Readers are invited to submit letters for publication in this department. Submit letters online at http://joem.edmgr.com. Choose “Submit New Manuscript.” A signed copyright assignment and financial disclosure form must be submitted with the letter. Form available at www.joem.org under Author and Reviewer information.
To the Editor:
The most widely utilized Nucleic Acid Amplification Test (NAAT) to detect SARS-CoV-2 RNA is the reverse transcriptase-polymerase chain reaction (RT-PCR) test, manufactured by many companies targeting one or more genomic regions of the virus. Although there is a several log difference in the sensitivity of the different RT-PCR tests to pick up viral RNA, many have sufficient analytical sensitivity to detect a viral load during the preinfectious stage in infected individuals.1–6 However, none of the tests have sufficient clinical sensitivity to detect virus during the first several days after infection, nor are they 100% sensitive at the time of peak infectiveness.7,8 Much has been written about the issue of false negative RT-PCR tests in symptomatic, presymptomatic, and asymptomatic persons infected with the virus.7,8 Less has been published about the problem of false positive RT-PCR or other NAAT tests.
In the United States, because of a shortage of tests and testing facilities during the early months of the pandemic tests were primarily used for diagnoses to identify a person with an active infection associated with signs or symptoms of COVID-19 or who had definite or suspected recent exposure to the virus.9 Later, the Federal Drug Administration (FDA) approved testing to be extended to screen for infection in individuals without known or suspected exposure to SARS-CoV-2 living in congregate settings, such as long-term care facilities or prisons.9 Finally, periodic screening programs have been developed for educational institutions, sport teams, and the workplace to detect asymptomatic, presymptomatic, and symptomatic infected individuals early and isolate them to reduce them infecting others.
The overall accuracy of a RT-PCR test is based upon its sensitivity representing the ability to detect infected individuals and the specificity, which is the percentage of uninfected people who test negative. The FDA has published recommendations concerning the data and information that test manufacturers should supply in their application for Emergency Use Authorization (EUA).10 For analytical specificity they ask for in vitro cross-reactivity studies to demonstrate that the test does not react with related pathogens, high prevalence disease agents, and normal or pathogenic flora that are reasonably likely to be encountered in a clinical specimen.11 Many of the RT-PCR assays have a 100% sensitivity in this analysis as reported by the manufacturers.12 For clinical evaluation, the FDA recommends testing 30 positive clinical samples and 30 individual negative samples and comparing the results of the test under consideration to an existing EUA RT-PCR test of high sensitivity. Acceptable clinical performance is defined as a minimum 95% positive and negative percent agreement (PPA and NPA). For a screening indication, the PPA recommendation remains at more than or equal to 95% and the NPA is raised to more than or equal to 98% to reduce false positive test results.11 In actual use, the clinical sensitivity and specificity of many of these tests is lower in part because of issues surrounding sample collection, handling, and analysis.8,12,13
The performance of these tests when deployed depends not only on their clinical sensitivities and specificities, but also the prevalence of SARS-CoV-2 infections in the setting in which the test is being used. If we consider a test that conforms to the FDA's recommendations for performance in a diagnostic (95% sensitivity and specificity) or screening setting (95% sensitivity, 98% specificity), we can compare its ideal clinical performance when the prevalence of active infection may be 10% (a diagnostic setting) and a prevalence of 1%, as may be found in a screening program.
In the diagnostic example, for every 10,000 individuals there will be 1000 infected and 9000 uninfected persons. Of the infected persons, 950 will be detected by the test (true positives) and 50 will be missed (false negatives). For the 9000 uninfected people, 8820 will correctly have negative tests (true negatives) and 180 will be positive (false positive). The positive predictive value (PPV) is the proportion of all positive tests that are true positives, in this case 950/(950 + 180) or 84%. Thus, most of the positive tests are true positives.
