Abstract
Objective:
To assess the diagnostic efficacy of contrast-enhanced digital mammography (CEDM) in breast cancer detection in comparison to synthetic two-dimensional mammography (s2D MG), digital breast tomosynthesis (DBT) alone and DBT supplemented with ultrasound examination in females with dense breast with histopathology as the gold-standard.
Methods:
It was a prospective study, where consecutive females presenting to symptomatic breast clinic between April 2019 and June 2020 were evaluated with DBT. Females who were found to have heterogeneously dense (ACR type C) or extremely dense (ACR type D) breast composition detected on s2D MG were further evaluated with high-resolution breast ultrasound and thereafter with CEDM, but before the core biopsy or surgical excision, were included in the study. s2D MG was derived from post-processing reconstruction of DBT data set. Females with pregnancy, renal insufficiency or prior allergic reaction to iodinated contrast agent were excluded from the study. Image interpretation was done by two experienced breast radiologists and both were blinded to histological diagnosis.
Results:
This study included 166 breast lesions in130 patients with mean age of 45 ± 12 years (age range 24–72 years). There were 87 (52.4%) malignant and 79 (47.6%) benign lesions. The sensitivity of CEDM was 96.5%, significantly higher than synthetic 2D MG (75.6%, p < 0.0001), DBT alone (82.8%, p < 0.0001) and DBT + ultrasound (88.5%, p = 0.0057); specificity of CEDM was 81%, significantly higher than s2D MG (63.3%, p = 0.0002) and comparable to DBT alone (84.4%, p = 0.3586) and DBT + ultrasound (79.7%, p = 0.4135). In receiver operating characteristic curve analysis, the area under the curve was of 0.896 for CEDM, 0.841 for DBT + ultrasound, 0.769 for DBT alone and 0.729 for s2D MG.
Conclusion:
CEDM is an accurate diagnostic technique for cancer detection in dense breast. CEDM allowed a significantly higher number of breast cancer detection than the s2D MG, DBT alone and DBT supplemented with ultrasonography in females with dense breast.
Advances in knowledge:
CEDM is a promising novel technology with higher sensitivity and negative predictive value for breast cancer detection in females with dense breast in comparison to DBT alone or DBT supplemented with ultrasound.
Introduction
Contrast-enhanced digital mammography (CEDM) is a newer technique based on the visualization of iodinated contrast within the tumor under mammography imaging. Females with dense breasts not only have increased risk of developing breast cancer but also have higher chances of missing the underlying tumor due to overlapping breast tissue, which may remain undetected on conventional mammography. 1 The sensitivity of two-dimensional (2D) mammography may be as low as 30–45% in females with dense breasts as opposed to 98% in mammographically fatty breasts and 75–85% of overall sensitivity. 2–5 Digital breast tomosynthesis (DBT) allows the breast to be imaged in multiple thin slices while reducing the obscuration due to breast tissue superimposition. Thus, the resultant imaging data set of DBT has the potential to improve the sensitivity and specificity of mammographic imaging for breast cancer detection in dense breast. 6 The synthetic 2D mammography (s2D MG) images derived from DBT may be utilized in place of full-field digital mammography (FFDM) to reduce radiation exposure to the breast. Several studies published in the last few years have shown that there is no significant difference in the sensitivity and negative predictive value of s2D MG and FFDM for breast cancer detection. 6–8 Ultrasound is another useful tool for screening of breast, which does not get influenced by breast density. It is an effective technology in identifying mammographically occult cancer in dense breasts and may detect up to three additional cancers per thousand females in comparison to the conventional 2D mammography. Studies have shown that ultrasound significantly increases the detection of clinically relevant, small invasive, early-stage and node-negative breast cancers. 9 However, microcalcifications or subtle distortion which can be detected on mammography may remain undetected on ultrasound. Currently, dynamic contrast-enhanced (DCE) MRI has the highest reported sensitivity among all the imaging modalities for breast cancer detection ranging between 79 and 98%. 10,11 However, due to lack of wide availability, high cost, long image acquisition time, claustrophobia and certain contraindications, e.g. patients with cardiac pacemakers, aneurysmal clips and other metallic hardware in the body, it may not be feasible to perform MRI in all females with dense breasts.
Similar to the basic principle of contrast-enhanced MRI, CEDM technique was recently developed to detect the tumor angiogenesis and to improve the diagnostic performance of mammography. The CEDM procedure is based on weighted subtraction processing after a dual-energy exposure (at low and high energy levels) in rapid succession to the compressed breast after intravenous administration of non-ionic iodinated contrast material. The low energy image is equivalent to the digital mammography image. The difference between the X-ray attenuation by the iodine and the breast tissue at two different energy levels are post-processed to suppress the density due to background parenchyma and to highlight the contrast enhancement within the hypervascular tumors. Thus, this newer technology has the potential to detect occult cancers in dense breasts. However, there are very limited studies published on the diagnostic efficiency of dual-energy CEDM in dense breasts.
The purpose of this study was to prospectively evaluate and compare the diagnostic accuracy of dual-energy CEDM, DBT (3D mammography), s2D mammography and DBT + ultrasound in detection of breast cancer in females with dense breasts with histopathology as the gold-standard.
Methods and materials
This single center prospective study was approved by the institutional review board. Informed written consent was obtained from all patients included in the study.
Patients
Consecutive females presenting to symptomatic breast clinic between April 2019 and June 2020, evaluated with DBT at our center, found to have either heterogeneously dense (ACR category C) or extremely dense (ACR category D) breast composition on DBT/s2D MG, as determined by experienced breast radiologists, subsequently evaluated with high-resolution whole-breast ultrasound and thereafter with CEDM, but before the core biopsy or surgical excision, were included in the study. Synthetic 2D MG was derived from post-processing reconstruction of DBT data set. Females with pregnancy, renal insufficiency, or prior allergic reaction to iodinated contrast agent and diagnosed cases of breast cancers treated with neoadjuvant chemotherapy were excluded from the study.
