Abstract
The evolutionarily conserved Ski2-Ski3-Ski8 (Ski) complex containing the 3′→5′ RNA helicase Ski2 binds to 80S ribosomes near the mRNA entrance and facilitates 3′→5′ exosomal degradation of mRNA during ribosome-associated mRNA surveillance pathways. Here, we assayed Ski’s activity using an in vitro reconstituted translation system and report that this complex efficiently extracts mRNA from 80S ribosomes in the 3′→5′ direction in a nucleotide-by-nucleotide manner. The process is ATP-dependent and can occur on pre- and post-translocation ribosomal complexes. The Ski complex can engage productively with mRNA and extract it from 80S complexes containing as few as 19 (but not 13) 3’-terminal mRNA nucleotides starting from the P site. The mRNA-extracting activity of the Ski complex suggests that its role in mRNA quality control pathways is not limited to acceleration of exosomal degradation, and could include clearance of stalled ribosomes from mRNA, poising mRNA for degradation and rendering stalled ribosomes recyclable by Pelota/Hbs1/ABCE1.
Keywords: Ski complex, Ski2 helicase, RNA exosome, ribosome-associated quality control, mRNA surveillance, No-Go decay
Graphical Abstract
eTOC Blurb
The evolutionarily conserved Ski complex containing the 3′→5′ RNA helicase Ski2 binds to 80S ribosomes near the mRNA entrance. Zinoviev et al. found that Ski extracts mRNA from 80S ribosomal complexes in the 3′→5′ direction in a nucleotide-by-nucleotide manner. Ski-mediated mRNA extraction renders ribosomal complexes susceptible to recycling by Pelota/Hbs1/ABCE1.
INTRODUCTION
To ensure the accuracy of gene expression, eukaryotic cells employ mRNA and protein quality control systems that are induced by interrupted translation. Thus, No-Go decay and non-stop decay surveillance mechanisms target mRNAs on which elongation complexes are stalled by structural defects, tandem rare codons, internal poly(A) sequences or the absence of a stop codon (Shoemaker and Green, 2012; Simms et al., 2017a; Ikeuchi et al., 2019a). The aberrant polypeptides arising from interrupted translation are degraded by the ribosome-associated quality control (RQC) pathway (Brandman and Hegde, 2016; Ikeuchi et al., 2019a; Joazeiro, 2019).
Collision of ribosomes at the stall site creates a unique interface between the small subunits of the stalled leading and the following colliding ribosome (Juszkiewicz et al., 2018; Ikeuchi et al., 2019b), which is recognized by the E3 ubiquitin ligase Hel2 (ZNF598 in mammals) that ubiquitinates collided ribosomes to trigger quality control events (Garzia et al., 2017; Juszkiewicz and Hegde, 2017; Matsuo et al., 2017; Simms et al., 2017b; Sundramoorthy et al., 2017; Ikeuchi et al., 2019b). Although endonucleolytic cleavage of mRNA upstream of the stall site is an established hallmark of No-Go decay (Doma and Parker, 2006), the nature of this process has remained obscure. Recent studies showed that ribosomal ubiquitination acts as a signal for recruitment to the stall site of an endonuclease, identified as Cue2 in Saccharomyces cerevisiae (D’Orazio et al., 2019) and NONU-1 in Caenorhabditis elegans (Glover et al., 2019). Cleavage is reported to occur in the A site of collided ribosomes (D’Orazio et al., 2019). The mechanism of the subsequent ribosomal disassembly is also not clear. Whereas the collided ribosome associated with the 5’-terminal mRNA fragment could be split by Pelota/Hbs1/ABCE1, which can act on ribosomal complexes containing only very few mRNA nucleotides after the P site (Pisareva et al., 2011), the mechanism of dissociation of the leading stalled ribosome bound to the 3’-terminal mRNA fragment is obscure. Although the RQC-trigger (RQT) complex containing the Slh1 helicase (ASCC3 in mammals) is implicated in dissociating stalled ribosomes (Brandmann et al., 2012; Matsuo et al., 2017; Sitron et al., 2017), the mechanism of dissociation and structural requirements for complexes to be susceptible to Slh1-mediated dissociation are not known. Ribosomal disassembly culminates in ubiquitination and proteasomal degradation of 60S-associated nascent chains (Brandman and Hegde, 2016; Ikeuchi et al., 2019a; Joazeiro, 2019), whereas the 3’ and 5’ mRNA cleavage products are degraded by Xrn1 and the RNA exosome, respectively (Doma and Parker, 2006).
The 3′→5′ exosomal degradation is facilitated by the Ski complex (Doma and Parker, 2006; van Hoof et al., 2002; Hashimoto et al., 2017; Arribere and Fire, 2018), which comprises the tetratricopeptide repeat protein Ski3 (TTC37 in humans), two copies of the WD repeat protein Ski8 (WDR61 in humans), and the DExH helicase Ski2 (SKIV2L in humans) that has a 3’–5’ unwinding activity (Synowsky and Heck, 2008; Halbach et al., 2013). This complex binds to 80S ribosomal complexes near the mRNA entrance via Ski2, Ski3 and one copy of Ski8, which leads to displacement of the autoinhibitory domain of Ski2, resulting in its adopting an open conformation and in threading of mRNA from the ribosome directly into Ski2 (Schmidt et al., 2016). The ribosomal surfaces involved in interactions with translational GTPases and ABCE1 remain accessible.
Regarding the importance of the Ski complex for quality control processes and its striking position on the ribosome, we investigated its activity using an in vitro reconstituted mammalian translation system.
RESULTS
The mammalian Ski complex extracts mRNAs from 80S ribosomal complexes
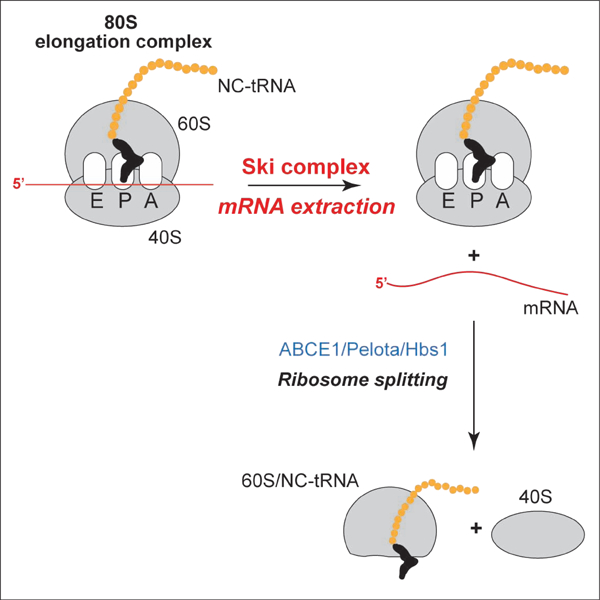
The Ski complex was purified from FLAG-Ski8 expressing HEK293T cells. The preparation (Figure 1A) contained a moderate excess of Ski8. To assay the activity of the Ski complex, we employed 80S initiation complexes (ICs) (with Met-tRNAiMet in the P site), elongation complexes (ECs) in the pre-translocation state (with deacylated tRNA in the P site and peptidyl-tRNA in the A site), ECs in the post-translocation state (with peptidyl-tRNA in the P site), and pre-termination complexes (pre-TCs) that are structurally identical to post-translocation ECs but contain a stop codon instead of a sense codon in the A site (Figure 1B). Reconstituted complexes were purified by sucrose density gradient (SDG) centrifugation, incubated with the Ski complex, and the ribosomal position was determined by toe-printing, which involves extension by reverse transcriptase of a primer annealed to the ribosome-bound mRNA (Figure S1A). cDNA synthesis is arrested by the leading edge of the 40S subunit yielding toe-prints +15–17 nt from the P site codon.
Figure 1. Ski-mediated extraction of mRNA from ribosomal complexes.
(A) Purified Ski complex, analyzed by SDS-PAGE followed by fluorescent SYPRO staining. (B) Schematic representation of 80S IC, EC and pre-TC complexes. (C) Schematic representation of MF-Stop, MLLSSF-Stop and MVLL-Stop mRNAs, showing sequences of their 5’UTRs and coding regions. Upstream codons that are cognate or near-cognate to the P site tRNAs in the assembled ECs and on which ribosomes were halted during Ski-mediated mRNA extraction are in red and bracketed. (D) The activity of the Ski complex on 80S ICs and pre- and post-translocation ECs formed on MF-Stop mRNA, assayed by toe-printing. (E) The model for testing of the mechanism of Ski’s action. (F-G) The activity of the Ski complex on ECs formed on MLLSSF-Stop mRNA and containing MLLSS-tRNASer and the UCU codon in the P site, depending on (F) ATP and (G) free [Mg2+], assayed by toe-printing. Red arrows indicate ribosomal complexes halted at upstream codons (shown in red on the left). Separation of lanes in panels D and F by white lines indicates that they were juxtaposed from the same gel. (H) Elongation by ribosomes halted at the upstream codon during Ski-mediated mRNA extraction from pre-TCs formed on MVLL-Stop mRNA, assayed by toe-printing. Red arrows indicate ribosomal complexes formed at upstream codons (shown in red on the left) before and after one round of elongation. (I) The influence of eRF1•eRF3 and the Ski complex on ECs formed on MLLSSF-Stop mRNA and containing [35S]MLLSS-tRNALeu in the P site and the UUC(Phe) codon in the A site, depending on Saa-tRNA, eEF1H and eEF2, assayed by SDS-PAGE. The positions of peptidyl-tRNAs are indicated. (J, K) Dissociation of ECs in post- (J) and pre- (K) translocation states formed on MF-Stop mRNA using [32P]60S subunits by combinations of the Ski complex and Pelota/Hbs1/ABCE1 in the presence of eIF6, assayed by SDG centrifugation.
The Ski complex strongly destabilized 80S ICs formed on MF-Stop mRNA (Figure 1C), evident by the weakened 80S IC toe-prints and the appearance of prominent full-length cDNA (Figure 1D, lane 2). Addition of eEF2 did not affect Ski’s activity (Figure 1D, lane 3). The Ski complex also efficiently destabilized ECs in pre- and post-translocation states (Figure 1D, lanes 4–7). The 5’-terminal cap did not interfere with Ski’s function, and release of full-length mRNA was also observed in complexes formed on capped mRNA (Figure S1B). Individual Ski8 did not affect the integrity of ECs (Figure S1C).
