Abstract
Despite recent advances, non-Hodgkin’s B cell lymphoma patients often relapse or remain refractory to therapy. Therapeutic resistance is often associated with survival signaling via nuclear factor κB (NF-κB) transcription factor, an attractive but undruggable molecular target. In this study, we describe a bipartite inhibitor comprising a NF-κB-specific decoy DNA tethered to a CpG oligodeoxynucleotide (ODN) targeting Toll-like receptor-9-expressing B cell lymphoma cells. The Bc-NFκBdODN showed efficient uptake by human diffuse large B cell (U2932, OCI-Ly3), Burkitt (RaJi), and mantle cell (Jeko1) lymphomas, respectively. We confirmed that Bc-NFκBdODN inhibited NF-κB nuclear translocation and DNA binding, resulting in CCND2 and MYC downregulation. Bc-NFκBdODN enhanced radiosensitivity of lymphoma cells in vitro. In xenotransplanted human lymphoma, local injections of Bc-NFκBdODN reduced NF-κB activity in whole tumors. When combined with a local 3-Gy dose of radiation, Bc-NFκBdODN effectively arrested OCI-Ly3 lymphoma progression. In immunocompetent mice, intratumoral injections of Bc-NFκBdODN suppressed growth of directly treated and distant A20 lymphomas, as a result of systemic CD8 T cell-dependent immune responses. Finally, systemic administration of Bc-NFκBdODN to mice bearing disseminated A20 lymphoma induced complete regression and extended survival of most of the treated mice. Our results underscore clinical relevance of this strategy as monotherapy and in support of radiation therapy to benefit patients with resistant or relapsed B cell lymphoma.
Keywords: F-κB, decoy oligodeoxynucleotides, TLR9, non-Hodgkin lymphoma, B-cell lymphoma, radiation therapy, cancer immunotherapy
Graphical Abstract
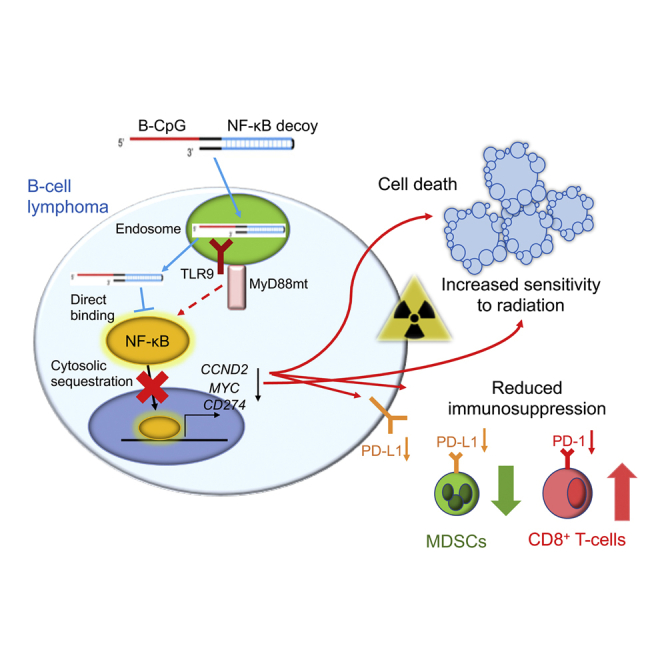
Despite recent advances, B cell lymphoma patients often relapse or remain refractory to therapies. Zhang et al. explore a new oligonucleotide strategy using targeted delivery of decoy DNA to inhibit NF-κB survival signaling, while stimulating T cell-mediated immune responses against disseminated B cell lymphoma.
