Abstract
Interventional radiology (IR) provides minimally invasive therapeutic and palliative options for the treatment of pancreatic cancer depending on the stage of the disease. IR plays a critical, and also a very effective role, in both pre- and post-operative care of the patients with early stage resectable disease and also in palliative treatment of the patients with locally advanced or metastatic disease. In this article, we aimed to present the capability and the limitations of IR procedures including: local treatment options of primary and metastatic pancreatic cancer, palliation of biliary and intestinal obstructions, minimally invasive treatment of post-operative complications, and pain management.
Introduction
Pancreatic adenocarcinoma (PA) accounts for 85% of all pancreatic cancers (PC) and is the fourth most common cause of cancer-related deaths. 1,2 PAs have a very poor prognosis with a 5 year survival rate of 5%. This poor prognosis is mostly related to aggressive biological nature and early local invasiveness of the tumor.
Surgical resection offers the only potential chance for cure; however, only 15–20% have resectable disease at the time of diagnosis. 3 Even patients who are surgical candidates, the overall 5 year survival rates are less than 20%. In addition, surgery-related morbidity rates range from 30 to 50%, often with life-threatening complications. 4 Palliative treatments may be required for locally advanced and metastatic disease. 3,5
Interventional Radiology (IR) plays a major role in treatment and palliation of PCs. Percutaneous procedures offer highly effective alternatives to other invasive techniques in this patient group from initial diagnosis to treatment and palliation in a safe and minimally invasive manner (Table 1). The purpose of this article is to provide a comprehensive overview of the role of IR in PC.
Table 1.
The role of interventional radiology in patients with pancreatic cancer
| Percutaneous biopsy | ||
| Management of post-operative complications | Drainage of fluid collections | |
| Percutaneous biliary drainage | ||
| Post-pancreatectomy hemorrhage | ||
| Palliative use of Interventional Radiology | Percutaneous biliary interventions | Percutaneous transhepatic biliary drainage |
| Biliary stent placement | ||
| Intraductal radiofrequency ablation | ||
| Management of the complications of biliary interventions | ||
| Palliation of malignant gastroduodenal obstruction | Duodenal stenting | |
| Pain management/Celiac ganglia blockage/Neurolysis | ||
| Locoregional therapies | Irreversible electroporation | |
| Thermal ablation | Radiofrequency ablation | |
| Microwave ablation | ||
| Cryoablation | ||
| Treatment of metastatic disease (Liver) | Percutaneous thermal ablation | |
| Radioembolization | ||
Percutaneous biopsy
Histopathological confirmation may not be necessary in patients with typical imaging findings of PCs who are amenable to potentially curative surgery. Patients with inoperable locally invasive tumors or distant organ metastases may benefit from percutaneous biopsy to confirm the diagnosis and help guide treatment planning. 6 In these situations, percutaneous or endoscopic image guided biopsy of pancreatic lesions may be highly effective and low risk in experienced hands. Additionally, as the liver is the most common site of metastasis and disease recurrence in patients with PC, percutaneous tru-cut biopsy from a more easily accessible distant metastasis (such as liver, peritoneum, etc.) should be preferred for histopathological confirmation. 7,8
CT and ultrasonography may both be used for imaging guidance in percutaneous biopsy. Although ultrasonography is generally the preferred modality for imaging guidance (Figure 1), CT guidance might be needed due to superior anatomic resolution compared to ultrasonography, particularly in patients with high body mass index. 6 Whenever possible, needle passage through other organs or healthy pancreatic parenchyma should be avoided. At times, it may be necessary to traverse stomach or small bowel, but colonic segments should be avoided. 9
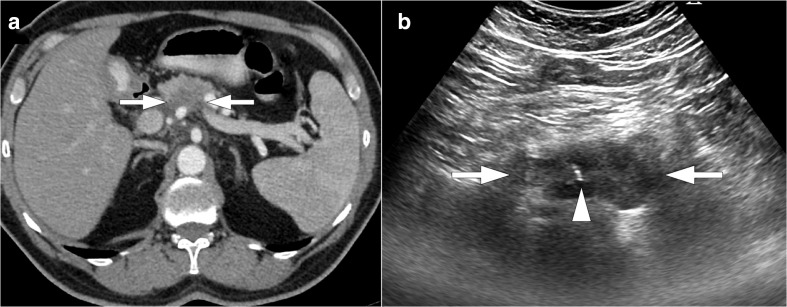
Figure 1.
54-year-old male with no known past medical history presented with recent onset epigastric pain and weight loss. (a) Axial plane post-contrast CT image shows hypodense pancreatic mass lesion (arrows) with encasement of SMA. (b) Percutaneous ultrasound-guided FNAB (arrowhead) confirmed pancreatic adenocarcinoma. FNAB, fine-needle aspiration biopsy; SMA, superior mesenteric artery.
Several parameters may affect the selection of the biopsy needle including lesion accessibility, vascularity and the amount of tissue required. 9 Coaxial systems are preferred to prevent tumor seeding along the biopsy tract. Fine-needle aspiration biopsy (FNAB) with 20–22 Gauge (G) needles may provide sufficient diagnostic material in solid tumors which may obviate the need for a tru-cut biopsy. 10 Tru-cut biopsies may cause local pancreatitis when normal parenchyma is included. In general, the increased needle caliber elevates the risk for potential complications. Therefore, tru-cut needles larger than 20G caliber should be avoided but small caliber (20G) tru-cut needles may be used with low complication rates if a larger tissue sample is required for histopathological analysis 6,9,10 (Figure 2).
Figure 2.
65-year-old female with no significant past medical history presented with epigastric pain and weight loss. (a) Axial plane post-contrast CT image shows hypodense mass lesion (arrows) located in the pancreatic tail. (b) CT-guided tru-cut biopsy was performed with 18G coaxial needle (arrow). Histopathologic examination confirmed pancreatic adenocarcinoma.
No significant difference was reported between endoscopic and percutaneous approaches in the diagnosis of PC, and both methods have similar low short-term complication rates (0%–4.9%). FNAB has similar diagnostic accuracy with tru-cut biopsy in pancreatic masses (91–99.4% and 86–93%, respectively), but has a lower risk of complications (0–4,9% vs 3.3–21%). 9,10
Acute pancreatitis is the most common major complication after pancreatic biopsy. Other complications of bleeding, sepsis, cholangitis, fistula formation and tumor seeding may also occur but are rare. 9
Management of post-operative complications
The most frequent complications after pancreatic surgery are pancreatic fistula, post-pancreatectomy hemorrhage (PPH), delayed gastric emptying, intra-abdominal collections and bile leak in the early post-operative period. 11 IR has an increasingly important role in the perioperative management of these early complications. 12,13 Percutaneous interventions may be required in up to 12% of the patients after pancreatic surgery. IR techniques for post-operative complications may obviate the need for surgery in up to 85–90% of patients. 12
Drainage of fluid collections
Anastomotic leaks are among the most common complications after pancreaticoduodenal surgery. Significant percentage of these leakage-related collections spontaneously regress; however, some collections may require drainage. 13 These persistent collections in the surgical bed can be effectively managed with percutaneous drainage under ultrasound or CT guidance (Figure 3). Large-bore catheters with multiple side holes are preferred for highly viscous collections, whereas small-bore catheters may effectively drain non-complicated serous collections. Sepsis, related to infection, typically regresses in 24–48 h after the drainage. The analysis of the drained fluid may also provide clues to the site of leakage, with high amylase levels typically seen in collections due to pancreaticoduodenal leakage and pancreatic fistula. Long-term drainage with periodic catheter exchanges may be needed in most patients. Endoscopic interventions may be used in conjunction with percutaneous procedures in select patients. 4,14
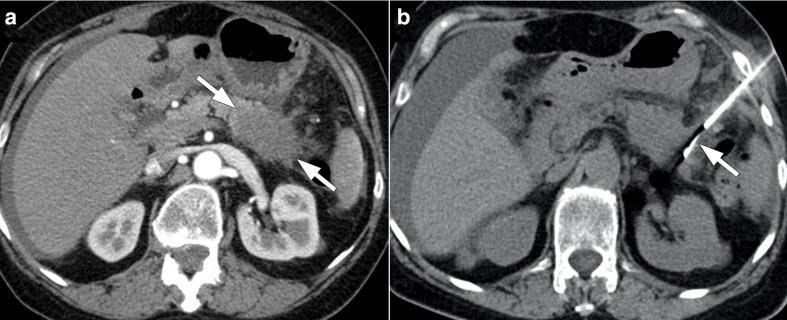
Figure 3.
