Main Text
Unimpeded progression of metastatic cancer is the principal cause of mortality in cancer patients. It occurs when every and all therapeutic options have been exhausted, necessitating an urgent search for novel therapies to slow cancer progression and educate the immune system to find and eliminate disseminated cancer cells. A promising approach to control disseminated cancer involves the administration of oncolytic virus, a novel cancer treatment modality that has proven efficacious at suppressing tumor growth in numerous pre-clinical models and in cancer patients with localized disease. To date, two oncolytic virus drugs have been approved for clinical use: Oncorine H101, an oncolytic adenovirus approved for treating patients with nasopharyngeal carcinoma by the Chinese Food and Drug Administration,1 and Imlygic, an oncolytic herpes simplex virus-1-based drug, approved for treating advanced melanoma by the US Food and Drug Administration.2 In both cases, therapeutic efficacy is observed upon injecting oncolytic viruses directly into tumors. Clinical trials have shown that, although direct intra-tumoral injection of Imlygic is well tolerated and suppresses the growth of local melanoma lesions, distant visceral metastases remain largely refractory to Imlygic.3 Two principal and complementary approaches are currently being explored to improve the efficacy of local virotherapy against disseminated disease. These include “arming” oncolytic viruses with immune-stimulatory transgenes and combining intra-tumoral virus administration with immune-checkpoint inhibitors4 to stimulate the so-called abscopal effect, whereby distant metastatic lesions undergo regression due to virus-mediated activation of systemic anti-tumor immunity. While both of these approaches improved systemic anti-tumor response after local administration of oncolytic viruses,5 the observation that tumor lesions subjected to direct virus injection typically undergo strongest regression (compared to non-injected distant metastatic nodules)3 suggests that systemic administration of therapeutic viruses (allowing virus access to all tumor lesions in the body) may be the most efficacious approach to controlling disseminated metastatic disease.
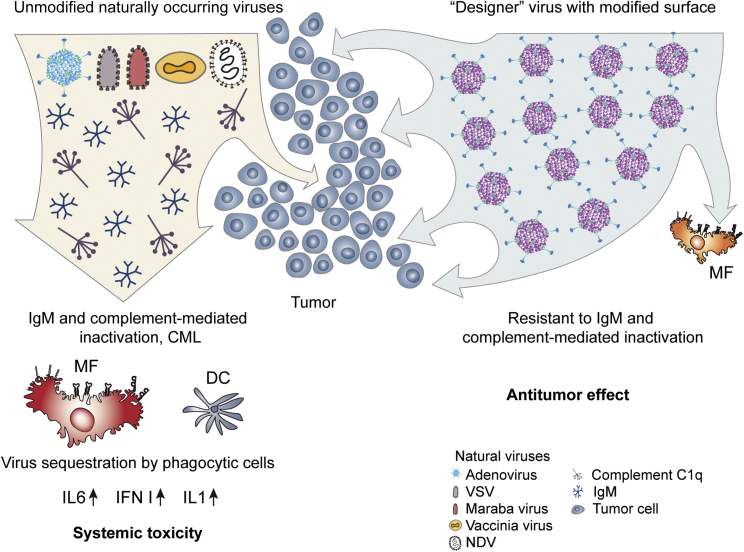
The unique advantage of oncolytic viruses as cancer therapeutics is that, unlike small molecule or antibody-based drugs, they represent a therapeutic platform that can be iteratively tailored for specific application though targeted engineering of their structural and regulatory elements, thus endowing them with ever-improved properties to reduce side effects and increase efficacy. As the list of desired properties for potential oncolytic virus-based drug candidates grows (Box 1), the complexity of designing oncolytic vectors that would be safe and effective upon systemic administration also becomes evident. Compared to oncolytic viruses injected directly into tumors, systemic administration leads to dilution of therapeutic virus in the blood stream and immediate exposure to blood components evolved to attack and inactivate invading pathogens. In addition to providing access to disseminated tumor cells, intravenous administration exposes the virus to the large pool of professional phagocytic cells of the innate immune system, namely tissue resident macrophages in the liver and macrophages and dendritic cells in the spleen. These cells specialize in sequestering pathogens from the blood and presenting their broken down components to cells of the adaptive immune system, which mount potent adaptive immune responses, further limiting the persistence of therapeutic viruses in the body. Natural immunoglobulin M (IgM) antibodies and complement components are abundant in the blood, and their principal function is to find and inactivate intruders, preventing pathogen spread through the circulation to vital organs.6 Natural IgM