Doing these same calculations for the screening scenario, 100 of the 10,000 individuals are infected and 9900 are not. The test will detect 95 of the infected persons and five will be falsely negative. For those who are not infected, 9702 will be correctly diagnosed and 198 will be false positives. The PPV is 95/95 + 198 or 32.4%. In this case, 2/3 of the positive results are false positives. For a prevalence of 0.1%, the PPV drops to 4.5%.
Table 1 lists various factors that have been documented to contribute to false-positive RT-PCR results.12,14–19 Based upon our own experience in investigating groups of false-positive RT-PCR results and discussions with laboratory directors, the two most common problems are contamination and determining the cut-off for stating that a specimen is positive with a low viral load versus being called indeterminate or equivocal. The WHO, and an international consortium of experts have addressed these issues and have produced a checklist for laboratories to reduce possible causes of false-positive RT-PCR results and how to handle equivocal results.19,20
TABLE 1.
| Contamination during |
| Sampling (eg, an infected worker or surfaces; aerosolization of virus during collection)15 |
| Extraction (eg, aerosolization in containment hood) |
| PCR amplification |
| Production of Lab Reagents (eg, manufacturers of the positive control may have contaminated other reagents produced in the same facility; contamination of other consumables)17–19 |
| Contamination of the equipment by high viral titer specimens (eg, sample carryover)16 |
| Cross-reaction with other viruses (eg, other coronaviruses) |
| Sample mix-ups |
| Software problems |
| Data entry or transmission errors |
| Miscommunicating results |
| Variations in parameters around the LOD and definition of an indeterminate result14,16,20 |
| Assuming that an indeterminate result is a positive |
| Non-specific reactions15 |
LOD, limit of detection; RT-PCR, reverse transcriptase-polymerase chain reaction.
The overdiagnosis of SARS-CoV-2 infection has multiple potential adverse effects (Table 2)12,21: the inconvenience, financial, and psychological issues affecting those misdiagnosed; the possible exposure of uninfected individuals to infected people in hospital or congregate living areas; misdiagnosed persons foregoing social distancing and the masks use because they think that they are immune from COVID-19; and temporary closure of a business because of the need to quarantine coworkers. In addition, the overdiagnosis can inflate the number of asymptomatic infections in public health statistics.
TABLE 2.
| Unnecessary isolation of individuals and quarantining of close contacts with financial and psychological strains16,22 |
| Unnecessary contact tracing and testing23 |
| Wasteful consumption of personal protective equipment |
| Delays in surgical or other procedures16,23 |
| Prolong hospital stays16,23 with wasteful consumption of PPE |
| Potentially harboring uninfected individuals with infected individuals in hospitals and congregate living areas with possible nosocomial infection16,22 |
| Possible exposure to inappropriate medical treatment |
| Individual given false sense of security about immunity so may not follow public health guidelines or receive vaccination |
| Impede correct diagnosis of patients with symptoms |
| Overdiagnosis may distort epidemiologic statistics by including false-positives to estimate prevalence, hospitalization, and death rates as well as modeling (eg, some individuals classified as asymptomatic carriers may actually had a false positive test) |
PPE, Personal Protective Equipment.