Introduction
B cell lymphoma (BCL) is the most common type of non-Hodgkin’s lymphoma (NHL), accounting for 85% of NHL cases in the United States.1 Despite recent advances in BCL therapies, 30%–40% of patients will relapse or develop resistance to treatment.2 Among different types of BCL, diffuse large BCL (DLBCL) is the most aggressive type of NHL, with 36% percent of patients dying within 5 years from diagnosis.3 An aberrant nuclear factor κB (NF-κB) signaling resulting from oncogenic mutations in upstream signaling regulators, such as B cell receptor (BCR), Toll-like receptor-9 (TLR9), MyD88, Bruton’s tyrosine kinase (BTK), or CARD11, occur frequently in the activated B cell (ABC) subtype of DLBCL.4, 5, 6 The most common are MyD88 mutations that constitutively activate NF-κB signaling in about 30% of ABC-DLBCL cases.4, 5, 6 Furthermore, NF-κB signaling is also associated with anti-apoptotic phosphatidylinositol 3-kinase (PI3K)/AKT signaling in ABC-DLBCL and other aggressive and therapy-resistant NHL subtypes, such as in Burkitt lymphoma and in mantle cell lymphoma (MCL).7, 8, 9 Canonical NF-κB transcription factors (TFs), including p65/RelA, c-Rel, or p50, play a critical role in pro-inflammatory cytokine production and immune cell functions.10,11 The activated NF-κB factors form homodimers or heterodimers, translocate into the nucleus, and regulate expression of target genes encoding proteins involved in a broad range of cellular functions from proliferation (cyclin D1/D2, c-Myc) and survival (c-Flip and Bcl-xL) to immune signaling (tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-10, and IL-12).12 NF-κB signaling is at least partly responsible for treatment resistance in NHL, as it is promoting expression of well-defined survival and immunosuppressive signals, such as IL-6, IL-10, or PD-L1.13, 14, 15 NF-κB has been long recognized as an important therapeutic target in cancer and inflammatory diseases.10,16, 17, 18 Despite high therapeutic potential of targeting NF-κB, there are currently no US Food and Drug Administration (FDA)-approved, direct pharmacological inhibitors of NF-κB.19 Clinically relevant strategies target upstream NF-κB regulators, such as BTK (ibrutinib), IKKβ (MLN120B), or proteasomal degradation of IκB as in the case of bortezomib.19,20 Bortezomib showed promise in the treatment of multiple myeloma,21 but as a monotherapy it has been less effective in DLBCL unless combined with chemotherapy.22 Major challenges of targeting NF-κB are potential toxicities resulting from the lack of molecular selectivity and/or off-target effects to non-malignant cells, especially in case of IKK inhibitors.20 Hence, there is an unmet need for alternative approaches permitting more precise NF-κB blockade primarily in lymphoma cells and in the tumor microenvironment. Decoy oligodeoxynucleotides (dODNs) are competitive inhibitors of TFs and comprise a consensus DNA sequence, which can bind and neutralize TF activity. Various types of NF-κB decoy DNAs showed promising effects in experimental models of inflammatory diseases, such as arthritis or asthma/pulmonary allergy.23 However, clinical translation of these strategies was hampered by the lack of cell-selective delivery methods for decoy molecules.24 We previously developed a myeloid cell and B cell targeting strategy adaptable for various oligonucleotide (ON) therapeutics, such as small interfering RNA (siRNA) as well as decoy DNA.25, 26, 27 In this study, we adapted this approach for targeting NF-κB survival signaling in BCL cells in vitro and in vivo.
