Abstract
Objective:
To assess whether intracranial vessel wall (IVW) MRI luminal measurements are more accurate than non-contrast 3D-TOF-MRA measurements for intracranial atherosclerotic stenosis, relative to CTA.
Methods:
Consecutive patients with non-calcified intracranial atherosclerotic stenosis seen on CTA, who had non-contrast 3D-TOF-MRA and IVW performed between 1 January 2013 and 20 April 2014 were selected, and images with stenosis were pre-selected by a single independent rater. The pre-selected CTA, MRA, and IVW (T 1-weighted) images were then reviewed by two independent raters blinded to the other measurements in random order. Measurements were made in a plane perpendicular to the lumen on each modality. MRA and IVW measurements were compared to CTA, to determine which more accurately matched the degree of stenosis.
Results:
18 patients with 33 intracranial atherosclerotic stenoses were included. Relative to CTA, IVW had 40% less variance than MRA (p = .004). IVW had a significantly higher concordance correlation coefficient (CCC) relative to CTA than MRA (.87 vs .68, p = .002). IVW and MRA did not have significant bias relative to CTA, however, 8/33 lesions showed >20% overestimation of the degree of stenosis on MRA, compared to 1/33 for IVW. CCC between raters were 0.84 (95% CI 0.67–0.93) for CTA, 0.83 (0.67–0.93) for TOF-MRA, and 0.85 (0.71–0.94) for IVW. For stenosis >50% sensitivity was 82% for IVW and 64% for MRA, while specificity was 73% for both.
Conclusion:
IVW provides more accurate stenosis measurements than MRA when compared to CTA.
Advances in knowledge:
Considering higher stenosis measurement accuracy of IVW, it can be more reliably used for quantitative evaluation relative to MRA.
Introduction
Currently, the evaluation of intracranial atherosclerotic arterial stenosis relies on luminal measurements using luminal imaging techniques, including digital subtraction catheter angiography (DSA), CT angiography (CTA), and MR angiography (MRA). While DSA is the gold standard in assessing luminal stenosis, most angiographic runs are performed as a 2D imaging acquisition and accurate assessment of stenosis may be difficult due to overlapping structures. 1,2 In addition, DSA is an invasive technique with a small risk of stroke. Patients frequently undergo both CTA and MRI/MRA, and at some institutions, high-resolution vessel wall (IVW) imaging. While CTA provides good spatial resolution and assessment of luminal stenosis, this technique also exposes the patient to radiation as well as iodinated contrast, which has a risk of contrast-induced nephropathy and allergic reactions. Non-contrast time-of-flight MRA is the most frequently utilized MR angiographic technique and provides good contrast resolution for luminal evaluation. In the setting of slow flow or high-grade stenosis, however, the degree of stenosis will frequently be overestimated due to flow dephasing artifacts and mild stenosis may also be underestimated due to bleeding effects of the vascular lumen. 3,4 IVW is an increasingly utilized and reliable technique that allows for assessment of vessel wall pathology, 5,6 including atherosclerosis, 7,8 and has shown value in disease characterization 9–11 and differentiation. 12,13 We hypothesize that luminal measurements on IVW are more accurate than those on 3D-TOF-MRA, relative to CTA.
Methods and materials
Patient population
After institutional review board approval, the radiology database was retrospectively queried for patients with intracranial arterial stenosis on CTA between the dates of 1 January 2013 and 20 April 2014. Inclusion criteria were: (1) CTA, TOF-MRA and pre- and post-contrast T1 IVW performed within 2 weeks of one another and available for review; (2) non-calcified intracranial arterial stenosis on CTA head; and 3) ≥2 vascular risk factors. Exclusion criteria were: (1) Lack of coverage of the target lesions on any of the imaging comparisons; (2) Lack of IVW acquisition in a plane perpendicular to the target lumen; (3) lack of post-contrast IVW sequences; and (4) any clinical or imaging evidence of other non-atherosclerotic vascular diseases (vasculitis, dissection, moyamoya disease, vasospasm, or reversible cerebral vasoconstriction syndrome).
Data abstraction
Electronic medical records were reviewed for patient data, specifically patient age, ethnicity, gender, vascular risk factors (dyslipidemia, hypertension, smoking, diabetes mellitus, age >50 for males and >55 for females, and obesity), and stroke history.