Recognizing that a positive RT-PCR result may be a false positive may be difficult. If a RT-PCR-positive individual has signs or symptoms of COVID-19 or has had exposure to somebody who has been shown or suspected of harboring the virus, it is prudent to assume that the result is a true positive, as has been the recommendation of the WHO and the Centers for Disease Control.24,25 However, in an asymptomatic individual without known close contact with an infectious individual, especially in a low prevalence setting, the finding of a positive RT-PCR test result should raise the possibility that the result is a false positive. “Red flags” that should alert the laboratory personnel include finding an acute rise in the percentage of positive results in comparison to the days and weeks before for all of the samples run in the lab or from a particular collection site, noting that multiple positive samples were in close proximity on the plates in the PCR platform, or finding that the high volume of positive samples exhibit high cycle time (Ct) values that could be associated with a low viral load or issues affecting the cut-off for calling a sample positive, indeterminate, or negative.19,20 In these situations, the laboratory should re-extract the original sample and rerun it on the original PCR platform or a different platform with a similar sensitivity. If this cannot be done, a new sample should be obtained and tested.19,20
We have examined the issue of false positive results in a screening setting for a segment of the entertainment industry. The various unions that represent members involved in studio and TV productions have provided guidelines for testing and other safety measures in a publication, The Safe Way Forward.26 They have divided productions into several zones each with their own PCR testing requirements from testing three times a week to testing every 2 weeks. From September 27 through December 5, 2020, The Walt Disney Company performed 122,300 PCR tests at TV production sites, of which 323 were positive (0.26%). After removing the 84 positive tests found during the pre-employment screening, which leads to the individual entering isolation and not working on a production, the positivity rate during production was 0.19%. This rate is low in comparison to the average US rate during that same period (4.1% to 10.5%)27 because members are in a screening program and tested frequently. Also, the studios have instituted strict safety measures.26
In some, but not all instances, an unexpected positive result in an asymptomatic cast or crew member who had prior negative PCR tests, led to an evaluation of whether the test was a false positive by retesting the person 24 or more hours after the positive test on at least two occasions. If both retests were negative, we considered the first test to be a false positive. Of the 239 positive tests found after the pre-employment tests were removed, 54 (22.6%) were deemed to be false positives, giving a positive predictive value of 77.4%. An important caveat to these numbers is that there was a selection bias in who was investigated for the possibility of a false positive result. As noted, all the individuals were asymptomatic and had at least a negative pre-employment test, and many had multiple negative PCR tests before a positive appeared. Also, there were “outbreaks” of positive tests due to documented contamination of reagents or mistakes in programing of the PCR platform. Finding multiple asymptomatic individuals who may not have been in contact with each other led to retesting of the original nasal swabs and testing of freshly obtained new specimens. Since we did not systematically reevaluate every positive test, we may have underestimated the false positive rate.
Our experience and the data reviewed above has led us to develop an algorithm for evaluating an unexpected positive result in an asymptomatic individual without known close contact with an actively infected person in a screening setting for the entertainment industry (Fig. 1). We feel that this algorithm should be applicable to any screening situation and conforms to the recommendation of the WHO, the United Kingdom, and the Norwegian Institute of Public Health,20,28,29 as well as multiple authors.12,15,21,30,31
FIGURE 1.
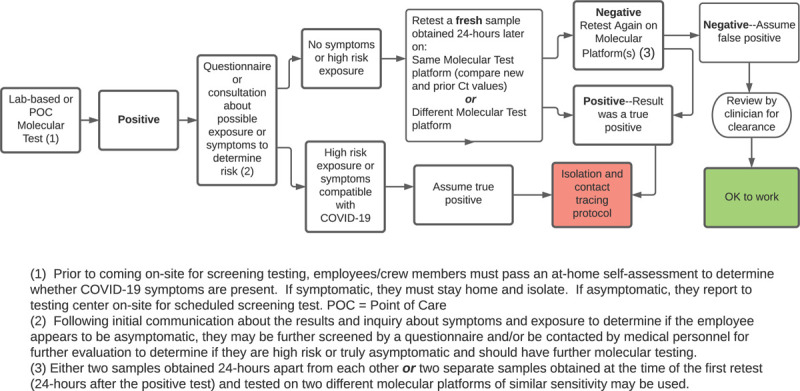
Management of a positive molecular test in a screening setting.
In summary, we have provided additional evidence that false positive SARS-CoV-2 PCR test results do occur in the clinical setting and are especially a problem in a low prevalence screening situation where the prior probability of a positive test is low. Although it is acknowledged that resource limitations may constrain the amount of retesting performed, we posit that the human and economic costs of considering all positive results to be definitive evidence of infection warrant an evaluation for the possibility that the result is falsely positive in an asymptomatic individual without known exposure to an actively infected person.
