Abstract
Renal cryoablation is a treatment option for early stage renal cell carcinomas with excellent oncological outcomes and low morbidity. This review outlines the technique of renal cryoablation and provides a guide for interventional radiologists on setting up an integrated service within a renal cancer network multidisciplinary setting. Patient selection and preparation, together with the technical aspects which ensure optimal oncological outcomes and avoid collateral damage to adjacent organs are highlighted.
1) Introduction
Renal cancer is the eighth commonest cancer in the USA 1 and the seventh commonest cancer in the UK 2 and the majority are discovered at an early stage. 3 The increased use of imaging has contributed to the increased detection of small renal masses (SRMs) 4,5 ; defined as tumours smaller than 4 cm.
Nephron-sparing therapeutic options, such as partial nephrectomy (PN) and thermal ablation, are the preferred treatments for renal cell carcinomas (RCC). Of these, renal cryoablation (RCYA) is a thermal ablative treatment, which has an advantage of visualising the ablation zone during treatment delivery. Whilst a randomised, prospective comparison with surgery is still awaited, 6 previous large series reporting cryoablation for early stage RCC, with long-term follow-up show comparable oncological outcomes to surgery 7–10 with fewer complications 8,9 and a shorter hospital stay. 9 Thermal ablation is now incorporated in the National Comprehensive Cancer Network guidelines for the treatment of SRMs 11 and by the American Urological Association regardless of age and performance status. 12
Based on the current available evidence and our own extensive experience, 10 this review aims to provide an overview of RCYA including patient selection, technique and follow-up, with the overall aim to help others develop a RCYA practice.
2) Initial requirements to commence a RCYA service
Figure 1 summaries the important perquisites in order to commence and sustain a successful RCYA service.
Figure 1.
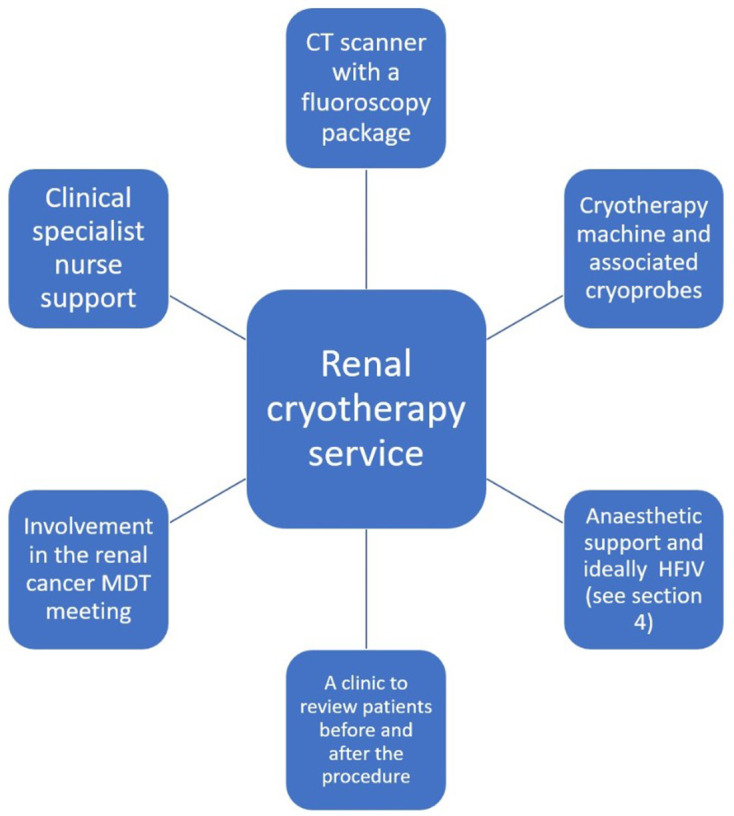
The important prerequisites to set up and maintain a renal cryotherapy service. HFJV, high frequency jet ventilation; MDT, multidisciplinary team.
3) Patient selection
Patient and lesion-specific factors are important considerations in multidisciplinary team (MDT) discussions and are considered below.
Patient-specific factors
Age and co-morbidities
Cryoablation has been suggested to be reserved for elderly or co-morbid patients with SRMs, 13 but the increasing evidence base suggests cryoablation can also be considered for younger, fitter patients who are still candidates for surgery. 14
Baseline renal function
Whilst a direct randomised comparison is awaited, 6 a previous non-randomised series demonstrated a 3-year freedom from chronic kidney disease of 70% for PN 15 and 95% for radiofrequency ablation. A meta-analysis indicated a non-significant trend to better preserved renal function with ablation relative to PN 16 and because there is generally no decline in renal function following thermal ablation, 17 RCYA is at least equivalent to surgery and may be superior in preserving renal function. RCYA therefore benefits those patients who may require repeat treatments (multiple SRMs or RCC syndromes, e.g. von Hippel-Lindau (VHL) or Birt-Hogg-Dubé) and those with poor renal function or solitary kidneys.
Lesion-specific factors
Size
There is now evidence that larger tumours can also be treated successfully, with T1b tumours (≤7 cm) demonstrating comparable outcomes to T1a tumours (≤4 cm). 9,18 However, the majority of the published literature focuses on T1a tumours so one can be more assured of the oncological efficacy in treating these smaller tumours. Larger tumours also require more needles and careful planning of needle positioning.
Location
Posterior, exophytic lesions are generally easier to target and less vulnerable to ‘freeze-sink,’ whereby the thermal energy is carried away by blood vessels adjacent to endophytic and central tumours. However, a recent large series comparing anterior vs posterior location, tumour centrality and polar location showed similar oncological outcomes and complication rates. 9 Anterior and central tumours can therefore be successfully treated, although when establishing a cryoablation service, the less technically challenging posterior and exophytic lesions should be prioritised.
Perinephric fat
Perinephric fat is an important consideration for urologists who can find it difficult to dissect through if it is adherent to the renal capsule and this results in increased operative times and blood loss. 19 Conversely for ablation, large volumes of fat are an advantage due to increased distance between the ice ball and surrounding non-target structures, sometimes negating the need for hydrodissection (section 6).
Histological subtypes of renal cancer
RCC histopathological subtype or Fuhrman grade do not routinely informthe decision to perform ablation, although this reflects a selection bias, as higher-grade lesions or aggressive subtypes are more likely to present with metastatic disease and be unsuitable for ablation. There is limited evidence to suggest worse oncological outcomes with RCYA in higher Fuhrman grade lesions. 20
RCYA is frequently performed to treat VHL syndrome, where 70% patients will develop an RCC by aged 60 21 and RCYA has been shown to be safe and highly effective in treating Birt-Hogg-Dubé syndrome-related RCC. 22 RCYA for metastases to the kidney is described with good technical efficacy. 18,23 However, given that renal metastases occur in less than 1% of patients with non-renal malignancy and there is frequently metastatic disease elsewhere, 24 the scenario of isolated or oligometastatic renal disease suitable for RCYA is not a common one and requires careful MDT discussion.
Other
The lesion vascularity or presence of calcification on imaging does not affect our decision to offer RCYA.
4) Patient preparation and periprocedural care
Clinic review
Following an MDTdiscussion, patients should be reviewed in a MDT clinic by the treating radiologist. The patient can be counselled regarding conservative, radiological and surgical interventions which allows a full medical history and preoperative assessment to be arranged.