CTA protocol
CTA head/neck scanning protocol for stroke evaluation was performed on the 128-channel Siemens Somatom AS + scanner (Siemens Healthineers; Erlangen, Germany). 100 ml Omnipaque 350 i.v. contrast is injected at 5 ml/s followed by 30 ml normal saline flush injected at 5 ml/s via 18-gauge cannula placed in the antecubital vein. After contrast injection, SmartPrep was used for bolus timing with monitoring at the aortic arch with a 7 s scanning delay. Scanning is performed from the aortic arch through the vertex. Scanning parameters are: 250 mAs, 120 kVp, 0.9 pitch, 0.5 rotation, 128×0.6 mm acquisition with 1 mm reconstructed slice thickness. Multiplanar reconstructions were performed with 1.0 mm thick slices.
Intracranial vessel wall imaging protocol
MRI was performed on a 3T Siemens Trio MRI system (Siemens Healthineers; Erglangen, Germany) with standard head coil. IVW MRI protocol consisted of 3D TOF-MRA, 2D T 2-weighted, 2D T 1-weighted pre- and post-contrast IVW performed in multiple planes, including axial planes and planes perpendicular to the course of the target lumen. For this study, the TOF-MRA and T 1-weighted IVW pre- and post-contrast sequences were used. TOF-MRA parameters are: in-plane resolution, 0.53×0.47 mm; slice thickness, 0.53 mm; repetition time/echo time, 20/3.69 ms; flip angle, 18; field of view, 205×184 mm; time, 5:40 min. T 1-weighted IVW parameters are: in-plane resolution, 0.4 × 0.35 mm; slice thickness, 2 mm; repetition time/echo time, 1000/10 ms; averages, 4; matrix, 448×448 pixels; field of view, 180×158 mm; GRAPPA factor, 2; turbo factor, 18; time, 36 s per slice.
Data analysis
An independent board-certified radiologist rater with 2 years of experience reviewed all CTA studies independent of clinical data or other imaging studies, to determine the location of intracranial arterial stenosis or irregularity, compatible with intracranial atherosclerotic disease. The rater identified and labeled images with stenosis, with the selected images being perpendicular to the plane of the targeted lumen. After the CTA images were selected, CTA, TOF-MRA and T 1-weighted pre- and post-contrast IVW sequences were registered at the selected images using the automated registration tool in RadiAnt Dicom viewer (Medixant Co, Poznan, Poland). Users could manually modify the registration if the automated registration did not align correctly. The selected images in addition to one image on each side-of the selected image were included.
Two separate board-certified radiologist raters with 6 and 8 years of experience, respectively, independently reviewed the selected images for each set (MRA, IVW or CTA) while blinded to the other imaging modalities for the patient and patient clinical information. The raters measured the maximum degree of stenosis for each stenosis selected on each modality. The stenosis measurement was calculated based on the following formula: {1-[D(stenosis)/D(normal)]} × 100 = stenosis. 14 D(stenosis) is the maximal point of stenosis, while D(normal) represents the normal arterial segment proximal to the stenosis, without any branching points between the normal segment and the stenosed segment. If there was no normal segment proximal to the stenosis on the ipsilateral side, the contralateral segment of the same artery was assessed. If none of these normal segment measurements could be made or stenosis involved the basilar artery, a normal segment distal to the stenosis was measured. Imaging modalities were reviewed in a random order and separate from the other imaging performed. All imaging studies were evaluated and reviewed on Radiant Dicom viewer (Medixant; Poznan, Poland). Luminal measurements were averaged between the two raters.
Statistics
All statistical calculations were conducted with the statistical computing language R (v. 2.14.1; R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-tailed tests were used with p < 0.05 denoting statistical significance.