Footnotes
Disclosures: G.D.B.: Consultant to The Walt Disney Company.
L.S. and P.H.: Employees of The Walt Disney Company.
J.F.: Consultant to SAG-AFTRA.
Funding Source: None specifically for this manuscript. The data reported from The Walt Disney Company was generated from the SARS-CoV-2 testing program in place at the Disney-affiliated studios and TV productions.
Contributor Information
Glenn D. Braunstein, Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, California.
Lori Schwartz, The Walt Disney Company, Burbank, California.
Pamela Hymel, The Walt Disney Company, Burbank, California.
Jonathan Fielding, The UCLA Fielding School of Public Health and The David Geffen School of Medicine at UCLA, Los Angeles, California..
REFERENCES
- 1. FDA, SARS-CoV-2 reference panel comparative data, current as of 12/2/2020. Available at: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-reference-panel-comparative-data. Accessed December 11, 2020. [Google Scholar]
- 2.Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis 2020; 71:2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaafar R, Aherfi S, Wurtz N, et al. Correlation between 3790 qPCR positive samples and positive cell cultures including 1941 SARS-CoV-2 isolates. Clin Infect Dis 2020; DOI: 10.1093/cid/ciaa 1491/5912603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singanayagam A, Patel M, Charlett A, et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020; 25:2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kissler SM, Fauver JR, Mack C, et al. SARS-CoV-2 viral dynamics in acute infections. MedRxiv . doi: [DOI] [Google Scholar]
- 6.Mina MJ, Parker R, Larremore DB. Rethinking Covid-19 test sensitivity—a strategy for containment. N Engl J Med 2020; 383:e120. [DOI] [PubMed] [Google Scholar]
- 7.Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Int Med 2020; 173:262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS-CoV-2 infection—challenges and implications. N Engl J Med 2020; 383:e38. [DOI] [PubMed] [Google Scholar]
- 9. CDC. Coronavirus disease 2019 (COVID-19). Testing overview; 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed December 11, 2020. [Google Scholar]
- 10. FDA. Policy for coronavirus disease-2019 tests during the Public Health Emergency (Revised). Immediately in effect guidance for clinical laboratories, commercial manufacturers, and Food and Drug Administration staff. Document issued on the web May 11, 2020. Available at: https://www.fda.gov/media/135659/download. Accessed December 11, 2020. [Google Scholar]
- 11. FDA. Molecular diagnostic template for commercial manufacturers. In In vitro Diagnostics EUAs; 2020. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas. Accessed December 11, 2020. [Google Scholar]
- 12. Cohen AN, Kessel B, Milgroom MG. Diagnosing SARS-CoV-2 infection: the danger of over-reliance on positive test results. False positive test results impact clinical and policy decisions (including Supplemental Material). Available at: https://www.medrxiv.org/content/10.1101/2020.04.26.20080911v4.full.pdf. Accessed December 11, 2020. [Google Scholar]
- 13. Mayers C, Baker K. Impact of false-positives and false-negatives in the UK's COVID-19 RT-PCR testing programme. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/895843/S0519_Impact_of_false_positives_and_negatives.pdf. Accessed December 11, 2020. [Google Scholar]