Antiplatelet and anticoagulant medications
Bridging plans regarding anticoagulant or antiplatelet medication can be instituted following the clinic review. Specifically, low dose aspirin is not routinely withheld, but other antiplatelet agents and anticoagulant agents are managed as per established guidelines. 25
Biopsy
SRMs are frequently benign. In one large study 19% were benign and this rose to 42% in patients less than 40 years old. 26 Unless the patient has a renal cancer syndrome with comprehensive imaging evidence of malignancy, a biopsy prior to RCYA is imperative to avoid treating benign lesions. As the evidence regarding whether size affects the ability to achieve a diagnostic biopsy is conflicting, 27,28 we tend to biopsy lesions once they are larger than 2 cm, but we will biopsy smaller lesions that are anatomically favourable (exophytic and posterior). On the rare occasion that a biopsy is taken at the time of treatment, we suggest it is performed after the cryoprobes are inserted to reduce the risk of biopsy-induced bleeding that may preclude probe placement.
Radiology planning meetings
Weekly interventional radiology planning meetings are performed to review the technical aspects of the procedure that might require specific additional arrangements, such as a preablation retrograde ureteric stent placement (section 6).
Urine culture and antibiotics
There is limited evidence regarding routine preprocedure urine cultures and prophylactic antibiotics, but the risk of infection related to renal ablation is low. 29 We employ a cautious approach and obtain midstream urine specimens for microscopy and culture from all patients even if they are asymptomatic. Any positive cultures are treated with appropriate antibiotics preoperatively. A prophylactic intravenous antibiotic dose is given at induction (gentamicin to cover urinary tract bacteria), but this practice varies across institutions. Antibiotics targeting the urinary tract microbes should however be given to patients with ureteric stents and in those with ileal conduits due to the risk of upper tract colonisation. 30
Anaesthesia
Nearly all procedures at our institution (University College Hospital) are performed under general anaesthesia using high-frequency jet ventilation (HFJV). Using HFJV reduces respiratory movement, procedural time and radiation dose. 31 We occasionally perform ablation under sedation and local anaesthesia if the patient is deemed unfit for a general anaesthetic. Other groups report performing RCYA under conscious sedation as their default, 8,32 but in our own experience needle placement and hydrodissection is more precise if general anaesthetic is employed. In the wider field of interventional radiology, interest in the use of non-pharmacological adjuncts, such as music or virtual reality headsets, 33 has increased in recent years, and these may prove helpful to those performing RCYA under local anaesthetic and sedation.
Most of our treatments are performed with the patient in a prone or prone oblique position (the latter means the patientis moved to approximately 20 degrees right or left side up from the prone position) and this has proved to be safe even with HFJV. 10,31 The patients arms are moved towards the head to ensure access to the lesion and prevent streak artefact, but shoulder abduction over 90 degrees is avoided to reduce the risk of brachial plexus injury. 34 It is also important to protect pressure points and peripheral nerves (e.g. ulnar nerve) with copious padding.
Procedural scan protocol
Once the anaesthetised patient is placed in a position likely to be suitable for the procedure, a low dose, unenhanced volumetric scan is performed prior to preparing the skin to confirm satisfactory lesion access.
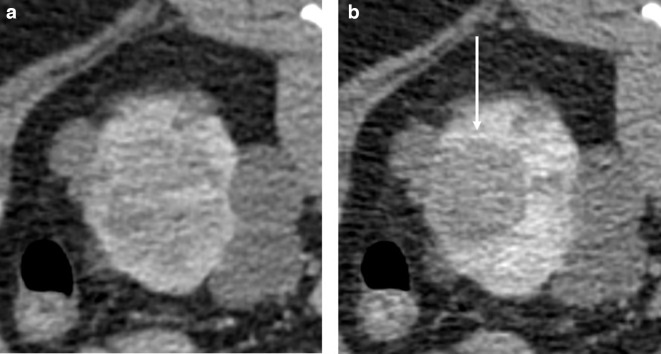
The skin is then prepared, the probes tested and patient warming blankets and intermittent pneumatic calf compression applied as per local hospital policy for surgical patients. A full dose contrast-enhanced scan limited to the kidneys (to minimise dose) is performed. This permits accurate identification of the lesion and a planning map to be drawn. A volumetric scan at 80 s post-contrast injection is performed, but occasionally a 35 s scan is also useful (preprocedural imaging should be reviewed in this respect). Quite frequently, the lesion ‘washes out’ and becomes more conspicuous with time, so a delayed phase scan can also help (Figure 2).
Figure 2.
Axial prone CT images of a left renal tumour in a 30-year-old male acquired at A) 80 s and B) 6 min post-contrast. The patient has von Hippel-Lindau syndrome, so there were multiple cyst and solid lesions elsewhere. The lesion was more conspicuous on the pre-operative MRI, but cannot be well defined in A), however treatment can be accurately planned from B) where the lesion has washed out (arrow) and is well seen.
The lesion can become more difficult to identify during the procedure due to the gradual loss of parenchymal contrast and later due to hydrodissection. In these cases, calyceal anatomy can be used as landmarks. Focal volumetric scans are performed to monitor cryoprobe positioning after each row of probe placement to confirm correct position within the lesion and inform subsequent needle placement.
Focal volumetric CT is also performed every 5 min during the freezing cycles to monitor the ice ball. The ice ball is seen as a well-defined low attenuation area relative to the renal parenchyma (Figure 3). If there is concern that an adequate margin will not be achieved, then further adjacent probes can be inserted. If vital structures are in danger, further protective methods (e.g. hydro-/pneumodissection) can be used or, depending on the specific machine settings, the ice ball can be sculpted by reducing the percentage at which the cryoprobe is driven.
Figure 3.
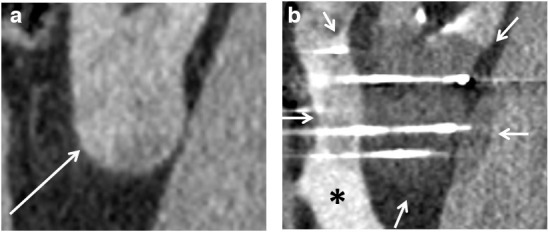
(A) A sagittal contrast-enhanced CT of an exophytic left lower pole clear cell renal cell carcinoma in a 60-year-old male (arrow). (B) Intraprocedural image with the ice ball highlighted (arrows) and hydrodissection (asterisk) to protect the colon. The ice ball is clearly seen as an area of relatively reduced attenuation. This permits monitoring of the adequacy of the treatment margin and whether any critical structures are at risk.
In a large series of 307 patients, MRI guidance demonstrated equivalent long-term overall and local progression free survival compared to CT guidance and cases were only slightly longer on average in the MRI group (mean duration 140 compared to 127 min). 35 Clearly, there is an advantage of avoiding radiation, but it is likely that most departments do not have access to interventional MRI suites currently. Ultrasound can be performed to assist with probe placement, but should not be used as the sole means of monitoring the ice ball as once formed, its highly reflective interface prevents assessment of the deep margin of the ice ball for adequacy and safety.
5) Probe choice and configuration
Treatment margins
One of the strengths of cryotherapy is that a visible ice ballis produced (Figure 3) and this can be monitored during treatment. The temperature of the edge of the ice ball is 0°C and because the lethal isotherm is deep to this, there must be a certain margin of coverage by the ice ball. The lethal temperature for normal kidney cells is -20°C, 36 but it has been suggested that renal tumours require an even lower temperature, 37 a finding mirrored in other malignancies, where a goal of -40°C is suggested. 38 A combination of cryoprobes results in a synergistic effect and a larger ice ball. The −40°C threshold is around 1 cm from the edge of the ice ball, the −20°C isotherm around 0.5 cm from the ice ball edge and the optimum distance between cryoprobesis 1.5–2 cm. 39 An ice ball margin of 0.5 cm predicts treatment efficacy 40 and a margin of 0.5–1 cm should be aimed for. In vitro and in vivo studies 39,41,42 show the size of the ice ball varies depending on the number or probes used and the length of the freezing component of the needle.
