Supplemental Digital Content is available in the text
Keywords: antibodies, caseworker, COVID-19, first responder, firefighter, police, SARS-CoV-2
Abstract
Objectives:
Define the seroprevalence and risk factors for SARS-CoV-2 antibodies in Arapahoe County, Colorado first responders (eg, law enforcement, human services, fire departments).
Methods:
Two hundred sixty four first responders were enrolled June to July 2020. SARS-CoV-2 seropositivity was defined as detection of immunoglobulin G (IgG) antibodies to both spike receptor binding domain and nucleocapsid in venous blood by validated enzyme-linked immunosorbent assay. We compared risk factors for being seropositive versus seronegative.
Results:
4% (11/264) were SARS-CoV-2 seropositive. Seropositive participants were significantly more likely to have lung disease (% seropositive, % seronegative; P-value) (36%, 8%; P = 0.01), prior SARS-CoV-2/COVID-19 testing (36%, 8%; P ≤ 0.01), a prior positive result (18%, less than 1%), and to believe they previously had COVID-19 (64%, 15%; P < 0.01). Only 15% of those believing they had COVID-19 had anti-SARS-CoV-2 antibodies.
Conclusions:
Human services employees and individuals with lung disease are at SARS-CoV-2 exposure risk. Few individuals believed they had COVID-19 had prior exposure.
The United States has become the epicenter of the coronavirus disease 2019 (COVID-19) pandemic, with over 6 million cases and 185,000 deaths as of September 2, 2020.1 Early in the pandemic, health care workers were identified as a higher risk population for COVID-19. Over 150,000 cases of COVID-19 have been identified among health care professionals in the United States2 and this population has also been found to have a high seroprevalence of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19.3 A less well understood, but potentially high-risk group, are first responders including firefighters, police and emergency medical service employees. In the United States, it is assumed that the risk of exposure to SARS-CoV-2 among first responders is similar to health care professionals, but few seroprevalence studies including this population have been conducted.
Information regarding the risk of SARS-CoV-2 exposure among first responders is limited. In New York City by the end of March 2020, it was reported that 11% of emergency medical staff and firefighters were on medical leave for confirmed COVID-19.4 A study of firefighters and paramedics in a South Florida fire department found that 9% of study participants were seropositive for SARS-CoV-2 antibodies in April 2020 and 4% were assumed to have had a recent infection.5 In addition, 7% of public safety, healthcare, and first response personnel in Detroit, Michigan, as of June 20206 and only 1.5% of first responders in Arizona in May 20207 were found to be seropositive. However, the risk factors for infection among first responders remain unclear. To ensure the health and safety of first responders, both at work and in the community, it is critical to understand exposures to SARS-CoV-2 and the risk factors for seroconversion within this population.
Arapahoe County is one of the largest counties in Colorado, consisting of both urban and rural regions with a population of more than 630,000. We invited first responders from Arapahoe County to participate in our study to assess the presence of SARS-CoV-2 antibodies and to identify risk factors for SARS-CoV-2 seropositivity among this potentially high-risk group.
METHODS
First responders (refer to Table 1 for full list of job titles) working in Arapahoe County were invited to participate in the study which took place between July and August 2020. Emails were sent to the chiefs of all agencies in the county asking for their agreement to participate at the agency level. They were asked to forward an email containing a web-based (REDCap) link to their employees who were then, individually, given the opportunity to indicate their desire to participate in the study. Individuals were eligible to participate in the study if they were employed by an Arapahoe County agency since January 2020, had direct contact with the public, and were 20 to 69 years old. Individuals were screened for COVID-19-related symptoms prior to study entry and would have been excluded from the study, if symptomatic, to protect study staff and participants. No individuals presenting to the study were symptomatic. Informed consent was obtained from each study participant. All protocol and consent forms were approved by the Colorado Multiple Institutional Review Board (COMIRB).
TABLE 1.
Demographics of CASES Project Participants (N = 264)
| Age—median [interquartile range] | 38 [32–48] |
| Gender | n (%) |
| Male | 121 (45.8) |
| Female | 143 (54.2) |
| Race/Ethnicity | n (%) |
| White, Non-Hispanic/Non-LatinX | 211 (79.9) |
| Black/African American, Non-Hispanic/Non-LatinX | 14 (5.3) |
| Other, Non-Hispanic/Non-LatinX∗ | 10 (3.8) |
| Hispanic/LatinX | 29 (11.0) |
| Agency | n (%) |
| Sherriff's Office or Police Department | 125 (47.3) |
| Department of Human Services | 91 (34.5) |
| Fire Department | 42 (15.9) |
| Other† | 6 (2.3) |
| Geographic designation of agency | n (%) |
| Urban | 239 (90.5) |
| Rural | 24 (9.1) |
| Employment status | n (%) |
| Full time | 246 (93.2) |
| Part time/Volunteer | 14 (5.3) |
| Agency role | n (%) |
| Human services/case workers | 57 (21.6) |
| Patrol | 51 (19.3) |
| Firefighter | 30 (11.4) |
| Supervision | 25 (9.5) |
| Investigations | 22 (8.3) |
| Support Services | 26 (9.8) |
| Dispatch | 15 (5.7) |
| Command | 10 (3.8) |
| Detentions/Courts | 13 (4.9) |
| Coroner Staff | 7 (2.7) |
| Medic/EMT | 4 (1.5) |
| Other‡ | 4 (1.5) |
| High-risk groups | n (%) |
| Age 65+ | 4 (1.5) |
| Chronic medical condition (including diabetes, high blood pressure, and/or kidney disease) | 39 (14.8) |
| Compromised immune system | 14 (5.3) |
| Heart disease | 4 (1.5) |
| Lung disease | 24 (9.1) |
| Cancer | 5 (1.9) |
| Overweight | 60 (23.0) |
| Obese | 10 (3.8) |
| None of the above | 154 (58.3) |
| Number of high-risk groups, among individuals reporting at least one group (n = 110) | |
| 1 | 56 (50.9) |
| 2 or more | 54 (49.1) |
All variables have complete data except (variable [missing n, %]): Age—[1, 0.4%], Geographic designation of agency [1, 0.4%], employment status [4, 1.5%].