To investigate the mechanism of Ski-mediated destabilization, we determined whether Ski acts by extracting mRNA in the 3′→5′ direction in a nucleotide-by-nucleotide manner. In this case, the Ski complex would extract mRNA until a cognate or near-cognate codon from the upstream mRNA region enters the P site, where it could stall the extraction by base-pairing with the P site tRNA that remains ribosome-associated (Figure 1E), yielding a new toe-print. To test this hypothesis, we assembled ECs with the P site MLLSS-tRNASer-AGA and the A site UUC(Phe) codon on MLLSSF-Stop mRNA containing upstream UCU(Ser) and UCC(Ser) codons that are cognate and near-cognate to the P site tRNASer-AGA (Figure 1C). Ski not only decreased toe-prints corresponding to original ECs and increased full-length cDNA, but also induced new toe-prints +16 nt from the upstream UCU and UCC codons (Figure 1F, lanes 1 and 2), confirming that the Ski complex extracts mRNA in the 3′→5′ direction in a nucleotide-by-nucleotide manner. Extraction was ATP-dependent and did not occur in the presence of AMPPNP (Figure 1F, lanes 3 and 4). Although elevation of [Mg2+] reduced Ski’s processivity, lowering the full-length cDNA and increasing toe-prints of original ECs and of ribosomes halted at the upstream codons, Ski retained substantial activity even at 7.5 mM free [Mg2+] (Figure 1G).
To determine whether ribosomes paused at upstream codons are elongation-competent, we employed MVLL-Stop mRNA with the 5’UTR containing a CUU(Leu) codon that is identical to the last codon of the open reading frame and is followed by a UCU(Ser) codon (Figure 1C). Incubation with Ski of pre-TCs formed on this mRNA yielded complexes arrested at the upstream CUU that underwent elongation upon addition of elongation factors and Ser-tRNASer-AGA (Figure 1H, lanes 2 and 4). We also assayed Ski-dependent elongation by following peptidyl-tRNA, using ECs assembled on MLLSSF-Stop mRNA and containing [35S]MLLSS-tRNASer in the P site and a UUC(Phe) codon in the A site. As expected, ECs could not undergo peptide release by eRF1•eRF3 irrespective of the presence of the Ski complex (Figure 1I, upper panel). Incubation of ECs with elongation factors and total amino-acylated tRNA (Σaa-tRNAs) converted them into pre-TCs, which could undergo peptide release (Figure 1I, lower panel, lanes 1 and 3). However, inclusion of Ski yielded not only pre-TCs, but also complexes with longer peptides that were not susceptible to eRF1•eRF3 (Figure 1I, lower panel, lanes 2 and 4). Taken together, these data indicate that ribosomes arrested at upstream codons are elongation-competent.
Ski’s activity did not depend on the length of the nascent peptide: it efficiently extracted mRNA from pre-TCs with a 75aa-long nascent chain (Figure S1D) and slightly reduced eRF1•eRF3-mediated peptide release on pre-TCs with a 25aa-long peptide (Figure S1E). However, Ski did not affect the integrity of 48S ICs (Figure S1F).
To test whether Ski-mediated mRNA extraction could render ribosomal complexes susceptible to dissociation by Pelota/Hbs1/ABCE1, pre- and post-translocation ECs were assembled on MF-Stop mRNA using 32P-labeled 60S subunits (Pisarev et al., 2007b). To prevent subunit reassociation, reaction mixtures were supplemented with eIF6. Consistent with the inability of Pelota/Hbs1/ABCE1 to split ribosomal complexes containing long mRNA regions downstream from the P site (Pisareva et al., 2011), incubation of pre- and post-translocation ECs with Pelota/Hbs1/ABCE1 alone yielded only small amounts of 60S subunits, which likely arose from dissociation of vacant 80S ribosomes present as minor contaminants in EC preparations (Figures 1J–K, green). However, in Ski’s presence, Pelota/Hbs1/ABCE1 promoted efficient splitting of pre- and post-translocation ECs (Figures 1J–K, red). Concomitant dissociation of mRNA was confirmed by toe-printing (Figure S1G). Ski alone did not induce ribosomal splitting (Figures 1J–K, blue). Although Ski-dependent splitting of post-translocation ECs was expected since these complexes do not have an A site tRNA, dissociation of the A site tRNA-containing pre-translocation ECs was more surprising. One possibility is that after mRNA extraction, these complexes can undergo eEF2-independent translocation (possibly stimulated by association with Pelota•Hbs1), which allows accommodation of Pelota in the A site. However, drop-off of peptidyl-tRNA from such complexes can also not be excluded, and experiments with pre-translocation ECs containing substantially longer peptides (which can currently not be reconstituted in vitro) are required to test this possibility.
Reconstitution of the mammalian cytoplasmic RNA exosome
Although the Ski complex extracted mRNA from complexes containing long 3’-terminal mRNA regions, mRNA that does not extend far enough from the ribosome might not bind productively to Ski. Since toe-printing cannot be done on complexes containing short overhanging mRNA regions, we determined the number of 3’-terminal mRNA nucleotides required for Ski’s activity by coupling mRNA extraction with its degradation by the exosome.
Eukaryotic RNA exosomes (Zinder and Lima, 2017) have a barrel-like nine-subunit (EXOSC1-EXOSC9) non-catalytic core (Exo9) containing a central channel that accommodates single-stranded RNA. The tenth subunit, DIS3, has a processive 3′→5′ exoribonuclease activity. In contrast to S. cerevisiae, mammals encode two exosome-associated DIS3 isoforms, nuclear DIS3 and cytoplasmic DIS3L1 (Tomecki et al., 2010; Staals et al., 2010). Dis3 contains a PilT N-terminal (PIN) domain and a RNAse II/R domain with processive 3’–5’ exonucleolytic activity (Lorentzen et al., 2008). The PIN domain has endonucleolytic activity in Dis3 (Dziembowski et al., 2007) but not in Dis3L1 (Staals et al., 2010). Dis3 attaches to the bottom of the exosome, so that access to it may be direct or involve passage through the Exo9 channel (Makino et al., 2015; Liu et al., 2016; Zinder et al., 2016). The nuclear exosome also associates with another catalytic subunit, EXOSC10, that has distributive 3′→5′ exoribonuclease activity (Lykke-Andersen et al., 2011).
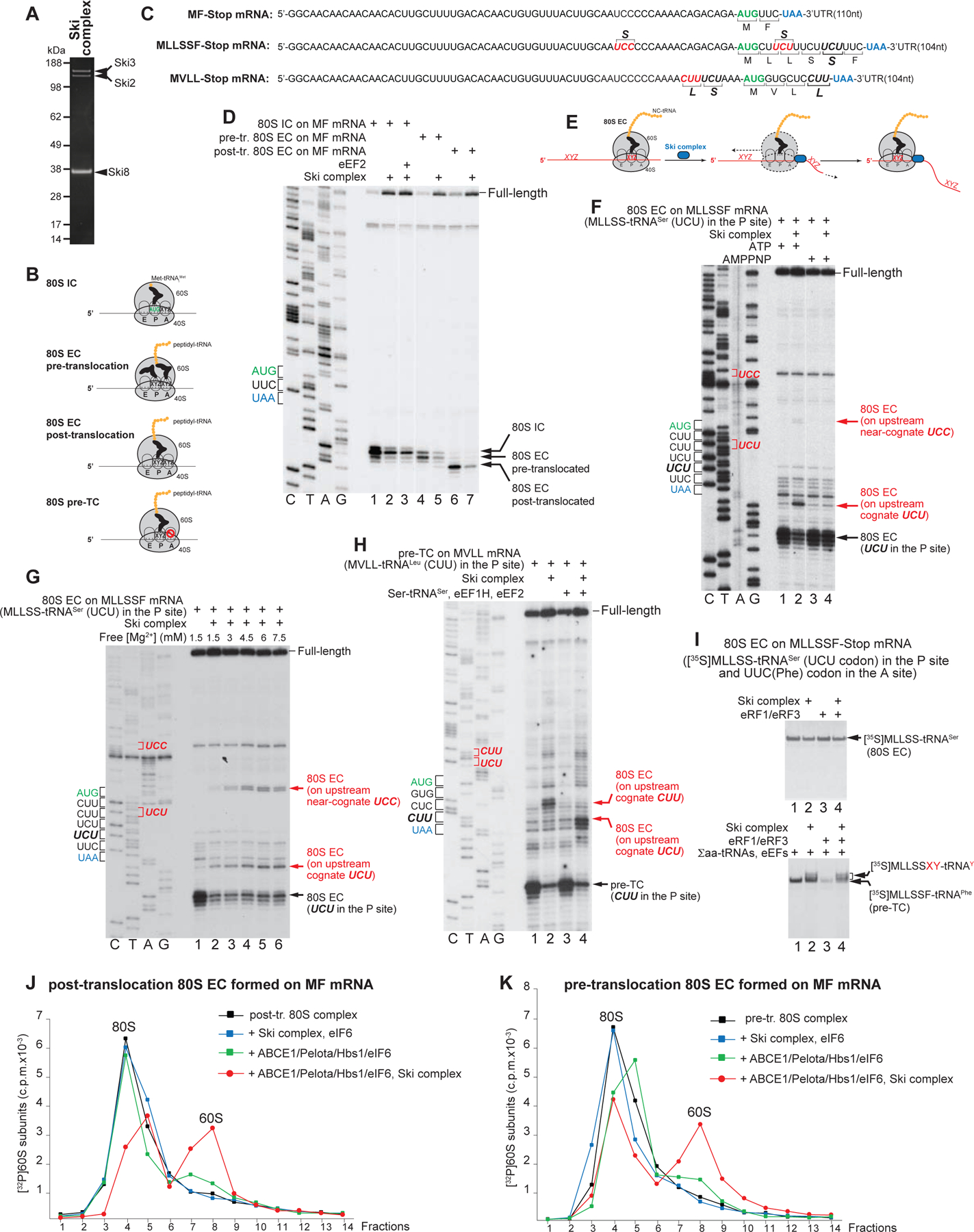
To reconstitute the human cytoplasmic RNA exosome in vitro, FLAG-tagged Dis3L1 was expressed in HEK293T cells (Figure 2A). The preparation contained only trace amounts of Exo9, indicating labile association of Dis3L1 with the core. The Exo9 subunits were expressed in E. coli (Figure 2B). To obtain Exo9 or the Exo9/Dis3L1 complex (Exo10Dis3L1), core subunits were combined and incubated with or without Dis3L1, and resulting complexes were resolved by gel-filtration (Figures 2C–D). Exosome activity was assayed using non-polyadenylated low-structured [32P]capped MLLFF-Stop mRNAs comprising a derivative of the β-globin 5’UTR, a MLLFF coding region, a UAA stop codon and a 7nt-, 27nt- or 47nt-long 3’UTR (Figure 2E). The same mRNAs were used for assembly of ribosomal complexes.