Probe choice and placement
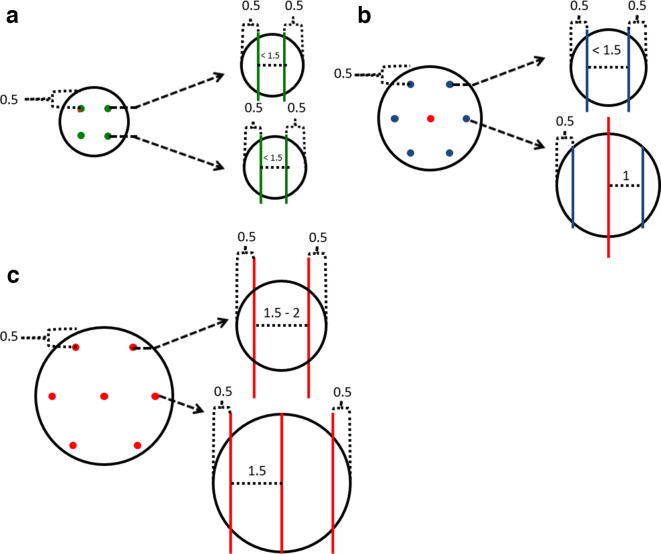
The cryoprobe placement guidelines in this paragraph and in Figure 4 are based on Galil Medical (Galil Medical Limited, Yokneam, Israel) isotherm data, but other systems, such as the one available from Endocare (Irvine, CA) are available. The superior and inferior cryoprobes are generally placed 0.5 cm from the top or bottom of the lesion respectively on coronal imaging. On axial imaging, the cryoprobes are placed 0.5 cm from the tumour edge and sufficient cryoprobes are placed so that they are ≤1.5 cm apart from each other. This usually produces a 0.5–1 cm margin and ensures the lesion is well encompassed by the lethal isotherm. More conservative treatment is performed when nephron sparing is particularly important (e.g. RCC syndromes needing repeat treatments or renal failure).The Galil Medical system includes cryoprobes that are 17–14 G. The newer CX needles have an electrical heating element that allows for helium-free thawing and tract ablation.
Figure 4.
A guide of where to place Galil cryoprobes and which probes to select for lesions of varying sizes. Images are not to scale and real-life measurements must be made to reflect the imperfect shape of in vivo tumors. Measurements are in cm. The IceSeed, IceSphere and IceRod probes are indicated by green, blue and red colors respectively. Spherical lesions of (A) 2 cm, (B) 3 cm and (C) 4 cm. Coronal images are on the left and the dashed arrows highlight the axial image at that specific level on the right. It is important to stress this is a guide and parallel probe placement at every level is frequently not possible due to anatomical constraints. It is also important to emphasise that the AP positioning of the cryoprobes requires knowledge of the AP length of the isotherm and consideration of whether there are critical structures anterior or posterior to the kidney. Measuring back the length of the expected isotherm along the cryoprobe helps placement in this regard. Some cryoprobes are placed over 1.5 cm from each other in (C) and additional cryoprobes may be required for a central tumour. AP, anteroposterior.
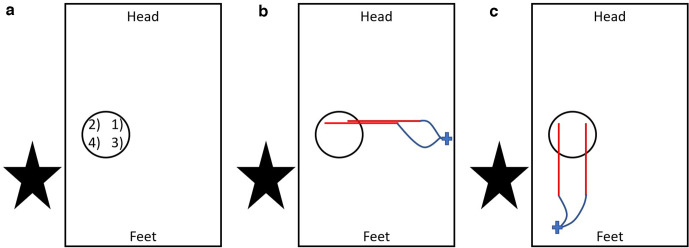
Once the choice of cryoprobes has been made, it is important that the order of placement is considered. This allows the cryoprobes to be plugged into the machine in a suitable sequence to avoid wire entanglement and to ensure that the operator is aware of the probe number assignment. Generally, the cryoprobes furthest away from the operator should be placed first (the most superior and on the opposite side to that which the operator is standing) to avoid having to reach over cryoprobes for subsequent placement. After having placed the probes, it is helpful to rotate them and clip the cables such that the hubs do not obstruct placement of the next row of needles. In rotating the needles, the tumour is often moved, so this step should be undertaken before the needle position is checked with volumetric CT and the next row of needles are planned. The probes are placed under CT fluoroscopic guidance to ensure accurate placement. Probe placement order and cable positioning is summarised in Figure 5.
Figure 5.
A diagram of probe placement order and suggested cable management. (A) A bird’s eye view of the patient (represented by rectangle) in a prone position with the operator (indicated by the star) standing to the left of the patient. The tumour is represented by the circle and the first probe to be placed (indicated by the number 1) should be that which is most superior and on the opposite side to the operator. Then probe 2 is placed without having to reach over probe 1. Placement of the lower row next (probes 3 and 4) means the operator avoids reaching over other probes to place these. (B) Probes 1 and 2 have now been placed. The red lines represent the plastic tubing on the outer portion of the probes. The wavy blue lines correspond to the probe cables and the probes have been clipped in position (cross) with the plastic tubing perpendicular to the long axis of the patient. The needle portion of the probes pass vertically in to the patient and cannot be seen on this bird’s eye view. By clipping the probes in this position, the more inferior row (probes 3 and 4) can be placed easily. After rotating the tubing and clipping, a volumetric scan should be performed as this rotation can move the tumour and affect the level at which robes 3 and 4 should be placed. (C) Probes 1 and 2 have been clipped with their tubing parallel to the patient and obscuring the entry points for probes 3 and 4. This should be avoided as it will now be more difficult to place probes 3 and 4.
Anatomical factors
It is important that anatomical factors are considered, e.g. placing cryoprobes closer together in more central lesions and close to the deep parenchymal margin due to the greater ‘freeze-sink’ effect. 42 Conversely, we find that the inter cryoprobe distance can be increased to 2 cm with large exophytic lesions which are less vulnerable to ‘freeze-sink’ and this falls in line with in vitro data. 39
6) Protecting important structures
Table 1 lists the anatomical structures that should be considered based on tumour location. Means of avoiding injury to these structures through patient positioning and ancillary techniques are also presented (summarised in Table 2). Cryoprobes are typically placed prior to employing the below techniques, with the exception of patient positioning and ureteric stent placement. Occasionally, hydrodissection is performed first to allow passage of the cryoprobes and to ensure that the bowel can be protected.
Table 1.
Anatomical structures to consider based on lesion location
| Lesion location | Anatomical structures at risk of injury |
|---|---|
Upper pole
|
|
Lower pole
|
|
Anterior
|
|
Posterior
|
|
Central
|
|
Table 2.
A summary of thermal sensitive structures and means of protection during renal tumour cryotherapy
| Structures requiring protection | Patient positioning | Primary protective methods | Secondary protective methods |
|---|---|---|---|
| Lung | Ipsilateral side-down | Artificial pneumothorax | Angle gantry/transhepatic treatment |
| Pleura | |||
| Bowel | Ipsilateral side-up | Hydrodissection | Balloon interposition/probe torque |
| Pancreatic tail | |||
| Rib neurovascular bundle, abdominal wall, genitofemoral nerve | |||
| Ureter | Stent | Pneumodissection/probe torque | |
| Collecting system | Renal sinus pneumodissection |
Patient position
Optimal patient positioning is a vital and should be considered in planning meetings. If there is concern over anadjacent structure’s proximity to the planned ablation zone or needle pathway, a positioning scan is performed prior before the procedure to avoid moving an anaesthetised patient.
The majority of our procedures are performed with the patient in a prone or an oblique prone position as this generally permits easy access to the lesion and is safe. 8,9,32 Supine positioning is generally avoided due to difficult lesion accessibility and the increased mobility of cryoprobes in the anterior abdominal wall, but can be useful to treat lower pole lesions and transplant kidneys.
