: fabiana.castiglione@unina.it.
Antonio Rispo, Email: antoniorispo@email.it.
Ettore Laccetti, Email: ettorelac@hotmail.it.
Salvatore Guarino, Email: sag1981@libero.it.
Valeria Romeo, Email: valeria.romeo@me.com.
Anna Testa, Email: annatesta82@virgilio.it.
Leonardo Pace, Email: lpace@unisa.it.
Simone Maurea, Email: maurea@unina.it.
REFERENCES
- 1. Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc 2017; 92: 1088–103. doi: 10.1016/j.mayocp.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 2. Chan WPW, Mourad F, Leong RW. Crohn's disease associated strictures. J Gastroenterol Hepatol 2018; 33: 998–1008. doi: 10.1111/jgh.14119 [DOI] [PubMed] [Google Scholar]
- 3. Kobayashi T, Hisamatsu T, Suzuki Y, Ogata H, Andoh A, Araki T, et al. Predicting outcomes to optimize disease management in inflammatory bowel disease in Japan: their differences and similarities to Western countries. Intest Res 2018; 16: 168–77. doi: 10.5217/ir.2018.16.2.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaugerie L, Seksik P, Nion-Larmurier I, Gendre J-P, Cosnes J. Predictors of Crohn's disease. Gastroenterology 2006; 130: 650–6. doi: 10.1053/j.gastro.2005.12.019 [DOI] [PubMed] [Google Scholar]
- 5. Loly C, Belaiche J, Louis E. Predictors of severe Crohn's disease. Scand J Gastroenterol 2008; 43: 948–54 10.1080/00365520801957149 [DOI] [PubMed] [Google Scholar]
- 6. Solberg IC, Vatn MH, Høie O, Stray N, Sauar J, Jahnsen J, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol 2007; 5: 1430–8. doi: 10.1016/j.cgh.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 7. Romberg-Camps MJL, Dagnelie PC, Kester ADM, Hesselink-van de Kruijs MAM, Cilissen M, Engels LGJB, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol 2009; 104: 371–83. doi: 10.1038/ajg.2008.38 [DOI] [PubMed] [Google Scholar]
- 8. Song X-ming, Gao X, Li M-zhe, Chen Z-hui, Chen S-cai, Hu P-J, et al. Clinical features and risk factors for primary surgery in 205 patients with Crohn’s disease: analysis of a South China cohort. Dis Colon Rectum 2011; 54: 1147–54. doi: 10.1097/DCR.0b013e318222ddc3 [DOI] [PubMed] [Google Scholar]
- 9. Seksik P, Nion-Larmurier I, Sokol H, Beaugerie L, Cosnes J. Effects of light smoking consumption on the clinical course of Crohn's disease. Inflamm Bowel Dis 2009; 15: 734–41 10.1002/ibd.20828 [DOI] [PubMed] [Google Scholar]
- 10. Rutgeerts P, Geboes K, Vantrappen G, Kerremans R, Coenegrachts JL, Coremans G. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984; 25: 665–72 10.1136/gut.25.6.665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rispo A, Imperatore N, Testa A, Bucci L, Luglio G, De Palma GD, et al. Combined Endoscopic/Sonographic-based risk matrix model for predicting one-year risk of surgery: a prospective observational study of a tertiary centre Severe/Refractory Crohn's disease cohort. J Crohns Colitis 2018; 12: 784–93 10.1093/ecco-jcc/jjy032 [DOI] [PubMed] [Google Scholar]
- 12. Jauregui-Amezaga A, Rimola J, Ordás I, Rodríguez S, Ramírez-Morros A, Gallego M, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn's disease in the era of biologics. Gut 2015; 64: 1397–402. doi: 10.1136/gutjnl-2014-308101 [DOI] [PubMed] [Google Scholar]
- 13. Rigazio C, Ercole E, Laudi C, Daperno M, Lavagna A, Crocella L, et al. Abdominal bowel ultrasound can predict the risk of surgery in Crohn's disease: proposal of an ultrasonographic score. Scand J Gastroenterol 2009; 44: 585–93 10.1080/00365520802705992 [DOI] [PubMed] [Google Scholar]
- 14. Castiglione F, de Sio I, Cozzolino A, Rispo A, Manguso F, Del Vecchio Blanco G, et al. Bowel wall thickness at abdominal ultrasound and the one-year-risk of surgery in patients with Crohn's disease. Am J Gastroenterol 2004; 99: 1977–83 10.1111/j.1572-0241.2004.40267.x [DOI] [PubMed] [Google Scholar]
- 15. Campos C, Perrey A, Lambert C, Pereira B, Goutte M, Dubois A, et al. Medical therapies for Stricturing Crohn's disease: efficacy and cross-sectional imaging predictors of therapeutic failure. Dig Dis Sci 2017; 62: 1628–36. doi: 10.1007/s10620-017-4572-4 [DOI] [PubMed] [Google Scholar]
- 16. Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27 10.1016/j.crohns.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 17. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis 2010; 4: 28–62. doi: 10.1016/j.crohns.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 18. Tolan DJM, Greenhalgh R, Zealley IA, Halligan S, Taylor SA. Mr enterographic manifestations of small bowel Crohn disease. Radiographics 2010; 30: 367–84. doi: 10.1148/rg.302095028 [DOI] [PubMed] [Google Scholar]