Image acquisition
DBT and synthetic 2D digital mammography
DBT was performed for each breast in craniocaudal (CC) and mediolateral oblique (MLO) positions and subsequently synthetic 2D mammography projections were derived from the logarithmic reconstruction.
Ultrasound
Ultrasound examination was performed after DBT examination on Siemen’s Acuson s2000 using a high-resolution linear probe (Frequency 12–18 MHz). Radial scanning of bilateral breast and the axillary tail was performed in a supine position, while the patient’s arms were relaxed and flexed behind the head. The lateral lesions including axilla were scanned in a contralateral oblique position. Longitudinal and transverse images of the breast lesion were obtained.
CEDM
CEDM was performed after the DBT and ultrasound examinations and prior to the breast biopsy or surgical excision. A wide bore cannula was inserted into the peripheral vein, preferably antecubital vein while the patient was sitting comfortably in an armchair. An intravenous injection of non-ionic iodinated contrast media (Omnipaque 350 mg ml−1; GE Healthcare, Inc. Cork, Ireland) was administered at the rate of 3 ml s−1 followed by 20 ml of the saline chase at the same rate. At 2 min, from the starting of contrast administration, the patient was positioned under the mammography for image acquisition of the compressed breast. For each breast, a pair of low and high energy images were obtained in CC and MLO views, and the image acquisition of both the breasts was completed within 5–6 min after the initiation of contrast administration. The low-energy (LE) image acquisition was done at 26–32 kV and high-energy (HE) image acquisition at 45-49 kV. After post-processing of low and high energy images, recombined images (subtracted contrast images) of each breast in CC and MLO views were derived and interpreted for the contrast enhancement.
Image interpretation
A dedicated workstation (Barco Mammography Diagnostic Workstation, GE Healthcare) was used for the image analysis. s2D MG and DBT images were independently read. Image interpretation of s2D MG was done first followed by the interpretation of DBT images, whole breast ultrasound and CEDM. All were evaluated in consensus by two experienced breast radiologists. Both the radiologists were blinded to final histopathological diagnosis.
On s2D MG, DBT and ultrasound, breast lesions were evaluated and lesions were categorized based on ACR Breast Imaging Reporting and Data System (BI-RADS) fifth edition. BI-RADS category 1, 2 and 3 were considered benign and category 4 and 5 were considered malignant.
On CEDM, recombined images were assessed for the contrast enhancement in correlation with the morphological features on low energy images. The enhancing areas were further classified as mass or non-mass enhancement. Enhancing mass was assessed for its margins (circumscribed or nor-circumscribed), the intensity of enhancement (mild, moderate or intense) and pattern of internal enhancement (homogenous, heterogeneous or ring enhancement). The non-mass enhancing areas were assessed for symmetry. Bilateral symmetrical non-mass enhancement with no morphological abnormality in LE image was considered background parenchymal enhancement (BPE). The asymmetric non-mass enhancing area was considered abnormal, and similar to MRI they were further assessed for distribution (focal, ductal, segmental or regional), the pattern of internal enhancement (homogenous or heterogeneous) and intensity of enhancement (mild, moderate and severe). Non-enhancing breast lesions were considered benign (BI-RADS ≤3) and enhancing lesions (mass and non-mass asymmetric enhancement) were considered suspicious (BI-RADS ≥4), although there is no standardized BI-RADS Lexicon yet for CEDM.
Pathological evaluation
The reference standard was the final histopathology of the surgical specimen for malignant breast lesions, and histopathology of the surgical specimen or core needle biopsy sample for the benign breast lesions. Ductal carcinoma in situ and invasive carcinomas were counted as malignant lesions. All other pathologies including lobular carcinoma in situ, fibroadenoma, fibroadenosis, ductal hyperplasia, sclerosing adenosis, cysts and non-invasive phyllodes tumor were considered non-malignant lesions.
Statistical analysis
Continuous variables were reported as mean and standard deviation value. Visualized lesions on DBT, s2D MG, ultrasound and CEDM were categorized into groups: true positive (BI-RADS ≥4 lesion on imaging and invasive or non-invasive breast cancer on histology); false positive (BI-RADS ≥4 lesion on imaging and proven benign on histology); false negative (BI-RADS ≤3 lesion on imaging and diagnosed breast cancer on histology); true negative (BI-RADS ≤3 on imaging and proven benign lesion on histology). Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated for each diagnostic modality. Diagnostic parameters were compared using the χ2 test and p-value < 0.05 was considered significant. Receiver operating characteristic (ROC) curve analysis was done for DBT, s2D MG, DBT + ultrasound and CEDM results and area under the curve (AUC) were calculated. All the statistical analyses were done using SPSS (IBM v. 22) software.
Results
This study cohort included 166 breast lesions in 130 patients with a mean age of 45 ± 12 years (age range 24–72 years). The detailed distribution of histological diagnosis of breast lesions is summarized in Table 1. Solitary breast lesion was diagnosed in 105 patients (80.8%), 2 or more lesions in the same breast in 16 patients (12.3%), and contralateral breast lesions in 9 patients (6.9%). Out of 166 breast lesions, 87 (52.4%) were malignant and 79 (47.6%) were benign lesions. Of 87 malignant masses, 73 (84%) were invasive carcinomas and 14 (16%) were ductal carcinoma in situ alone. Of 73 invasive cancers, 65 infiltrating ductal carcinomas (IDCs), 3 invasive lobular carcinomas (ILCs), 2 papillary carcinomas, 1 mucinous carcinoma and 2 metastases. Benign lesions included fibroadenomas (35), adenosis (17), mastitis (6), sclerosing adenosis (3), benign phyllodes tumor (5), papillomas (4), ductal hyperplasias (5) and fibrosis (4).