Mean stenosis and standard deviation of each imaging technique were calculated. Agreement between stenosis and lumen diameter measurements by MRA or IVW with CTA was assessed qualitatively using Bland-Altman plots. Agreement was also summarized quantitatively by the mean difference, the SD of differences and the concordance correlation coefficient (CCC). 15 Generalized estimated equations (GEEs) were used to test for bias (mean difference not equal to zero). Sensitivity and specificity for >50% stenosis were estimated for MRA and IVW, using CTA as the reference. Inter-rater agreement was assessed using the CCC. The non-parametric bootstrap was used to compute 95% CIs and to compare the agreement statistics for MRA and IVW measurements. To account for the clustering of lesions, resampling was done at the patient level. 16
Results
Patient demographics
Eighteen total patients with 33 atherosclerotic lesions were included in the study. Of the 18 patients, 13 were imaged within 3 months of stroke, while two additional patients had strokes greater than 6 months after an ischemic event. Three patients had no history of stroke or transient ischemic attack. No patients presented with intracranial hemorrhage. Patients had 2–6 vascular risk factors (mean: 4.1, std. dev. 1.3). The most common vascular risk factors were hypertension (17), dyslipidemia (15), and diabetes mellitus (11). Mean patient age was 53.1 years (std. dev. 10.4). Seven patients were white, 5 Hispanic, 4 Asian, 1 black, and 1 Pacific Islander. There were 6 males and 12 females in the cohort. Ten patients were receiving statin therapy, and nine patients were receiving anti-platelet agents. Only one patient had history of myocardial infarction
Segments of involvement
Arterial lesion distribution was: M1 middle cerebral artery (MCA) (14), supraclinoid internal carotid artery (ICA) (6), A1 anterior cerebral artery (ACA) (4), V4 vertebral artery, basilar artery, P1 posterior cerebral artery (PCA), A2 ACA and P2 PCA (two each), and M2 MCA (1), for a total of 33 stenotic atherosclerotic lesions.
Inter-rater agreement
The CCC between the stenosis measurements made by the 2 raters was 0.84 [95% CI (0.67–0.93)] for CTA, 0.83 [95% CI (0.67–0.93)] for TOF-MRA, and 0.85 [95% CI (0.71–0.94)] for IVW.
Comparison of stenosis measurements
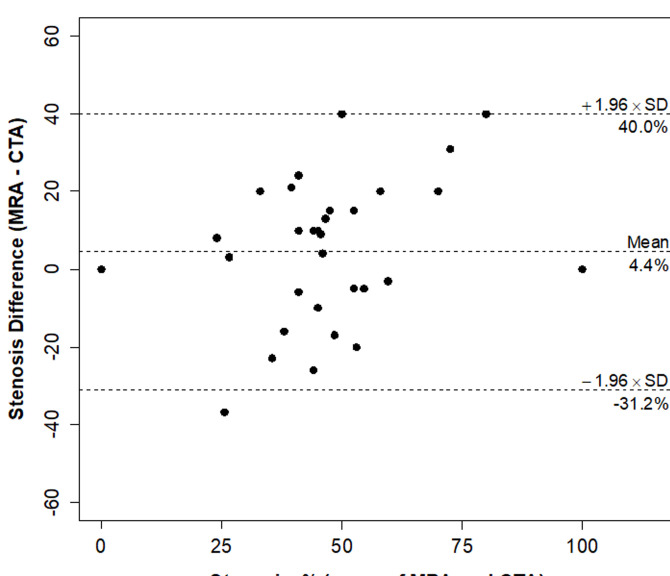
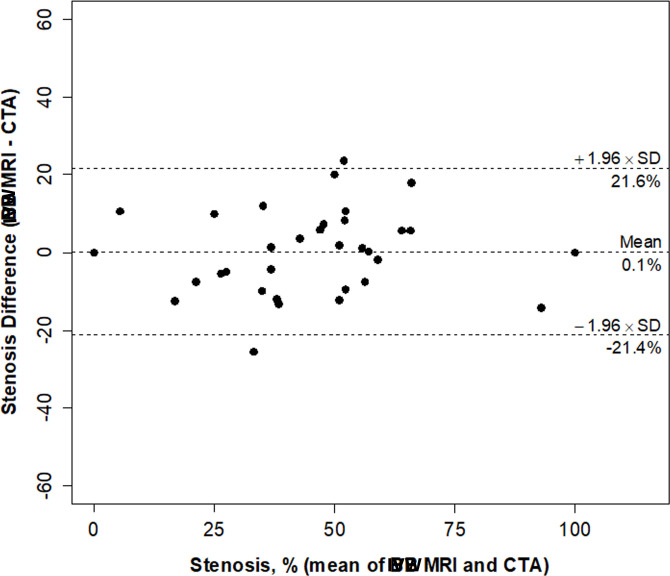
Neither IVW nor MRA had any significant bias relative to CTA for stenosis and lumen diameter measurements (Table 1). IVW stenosis measurements were found to have nearly 40% lower variability (p = 0.004) and a significantly higher CCC (0.87 vs 0.68; p = 0.002) than MRA measurements with respect to the standard used, CTA (Figures 1 and 2, Table 1). Figures 3 and 4 demonstrate the corresponding Bland-Altman plots for MRA-CTA and IVW MRI-CTA stenosis comparisons, respectively, indicating a distinctly superior agreement of IVW with CTA, when compared to MRA. IVW lumen diameter measurements also had significantly higher agreement with CTA than MRA lumen diameter measurement (CCC: 0.64 vs 0.90, p < 0.001; Table 1).