56-year-old male with pancreatic adenocarcinoma underwent Whipple surgery. The patient presented with high fever and leukocytosis 7 days after the surgery. (a) Axial plane post-contrast CT image shows a large collection (arrows) within the surgical bed. Percutaneous CT-guided drainage was performed (not shown). (b) Follow-up CT image showed almost complete resolution of the collection.
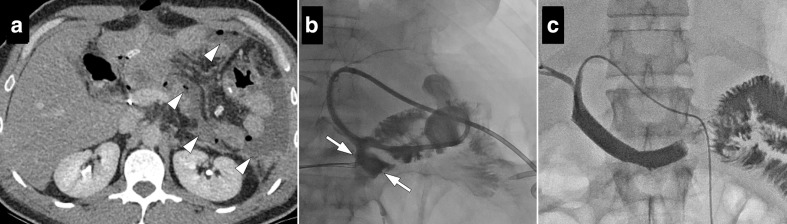
Post-operative abscesses may also develop after Whipple surgery either in the surgical bed or far from the surgical bed (e.g. pelvis) due to the extensive surgical procedure comprising multiple organs. Percutaneous drainage may be performed under ultrasound or CT guidance. A transhepatic approach may be needed due to the deep location of the abscesses in the surgical bed 15,16 (Figure 4). Amylase and bilirubin levels should be analyzed within the abscess sample to evaluate the possible communication with pancreatic duct and biliary leakage.
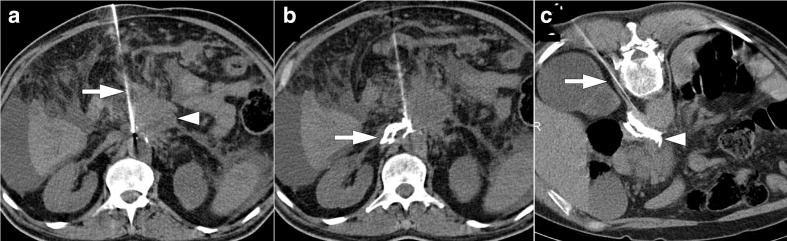
Figure 4.
56-year-old male with pancreatic adenocarcinoma underwent Whipple surgery. The patient had fever and leukocytosis on post-operative third day. (a) Axial postcontrast CT image shows a collection (arrows) within the surgical bed. (b) Percutaneous ultrasound-guided transhepatic (arrowhead) drainage was performed. (c) Follow-up CT image acquired 10 days after the initial study demonstrated complete resolution of the collection.
Daily catheter output and the patient’s clinical response should be monitored. The drainage catheter may be removed when the output drops below 10–20 ml per day with the clinical improvement of the patient. 17
Percutaneous biliary drainage
Biliary leakage is another important complication after hepatopancreatobiliary surgery that is characterized by elevated levels of bilirubin in the drainage fluid. 18
Ultrasound and CT are commonly used for detection of these collections. Peritoneal thickening and enhancement on contrast-enhanced CT may indicate biliary peritonitis. Magnetic resonance cholangiopancreatography with hepatobiliary specific agents may pinpoint the site of leakage (Figure 5).
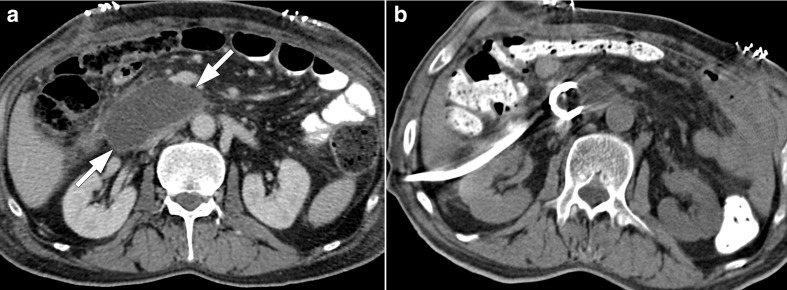
Figure 5.
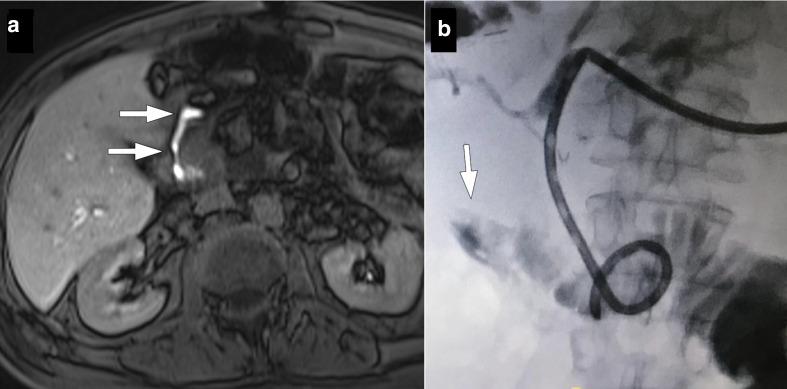
57-year-old male recently underwent Whipple surgery for pancreatic cancer now presented with fever, elevated CRP levels and bilious discharge from surgical drains 7 days after the surgery. (a) Axial plane T 1W late phase MRI after gadoxetic acid injection shows contrast extravasation at the choledochojejunostomy site (arrows). Percutaneous diversionary biliary drainage was performed and an internal–external biliary drainage catheter was placed. (b) Fluoroscopic image again confirmed contrast extravasation through the anastomosis site (arrow).
For patients with biliary leakage, optimal treatment involves both diversionary percutaneous biliary drainage and drainage of abdominal collection for effective management. 13 Technical difficulties are common in these patients as non-dilated intrahepatic bile ducts may pose significant challenges for percutaneous access. With non-dilated biliary systems, the technical success rates are 65–75% as compared to success rates of more than 95% in the dilated system. 19 Several attempts may be needed for successful biliary drainage in these patients. For facilitating access, the fine needle may be withdrawn and simultaneous injection of contrast to opacify the bile ducts. Once a bile duct is accessed and opacified, a microwire may be sent through the needle to secure percutaneous access. Biliary anatomy and the leakage site may easily be demonstrated with a cholangiogram. If an anastomotic leakage is observed, an internal–external biliary drainage catheter can be placed through hepaticoenterostomy to divert the bile flow from the site of leakage in order to allow healing while preserving the anastomosis (Figure 6). An external drainage catheter may also be placed above the leakage site in patients with suspected intestinal leakage. Follow-up injections through this catheter are helpful to monitor the patency of the anastomosis site and may be withdrawn after confirmation of healing of the site of leakage. 4,20
Figure 6.
34-year-old male with recently diagnosed pancreatic adenocarcinoma located in the pancreatic head underwent pancreaticoduodenectomy. The patient presented with abdominal distention and fever 5 days after the surgery. Also noted was bilious fluid drainage from the surgical drain. (a) Axial plane post-contrast CT image shows a collection in the surgical bed extending to the left side wall of the abdomen (arrowheads). Subsequent percutaneous cholangiography and drainage were performed. (b) Cholangiography demonstrated anastomotic leakage at the choledocojejunostomy site (arrows). On 2 weeks of follow-up after external drainage, the drainage continued but the amount of bilious fluid coming from the surgical drain dropped significantly. (c) A covered biliary stent implanted and cholangiography showed no biliary leakage.