binding to the virus triggers activation of a complement cascade that leads to virus inactivation in a process known as complement-mediated lysis (CML).7 Furthermore, IgM and complement binding to pathogens facilitates their rapid sequestration in immune phagocytic cells via interaction with scavenger,8 Fc, and complement receptors.9 Shielding virus particles from blood components by chemical coupling to polyethylene glycol,10 depletion of immune phagocytic cells,11 and even loading oncolytic viruses into live carrier cells that may home to disseminated tumor nodules12 improve the stability of oncolytic viruses in the blood and their anti-tumor activity in pre-clinical models. However, the safety and efficacy of these approaches in clinical settings remain unclear. Therapeutic viruses are perceived by the immune system as genuine pathogens due to their highly regular virion structure (which is distinct from the typical host cell organization) as well as disturbance of tissue homeostasis, which occurs upon virus replication and spread. A consequence of the therapeutic virus recognition by immune phagocytic cells is activation of local and systemic inflammation. Whereas the induction of certain cytokines, e.g., interleukin 12 (IL-12) and interferon (IFN)-γ, within the tumor microenvironment is thought to be essential for promoting potent anti-tumor immunity, elevated blood levels of IL-6 and tumor necrosis factor alpha (TNF-α) are associated with systemic toxicities, limiting the therapeutic virus dose that can be safely administered to patients (Figure 1). Collectively, both humoral and cellular arms of the innate immune system work in concert to ensure that little to no pathogen spread occurs via the bloodstream, a barrier that, thus far, has proven formidable for oncolytic virus platforms.
Box 1. Desirable Features of the Oncolytic Virus Platform for Systemic Cancer Therapy.
-
1.
Resistance to inactivation by humoral factors
-
2.
Low toxicity after intravenous administration
-
3.
Feasibility of virus targeting to tumor cells
-
4.
Feasibility of changing unfavorable virus biodistribution
-
5.
Effective machinery for immune modulation in human hosts for persistence
-
6.
Feasibility of engineering mechanisms for tumor cell-selective cytotoxicity
-
7.
High fidelity of replication and stability of the genome
-
8.
Low sensitivity to IFN
-
9.
Capacity for therapeutic transgenes
-
10.
Mechanistic evidence underlying therapeutic efficacy
Figure 1.
Interaction of Naturally Occurring, Unmodified Viruses and “Designer” Oncolytic Viruses with Blood Factors and Cells of Innate Immunity after Intravenous Administration
Engineering the virion surface of oncolytic viruses enables their resistance to inactivation by blood factors, reduces virus sequestration in phagocytic cells of the innate immune system, and improves their safety profile and therapeutic efficacy after intravenous administration.
Engineering viruses to modify their interactions with host factors is a major undertaking due their highly structured assembly. Virions display only a small number of proteins (frequently only two or three), which multimerize to form the exposed exterior surface of the virus. The structural proteins of the virion surface are critical for virus attachment to host cells and mediating virus cell entry via fusogenic activity of envelope glycoproteins (lipid membrane-enveloped viruses) or by promoting endosome formation (non-enveloped viruses). As such, the structure of these multifunctional proteins is finely tuned to perform these functions and antibody binding, which interfere with virus attachment or internalization, blocking virus infectivity. Furthermore, both natural IgM and virus-specific antibody (Ab) binding mediate complement activation and lysis of viruses with enveloped virions via CML,13 thus inactivating viruses independently of preventing their attachment and entry into cells.