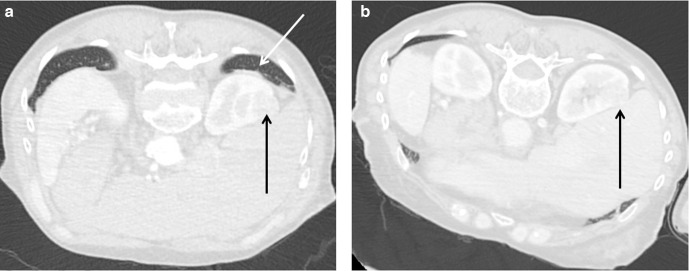
Patient positioning is particularly important for upper pole lesions. In the prone position, the lung bases frequently overlie upper pole lesions. By elevating the contralateral side, the ipsilateral lung is relatively deflated and often permits access (Figure 6). If the bowel is close to an anterior lesion, elevating the ipsilateral side may cause the bowel to fall away medially.
Figure 6.
(A) Prone contrast-enhanced CT on lung windows shows the right upper pole clear cell renal cell carcinoma (black arrow) in a 48-year-old male to be obscured by the overlying lung (white arrow). (B) Contrast-enhanced CT in the prone oblique (right side-down). The mass (black arrow) is no longer obscured by the lung and was treated without transgressing the lung parenchyma.
Iatrogenic pneumothorax
The parietal pleural reflection is at the level of the 10th rib in the mid axillary line and crosses the 11th and 12th ribs to meet the top of L1 at its most medial aspect. 43 Given that the upper poles are usually supracostal, treatment through the pleura is often necessary and can be safely performed. 8,9,44 Puncturing the visceral pleura will result in a higher risk of pneumothorax, so if patient positioning does not protect the lung and more than one cryoprobe is going to traverse it, an iatrogenic pneumothorax can be created 45 (Figure 7).
Figure 7.
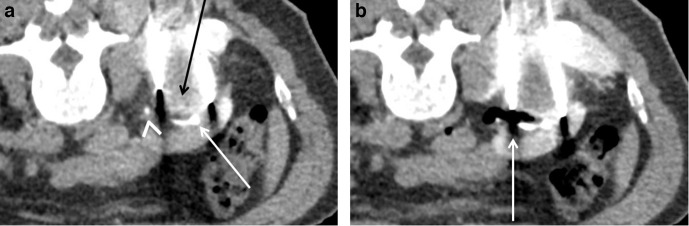
(A) Despite an ipsilateral side-down prone position the right posterior chromophobe renal cell carcinoma (arrow) in this 64-year-old female cannot be accessed without traversing the lung. (B) A 22G needle was passed into the pleural space under CT guidance and carbon dioxide injected to create a pneumothorax. A chest drain was inserted and clamped (not shown). (C) Cryoablation treatment probes were passed through the pneumothorax without traversing the lung. The pneumothorax was aspirated at the end of the procedure.
A 22G needle is passed into the pleural space under CT guidance and carbon dioxide injected. A 6-8 Fr chest drain can then be placed and clamped. After the ablation, the pneumothorax is aspirated, the drain clamped and a chest radiograph performed at 2–4 h. If air has accumulated a Heimlich valve or underwater seal can be attached, but otherwise the drain can be removed.
Hydrodissection
Why and how?
The aim is predominantly that of organ displacement with a secondary effect of insulating the thermal energy. A needle (e.g. 22G spinal needle) of the required length is placed into the area of intended hydrodissection and a solution of normal saline with a small amount of iodinated contrast (20 ml of Omnipaque 300 (GE Healthcare, Milwaukee, WI) per 1 litre normal saline) is instilled via a three-way tap till the desired effect is achieved (Figure 8). Further injections can be performed during treatment.
Figure 8.
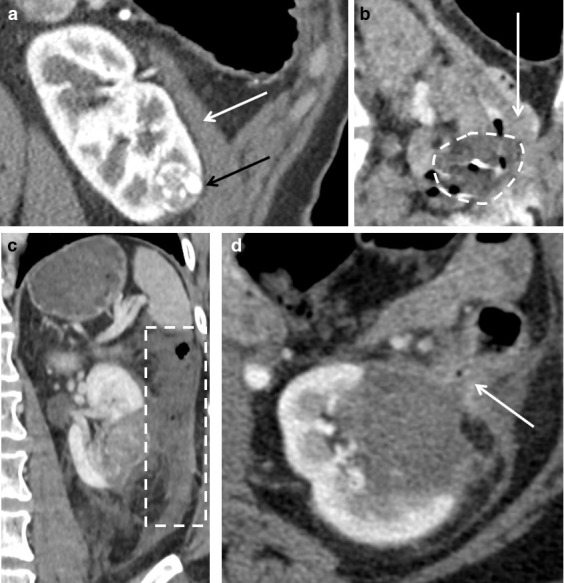
(A) The close proximity of the descending colon (white arrow) to the clear cell renal carcinoma (black arrow) in this 62-year-old male. (B) Hydrodissection was performed prior to the contrast examination and cryoprobe placement as it was not clear whether adequate dissection would be possible. A posterior 22G spinal needle (arrowhead) was used to displace the colon anteriorly which subsequently permitted insertion of a second needle (arrow) via a lateral approach to further increase colonic displacement. Ablation was then safely performed.
Which structures to protect?
Hydrodissection is most effective with mobile structures and most commonly performed to protect the bowel with anterior and lower pole tumours. 46
When assessing the need for hydrodissection (or other organ-protective techniques), it is important to be aware of the adjacent organs’ thermal sensitivities. The bowel is liable to damage 47 (Figure 9) and if a bowel injury does occur, then conservative management may be successful, 48 but an immediate surgical consult is mandatory. A cut-off distance where protective measures should be employed has not been defined. However, if the bowel is 1 cm or less from the tumour edge, then protective measures should be employed and the edge of the ice ball should be kept a minimum of 5 mm from sensitive structures.
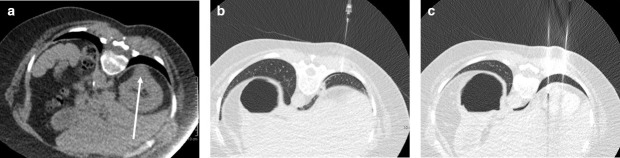
Figure 9.
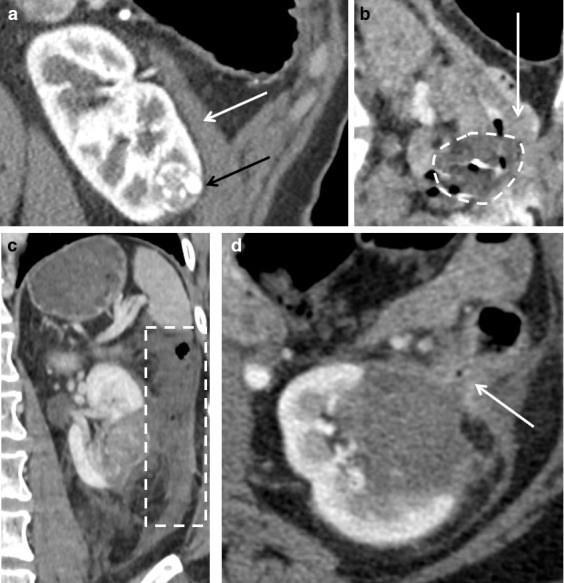
(A) A coronal oblique post-contrast CT of a 45-year-old female indicates very close proximity of a lower pole clear cell renal carcinoma (black arrow) to the descending colon (white arrow). (B) An intraprocedural image shows the ice ball (dashed circle) abutting the colon (arrow), despite the use of hydrodissection. (C) A post-contrast CT obtained 1 day after treatment following abdominal pain shows the descending colon to be poorly enhancing and thick walled (dotted rectangle), but there was no evidence of perforation. (D) A post-contrast CT on day 2 post-procedure shows improved enhancement of the bowel, but tethering to ablation zone and a locule of gas in the bowel wall (arrow). The patient improved with conservative measures and further follow-up imaging (not shown) showed persistence of this tethering, but no gas in the involuting ablation zone to suggest fistulation and the patient remained clinically well.