Other Race/Ethnicities Reported include: Mixed, White/Asian, White/East Indian, White/Native American/ American Indian, White/Pacific Islander, Asian, Asian/Pacific Islander.
Other agencies included: Coroner's Office, Health Department, Office of Emergency Management, Attorney's Office.
Other agency roles include: Emergency Management, Victim Assistance Coordinator, Paralegal, Staff trainer/Coach.
Interested individuals were asked to come to a central location where they completed a questionnaire. Data on demographics, employment characteristics, high-risk group characteristics, potential SARS-CoV-2 exposures, prior COVID-19 related symptoms, and prior COVID-19/SARS-CoV-2 testing were collected at enrollment (Supplement 1). Participants provided a venous blood draw of up to 3 mL which was collected in BD vacutainer SST tubes (Franklin Lake, NJ). Whole blood was allowed to clot at room temperature. Samples were centrifuged at ∼2700 RPM (1300 ± 100 × g), nine acceleration, nine deceleration, for 10 minutes at 25 °C and the serum removed and stored at –20 °C until testing was completed. Centrifugation never occurred more than 8 hours after sample collection.
Venous blood draws were tested for immunoglobulin G (IgG) antibodies using an enzyme-linked immunosorbent assay (ELISA) developed at the University of Colorado at Exsera BioLabs. The assay was validated to EUA and CAP/CLIA standards in a compliant laboratory. Validation included testing of over 1000 pre-pandemic normal and 100 PCR-confirmed positive sera. ELISA development was based on the work of Stadlbauer et al8 with some modification. In brief, the SARS-CoV-2 receptor binding domain (RBD) was grown in HEK293T cells and the second antigen utilized was Nucleocapsid purified from Escherichia coli. All specimens were tested for reactivity to both antigens. To be considered seropositive, antibodies to both the spike RBD and nucleocapsid of SARS-CoV-2 had to be detected. The cut-off for positivity was based on the aforementioned validation which yielded a specificity of 98.6% and sensitivity of 85%.
To identify potential risk factors for SARS-CoV-2 seropositivity, we compared the seropositive and seronegative groups to test whether pre-identified risk factors were more or less likely to be associated with the presence of anti-SARS-CoV-2 antibodies. Exposures of interest included age, sex, race/ethnicity, whether an individual self-identified as being in a high-risk group, frequency of interactions with the public, contact with confirmed or suspected COVID-19 cases, use of personal protective equipment (PPE), prior SARS-CoV-2 testing and results, belief of a prior SARS-CoV-2 infection, and existence and timing of COVID-19 related symptoms. Mann–Whitney U test was used to compare median age in seropositive versus seronegative individuals. All other comparisons were performed using a chi-squared or Fisher exact test, where appropriate. Statistical significance was defined as a P < 0.05. All analyses were completed using SAS® software, Version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Participant Characteristics
We enrolled 264 first responders from Arapahoe County. The median age of participants was 38 years old. Slightly more women than men enrolled, and the majority of individuals identified as White, Non-Hispanic/Non-Latinx (Table 1).
Staff from a sheriff's office or police department represented the largest number of participants followed by those from the Department of Human Services. Less than 10% of participants worked in a rural area and 95% were employed full-time. In addition to individuals traditionally considered first responders (police, firefighters, EMTs, etc), we allowed enrollment of individuals who worked for the county that had direct contact with the public. As such, over 20% of our enrollees worked as caseworkers or in human services, whom provide services to the county including: adult and senior services, child and adult protective services, food, financial, and medical benefits, as well as other child and community services. The next most common roles included those working in police patrol, and those working as firefighters (Table 1).
To identify whether characteristics of patients considered high-risk for COVID-19 were also associated with SARS-CoV-2 seropositivity among first responders, we asked participants to identify if they had any comorbidities based on previously identified categories. More than half did not identify as belonging to any high-risk group. Among individuals that did report a comorbidity, the most frequently reported were being overweight, having a chronic medical condition including diabetes, high blood pressure, and/or kidney disease, followed by those with lung disease (Table 1).
