Abstract
Background
Placebo can have a significant therapeutic effect in patients with hand osteoarthritis (OA). This aim of the study is to identify factors associated with a clinically meaningful placebo response in patients with hand OA.
Methods
This post-hoc analysis of two double-blind, placebo-controlled, randomized trials (RCTs) investigating the efficacy of GCSB-5 or diacerein as treatments for hand OA analyzed the efficacy of a placebo. Clinical and laboratory factors associated with a clinically meaningful response, defined as an improvement in the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) pain score > 10 at 4 weeks relative to baseline, were identified.
Results
The mean improvement in the AUSCAN pain score was − 6.0 ± 20.3, with marked variation between 143 hand OA patients (range: − 76.4 to 33.2). A clinically meaningful improvement was observed in 54 (37.8%) patients. Placebo responders had worse AUSCAN pain scores (55.7 ± 19.7 vs. 43.6 ± 21.6, p = 0.001) and a worse AUSCAN stiffness (68.2 ± 20.5 vs. 57.5 ± 24.5, p = 0.008) at baseline than non-responders. Improvements in pain correlated with the baseline pain level (Pearson r = − 427, p < 0.001). Structural joint changes such as tender, swollen, enlarged, or deformed joint counts did not differ between placebo responders and non-responders. In a multivariable analysis, only baseline AUSCAN pain was associated with a clinically meaningful placebo response (OR: 1.054, 95% CI [1.019–1.089], p = 0.002).
Conclusions
High levels of pain at baseline are predictive of a clinically meaningful placebo response in patients with hand OA. Further studies are needed to optimize and utilize the benefit of placebo responses in patients with hand OA.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12891-021-04089-9.
Keywords: Osteoarthritis, Hand, Placebo
Background
Osteoarthritis (OA) of the hands is common in middle-aged and elderly populations, especially women [1]. The marked disability and reduced quality of life caused by the disease are comparable with those caused by rheumatoid arthritis (RA) [2, 3]. Pain can be especially debilitating in patients with erosive hand OA, which is characterized by painful swelling, and joint inflammation as well as the subchondral bone erosions and marginal osteophyte formation on radiographic images. The main therapeutic approach to hand OA is to control symptoms by using a combination of non-pharmacological and pharmacological interventions [4]; this is because, unlike for RA, there are no effective disease-modifying osteoarthritis drugs (DMOADs). To date, oral non-steroidal anti-inflammatory drugs (NSAIDs), acetaminophen, or opioid-based analgesics constitute the mainstay of treatment targeting pain control. Inflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor alpha (TNF-α) may contribute to the degeneration of articular cartilage matrix [5]. Therefore, treatment targeting inflammation and pro-inflammatory cytokines were attempted. In a recent study, a short-term treatment with low dose corticosteroid improved pain and signs of inflammation in patients who experience a flare-up of hand OA [6]. However, they and other medications, including anti-tumor necrosis factor inhibitors and anti-interleukin-1 antibody, show only minimal to moderate effect sizes, emphasizing an unmet need for better treatment modalities for hand OA [7–9].
A previous randomized clinical placebo-controlled trial (RCT) involving patients with hand OA reported that 30.2% of patients in the placebo group demonstrated a positive Outcome Measures in Rheumatology-OA Research Society International (OMERACT-OARSI) response at Week 4 [10], suggesting that placebo can have a significant therapeutic effect [11]. In one meta-analysis, the placebo response might account for about 75% of response to drugs commonly used in OA [12]. Boosting the intrinsic placebo response in OA treatment might improve clinical care. For this, it might be important to identify factors associated with a susceptibility to placebo effect [13]. However, it is unclear which patients with hand OA will benefit most from this placebo effect. In this post-hoc analysis of two RCTs, we aimed to identify factors associated with a clinically meaningful placebo response in patients with hand OA.