Figure 2. Reconstitution and activity of the mammalian cytoplasmic RNA exosome.
(A) Dis3L1 purified from HEK293T cells, (B) Exo9 subunits purified from E. coli, (C) assembled Exo9 and (D) assembled Exo10Dis3L1, analyzed by SDS-PAGE followed by fluorescent SYPRO staining. (E) Sequences of MLLFF-Stop mRNAs containing 7nt-, 27nt- and 47nt-long 3’UTRs, and the Mfold stem-loop in the 47nt-long 3’UTR. (F) Time courses of degradation of [ 32P]cap-27nt-3’UTR MLLFF-Stop mRNA by Dis3L1, Exo10Dis3L1 and Dis3L1 with Exo9 at 1.5 mM free [Mg2+]. Lane 1 contains RNA markers. Separation of lanes by white lines indicates that they were juxtaposed from the same gel. (G, H) The activities of Dis3L1 and Exo10Dis3L1 on (G) [32P]cap-7nt-3’UTR and (H) [ 32P]cap-47-nt-3’UTR MLLFF-Stop mRNAs after 30 min incubation at 1.5 mM free [Mg2+]. (I) [Mg2+]-dependence of the activity of Exo10Dis3L1 on [32P]cap-27nt-3’UTR MLLFF-Stop mRNA after 30 min incubation. Lane 6 contains RNA markers.
Exo10Dis3L1 efficiently degraded mRNA with a 27nt-long 3’UTR, yielding a ~9nt-long final product (Figure 2F, lanes 7–10). A similar product was obtained during degradation of 5’[32P]-phosphorylated mRNA (Figure S2). Individual Dis3L1 had substantially lower activity, evidenced by the persistence of full-length mRNA and only slightly truncated degradation intermediates (Figure 2F, lanes 3–6), but addition of Exo9 strongly increased the yield of the 9nt-long product (Figure 2F, lanes 11–14). Qualitatively similar activities of Dis3L1 and Exo10Dis3L1 were observed on mRNAs containing 7nt- and 47nt-long 3’UTRs: in both cases Exo10Dis3L1 yielded the 9nt-long final product, whereas Dis3L1 produced long degradation intermediates (Figures 2G–H). Degradation of mRNA containing a 47nt-long 3’UTR was less efficient consistent with the presence of a structured element at its 3’-end (Figure 2E). Exosome activity was sensitive to elevation of [Mg2+] (Figure 2I).
Individually, human Dis3L1 and yeast Rrp44 (Dis3p) were shown to generate 4–5nt-long final products (Tomecki et al., 2010; Lorentzen et al., 2008), and binding of Exo9 to Rrp44 did not change the product’s size (Wasmuth and Lima, 2012). Generation of longer cleavage products by Exo10 Dis3L1 could reflect Exo9-induced changes that are specific to Dis3L1, but may also be determined by the 5’-terminal nucleotide sequence that is common to all employed mRNAs. Another distinctive characteristic of the human cytoplasmic exosome is that Exo9 enhanced Dis3L1’s processivity on relatively long mRNAs employed here, whereas binding to Exo9 suppressed Dis3’s activity, at least when tested using shorter substrates (Wasmuth and Lima, 2012; Drazkowska et al., 2013; Zinder et al., 2016). Future analysis of the human cytoplasmic exosome should include systematic variation of the length, sequence and secondary structure of RNA substrates.
Determination of the number of mRNA nucleotides downstream from the P site required for Ski-mediated mRNA extraction
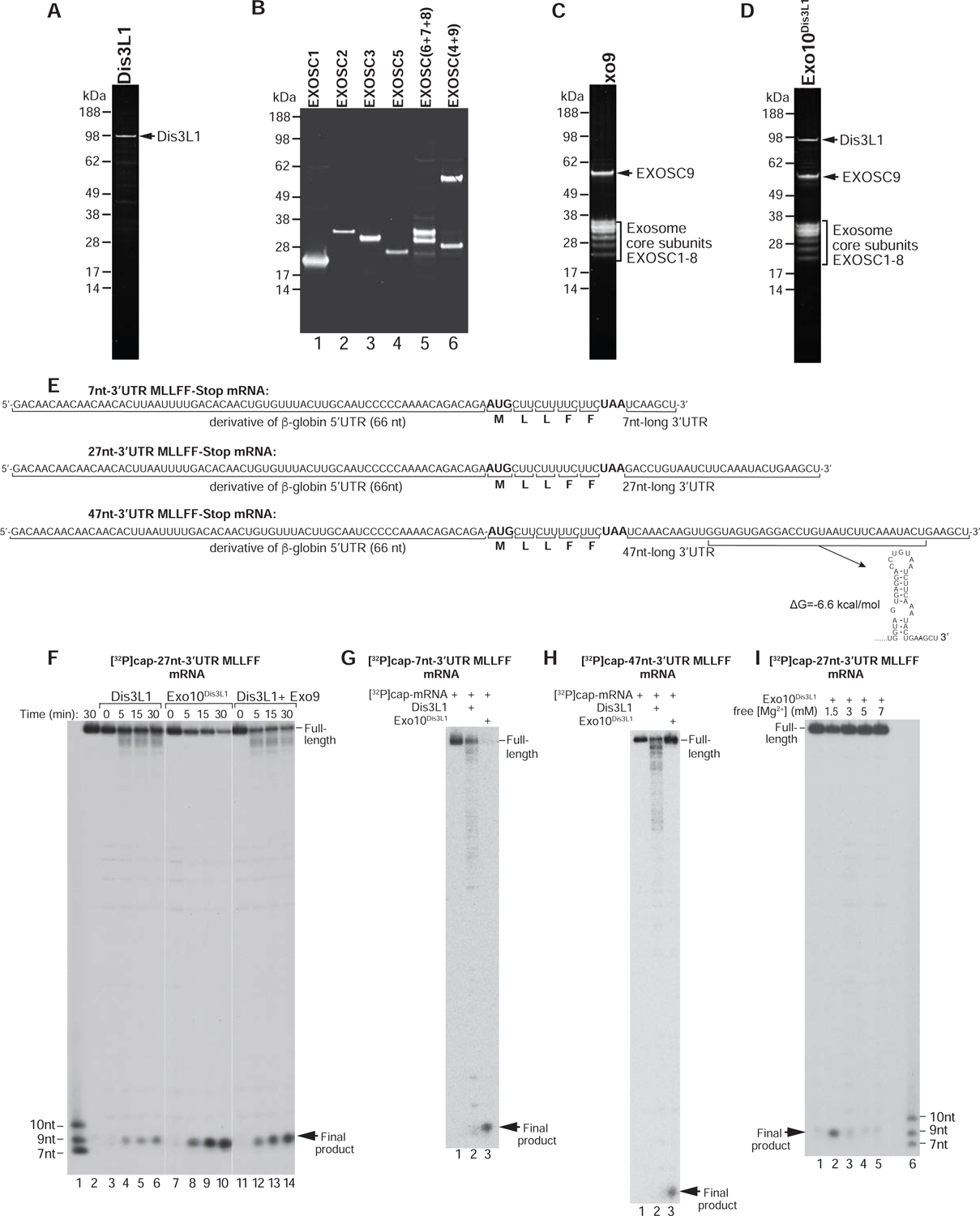
To investigate Ski’s activity as a function of the length of mRNA downstream of the P site, we employed post-translocation ECs and pre-TCs assembled on [32P]capped MLLFF-Stop mRNAs (Figure 2E). ECs formed on mRNAs with 7nt-, 27nt- and 47nt-long 3’UTRs contained MLL-tRNALeu in the P site, the UUC(Phe) codon in the A site, and 19, 39 or 59 mRNA nucleotides starting from the P site, whereas corresponding pre-TCs contained MLLFF-tRNAPhe in the P site, the UAA stop codon in the A site, and 13, 33 or 53 mRNA nucleotides starting from the P site.
To verify this approach, we first assayed Dis3L1-mediated degradation in ECs formed on mRNA with a 27nt-long 3’UTR, depending on the presence of Ski and Exo9. In Ski’s absence, Dis3L1 (with or without Exo9) cleaved only a few 3’-terminal mRNA nucleotides, whereas addition of Ski resulted in efficient degradation (Figure 3A, lanes 2–5). Exo9 enhanced the activity of Dis3L1. No degradation occurred with the Ski complex alone (Figure 3A, lane 6).
Figure 3. Dependence of the activity of the Ski complex on the length of mRNA downstream of the P site.

(A) mRNA degradation in ECs assembled on [32P]cap-27nt-3’UTR MLLFF-Stop mRNA and containing MLL-tRNALeu in the P site and 39 mRNA nucleotides downstream from the P site after 30 min incubation with Dis3L1 or Dis3L1+Exo9 in the presence/absence of the Ski complex. Separation of lanes by white lines indicates that they were juxtaposed from the same gel. (B-E) mRNA degradation in (B, D) ECs and (C, E) pre-TCs assembled on [32P]cap-labeled 47nt-3’UTR, 27nt-3’UTR and 7nt-3’UTR MLLFF-Stop mRNAs and containing (B, D) 59, 39 or 19, and (C, E) 53, 33 or 13 mRNA nucleotides downstream from the P site after 30 min incubation with (B, C) Dis3L1+Exo9 or (D, E) Exo12EXOSC10/SKIV2L2/C1D in the presence/absence of the Ski complex. (F) mRNA degradation in ECs and pre-TCs assembled on [32P]cap-47nt-3’UTR after 30 min incubation with Exo10Dis3L1 or Exo12EXOSC10/SKIV2L2/C1D.
In Ski’s absence, Dis3L1•Exo9 cleaved not more than a few 3’-terminal mRNA nucleotides in all ECs and post-TCs (Figures 3B–C, lanes 2, 5 and 8). Ski enabled efficient degradation in all ECs containing as few as 19 mRNA nucleotides downstream of the P site (Figure 3B, lanes 3, 6 and 9) and in pre-TCs containing 53 or 33 mRNA nucleotides (Figure 3C, lanes 3 and 6). However, Ski did not promote degradation in pre-TCs formed on mRNA with a 7nt-long 3’UTR and containing 13 nucleotides from the P site (Figure 3C, lane 9). The same dependence of Ski’s activity on the length of the 3’-terminal region of mRNA was observed using the nuclear Exo12EXOSC10/SKIV2L2/C1D exosome containing the catalytic EXOSC10 instead of Dis3 (Domanski et al., 2016; Zinoviev et al., 2018) (Figures 3D–E). Consistent with EXOSC10 having distributive exoribonuclease activity, Exo12EXOSC10/SKIV2L2/C1D yielded relatively long degradation intermediates instead of the ~9nt-long product.