Participant SARS-CoV-2 Exposures
To identify potential SARS-CoV-2 exposure and behavioral risk factors among first responders, respondents were asked about their interactions with the public, potential exposures to individuals with COVID-19, use of PPE, prior SARS-CoV-2 testing, and belief about the likelihood they had been previously infected. Most individuals interacted with the public either sometimes, often, or always and at least 1 or more days per week. Around one-third of individuals reported close contact with someone with laboratory-confirmed COVID-19 or possible COVID-19 in the two months prior to enrollment and the majority of individuals who were exposed reported using PPE either often or always (Table 2).
TABLE 2.
Potential COVID-19 Exposures reported by CASES Project Participants (N = 264)
| As part of daily responsibilities, respondents interact with public: | n (%) |
| Always | 90 (34.1) |
| Often | 76 (28.8) |
| Sometimes | 70 (26.5) |
| Rarely | 21 (8.0) |
| Never | 7 (2.7) |
| Times respondents interact with the public for job in an average week? | n (%) |
| 5–7 d/wk | 47 (17.8) |
| 3–4 d/wk | 129 (48.9) |
| 1–2 d/wk | 53 (20.1) |
| <1 d/wk | 35 (13.3) |
| In past 2 months, close contact with laboratory-confirmed COVID-19 case. | n (%) |
| Yes | 79 (29.9) |
| No | 181 (68.6) |
| Unknown | 4 (1.5) |
| In past 2 months, close contact with possible but untested COVID-19 case | n (%) |
| Yes | 90 (34.1) |
| No | 167 (63.3) |
| Unknown | 7 (2.7) |
| If in direct contact with another person possibly exposed to the coronavirus, how often do you use personal protective equipment? | n (%) |
| Always | 157 (59.5) |
| Often | 50 (18.9) |
| Sometimes | 13 (4.9) |
| Rarely | 4 (1.5) |
| Never | 1 (0.4) |
| Unknown | 1 (0.4) |
| I have not had direct contact | 38 (14.4) |
| COVID-19 test results. | n (%) |
| Tested positive | 3 (1.1) |
| Tested negative | 22 (8.3) |
| Tested unknown | 5 (1.9) |
| Never tested | 234 (88.6) |
| Regardless of test results, the belief that respondent has had COVID-19. | n (%) |
| Definitely | 9 (3.4) |
| Very probably | 16 (6.1) |
| Probably | 21 (8.0) |
| Possibly | 67 (25.4) |
| Probably not | 110 (41.7) |
| Definitely not | 41 (15.5) |
All variables have complete data except where specified.
For 89% of participants, the testing done during our study was their first SARS-CoV-2 test of any type, and only three individuals (1%) had previously tested positive. Regardless of test results, around 15% of individuals thought their likelihood of having had COVID-19 was probable, very probable, or definite (Table 2).
Risk Factors for SARS-CoV-2 Seropositivity
We chose to use a dual-antigen ELISA to assess antibodies to the SARS-CoV-2 spike receptor binding domain (RBD) and nucleocapsid protein. Of the 264 individuals enrolled, 11 (4%) were reactive for antibodies to both the RBD and nucleocapsid of SARS-CoV-2 (Fig. 1). Of those, five (46%) worked in human services/casework, five (46%) worked in a sheriff's office or police department, and one (9%) worked in a fire department. Among human services employees and caseworkers included in our study, the seroprevalence for SARS-CoV-2 was 6%, while the SARS-CoV-2 seroprevalence for sheriff's office or police department employees and fire department employees was 4% and 2%, respectively. These differences in seroprevalence by employment type were not significant. We compared demographics, health characteristics, and potential SARS-CoV-2 exposure and explanatory variables in seropositive and seronegative individuals and found that there was no association between reactivity and age, sex, or race/ethnicity. Seropositive individuals were significantly more likely to report lung disease (P = 0.0114) and marginally more likely to be obese (P = 0.0563) (Table 3). Among individuals who reported being in a high-risk group, there was no significant difference in the number of individuals reporting being in one compared with two or more high-risk groups in seropositive compared with seronegative participants (P = 0.1579).
FIGURE 1.
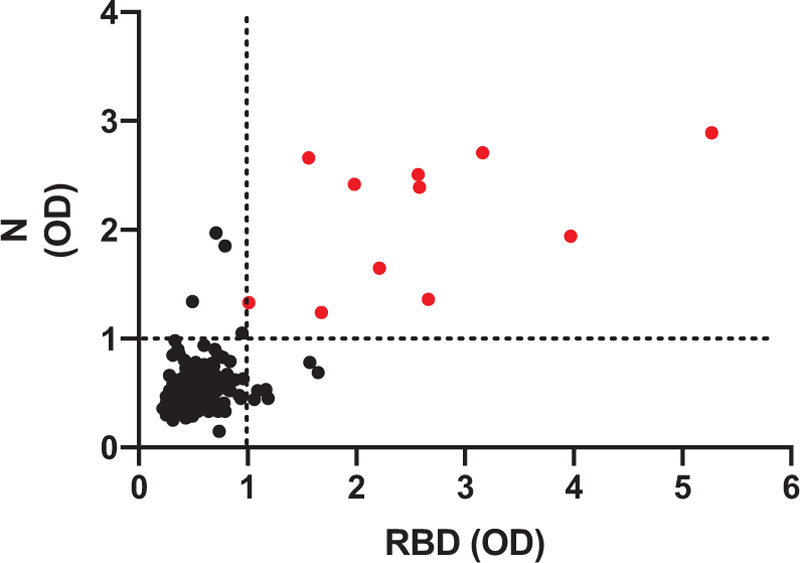
Anti-SARS CoV-2 antibodies response. Serum samples from study participants were analyzed for antibodies to the receptor binding domain (RBD) of the spike protein and the nucleocapsid (N) protein by enzyme-linked immunosorbent assay. Shown are the optical density (OD) values for the individual study participants. Only those samples which had an OD above the cut-off were considered positive and are indicated in red.