Methods
Study design
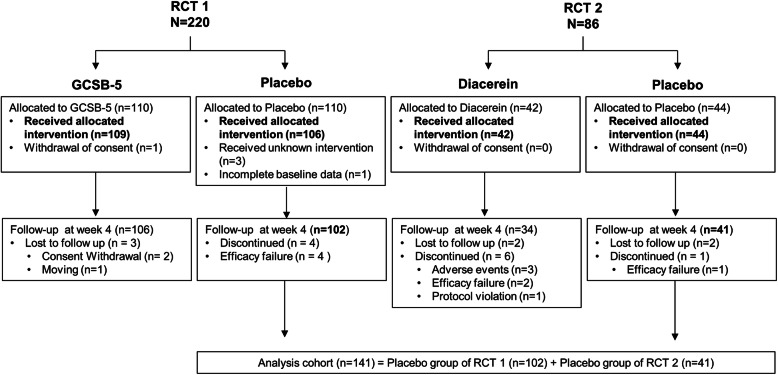
This post-hoc analysis was based on clinical and laboratory data from two prospective, double-blind, randomized, placebo-controlled trials designed to investigate the efficacy and safety of GCSB-5 or diacerein for treating hand OA; both studies were conducted in accordance with the Declaration of Helsinki [10, 14]. In the first RCT, 220 patients with hand OA according to the 1990 American College of Rheumatology (ACR) criteria for hand OA [15], all of whom were aged > 40 years and had pain exceeding 30/100 mm on a visual analog scale in the preceding 48 h, were randomly assigned to receive oral GCSB-5 (600 mg) or placebo twice a day for 12 weeks [10]. In the second RCT, 86 patients with hand OA according to the 1990 ACR for hand OA were randomized to receive diacerein (50 mg) or placebo twice a day [14]. All participating patients provided written informed consent. The study was approved by the institutional review boards of all participating centers and was registered at ClinicalTrials.gov (study no: NCT01910116 and NCT00685542). The post-hoc analysis included 102 patients with hand OA that were in the placebo group of the first RCT and 41 patients that were in the placebo group of the second RCT; patients with available clinical and laboratory parameters at baseline and at Week 4 were included in the analysis group (n = 143) (Fig. 1).
Fig. 1.
Flow diagram. RCT, randomized controlled trial
Outcome
The efficacy endpoints included changes in the following variable from baseline: the AUSCAN pain score (0–100), the AUSCAN stiffness score (0–100), the AUSCAN function score (0–100), a patient global assessment (0–100), a physician global assessment (0–100), and the OMERACT-OARSI response criteria. A clinically significant improvement in pain was defined as an improvement in the AUCAN pain score of 10 (0–100) or more [16]. Patients deemed to be OMERACT-OARSI responders when they showed an improvement relative to baseline in pain or function domains of ≥50% with an absolute change of ≥20, or an improvement relative to baseline in at least two of three (pain, function, and patient global assessment) domains of ≥20% with an absolute change of ≥10 [17].
Statistical analysis
An independent t-test and the Chi-squared test or Fisher’s exact test (as appropriate) were used to compare placebo responders and non-responders in terms of demographics and clinical variables. Normality of variables was examined using Kolmogorov-Smirnov test. Correlations between pain and clinical parameters were assessed using Pearson’s correlation. Multivariable logistic regression analysis was performed to identify factors associated with a clinically meaningful response. P < 0.05 was considered to indicate statistical significance. All analyses were performed by using IBM SPSS Statistics 22 software. All statistical analyses were performed by the authors.
Results
Patients’ characteristics
The mean age of the 102 patients with hand OA in RCT 1 and 41 patients in RCT 2 were 59.4 ± 8.0 years and 61.7 ± 18.9 years, respectively. Women were dominant in both RCTs. The mean disease duration of patients in RCT 1 and those in RCT 2 were 31.5 ± 47.7 months and 60.4 ± 61.1 months, respectively. Baseline characteristics of patients including AUSCAN pain, stiffness and function score were comparable between both groups (Table 1).
Table 1.
Baseline characteristics of patients with hand OA who received placebo in the two randomized controlled trials
| Baseline characteristics | RCT 1 (n = 102) |
RCT 2 (n = 41) |
p-value |
|---|---|---|---|
| Age, years | 59.2 ± 8.0 | 61.7 ± 18.9 | 0.305 |
| Female, n (%) | 95 (93.1) | 39 (95.1) | 0.496 |
| Weight, kg | 59.1 ± 8.0 | 60.4 ± 8.9 | 0.398 |
| Height, cm | 157.0 ± 6.0 | 156.0 ± 6.3 | 0.348 |
| Body mass index, kg/m2 | 23.9 ± 2.9 | 24.7 ± 2.8 | 0.129 |
| Duration of hand OA, months | 31.5 ± 47.7 | 60.4 ± 61.1 | 0.009 |
| Baseline | |||
| AUSCAN pain score (1–100) | 47.8 ± 19.8 | 48.9 ± 25.9 | 0.788 |
| AUSCAN stiffness score (1–100) | 60.6 ± 21.7 | 63.9 ± 27.6 | 0.504 |
| AUSCAN function score (1–100) | 45.7 ± 23.7 | 41.7 ± 27.1 | 0.382 |
| Patient global assessment (1–100) | 49.6 ± 15.9 | 60.8 ± 19.4 | 0.002 |
| Physician global assessment (1–100) | 41.0 ± 13.0 | 42.6 ± 10.5 | 0.471 |
| Tender joint count | 6.3 ± 5.1 | 5.5 ± 5 | 0.400 |
| Swollen joint count | 0.9 ± 2.4 | 0.0 ± 0.3 | 0.000 |
| Palpable node count | 5.2 ± 2.5 | N/A | |
| Deformed joint count | 2.0 ± 1.7 | N/A | |
| Erythrocyte sedimentation rate, mm/hr | 12.9 ± 9.4 | 16.1 ± 11.6 | 0.086 |
| hs-CRP, mg/dL (normal < 0.5 mg/dL) | 0.12 ± 0.26 | 0.21 ± 0.76 | 0.317 |
| Prior treatment, n (%) | N/A | ||
| Acetaminophen/Tramadol | 10 (9.8) | ||
| Acetaminophen | 1 (1.0) | ||
| NSAIDs | 36 (35.3) | ||
| Glucosamine | 13 (12.7) | ||
| Diacerein | 3 (2.9) | ||
| Others | 3 (2.9) | ||
Data are presented as the mean (SD) or n (%). AUSCAN Australian/Canadian Osteoarthritis Hand Index; CRP C-reactive protein, ESR Erythrocyte sedimentation rate, N/A Not available, NSAID Non-steroidal anti-inflammatory drug, OA Osteoarthritis, RCT Randomized controlled trial.