Table 1.
Histopathological diagnosis of breast lesions
| Breast pathologies | Number (n = 166) |
% |
|---|---|---|
| Malignant lesions | 87 | 52.4% |
| DCIS | 14 | 16% |
| Invasive carcinomas | 73 | 84% |
| IDC | 60 | 82.2% |
| ILC | 4 | 5.5% |
| Papillary carcinoma | 3 | 4.1% |
| Mucinous carcinoma | 2 | 2.7% |
| Malignant phyllodes | 2 | 2.7% |
| Metastases | 2 | 2.7% |
| Benign | 79 | (47.6%) |
| Fibroadenoma | 35 | 44.3% |
| Adenosis | 17 | 21.5% |
| Mastitis | 6 | 7.6% |
| Ductal hyperplasia | 5 | 6.4% |
| Sclerosing adenosis | 3 | 3.8% |
| Phyllodes | 5 | 6.4% |
| Papillomas | 4 | 5% |
| Fibrosis | 4 | 5% |
DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma.
The distribution of breast lesions detected on s2D MG, DBT alone, DBT + ultrasound and CEDM are summarized in Table 2. The s2D MG identified 108 lesions in 98 patients. Of 108 lesions, 99 (91.7%) lesions in a single breast and 9 lesions (8.3%) in the contralateral breast; 95 (88%) were BI-RADS ≥4 and 13 (12%) were BI-RADS ≤3 lesions. Of 95 suspicious lesions, 66 lesions (69.5%) were histologically malignant and 29 (30.5%) were benign. Of 95 suspected lesions detected on MG, 88 lesions (92.6%) were detected in the single breast and 7 lesions (7.4%) in the contralateral breast. Of 66 malignant lesions detected on MG, 54 lesions were invasive carcinomas (81.8%) and 12 were ductal carcinomas in situ (18.2%).
Table 2.
Distribution of breast lesions detected on s2D MG, DBT (3D), ultrasound combined with DBT and CEDM
| Examinations | Lesions with pathological diagnosis n = 166 (patients, 130) |
Single breast (151) |
Contralateral breast lesion (15) | Suspicious Lesions (BI-RADS ≥4) |
Malignant (87) | Benign (79) |
Invasive cancers (73) | DCIS (14) |
|---|---|---|---|---|---|---|---|---|
| s2D MG | 108 (98) | 99 | 9 | 95 | 66 | 29 | 54 | 12 |
| DBT (3D) | 134 (120) | 118 | 16 | 84 | 72 | 12 | 60 | 12 |
| Ultrasound + DBT | 144 (125) | 122 | 22 | 95 | 77 | 18 | 64 | 13 |
| CEDM | [td] | 90 | 9 | 99 | 84 | 15 | 72 | 12 |
CEDM, contrast-enhanced digital mammography; DBT, digital breast tomosynthesis; DCIS, ductal carcinoma in situ; s2D MG, synthetic two-dimensional mammography.
DBT identified 134 lesions in 120 patients of which 118 in a single breast and 16 in the contralateral breast. Of 134 lesions, 84 (62.7%) were suspicious lesions (BI-RADS ≥4) and 50 (37.3%) were BI-RADS ≤3 lesions. Of 84 suspicious lesions, 72 (85.7%) were histologically malignant and 12 (14.3%) were benign breast lesions. Of 72 malignant lesions, 60 (83.3%) invasive cancers and 12 (16.7%) DCIS.
DBT + ultrasound examination identified 144 lesions in 125 patients, 122 (84.7%) lesions in single breast and 22 (15.3%) lesions in contralateral breast. Of 144 lesions, 95 (66%) were suspicious (BI-RADS ≥4) of which 77 (81%) were histologically malignant and 18 were benign lesions (19%). Of 77 malignant lesions, 64 (83.1%) were histologically invasive carcinomas and 13 (16.9%) were DCIS.
CEDM detected 99 enhancing lesions in 127 patients, 90 (90.9%) in a single breast and 9 (9.1%) in the contralateral breast. Of 99 enhancing lesions, 84 (84.8%) were histologically malignant and 15 (15.2%) were benign breast lesions. Of 84 malignant lesions, 72 (85.7%) were invasive breast cancers and 12 (14.3%) were DCIS.
The breast cancers, which were missed on imaging, are summarized in Table 3. The lesions missed on s2D MG or DBT were either small invasive cancers with a mean size of 7.2 mm (range 5–16 mm) or DCIS without microcalcifications. The missed DCIS and one of the ILCs were seen as non-mass enhancement on CEDM, and the other invasive cancers (IDC and papillary carcinomas) as small enhancing masses. CEDM did not show enhancement in two cases of DCIS (low/intermediate nuclear grade) which were detected as grouped amorphous calcifications on s2D MG and DBT, and in one case of well-differentiated invasive mucinous carcinoma which was detected on breast ultrasound as non-circumscribed mixed echogenic mass.
Table 3.