TABLE 3.
Characteristics and Potential COVID-19 Exposures for CASES Project Participants by Reactivity Versus Non-Reactivity to Anti-SARS-CoV-2 Antibodies (N=264)
| COVID-19 Antibody Status | |||
| Non-Reactives (n = 253) | Reactive (n = 11) | P-Value | |
| Participant characteristics | |||
| Age—median[interquartile range] | 39 [32,48] | 32 [29,45] | 0.0963 |
| Sex—male versus female | 116 (45.8) | 5 (45.5) | 0.9795 |
| Race/Ethnicity | 0.4894 | ||
| Black/African American, Non-Hispanic/Non-LatinX | 13 (5.1) | 1 (9.1) | |
| Hispanic/LatinX | 27 (10.7) | 2 (18.2) | |
| Other, Non-Hispanic/Non-LatinX | 10 (4.0) | 0 (0.0) | |
| White, Non-Hispanic/Non-LatinX | 203 (80.2) | 8 (72.7) | |
| High Risk Groups | |||
| Age 65+ | 4 (1.6) | 0 (0.0) | 1.0000 |
| Chronic medical condition (including high blood pressure and/or diabetes) | 36 (14.2) | 3 (27.3) | 0.2112 |
| Compromised immune system | 14 (5.5) | 0 (0.0) | 1.0000 |
| Heart disease | 4 (1.6) | 0 (0.0) | 1.0000 |
| Lung disease | 20 (7.9) | 4 (36.4) | 0.0114∗ |
| Cancer | 5 (2.0) | 0 (0.0) | 1.0000 |
| Overweight | 56 (22.1) | 4 (36.4) | 0.2777 |
| Obese | 8 (3.2) | 2 (18.2) | 0.0592 |
| None of the above | 151 (59.7) | 3 (27.3) | 0.0563 |
| Number of high-risk groups, among individuals reporting at least one group (n = 110)—two or more versus one | 48 (52.9) | 6 (75.0) | 0.1579 |
| COVID-19 exposures | |||
| As part of daily responsibilities, respondents interact with public—often/always versus sometimes/rarely/never | 159 (62.8) | 7 (63.6) | 1.0000 |
| Times respondents interact with the public for job in an average week—3+ d/wk versus <3 d/wk | 169 (66.8) | 7 (63.6) | 1.0000 |
| In the past 2 months, close contact with someone with laboratory-confirmed COVID-19 diagnosis. Yes versus Noa | 75 (30.1) | 4 (36.4) | 0.7397 |
| In the past 2 months, close contact with someone ill with possibly COVID-19 but not tested. Yes versus Noa | 87 (35.4) | 3 (27.3) | 0.7518 |
| If in direct contact with another person possibly exposed to the coronavirus, how often do you use personal protective equipment?a | 0.6736 | ||
| Always | 151 (59.9) | 6 (54.5) | |
| Often/Sometimes/Rarely/Never | 37 (14.7) | 1 (9.1) | |
| No contact | 64 (25.4) | 4 (36.4) | |
| COVID-19 test results | 0.0016∗ | ||
| Not tested/Unknown test result | 232 (91.7) | 7 (63.6) | |
| Tested, negative | 20 (7.9) | 2 (18.2) | |
| Tested, positive | 1 (0.4) | 2 (18.2) | |
| Regardless of test results, the belief that respondent has had COVID-19—definitely/very probably/probably versus possibly/probably not/definitely not | 39 (15.4) | 7 (63.6) | 0.0006∗ |
All values are given as n (%) unless otherwise specified.
All variables have complete data except (variable [missing no. non-reactive, missing no. reactive]): contact with someone with lab-confirmed COVID-19 [4,0], contact with someone with lab-confirmed COVID-19 [7,0], Use of personal protective equipment [1,0].
Chi-square, Fishers Exact, and Mann–Whitney U tests used for comparisons.
Unknown responses excluded.
P-values <0.05 are considered statistically significant.
There did not appear to be differences between seropositive and seronegative individuals in how regularly they had contact with the public nor their contact with a known or suspected COVID-19 case. Seropositive individuals were significantly more likely to report prior SARS-CoV-2 testing, and a prior positive SARS-CoV-2 test. They were also significantly more likely to report that they believed they probably, very probably, or definitely had COVID-19 prior to enrollment compared with seronegative individuals (Table 3). Among individuals seropositive for anti-SARS-CoV-2 antibodies who believed they probably, very probably, or definitely had COVID-19 previously, two had a prior positive COVID-19 test.