Post-pancreatectomy hemorrhage
Post-pancreatectomy hemorrhage (PPH) is one of the most severe and dreaded complications of pancreaticoduodenectomy. PPH occurs in less than 10% of the patients but accounts for 11–38% of the mortality related to surgery. 21 It is an independent major risk factor for mortality and re-operation due to bleeding significantly increases the risk of mortality even higher (ranging from 29 to 58%). 22
In 2007, the International Study Group of Pancreatic Surgery has clinically graded PPH on the basis of onset, location, and severity (Table 2). Early PPHs are often caused by leakage from gastroduodenal artery (GDA) stump related to technical failure and may require additional surgery to maintain homeostasis. Late PPHs may be associated with vascular erosion from pancreatic leak, fistula, pseudoaneurysm, or anastomotic dehiscence. 11
Table 2.
Grading of post-pancreatectomy hemorrhage (International Study Group of Pancreatic Surgery)
| Time of onset | Early | ≤24 h after operation |
| Late | >24 h after operation | |
| Location | Intraluminal | Gastrointestinal bleeding representing as hematemesis, melena or bleeding from nasogastric tube |
| Extraluminal | Intraperitoneal or bleeding from surgical drains | |
| Severity | Mild | The level of hemoglobin drop is ≤ 3 g dl−1 |
| Severe | The level of hemoglobin drop is > 3 g dl−1 |
Multidisciplinary approach is crucial for effective and early management of PPH. The clinical condition of the patient is the main parameter for decision-making process. Endovascular treatment is the preferred option with lower mortality rates and prevents an already fragile patient from the stress of repeat surgery. 22
Late PPHs may sometimes stop spontaneously, however a major bleeding episode may follow this early bleeding (i.e. sentinel bleeding). The late major bleeding episode happens in 76% of the cases within 14–85 h from the initial episode. 4 Multiphase contrast-enhanced CT angiography is the imaging modality of choice for early detection. Emergent laparotomy may be indicated in patients with severe hemodynamic instability. Transarterial interventions may also be helpful in relatively more stable patients 22 (Figure 7).
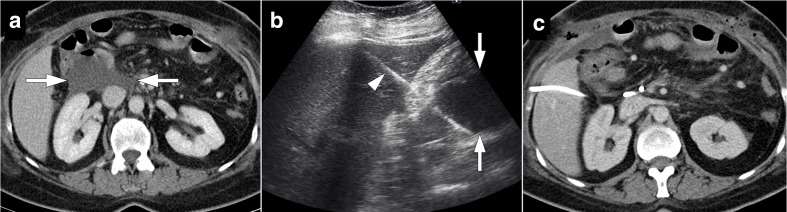
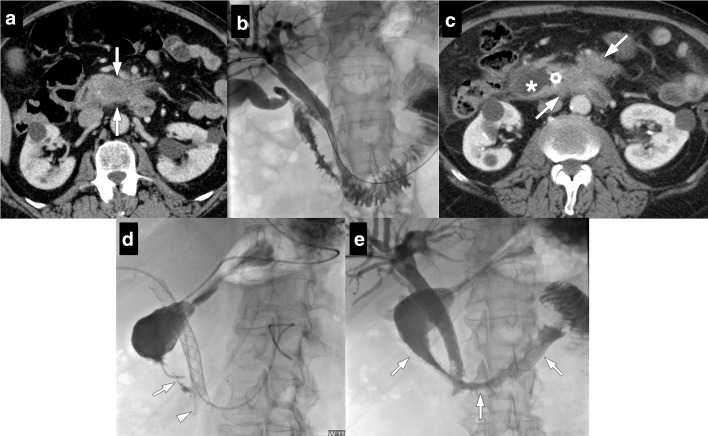
Figure 7.
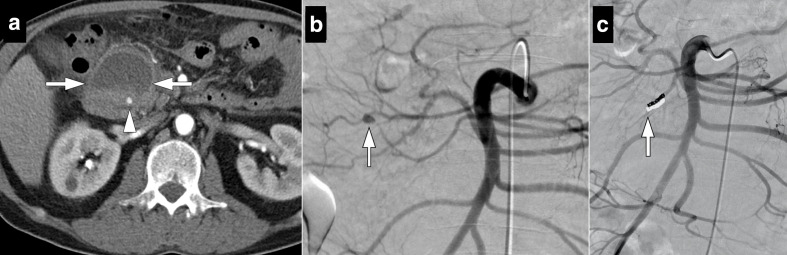
54-year-old male who underwent Whipple surgery for pancreatic adenocarcinoma presented with acute onset abdominal pain and hypotension 3 days after surgery. (a) Axial post-contrast CT image shows heterogeneous solid appearing lesion with peripheral fat stranding. Findings were considered to represent surgical site hematoma (arrows). Also note is made of a small contrast filling nodular structure within the hematoma (arrowhead), consistent with a pseudoaneurysm. (b) Subsequent emergent catheter angiography confirmed the pseudoaneursym (arrow) originating from inferior pancreaticoduodenal artery stump (c). This pseudoaneurysm was successfully coil embolized (arrow).
Glue or endovascular coils may be used to embolize the GDA stump. In certain patients, covered stents may be utilized to exclude the stump from systemic circulation when there is not enough distance between the vessel ostium and the extravasation site. 2
Palliative use of interventional radiology
The great majority of the PC patients are inoperable at the time of diagnosis, with slim chance for long-term survival. For these patients, IR plays a fundamental role in palliation. 2,4
Percutaneous biliary interventions
Percutaneous transhepatic biliary drainage
Percutaneous stenting of the common bile duct is among the most common procedures for palliative purposes in patients with inoperable pancreaticobiliary tumors. The initial palliative diversionary biliary drainage is performed either endoscopically or percutaneously. Percutaneous transhepatic biliary drainage (PTBD) is selected typically for segmental intrahepatic biliary obstructions and in patients with post-operative biliary-enteric anastomosis in which endoscopic approach is technically not feasible. 23
The presence of biliary obstruction is indicative of poor prognosis and associated jaundice and treatment resistant pruritus markedly reduce the overall quality of life. In addition, high serum bilirubin levels may preclude surgery or chemotherapy. In these patients, local intra-arterial treatment options for liver metastases are also limited. Therefore, reduction of serum bilirubin levels is of critical importance for treatment planning. 4
The site of biliary obstruction in patients with pancreatic carcinomas are typically below the confluence of the right and left hepatic bile ducts. Therefore, single drainage catheter is typically adequate for sufficient biliary drainage. The drainage catheter may be advanced either via the right lobe (subcostal or intercostal) or the left lobe (sub xiphoid approach). Drainage may be more effective via the right lobe as it provides prompt decompression due to the larger portion of the liver. 4,24
Initial external drainage catheters should be performed to acutely decompress the biliary system and attempts to cross the stenotic segment should be avoided in patients with ongoing cholangitis and/or massive biliary dilatation to prevent bacteremia and potential sepsis. Internalization may be attempted after 3–7 days of decompression.
Eventual internalization of the drainage catheters is mandatory and every effort should be spent to achieve this goal. Internalization provides enterohepatic circulation of the bile and prevents the loss of bile salts (and subsequent malabsorption) and dehydration. 23,24
Biliary stent placement
Biliary stent placement in inoperable PC patients provides more physiologic flow of bile and may significantly improve the patients’ quality of life. Metallic stents are generally preferred for this purpose due to their higher long-term patency and requirement for less re-intervention as compared to plastic stents, which are more likely to occlude or migrate and require periodic exchanges. 25 Ideally, the time of stent patency should be longer than the expected lifetime of the patient to lower the clinical need for repetitive procedures. The median survival of patients with inoperable PC is between 6 and 11 months and this period drops down to 2 and 6 months in patients with metastatic disease. 23 The average reported patency of metallic stents ranges from 43 to 81% at 6 months, 26 and based on these data, metallic stents should provide patency for most of the patients with inoperable PC.
Decompression of the biliary system with biliary drainage is the generally preferred approach before metallic stent placement. By doing this, the stent may be more precisely deployed through the obstructed segment with less risk for migration. The occluded segment should be covered completely with the stent with its free margins exceeding beyond the obstructing lesion in both proximal and distal ends, to prevent occlusion by tumor overgrowth 4 (Figure 8). Balloon dilatation may be preferred to better embed the stent into the duct wall but some operators may not do this based on local preferences.
Figure 8.