AUSCAN pain
The mean AUSCAN pain score at baseline was 47.8 ± 19.8 in RCT 1 and 48.9 ± 25.9 in RCT 2 (Table 1). In RCT 1, the AUSCAN pain score was associated with the AUSCAN stiffness score (r = 0.312, p < 0.001), the AUSCAN function score (r = 0.743, p < 0.001), the patient global assessment (r = 0.393, p < 0.001), and the physician global assessment (r = 0.205, p = 0.039). However, the AUSCAN pain score was not associated with the tender joint count (r = 0.057, p = 0.567), the swollen joint count (r = 0.032, p = 0.749), the enlarged joint count (r = − 0.044, p = 0.659), or the deformed joint count (r = − 0.065, p = 0.515) at baseline. Similar correlations between baseline AUSCAN pain and other clinical characteristics were observed in RCT 2, except for TJC, which correlated with baseline AUSCAN-pain (r = 0.506, p = 0.001) (Supplementary Table S1).
Factors associated with a significant placebo response
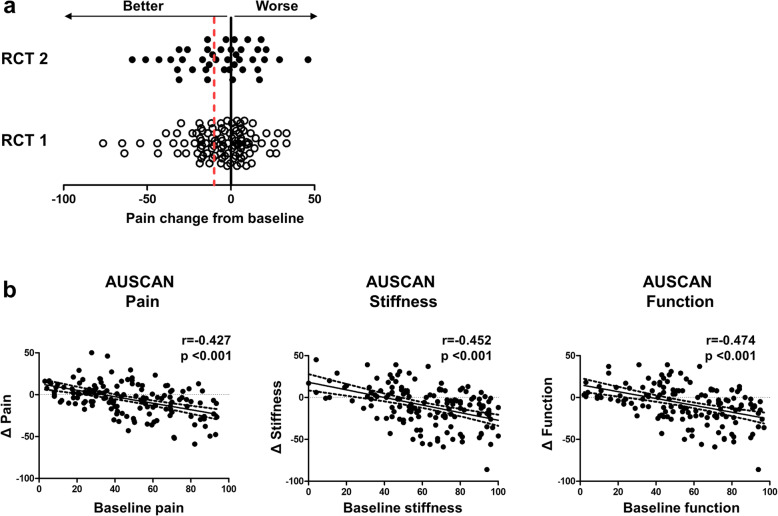
The overall improvement in the AUSCAN pain in 143 patients was − 6.0 ± 20.3. The mean improvement in the AUSCAN pain score did not differ between RCT 1 and RCT 2 (− 6.0 ± 19.7 vs. -6.1 ± 22.1, p = 0.944). The change in pain varied markedly between patients, ranging from − 76.4 to 33.2 in RCT 1 and − 59 to 46.0 in RCT 2 from baseline (Fig. 2a). Patients in RCT 1 and RCT 2 who received placebo showed a similar response with respect to improved pain, stiffness, and function scores. In addition, change in patient and physician global assessments at week 4 were similar between RCTs.
Fig. 2.