Finally, we asked participants about any symptoms they had experienced in the 3 months prior to antibody testing and compared COVID-19 related symptoms between SARS-CoV-2 seropositive and seronegative participants. Of the 11 seropositive individuals, three (27%) reported never having symptoms. Seropositive participants were significantly more likely to report having any symptoms and were also significantly more likely to report ever having shortness of breath or difficulty breathing, coughing, fever more than 100 °F, chills, rigors, sore throat, loss of taste or smell, gastrointestinal symptoms, and other symptoms within the 3 months prior to testing. Myalgias were also more commonly reported by SARS-CoV-2 seropositive individuals though this was not statistically significant at the P < 0.05 level (Table 4).
TABLE 4.
Symptoms Reported by CASES Project Participants by Reactivity Versus Non-Reactivity to Anti-SARS-CoV-2 Antibodies (N=264)
| Non-Reactive (n = 253) | Antibody Reactive (n = 11) | P-Value | |
| Ever any symptomsa | 116 (45.8) | 8 (72.7) | 0.0804 |
| Any symptoms 3 months ago (April) | 57 (22.5) | 6 (54.5) | 0.0249∗ |
| Any symptoms 2 months ago (May) | 39 (15.4) | 4 (36.4) | 0.0849 |
| Any symptoms 1 month ago (June) | 74 (29.2) | 5 (45.5) | 0.3132 |
| Any of the following symptoms (ever vs never) | |||
| Shortness of breath or difficulty breathing | 18 (7.1) | 5 (45.5) | 0.0011∗ |
| Coughing | 38 (15.0) | 6 (54.5) | 0.0037∗ |
| Headache | 71 (28.2) | 5 (50.0) | 0.1597 |
| Fever >100 °F | 14 (5.6) | 6 (54.5) | <0.0001∗ |
| Chills | 16 (6.3) | 4 (36.4) | 0.0057∗ |
| Repeated shaking with chills (rigors) | 7 (2.8) | 2 (18.2) | 0.0485∗ |
| Muscle pain (unrelated to exercise or vigorous activities) | 21 (8.5) | 3 (27.3) | 0.0678 |
| Sore throat | 47 (19.0) | 5 (45.5) | 0.0446∗ |
| Loss of taste or smell | 8 (3.2) | 5 (45.5) | <0.0001∗ |
| Gastrointestinal symptoms, such as nausea or diarrhea | 43 (17.0) | 5 (45.5) | 0.0316∗ |
| Other symptoms2 | 9 (3.6) | 3 (27.3) | 0.0097∗ |
All values are given as n (%) unless otherwise specified.
All variables have complete data except (variable [missing no. non-reactive, missing no. reactive]): headache [1,1]; fever more than 100° [2,0]; muscle pain [1,0]; sore throat [1,0]; loss of taste or smell [2,0].
Excludes “Other symptoms”.
Other symptoms include: rash, allergies, sore neck, shingles, light-headedness, acid reflux, nasal drip, fatigue, high pitch ringing in ears, burning/red eyes, lung rattling during inhalation.
P-values <0.05 considered statistically significant.
DISCUSSION
In this study we aimed to identify the seroprevalence and risk factors for detection of anti-SARS-CoV-2 antibodies in Arapahoe County first responders. We found a 4% seropositivity for SARS-CoV-2 antibodies among our participants and almost half of those individuals worked in human services/casework, an under-investigated group that is potentially at high-risk of SARS-CoV-2 exposure. Approximately one-third of first responders reported contact with a confirmed or suspected COVID-19 case but most appeared to be taking steps to protect themselves by using PPE. We sought to identify possible risk factors for SARS-CoV-2 exposure among first responders and found that those with anti-SARS-CoV-2 antibodies were more likely to report lung disease and obesity, were more likely to have been previously tested for SARS-CoV-2 infection, have a previous positive test result, believe they had already had COVID-19, and previously have COVID-19 related symptoms. Understanding the seroprevalence and risk factors for SARS-CoV-2 among this high-risk group is paramount to ensuring their health and safety.
The 4% SARS-CoV-2 seroprevalence in our first responder participants was higher than the 1.5% seroprevalence reported among Arizona first responders,7 similar to that of non-healthcare related first responders in Detroit, Michigan which found 5% seropositivity for SARS-CoV-26 but was lower than those found in other studies of US first responders. In New York City as of March 31, 11% of emergency medical staff and firefighters were on medical leave for confirmed COVID-194 and in Florida first responders in April 2020, there was a 9% anti-SARS-CoV-2 seropositivity rate when using an assay that measured both IgM and IgG.5 Both regions have some of the highest reported COVID-19 case counts in the country.1 In addition, first responders in the Florida study were tested for both IgM and IgG anti-SARS-CoV-2 antibodies whereas the assay used to test our participants detected anti-SARS-CoV-2 IgG only. Most individuals will have detectable anti-SARS-CoV-2 IgG within 14 days after initial symptom onset.9–11 but by not testing for IgM, we may have missed seropositive individuals with more recent infection. IgM testing in this population should be considered in future studies to adequately determine overall recent and long-term seroconversion. We may also be underestimating the number of individuals who had a previous exposure because some individuals may have lost their antibodies over time.10,12 We were unable to explore the concept of antibody decay in this study, but it will be the subject of future follow-up work in this cohort.