We also noticed that incubation with Dis3L1•Exo9 or Exo12 EXOSC10/SKIV2L2/C1D of ECs or pre-TCs formed on mRNA containing a 47nt-long 3’UTR yielded distinct products (Figure 3B–C, lanes 2, and Figure 3C–D, lanes 3), which were identified as mRNA truncated by 4–5 nt (Figure 3F). This would be consistent with the stem-loop (Figure 2E) that would be present directly outside the mRNA entrance.
In conclusion, 19 but not 13 mRNA nucleotides downstream from the P site are sufficient for Ski-mediated extraction of mRNA.
Exosomal degradation of polyadenylated mRNA
Since 3’-polyadenylation protects mRNAs from degradation, we investigated exosomal activity on MLLFF-Stop-(A)50 mRNA with a 50nt-long poly(A) tail (Figure 4A). Whereas Dis3L1 degraded polyadenylated mRNA, yielding 3’-terminally truncated intermediates, almost no degradation occurred in the presence of Exo10Dis3L1 (Figure 4B). Consistently, addition of Exo9 inhibited Dis3L1’s activity, leading to longer degradation products and more full-length mRNA (Figure 4C). Exo9 had a similar effect on Dis3L1-mediated degradation of MLLFF-Stop-(A)50 mRNA engaged in ECs (Figure 4D). Thus, association with Exo9 specifically inhibited Dis3L1 activity on a poly(A) tract, which has a distinct stacked helical structure (e.g. Tang et al., 2019). Similar attenuation of degradation of poly(A)-containing mRNA by fungal Dis3 occurred on its binding to Exo9 (Wasmuth and Lima, 2012; Drazkowska et al., 2013), likely due to imposition of a requirement for RNA to pass through the central channel of Exo9 to access the Dis3 active site. However, it might not be possible to extrapolate these studies of the yeast RNA exosome to mammalian cytoplasmic complexes directly since the length of the central Exo9 channel, the conformation of Dis3 and the interaction of Dis3 with RNA substrate in human and yeast exosomes differ (Gerlach et al., 2018; Weick et al., 2018), and moreover, the structure of Dis3L-containing exosomes has not yet been determined.
Figure 4. The activity of the cytoplasmic RNA exosome on polyadenylated mRNA.
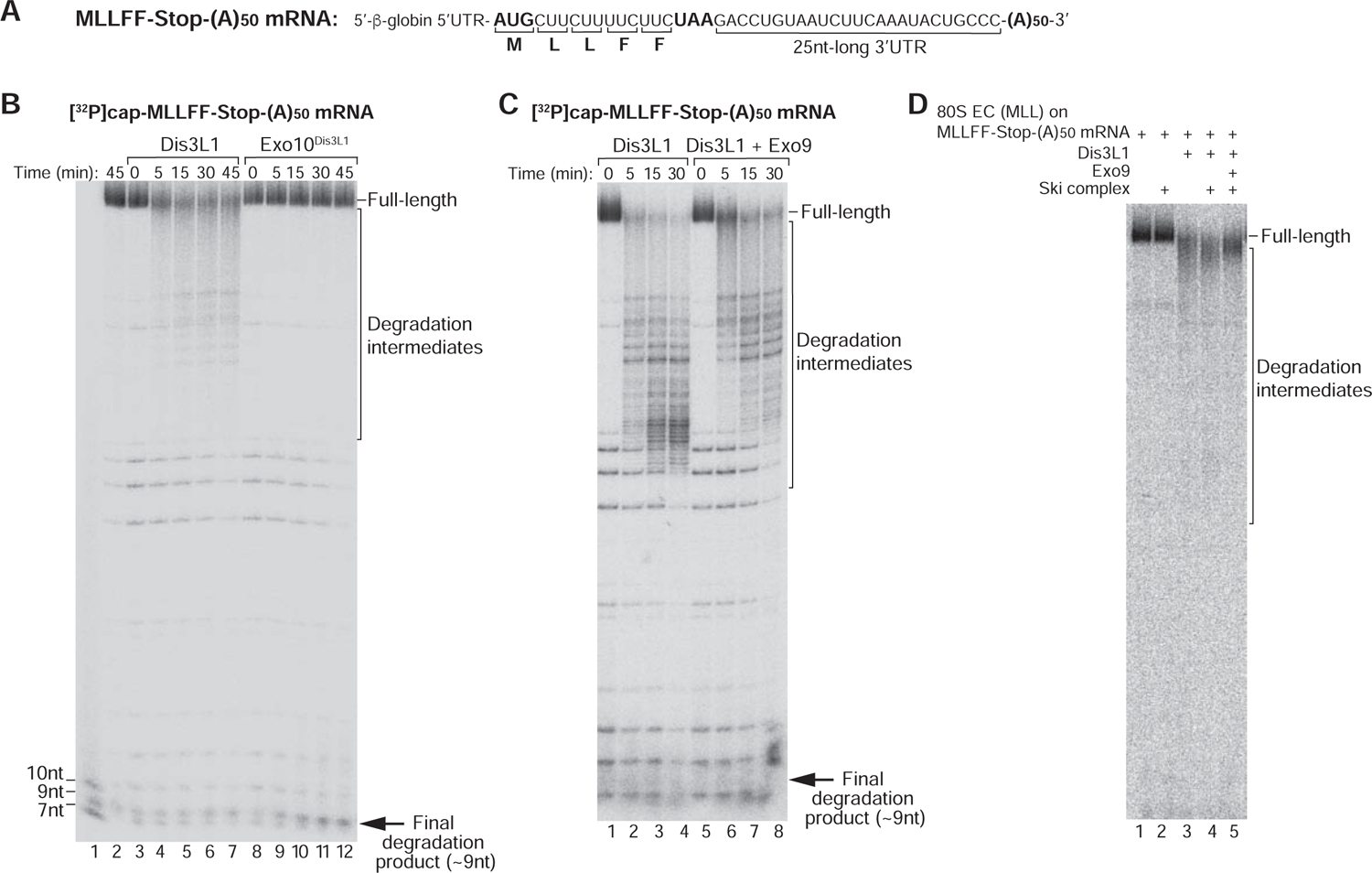
(A) Schematic representation of MLLFF-Stop-(A)50 mRNA. (B) Time courses of degradation of [32P]cap-MLLFF-Stop-(A)50 mRNA by Dis3L1 and Exo10Dis3L1 at 1.5 mM [Mg2+]. Lane 1 contains RNA markers. (C) Time courses of degradation of [32P]cap-MLLFF-Stop-(A)50 mRNA by Dis3L1 with/without Exo9 at 1.5 mM [Mg2+]. (D) mRNA degradation in ECs assembled on [32P]cap-MLLFF-Stop-(A)50 mRNA and containing MLL-tRNALeu in the P site after 30 min incubation with Dis3L1 in the presence/absence of the Ski complex and Exo9.
DISCUSSION
We report that the mammalian Ski complex extracts mRNAs from 80S ribosomes in the 3′→5′ direction in a nucleotide-by-nucleotide manner. The process is ATP-dependent and can occur on pre- and post-translocation ribosomal complexes. This activity is consistent with the structure of the S. cerevisiae 80S-bound Ski complex, in which Ski2 is located at the mRNA entrance and the 3’-terminal mRNA nucleotides are threaded directly into the Ski2 RNA channel (Schmidt et al., 2016). Strikingly, the ribosomal position of Ski2’s helicase core is similar to that of DHX29, a Ski2-like DExH-protein that performs the opposite function, promoting feeding of mRNA into the mRNA-binding channel during initiation on structured 5’UTRs (Pisareva et al., 2008; Hashem et al., 2013; des Georges et al., 2015). However, whereas in both cases RecA2 domains interact with helix 16, the orientation of RecA1 domains is different. In the S. cerevisiae complex containing A- and P-site tRNAs and E-site eIF5A, Ski2 contacts mRNA at positions +16–22 starting from the P site (Schmidt et al., 2016). We found that at least in post-translocation complexes containing only P site tRNA, 19 (but not 13) mRNA nucleotides from the P site are sufficient for Ski2 to engage productively with mRNA and to extract it from ribosomes. The Ski complex also efficiently extracted ribosome-bound mRNAs with long overhanging 3’-terminal regions.
We suggest that the mRNA-extracting activity of the Ski complex provides the mechanism by which it could perform its role in mRNA surveillance and protein quality control pathways. A recent report indicated that S. cerevisiae Cue2 is recruited to stalled ribosomes to initiate No-Go mRNA decay by cleaving mRNA within the A site of the collided ribosome (D’Orazio et al., 2019). After translocational rearrangement, which would also free the A site of the collided ribosome from tRNA, the collided ribosome would become susceptible to dissociation by Pelota/Hbs1/ABCE1 (Pisareva et al., 2011), whereas the leading stalled ribosome associated with the 3’-terminal mRNA cleavage product would still not be susceptible to splitting. However, the stalled leading ribosomes would be converted to monosomes with a relatively short 5’-terminal mRNA region and would be ideal substrates for Ski-mediated mRNA extraction, which would make them susceptible to splitting. Thus, the role of the Ski complex would be to render the leading stalled ribosome susceptible to recycling by Pelota/Hbs1/ABCE1 and to free the 3’-terminal mRNA cleavage product for degradation by Xrn1. Notably, in S. cerevisiae, Ski2, Ski3 and Ski8 mutants are synthetically lethal with Xrn1 null mutants (Johnson and Kolodner, 1995; Anderson and Parker, 1998).
Interestingly, the Cue2-mediated pathway is activated in a genetic background lacking Slh1, which emphasizes the existence of multiple surveillance pathways possibly targeting mRNAs with different features (D’Orazio et al., 2019). The mechanism of action of Slh1 and its exact role in mRNA surveillance and protein quality control are not clear. If Slh1 indeed promotes dissociation of stalled ribosomes (Matsuo et al., 2017), the question remains whether it can act on the leading stalled ribosome after endonucleolytic cleavage, or if the leading ribosome could be dissociated only by Pelota/Hbs1/ABCE1 following mRNA extraction. Another important question is whether the mRNA-extracting function of the Ski complex could play a role in the RQT-mediated or any other surveillance and quality control mechanism. Notably, Nonstop suppression in a C. elegans skih-2 nonu-1 double mutant was greater than in either single mutant, suggesting that the activities of NONU-1 and the Ski complex may not be strongly interdependent (Glover et al., 2019).