Histopathological distribution of missed breast cancers on s2D MG, DBT, DBT + ultrasound and CEDM
| Missed cancers on imaging |
Histopathological diagnosis of missed breast cancers | |
|---|---|---|
| s2D MG | 21 (24.1%) | ILC (3), DCIS without calcifications (2), Papillary carcinoma (2), Mucinous carcinoma (1), IDC (13) |
| DBT | 15 (17.2%) | ILC (1), DCIS without calcifications (2) Papillary carcinoma (2) Mucinous carcinoma (1) IDC (9) |
| DBT + ultrasound | 10 (11.5%) | DCIS without calcifications (1) ILC (1) IDC (8) |
| CEDM | 3 (3.4%) | DCIS with amorphous calcifications (2) Mucinous carcinoma(1) |
CEDM, contrast-enhanced digital mammography; DBT, digital breast tomosynthesis; DCIS, ductal carcinoma in situ; IDC, Invasive ductal carcinoma; ILC, Invasive lobular carcinoma;s2D MG, synthetic two-dimensional mammography.
Accuracies of BI-RADS assessments on all four modalities with histological diagnosis as the reference standard are summarized in Figure 1. The highest rate of true-positive diagnosis was made on CEDM (96.5%), followed by 87.4% on DBT + ultrasound, 82.8% on DBT alone, and 71.3% on MG. The largest rate of false-negative diagnoses was made on MG (28.7%) followed by DBT (17.2%), ultrasound (12.6%) and CEDM (3.4%). The sensitivity, specificity, PPV, NPV and accuracy of MG, DBT, DBT + ultrasound and CEDM are summarized in Table 4. ROC curve analysis based on the BI-RADS assessment for differentiating benign and malignant lesions revealed AUC of 0.896 for CEDM, 0.841 for DBT + ultrasound, 0.769 for DBT alone and 0.729 for s2D MG (Figure 2). An example of breast cancer identified on s2D MG, DBT, ultrasound and CEDM in a female with the dense breast is demonstrated in Figures 3–6.
Figure 1.
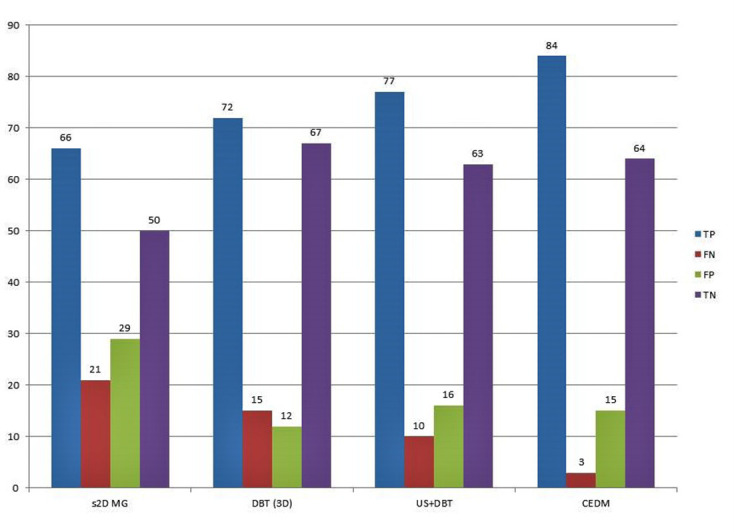
Accuracy results of BI-RADS assessments on s2D MG, DBT (3D), ultrasound and CEDM with reference to histopathological diagnosis. BI-RADS, Breast Imaging Reporting and Data System; CEDM, contrast-enhanced digital mammography; DBT, digital breast tomosynthesis; s2D MG, synthetic two-dimensional mammography.
Table 4.
Sensitivity, specificity, PPV, NPV and accuracy of MG, DBT, ultrasound and CEDM
| Imaging modalities | Sensitivity | Specificity | PPV | NPV | Accuracy |
|---|---|---|---|---|---|
| MG (s2D) | 75.6% | 63.3% | 69.1% | 70.4% | 69.7% |
| DBT (3D) | 82.8% | 84.8% | 85.7% | 81.7% | 83.7% |
| Ultrasound + DBT | 88.5% | 79.7% | 82.8% | 86.3% | 84.3% |
| CEDM | 96.5% | 81% | 84.8% | 95.5% | 89.2% |
| p-value: | |||||
| CEDM vs .MG | <0.0001 | 0.0002 | 0.0007 | <0.0001 | <0.0001 |
| CEDM vs DBT | <0.0001 | 0.3586 | 0.8174 | 0.0001 | 0.1438 |
| CEDM vs ultrasound + DBT | 0.0057 | 0.4135 | 0.6215 | 0.0036 | 0.1886 |
| DBT vs MG | 0.1066 | <0.0001 | 0.0003 | 0.0160 | 0.0026 |
| DBT vs ultrasound + DBT | 0.1399 | 0.2654 | 0.4689 | 0.2537 | 0.8816 |
| Ultrasound + DBT vs. MG | 0.0022 | 0.0007 | 0.0159 | 0.0004 | 0.0016 |
CEDM, contrast-enhanced digital mammography; DBT, digital breast tomosynthesis; MG, mammography; NPV, negative predictive value; PPV, positive predictive value.
Figure 2.