Among SARS-CoV-2 seropositive individuals, almost half were employed in human services or casework and the rest in protective services (police, firefighters, etc). In the United States, it is estimated that 3.4 million individuals work in protective services (police officers, firefighters, etc) and another 2.1 million in community and social services occupations (probation officers, community health workers, etc), representing a significant number of first line workers at risk of SARS-CoV-2 exposure and COVID-19 development.13 Identification of seropositive human service or social workers speaks to the close proximity with which they work with the public. Forms of transmission of SARS-CoV-2 include respiratory droplets which can be spread when an individual coughs, sneezes, or speaks, transmission through airborne aerosols and indirect transmission through contact with a contaminated surface or object.14 As part of their occupation, caseworkers may have closer contact with individuals that last for longer periods of time and may be more likely to be exposed to others in an indoor setting, say in the instance of home inspections, compared with other occupations. There was no difference in the frequency of public interaction for caseworkers compared with other occupations among seropositive participants in our study, which is not unexpected as all individuals received training on safety and use of PPE. In addition to individuals who would classically be considered first responders, studies of COVID-19 high-risk groups, as well as policies created to protect at-risk employees, should include individuals working in human services occupations with frequent public contact.
Older age, as well as several medical conditions, including cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), immunocompromised state, heart disease, diabetes, and obesity, have been suggested as underlying conditions that increase risk for development of severe COVID-19.15 We aimed to identify whether these conditions were also associated with being SARS-CoV-2 seropositive in first responders. We did not see a difference in seropositivity among individuals with and without cancer, immunocompromised state, heart disease, or diabetes and we did not collect data on chronic kidney disease or COPD, though we did ask about general lung disease. Seropositive individuals in our study were more likely to report having lung disease, which is surprising given that these individuals might perceive themselves to be at higher risk for severe disease and therefore more readily protect themselves. In COVID-19 patients, mixed results have been found with regards to lung disease such as COPD or asthma and risk of more severe disease.16–21 Multiple reports of clinical characteristics among COVID-19 patients listed individuals with underlying lung disease, such as COPD and asthma,22–24 but whether chronic lung diseases are associated with worse outcomes among COVID-19 patients remains unclear.16–21 Unfortunately, we did not collect information about the type, history, or severity of lung disease so were not able to describe this relationship further. We also found that individuals who had anti-SARS-CoV-2 antibodies were more likely to report being obese, though this association was not statistically significant. Our findings are in contrast with a study of first responders in Florida that reported no association between seropositivity for SARS-CoV-2 and self-reported body mass index (BMI). Although we found that obesity was more often reported in SARS-CoV-2 seropositive individuals, only 4% of respondents overall reported being obese. This is substantially lower than national estimates that almost 40% of adults are obese in the United States.25 By relying on individuals to self-identify as obese we may be underestimating the true prevalence of obesity in our study population. It is likely that we had non-differential misclassification, where most individuals reported less obesity, regardless of their blood test result (which they were unaware of when they reported their obesity status), meaning the true association is likely stronger than what we report here. However, it is also possible that the association between obesity and antibody reactivity may be a result of reporting bias where individuals who were seronegative were less likely to report being obese. The limited number of cases (11 out of 264) did not allow for investigating the association between lung disease or obesity and anti-SARS-2 reactivity in a multivariable regression, potentially including other factors, as for example socio-economic status or age. When investigating associations between BMI and COVID-19, future work should rely on physical measurements rather than self-report of height and weight.
Participants who were SARS-CoV-2 seropositive were more likely to have been tested and to have a positive result, which is in contrast with the study of Arizona first responders that reported no difference between seropositive and seronegative individuals and prior testing.7 However, testing overall in this population prior to our study was low. This may have resulted from expensive or hard to find SARS-CoV-2 tests. This may also represent a recruitment bias in our study participants. Individuals with a previous positive SARS-CoV-2 or COVID-19 test may not have felt as inclined to participate in our study, as one of the main benefits to the participant was a free test result. In addition, individuals may have been more likely to have a previous test if they had any COVID-19-like symptoms. If that is the case, we may be underestimating the seroprevalence of SARS-CoV-2 antibodies in first responders in the region. Interestingly, two of the SARS-CoV-2 seropositive individuals had a prior negative and one of the seronegative individuals had a prior positive SARS-CoV-2 test. Unfortunately, we did not collect data on the type or timing of prior testing and therefore cannot make any direct comparisons to our assay.
As part of our survey, we asked first responders to define how likely they thought they were to have already had COVID-19. We found that SARS-CoV-2 seropositive participants were more likely to believe they had a prior COVID-19 infection when compared with seronegative participants. Overall, 46 (17%) participants thought they probably to definitely had previously had COVID-19, but only seven (15%) were seropositive for anti-SARS-CoV-2 antibodies, denoting a previous infection. An individual's perception of their risk of infection with SARS-CoV-2 has been associated with adoption of protective behaviors (eg, wearing masks, social distancing, improved hand washing).26–28 It is possible an individual's perception of past infection may also influence changes in behavior. Individuals who believe they already had COVID-19 may be less likely to follow preventive protocols, assuming protection through immunity. However, we found that a small percentage of individuals who believe they already had COVID-19 had detectable antibodies. It is also unclear at this time how long immunity after infection lasts but there is some evidence that immunity wanes over time10 and this waning may occur more quickly in individuals with mild illness29 or asymptomatic individuals.12 Unfortunately, we did not ask about perceptions of future risk nor did we ask about infection prevention behaviors outside of PPE use. It will be important moving forward to remind and support individuals to practice safety measures both at home and in the workplace, regardless of their past infection status.