The binding of natural IgM to structurally repeating virion surface was thought to occur via “non-specific” low affinity-high avidity interactions,9 which are not amenable to engineering without negatively affecting virus cell entry. Although widely accepted, the notion that natural IgM binding to the virion surface is “non-specific” may need to be revised based on evidence that the envelope glycoproteins within a closely related family of viruses vary greatly in their sensitivity to CML. These findings indicate that natural IgM bind to different envelope glycoproteins, even when arranged in a similar repeated pattern, with different efficacy. Specifically, whereas exposure of vesicular stomatitis virus (VSV) to undiluted human sera leads to a five-orders-of-magnitude reduction in virus infectivity, a VSV virus pseudotyped with glycoprotein from a Maraba virus loses infectivity by only two orders of magnitude.14 These data suggest that natural IgM binding to VSV glycoprotein G and glycoprotein G from a closely-related Maraba virus occurs with different efficacy. Direct evidence for the feasibility of engineering oncolytic viruses with resistance to IgM and complement came from a recent study. Atasheva et al.8 found that the large negatively charged hyper-variable loop 1 (HVR1) in the adenovirus capsid protein hexon is the principal site mediating natural IgM binding to adenovirus HAdv-C5. Analysis of a set of viruses with mutated HVR1 loops of various lengths and charges showed that removal of all negatively charged amino acid was necessary to prevent IgM and complement binding to the virus. A mutant virus with a short HVR1 loop that lacked any negatively charged amino acids avoided interactions with IgM and complement and resisted inactivation in undiluted mouse and human sera.8 In addition to demonstrating the feasibility of engineering a large virus that avoids interactions with IgMs, this study also showed that liver macrophages sequester adenovirus after intravenous administration via an IgM-dependent manner, even without a complement-mediated virus inactivation. Therefore, virus resistance to CML or other forms of complement-mediated inactivation may not serve as a surrogate readout for the lack of IgM binding to the virus surface. This notion is important because IgM binding to the virus tags it for sequestration in immune phagocytic cells, even without complement-mediated loss of virus infectivity, limiting the virus dose that can reach tumor sites after intravenous administration. A reduced accumulation of a virus in immune phagocytic cells after intravenous administration is an important goal as it may lead to reduced systemic inflammatory host responses, thus improving the overall safety of virotherapy (Figure 1).
Systemic delivery of any oncolytic virus will undoubtedly trigger generation of neutralizing virus-specific IgM and IgG antibodies and cytotoxic T lymphocytes, which will limit virus infectivity and intra-tumoral persistence and reduce the efficacy of repeated rounds of systemic therapy with the same virus variant, independent of the virus’s ability to escape natural IgM recognition. Because clinical studies show that the most durable anti-tumor responses have been observed only after repeated rounds of intra-tumoral virus administration, repeated rounds of systemic virotherapy may also be required to achieve control of disseminated disease. Although virus particle shielding with polymers or encapsulation of the oncolytic virus genome in liposomes allows for repeated systemic administration of these therapeutic platforms, opportunities to modify their unfavorable bio-distribution, e.g., to de-target liposomes and polymer-shielded viruses from liver phagocytes, are limited. In contrast, oncolytic viruses that lack lipid envelopes, such as adenovirus, coxsackievirus, or reovirus, are more amenable to modifications to prevent virus neutralization by pre-existing humoral immunity. To this end, targeted engineering of virus surface by swapping surface-localized loops of human adenovirus HAdv-C5 hexon for surface-localized hexon loops of human adenovirus HAdv-D48 was sufficient to avoid neutralization of HAdv-C5-based vaccines by pre-existing immunity.15 Based on these data, it is conceivable that a combination of targeted mutations preventing natural IgM and virus-specific IgM and IgG binding to the virus could be introduced into structural adenovirus proteins to generate a panel of oncolytic viruses that resist both innate and pre-existing adaptive immunity and are suitable for sequential rounds of systemic therapy.
Taken together, new data indicate that natural IgM binding to viruses with both enveloped and non-enveloped virions has a considerable degree of specificity and one can identify and mutate the IgM binding site(s) at the virus surface through well-established targeted mutagenesis approaches. With the understanding that natural IgM binding to viruses can be avoided via targeted mutagenesis, a new generation of “designer” oncolytic viruses for systemic administration can now be developed. These viruses will have improved safety by avoiding IgM-dependent sequestration in immune phagocytic cells and will have improved therapeutic efficacy through resistance to complement-mediated inactivation in human blood. These “designer” viruses for systemic administration may be a useful platform for the development of a panel of novel oncolytic viruses “armed” with immune-stimulatory or anti-cancer transgenes and could be used as monotherapy or in combination with immune-oncology or other treatment modalities4 to stimulate host immunity to find and eliminate cancer cells disseminated throughout the body, thus addressing an urgent need for patients with metastatic disease.