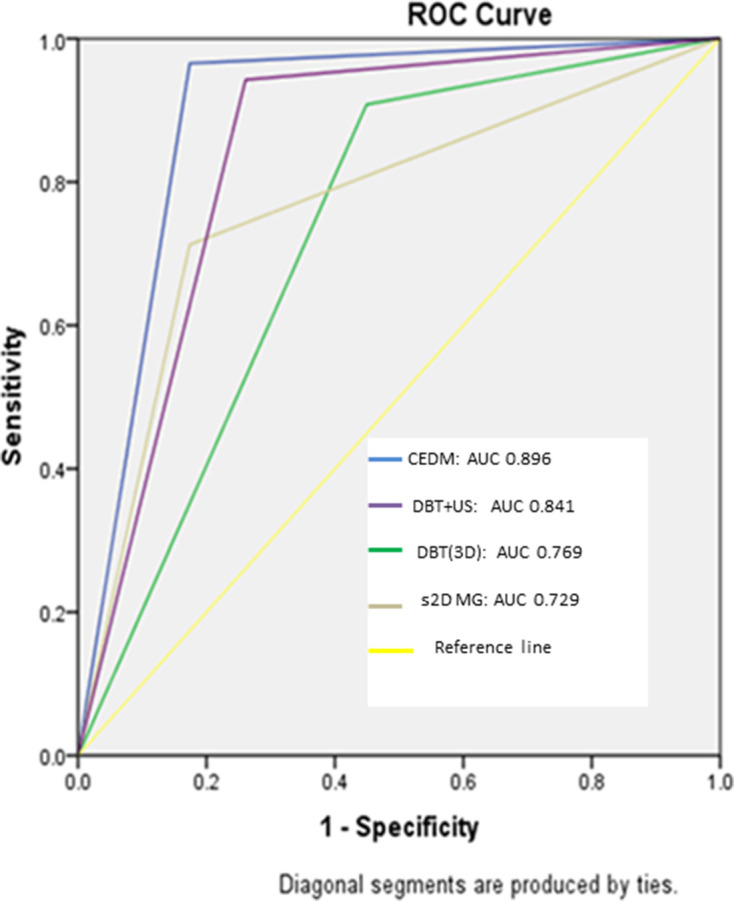
.ROC curves with AUC for s2D MG, DBT (3D) alone, DBT + ultrasound and CEDM for detecting cancer in dense breast. AUC, area under thecurve; BI-RADS, Breast Imaging Reporting and Data System; CEDM, contrast-enhanced digital mammography; DBT, digital breast tomosynthesis; ROC, receiver operating characteristic; s2D MG, synthetic two-dimensional mammography.
Figure 3.
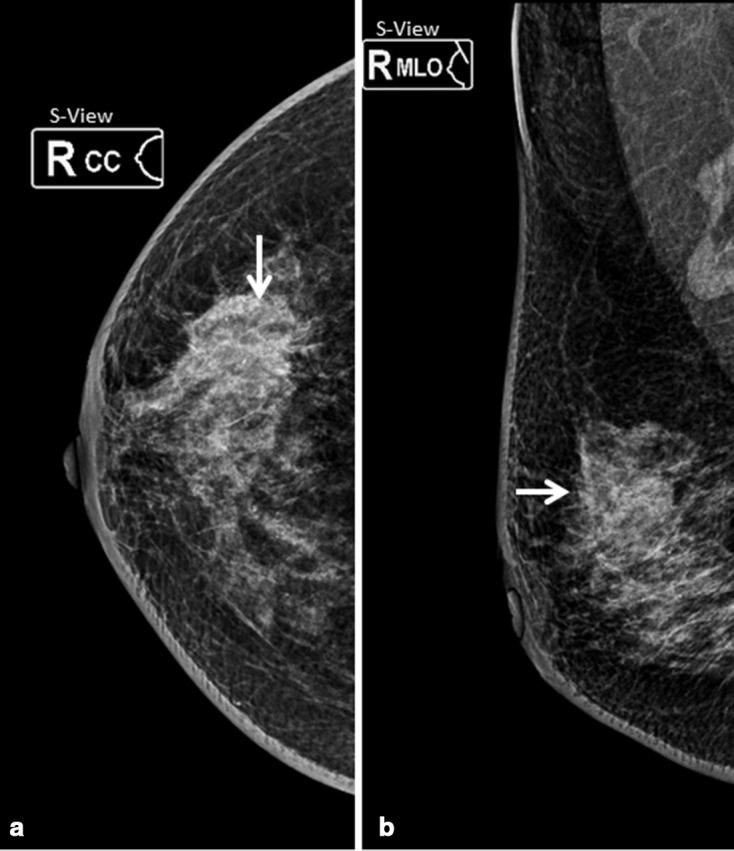
Synthetic 2D mammography of right breast, cranio-caudal and medio-lateral oblique projections showed heterogeneously dense breast tissue (ACR type C) with a non-circumscribed isodense irregular mass in upper outer quadrant (arrow in a and b).
Figure 4.
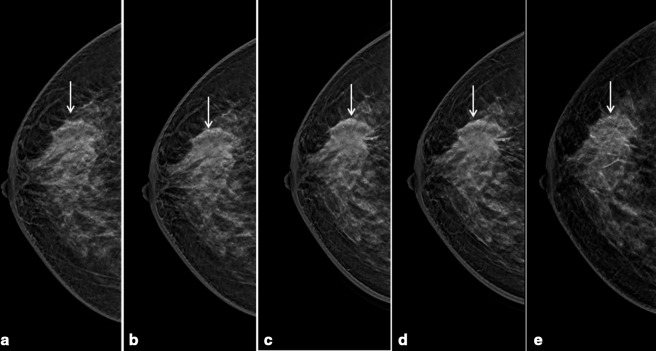
Digital breast tomosynthesis right breast cranio-caudal slices of the same patient in Figure 3, showed better margin characterization (spiculated margins) of the corresponding mass (arrows in a - e). No other lesions were evident.
Figure 5.