When asked about the presence of COVID-19 associated symptoms, SARS-CoV-2 seropositive first responders were more likely to report ever having symptoms 3 months prior to testing. Interestingly, there were no associations seen with symptoms present within 1 or 2 months of testing and being SARS-CoV-2 seropositive. This suggests that individuals in our study may have been infected early in the pandemic potentially because of increased risk of infection due to a lack of recognition of the severity of the pandemic, an inadequate supply of PPE, and/or delays in the implementation of prevention protocols for the general population (eg., stay at home order, wearing masks, etc). When broken down by symptom we found that SARS-CoV-2 seropositive participants were more likely to report ever having any of the COVID-19 related symptoms asked about, except for headaches. Headache was the most reported symptom among participants, regardless of their SARS-CoV-2 serostatus, which is consistent with studies of COVID-19 symptoms among health care workers.30 Headaches have been found to be associated with wearing PPE.31 Since first responders in our study were all trained on and were, presumptively, wearing proper PPE, we would expect to see similar frequencies of headaches regardless of an individual's previous SARS-CoV-2 exposure status. We also found that 27% of SARS-CoV-2 seropositive participants reported no symptoms in the 3 months prior to testing. This is slightly lower than the current centers for disease control and prevention estimates that 40% of SARS-CoV-2 infections are asymptomatic32 but similar to reports that 29% of frontline health care personnel with SARS-CoV-2 antibodies were asymptomatic.33 In comparison, over 70% of first responders in Arizona with detection of anti-SARS-CoV-2 IgG antibodies reported having no COVID-19 symptoms.7 There is strong evidence that supports presymptomatic transmission of SARS-CoV-234–37 but whether truly asymptomatic individuals can infect others is still up for debate.38,39 Regardless, proper training on and continued use of PPE is important for protecting first responders as well as the general population.
One strength of our study was enrollment of individuals from a variety of first responder roles and agencies within Arapahoe County, including those working in both rural and urban areas. We used a validated ELISA assay with a high sensitivity and specificity to test for anti-SARS-CoV-2 IgG antibodies to measure prior infection and were able to report on SARS-CoV-2 serology among human services workers, a previously unidentified potentially high-risk group for exposure to SARS-CoV-2. There were also limitations. We did not collect data on smoking status, a potentially important risk factor for COVID-19. Enrolled individuals identified predominately as White, Non-Hispanic/Non-Latinx which may have reduced the generalizability of our results, though it is in line with the demographics of individuals employed in protective service and community and social service occupations in the United States.40 In addition, our study may have had some selection bias as we had low acceptance of the study in rural areas and so may have an underrepresentation of SARS-CoV-2 seroprevalence in that subregion.
CONCLUSION
We were able to estimate the seroprevalence of SARS-CoV-2 in Arapahoe County first responders and to identify factors that may be driving seropositivity and SARS-CoV-2 exposure in this population. The identification of caseworkers as having similar risk of SARS-CoV-2 exposure compared with other first responders points to a need to analyze types of exposure (eg, limited outdoors vs extensive indoor) among first responder populations, and to ensure their inclusion in future risk assessments. Our findings will help with public health strategies regarding the COVID-19 response and provide guidance towards risk assessments of first responders during this pandemic. To ensure the health and safety of first responders, both at work and in the community, future studies need to continue to identify risk factors for transmission both among first responders and the general population, identify the transmission dynamics of SARS-CoV-2, and elucidate the immune systems response to this virus.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the support provided by the Arapahoe County Office of Emergency Management, the University of Colorado Graduate Experience for Multicultural Students For Health Professional Students (GEMS-HP) Program, the staff and facilities of Center Pointe Plaza, our field staff, and all study participants, without whom this work would not have been possible.
Footnotes
Funding Sources: Funding for this study was provided by the Arapahoe County CARES Act. The funders had no role in study design, data collection, or interpretation of the data. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the government of Arapahoe County.
Conflict of Interest: None declared.
Ethical Considerations & Disclosure(s): Informed consent was obtained from each study participant. All protocol and consent forms were approved by the Colorado Multiple Institutional Review Board (COMIRB# 20-1279).
Clinical Significance: First responders are high risk for SARS-CoV-2 exposure, but few studies have focused on this population. To ensure the health and safety of first responders and the community, factors driving SARS-CoV-2 seropositivity must be investigated. Our findings will inform policies to ensure first responder safety during the COVID-19 pandemic.
Supplemental digital contents are available for this article.