Results
Cell-Selective Delivery of NF-κB dODNs to BCL
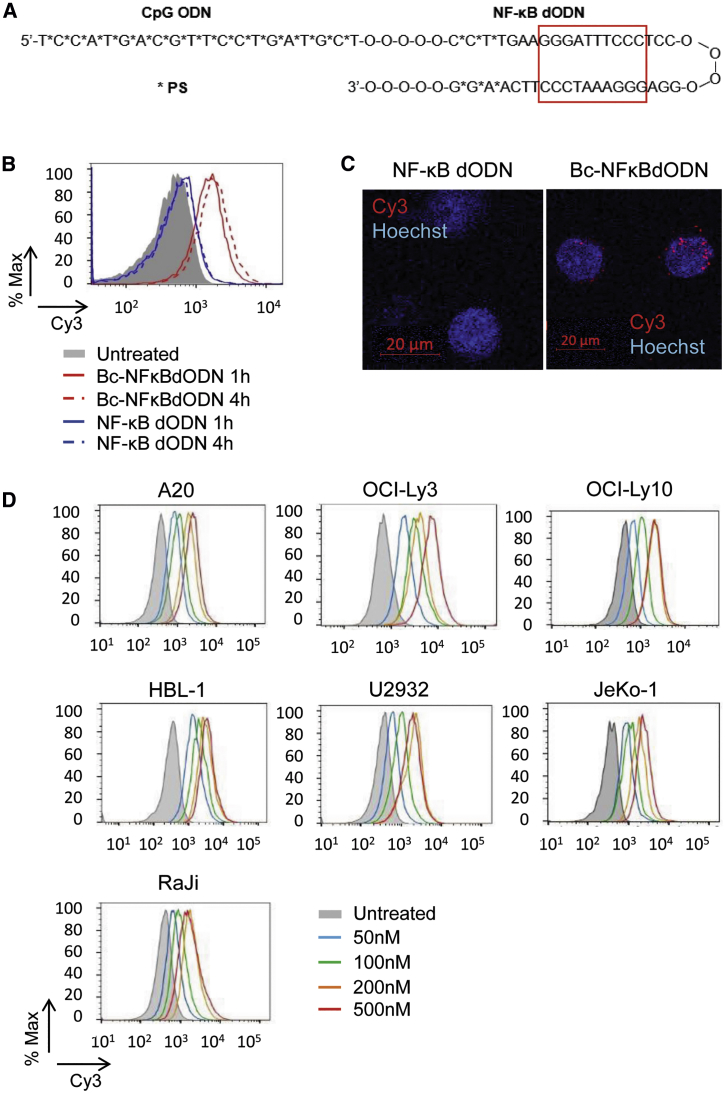
The synthetic phosphorothioated ODNs (PS-ODNs) stimulate the scavenger receptor (SR)- and TLR9-mediated delivery of therapeutic molecules into human and mouse myeloid cells and B cells, including malignant BCL.25 To adapt this strategy for targeting NF-κB, we selected four previously described dODN sequences, which span the consensus motif for NF-κB binding, and converted them into a chemically modified hairpin design (Figure S1).28 The dODN sequence with the maximal NF-κB inhibitory effect in target mouse macrophages on the transcriptional activity of NF-κB was then conjugated via a synthetic linker to the 3′ end of a single-stranded and fully PS-modified ODN (CpG1668) as a B cell targeting domain, creating a Bc-NFκBdODN conjugate (Figure 1A). Next, we compared the internalization of Bc-NFκBdODN with the unconjugated NFκBdODN by target BCL cells. Consistent with previous studies, the mouse A20 BCL cells rapidly internalized the fluorescently labeled Bc-NFκBdODNCy3, but not the NFκBdODNCy3 alone, as detected by flow cytometry (Figure 1B). Confocal microscopy confirmed intracellular, likely endosomal localization of the conjugate in A20 cells (Figure 1C). In addition to A20 cells, Bc-NFκBdODN showed robust uptake by a panel of human DLBCL cell lines, such as OCI-Ly3, OCI-Ly10, HBL-1, U2932, and also MCL JeKo-1 cells and Burkitt lymphoma RaJi cells at concentrations as low as 50 nM (Figure 1D). Bc-NFκBdODN was selectively internalized by primary mouse immune cells such as dendritic cells (DCs), granulocytic cells, macrophages, and B cells, but not by T cells after overnight incubation (Figure S2A). Overall, our results demonstrated a pattern of cell-selective Bc-NFκBdODN uptake consistent with the SR-/TLR9-dependent mechanism of internalization/cytoplasmic release of other CpG conjugates by myeloid and B cell lineage cells.25
Figure 1.
Design of the Bc-NFκBdODN Conjugate for Targeting B Cell Lymphoma Cells
(A) Predicted hairpin structure of the synthetic Bc-NFκBdODN conjugate. The consensus NF-κB DNA-binding sequence is marked by a red box. (B and C) Mouse A20 lymphoma cells were incubated with 500 nM Cy3-labeled Bc-NFκBdODN or NF-κB dODN for 1–4 h (B) or 4 h (C) and the oligonucleotide uptake (B) or intracellular localization (C) were examined by flow cytometry or confocal microscopy, respectively. Scale bars, 20 μm. (D) Human NHL lymphoma cells were incubated for 4 h in the presence of Cy3-labeled Bc-NFκBdODN at various concentrations. The oligonucleotide uptake was assessed by flow cytometry.