68-year-old female with known unresectable pancreatic adenocarcinoma located within the pancreatic head presented with elevated bilirubin levels and medically unresponsive pruritus. (a) Axial plane postcontrast CT image shows PC (arrows) encircling and obliterating the distal common bile duct. (b) MRCP image shows dilation of the proximal intra- and extrahepatic bile ducts (arrowheads). (c) Percutaneous biliary drainage was performed through right liver lobe and the occlusion caused by the mass (arrow) and a bare metal stent was implanted (d) Follow-up cholangiography from the safety catheter showed satisfactory flow through the stent. The safety catheter was removed at the same session. MRCP, magnetic resonance cholangiopancreatography; PC, prostate cancer;
Tumor or epithelial overgrowth may occlude metal stents in some patients. In order to prevent this, potential complication covered metal stents (CMS) were developed. 23 The patency rates of CMS were reported to be higher by Krokidis et al 27 in a randomized prospective trial. In their trial, the reported patency rates at 6 and 12 months were 92.2 and 87.6% with CMS as compared to 69.8 and 69.8% for bare metal stents. Similar observations were also made by Isayama et al 28 in a different randomized prospective study.
Restenosis is the main problem that limits the survival benefit after stent placement in patients with malignant biliary obstruction. Percutaneous intraductal RFA may be utilized before stent placement to delay tumor growth, prolong stent patency and improve overall survival. It provides local thermal tumor destruction and reduces tumor burden inside the biliary tract. 29 Furthermore, intraductal RFA may also be used in cases of obstruction of the previously deployed metal stent. It can clear the occlusion and restore biliary flow without the need for a new stent insertion inside the obstructed stent. 30
Despite their advantage in terms of patency, the main limitation of CMS is the increased risk of stent migration and tumor overgrowth. The risks of cholecystitis and acute pancreatitis were also reported to increase in these patients. 2,23
Complications of biliary interventions
Major procedure related complications may be at a rate of 4–7%, even in experienced hands. Sepsis, hemorrhage, abscess formation, peritonitis, pleural effusion/empyema and acute pancreatitis are among the most common complications. Pre-existing cholangitis or patients who underwent ERCP prior to stenting are more prone to develop sepsis. 19,23,24
Hemobilia is among the serious complications, and commonly occurs when the drainage catheter intersects a portal vein branch. This may cause subsequent catheter or stent obstruction after clot formation within the lumen. 31 Repositioning or upsizing of the catheter may provide relief. The drainage of bright red blood from the catheter in a patient who is hemodynamically unstable should raise suspicion for bleeding from an arterial origin. CT angiography is often helpful to identify the presence of a pseudoaneurysm or extravasation and can provide effective guidance for an angiographic procedure. 2,4 The most common origin is typically an intraparenchymal branch of the hepatic artery and this complication may be seen in 2.2% of drainage procedures. 2 The detection of the bleeding site may be difficult to visualize with catheter angiography because of the tamponade effect of the drainage catheter and controlled removal of the catheter over a guidewire followed by selective angiography may be necessary to precisely locate the bleeding source.
Palliation of malignant gastroduodenal obstruction
Malignant gastroduodenal obstruction may be seen in advanced PCs. Abdominal pain, nausea, vomiting and malnutrition with associated weight loss are typical features. Surgical palliation is often not considered due to associated high morbidity and mortality. Duodenal stenting may be done via transoral, transgastric or transhepatic (afferent loop obstruction) approaches under fluoroscopic guidance. A combined procedure (simultaneous metallic biliary and duodenal stenting) may also be performed 32 (Figure 9). Technical and clinical success rates are high (92–100% and 76–94%, respectively). Immediate major complications and mortality related to procedure are highly unusual, but recurrent obstruction due to tumor ingrowth and stent migration may happen in 2–4% of the patients which may necessitate repeat intervention. 33
Figure 9.
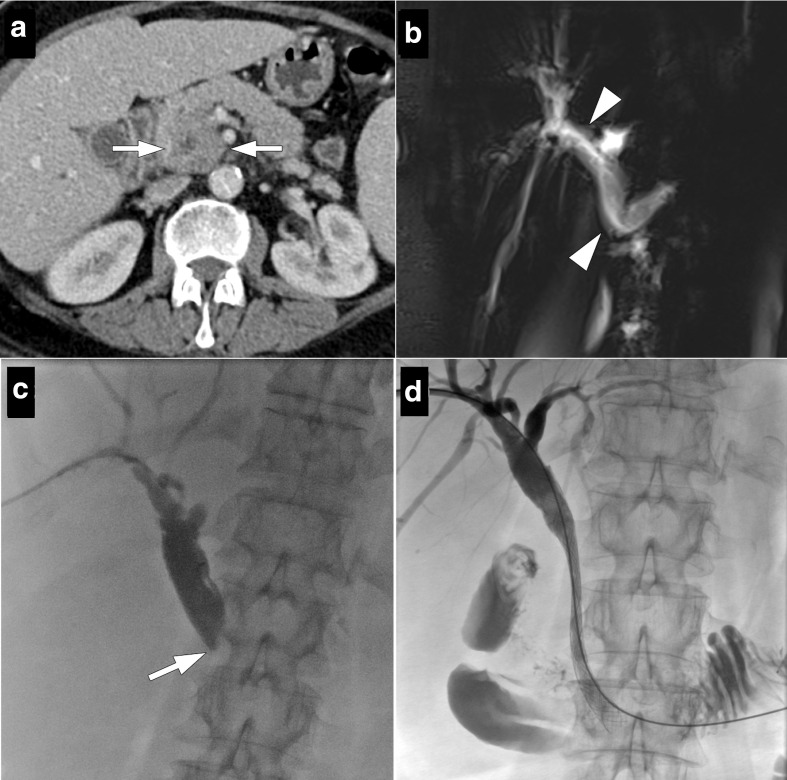
71-year-old male with no significant past medical history presented with jaundice. (a) Axial plane post-contrast CT image shows a hypoechoic mass lesion (arrows) in the uncinate process of pancreas infiltrating SMA and duodenal wall. (b) Percutaneous biliary drainage and subsequent stenting were performed. Four months after the procedure the patient presented with nausea, vomiting and jaundice. (c) Axial plane post-contrast CT image shows biliary dilatation with progression of the tumor (arrows) with further infiltration of duodenum and mesentery. Proximal duodenum was dilated (asterisk). Percutaneous biliary drainage was performed. (d) Biliary stent was found to be occluded due to tumor ingrowth and an additional metal stent (arrowhead) was implanted with a stent-in-stent placement method. Also, an angiographic catheter was placed into duodenum lumen via nasogastric path and severe duodenal stenosis was demonstrated (arrow). (e) A metal stent was also implanted into stenotic duodenal segment in the same procedure (arrows). Follow-up cholangiography showed satisfactory flow through the stents. SMA, superior mesenteric artery.
Pain management/Celiac ganglia blockage/Neurolysis
Abdominal pain is a very common symptom and 70–80% of PC patients report significant pain during the course of the disease. Although pain is multifactorial, it appears like perineural invasion by the local invasive tumor is the main contributor. Systemic treatment with opioid analgesics is generally first approach to alleviate the pain. 34,35
Celiac plexus neurolysis (CPN) is typically reserved for patients who are unresponsive to systemic analgesic treatment. 34 Celiac plexus (CP) is localized around the origin of the celiac trunk and extends to the anterolateral surface of the abdominal aorta. Permanent destruction of this structure may provide significant pain relief in patients with locally advanced PCs.
The celiac artery (CA) may serve as an effective anatomic landmark for localizing the CP as it is typically located immediately caudal to the origin of CA. CP appears as a linear soft tissue structure wrapping around the anterolateral surface of the aorta.