- 19. Punwani S, Rodriguez-Justo M, Bainbridge A, Greenhalgh R, De Vita E, Bloom S, et al. Mural inflammation in Crohn disease: location-matched histologic validation of Mr imaging features. Radiology 2009; 252: 712–20. doi: 10.1148/radiol.2523082167 [DOI] [PubMed] [Google Scholar]
- 20. Bruining DH, Zimmermann EM, Loftus EV, Sandborn WJ, Sauer CG, Strong SA, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn's disease. Radiology 2018; 286: 776–99. doi: 10.1148/radiol.2018171737 [DOI] [PubMed] [Google Scholar]
- 21. Oto A, Zhu F, Kulkarni K, Karczmar GS, Turner JR, Rubin D. Evaluation of diffusion-weighted MR imaging for detection of bowel inflammation in patients with Crohn’s disease. Acad Radiol 2009; 16: 597–603. doi: 10.1016/j.acra.2008.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hordonneau C, Buisson A, Scanzi J, Goutorbe F, Pereira B, Borderon C, et al. Diffusion-weighted magnetic resonance imaging in ileocolonic Crohn’s disease: validation of quantitative index of activity. Am J Gastroenterol 2014; 109: 89–98. doi: 10.1038/ajg.2013.385 [DOI] [PubMed] [Google Scholar]
- 23. Li X-H, Sun C-H, Mao R, Zhang Z-W, Jiang X-S, Pui MH, et al. Assessment of activity of Crohn disease by diffusion-weighted magnetic resonance imaging. Medicine 2015; 94: e1819. doi: 10.1097/MD.0000000000001819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kiryu S, Dodanuki K, Takao H, Watanabe M, Inoue Y, Takazoe M, et al. Free-breathing diffusion-weighted imaging for the assessment of inflammatory activity in Crohn's disease. J Magn Reson Imaging 2009; 29: 880–6. doi: 10.1002/jmri.21725 [DOI] [PubMed] [Google Scholar]
- 25. Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard M-A, Régent D, et al. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut 2010; 59: 1056–65 10.1136/gut.2009.197665 [DOI] [PubMed] [Google Scholar]
- 26. Oto A, Kayhan A, Williams JTB, Fan X, Yun L, Arkani S, et al. Active Crohn's disease in the small bowel: evaluation by diffusion weighted imaging and quantitative dynamic contrast enhanced MR imaging. J Magn Reson Imaging 2011; 33: 615–24 10.1002/jmri.22435 [DOI] [PubMed] [Google Scholar]
- 27. Buisson A, Joubert A, Montoriol P-F, Da Ines D, Ines DD, Hordonneau C, et al. Diffusion-weighted magnetic resonance imaging for detecting and assessing ileal inflammation in Crohn’s disease. Aliment Pharmacol Ther 2013; 37: 537–45. doi: 10.1111/apt.12201 [DOI] [PubMed] [Google Scholar]
- 28. Seo N, Park SH, Kim K-J, Kang B-K, Lee Y, Yang S-K, et al. Mr Enterography for the evaluation of small-bowel inflammation in Crohn disease by using diffusion-weighted imaging without intravenous contrast material: a prospective Noninferiority study. Radiology 2016; 278: 762–72 10.1148/radiol.2015150809 [DOI] [PubMed] [Google Scholar]
- 29. Catalano OA, Gee MS, Nicolai E, Selvaggi F, Pellino G, Cuocolo A, et al. Evaluation of quantitative PET/MR Enterography biomarkers for discrimination of inflammatory strictures from fibrotic strictures in Crohn disease. Radiology 2016; 278: 792–800. doi: 10.1148/radiol.2015150566 [DOI] [PubMed] [Google Scholar]
- 30. Tielbeek JAW, Ziech MLW, Li Z, Lavini C, Bipat S, Bemelman WA, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn’s disease assessment with histopathology of surgical specimens. Eur Radiol 2014; 24: 619–29 10.1007/s00330-013-3015-7 [DOI] [PubMed] [Google Scholar]
- 31. Huh J, Kim KJ, Park SH, Park SH, Yang S-K, Ye BD, et al. Diffusion-weighted Mr enterography to monitor bowel inflammation after medical therapy in Crohn's disease: a prospective longitudinal study. Korean J Radiol 2017; 18: 162–72 10.3348/kjr.2017.18.1.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins PDR. Measurement of fibrosis in Crohn's disease strictures with imaging and blood biomarkers to inform clinical decisions. Dig Dis 2017; 35(1-2): 32–7. doi: 10.1159/000449080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fiorino G, Morin M, Bonovas S, Bonifacio C, Spinelli A, Germain A, Laurent V, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn's disease and its impact on disease outcome. J Crohns Colitis 2017; 11: 274–80 10.1093/ecco-jcc/jjw185 [DOI] [PubMed] [Google Scholar]
- 34. Pendsé DA, Makanyanga JC, Plumb AA, Bhatnagar G, Atkinson D, Rodriguez-Justo M, et al. Diffusion-weighted imaging for evaluating inflammatory activity in Crohn's disease: comparison with histopathology, conventional MRI activity scores, and faecal calprotectin. Abdom Radiol 2017; 42: 115–23 10.1007/s00261-016-0863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watson T, Calder A, Barber JL. Quantitative bowel apparent diffusion coefficient measurements in children with inflammatory bowel disease are not reproducible. Clin Radiol 2018; 73: 574–9 10.1016/j.crad.2018.01.015 [DOI] [PubMed] [Google Scholar]