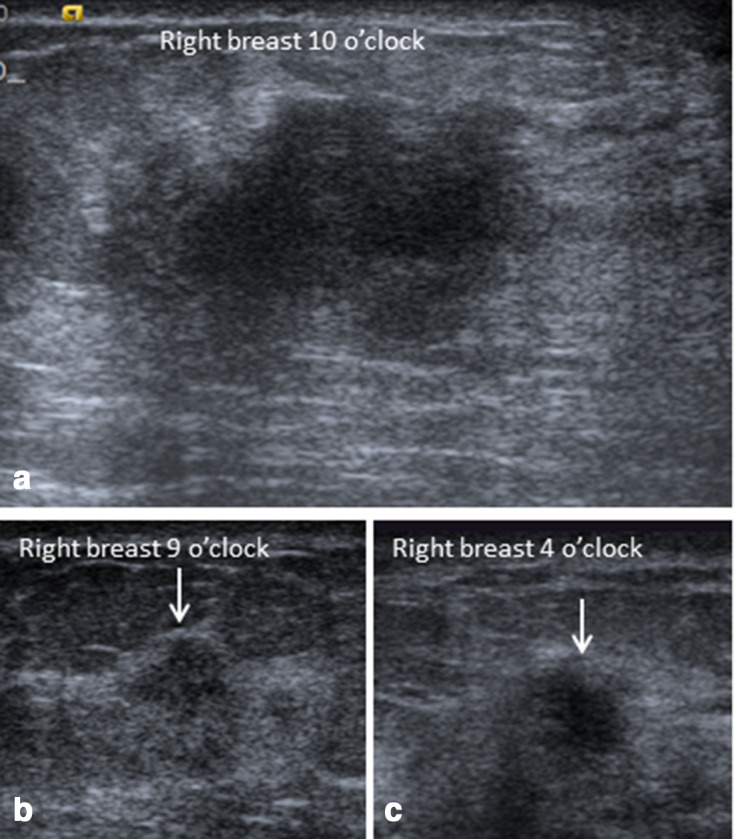
Ultrasonography of the right breast of the same patient in Figures 3 and 4, showed hypoechoic irregular primary mass at 10 o’clock with two additional smaller masses at 9 and 4 o’clock (arrows in b and c).
Figure 6.
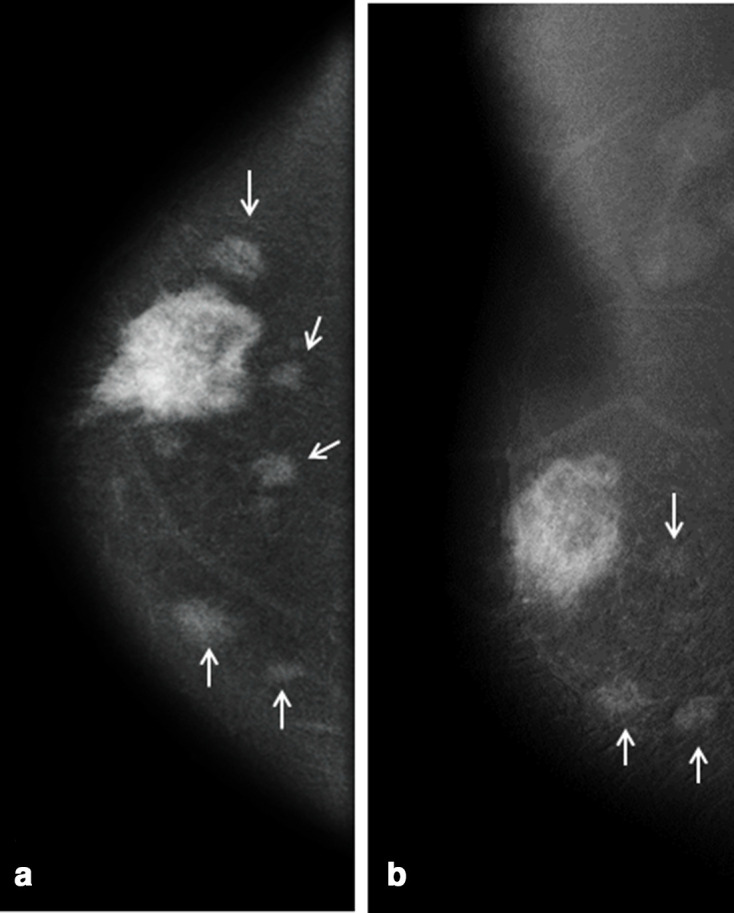
CEDM of the same patient in Figures 3–5 showed heterogeneous intense enhancement of the primary mass with multiple additional small enhancing masses (arrows in a and b) in different quadrants of the same breast which were occult on s2D MG and DBT, and only 2 of them were identified on ultrasonography.