Decoy-Mediated Inhibition of NF-κB Activity in Target Cells
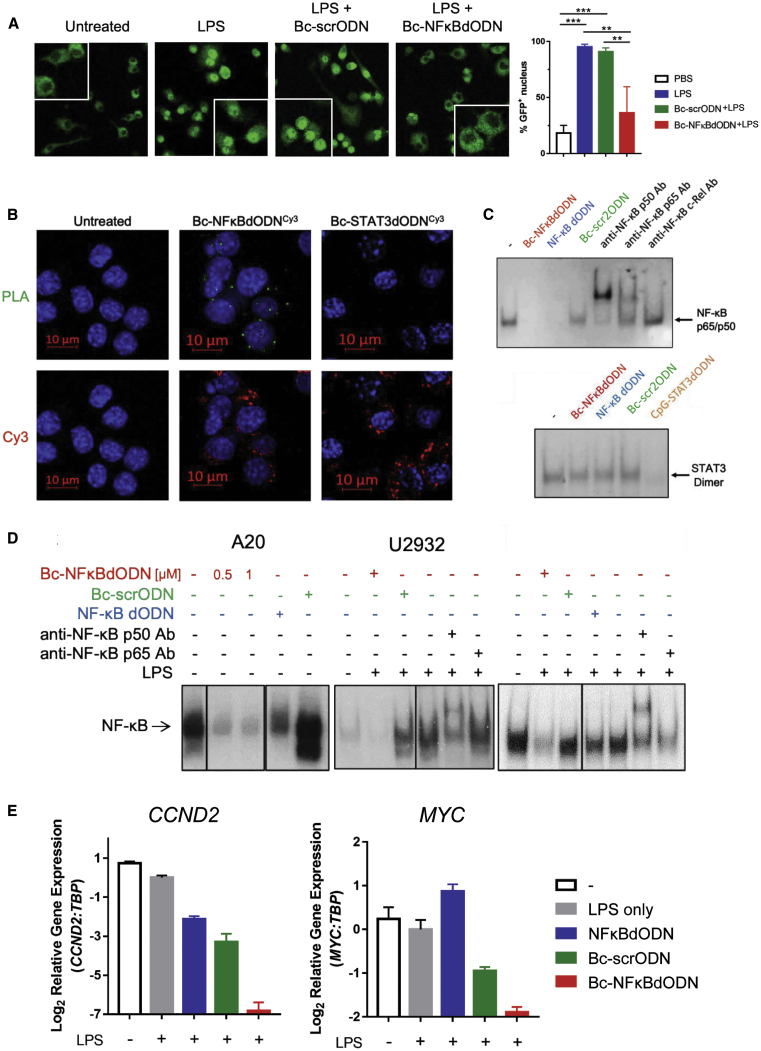
To characterize the effect of Bc-NFκBdODN on target cells, we assessed intracellular trafficking, DNA binding, and the transcriptional activity of NF-κB. First, we used confocal microscopy to assess the effect of the free oligonucleotide uptake on the nuclear translocation of NF-κB using a fluorescent RelA/p65-GFP fusion protein expressed in SR/TLR9-positive mouse RAW264.7 cells. The cells were incubated with Bc-NFκBdODN or with a control scrambled ODN (Bc-scrODN) before stimulation with lipopolysaccharide (LPS) to activate NF-κB. The confocal imaging revealed that LPS-induced nuclear translocation of p65-GFP was completely prevented by Bc-NFκBdODN, but not by a scrambled ODN (Bc-scrODN) (Figure 2A). To assess whether Bc-NFκBdODN interacts directly with NF-κB proteins in target cells, we performed an in situ proximity ligation assay (PLA). Using the PLA with a set of Cy3- and p50-specific antibodies, we confirmed the interaction between Cy3-labeled Bc-NFκBdODN and NF-κB/p50 complexes in cytosol of target macrophages as indicated by green fluorescent spot formation (Figure 2B). Next, we measured the NF-κB DNA-binding activity using an electrophoretic mobility shift assay (EMSA) in the nuclear extracts from RAW264.7 macrophages (Figure 2C) in mouse bone marrow cells or splenocytes (Figure S2B). In cell-free nuclear extracts, Bc-NFκBdODN significantly reduced DNA binding of LPS-induced NF-κB complexes, mainly representing p65/p50 heterodimers to the same extent as the unconjugated NFκBdODN (Figure 2C, top). The control Bc-scrODN had minimal inhibitory effect on NF-κB activity. Importantly, neither oligonucleotide inhibited activity of STAT3 TF, suggesting specificity of Bc-NFκBdODN in targeting NF-κB (Figure 2C, bottom). When tested on intact human and mouse lymphoma cells, Bc-NFκBdODN almost completely abrogated the constitutive nuclear activity of NF-κB in mouse A20 cells, human U2932 cells, and RaJi lymphoma cells (Figure 2D). The negative control Bc-scrODN slightly enhanced NF-κB activity in A20 and U2932 cells as well as in mouse splenocytes and bone marrow cells (Figure S2B), likely due to TLR9 stimulation.13 The unconjugated NF-κB dODN showed minimal inhibitory effects corresponding to low cellular uptake (Figure 1B). Finally, only Bc-NFκBdODN, but not the control ODNs, significantly inhibited the expression of NF-κB downstream target genes, CCND2 and MYC, in U2932 cells as measured using quantitative real-time PCR (Figure 2E). Taken together, these results provide evidence that Bc-NFκBdODN effectively inhibits NF-κB activity by specific and direct interaction with NF-κB in the cytoplasm of target SR/TLR9-positive cells, such as BCL cells.