Table 1.
Comparison of stenosis and lumen diameter measurements by MRA and IVW MRI with CTA (reference) ( n = 33 lesions)
| Modality a | Difference | ||||||
|---|---|---|---|---|---|---|---|
| Stenosis | MRI | CTA | Mean | SD | p value b | CCC | (95% CI) |
| MRA, % | 49 ± 25 | 45 ± 21 | 4.4 | 18 | 0.19 | 0.68 | (0.57, 0.76) |
| IVW, % | 45 ± 22 | 45 ± 21 | 0.1 | 11 | 0.94 | 0.87 | (0.81, 0.92) |
| p value c | 0.24 | 0.004 | 0.002 | ||||
| Difference | |||||||
| Lumen Diameter | MRI | CTA | Mean | SD | p value b | CCC | (95% CI) |
| MRA, mm | 1.14 ± 0.65 | 1.05 ± 49 | 0.09 | 0.49 | 0.41 | 0.64 | (0.49, 0.74) |
| IVW, mm | 1.08 ± 0.54 | 1.05 ± 49 | 0.03 | 0.23 | 0.56 | 0.90 | (0.81, 0.96) |
| p value c | 0.45 | <0.001 | – | <0.001 | |||
CCC, Concordance correlation coefficient; CI, Confidence interval; CTA, CT angiography; IVW, Intracranial vessel wall MRI; MRA, MR angiography; SD, Standard deviation.
Values are mean ± SD.
Test of mean difference between MRI and CTA = 0.
Test of difference between MRA and IVW, mean difference, SD of differences, and CCC.
Figure 1.
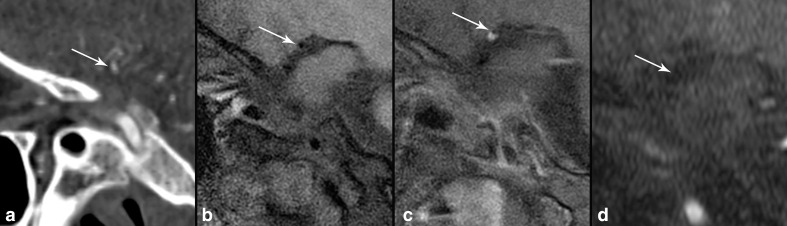
40-year-old female with right MCA territory stroke and infarct (not shown), with enhancing, eccentric atherosclerosis involving the right carotid terminus (Figure 1c, arrow). On sagittal CTA (a), there is narrowing of 60% of the right carotid terminus (arrow). On sagittal T1 IVW (b), the right carotid terminus has 58% narrowing (arrow). Sagittal T1 post-contrast IVW (c) shows an eccentric enhancing lesion with outer wall remodeling (arrow) consistent with atherosclerosis. On sagittal reconstructed 3D TOF MRA (d), there is no evidence of flow-related enhancement in the expected location of the right carotid terminus (arrow) corresponding to 100% stenosis.
Figure 2.
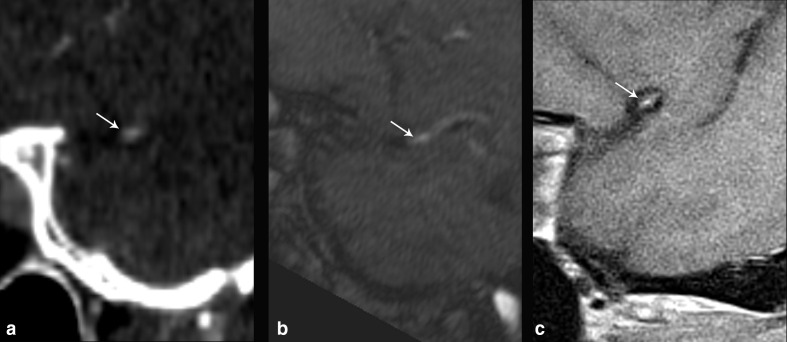
53-year-old male with multiple vascular risk factors presenting with worsening dementia and multiple infarcts on CT of different stages of evolution (not shown), including right MCA territory acute infarct on MRI (not shown). CTA of the head (a) shows narrowing of the distal right M1 MCA, which measured 39% stenosis (arrow). TOF-MRA (b) showed narrowing at the same location (arrow), with 50% stenosis, while sagittal T 1-weighted IVW (c) showed atherosclerotic plaque along the superior wall of the arterial segment which showed partial enhancement (arrow), resulting in 35% stenosis, which more closely approximated the degree of stenosis seen on CTA relative to MRA.