The ability of the Ski complex to act on complexes with long 3’-terminal mRNA regions raises the question of whether it can interfere with elongation. The structure of the S. cerevisiae Ski-bound 80S ribosome indicated that the Ski complex leaves the A site accessible to translational GTPases (Schmidt et al., 2016). Consistently, we found that ribosomes arrested at upstream codons during Ski-mediated mRNA extraction are elongation-competent. Thus, occasional binding of the Ski complex would not prevent the ribosomes from interacting functionally with elongation factors and likely would not be able to compete with the efficiently proceeding elongation. Moreover, the mRNA-extracting activity of the Ski complex on polysomes is probably lower than on monosomes. However, if the ribosomal stall were to occur in conditions of reduced protein synthesis with low polysomal density, the Ski complex might be able to extract the mRNA, after which the stalled ribosome would be recycled by Pelota/Hbs1/ABCE1. If the extraction was partial and if ribosomes were consequently halted at upstream codons, protein synthesis might resume in a new reading frame with subsequent termination on stop codons that were previously not in frame, which would again result in resolution of the stall and freeing of translational components. If for some reason endonucleolytic cleavage were to occur downstream from the stalled ribosome (e.g. Boehm et al., 2014), mRNA extraction by the Ski complex could be directly coupled with the exosomal degradation of the 5’-terminal mRNA cleavage product.
STAR METHODS
LEAD CONTACT AND MATERIALS AVAILABILITY
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Tatyana Pestova (tatyana.pestova@downstate.edu). All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
HEK293T cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Gibco), supplemented with 10% FBS (Gibco), 1X Glutamax (Gibco) and 1x penicillin/streptomycin (Gibco) at 37°C with 5% CO2. Recombinant proteins were expressed in Escherichia coli BL21 Star (DE3) cells grown in LB medium.
METHOD DETAILS
Construction of plasmids
Vectors for bacterial expression of N-terminally His6-tagged EXOSC1, EXOSC3 and EXOSC5, and C-terminally His6-tagged EXOSC8 were prepared by inserting appropriate ORFs into pET28a between NcoI/NotI, NcoI/XhoI, NcoI/Not1 and NcoI/NotI sites, respectively (GenScript, Piscataway, NJ). The vector for bacterial expression of C-terminally His6-tagged EXOSC2 was prepared by inserting the His6-tagged ORF into pET3a between NdeI/BamHI restriction sites (GenScript). The vector for bacterial co-expression of EXOSC7 and C-terminally Flag-tagged EXOSC6 was prepared by inserting the appropriately tagged ORFs into pET-Duet between NdeI/KpnI and NcoI/NotI restriction sites, respectively. The vector for bacterial co-expression of C-terminally His6-tagged EXOSC4 and C-terminally Flag-tagged EXOSC9 was prepared by inserting the appropriately tagged ORFs into pET-Duet between NdeI/KpnI and NcoI/NotI restriction sites, respectively. A vector for bacterial expression of N-terminally His-tagged and C-terminally FLAG- and His-tagged human WDR61 (Ski8) was prepared by inserting the appropriate ORF into pET28a between NdeI/XhoI restriction sites (GenScript). Mammalian expression vectors for preparation of C-terminally FLAG-tagged human Dis3L1 and N-terminally FLAG-tagged human Ski8 were prepared by inserting the appropriate ORF into pcDNA3.1+/C-(K)DYK and pcDNA3.1(+)-N-DYK, respectively (GenScript). The vector for bacterial expression of N-terminally His6-tagged eIF6 was prepared by inserting the appropriate ORF into pET14b between Nde1 and BamH1 sites (GenScript).
Transcription vectors for MF-Stop and MLLSSF-Stop were previously described (Zinoviev et al, 2018). Transcription vectors for MVLL-Stop, 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop, 47nt-3’UTR MLLFF-Stop and MLLFF-Stop-(A) 50 mRNAs were made by GeneWiz (South Plainfield, NJ) analogously to the MVHC-STOP vectors (Pisareva et al., 2011) by insertion of a T7 promoter and appropriate downstream sequences into pUC57. The transcription vector for β-VHP(25aa) mRNA (GeneWiz) was based on the pUC57-β-VHP(75aa) vector (Kuroha et al., 2018) and contained the ORF comprising N-terminal Met followed by the coding region for the last 24 C-terminal amino acids of the β-VHP(75aa) ORF.
mRNA and tRNA preparation
The β-VHP(75aa) and β-VHP(25aa) plasmids were linearized with EcoRI. Transcription vectors for MF-Stop, MLLSSF-Stop, MVLL-Stop, 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop and 47nt-3’UTR MLLFF-Stop mRNAs were linearized with HindIII. The transcription vector for MLLFF-Stop-(A)50 mRNA was linearized with BspQI. tRNASer-AGA, tRNALeu-AAG, tRNAPhe-GAA and tRNAiMet plasmids were linearized with BstNI. All mRNAs and tRNAs were transcribed using T7 RNA polymerase. For mRNA extraction experiments done on capped mRNA, MF-Stop mRNA was capped using the T7 mScript Standard mRNA Production System (Cellscript, Madison, WI) according to the manufacturer’s protocol. For exosomal degradation experiments, 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop, 47nt-3’UTR MLLFF-Stop and MLLFF-Stop-(A)50 mRNAs were capped with [α−32P]GTP using the same protocol. For the experiment shown in Figure S2, 27nt-3’UTR MLLFF-Stop mRNA was also 5’-phosphorylated with [γ−32P]ATP by T4 polynucleotide kinase.
Purification of factors and ribosomal subunits, and aminoacylation of tRNA
Native 40S and 60S subunits, eIF2, eIF3, eIF5B, eEF1H, eEF2 and total aminoacyl-tRNA synthetases were purified from RRL as described (Pestova and Hellen, 2003; Pisarev et al., 2007a, and references therein). Recombinant His6-tagged eIFs 1, 1A, 4A, 4B, 4G736–1115 and 5, eRF1, eRF1AGQ (eRF1 with a G183A substitution in the GGQ motif), eRF3, Pelota and Hbs1 were expressed in E. coli and purified as described (Pisarev et al., 2007a and referenced therein; Alkalaeva et al., 2006 and references therein; Pisareva et al., 2011). Nuclear RNA exosome (Exo12EXOSC10/SKIV2L2/C1D) was purified by a single step FLAG affinity chromatography from EXOSC10–3xFLAG expressing HEK293 cells using “ExoI extraction solution” (20 mM Hepes-KOH, pH 7.4, 300 mM NaCl, 1% Triton X-100, 1x protease inhibitor cocktail, 5% glycerol), as described (Domanski et al, 2016). For purification of FLAG-tagged ABCE1 from mammalian cells, it was transiently expressed in HEK293T cells and isolated by FLAG affinity chromatography as described (Shao and Hegde, 2014). eIF6 was expressed in 4 L E. coli BL21(DE3). Protein expression was induced with 1 mM IPTG, and cells were grown for 16 h at 16°C. eIF6 was purified by Ni-NTA chromatography followed by FPLC on a MonoQ 5/50 GL.
Aminoacylation of tRNASer-AGA, tRNALeu-AAG, tRNAPhe-GAA, tRNAiMet and native ΣtRNA was done in the presence of serine, leucine, phenylalanine, methionine or all amino acids, respectively, using total native aminoacyl-tRNA synthetases as described (Pisarev et al., 2007a). For experiments shown in Figure 1I, tRNAiMet was aminoacylated in the presence of [35S]-methionine (>1000Ci (37.0TBq)/mmol).
Purification of the Ski complex and individual Ski8
For purification of the Ski complex, FLAG-tagged Ski8 was expressed in HEK293T cells. Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, Gibco), supplemented with 10% FBS (Gibco), 1X Glutamax (Gibco) and 1x penicillin/streptomycin (Gibco) at 37°C with 5% CO2 to 70%–90% confluency and transfected with an expression plasmid using Lipofectamine 3000 (Invitrogen). After 72 hours, cells were harvested and lysed on ice in buffer A (50 mM Hepes pH 7.4, 150 mM KCl, 1x protease inhibitor cocktail, 2 mM DTT, 10% glycerol, 1% Triton X-100). Cell debris was pelleted by centrifugation at 20,000 g for 10 min. The supernatants were loaded twice on a 100 µl anti-FLAG M2 affinity agarose gel (Sigma-Aldrich), washed with 6 ml buffer A, 6 ml buffer B (50 mM Hepes pH 7.4, 2 mM DTT, 10% glycerol) + 300 mM KCl, 6ml buffer B + 100 mM KCl and eluted by incubation with buffer B + 100 mM KCl supplemented with 0.2 mg/ml FLAG peptide for 30 min at room temperature. The resulting eluate was applied to a FPLC MonoQ 5/50 GL column. Fractions were collected across a 100–500 mM KCl gradient, and the Ski complex was eluted at ~270 mM KCl. The identity of the Ski2, Ski3 and Ski8 subunits was confirmed by western blotting, as well as by mass spectrometry.
For purification of individual Ski8, the recombinant His-tagged protein was expressed by growing E. coli BL21 Star DE3 (Invitrogen) in 4 L of LB medium. Protein production was induced by addition of 0.5 mM IPTG, after which cells were grown for 16 hours at 16°C. Ski8 was isolated by affinity chromatography on Ni-NTA agarose followed by affinity chromatography on anti-FLAG M2 affinity agarose gel (Sigma-Aldrich) column.
Reconstitution of the mammalian cytoplasmic RNA exosome
Recombinant EXOSC1, EXOSC2, EXOSC3, EXOSC5 were expressed individually by growing E. coli BL21 Star DE3 (Invitrogen) in 4 L of LB medium. Protein production was induced by addition of 1 mM IPTG, after which cells were grown for 4 h at 37°C. All proteins were isolated by affinity chromatography on Ni-NTA agarose followed by FPLC on a MonoS 5/50 GL column with 50–500 mM KCl gradient for EXOSC2 and on a Superdex 200 5/150 GL column for EXOSC3 and EXOSC5. EXOSC4/EXOSC9 and EXOSC6/EXOSC7/EXOSC8 were co-expressed in 8 L of E. coli BL21 Star DE3 (Invitrogen). Protein production was induced by addition of 0.5 mM IPTG, after which cells were grown for 16 h at 16°C. All proteins were isolated by affinity chromatography on FLAG-agarose. The proteins were eluted from the resin with buffer C (20 mM Tris-HCl pH 7.5, 100 mM KCl, 0.1 mM EDTA, 2 mM DTT, 5% glycerol) supplemented with 0.2 mg/ml FLAG-peptide.