REFERENCES
- 1. CDC Centers for Disease Control and Prevention. United States COVID-19 Cases and Deaths by State; 2020. Available at: https://covid.cdc.gov/covid-data-tracker/#cases. Accessed September 23, 2020. [Google Scholar]
- 2. CDC Centers for Disease Control and Prevention. CDC COVID Data Tracker - Cases & Deaths among Healthcare Personnel; 2020. Available at: https://covid.cdc.gov/covid-data-tracker/#health-care-personnel. Accessed September 23, 2020. [Google Scholar]
- 3.Madsen T, Levin N, Niehus K, et al. Prevalence of IgG antibodies to SARS-CoV-2 among emergency department employees. Am J Emerg Med 2020; 38:2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prezant DJ, Zeig-Owens R, Schwartz T, et al. Medical leave associated with COVID-19 among emergency medical system responders and firefighters in New York City. JAMA Netw Open 2020; 3:e2016094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caban-Martinez AJ, Schaefer-Solle N, Santiago K, et al. Epidemiology of SARS-CoV-2 antibodies among firefighters/paramedics of a US fire department: a cross-sectional study. Occup Environ Med 2020; 77:857–861. [DOI] [PubMed] [Google Scholar]
- 6.Akinbami LJ, Vuong N, Petersen LR, et al. SARS-CoV-2 seroprevalence among healthcare, first response, and public safety personnel, detroit metropolitan area, Michigan, USA, May-June 2020. Emerg Infect Dis 2020; 26:2863–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla V, Lau CSM, Towns M, et al. COVID-19 exposure among first responders in Arizona. J Occup Environ Med 2020; 62:981–985. [DOI] [PubMed] [Google Scholar]
- 8.Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol 2020; 57:e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer AS, Jones FK, Nodoushani A, et al. Dynamics and significance of the antibody response to SARS-CoV-2 infection. Preprint medRxiv 2020. [Google Scholar]
- 11.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–1204. [DOI] [PubMed] [Google Scholar]
- 13.Baker MG, Peckham TK, Seixas NS. Estimating the burden of United States workers exposed to infection or disease: a key factor in containing risk of COVID-19 infection. PLoS One 2020; 15:e0232452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. WHO World Health Organization. Transmission of SARS-CoV-2 – implications for infection prevention precautions: Scientific brief; 2020. [Google Scholar]
- 15. CDC Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19)/People with Certain Medical Conditions; 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fneed-extra-precautions%2Fgroups-at-higher-risk.html. Accessed September 2, 2020. [Google Scholar]
- 16.Chhiba KD, Patel GB, Vu THT, et al. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J Allergy Clin Immunol 2020; 146:307.e4–314.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol 2020; 92:1915–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020; 323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020; 133:1032–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang P, Wang P, Song Y, Zhang A, Yuan G, Cui Y. A retrospective study on the epidemiological characteristics and establishment of an early warning system of severe COVID-19 patients. J Med Virol 2020; 92:2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020; 382:2372–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg SK, Whitaker M, O’Halloran A, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 States, March 1-30, 2020. MMWR Morb Mortal Wkly Rep 20202020; 69:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; 75:1730–1741. [DOI] [PubMed] [Google Scholar]
- 25. National Center for Health Statistics. Table 21, Selected health conditions and risk factors, by age: United States, selected years 1988–1994 through 2015–2016; 2019. Available at: https://www.cdc.gov/nchs/hus/contents2018.htm#Table_021. Accessed September 2, 2020. [Google Scholar]
- 26.Bruine de Bruin W, Bennett D. Relationships between initial COVID-19 risk perceptions and protective health behaviors: a national survey. Am J Prev Med 2020; 59:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerhold L. COVID-19: risk perception and Coping strategies. PsyArXiv 2020; [Epub ahead of print]. [Google Scholar]
- 28.Harper CA, Satchell LP, Fido D, Latzman RD. Functional fear predicts public health compliance in the COVID-19 pandemic. Int J Ment Health Addict 2020; 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid Decay of Anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med 2020; 383:1085–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chew NWS, Lee GKH, Tan BYQ, et al. A multinational, multicentre study on the psychological outcomes and associated physical symptoms amongst healthcare workers during COVID-19 outbreak. Brain Behav Immun 2020; 88:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong JJY, Bharatendu C, Goh Y, et al. Headaches associated with personal protective equipment - a cross-sectional study among frontline healthcare workers during COVID-19. Headache 2020; 60:864–877. [DOI] [PubMed] [Google Scholar]
- 32. CDC Centers for Disease Control and Prevention. Coronavirus Disease/Healthcare Workers/Pandemic Planning Scenarios. Web: Centers for Disease Control and Prevention; 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html. Accessed September 2, 2020. [Google Scholar]
- 33.Self WH, Tenforde MW, Stubblefield WB, et al. CDC COVID-19 response team; IVY network. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network — 13 Academic Medical Centers, April–June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis 2020; 26:1341–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qian G, Yang N, Ma AHY, et al. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis 2020; 71:861–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020; 69:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei WE, Zongbin L, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bae SH, Shin H, Koo H-Y, Lee SW, Yang JM, Yon DK. Asymptomatic transmission of SARS-CoV-2 on evacuation flight. Emerg Infect Dis 2020; 26:2705–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao M, Yang L, Chen X, et al. A study on infectivity of asymptomatic SARS-CoV-2 carriers. Respir Med 2020; 169:106026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. U.S. Bureau of Labor Statistics. Labor Force Statistics from the Current Populatoin Survey – Employed persons by detailed occupation, sex, race, and Hispanic or Latino ethnicity 2019. Web: U.S. Bureau of Labor Statistics; 2020. Available at: https://www.bls.gov/cps/cpsaat11.htm. Accessed November 17, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