Figure 3.
Bland-Altman plot of stenosis by MRA and CTA in 33 lesions.
Figure 4.
Bland-Altman plot of stenosis by IVW MRI and CTA in 33 lesions.
There were 11 lesions with stenosis >50% on CTA. Sensitivity for >50% stenosis was 64% [7/11, 95% CI (39–83%)] on MRA and 82% [9/11, 95% CI (53–95%)] on IVW. Specificity was the same for both sequences at 73% [16/22, 95% (CI 52–87%)).
Discussion
Conventional luminal imaging techniques are the first-line approach to the evaluation and characterization of neurovascular disease, with TOF-MRA frequently used due to its non-invasive nature, lack of ionizing radiation and iodinated contrast injection. Questions exist about the accuracy of luminal measurements on MRA, however. In the current study, we compared luminal measurements on TOF-MRA and IVW relative to the CTA reference standard and found significantly greater agreement between IVW and CTA compared to MRA and CTA (CCC of 0.87 vs 0.68, p = .002) when evaluating continuous stenosis measurements. IVW had a sensitivity of 82% compared to the MRA value of 64% while both had 73% specificity.
CTA has previously shown strong agreement in luminal measurements relative to DSA. Nguyen-Huynh et al 17 compared CTA and DSA findings in 41 patients and 475 arterial segments. They found the stenosis intra-class correlation coefficient between CTA and DSA to be 0.98. CTA detected large arterial occlusion with 100% sensitivity and specificity and for detection of ≥50% stenosis, CTA had 97.1% sensitivity and 99.5% specificity. This, in addition to the easier access, increased utilization and first-line use of CTA for stenosis evaluation justified its implementation as the reference standard in our study.
While TOF-MRA has the advantage of not exposing patients to i.v. contrast or ionizing radiation, its lower spatial resolution as compared to CTA or DSA limits its utility in smaller arterial segments. Furthermore, as a result of dependence on vascular flow for the maintenance blood signal, high-grade stenosis, turbulent or in-plane flow lead to proton spin-dephasing and downstream flow signal intensity loss. Due to these limitations, TOF-MRA has lost favor as the primary neurovascular imaging modality. The SONIA study 18 found that MRA has modest correlations with DSA and concluded that abnormal findings on MRA require confirmation with DSA.
There are a few published studies evaluating the accuracy of IVW for luminal measurement assessment relative to other luminal imaging modalities. Liu et al 19 compared 2D T 2-weighted black blood imaging with CTA against 2D DSA for the evaluation of MCA steno-occlusive disease in 28 patients. IVW-derived stenosis values correlated better with DSA (Spearman correlation R = 0.68, p = 0.01) than CTA MIP or VR reconstructions (Spearman correlation R = 0.45, 0.22; p = 0.02, 0.24, respectively). In 10 patients with large vessel occlusion, Hui et al 20 compared findings of pre- and post-contrast T1 IVW with 3D-TOF-MRA and 2D DSA. Apart from 10 occluded vessels in which there was full agreement among all imaging methods, IVW showed seven other stenotic vessel segments versus only two demonstrated by MRA and DSA. IVW showed a higher frequency of wall abnormalities (thickening and enhancement) compared with DSA and MRA (p = 0.016 and p = 0.008, respectively). Lee et al 21 compared 2D pre- and post-contrast IVW with 3D DSA in 37 patients, and found moderate-to-excellent agreement (interclass correlation coefficient = 0.892–0.949; Κ = 0.548–0.614) and significant correlations (R = 0.766–892) between IVW and DSA on the degree of stenosis and minimal luminal diameter. The inter-observer diagnostic agreement was good for DSA (Κ = 0.643) and excellent for IVW (Κ = 0.818). Park et al 22 also compared 3D IVW with 2D DSA in 43 patients and found a similar degree of stenosis (p > .05) and higher luminal diameter (p < .05) on IVW compared to DSA. Kim et al 23 evaluated 286 segments in 17 patients with both IVW and MRA, for stenosis and atherosclerosis presence, with IVW as the reference standard for atherosclerosis presence and MRA as the reference standard for luminal stenosis. IVW had a 92.5% sensitivity and 82.1% specificity for stenosis relative to MRA, while MRA had a sensitivity of 59.4% and a specificity of 98.3%, respectively, relative to IVW. This study shows the value of IVW for stenosis measurements, however, the current and other studies demonstrate limitations of MRA in stenosis assessments. Bai et al 24 developed a luminal imaging technique from IVW acquisitions and found this new technique showed significantly higher sensitivity for detection of severe stenosis (89.3% vs 64.3%, p = .039) relative to MRA and comparable to the sensitivity of IVW. The results of the present study are in line with the above-discussed literature suggesting superior correlation of IVW with the reference standard test for the evaluation of steno-occlusive disease; however, this study is the only one to compare stenosis measurements on IVW and MRA relative to the front-line imaging modality for intracranial stenotic disease, CTA.