CPN is most commonly performed with absolute alcohol injection through a fine needle after confirmation of its correct localization under CT guidance (Figure 10). The patient is typically placed in prone position and two needles are placed bilaterally at each sites of the abdominal aorta where the CP is localized. Before final injection of alcohol diluted contrast may be injected for confirmation of correct positioning of the needles. For the success of the procedure, alcohol should diffuse along the anterolateral wall of the aorta. In patients who have large tumors alcohol may be directly injected into the mass itself in addition to celiac ganglion injections. 36,37
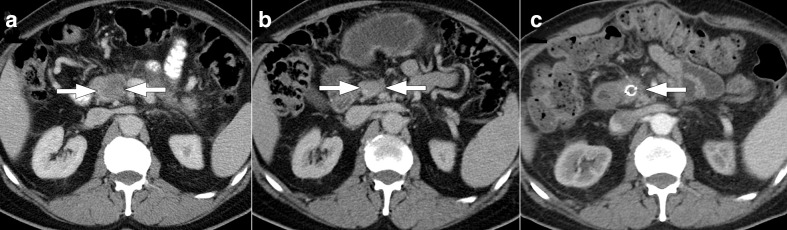
Figure 10.
56-year-old female with surgically unresectable pancreatic adenocarcinoma presented with intractable abdominal pain which was unresponsive to maximum pain palliation. The patient was planned for CT-guided celiac plexus neurolysis. (a) Axial non-enhanced CT image demonstrates pancreatic mass lesion (arrowhead) and the anterior approach to celiac plexus with 20G needle (arrow) in the supine position. (b) CT image acquired after contrast material injection showed the optimal location of the needle (arrow) before alcohol injection. (c) Axial non-enhanced CT image obtained from another patient demonstrates placement of needle (arrow) with posterior paravertebral approach.
Anterior approach may be advantageous in patients who cannot tolerate lying in a prone position or in the presence of ostomies. In such cases, patient in supine position may also be used. 36
The efficacy of CPN is basically determined by the invasion grade of CP which is classified by Akhan et al into four grades considering the tumoral invasion of paraaortic and paracaval fat planes on CT. These fatty tissues are almost completely preserved in Grade 1 invasion. Most (>50%) of them are preserved in Grade 2 (with some infiltrated areas), on the contrary most (>50%) of them are infiltrated in Grade 3 (with minimally preserved fat planes). Finally, the fat planes are always almost completely infiltrated in Grade 4 invasion. 38,39 A significant relationship was defined between this level of classification and pain relief after celiac blockage. The effectiveness of the CPN decreases as the grade of CP invasion increases. 40 The effectiveness of CPN (pain relief) may be determined in a subjective way based on baseline pain scores (0 = no pain, 10 = worst pain) obtained from patients or in an objective way based on the alteration of daily analgesic doses.
The success rate of the CPN has been reported to be between 70 and 100% when coupled with other treatment for analgesia. 37,38,41 With CPN, the dose of opioid used for pain relief may significantly be reduced with highly satisfactory pain control. Complications related to procedure are mostly minor and transient and can involve hypotension or diarrhea and tend to regress in a few days after the procedure. 37
Locoregional therapies
Irreversible electroporation
As stated above, most patients with PC have locally advanced disease at the time of diagnosis and only 15–20% are amenable to surgical resection. Locally advanced pancreatic adenocarcinoma (LAPC) may be non-metastatic but even at this locally unresectable stage the overall survival is around 12 months. 42 Despite chemoradiotherapy, less than 40% of LAPC patients may achieve adequate tumor regression for curative surgical attempt. 2 Irreversible electroporation (IRE) appears to be a promising treatment method for LAPCs, which still remain unresectable even after neoadjuvant chemo (radio) therapy.
IRE is based on the application of pulsatile and targeted high-voltage electric energy to disrupt tumor cell membranes. 43 The electrical pulses alter the current potential of the cellular membrane, and subsequently create multiple holes on a nanoscale within the lipid bilayer of the cell membrane. This membranous disruption results in altered cellular homeostasis and finally leads to apoptotic cell death. With IRE, extracellular matrix structures are typically preserved with simultaneous tumor destruction. This feature makes IRE an attractive alternative for local tumor ablation with associated vascular invasion (Figure 11). Although the ideal tumor size is preferred to be under 3 cm, tumor size between 3 and 5 cm is also possible for IRE treatment. Histopathological diagnosis is expected to obtain before the procedure. 42,44
Figure 11.
65-year-old male with no significant past medical history presented with weight loss and jaundice. Abdominal ultrasound study showed a hypoechoic pancreatic mass lesion with intra- and extrahepatic bile duct dilatation. (a) Axial plane post-contrast CT image shows a hypoechoic mass (arrows) located in the pancreatic head infiltrating SMV. Percutaneous ultrasound-guided FNAB revealed pancreatic adenocarcinoma. The patient underwent chemo-radiotherapy. (b) Axial plane post-contrast CT image one year after the initial diagnosis showed partial regression of the tumor (arrows). Surgical resection was planned for curative intend; however, metastatic nodules were found on liver capsule after laparotomy. Intraoperative IRE was performed. A metallic biliary stent was implanted endoscopically immediately after the procedure. (c) Axial plane post-contrast CT image six months after intraoperative IRE shows almost complete regression of the pancreatic mass (arrow). SMV, superior mesenteric vein; FNAB, fine-needle aspiration biopsy; IRE, irreversible electroporation
IRE is typically performed under general anesthesia to provide complete muscle paralysis. Gastric decompression with nasogastric drainage and cardiac monitoring are also required. 45
The 19-Gauge needle electrodes may be placed either percutaneously with CT guidance or during open surgery within and around the tumor. The distance between the electrodes is between 1.5 and 2.4 cm and a 5 mm tumor free margin should be obtained to provide adequate coverage. The number of the electrodes and the configuration of the ablation zone are determined mainly by the three-dimensional shape of the tumor. High-voltage direct-current electrical pulses are produced by the generator and delivered to the electrodes to achieve complete tumor ablation. Electrodes can be repositioned during the procedure for large-sized tumors. 42,44
A contrast-enhanced CT scan is performed immediately after the procedure to confirm the correct ablation zone. With this study, adjacent vascular structures and other potentially immediate post-procedure complications may effectively and promptly be evaluated. Patients are admitted for overnight observation after the procedure. Routine laboratory tests (CBC, serum chemistry profile including amylase, and lipase levels) and imaging may be required to monitor the patient during the admission. The initial follow-up imaging and laboratory tests (CA 19–9, amylase, and lipase levels) are typically performed 4–6 weeks after the procedure. 4
The overall complication rates are reported to be between 10 and 37%. Severe complications were reported to be 21% and most complications are relatively minor and respond to conservative approach. The most common complications are associated with the gastrointestinal system, such as nausea, vomiting, loss of appetite and gastroparesis. Cholangitis, bilioma formation and severe pancreatitis, the most commonly encountered major complications, are rare but may be observed after the procedure, therefore biliary protection with placement of plastic stent was recommended before the procedure to protect the patient from these complications. 42,46 Vascular complications such as SMA obstruction and bleeding were also reported. The median overall survival was reported to be 11 months after CT-guided IRE treatment in 25 patients. Complete remission is rare and was seen in 16% of the patients with a partial response rate of 38%. 46
IRE may also be performed during open surgery and this approach has been advocated by some authors as IRE needles may be more accurately located with direct visualization. Another advantage of this approach was reported to be potential direct visualization of distant metastatic disease which may be occult to imaging in 50% of the patients. Despite this potential benefit, the main disadvantage of this approach is relatively higher rates of morbidity as compared to the percutaneous approach (36% vs 24.3%, respectively). Therefore, percutaneous approach may be more preferable to surgical approach in patients with poor performance status. 46
Tumor size is another important factor to mention as overall survival after IRE treatment appears to be better in patients who have tumors less than 3 cm. 44
Thermal ablation
Thermal ablation techniques have also been investigated in the treatment of these patients. Due to close proximity of pancreas to several vital structures, complete ablation may be impossible. However, even in these cases, cytoreduction may be beneficial for patients’ survival. Thermal ablation may also stimulate immune response, which may be another indirect benefit. Pancreatitis, pancreatic fistula, hollow viscus perforation are among the common complications that may be encountered.