FLAG-tagged Dis3L1 was expressed in HEK293T cells. Cells were grown in DMEM (Gibco), supplemented with 10% FBS (Gibco), 1X Glutamax (Gibco) and 1x penicillin/streptomycin (Gibco) at 37°C with 5% CO2 to 70%–90% confluency and transfected with an expression plasmid using Lipofectamine 3000 (Invitrogen). After 72 hours, cells were harvested and lysed on ice in buffer A. Cell debris was pelleted by centrifugation at 20,000 g for 10 min. The supernatants were loaded twice on a 100 µl anti-FLAG M2 affinity agarose gel (Sigma-Aldrich), washed with 6 ml buffer A, 6 ml buffer D (50 mM Hepes pH 7.4, 2.5 mM MgCl2, 2 mM DTT, 10% glycerol) + 400 mM KCl, 6ml buffer D + 100 mM KCl and eluted by incubation with buffer D + 100 mM KCl supplemented with 0.2 mg/ml FLAG peptide for 30 min at room temperature.
To assemble Exo9 and Exo10Dis3L1, all individual proteins were combined in equimolar ratios, incubated overnight at 4°C in buffer E (20 mM Tris-HCl pH 7.5, 100 mM KCl, 0.1 mM EDTA, 2 mM DTT, 5% glycerol) for Exo10Dis3L1 complex and buffer F (20 mM Tris-HCl pH 7.5, 300 mM KCl, 0.1 mM EDTA, 2 mM DTT, 5% glycerol) for Exo9, and the assembled complex was purified by gel-filtration chromatography on a Superdex 200 Increase 5/150 GL column in the same buffer.
Preparation of ribosomal complexes
To reconstitute ECs and pre-TCs on MF-Stop, MLLSSF-Stop, MVLL-Stop, 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop, 47nt-3’UTR MLLFF-Stop and MLLFF-Stop-(A) 50 mRNAs, 75 nM unlabeled or [32P]cap-labeled mRNAs were incubated with 125 nM 40S ribosomal subunits, 500 nM eIF1, 500 nM eIF1A, 150 nM eIF2, 150 nM eIF3, 500 nM eIF4A, 250 nM eIF4B, 500 nM ΔG736–1115 and 250 nM Met-tRNAiMet (or [35S]Met-tRNAiMet) in 400 ml buffer G (20mM Tris-HCl pH7.5, 100mM KCl, 2.5mM MgCl2, 0.25mM spermidine, 2mM DTT) supplemented with 0.2 mM GTP, 1mM ATP and 2 U/μl RiboLock RNase inhibitor for 10 min at 37°C. Assembled 48S initiation complexes were then incubated with 170 nM 60S subunits, 430 nM eIF5 and 65 nM eIF5B for 10 min at 37°C to form 80S initiation complexes (80S ICs). To obtain pre-termination complexes (pre-TCs) and elongation complexes (ECs) in the post-translocation state, 80S ICs were mixed with 120 nM eEF1H, 120 nM eEF2 and appropriate aminoacylated tRNAs, and incubation continued for another 10 min. To obtain ECs in the pre-translocation state on MF-Stop mRNA, 80S ICs were mixed with 120 nM eEF1H and Phe-tRNAPhe (in the absence of eEF2), and incubation continued for another 10 min. The resulting 80S ICs, ECs (in both pre- and post-translocation states) and pre-TCs were purified by centrifugation in a Beckman SW55 rotor at 53,000 rpm for 95 min at 4°C in 10%–30% linear sucrose density gradients (SDG) prepared in buffer H (20 mM Tris pH 7.5, 100 mM KCl, 1.5 mM MgCl2, 2 mM DTT and 0.25 mM spermidine).
To prepare pre-TCs on β-VHP(75aa)-Stop and β-VHP(25aa)-Stop mRNAs, mRNAs were translated using the Flexi RRL system (Promega) supplemented with 1.5 mM eRF1AGQ and 1.5 mM eRF3 in the presence of [35S]-methionine (>1000Ci (37.0TBq)/mmol). eRF1AGQ can bind to pre-TCs but cannot trigger peptide release and, therefore, arrests ribosomal complexes at the pre-termination stage. Translation mixtures were incubated for 50 min at 32°C and subjected to 10%–30% SDG centrifugation as described above. Centrifugation dissociated eRF1AGQ, yielding factor-free pre-termination complexes (Zinoviev et al., 2015).
Assays for the mRNA-extracting activity of the Ski complex
For examination of Ski complex activity in extraction of mRNAs engaged into ribosomal complexes, 3 nM of purified 80S ICs or ECs (in pre- and post-termination states) assembled on MF-Stop mRNA (capped or uncapped), ECs assembled on MLLSSF-Stop mRNA, 48S or pre-TCs assembled on MVLL-Stop mRNA, or pre-TCs assembled on β-VHP(75aa) mRNA were incubated in 20 ml reactions in buffer I (20 mM Tris-HCl pH 7.5, 100 mM KCl, 3 mM MgCl2, 2 mM DTT, 0.5 mM GTP, 2 U/μl RiboLock RNase inhibitor, 0.25mM spermidine) supplemented with 1 mM ATP or 1 mM AMPPNP (in Figure 1F) with indicated combinations of 8 nM Ski complex, 50 nM Ski8, 20 nM eEF2, 20 nM eEF1H, 30 nM Ser-tRNASer at 37°C for 15 minutes. In experiments shown in Figure 1G, the concentration of MgCl2 was elevated to achieve the indicated free [Mg2+]. All resulting ribosomal complexes were analyzed by primer extension (Pisarev et al., 2007a) using AMV reverse transcriptase (Promega) and [32P]-labeled primers. After primer extension, the labeled cDNA was phenol-extracted, ethanol-precipitated and resolved on 6% polyacrylamide sequencing gels followed by autoradiography or phosphoimager analysis.
In Figures 1I and S1E, Ski complex activity was assayed by incubation of 3 nM of ECs assembled on MLLSSF-Stop mRNA or pre-TCs assembled on β-VHP(25aa)-Stop mRNA, in which the nascent peptide was labeled by [35S]Met, with the indicated combinations of 8 nM Ski complex, 50 nM eEF1H, 50 nM eEF2, 500 nM native aa-StRNA at 37°C for 30 minutes. Where indicated 50 nM eRF1 and 50 nM eRF3 were added into the reaction for the last 3 minutes of incubation. The resulting labeled peptides were resolved on 4–12% SDS-PAGE and visualized by autoradiography or phosphoimager analysis.
Ski-dependent recycling of elongation complexes by Pelota/Hbs1/ABCE1
Phosphorylation of 60S ribosomal subunits.
500 pmol 60S subunits were incubated with 5000 units of Casein kinase II (NEB) and 40 pmol [γ−32P]ATP (Perkin Elmer) in 300 ml buffer J (20 mM Tris-HCl pH 7.5, 100 mM KCl, 10 mM MgCl2, 2 mM DTT and 0.25 mM spermidine) for 30 min at 30°C and purified by centrifugation in a Beckman SW55 rotor at 53,000 rpm for 80 min at 4°C in 10%–30% linear SDG prepared in buffer H. 32P-labeled 60S subunits were detected by Cherenkov counting and by measuring OD260, and concentrated using Amicon Centrifugal Filter Units (MilliporeSigma).
Preparation of 32P-labeled ECs in pre- and post-translocation states.
To reconstitute 32P-labeled ECs in pre-and post-translocation states, 120 nM MF-Stop mRNA was first incubated with 50 nM 40S subunits, 150 nM eIF2, 150 nM eIF3, 300 nM eIF1, 300 nM eIF1A, 400 nM eIF4A, 200 nM eIF4B, 300 nM 4G736–1115 and 200 nM Met-tRNAiMet in 400 ml buffer G supplemented with 0.2 mM GTP, 1mM ATP and 2 U/μl RiboLock RNase inhibitor for 10 min at 37°C. Assembled 48S initiation complexes were then incubated with 70 nM 32P-labeled 60S subunits, 400 nM eIF5 and 65 nM eIF5B for 10 min at 37°C to form 80S ICs. To obtain ECs in the pre-translocation state, 80S ICs were mixed with 120 nM eEF1H and Phe-tRNAPhe, and incubation continued for another 10 min. To obtain ECs in the post-translocation state, 80S ICs were mixed with 120 nM eEF1H, Phe-tRNAPhe and 120 nM eEF2, and incubation continued for another 10 min. The resulting ECs (in both pre- and post-translocation states) were purified by SDG centrifugation as described above. Ribosomal complexes were detected by Cherenkov counting.
Ski-dependent dissociation of ECs by Pelota/Hbs1/ABCE1.
2 nM 32P-labeled ECs were incubated with different combination of 15 nM Ski, 50 nM Pelota, 50 nM Hbs1, 50 nM ABCE1 and 50 nM eIF6 in 300 ml buffer G supplemented with 1mM ATP and 0.5 mM GTP for 15 min at 37°C. The reaction mixtures were then subjected centrifugation in a Beckman SW55 rotor at 53,000 rpm for 80 min at 4°C in 10%–30% linear SDG prepared in buffer H. 32P-labeled ECs and 60S subunits were detected by Cherenkov counting of aliquots of gradient fractions. Release of mRNA was confirmed by toe-printing of 10 ml aliquots taken from reaction mixtures before centrifugation.
Exosomal degradation of mRNA
For exosomal degradation of individual mRNAs, 2.5 nM [32P]cap-labeled or [32P]5’-phosphorylated 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop, 47nt-3’UTR MLLFF-Stop or MLLFF-Stop-(A)50 mRNA were incubated with 6.25 nM Exo10Dis3L1, 6.25 mM Dis3L1, or 6.25 nM Dis3L1 + 20 nM Exo9 in 40 ml buffer K (20 mM Tris-HCl pH 7.5, 100 mM KCl, 3.5 mM MgCl2, 2 mM DTT, 1 mM ATP, 1 mM GTP, 2 U/μl RiboLock RNase inhibitor, 0.18 μg/μl casein) at 37°C for the indicated times. In experiments shown in Figure 2I, the concentration of MgCl2 was elevated to achieve the indicated free [Mg2+]. Reactions were stopped by addition of an equal volume of Stop solution containing 95% formamide, 0.125% bromophenol blue and 0.125% xylene cyanol. Samples were analyzed in 6% polyacrylamide sequencing gels followed by phosphoimager analysis.