To our knowledge, there are no published reports of RCYA-induced pancreatitis, but pancreatitis is a complication of direct cryotherapy treatment of the pancreas 49 and it is rational to treat the pancreas with the same caution as the bowel. The collecting system and ureter are also vulnerable to cryotherapy injury 8,9 (but less so than with other thermal ablative modalities), but are better protected with a ureteric stent (see below). Extending the ice ball by small amounts into the liver and spleen does not appear to cause significant complications. Nerve injury is less frequent with cryoablation compared to radiofrequency ablation and is usually transient. 29 Hydrodissection should however be performed for lesions close to the psoas muscle to protect the genitofemoral nerve. Intercostal and abdominal wall nerves should be protected with hydrodissection to prevent paresthesia or abdominal wall bulge.
When performing hydrodissection, awareness of the retroperitoneal planes and spaces is helpful to maximise efficacy (Figure 10). It is also important to remember that hydrodissection can dissipate and continued monitoring, with or without further infusion of fluid, is required throughout the treatment cycle.
Figure 10.
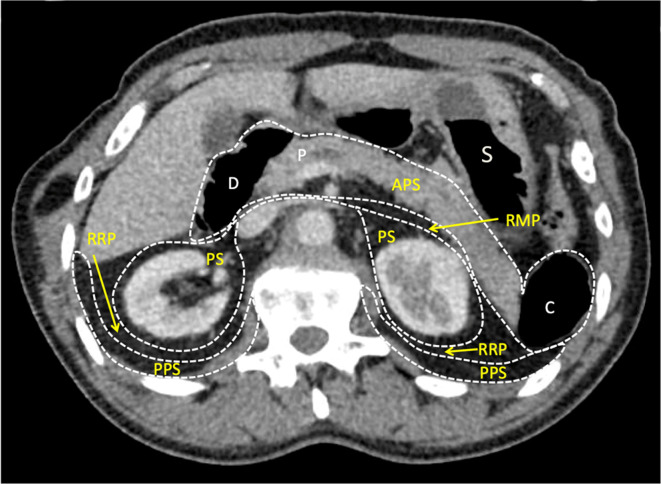
Knowledge of the retroperitoneal anatomy helps target specific spaces to optimise hydrodissection. The RRP lies between the PPS and the PS. The APS contains the retroperitoneal portion of the duodenum (D), the pancreas (P) and the descending (and ascending) colon (C). The RMP separate the APS from the PS and is continuous with the RRP. The stomach (S) is an intraperitoneal organ. APS, anterior pararenalspace; PPS, posterior pararenal space; PS, perinephric space; RMP, retromesentericplane; RRP, retrorenal plane.
Ureteric stent
The edge of the ice ball can extend into the renal sinus by up to 1 cm without causinginjury to the collecting system, 50 but the ureter is less forgiving and injury can result in a stricture. 8,9 Ureteric stents are placed pre-procedure when the ureter is within 1 cm of the tumour for protection. This allows excellent visualisation of the ureter during treatment (Figure 11) and the urothelium to recover, thus preventing stricturing. 8
Figure 11.
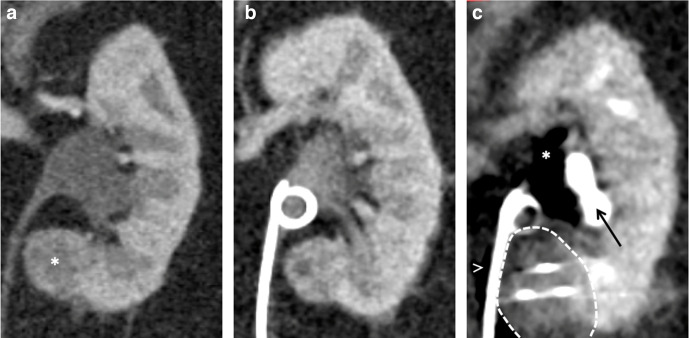
(A) Coronal contrast-enhanced CT of a 68-year-old female demonstrating the proximity of the medial left lower pole papillary renal cell carcinoma (asterisk) to the ureter. (B) Prior to treatment, a ureteric stent was placed in a retrograde manner. (C) Intraprocedural image demonstrating the ice ball (dashed line) encompassing the lesion and extending right up to the ureter. Carbon dioxide (asterisk) was also injected into the renal sinus to protect the calyces that are opacified and displaced laterally (arrow) and carbon dioxide is also tracking adjacent to the ureter (arrowhead). The stent was removed at 6 weeks post-procedure, with no evidence of a collecting system or ureteric injury.
The use of pyeloperfusion with warmed saline is also described for RCYA, but we have not found this necessary.
Pneumodissection
A further means of protecting the ureter or collecting system is injection of carbon dioxide 51 with a fine needle (e.g. 22G spinal needle) around the ureter or directly into the renal sinus fat respectively (Figures 11 and 12) and this provides excellent insulation. Care should be taken not to inject carbon dioxide into the collecting system itself, which may shorten the distance to the tumour. Intravascular injection should also be avoided, but the use of carbon dioxide is safer than air in this respect.
Figure 12.
(A) Intraprocedural CT demonstrating a right posterior tumor (black arrow), close to the ureter (arrowhead) and a major calyx (white arrow). (B) A 22G spinal needle (not shown) was passed into the renal sinus fat just superior to the tumour and carbon dioxide instilled (arrow). This has provided insulation of the calyx and ureter and freezing could be safely performed. Hydrodissection has also been performed to protect the posterior abdominal wall.
Pneumodissection can also be employed around the kidney to protect the bowel. We have noted more haemorrhage around the kidney when using this, presumably due to a reduced tamponade effect, although other groups have shown it to be safe. 52 The time to complete carbon dioxide reabsorption has not been documented in the context of ablation, but carbon dioxide is a highly soluble gas and further injections may be required during the procedure to maintain a safe distance between the ice ball and the critical structure.
Probe torque
If a further adjunctive thermal protective method is required, a ‘stick-freeze’ (freeze for 1 min or use the specific machine function if available) can be performed in order to anchor the probes in the tumour and torque it away from vital structures during treatment. This technique should be used with caution due to the theoretical risk of vascular avulsion.
Other
Other techniques to avoid the lung in treating upper pole tumours include angling the gantry or performing transhepatic treatment. 53 If probes are placed through the pleural cavity, it is important to ensure the ice ball is away from the lung and diaphragm, to reduce injury risk and post-procedural pain. Inflation of a balloon between the tumour and critical structure is also described. 54
7) Treatment
Cryoablation produces extra- and intracellular ice formation which results in cell membrane and intracellular damage. 38 The rapidity of cooling is important in order to ensure intracellular ice formation which is usually lethal to cells. Thawing is required, not only to remove the probes from the patient, but also for a therapeutic effect as it results in endothelial damage, vascular thrombosis, tissue hypoxia and cell death. 38 Passive thawing is thought to result in more cellular damage, as it permits to maximal growth of ice crystals and secondary cell membrane damage, but the choice of active or passive thawing did not matter in an in vivo study. 55 It is however clear that a double freeze-thaw cycle results in significantly more tissue damage. 55 Our standard treatment protocol (Table 3) reflects the above evidence, allows for efficient treatment and falls in line with other described protocols. 8,9,32
Table 3.
Standard cryotherapy treatment protocol
| First cycle: | Second cycle: |
|---|---|
|
|
The active thaw in the second cycle often needs to be increased if there are multiple cryoprobes (e.g. more than around 6) in order to remove them.