Figure 2.
Bc-NFκBdODN Inhibits NF-κB Activity in Human and Mouse Target Cells In Vitro
(A) RAW264.7 macrophages stably expressing NF-κB/p65-GFP fusion protein were treated 18 h using 1 μM Bc-NFκBdODN or Bc-scrODN, then stimulated for 30 min with 100 ng/mL LPS. Localization of p65-GFP fusion protein was examined using confocal microscopy (right) and the percentage of cells with nuclear NF-κB signal was quantified (left). Counted were >40 cells/area in triplicate per experimental group. Means ± SD are shown. (B) RAW264.7 macrophages were incubated with fluorescently labeled Bc-NFκBdODNCy3 or CpG-STAT3dODNCy3 (200 nM) for 18 h and then stimulated using LPS (100 ng/mL) for the last 30 min. Cells were fixed, permeabilized, and the interaction between the oligonucleotides and NF-κB was assessed using PLA with Cy3- and NF-κB/p50-specific antibodies or control isotype antibodies followed by secondary DuoLink antibodies with PLA probes. The close proximity of both tested epitopes is indicated by a cyclic polymerase reaction producing green fluorescent spots in the cytosol. (C) Target specificity of Bc-NFκBdODN in cell-free EMSA detecting the effect on the DNA binding by NF-κB (top) or STAT3 (bottom). RAW264.7 cells were stimulated using LPS (100 ng/mL) for 30 min before collecting nuclear extracts, which were then incubated in the presence of 30 nM Bc-NFκBdODN, NF-κB dODN, Bc-scrODN, or CpG-STAT3dODN for 30 min before adding radiolabeled probes for NF-κB (B) or STAT3 (C) and gel shift assays. (D) Bc-NFκBdODN reduces NF-κB activity in lymphoma cells. The A20, U2932, and RaJi cells were treated 18 h using 1 μM Bc-NFκBdODN, Bc-scrODN, or NF-κB dODN, before collection of nuclear extracts and testing by EMSA. (E) Bc-NFκBdODN prevents expression of NF-κB target genes in BCL cells. Human U2932 cells were treated for 3 days using 1 μM Bc-NFκBdODN, Bc-scrODN, or NF-κB dODN, then stimulated with 100 ng/mL LPS for 3 h. The expression of CCND2 and MYC genes was assessed using quantitative real-time PCR; experiments were repeated twice in triplicates. Means ± SEM are shown.