Placebo response. a Change in AUSCAN-pain at 4 weeks from the baseline in 102 patients in RCT 1 and 41 patients in RCT 2 were depicted. Red dotted line marks the clinically meaningful response. b Correlation between pain, stiffness, and function at baseline and placebo responses in 143 patients with hand osteoarthritis. Scatterplot represents the relation between the change in pain, stiffness and function and their respective baseline value. Correlations were examined by using Pearson’s correlation analysis
At 4 weeks, 54 (37.8%) of the 143 patients showed a clinically meaningful improvement (i.e., pain reduction > 10) (Table 2). These patients had a worse AUSCAN pain score at baseline (55.7 ± 19.7 vs. 43.6 ± 21.6, p = 0.001), a worse AUSCAN stiffness (68.2 ± 20.5 vs. 57.5 ± 24.5, p = 0.008) than patients without clinical improvement. The tender joint count (TJC), the swollen joint count (SJC), the enlarged joint count, and the deformed joint count did not differ between patients with or without clinically meaningful improvement. More patients showing clinically meaningful improvement used tramadol-AAP at baseline than those not showing clinical improvement (18.9% vs. 1.5%, respectively; p = 0.034). There was no difference between groups with respect to other medications, including NSAIDs and glucosamine (Table 2).
Table 2.
Demographic and clinical characteristics of the 143 hand OA patients in randomized controlled trial 1 and 2 according to clinically significant improvement
| Response (−) (n = 89) |
Response (+) (n = 54) |
P-value | |
|---|---|---|---|
| Age, years | 58.1 ± 7.5 | 60 ± 8.2 | 0.164 |
| Female | 81 (91.0) | 53 (98.1) | 0.153 |
| Weight, kg | 59.1 ± 8.3 | 59.9 ± 8.3 | 0.606 |
| Height, cm | 157.3 ± 5.9 | 155.8 ± 6.2 | 0.150 |
| BMI, kg/m2 | 23.9 ± 2.7 | 24.7 ± 3.1 | 0.105 |
| OA duration, month | 3.2 ± 4 | 3.5 ± 5.1 | 0.657 |
| AUSCAN Pain | 43.6 ± 21.6 | 55.7 ± 19.7 | 0.001 |
| AUSCAN Stiffness | 57.5 ± 24.5 | 68.2 ± 20.5 | 0.008 |
| AUSCAN Function | 41.7 ± 23.2 | 49.1 ± 26.6 | 0.083 |
| Patient GA | 51.1 ± 16 | 55.6 ± 19.9 | 0.158 |
| Physician GA | 40.1 ± 11.3 | 43.7 ± 13.6 | 0.084 |
| Tender JC | 5.8 ± 4.7 | 6.5 ± 5.7 | 0.426 |
| Swollen JC | 0.8 ± 2.4 | 0.5 ± 1.5 | 0.328 |
| Enlarged JC a | 5.1 ± 2.7 | 5.4 ± 2.2 | 0.622 |
| Deformity JC a | 2 ± 1.6 | 1.9 ± 1.9 | 0.658 |
| ESR | 0.17 ± 0.52 | 0.09 ± 0.13 | 0.291 |
| CRP | 13.4 ± 10.4 | 14.5 ± 9.6 | 0.533 |
| Prior treatment | |||
| Tramadol-AAP a | 3 (4.6) | 7 (18.9) | 0.034 |
| Tramadol a | 1 (1.5) | 0 (0) | 1.000 |
| NSAIDs a | 22 (33.8) | 14 (37.8) | 0.685 |
| Diacerin a | 2 (3.1) | 1 (2.7) | 1.000 |
| Glucosamine a | 9 (13.8) | 4 (10.8) | 0.765 |
| Others a | 1 (1.5) | 2 (5.4) | 0.297 |
Data are presented as the mean (SD) or n (%). P values were generated by using an independent t-test (continuous variables) or the Chi-squared test (categorical variables). a Data were not available in the placebo group of RCT 2. AUSCAN Australian/Canadian Osteoarthritis Hand Index, CRP C-reactive protein, ESR Erythrocyte sedimentation rate, NSAID Non-steroidal anti-inflammatory drug, OA Osteoarthritis. Joints according to ACR OA classification criteria were evaluated.
Strikingly, there was a correlation between improvement in pain and level of pain at baseline (Pearson r = − 0.427, p < 0.001). In addition, change in stiffness and function from baseline correlated with baseline stiffness (Pearson r = − 0.425, p < 0.001) and baseline function (Pearson r = − 0.474, p < 0.001), respectively (Fig. 2b).
Factors associated with a clinically meaningful placebo response
A logistic regression analysis was performed to identify factors associated with a clinically meaningful placebo response. In a univariable analysis, baseline AUSCAN pain (OR [95% CI] 1.028, [1.0105–1.0458], p = 0.002) and baseline AUSCAN function (1.021 [1.005–1.0371], p = 0.010) were associated with a better placebo response. In a multivariable analysis, only baseline AUSCAN pain was associated with clinically meaningful placebo response (1.054 [1.019–1.089], p = 0.002).