Radiofrequency ablation
Radiofrequency ablation (RFA) may be used for the treatment of LAPCs or in PA patients unfit-for-surgery. 47 Technically, RF ablation may be performed during surgery or percutaneously. The procedure is mostly performed with US guidance during open laparotomy. As for percutaneous approach, both CT or US guidance may be used for ablation. 48
RFA is typically performed with insertion of a monopolar electrode into the target lesion under imaging guidance. An alternating current is applied to the electrode and the target tissue is destroyed with thermal damage. The electrode may need to be repositioned to acquire overlapping ablation zones depending on the morphology and the size of the tumor. The ablation parameters, current (Amperes) and the time in seconds (s), are adjusted accordingly in relation to the lesion size and the tissue impedance recorded by the needle tip. The typical target temperature is between 90°C and 100°C. The thermal damage may be observed real-time with US-guidance. 49
Attention must be paid during ablation to prevent collateral damage to adjacent vital anatomic structures close to the tumor. Typically, a safety margin of 5 mm should be aimed. Heat sink effect may be a limitation of RFA in certain patients. 48,49
The RFA related morbidity and mortality rates are relatively high and range between 3.5–28% and 1.8–25%, respectively, in LAPC patient series. 50
Microwave ablation
Electromagnetic microwaves heat biological tissues by excitation of water molecules and induce cellular death via coagulation necrosis. With microwave energy, larger kill zones may achieved in less time as compared to RFA. With these advantages, microwave ablation is gaining wide acceptance over RFA for ablative purposes. The technique is not different from RFA and both open and percutaneous approaches may be preferred depending on the lesion characteristics and local expertise. 48,49
Carrafiello et al 51 reported the overall survival rate in their study at 1 year as 80% and none of the patients in their series showed a complete response to ablative treatment. The morbidity rate was reported to be 30% (Pancreatitis developed in two patients and a GDA pseudoaneurysm developed in one patient which is treated with endovascular approach).
Cryoablation
Cryoablation is also a thermal ablation technique using cold instead of heat. The mechanism of cell death in cryoablation is the creation of ice in both intra- and extracellular spaces. This freezing process eventually causes cell death. An “ice ball” is created around the electrodes which is helpful for relatively precise direct observation of the kill zone. 48,49
Cryoablation may be performed percutaneously with ultrasound or CT guidance, however open surgical approach is more commonly preferred. Small tumors (<3 cm) may be treated with a single, centrally placed probe but larger tumors may require placement of multiple probes or sequential treatments with a safety margin of at least 5 mm. After the creation of the ice ball, the tissue gradually thaws to 0°C, and then a second freezing process is started after repositioning of the probes if necessary. 49
Cryoshock is an extremely rare but potentially life-threating complication of cryoablation. It is a cytokine-mediated biological process and clinically presents with multiorgan failure, severe coagulopathy, and disseminated intravascular coagulation. 52
Niu et al 53 reported at least 50% decrease in pain score in 84% of the patients and 50% decrease in analgesic consumption in 69% of the patients with no severe post-operative complications. The median overall survival was reported to be 7 months in their pure cryoablation group, whereas it was 13 months in cryoablation plus immunotherapy receiving group.
Treatment of metastatic disease
Liver metastases are especially important as they indicate poor survival rates. Systemic chemotherapy has limited efficiency in liver metastases; therefore, second line strategies are necessary. 54,55
Percutaneous heat-based thermal ablation techniques can be used in some patients who have limited metastatic liver disease 2 (Figure 12). Ablation procedure may be performed percutaneously or intraoperatively. Park et al 54 evaluated the efficacy of RFA for liver metastases (<5 lesions and <3 cm in size) in PC patients. The median survival after ablation was found to be 15 months and even better survival rates were reported in patients with solitary liver metastases and smaller lesions (less than 2 cm in size).
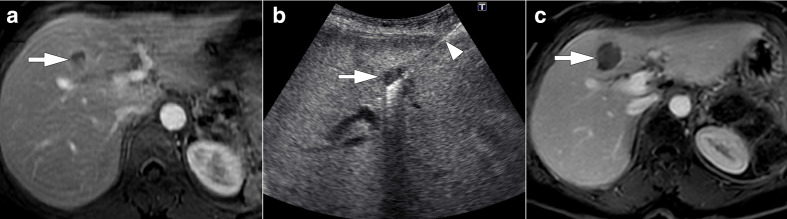
Figure 12.
54-year-old female with surgically treated pancreatic cancer presented with solitary liver metastasis. (a) Axial post-contrast T 1W MR image shows hypointense, peripherally enhancing mass lesion (arrow) highly concerning for metastasis which was subsequently confirmed with ultrasound-guided percutaneous biopsy. (b) As this lesion was the only radiologically detectable lesion (arrow), ultrasound-guided percutaneous RF ablation was performed (arrowhead). (c) Follow-up post-contrast T 1W MR image two months after the procedure demonstrates the ablation zone (arrow) with no evidence of residual or local recurrent disease.
Intra-arterial therapeutic approaches are typically reserved for patients with diffuse liver metastases (Figure 13). Radioembolization may provide benefit in prolonging survival and improving quality of life in these patients. Kim et al 55 retrospectively evaluated the efficacy and safety of yttrium-90 (90Y) radioembolization (RE) in 33 patients with liver metastases from PA. The median overall survival (8,1 months after RE) improved after RE and follow-up imaging demonstrated partial response in 42% and stable disease in 37% of the patients. Progressive disease was observed in 21% of the patients in their cohort. Major liver toxicity is not expected in carefully selected patients after RE.
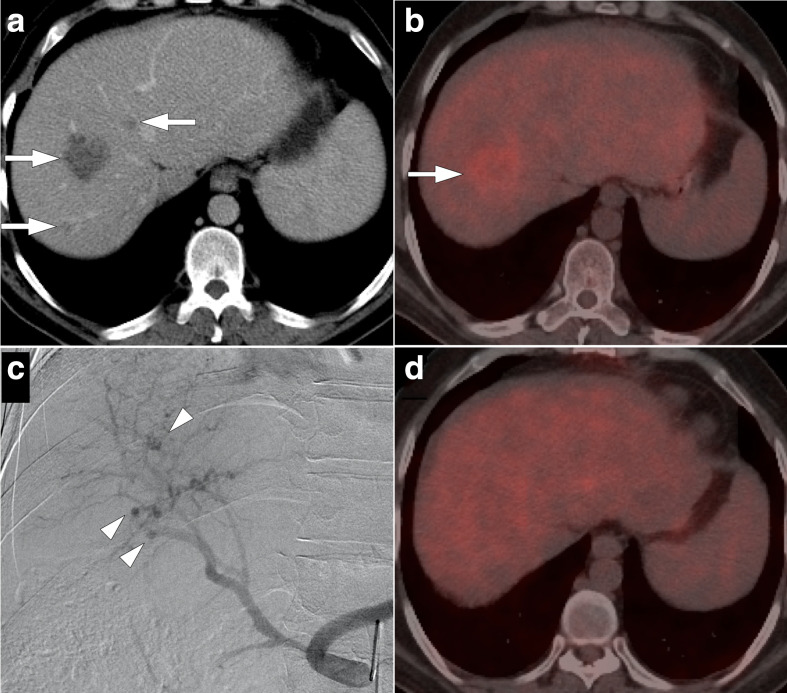
Figure 13.
41-year-old male with a history of Whipple surgery for pancreatic adenocarcinoma presented with liver metastasis on his most recent follow-up CT scan. (a) Axial post-contrast CT image shows hypodense lesions within the right liver lobe, highly suggestive for metastases (arrows). Subsequent percutaneous ultrasound-guided biopsy confirmed this clinical suspicion. (b) Axial plane fused PET/CT image demonstrates, mostly peripheral, intense FDG uptake in the largest lesion (arrow). There were no other suspicious foci in the left liver lobe. There was also no evidence of metastatic disease elsewhere in the chest and abdomen. With these findings the patient underwent right liver lobe radioembolization. (c) Celiac arteriogram image acquired during the procedure shows multiple hypervascular lesions (arrowheads) within the right liver lobe. (d) Axial plane fused PET/CT image four months after the procedure shows no evidence of active disease within the liver or elsewhere in the body. FDG, fludeoxyglucose; PET, positron emission tomography.