For exosomal degradation of mRNAs engaged into ribosomal complexes, 3 nM of purified 80S ECs or pre-TCs assembled on [32P]cap-labeled 7nt-3’UTR MLLFF-Stop, 27nt-3’UTR MLLFF-Stop, 47nt-3’UTR MLLFF-Stop or MLLFF-Stop-(A)50 mRNA were incubated in 20 μl buffer L (20 mM Tris-HCl pH 7.5, 100 mM KCl, 3.5 mM MgCl2, 2 mM DTT, 1 mM ATP, 1 mM GTP, 2 U/μl RiboLock RNase inhibitor, 0.25mM spermidine) with indicated combinations of 8 nM Ski complex, 6.25 nM Dis3L1, 20 nM Exo9 and 15 nM Exo12EXOSC10/SKIV2L2/C1D at 37°C for 30 minutes. After incubation, mRNA was phenol-extracted, ethanol-precipitated and resolved on 6% polyacrylamide sequencing gels followed by autoradiography or phosphoimager analysis.
QUANTIFICATION AND STATISTICAL ANALYSIS
All in vitro experiments were repeated at least three times, and representative gel images and sucrose density gradient graphs were shown.
DATA AND CODE AVAILABILITY
Gel images are available at https://data.mendeley.com/datasets/k5s73hjmkj/draft?a=188bd905-9c0c-424c-9856-e9f4563bffa2
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-6xHis-tag | Abcam | ab18184; RRID:AB_444306 |
| Anti-FLAG | Sigma-Aldrich | F7425; RRID:AB_439687 |
| Anti-RRP4 (EXOSC2) | Abcam | ab111810; RRID:AB_10862519 |
| Anti-EXOSC3 | Abcam | ab156683; RRID:AB_2619635 |
| Anti-RRP41 (EXOSC4) | Abcam | ab137250 |
| Anti-EXOSC5 | Abcam | ab69699; RRID:AB_1268842 |
| Anti-EXOSC9 | Abcam | ab176802 |
| Anti-Dis3L1 | Abcam | ab89042; RRID:AB_2040576 |
| Anti-WDR61 antibody | Abcam | ab57840; RRID:AB_946149 |
| Anti-SKI2W antibody | MilliporeSigma | MABE1048 |
| Anti-SKI3 antibody | ThermoFisher Scientific | PA5-40078 RRID:AB_2607233 |
| ECL anti-Rabbit IgG HRP-linked whole Ab | GE Healthcare | NA934; RRID:AB_772206 |
| Anti-Mouse IgG-Peroxidase Ab | Sigma-Aldrich | A9044; RRID:AB_258431 |
| Bacterial and Virus Strains | ||
| E.coli DH5α | Invitrogen | 18265017 |
| E.coli BL21 Star (DE3) | Invitrogen | C601003 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Rabbit reticulocyte lysate | Green Hectares | n/a |
| Flexi Rabbit Reticulocyte Lysate System | Promega | L4540 |
| tRNA calf liver | Promega | Y209X |
| Gibco-Penicillin-Streptomycin | Fisher Scientific/Life Technologies | 15-140-163 |
| Gibco-GlutaMAX-Supplement | Fisher Scientific/Life Technologies | 35 050 079 |
| Gibco-Dulbecco’s Modified Eagle Medium | Fisher Scientific/Life Technologies | 11 960 069 |
| Gibco-Fetal Bovine Serum | Fisher Scientific/Life Technologies | 26-140-079 |
| Lipofectamine 3000 | Fisher Scientific/Life Technologies | L3000075 |
| Spermidine Trihydrochloride | Fisher Scientific | AC21510-0050 |
| Isopropyl-beta-D-thiogalactoside | Gold Biotechnology | I2481C100 |
| Anti-FLAG M2 Affinity Gel | Sigma-Aldrich | A2220 |
| 3x Flag peptide | Sigma-Aldrich | F4799 |
| BM Chemiluminescence Blotting Substrate | Roche | 11500694001 |
| Ni-NTA Agarose | QIAGEN | 30230 |
| MonoS 5/50 GL column | GE Healthcare Life Sciences | 17516801 |
| MonoQ 5/50 GL column | GE Healthcare Life Sciences | 17516601 |
| Superdex 200 Increase 5/150 GL | GE Healthcare Life Sciences | 28990945 |
| RiboLock RNase inhibitor | Thermo Scientific | EO0381 |
| T7 RNA polymerase | Thermo Scientific | EP0111 |
| Avian Myeloblastosis Virus Reverse Transcriptase | Promega | M5108 |
| T7 mScript-Standard mRNA Production System | Cellscript | C-MSC100625 |
| BspQI | New England Biolabs | R0712S |
| BstNI | New England Biolabs | R0607S |
| EcoRI | New England Biolabs | R0101S |
| HindIII | New England Biolabs | R0104S |
| T4 Polynucleotide Kinase | New England Biolabs | M0201S |
| Casein Kinase II | New England Biolabs | P6010S |
| [γ32P]ATP | Perkin Elmer | BLU002Z |
| [α32P]GTP | Perkin Elmer | BLU006H500UC |
| [35S]-methionine | Perkin Elmer | NEG009T |
| Deposited Data | ||
| Raw data | Mendeley data | https://data.mendeley.com/datasets/k5s73hjmkj/draft?a=188bd905-9c0c-424c-9856-e9f4563bffa2 |
| Experimental Models: Cell Lines | ||
| HEK293T cells | ATCC | CRL-3216; RRID:CVCL_0063 |
| Oligonucleotides | ||
| ssRNA (CUGAGGACUU) | Dharmacon | n/a |
| ssRNA (CCGCAGCCC) | Dharmacon | n/a |
| ssRNA (GCAGCCC) | Dharmacon | n/a |
| Recombinant DNA | ||
| pET28a-His6-EXOSC1 | This study | |
| pET3a-EXOSC2-His6 | This study | |
| pET28a-His6-EXOSC3 | This study | |
| pET28a-His6-EXOSC5 | This study | |
| pET28a-EXOSC8-His6 | This study | |
| pETDuet-ExoSc9-Flag-EXOSC4-His6 | This study | |
| pETDuet-ExoSc6-Flag-EXOSC7 | This study | |
| pcDNA3.1-Dis3L1-FLAG | This study | |
| pcDNA3.1-FLAG-WDR61 | This study | |
| pET28a-His6-WDR61-FLAG-His6 | This study | |
| pET14b-His6-eIF6 | This study | |
| pQE31-His6-eIF1 | Pisarev et al., 2007a | |
| pET28-His6-eIF1A | Pisarev et al., 2007a | |
| pET15b-His6-eIF4A | Pisarev et al., 2007a | |
| pET15b-His6-eIF4B | Pisarev et al., 2007a | |
| pET28-His6-eIF4G736-1115 | Pisarev et al., 2007a | |
| pET19b-His6-eIF5 | Pisarev et al., 2007a | |
| pET23b-eRF1-His6 | Alkalaeva et al., 2006 | |
| pQE-30-His6-eRF1(AGQ) | Alkalaeva et al., 2006 | |
| pET23b-eRF3Cp-His6 | Alkalaeva et al., 2006 | |
| pFL-B31cl-Pelota-His6 | Pisareva et al., 2011 | |
| pFL-B31cl-Hbs1-His6 | Pisareva et al., 2011 | |
| pcDNA-3xFLAG-ABCE1 | Shao and Hegde, 2014 | |
| pUC57-β-globin-30 bases | This study | |
| pUC57-β-globin-30-(A)50 bases | This study | |
| pUC57-β-globin-10 bases | This study | |
| pUC57-β-globin-50 bases | This study | |
| pUC57-MF-Stop | Zinoviev et al., 2018 | |
| pUC57-MLLSSF-Stop | Zinoviev et al., 2018 | |
| pUC57-MVLL-Stop | This study | |
| pUC57-MLLFF-Stop-(A50) | This study | |
| pUC57-[7nt-3’UTR MLLFF-Stop] | This study | |
| pUC57-[27nt-3’UTR MLLFF-Stop] | This study | |
| pUC57-[47nt-3’UTR MLLFF-Stop] | This study | |
| pUC57-β-VHP(75aa) | Kuroha et al., 2018 | |
| pUC57-β-VHP(25aa) | This study | |
| pTRM1(pBR322-tRNAiMet) | Pestova and Hellen, 2001 | |
| pUC57-[tRNASer-AGA] | Zinoviev et al., 2015 | |
| pUC57-[tRNALeu-AAG] | Pisarev et al., 2010 | |
| pUC57-[tRNAPhe-GAA] | Zinoviev et al., 2018 | |
| Software and Algorithms | ||
| ImageQuant TL version 8.1 | GE Healthcare | RRID:SCR_014246 |
| Mfold | http://unafold.rna.albany.edu/?q=mfold/download-mfold | RRID:SCR_008543 |
Highlights.