Discussion
This post-hoc analysis of two prospective, double-blind, randomized, placebo-controlled studies shows that placebo yielded a clinically meaningful improvement in about one third of patients with hand OA. This placebo response was associated significantly with baseline pain, but not with structural changes such as joint swelling or osteophyte formation.
Hand OA is common, with a prevalence ranging from 29 to 76% [1, 18]. In half of patients, the disease will progress, leading to severe functional limitation and a serious disease burden [19]. In the absence of effective DMOADs, symptoms (i.e., pain, function and stiffness) are controlled by NSAIDs, tramadol, and opioid analgesics. However, the potential gastrointestinal and cardiovascular side effects of these drugs limit long-term use [20–23].
Although pain associated with OA is caused by structural changes due to accelerated degeneration of articular cartilage and secondary bone remodeling, pain signals are ultimately perceived by the brain after intensive central pain processing at multiple levels [24]. Consistent with this, we found that pain at baseline was not associated with structural changes such as swollen joints, nor was it associated with osteophyte formation and joint deformity (Supplementary Table S1). Pain correlated with the tender joint count only in RCT 2. Rather, pain was more closely associated with subjective parameters such as the AUCAN stiffness and function scores. Similarly, improvements in AUSCAN stiffness and function scores correlated with baseline stiffness and function, respectively. Taken together, not only pain generation in joints, but also central pain processing, might ultimately determine the level of pain and functional impairment experienced by patients with hand OA.
Although the mean improvement in AUSCAN pain was low, pain responses to placebo varied markedly among the OA patients, ranging from − 76.4 - 33.2 from baseline. Strikingly, high baseline pain, but not the severity of structural joint changes, was associated with a better placebo response (Table 1) [25]. This is consistent with a prior observation demonstrating that neither structural damage observed on ultrasound nor clinical severity of OA are predictive of treatment response [26]; this further supports the dissociation between treatment response and structural joint changes in those with hand OA. Rather, we found that improvements in pain, function, and stiffness correlated significantly with their respective baseline levels (Fig. 1).
While baseline AUSCAN pain and function were associated with a clinically meaningful placebo response, the multivariable analysis identified only the baseline AUSCAN pain as the factor for the placebo response. Interestingly, women with hand OA were 10 fold more likely to have a positive placebo response (Table 3), consistent with sex difference in the placebo response [27]. While OA affects both men and women, women were dominant in both RCTs, consistent with female dominance in the recent trials with hand OA [6, 9]. This suggests that women might suffer more from hand OA than men and they, therefore, are more likely to seek medical attention. Whether women with hand OA are more susceptible to pain, placebo response or both needs further investigation.
Table 3.
Factors associated with a clinically meaningful placebo response
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | OR | 95% CI | P value | OR | 95% CI | P value |
| Age | 1.031 | 0.987–1.077 | 0.164 | 1.039 | 0.987–1.094 | 0.141 |
| Gender (female) | 5.235 | 0.636–43.068 | 0.124 | 10.552 | 0.931–119.633 | 0.057 |
| Weight, kg | 1.011 | 0.970–1.053 | 0.603 | |||
| Height, cm | 0.958 | 0.902–1.016 | 0.152 | 1.010 | 0.934–1.091 | 0.811 |
| BMI | 1.104 | 0.979–1.245 | 0.108 | 1.104 | 0.969–1.258 | 0.136 |
| Ds duration | 1.017 | 0.944–1.097 | 0.655 | |||
| AUSCAN-Pain | 1.028 | 1.010–1.046 | 0.002 | 1.054 | 1.019–1.089 | 0.002 |
| AUSCAN-Function | 1.021 | 1.005–1.037 | 0.010 | 0.974 | 0.947–1.001 | 0.058 |
| AUSCAN-Stiffness | 1.028 | 0.998–1.027 | 0.423 | |||
| Physician global assessment | 1.025 | 0.996–1.054 | 0.088 | 1.018 | 0.986–1.052 | 0.270 |
| Patient global assessment | 1.015 | 0.995–1.035 | 0.137 | 0.998 | 0.972–1.024 | 0.872 |
| Tender JC | 1.028 | 0.961–1.098 | 0.423 | |||
| Swollen JC | 0.914 | 0.762–1.097 | 0.334 | |||
| CRP | 0.444 | 0.08–2.474 | 0.355 | |||
| ESR | 1.011 | 0.978–1.045 | 0.531 | |||
Multivariate logistic regression was performed. Variables that showed association (p < 0.2) in the univariable analysis were included in the multivariable analysis. AUSCAN Australian/Canadian Osteoarthritis Hand Index, BMI Body mass index, CI Confidence interval, CRP C-reactive protein, Ds Disease, ESR Erythrocyte sedimentation rate, JC Joint count, NSAID Non-steroidal anti-inflammatory drug, OR Odds ratio, OA Osteoarthritis.