Conclusion
IR offers a wide range of effective and safe treatment modalities for PC patients in every steps of the disease processes starting from the initial diagnosis. Despite the poor prognosis and high mortality of the disease, IR provides highly effective minimally invasive solutions for PC patients to improve their quality of life.
Contributor Information
Aycan Uysal, Email: aycanuysal@hotmail.com.
Emre Unal, Email: emreunal.rad@gmail.com.
Ali Devrim Karaosmanoglu, Email: alidevrim76@yahoo.com.
Ronald Arellano, Email: rarellano@mgh.harvard.edu.
Turkmen Turan Ciftci, Email: turkmenciftci@yahoo.com.
Devrim Akinci, Email: akincid@hotmail.com.
Okan Akhan, Email: akhano@tr.net.
REFERENCES
- 1. Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–49. doi: 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- 2. Hevert EAC, Howser CG, Gould ML, Brown DB, Ablative BDB. Ablative, endovascular, and biliary interventions for patients with pancreatic cancer. Semin Intervent Radiol 2019; 36: 203–12. doi: 10.1055/s-0039-1693118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Taieb J, Prager GW, Melisi D, Westphalen CB, D'Esquermes N, Ferreras A, et al. First-Line and second-line treatment of patients with metastatic pancreatic adenocarcinoma in routine clinical practice across Europe: a retrospective, observational chart review study. ESMO Open 2020; 5: e000587. doi: 10.1136/esmoopen-2019-000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown DB, Narayanan G. Interventional radiology and the pancreatic cancer patient. Cancer J 2012; 18: 591–601. doi: 10.1097/PPO.0b013e3182745bee [DOI] [PubMed] [Google Scholar]
- 5. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. Folfirinox versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011; 364: 1817–25. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 6. Tyng CJ, Almeida MFA, Barbosa PNV, Bitencourt AGV, Berg JAAG, Maciel MS, et al. Computed tomography-guided percutaneous core needle biopsy in pancreatic tumor diagnosis. World J Gastroenterol 2015; 21: 3579–86. doi: 10.3748/wjg.v21.i12.3579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pucci MJ, Kennedy EP, Yeo CJ. Chapter 62 - Pancreatic cancer: clinical aspects, assessment, and management. In: Jarnagin W. R, ed. Blumgart’s surgery of the liver, biliary tract and pancreas, volume 2. 6th edition Philadelphia: WB Saunders; 2017. pp. 979–87. [Google Scholar]
- 8. Matsubara J, Okusaka T, Morizane C, Ikeda M, Ueno H. Ultrasound-Guided percutaneous pancreatic tumor biopsy in pancreatic cancer: a comparison with metastatic liver tumor biopsy, including sensitivity, specificity, and complications. J Gastroenterol 2008; 43: 225–32. doi: 10.1007/s00535-007-2142-9 [DOI] [PubMed] [Google Scholar]
- 9. Huang Y, Shi J, Chen Y-Y, Li K. Ultrasound-guided percutaneous core needle biopsy for the diagnosis of pancreatic disease. Ultrasound Med Biol 2018; 44: 1145–54. doi: 10.1016/j.ultrasmedbio.2018.02.016 [DOI] [PubMed] [Google Scholar]
- 10. D'Onofrio M, De Robertis R, Barbi E, Martone E, Manfrin E, Gobbo S, et al. Ultrasound-guided percutaneous fine-needle aspiration of solid pancreatic neoplasms: 10-year experience with more than 2,000 cases and a review of the literature. Eur Radiol 2016; 26: 1801–7. doi: 10.1007/s00330-015-4003-x [DOI] [PubMed] [Google Scholar]
- 11. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an international Study group of pancreatic surgery (ISGPS) definition. Surgery 2007; 142: 20–5. doi: 10.1016/j.surg.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 12. Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, et al. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg 2003; 7: 209–19. doi: 10.1016/S1091-255X(02)00193-2 [DOI] [PubMed] [Google Scholar]
- 13. Baker TA, Aaron JM, Borge M, Pierce K, Shoup M, Aranha GV. Role of interventional radiology in the management of complications after pancreaticoduodenectomy. Am J Surg 2008; 195: 386–90. doi: 10.1016/j.amjsurg.2007.12.026 [DOI] [PubMed] [Google Scholar]
- 14. Cronin CG, Gervais DA, Castillo CF-D, Mueller PR, Arellano RS. Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review. AJR Am J Roentgenol 2011; 197: 241–6. doi: 10.2214/AJR.10.5447 [DOI] [PubMed] [Google Scholar]
- 15. Ciftci TT, Akinci D, Akhan O. Percutaneous transhepatic drainage of inaccessible postoperative abdominal abscesses. AJR Am J Roentgenol 2012; 198: 477–81. doi: 10.2214/AJR.11.6680 [DOI] [PubMed] [Google Scholar]
- 16. Akinci D, Akhan O, Ozmen MN, Karabulut N, Ozkan O, Cil BE, et al. Percutaneous drainage of 300 intraperitoneal abscesses with long-term follow-up. Cardiovasc Intervent Radiol 2005; 28: 744–50. doi: 10.1007/s00270-004-0281-4 [DOI] [PubMed] [Google Scholar]
- 17. Gervais DA, Fernandez-del Castillo C, O'Neill MJ, Hahn PF, Mueller PR. Complications after pancreatoduodenectomy: imaging and imaging-guided interventional procedures. Radiographics 2001; 21: 673–90. doi: 10.1148/radiographics.21.3.g01ma16673 [DOI] [PubMed] [Google Scholar]
- 18. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International study group of liver surgery. Surgery 2011; 149: 680–8. doi: 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 19. Saad WEA, Wallace MJ, Wojak JC, Kundu S, Cardella JF. Quality improvement guidelines for percutaneous transhepatic cholangiography, biliary drainage, and percutaneous cholecystostomy. J Vasc Interv Radiol 2010; 21: 789–95. doi: 10.1016/j.jvir.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 20. Nikpour AM, Knebel RJ, Cheng D. Diagnosis and management of postoperative biliary leaks. Semin Intervent Radiol 2016; 33: 307–12. doi: 10.1055/s-0036-1592324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jagad RB, Koshariya M, Kawamoto J, Chude GS, Neeraj RV, Lygidakis NJ. Postoperative hemorrhage after major pancreatobiliary surgery: an update. Hepatogastroenterology 2008; 55: 729–37. [PubMed] [Google Scholar]
- 22. Puppala S, Patel J, McPherson S, Nicholson A, Kessel D. Hemorrhagic complications after Whipple surgery: imaging and radiologic intervention. AJR Am J Roentgenol 2011; 196: 192–7. doi: 10.2214/AJR.10.4727 [DOI] [PubMed] [Google Scholar]
- 23. Lorenz JM. Management of malignant biliary obstruction. Semin Intervent Radiol 2016; 33: 259–67. doi: 10.1055/s-0036-1592330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Delden OM, Laméris JS. Percutaneous drainage and stenting for palliation of malignant bile duct obstruction. Eur Radiol 2008; 18: 448–56. doi: 10.1007/s00330-007-0796-6 [DOI] [PubMed] [Google Scholar]
- 25. Lammer J, Hausegger KA, Flückiger F, Winkelbauer FW, Wildling R, Klein GE, et al. Common bile duct obstruction due to malignancy: treatment with plastic versus metal stents. Radiology 1996; 201: 167–72. doi: 10.1148/radiology.201.1.8816539 [DOI] [PubMed] [Google Scholar]
- 26. Bezzi M, Zolovkins A, Cantisani V, Salvatori FM, Rossi M, Fanelli F, et al. New ePTFE/FEP-covered stent in the palliative treatment of malignant biliary obstruction. J Vasc Interv Radiol 2002; 13: 581–9. doi: 10.1016/S1051-0443(07)61651-0 [DOI] [PubMed] [Google Scholar]