Discussion
Dual-energy CEDM is a newer diagnostic technique, approved by U.S. FDA in 2011, which identifies cancers in the breast based on tumor angiogenesis assessment. This technology provides morphological features of the tumor on the 2D standard mammogram (low energy image) as well as the enhancement characteristics related to angiogenesis on post-processed contrast subtracted or recombined images. In this study cohort, four diagnostic imaging techniques: DBT (3D mammogram), synthetic 2D mammography, ultrasound and CEDM were evaluated and their diagnostic performances were compared among 130 patients with dense breast tissue (ACR type C and D) and 166 breast lesions. The sensitivity of CEDM was 96.5%, which was significantly higher than the s2D MG (75.6%, p < 0.0001), DBT (82.8%, p < 0.0001) and DBT + ultrasound (88.5%, p = 0.0057). Besides, CEDM detected many subcentimeter masses which were missed either on mammography or ultrasound examinations or both. Hence, CEDM may have added value in the screening of females with dense breast. CEDM also showed significantly higher specificity (81%) than the s2D MG (63.3%, p value = 0.0002) and comparable to DBT alone (84.4%, p = 0.3586) and DBT + ultrasound (79.7%, p = 0.4135). Thus, CEDM improved the sensitivity of s2D mammography and DBT without compromising on specificity. CEDM detected more number of cases with multifocal or multicentric or contralateral breast cancers than the s2D MG, DBT and ultrasound, which was important for the therapeutic planning. The sensitivity of CEDM reported by previous authors ranged between 63.5 and 100%. 12,13 The study published by Lusczynska et al compared MG, CEDM and ultrasound in 116 patients with 137 lesions and reported 100% sensitivity of CEDM, 10% higher than that of MG (p-value < 0.004) and 8% higher than ultrasound (p value < 0.01). 13 The meta-analysis summarizing the diagnostic performance of CEDM in eight eligible studies reported a pooled sensitivity of 98% [95% CI (96–100%)]. 14–18 The present study also showed higher sensitivity of CEDM as compared to DBT because of suppression of the normal breast parenchymal tissue, while the margin characterization and precise localization of the breast lesions were better appreciated on DBT. The accuracy of CEDM was 89.2%, which was also significantly higher than s2D MG (69.7%, p < 0.0001). Comparing the accuracy of CEDM, DBT alone, and DBT + ultrasound, CEDM showed higher accuracy than both, but the difference was not statistically significant. Petrillo et al studied 134 breast lesions in 100 patients and found DBT and CEDM to have superior accuracy than synthetic 2D mammography and better diagnostic performance than DBT alone. 18 In the current study, the false-positive rate of CEDM was 9% because of enhancing benign lesions including fibroadenomas, adenosis, mastitis, hyperplasia, phyllodes, papillomas and few other benign pathologies due to hypervascularity. The false-positive rate was comparable to DBT and ultrasound and significantly less than s2D MG. A study comparing CEDM and MRI, Xing et al found CEDM had a false-positive rate of 10.5% in 235 patients with 263 breast lesions as opposed to 19.8% with MRI. 19 In this study, the NPV of CEDM was 95.5%, significantly higher than s2D MG (70.4%, p < 0.0001), ultrasound (86.4%, p = 0.0039) and DBT (81.7%, p = 0.0001). The present study showed that the false-negative rate of CEDM was significantly lower than the s2D MG and DBT alone or combined with ultrasound. Three out of 87 malignant breast lesions did not show enhancement, which included two cases of DCIS, identified on s2D MG and DBT as grouped amorphous microcalcifications, and one case of well-differentiated invasive mucinous carcinoma identified on ultrasound examination. Addition of ultrasound examination to DBT, further improved the sensitivity of DBT by 4.6% (p = 0.2399). Well-differentiated cancers, e.g. small invasive papillary and mucinous carcinomas were identified on ultrasound which were otherwise missed on s2D MG and DBT. A study comparing ultrasound, digital mammogram and DBT by Elizabeth et al reported that additional cancer detection rate by ultrasound after digital mammogram was 3.5 per 1000 females screened and after DBT was 3.0 per 1000 females screened with no significant difference in additional cancer detection rate with ultrasound screening after mammography or DBT (p = 0.0999). 20
Comparing the DBT and s2D MG, DBT detected 7.2% higher breast cancers than the s2D MG (p = 0.1066) with significantly higher specificity (84.8% vs 63.3% with p < 0.0001) and diagnostic accuracy (83.7 and 69.7% with p < 0.0020). These findings were comparable with the findings of the meta-analysis published by Lei et al comprising seven studies involving 2014 patients and 2666 breast lesions with the higher pooled sensitivity and specificity of DBT (90.0 and 79.0%) than the MG (89.0 and 72.0%). 21 The studies published by Skanne et al 22 and Michell et al 23 reported significantly higher sensitivity, specificity and diagnostic accuracy of DBT combined with MG than the MG alone.
In ROC curve analysis, the AUC for CEDM was higher (0.896) than the MG (0.729), DBT alone (0.769) and DBT combined with ultrasound (0.841).
There were a few limitations in the current study, which included the following: a synthetic 2D mammography derived from DBT study was included in place of independent full-field digital mammography; CE-MRI was not included in the study to compare the relative accuracy of CEDM, and as the CEDM is a newer technology, it still does not have dedicated BI-RADS lexicon descriptors.
Conclusions
Our data suggest that CEDM is an accurate diagnostic technique for breast cancer detection in females with dense breasts. CEDM detects significantly more number of cancers in the dense breast with high NPV than s2D mammography, DBT alone and DBT + ultrasound combined. Hence, CEDM could be considered as an alternative modality to DBT and ultrasound for cancer evaluation in dense breasts. However, larger multicenter trials are recommended for validation of these observations.
Footnotes
Basavatarakam Indo-American Cancer hospital and research InstituteHyderabad, India
Basavatarakam Indo-American Cancer hospital and research instituteHyderabad, India
Funding: None.
Patient consent: Written consent were obtained.
Contributor Information
Rashmi Sudhir, Email: rashmi4210@gmail.com.
Kamala Sannapareddy, Email: Kamalareddy2001@yahoo.co.in.
Alekya Potlapalli, Email: P.alekya@gmail.com.
Pooja Boggaram Krishnamurthy, Email: drpoojabk@gmail.com.