Placebo effect is not limited to hand OA and it depends on the mode of delivery. In knee OA, intra-articular and topical placebo elicited a greater placebo response than oral placebo [28]. The placebo effect can vary among OA sites since it was greater in knee OA than in hip OA [29]. Taken together, all placebo are not equal. However, it is still important to identify additional factors associated with a treatment response to optimize clinical care of OA patients. As example, early radiographic features such as congruent articular reduction and tiabial plateau alignment were associated with a better pain improvement after surgical treatment of displaced tibial plateau fractures [30]. It is interesting that use of tramadol/AAP was also associated with a better placebo response, whereas NSAIDs and other medications were not. Tramadol acts on central pain processing; it is a weak agonist of the mu opiate receptor and inhibits both serotonin and norepinephrine reuptake, thereby exerting anti-nociceptive effects [31].
A previous study shows that in patients with chronic pain and associated pain sensitization (such as those with fibromyalgia), the retention rate for tramadol/AAP is higher than that for placebo [32, 33]. Therefore, OA patients with severe pain might have developed aberrant central pain processing over time, resulting in increased central sensitization [34]. OA patients who were taking tramadol/AAP at baseline might benefit from anti-nociceptive effects on central pain processing, making them more susceptible to placebo effects. Indeed, duloxetine, which modifies central pain sensitization, is an effective treatment for knee OA [35]. The mechanism underlying central pain processing in OA requires further investigation.
It might be unethical to prescribe a placebo in routine clinical practice. However, the inherent placebo effect of any pharmacological and non-pharmacological treatment could be optimized in routine clinical practice. This is of particular importance since the placebo response might account for about 75% of response to drugs that are commonly used in OA treatment [12]. To optimize this placebo effect, it might be crucial to identify patients who are more susceptible to a placebo response. In this study, female gender and high baseline pain were associated with a clinically significant placebo response (Table 3). The question of whether a warm and reassuring consultation, optimistic attitudes of healthcare providers, and positive relationships between patient and physician improve OA outcomes should be investigated in future.
This study has several limitations. First, the lack of a control group that did not receive any treatment (even placebo) makes estimating the placebo effect difficult. Second, we did not consider depressive mood disorders and/or emotional or physical stress, which might influence pain processing and so placebo responses. Third, RCT 2 (41 patients in the placebo arm) is too small to enable comparison of clinical parameters between clinical responders and non-responders. However, both RCT 1 and RCT 2 showed remarkably similar placebo responses (Supplementary Table S2). Further studies are needed to identify therapeutic and situational factors that improve placebo responses.
Conclusions
The placebo effect can be significant in patients with hand OA who have high pain levels at baseline. Further studies are needed to understand the pathophysiology and underlying mechanisms, and to optimize the placebo effect as an OA treatment in clinical practice.
Supplementary Information
Additional file 1: Table S1. Correlation between AUSCAN pain with baseline characteristics. Table S2. Placebo response at 4 weeks in RCT 1 and RCT 2.
Acknowledgments
The original study was funded by GC pharma, Korea and Myungmmon Pharm Co, Korea. As an investigator-initiated trial, the companies were s not involved in the study design, data acquisition and interpretation, or manuscript preparation. The corresponding author had full access to all the study data and bears ultimate responsibility for the decision to submit for publication.
Abbreviations
- ACR
American College of Rheumatology
- AUSCAN
Australian/Canadian Osteoarthritis Hand Index
- DMOADs
Disease-modifying osteoarthritis drug
- NSAID
Non-steroidal anti-inflammatory drug
- OA
Osteoarthritis
- OMERACT-OARSI
Outcome Measures in Rheumatology-OA Research Society International
- RCT
Randomized trial
- RA
Rheumatoid arthritis
- SJC
Swollen joint count
- TJC
Tender joint count
Authors’ contributions
JKP and EBL had full access to all of the study data and take full responsibility for the integrity of the data and the accuracy of the data analysis. JKP, SHA, KS, YJL, YWS, and EBL designed the experiments, analyzed and interpreted the results, and wrote the manuscript. All authors approved the final version.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1136, HC17C0069).
Availability of data and materials
The data that support the results of this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
The informed consent was received by all participants enrolled in the study. Institutional review boards of all participating centers approved the study.