- 27. Krokidis M, Fanelli F, Orgera G, Tsetis D, Mouzas I, Bezzi M, et al. Percutaneous palliation of pancreatic head cancer: randomized comparison of ePTFE/FEP-covered versus uncovered nitinol biliary stents. Cardiovasc Intervent Radiol 2011; 34: 352–61. doi: 10.1007/s00270-010-9880-4 [DOI] [PubMed] [Google Scholar]
- 28. Isayama H, Komatsu Y, Tsujino T, Sasahira N, Hirano K, Toda N, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut 2004; 53: 729–34. doi: 10.1136/gut.2003.018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yu T, Zhang W, Li C, Wang C, Gong G, Wang L, et al. Percutaneous intraductal radiofrequency ablation combined with biliary stent placement for treatment of malignant biliary obstruction. Abdom Radiol 2020; 45: 3690–7. doi: 10.1007/s00261-020-02516-4 [DOI] [PubMed] [Google Scholar]
- 30. Xia N, Gong J, Lu J, Chen Z-J, Zhang L-Y, Wang Z-M. Percutaneous intraductal radiofrequency ablation for treatment of biliary stent occlusion: a preliminary result. World J Gastroenterol 2017; 23: 1851–6. doi: 10.3748/wjg.v23.i10.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cumhur T, Ösmen MN, Akhan O, Ölçer T, Çekirge S, Özdemir E. Malignant biliary obstruction: treatment with self-expandable metallic stents. Eur Radiol 1995; 5: 6–12. doi: 10.1007/BF00178073 [DOI] [Google Scholar]
- 32. Akinci D, Akhan O, Ozkan F, Ciftci T, Ozkan OS, Karcaaltincaba M, et al. Palliation of malignant biliary and duodenal obstruction with combined metallic stenting. Cardiovasc Intervent Radiol 2007; 30: 1173–7. doi: 10.1007/s00270-007-9045-2 [DOI] [PubMed] [Google Scholar]
- 33. Bulut E, Çiftçi T, Akhan O, Akıncı D. Palliation of malignant gastroduodenal obstruction: fluoroscopic metallic stent placement with different approaches. Diagn Interv Radiol 2017; 23: 211–6. doi: 10.5152/dir.2016.16165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Drewes AM, Campbell CM, Ceyhan GO, Delhaye M, Garg PK, van Goor H, et al. Pain in pancreatic ductal adenocarcinoma: a multidisciplinary, International guideline for optimized management. Pancreatology 2018; 18: 446–57. doi: 10.1016/j.pan.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 35. Wyse JM, Chen Y-I, Sahai AV. Celiac plexus neurolysis in the management of unresectable pancreatic cancer: when and how? World J Gastroenterol 2014; 20: 2186–92. doi: 10.3748/wjg.v20.i9.2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, Zhao XH. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging 2006; 31: 710–8. doi: 10.1007/s00261-006-9153-5 [DOI] [PubMed] [Google Scholar]
- 37. Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS. CT-guided celiac plexus neurolysis: a review of anatomy, indications, technique, and tips for successful treatment. Radiographics 2011; 31: 1599–621. doi: 10.1148/rg.316115526 [DOI] [PubMed] [Google Scholar]
- 38. Akhan O, Ozmen MN, Basgun N, Akinci D, Oguz O, Koroglu M, et al. Long-term results of celiac ganglia block: correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol 2004; 182: 891–6. doi: 10.2214/ajr.182.4.1820891 [DOI] [PubMed] [Google Scholar]
- 39. Akhan O, Altinok D, Ozmen MN, Oguzkurt L, Besim A. Correlation between the grade of tumoral invasion and pain relief in patients with celiac ganglia block. AJR Am J Roentgenol 1997; 168: 1565–7. doi: 10.2214/ajr.168.6.9168726 [DOI] [PubMed] [Google Scholar]
- 40. Akinci D, Akhan O. Celiac ganglia block. Eur J Radiol 2005; 55: 355–61. doi: 10.1016/j.ejrad.2005.03.008 [DOI] [PubMed] [Google Scholar]
- 41. Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg 1995; 80: 290–5. doi: 10.1097/00000539-199502000-00015 [DOI] [PubMed] [Google Scholar]
- 42. Scheffer HJ, Vroomen LGPH, de Jong MC, Melenhorst MCAM, Zonderhuis BM, Daams F, et al. Ablation of locally advanced pancreatic cancer with percutaneous irreversible electroporation: results of the phase I/II PANFIRE study. Radiology 2017; 282: 585–97. doi: 10.1148/radiol.2016152835 [DOI] [PubMed] [Google Scholar]
- 43. Narayanan G. Irreversible electroporation. Semin Intervent Radiol 2015; 32: 349–55. doi: 10.1055/s-0035-1564706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Narayanan G, Hosein PJ, Beulaygue IC, Froud T, Scheffer HJ, Venkat SR, et al. Percutaneous image-guided irreversible electroporation for the treatment of unresectable, locally advanced pancreatic adenocarcinoma. J Vasc Interv Radiol 2017; 28: 342–8. doi: 10.1016/j.jvir.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 45. Rashid MF, Hecht EM, Steinman JA, Kluger MD. Irreversible electroporation of pancreatic adenocarcinoma: a primer for the radiologist. Abdom Radiol 2018; 43: 457–66. doi: 10.1007/s00261-017-1349-3 [DOI] [PubMed] [Google Scholar]
- 46. Moris D, Machairas N, Tsilimigras DI, Prodromidou A, Ejaz A, Weiss M, et al. Systematic review of surgical and percutaneous irreversible electroporation in the treatment of locally advanced pancreatic cancer. Ann Surg Oncol 2019; 26: 1657–68. doi: 10.1245/s10434-019-07261-7 [DOI] [PubMed] [Google Scholar]
- 47. D'Onofrio M, Crosara S, De Robertis R, Butturini G, Salvia R, Paiella S, et al. Percutaneous radiofrequency ablation of unresectable locally advanced pancreatic cancer: preliminary results. Technol Cancer Res Treat 2017; 16: 285–94. doi: 10.1177/1533034616649292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saccomandi P, Lapergola A, Longo F, Schena E, Quero G. Thermal ablation of pancreatic cancer: a systematic literature review of clinical practice and pre-clinical studies. Int J Hyperthermia 2018; 35: 398–418. doi: 10.1080/02656736.2018.1506165 [DOI] [PubMed] [Google Scholar]
- 49. D'Onofrio M, Ciaravino V, De Robertis R, Barbi E, Salvia R, Girelli R, et al. Percutaneous ablation of pancreatic cancer. World J Gastroenterol 2016; 22: 9661–73. doi: 10.3748/wjg.v22.i44.9661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Linecker M, Pfammatter T, Kambakamba P, DeOliveira ML. Ablation strategies for locally advanced pancreatic cancer. Dig Surg 2016; 33: 351–9. doi: 10.1159/000445021 [DOI] [PubMed] [Google Scholar]
- 51. Carrafiello G, Ierardi AM, Fontana F, Petrillo M, Floridi C, Lucchina N, et al. Microwave ablation of pancreatic head cancer: safety and efficacy. J Vasc Interv Radiol 2013; 24: 1513–20. doi: 10.1016/j.jvir.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 52. Rong G, Bai W, Dong Z, Wang C, Lu Y, Zeng Z, et al. Long-term outcomes of percutaneous cryoablation for patients with hepatocellular carcinoma within Milan criteria. PLoS One 2015; 10: e0123065. doi: 10.1371/journal.pone.0123065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Niu L, He L, Zhou L, Mu F, Wu B, Li H, et al. Percutaneous ultrasonography and computed tomography guided pancreatic cryoablation: feasibility and safety assessment. Cryobiology 2012; 65: 301–7. doi: 10.1016/j.cryobiol.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 54. Park JB, Kim YH, Kim J, Chang H-M, Kim TW, Kim S-C, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol 2012; 23: 635–41. doi: 10.1016/j.jvir.2012.01.080 [DOI] [PubMed] [Google Scholar]
- 55. Kim AY, Frantz S, Brower J, Akhter N. Radioembolization with yttrium-90 microspheres for the treatment of liver metastases of pancreatic adenocarcinoma: a multicenter analysis. J Vasc Interv Radiol 2019; 30: 298–304. doi: 10.1016/j.jvir.2018.09.020 [DOI] [PubMed] [Google Scholar]