REFERENCES
- 1. Buist DSM, Porter PL, Lehman C, Taplin SH, White E. Factors contributing to mammography failure in women aged 40-49 years. J Natl Cancer Inst 2004; 96: 1432–40. doi: 10.1093/jnci/djh269 [DOI] [PubMed] [Google Scholar]
- 2. Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology 2002; 225: 165–75. doi: 10.1148/radiol.2251011667 [DOI] [PubMed] [Google Scholar]
- 3. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005; 23: 8469–76. doi: 10.1200/JCO.2004.00.4960 [DOI] [PubMed] [Google Scholar]
- 4. Lee EH, Kim KW, Kim YJ, Shin D-R, Park YM, Lim HS, et al. Performance of screening mammography: a report of the alliance for breast cancer screening in Korea. Korean J Radiol 2016; 17: 489–96. doi: 10.3348/kjr.2016.17.4.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sardanelli F, Podo F, D'Agnolo G, Verdecchia A, Santaquilani M, Musumeci R, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology 2007; 242: 698–715. doi: 10.1148/radiol.2423051965 [DOI] [PubMed] [Google Scholar]
- 6. Dodelzon K, Simon K, Dou E, et al. Performance of 2D synthetic mammography versus digital mammography in the detection of microcalcifications at the screening. AJR Am J Roentgenol 2020; 7: 1–9. [DOI] [PubMed] [Google Scholar]
- 7. Durand MA. Synthesized mammography: clinical evidence, appearance, and implementation. Diagnostics 2018; 8: 22. doi: 10.3390/diagnostics8020022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miglioretti DL, Abraham L, Lee CI, Buist DSM, Herschorn SD, Sprague BL, et al. Digital breast Tomosynthesis: radiologist learning curve. Radiology 2019; 291: 34–42. doi: 10.1148/radiol.2019182305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaplan SS. Clinical utility of bilateral whole-breast us in the evaluation of women with dense breast tissue. Radiology 2001; 221: 641–9. doi: 10.1148/radiol.2213010364 [DOI] [PubMed] [Google Scholar]
- 10. Petrillo A, Fusco R, Petrillo M, Triunfo F, Filice S, Vallone P, et al. Added value of breast MRI for preoperative diagnosis of ductal carcinoma in situ: diagnostic performance on 362 patients. Clin Breast Cancer 2017; 17: e127–34. doi: 10.1016/j.clbc.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 11. Petrillo A, Porto A, Fusco R, Filice S, Vallone P, Rubulotta MR, et al. Surgical impact of preoperative breast MRI in women below 40 years of age. Breast Cancer Res Treat 2013; 140: 527–33. doi: 10.1007/s10549-013-2651-6 [DOI] [PubMed] [Google Scholar]
- 12. Tagliafico AS, Bignotti B, Rossi F, Signori A, Sormani MP, Valdora F, et al. Diagnostic performance of contrast-enhanced spectral mammography: systematic review and meta-analysis. Breast 2016; 28: 13–19. doi: 10.1016/j.breast.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 13. Luczyńska E, Heinze S, Adamczyk A, Rys J, Mitus JW, Hendrick E, et al. Comparison of the mammography, contrast-enhanced spectral mammography and ultrasonography in a group of 116 patients. Anticancer Res 2016; 36: 4359–66. [PubMed] [Google Scholar]
- 14. Lobbes MBI, Lalji U, Houwers J, Nijssen EC, Nelemans PJ, van Roozendaal L, et al. Contrast-Enhanced spectral mammography in patients referred from the breast cancer screening programme. Eur Radiol 2014; 24: 1668e76. doi: 10.1007/s00330-014-3154-5 [DOI] [PubMed] [Google Scholar]
- 15. Łuczynska E, Heinze-Paluchowska S, Hendrick E, et al. Comparison between breast MRI and contrast-enhanced spectral mammography. Med Sci Monit 2015; 12: 1358e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luczyńska E, Heinze-Paluchowska S, Dyczek S, Blecharz P, Rys J, Reinfuss M. Contrast-enhanced spectral mammography: comparison with conventional mammography and histopathology in 152 women. Korean J Radiol 2014; 15: 689. doi: 10.3348/kjr.2014.15.6.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jochelson MS, Dershaw DD, Sung JS, Heerdt AS, Thornton C, Moskowitz CS, et al. Bilateral contrast-enhanced dual-energy digital mammography: feasibility and comparison with conventional digital mammography and MR imaging in women with known breast carcinoma. Radiology 2013; 266: 743–51. doi: 10.1148/radiol.12121084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petrillo A, Fusco R, Vallone P, et al. Digital breast tomosynthesis and contrast-enhanced dual-energy digital mammography alone and in combination compared to 2D digital synthesized mammography and MR imaging in breast cancer detection and classification. Breast J 2020; 26: 860–72. [DOI] [PubMed] [Google Scholar]
- 19. Xing D, Lv Y, Sun B, Xie H, Dong J, Hao C, et al. Diagnostic value of contrast-enhanced spectral mammography in comparison to magnetic resonance imaging in breast lesions. J Comput Assist Tomogr 2019; 43: 245–51. doi: 10.1097/RCT.0000000000000832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dibble EH, Singer TM, Jimoh N, Baird GL, Lourenco AP. Dense breast ultrasound screening after digital mammography versus after digital breast tomosynthesis. AJR Am J Roentgenol 2019; 213: 1397–402. doi: 10.2214/AJR.18.20748 [DOI] [PubMed] [Google Scholar]
- 21. Lei J, Yang P, Zhang L, Wang Y, Yang K. Diagnostic accuracy of digital breast tomosynthesis versus digital mammography for benign and malignant lesions in breasts: a meta-analysis. Eur Radiol 2014; 24: 595–602. doi: 10.1007/s00330-013-3012-x [DOI] [PubMed] [Google Scholar]
- 22. Skaane P, Skjennald A. Screen-film mammography versus full-field digital mammography with soft-copy reading: randomized trial in a population-based screening program--the Oslo II Study. Radiology 2004; 232: 197–204. doi: 10.1148/radiol.2321031624 [DOI] [PubMed] [Google Scholar]
- 23. Michell MJ, Iqbal A, Wasan RK, Evans DR, Peacock C, Lawinski CP, et al. A comparison of the accuracy of film-screen mammography, full-field digital mammography, and digital breast tomosynthesis. Clin Radiol 2012; 67: 976–81. doi: 10.1016/j.crad.2012.03.009 [DOI] [PubMed] [Google Scholar]