There are several limitations with the current study, including retrospective study design and limited number of subjects. Studies with larger cohorts and lesion counts, additional readers and evaluating varied luminal techniques are needed to further validate these findings. The study also utilized 2D-IVW techniques, although only lesions involving arteries coursing perpendicular to the plane of imaging were included in order to minimize volume averaging and wall thickness overestimation effects. Further comparisons with additional IVW techniques is necessary to also confirm these findings, as these results may differ with technique differences.
Conclusion
IVW can more accurately assess atherosclerotic luminal measurements relative to TOF-MRA, as compared to CTA. TOF-MRA is frequently performed as part of IVW protocols, for rapid identification of intracranial stenosis; however, these stenoses should be confirmed with IVW, and IVW can be used to quantify more accurately the degree of stenosis.
Contributor Information
Basar Sarikaya, Email: basar@uw.edu.
Charles Colip, Email: charcoli@uw.edu.
William D Hwang, Email: bhwang44@hotmail.com.
Daniel S Hippe, Email: dhippe@uw.edu.
Chengcheng Zhu, Email: zhucheng@uw.edu.
Jie Sun, Email: sunjie@uw.edu.
Niranjan Balu, Email: ninja@uw.edu.
Chun Yuan, Email: cyuan@uw.edu.
Mahmud Mossa-Basha, Email: mmossab@uw.edu.
REFERENCES
- 1. Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke 2009; 40: 1999–2003. doi: 10.1161/STROKEAHA.108.546150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006; 113: 555–63. doi: 10.1161/CIRCULATIONAHA.105.578229 [DOI] [PubMed] [Google Scholar]
- 3. Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: evaluation with CT angiography, Mr angiography, and digital subtraction angiography. AJNR Am J Neuroradiol 2005; 26: 1012–21. [PMC free article] [PubMed] [Google Scholar]
- 4. van den Wijngaard IR, Holswilder G, van Walderveen MAA, Algra A, Wermer MJH, Zaidat OO, et al. Treatment and imaging of intracranial atherosclerotic stenosis: current perspectives and future directions. Brain Behav 2016; 6: e00536. doi: 10.1002/brb3.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mossa-Basha M, Watase H, Sun J, Shibata DK, Hippe DS, Balu N, et al. Inter-rater and scan-rescan reproducibility of the detection of intracranial atherosclerosis on contrast-enhanced 3D vessel wall MRI. Br J Radiol 2019; 92: 20180973. doi: 10.1259/bjr.20180973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schaafsma JD, Rawal S, Coutinho JM, Rasheedi J, Mikulis DJ, Jaigobin C, et al. Diagnostic impact of intracranial vessel wall MRI in 205 patients with ischemic stroke or TIA. AJNR Am J Neuroradiol 2019; 40: 1701–6. doi: 10.3174/ajnr.A6202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Havenon A, Yuan C, Tirschwell D, Hatsukami T, Anzai Y, Becker K, et al. Nonstenotic culprit plaque: the utility of high-resolution vessel wall MRI of intracranial vessels after ischemic stroke. Case Rep Radiol 2015; 2015: 1–4. doi: 10.1155/2015/356582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke 2015; 46: 1567–73. doi: 10.1161/STROKEAHA.115.009037 [DOI] [PubMed] [Google Scholar]
- 9. Edjlali M, Guédon A, Ben Hassen W, Boulouis G, Benzakoun J, Rodriguez-Régent C, et al. Circumferential thick enhancement at vessel wall MRI has high specificity for intracranial aneurysm instability. Radiology 2018; 289: 181–7. doi: 10.1148/radiol.2018172879 [DOI] [PubMed] [Google Scholar]