8) Post-procedural care
Upon needle removal, an unenhanced CT is performed to assess for any significant haemorrhage or a pneumothorax when treating upper pole tumours. Bed rest for 4 h is employed, with patients being permitted to eat and drink at 1 h, if there are no clinical concerns for an immediate complication. Low molecular weight heparin (LMWH) is routinely given 6 h after the procedure is completed, 56 providing there is no evidence of bleeding or heavy haematuria. LMWH is continued whilst an in-patient.
Routine day 1 CT is not performed unless there is concern for an early complication (bleeding, obstruction due to collecting system haematoma, non-target injury or urine leak). Most patients require no more than simple analgesia and are routinely discharged the next day. Other centres routinely discharge the patient on the same day of the procedure if their pain is controlled. 8,20 Either strategy compares favourably to surgical lengths of admission of between 2.5 and 4.5 days. 57 This likely contributes to the cost effectiveness of RCYA at up to 50% less than surgery. 57,58
9) Follow-up
Patients are reviewed in clinic at 1 month where CT imaging (MRI if young or poor renal function) and blood tests, including renal function, are performed. In the rare circumstance where residual tumour is detected at the initial follow-up, this should be defined as residual unablated tumour. 59 Over 50% of residual unablated tumour is evident on imaging at 1 month and nearly 70% at 3 months. 60 By imaging at 1 month therefore, much of the residual unablated disease is detected, but if there is equivocal change, then an early repeat follow-up scan is performed.
Given the high specificity for cross-sectional imaging to detect disease 6 months following RCYA, 61 with classical imaging appearances and a high pre-test probability (e.g. nodular enhancement at the deep margin of the ablation zone where there may have been a small margin) we do not routinely perform a biopsy before offering further ablation. There are however several reports of fat necrosis mimicking nodular seeding along the cryoprobe path 62,63 and we would advocate biopsy for nodular perinephric change prior to further definitive management.
If there has been adequate treatment at the initial follow-up, then the patients are followed up, with a renal function test and with annual imaging. Recurrent disease detected after at least one post-procedural scan documenting a technically successful ablation is known as local tumour progression, regardless of whether this is early or late. 59
One study reports outcomes specifically for treatment of 21 cases of RCYA recurrence with further RCYA and describes a local recurrence free survival rate of 85% at a median of 30 months. 64 Other large series report successfully treating local tumour progression with repeat RCYA, 9,20,32 so this is usually a therapeutic option, but requires further MDT involvement.
The length of follow-up required is not clear, but because of the very low risk of late local tumour progression, a follow-up duration of at least 5 years is suggested. 65,66 The post-cryoablation imaging appearances are reviewed elsewhere. 67
10) Conclusion
RCYA is a widely accepted, safe and efficacious treatment for early stage RCC. A pre-procedural MDT discussion is important in order to ensure appropriate patients are selected and lesion-specific characteristics are considered. Probe selection and configuration should be based on manufacturer-specific guidance, lesion size and other anatomical factors. This optimises oncological efficacy, but to perform safe treatments, vital structures should be protected by means of patient position and adjunctive methods, such as hydrodissection. Clinical, laboratory and imaging follow-up should then be extended to at least 5 years in order to ensure treatment success and plan any further intervention in the rare instances of local tumour progression.
It is imperative that the treating radiologist leads in each of the steps outlined above and, whilst there is a short learning curve for RCYA, an interventional radiology lead service should mean that results comparable to surgery are attainable.
Contributor Information
Matthew Seager, Email: matthew.seager1@nhs.net.
Shankar Kumar, Email: shankar.kumar@nhs.net.
Emma Lim, Email: emma.lim@nhs.net.
Graham Munneke, Email: graham.munneke@nhs.net.
Steve Bandula, Email: sbandula@nhs.net.
Miles Walkden, Email: miles.walkden@nhs.net.
REFERENCES
- 1. Surveillance, Epidemiology, and End Results (SEER) Program SEER statistics database: Incidence. 2019. Available from: https://seer.cancer.gov/statfacts/html/kidrp.html [cited 2020 July 12].
- 2. Cancer Research UK Cancer incidence for common cancers. 2020. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#collapseZero [cited 2020 July 12].
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; ; 69: 7–34 2019. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 4. Gill IS, Aron M, Gervais DA, Jewett MA. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–34. [DOI] [PubMed] [Google Scholar]
- 5. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst 2006; 98: 1331–4. doi: 10.1093/jnci/djj362 [DOI] [PubMed] [Google Scholar]
- 6. Neves JB, Cullen D, Grant L, Walkden M, Bandula S, Patki P, et al. Protocol for a feasibility study of a cohort embedded randomised controlled trial comparing NEphron Sparing Treatment (NEST) for small renal masses . BMJ Open 2019; 9: e030965. doi: 10.1136/bmjopen-2019-030965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015; 67: 252–9. doi: 10.1016/j.eururo.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 8. Georgiades CS, Rodriguez R. Efficacy and safety of percutaneous cryoablation for stage 1A/B renal cell carcinoma: results of a prospective, single-arm, 5-year study. Cardiovasc Intervent Radiol 2014; 37: 1494–9. doi: 10.1007/s00270-013-0831-8 [DOI] [PubMed] [Google Scholar]
- 9. Breen DJ, King AJ, Patel N, Lockyer R, Hayes M. Image-Guided cryoablation for sporadic renal cell carcinoma: three- and 5-year outcomes in 220 patients with biopsy-proven renal cell carcinoma. Radiology 2018; 289: 554–61. doi: 10.1148/radiol.2018180249 [DOI] [PubMed] [Google Scholar]
- 10. Lim E, Kumar S, Seager M, Modi S, Mandal I, Neves JB, et al. Outcomes of renal tumors treated by image-guided percutaneous cryoablation: immediate and 3- and 5-year outcomes at a regional center. AJR Am J Roentgenol 2020;: 1–6. [DOI] [PubMed] [Google Scholar]
- 11. National Comprehensive Cancer Network Kidney cancer. 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [cited 2020 July 12].
- 12. American Urological Association Renal mass and localized renal cancer: AUA guideline. 2017. Available from: https://www.auanet.org/guidelines/renal-cancer-renal-mass-and-localized-renal-cancer-guideline#x7065 [cited 2020 July 12].
- 13. European Association of Urology Renal cell carcinoma. 2018. Available from: https://uroweb.org/guideline/renal-cell-carcinoma/#11 [cited 2020 July 12].