- 14.Matheeussen V, Corman VM, Mantke OD, et al. On behalf of the RECOVER project and collaborating networks. International external quality assessment for SARS-CoV-2 molecular detection and survey on clinical laboratory preparedness during the COVID-19 pandemic, April/May 2020. Euro Surveill 2020; 25:pii=2001223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skittral JP, Wilson M, Smielewska AA, et al. Specificity and positive predictive value of SARS-CV-2 nucleic acid amplification testing in a low-prevalence setting. Clin Microbiol Infect 2020; 10.1016/j.cmi.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz AP, Civantos FJ, Sargi Z, et al. False-positive reverse transcriptase polymerase chain reaction screening for SARS-CoV-2 in the setting of urgent head and neck surgery and otolaryngologic emergencies during the pandemic: clinical implications. Head Neck 2020; 42:1621–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wernike K, Keller M, Conraths FJ, Mettenleiter TC, Groschup MH, Beer M. Pitfalls in SARS-CoV-2 diagnostics. Transbound Emerg Dis 2020; DOI: 10.1111/tbed.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mogling R, Meijer A, Berginc N, et al. Delayed laboratory response to COVID-19 caused by molecular diagnostic contamination. Emerg Infect Dis 2020; 26:1944–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huggett JF, Benes V, Bustin SA, et al. Cautionary note on contamination of reagents used for molecular detection of SARS-CoV-2. Clin Chem 2020; 66:1369–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization. Diagnostic testing for SARS-CoV-2. Interim guidance; 2020. Available at: https://www.who.int/publications/i/item/diagnostic-testing-for-sars-cov-2. Accessed December 11, 2020. [Google Scholar]
- 21.Surkova E, Nikolayevskyy V, Drobniewski F. False-positive COVID-19 results: hidden problems and costs. Lancet Resp Med 2020; 8:1167–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Linder R, Cohen S, Zikri AB. Israeli lab to stop coronavirus testing after dozens misdiagnosed. Dozens of elderly people from two different nursing homes received false positives; at least 16 were taken to coronavirus wards, where they were heavily exposed to the virus. Haaretz; 2020. Available at: https://www.haaretz.com/israel-news/.premium-israeli-lab-to-stop-testing-after-dozens-misdiagnosed-with-coronavirus-1.8777241. Accessed December 11, 2020. [Google Scholar]
- 23.Healy B, Khan A, Metezai H, et al. Covid-19 testing, low prevalence, and the impact of false positive results. BMJ 2020; 369:m1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Who COVID-19 case definition. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1. Accessed December 11, 2020. [Google Scholar]
- 25. Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19). 2020 interim case definition, approved August 5, 2020. Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Surveillance_Case_Definition-2020.1. Accessed December 11, 2020. [Google Scholar]
- 26. The Safe Way Forward. A joint report of the DGA, SAG-AFTRA, IATSE and Teamsters’ Committees for COVID-19 safety Guidelines. Available at: https://www.sagaftra.org/files/sa_documents/ProductionSafetyGuidelines_June2020EditedP.pdf. Accessed December 11, 2020. [Google Scholar]
- 27. Available at: https://coronavirus.jhu.edu/data. Accessed December 11, 2020. [Google Scholar]
- 28. Public Health England. Assurance of SARS-CoV-2 RNA positive results during periods of low prevalence; 2020. Available at: https://www.gov.uk/government/publications/sars-cov-2-rna-testing-assurance-of-positive-results-during-periods-of-low-prevalence/assurance-of-sars-cov-2-rna-positive-results-during-periods-of-low-prevalence. Accessed December 11, 2020. [Google Scholar]
- 29. Norwegian Health Network. Test criteria for coronavirus; 2020. Available at: https://www.fhi.no/nettpub/coronavirus/testing-og-oppfolging-av-smittede/testkriterier/?term=&h=1. Accessed December 11, 2020. [Google Scholar]
- 30. Sudlow C, Diggle P, Warlow O, et al. Testing for coronavirus (SARS-CoV-2) infection in populations with low infection prevalence: the largely ignored problem of false positives and the value of repeat testing. Available at: https://www.medrxiv.org/content/10.1101/2020.08.19.20178137v1. Accessed December 11, 2020. [Google Scholar]
- 31. Cohen AN, Kessel B, Milgroom MG. SARS-CoV-2 mass testing endangers residents of long-term care facilities. Available at: . Accessed December 11, 2020. [DOI] [Google Scholar]