- 10. Hartman JB, Watase H, Sun J, Hippe DS, Kim L, Levitt M, et al. Intracranial aneurysms at higher clinical risk for rupture demonstrate increased wall enhancement and thinning on multicontrast 3D vessel wall MRI. Br J Radiol 2019; 92: 20180950. doi: 10.1259/bjr.20180950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiao Y, Guallar E, Suri FK, Liu L, Zhang Y, Anwar Z, et al. Mr imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016; 280: 860–8. doi: 10.1148/radiol.2016151124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mossa-Basha M, de Havenon A, Becker KJ, Hallam DK, Levitt MR, Cohen WA, et al. Added value of vessel wall magnetic resonance imaging in the differentiation of moyamoya vasculopathies in a non-Asian cohort. Stroke 2016; 47: 1782–8. doi: 10.1161/STROKEAHA.116.013320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mossa-Basha M, Shibata DK, Hallam DK, de Havenon A, Hippe DS, Becker KJ, et al. Added value of vessel wall magnetic resonance imaging for differentiation of Nonocclusive intracranial vasculopathies. Stroke 2017; 48: 3026–33. doi: 10.1161/STROKEAHA.117.018227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989; 45: 255–68. doi: 10.2307/2532051 [DOI] [PubMed] [Google Scholar]
- 16. Davidson DH AC. Bootstrap methods and their application. Cambridge, UK: University of Cambridge; 1997. [Google Scholar]
- 17. Nguyen-Huynh MN, Wintermark M, English J, Lam J, Vittinghoff E, Smith WS, et al. How accurate is CT angiography in evaluating intracranial atherosclerotic disease? Stroke 2008; 39: 1184–8. doi: 10.1161/STROKEAHA.107.502906 [DOI] [PubMed] [Google Scholar]
- 18. Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The stroke outcomes and neuroimaging of intracranial atherosclerosis (SONIA) trial. Neurology 2007; 68: 2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1 [DOI] [PubMed] [Google Scholar]
- 19. Liu Q, Huang J, Degnan AJ, Chen S, Gillard JH, Teng Z, et al. Comparison of high-resolution MRI with CT angiography and digital subtraction angiography for the evaluation of middle cerebral artery atherosclerotic steno-occlusive disease. Int J Cardiovasc Imaging 2013; 29: 1491–8. doi: 10.1007/s10554-013-0237-3 [DOI] [PubMed] [Google Scholar]
- 20. Hui FK, Zhu X, Jones SE, Uchino K, Bullen JA, Hussain MS, et al. Early experience in high-resolution MRI for large vessel occlusions. J Neurointerv Surg 2015; 7: 509–16. doi: 10.1136/neurintsurg-2014-011142 [DOI] [PubMed] [Google Scholar]
- 21. Lee NJ, Chung MS, Jung SC, Kim HS, Choi C-G, Kim SJ, et al. Comparison of high-resolution MR imaging and digital subtraction angiography for the characterization and diagnosis of intracranial artery disease. AJNR Am J Neuroradiol 2016; 37: 2245–50. doi: 10.3174/ajnr.A4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JE, Jung SC, Lee SH, Jeon JY, Lee JY, Kim HS, et al. Comparison of 3D magnetic resonance imaging and digital subtraction angiography for intracranial artery stenosis. Eur Radiol 2017; 27: 4737–46. doi: 10.1007/s00330-017-4860-6 [DOI] [PubMed] [Google Scholar]
- 23. Kim DK, Verdoorn JT, Gunderson TM, Huston Iii J, Brinjikji W, Lanzino G, et al. Comparison of non-contrast vessel wall imaging and 3-D time-of-flight MRA for atherosclerotic stenosis and plaque characterization within intracranial arteries. J Neuroradiol 2020; 47: 266–71. doi: 10.1016/j.neurad.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 24. Bai X, Lv P, Liu K, Li Q, Ding J, Qu J, et al. 3D Black-Blood luminal angiography derived from high-resolution Mr vessel wall imaging in detecting MCA stenosis: a preliminary study. AJNR Am J Neuroradiol 2018; 39: 1827–32. doi: 10.3174/ajnr.A5770 [DOI] [PMC free article] [PubMed] [Google Scholar]