Consent for publication
Not applicable.
Competing interests
EBL has acted as a consultant to Pfizer and research grants from GC Pharma Korea and Handok inc. The other authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kalichman L, Hernandez-Molina G. Hand osteoarthritis: an epidemiological perspective. Semin Arthritis Rheum. 2010;39(6):465–476. doi: 10.1016/j.semarthrit.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Michon M, Maheu E, Berenbaum F. Assessing health-related quality of life in hand osteoarthritis: a literature review. Ann Rheum Dis. 2011;70(6):921–928. doi: 10.1136/ard.2010.131151. [DOI] [PubMed] [Google Scholar]
- 3.Slatkowsky-Christensen B, Mowinckel P, Loge JH, Kvien TK. Health-related quality of life in women with symptomatic hand osteoarthritis: a comparison with rheumatoid arthritis patients, healthy controls, and normative data. Arthritis Rheum. 2007;57(8):1404–1409. doi: 10.1002/art.23079. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 6.Kroon FPB, Kortekaas MC, Boonen A, Bohringer S, Reijnierse M, Rosendaal FR, Riyazi N, Starmans M, Turkstra F, van Zeben J, et al. Results of a 6-week treatment with 10 mg prednisolone in patients with hand osteoarthritis (HOPE): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;394(10213):1993–2001. doi: 10.1016/S0140-6736(19)32489-4. [DOI] [PubMed] [Google Scholar]
- 7.Kloppenburg M. Hand osteoarthritis-nonpharmacological and pharmacological treatments. Nat Rev Rheumatol. 2014;10(4):242–251. doi: 10.1038/nrrheum.2013.214. [DOI] [PubMed] [Google Scholar]
- 8.Cai G, Aitken D, Laslett LL, Pelletier JP, Martel-Pelletier J, Hill C, March L, Wluka AE, Wang Y, Antony B, et al. Effect of Intravenous Zoledronic Acid on Tibiofemoral Cartilage Volume Among Patients With Knee Osteoarthritis With Bone Marrow Lesions: A Randomized Clinical Trial. JAMA. 2020;323(15):1456–1466. doi: 10.1001/jama.2020.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloppenburg M, Peterfy C, Haugen IK, Kroon F, Chen S, Wang L, Liu W, Levy G, Fleischmann RM, Berenbaum F, et al. Phase IIa, placebo-controlled, randomised study of lutikizumab, an anti-interleukin-1alpha and anti-interleukin-1beta dual variable domain immunoglobulin, in patients with erosive hand osteoarthritis. Ann Rheum Dis. 2019;78(3):413–420. doi: 10.1136/annrheumdis-2018-213336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JK, Shin K, Kang EH, Ha YJ, Lee YJ, Lee KH, Lee EY, Song YW, Choi Y, Lee EB. Efficacy and Tolerability of GCSB-5 for Hand Osteoarthritis: A Randomized, Controlled Trial. Clin Ther. 2016;38(8):1858–1868. doi: 10.1016/j.clinthera.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Abhishek A, Doherty M. Mechanisms of the placebo response in pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1229–1235. doi: 10.1016/j.joca.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Zou K, Wong J, Abdullah N, Chen X, Smith T, Doherty M, Zhang W. Examination of overall treatment effect and the proportion attributable to contextual effect in osteoarthritis: meta-analysis of randomised controlled trials. Ann Rheum Dis. 2016;75(11):1964–1970. doi: 10.1136/annrheumdis-2015-208387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaptchuk TJ, Hemond CC, Miller FG. Placebos in chronic pain: evidence, theory, ethics, and use in clinical practice. BMJ. 2020;370:m1668. doi: 10.1136/bmj.m1668. [DOI] [PubMed] [Google Scholar]
- 14.Shin K, Kim JW, Moon KW, Yang JA, Lee EY, Song YW, Lee EB. The Efficacy of Diacerein in Hand Osteoarthritis: A Double-Blind, Randomized, Placebo-Controlled Study. Clin Ther. 2013;35:431. doi: 10.1016/j.clinthera.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, Brown C, Cooke TD, Daniel W, Gray R, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–1610. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 16.Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol. 2000;27(11):2635–2641. [PubMed] [Google Scholar]
- 17.Pham T, van der Heijde D, Altman RD, Anderson JJ, Bellamy N, Hochberg M, Simon L, Strand V, Woodworth T, Dougados M. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12(5):389–399. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 18.Haugen IK, Englund M, Aliabadi P, Niu J, Clancy M, Kvien TK, Felson DT. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70(9):1581–1586. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bijsterbosch J, Watt I, Meulenbelt I, Rosendaal FR, Huizinga TW, Kloppenburg M. Clinical and radiographic disease course of hand osteoarthritis and determinants of outcome after 6 years. Ann Rheum Dis. 2011;70(1):68–73. doi: 10.1136/ard.2010.133017. [DOI] [PubMed] [Google Scholar]