The evolutionarily conserved Ski complex extracts mRNA from 80S ribosomal complexes
mRNA extraction occurs in the 3′→5′ direction in a nucleotide-by-nucleotide manner
The process is ATP-dependent and can occur on pre- and post-translocation ribosomes
19 mRNA nucleotides from the P site are sufficient for Ski-mediated mRNA extraction
ACKNOWLEDGMENTS
We thank John LaCava for the pellets of EXOSC10-3xFLAG expressing HEK293 cells, Sichen Shao for the ABCE1 expression vector, and Andrew Tcherepanov for expert technical assistance. This work was supported by NIH grant GM122602 to T.V.P and NIH grant AI123406 to C.U.T.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, and Pestova TV (2006). In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell 125, 1125–1136. [DOI] [PubMed] [Google Scholar]
- Anderson JSJ, and Parker R (1998). The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J 17, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arribere JA, and Fire AZ (2018). Nonsense mRNA suppression via nonstop decay. Elife 7, pii: e33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm V, Haberman N, Ottens F, Ule J, and Gehring NH (2014). 3’ UTR length and messenger ribonucleoprotein composition determine endocleavage efficiencies at termination codons. Cell Rep 9, 555–568. [DOI] [PubMed] [Google Scholar]
- Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D, Shen PS, Weibezahn J et al. (2012). A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress. Cell 151, 1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandman O, and Hegde RS (2016). Ribosome-associated protein quality control. Nat. Struct. Mol. Biol 23, 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- des Georges A, Dhote V, Kuhn L, Hellen CU, Pestova TV, Frank J, and Hashem Y (2015). Structure of mammalian eIF3 in the context of the 43S preinitiation complex. Nature 525, 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doma MK, and Parker R (2006). Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature 440, 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski M, Upla P, Rice WJ, Molloy KR, Ketaren NE, Stokes DL, Jensen TH, Rout MP, and LaCava J (2016). Purification and analysis of endogenous human RNA exosome complexes. RNA 22, 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Orazio KN, Wu CC, Sinha N, Loll-Krippleber R, Brown GW, and Green R (2019). The endonuclease Cue2 cleaves mRNAs at stalled ribosomes during No Go Decay. Elife 8, pii: e49117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazkowska K, Tomecki R, Stodus K, Kowalska K, Czarnocki-Cieciura M, and Dziembowski A (2013). The RNA exosome complex central channel controls both exonuclease and endonuclease Dis3 activities in vivo and in vitro. Nucleic Acids Res 41, 3845–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowski A, Lorentzen E, Conti E, and Séraphin B (2007). A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol 14, 15–22. [DOI] [PubMed] [Google Scholar]
- Garzia A, Jafarnejad SM, Meyer C, Chapat C, Gogakos T, Morozov P, Amiri M, Shapiro M, Molina H, Tuschl T et al. (2017). The E3 ubiquitin ligase and RNA-binding protein ZNF598 orchestrates ribosome quality control of premature polyadenylated mRNAs. Nat. Commun 8, 16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach P, Schuller JM, Bonneau F, Basquin J, Reichelt P, Falk S, and Conti E (2018). Distinct and evolutionary conserved structural features of the human nuclear exosome complex. Elife 7, pii: e38686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover ML, Burroughs AM, Egelhofer TA, Pule MN, Aravind L, and Arribere JA (2019). NONU-1 encodes a conserved endonuclease required for mRNA translation surveillance. bioRxiv, 10.1101/674358. [DOI] [PMC free article] [PubMed]
- Halbach F, Reichelt P, Rode M, and Conti E (2013). The yeast ski complex: crystal structure and RNA channeling to the exosome complex. Cell 154, 814–826. [DOI] [PubMed] [Google Scholar]
- Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Hellen CU, Pestova TV, and Frank J (2013). Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 153, 1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Takahashi M, Sakota E, and Nakamura Y (2017). Nonstop-mRNA decay machinery is involved in the clearance of mRNA 5’-fragments produced by RNAi and NMD in Drosophila melanogaster cells. Biochem. Biophys. Res. Commun 484, 1–7. [DOI] [PubMed] [Google Scholar]
- Ikeuchi K, Izawa T, and Inada T (2019a). Recent progress on the molecular mechanism of quality controls induced by ribosome stalling. Front. Genet 9, 743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi K, Tesina P, Matsuo Y, Sugiyama T, Cheng J, Saeki Y, Tanaka K, Becker T, Beckmann R, and Inada T (2019b). Collided ribosomes form a unique structural interface to induce Hel2-driven quality control pathways. EMBO J 38, pii: e100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CAP (2019). Mechanisms and functions of ribosome-associated protein quality control. Nat. Rev. Mol. Cell. Biol 20, 368–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AW, and Kolodner RD (1995). Synthetic lethality of sep1 (xrn1) ski2 and sep1 (xrn1) ski3 mutants of Saccharomyces cerevisiae is independent of killer virus and suggests a general role for these genes in translation control. Mol. Cell. Biol 15, 2719–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz S, and Hegde RS (2017). Initiation of quality control during poly(A) translation requires site-specific ribosome ubiquitination. Mol. Cell 65, 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juszkiewicz S, Chandrasekaran V, Lin Z, Kraatz S, Ramakrishnan V, and Hegde RS (2018). ZNF598 is a quality control sensor of collided ribosomes. Mol. Cell 72, 469–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroha K, Zinoviev A, Hellen CUT, and Pestova TV (2018). Release of ubiquitinated and non-ubiquitinated nascent chains from stalled mammalian ribosomal complexes by ANKZF1 and Ptrh1. Mol. Cell 72, 286–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Niu CY, Wu Y, Tan D, Wang Y, Ye MD, Liu Y, Zhao W, Zhou K, Liu QS et al. (2016). CryoEM structure of yeast cytoplasmic exosome complex. Cell Res 26, 822–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentzen E, Basquin J, Tomecki R, Dziembowski A, and Conti E (2008). Structure of the active subunit of the yeast exosome core, Rrp44: diverse modes of substrate recruitment in the RNase II nuclease family. Mol. Cell 29, 717–728. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen S, Tomecki R, Jensen TH, and Dziembowski A (2011). The eukaryotic RNA exosome: same scaffold but variable catalytic subunits. RNA Biol 8, 61–66. [DOI] [PubMed] [Google Scholar]
- Makino DL, Schuch B, Stegmann E, Baumgärtner M, Basquin C, and Conti E (2015). RNA degradation paths in a 12-subunit nuclear exosome complex. Nature 524, 54–58. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Ikeuchi K, Saeki Y, Iwasaki S, Schmidt C, Udagawa T, Sato F, Tsuchiya H, Becker T, Tanaka K et al. (2017). Ubiquitination of stalled ribosome triggers ribosome-associated quality control. Nat. Commun 8, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, and Hellen CU (2001). Preparation and activity of synthetic unmodified mammalian tRNAiMet in initiation of translation in vitro. RNA 7, 1496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, and Hellen CU (2003). Translation elongation after assembly of ribosomes on the Cricket paralysis virus internal ribosomal entry site without initiation factors or initiator tRNA. Genes Dev 17, 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev AV, Unbehaun A, Hellen CU, and Pestova TV (2007a). Assembly and analysis of eukaryotic translation initiation complexes. Methods Enzymol 430, 147–177. [DOI] [PubMed] [Google Scholar]
- Pisarev AV, Skabkin MA, Pisareva VP, Skabkina OV, Rakotondrafara AM, Hentze MW, Hellen CU, and Pestova TV (2010). The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell 37, 196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev AV, Hellen CU, and Pestova TV (2007b). Recycling of eukaryotic posttermination ribosomal complexes. Cell 131, 286–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisareva VP, Pisarev AV, Komar AA, Hellen CU, and Pestova TV (2008). Translation initiation on mammalian mRNAs with structured 5’UTRs requires DExH-box protein DHX29. Cell 135, 1237–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisareva VP, Skabkin MA, Hellen CU, Pestova TV, and Pisarev AV (2011). Dissociation by Pelota, Hbs1 and ABCE1 of mammalian vacant 80S ribosomes and stalled elongation complexes. EMBO J 30, 1804–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Kowalinski E, Shanmuganathan V, Defenouillère Q, Braunger K, Heuer A, Pech M, Namane A, Berninghausen O, Fromont-Racine M et al. (2016). The cryo-EM structure of a ribosome-Ski2-Ski3-Ski8 helicase complex. Science 354, 1431–1433. [DOI] [PubMed] [Google Scholar]
- Shao S, and Hegde RS (2014). Reconstitution of a minimal ribosome-associated ubiquitination pathway with purified factors. Mol. Cell 55, 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker CJ, and Green R (2012). Translation drives mRNA quality control. Nat. Struct. Mol. Biol 19, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Thomas EN, and Zaher HS (2017a). Ribosome-based quality control of mRNA and nascent peptides. Wiley Interdiscip. Rev. RNA 8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms CL, Yan LL, and Zaher HS (2017b). Ribosome collision is critical for quality control during No-Go decay. Mol. Cell 68, 361–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitron CS, Park JH, and Brandman O (2017). Asc1, Hel2, and Slh1 couple translation arrest to nascent chain degradation. RNA 23, 798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staals RH, Bronkhorst AW, Schilders G, Slomovic S, Schuster G, Heck AJ, Raijmakers R, and Pruijn GJ (2010). Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J 29, 2358–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy E, Leonard M, Mak R, Liao J, Fulzele A, and Bennett EJ (2017). ZNF598 and RACK1 regulate mammalian ribosome-associated quality control function by mediating regulatory 40S ribosomal ubiquitylation. Mol. Cell 65, 751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synowsky SA, and Heck AJ (2008). The yeast Ski complex is a hetero-tetramer. Protein Sci 17, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TTL, Stowell JAW, Hill CH, and Passmore LA (2019). The intrinsic structure of poly(A) RNA determines the specificity of Pan2 and Caf1 deadenylases. Nat. Struct. Mol. Biol 26, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomecki R, Kristiansen MS, Lykke-Andersen S, Chlebowski A, Larsen KM, Szczesny RJ, Drazkowska K, Pastula A, Andersen JS, Stepien PP et al. (2010). The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J 29, 2342–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof A, Frischmeyer PA, Dietz HC, and Parker R (2002). Exosome-mediated recognition and degradation of mRNAs lacking a termination codon. Science 295, 2262–2264. [DOI] [PubMed] [Google Scholar]
- Wasmuth EV, and Lima CD (2012). Exo- and endoribonucleolytic activities of yeast cytoplasmic and nuclear RNA exosomes are dependent on the noncatalytic core and central channel. Mol. Cell 48, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weick EM, Puno MR, Januszyk K, Zinder JC, DiMattia MA, and Lima CD (2018). Helicase-dependent RNA decay illuminated by a cryo-EM structure of a human nuclear RNA exosome-MTR4 complex. Cell 173, 1663–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder JC, Wasmuth EV, and Lima CD (2016). Nuclear RNA exosome at 3.1 Å reveals substrate specificities, RNA paths, and allosteric inhibition of Rrp44/Dis3. Mol. Cell 64, 734–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder JC, and Lima CD (2017). Targeting RNA for processing or destruction by the eukaryotic RNA exosome and its cofactors. Genes Dev. 31, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoviev A, Hellen CUT, and Pestova TV (2015). Multiple mechanisms of reinitiation on bicistronic calicivirus mRNAs. Mol. Cell 57,1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinoviev A, Goyal A, Jindal S, LaCava J, Komar AA, Rodnina MV, Hellen CUT, and Pestova TV (2018). Functions of unconventional mammalian translational GTPases GTPBP1 and GTPBP2. Genes Dev 32, 1226–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Gel images are available at https://data.mendeley.com/datasets/k5s73hjmkj/draft?a=188bd905-9c0c-424c-9856-e9f4563bffa2