Acknowledgments
This work was supported by NIH grant AI107960 and the Children’s Healthcare of Atlanta Research Trust to D.M.S.
Declaration of Interests
D.M.S. serves as a paid consultant of Merck and Co. D.M.S. has equity interest in and is an officer of AdCure Bio, which develops adenovirus technologies for therapeutic use. D.M.S. is an inventor on issued US patent No. 10,376,549 and issued European patent No. 3247807 (Detargeted adenovirus variants and related methods) and a pending US patent application 16/460,160, submitted by AdCure Bio. S.A. declares no competing interests.
Contributor Information
Svetlana Atasheva, Email: svetlana.atasheva@emory.edu.
Dmitry M. Shayakhmetov, Email: dmitryshay@emory.edu.
References
- 1.Liang M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets. 2018;18:171–176. doi: 10.2174/1568009618666171129221503. [DOI] [PubMed] [Google Scholar]
- 2.Pol J., Kroemer G., Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. OncoImmunology. 2015;5:e1115641. doi: 10.1080/2162402X.2015.1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andtbacka R.H., Ross M., Puzanov I., Milhem M., Collichio F., Delman K.A., Amatruda T., Zager J.S., Cranmer L., Hsueh E. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016;23:4169–4177. doi: 10.1245/s10434-016-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Senior M. Checkpoint inhibitors go viral. Nat. Biotechnol. 2019;37:12–17. doi: 10.1038/nbt.4327. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ochsenbein A.F., Fehr T., Lutz C., Suter M., Brombacher F., Hengartner H., Zinkernagel R.M. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 7.Stoermer K.A., Morrison T.E. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atasheva S., Emerson C.C., Yao J., Young C., Stewart P.L., Shayakhmetov D.M. Systemic cancer therapy with engineered adenovirus that evades innate immunity. Sci. Transl. Med. 2020;12:eabc6659. doi: 10.1126/scitranslmed.abc6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein M.R., Notley C.A. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 10.Tesfay M.Z., Kirk A.C., Hadac E.M., Griesmann G.E., Federspiel M.J., Barber G.N., Henry S.M., Peng K.W., Russell S.J. PEGylation of vesicular stomatitis virus extends virus persistence in blood circulation of passively immunized mice. J. Virol. 2013;87:3752–3759. doi: 10.1128/JVI.02832-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shashkova E.V., Doronin K., Senac J.S., Barry M.A. Macrophage depletion combined with anticoagulant therapy increases therapeutic window of systemic treatment with oncolytic adenovirus. Cancer Res. 2008;68:5896–5904. doi: 10.1158/0008-5472.CAN-08-0488. [DOI] [PubMed] [Google Scholar]
- 12.Mahasa K.J., de Pillis L., Ouifki R., Eladdadi A., Maini P., Yoon A.R., Yun C.O. Mesenchymal stem cells used as carrier cells of oncolytic adenovirus results in enhanced oncolytic virotherapy. Sci. Rep. 2020;10:425. doi: 10.1038/s41598-019-57240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wakimoto H., Ikeda K., Abe T., Ichikawa T., Hochberg F.H., Ezekowitz R.A., Pasternack M.S., Chiocca E.A. The complement response against an oncolytic virus is species-specific in its activation pathways. Mol. Ther. 2002;5:275–282. doi: 10.1006/mthe.2002.0547. [DOI] [PubMed] [Google Scholar]
- 14.Tesfay M.Z., Ammayappan A., Federspiel M.J., Barber G.N., Stojdl D., Peng K.W., Russell S.J. Vesiculovirus neutralization by natural IgM and complement. J. Virol. 2014;88:6148–6157. doi: 10.1128/JVI.00074-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts D.M., Nanda A., Havenga M.J.E., Abbink P., Lynch D.M., Ewald B.A., Liu J., Thorner A.R., Swanson P.E., Gorgone D.A. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–243. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]