- 20.Silverstein FE, Faich G, Goldstein JL, Simon LS, Pincus T, Whelton A, Makuch R, Eisen G, Agrawal NM, Stenson WF, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA. 2000;284(10):1247–1255. doi: 10.1001/jama.284.10.1247. [DOI] [PubMed] [Google Scholar]
- 21.Kvien TK, Fjeld E, Slatkowsky-Christensen B, Nichols M, Zhang Y, Proven A, Mikkelsen K, Palm O, Borisy AA, Lessem J. Efficacy and safety of a novel synergistic drug candidate, CRx-102, in hand osteoarthritis. Ann Rheum Dis. 2008;67(7):942–948. doi: 10.1136/ard.2007.074401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang DO, An H, Park GU, Yum Y, Park EJ, Park Y, Jang WY, Kim W, Choi JY, Roh SY, et al. Cardiovascular and Bleeding Risks Associated With Nonsteroidal Anti-Inflammatory Drugs After Myocardial Infarction. J Am Coll Cardiol. 2020;76(5):518–529. doi: 10.1016/j.jacc.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Chang CH, Shau WY, Kuo CW, Chen ST, Lai MS. Increased risk of stroke associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study. Stroke. 2010;41(9):1884–1890. doi: 10.1161/STROKEAHA.110.585828. [DOI] [PubMed] [Google Scholar]
- 24.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatol (Oxford) 2011;50(12):2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- 25.Cubukcu D, Sarsan A, Alkan H. Relationships between Pain, Function and Radiographic Findings in Osteoarthritis of the Knee: A Cross-Sectional Study. Arthritis. 2012;2012:984060. doi: 10.1155/2012/984060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallinson PI, Tun JK, Farnell RD, Campbell DA, Robinson P. Osteoarthritis of the thumb carpometacarpal joint: correlation of ultrasound appearances to disability and treatment response. Clin Radiol. 2013;68(5):461–465. doi: 10.1016/j.crad.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Vambheim SM, Flaten MA. A systematic review of sex differences in the placebo and the nocebo effect. J Pain Res. 2017;10:1831–1839. doi: 10.2147/JPR.S134745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bannuru RR, McAlindon TE, Sullivan MC, Wong JB, Kent DM, Schmid CH. Effectiveness and Implications of Alternative Placebo Treatments: A Systematic Review and Network Meta-analysis of Osteoarthritis Trials. Ann Intern Med. 2015;163(5):365–372. doi: 10.7326/M15-0623. [DOI] [PubMed] [Google Scholar]
- 29.Reiter-Niesert S, Boers M, Detert J. Short-term placebo response in trials of patients with symptomatic osteoarthritis: differences between hip and knee. Osteoarthritis Cartilage. 2016;24(6):1007–1011. doi: 10.1016/j.joca.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Biz C, Maso G, Gambato M, Belluzzi E, Pozzuoli A, Favero M, Vigo M, Ruggieri P. Challenging Surgical Treatment of Displaced Articular Tibial Plateau Fractures: Do Early Knee Radiographic Features Have a Predictive Value of the Mid-Term Clinical Functional Outcomes? Orthop Surg. 2019;11(6):1149–1162. doi: 10.1111/os.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold LM. Biology and therapy of fibromyalgia. New therapies in fibromyalgia. Arthritis Res Ther. 2006;8(4):212. doi: 10.1186/ar1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–545. doi: 10.1016/S0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 33.Bennett RM, Schein J, Kosinski MR, Hewitt DJ, Jordan DM, Rosenthal NR. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum. 2005;53(4):519–527. doi: 10.1002/art.21319. [DOI] [PubMed] [Google Scholar]
- 34.Ramaswamy S, Wodehouse T. Conditioned pain modulation-A comprehensive review. Neurophysiol Clin. 2020;S0987-7053:30146. doi: 10.1016/j.neucli.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Chappell AS, Ossanna MJ, Liu-Seifert H, Iyengar S, Skljarevski V, Li LC, Bennett RM, Collins H. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146(3):253–260. doi: 10.1016/j.pain.2009.06.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Correlation between AUSCAN pain with baseline characteristics. Table S2. Placebo response at 4 weeks in RCT 1 and RCT 2.
Data Availability Statement
The data that support the results of this study are available from the corresponding author upon reasonable request.