- 14. Zaid HB, Atwell TD, Schmit G, Boorjian SA, Parker WP, Cheville JC, et al. Cryoablation of cT1 renal masses in the “healthy” patient: Early outcomes from mayo clinic. J Clin Oncol 2017; 35(6_suppl): 433. [Google Scholar]
- 15. Lucas SM, Stern JM, Adibi M, Zeltser IS, Cadeddu JA, Raj GV. Renal function outcomes in patients treated for renal masses smaller than 4 cm by ablative and extirpative techniques. J Urol 2008; 179: 75–9 discussion 9-80. doi: 10.1016/j.juro.2007.08.156 [DOI] [PubMed] [Google Scholar]
- 16. Patel HD, Pierorazio PM, Johnson MH, Sharma R, Iyoha E, Allaf ME, et al. Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: a systematic review and meta-analysis. Clin J Am Soc Nephrol 2017; 12: 1057–69. doi: 10.2215/CJN.11941116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raman JD, Jafri SM, Qi D. Kidney function outcomes following thermal ablation of small renal masses. World J Nephrol 2016; 5: 283–7. doi: 10.5527/wjn.v5.i3.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoun HD, Littrup PJ, Jaber M, Memon F, Adam B, Krycia M, et al. Percutaneous Cryoablation of Renal Tumors: Is It Time for a New Paradigm Shift? J Vasc Interv Radiol 2017; 28: 1363–70. doi: 10.1016/j.jvir.2017.07.013 [DOI] [PubMed] [Google Scholar]
- 19. Kocher NJ, Kunchala S, Reynolds C, Lehman E, Nie S, Raman JD. Adherent perinephric fat at minimally invasive partial nephrectomy is associated with adverse peri-operative outcomes and malignant renal histology. BJU Int 2016; 117: 636–41. doi: 10.1111/bju.13378 [DOI] [PubMed] [Google Scholar]
- 20. Lang EK, Zhang KK, Nguyen Q, Myers L, Allaf M, Colon I. Efficacy of percutaneous cryoablation of renal cell carcinoma in older patients with medical comorbidities: outcome study in 70 patients. Can Urol Assoc J 2015; 9(5-6): E256–61. doi: 10.5489/cuaj.2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med 1990; 77: 1151–63. doi: 10.1093/qjmed/77.2.1151 [DOI] [PubMed] [Google Scholar]
- 22. Matsui Y, Hiraki T, Gobara H, Iguchi T, Tomita K, Uka M, et al. Percutaneous thermal ablation for renal cell carcinoma in patients with Birt-Hogg-Dubé syndrome. Diagn Interv Imaging 2019; 100: 671–7. doi: 10.1016/j.diii.2019.06.009 [DOI] [PubMed] [Google Scholar]
- 23. Degn S, Davidsen JR, Graumann O. Cryoablation: a potential treatment option for renal metastasis from lung cancer? BMJ Case Rep 2018; 2018: bcr-2018-22584101 Nov 2018. doi: 10.1136/bcr-2018-225841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel U, Ramachandran N, Halls J, Parthipun A, Slide C. Synchronous renal masses in patients with a nonrenal malignancy: incidence of metastasis to the kidney versus primary renal neoplasia and differentiating features on CT. AJR Am J Roentgenol 2011; 197: W680–6. doi: 10.2214/AJR.11.6518 [DOI] [PubMed] [Google Scholar]
- 25. Patel IJ, Rahim S, Davidson JC, Hanks SE, Tam AL, Walker TG, et al. Society of interventional radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions-part II: recommendations: endorsed by the Canadian association for interventional radiology and the cardiovascular and interventional radiological Society of Europe. J Vasc Interv Radiol 2019; 30: 1168–84. doi: 10.1016/j.jvir.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 26. Fernando A, Fowler S, O'Brien T, .British Association of Urological Surgeons (BAUS) . Nephron-sparing surgery across a nation - outcomes from the British Association of Urological Surgeons 2012 national partial nephrectomy audit. BJU Int 2016; 117: 874–82. doi: 10.1111/bju.13353 [DOI] [PubMed] [Google Scholar]
- 27. Seager MJ, Patel U, Anderson CJ, Gonsalves M. Image-Guided biopsy of small (≤4 cm) renal masses: the effect of size and anatomical location on biopsy success rate and complications. Br J Radiol 2018; 91: 20170666. doi: 10.1259/bjr.20170666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Richard PO, Jewett MAS, Bhatt JR, Kachura JR, Evans AJ, Zlotta AR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol 2015; 68: 1007–13. doi: 10.1016/j.eururo.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 29. Atwell TD, Carter RE, Schmit GD, Carr CM, Boorjian SA, Curry TB, et al. Complications following 573 percutaneous renal radiofrequency and cryoablation procedures. J Vasc Interv Radiol 2012; 23: 48–54. doi: 10.1016/j.jvir.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 30. Wah TM, Irving HC. Infectious complications after percutaneous radiofrequency ablation of renal cell carcinoma in patients with ileal conduit. J Vasc Interv Radiol 2008; 19: 1382–5. doi: 10.1016/j.jvir.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 31. Buchan T, Walkden M, Jenkins K, Sultan P, Bandula S. High-Frequency jet ventilation during cryoablation of small renal tumours. Cardiovasc Intervent Radiol 2018; 41: 1067–73. doi: 10.1007/s00270-018-1921-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buy X, Lang H, Garnon J, Sauleau E, Roy C, Gangi A. Percutaneous renal cryoablation: prospective experience treating 120 consecutive tumors. AJR Am J Roentgenol 2013; 201: 1353–61. doi: 10.2214/AJR.13.11084 [DOI] [PubMed] [Google Scholar]
- 33. Cornelis FH, Monard E, Moulin MA, Vignaud E, Laveissiere F, Ben Ammar M, et al. Sedation and analgesia in interventional radiology: where do we stand. where are we heading and why does it matter? Diagn Interv Imaging 2019; 100: 753–62. [DOI] [PubMed] [Google Scholar]
- 34. Edgcombe H, Carter K, Yarrow S. Anaesthesia in the prone position. Br J Anaesth 2008; 100: 165–83. doi: 10.1093/bja/aem380 [DOI] [PubMed] [Google Scholar]
- 35. Bhagavatula SK, Tuncali K, Shyn PB, Levesque VM, Chang SL, Silverman SG. Percutaneous CT- and MRI-guided cryoablation of cT1 renal cell carcinoma: intermediate- to long-term outcomes in 307 patients. Radiology 2020; 296: 687–95. doi: 10.1148/radiol.2020200149 [DOI] [PubMed] [Google Scholar]
- 36. Rupp CC, Hoffmann NE, Schmidlin FR, Swanlund DJ, Bischof JC, Coad JE. Cryosurgical changes in the porcine kidney: histologic analysis with thermal history correlation. Cryobiology 2002; 45: 167–82. doi: 10.1016/s0011-2240(02)00125-6 [DOI] [PubMed] [Google Scholar]
- 37. Uchida M, Imaide Y, Sugimoto K, Uehara H, Watanabe H. Percutaneous cryosurgery for renal tumours. Br J Urol 1995; 75: 132–6 discussion 6-7. doi: 10.1111/j.1464-410x.1995.tb07297.x [DOI] [PubMed] [Google Scholar]
- 38. Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology 1998; 37: 171–86. doi: 10.1006/cryo.1998.2115 [DOI] [PubMed] [Google Scholar]
- 39. Shah TT, Arbel U, Foss S, Zachman A, Rodney S, Ahmed HU, et al. Modeling cryotherapy ice ball dimensions and isotherms in a novel gel-based model to determine optimal cryo-needle configurations and settings for potential use in clinical practice. Urology 2016; 91: 234–40. doi: 10.1016/j.urology.2016.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ge BH, Guzzo TJ, Nadolski GJ, Soulen MC, Clark TWI, Malkowicz SB, et al. Percutaneous renal cryoablation: Short-axis ice-ball margin as a predictor of outcome. J Vasc Interv Radiol 2016; 27: 403–9. doi: 10.1016/j.jvir.2015.11.035 [DOI] [PubMed] [Google Scholar]
- 41. Breda A, Lam JS, Riggs S, Leppert JT, Gui D, Said JW, et al. In vivo efficacy of laparoscopic assisted percutaneous renal cryotherapy: evidence based guidelines for the practicing urologist. J Urol 2008; 179: 333–7. doi: 10.1016/j.juro.2007.08.089 [DOI] [PubMed] [Google Scholar]
- 42. Weld KJ, Hruby G, Humphrey PA, Ames CD, Landman J. Precise characterization of renal parenchymal response to single and multiple cryoablation probes. J Urol 2006; 176: 784–6. doi: 10.1016/j.juro.2006.03.084 [DOI] [PubMed] [Google Scholar]
- 43. Nichols DM, Cooperberg PL, Golding RH, Burhenne HJ. The safe intercostal approach? pleural complications in abdominal interventional radiology. AJR Am J Roentgenol 1984; 142: 1013–8. doi: 10.2214/ajr.142.5.1013 [DOI] [PubMed] [Google Scholar]
- 44. Atwell TD, Schultz GG, Leibovich BC, Schmit GD, Boorjian SA, Kurup AN, et al. Patient-Reported outcomes after percutaneous renal ablation: initial experience. AJR Am J Roentgenol 2019; 212: 672–6. doi: 10.2214/AJR.17.19463 [DOI] [PubMed] [Google Scholar]
- 45. Ahrar K, Matin S, Wallace MJ, Gupta S, Hicks ME. Percutaneous transthoracic radiofrequency ablation of renal tumors using an iatrogenic pneumothorax. AJR Am J Roentgenol 2005; 185: 86–8. doi: 10.2214/ajr.185.1.01850086 [DOI] [PubMed] [Google Scholar]
- 46. Bodily KD, Atwell TD, Mandrekar JN, Farrell MA, Callstrom MR, Schmit GD, et al. Hydrodisplacement in the percutaneous cryoablation of 50 renal tumors. AJR Am J Roentgenol 2010; 194: 779–83. doi: 10.2214/AJR.08.1570 [DOI] [PubMed] [Google Scholar]
- 47. Silverman SG, Tuncali K, vanSonnenberg E, Morrison PR, Shankar S, Ramaiya N, et al. Renal tumors: MR imaging-guided percutaneous cryotherapy--initial experience in 23 patients. Radiology 2005; 236: 716–24. doi: 10.1148/radiol.2362041107 [DOI] [PubMed] [Google Scholar]
- 48. Shimizu K, Mogami T, Michimoto K, Kameoka Y, Tokashiki T, Kurata N, et al. Digestive tract complications of renal cryoablation. Cardiovasc Intervent Radiol 2016; 39: 122–6. doi: 10.1007/s00270-015-1110-7 [DOI] [PubMed] [Google Scholar]
- 49. Xu KC, Niu LZ, Hu YZ, He WB, He YS, Zuo JS. Cryosurgery with combination of (125)iodine seed implantation for the treatment of locally advanced pancreatic cancer. J Dig Dis 2008; 9: 32–40. doi: 10.1111/j.1443-9573.2007.00322.x [DOI] [PubMed] [Google Scholar]
- 50. Rosenberg MD, Kim CY, Tsivian M, Suberlak MN, Sopko DR, Polascik TJ, et al. Percutaneous cryoablation of renal lesions with radiographic ice ball involvement of the renal sinus: analysis of hemorrhagic and collecting system complications. AJR Am J Roentgenol 2011; 196: 935–9. doi: 10.2214/AJR.10.5182 [DOI] [PubMed] [Google Scholar]
- 51. Buy X, Tok C-H, Szwarc D, Bierry G, Gangi A. Thermal protection during percutaneous thermal ablation procedures: interest of carbon dioxide dissection and temperature monitoring. Cardiovasc Intervent Radiol 2009; 32: 529–34. doi: 10.1007/s00270-009-9524-8 [DOI] [PubMed] [Google Scholar]
- 52. Maurice MJ, Haaga JR, Nakamoto DA, Ponsky LE. Pneumodissection: an alternative protective technique for the percutaneous cryoablation of small renal masses. Urol Int 2013; 90: 381–3. doi: 10.1159/000346332 [DOI] [PubMed] [Google Scholar]
- 53. Malcolm JB, Gold R, Derweesh IH. Pilot experience with transhepatic percutaneous renal cryoablation. J Endourol 2007; 21: 721–5. doi: 10.1089/end.2006.0388 [DOI] [PubMed] [Google Scholar]
- 54. Sone M, Arai Y, Sugawara S, Tomita K, Fujiwara K, Ishii H, et al. Angio-CT-assisted balloon dissection: protection of the adjacent intestine during cryoablation for patients with renal cancer. J Vasc Interv Radiol 2016; 27: 1414–9. doi: 10.1016/j.jvir.2016.02.028 [DOI] [PubMed] [Google Scholar]
- 55. Woolley ML, Schulsinger DA, Durand DB, Zeltser IS, Waltzer WC. Effect of freezing parameters (freeze cycle and thaw process) on tissue destruction following renal cryoablation. J Endourol 2002; 16: 519–22. doi: 10.1089/089277902760367494 [DOI] [PubMed] [Google Scholar]
- 56. Jaffe TA, Raiff D, Ho LM, Kim CY. Management of anticoagulant and antiplatelet medications in adults undergoing percutaneous interventions. AJR Am J Roentgenol 2015; 205: 421–8. doi: 10.2214/AJR.14.13342 [DOI] [PubMed] [Google Scholar]
- 57. Chehab M, Friedlander JA, Handel J, Vartanian S, Krishnan A, Wong C-YO, et al. Percutaneous cryoablation vs partial nephrectomy: cost comparison of T1a tumors. J Endourol 2016; 30: 170–6. doi: 10.1089/end.2015.0183 [DOI] [PubMed] [Google Scholar]
- 58. Xing M, Kokabi N, Kim H. Cost-utility analysis of surgery vs. Ablation in stage T1a renal cell carcinoma: A surveillance, epidemiology, and end results (SEER)-medicare study. J Vasc Interv Radiol 2016; 27: S132. [Google Scholar]
- 59. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 2014; 273: 241–60. doi: 10.1148/radiol.14132958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Matin SF, Ahrar K, Cadeddu JA, Gervais DA, McGovern FJ, Zagoria RJ, et al. Residual and recurrent disease following renal energy ablative therapy: a multi-institutional study. J Urol 2006; 176: 1973–7. doi: 10.1016/j.juro.2006.07.016 [DOI] [PubMed] [Google Scholar]
- 61. Weight CJ, Kaouk JH, Hegarty NJ, Remer EM, O'Malley CM, Lane BR, et al. Correlation of radiographic imaging and histopathology following cryoablation and radio frequency ablation for renal tumors. J Urol 2008; 179: 1277–81 discussion 81-3. doi: 10.1016/j.juro.2007.11.075 [DOI] [PubMed] [Google Scholar]
- 62. Kahn AE, Wu KJ, Thiel DD. Tumefactive fat necrosis with multinucleate giant cell reaction mimicking recurrent renal cell carcinoma following percutaneous renal cryoablation. Case Rep Urol 2019; 2019: 1678193. doi: 10.1155/2019/1678193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Durack JC, Richioud B, Lyon J, Solomon SB. Late emergence of contrast-enhancing fat necrosis mimicking tumor seeding after renal cryoablation. J Vasc Interv Radiol 2014; 25: 133–7. doi: 10.1016/j.jvir.2013.07.006 [DOI] [PubMed] [Google Scholar]
- 64. Okhunov Z, Chamberlin J, Moreira DM, George A, Babaian K, Shah P, et al. Salvage percutaneous cryoablation for locally recurrent renal-cell carcinoma after primary cryoablation. J Endourol 2016; 30: 632–7. doi: 10.1089/end.2016.0088 [DOI] [PubMed] [Google Scholar]
- 65. Clark TWI, Millward SF, Gervais DA, Goldberg SN, Grassi CJ, Kinney TB, et al. Reporting standards for percutaneous thermal ablation of renal cell carcinoma. J Vasc Interv Radiol 2009; 20(7 Suppl): S409–16. doi: 10.1016/j.jvir.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 66. Krokidis ME, Orsi F, Katsanos K, Helmberger T, Adam A. Cirse guidelines on percutaneous ablation of small renal cell carcinoma. Cardiovasc Intervent Radiol 2017; 40: 177–91. doi: 10.1007/s00270-016-1531-y [DOI] [PubMed] [Google Scholar]
- 67. Patel N, King AJ, Breen DJ. Imaging appearances at follow-up after image-guided solid-organ abdominal tumour ablation. Clin Radiol 2017; 72: 680–90. doi: 10.1016/j.crad.2017.01.014 [DOI] [PubMed] [Google Scholar]