Bc-NFκBdODN Blocks NF-κB-Driven Survival Signaling and Induces Lymphoma Cell Death In Vitro and In Vivo
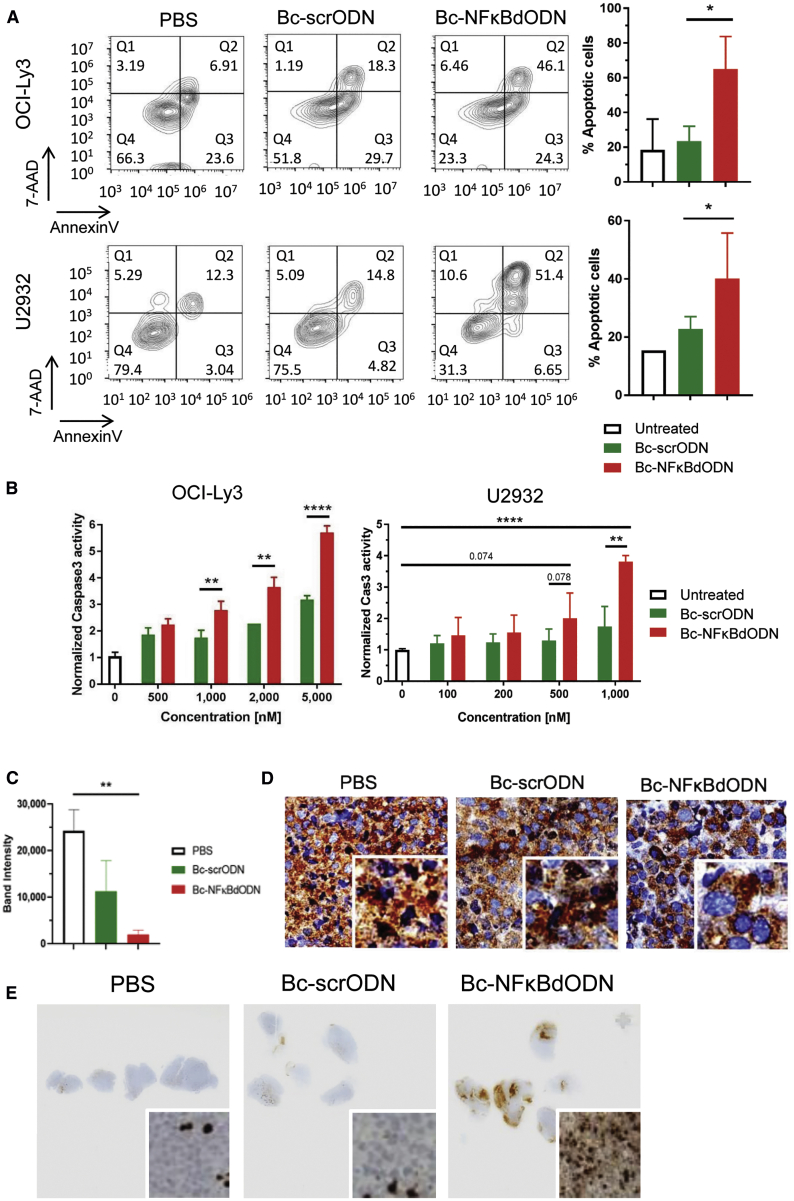
BCL cells rely on NF-κB signaling for their survival and therapeutic resistance.5,13,29 Thus, we tested whether Bc-NFκBdODN will trigger direct cytotoxicity in human ABC-DLBCL cells, such as OCI-Ly3 and U2932 cells. As shown in Figure 3A, Bc-NFκBdODN but not control Bc-scrODN induced apoptosis in ∼60% of OCI-Ly3 cells and ∼40% of U2932 cells after 3 days of culture as measured using flow cytometry after annexin V and 7-aminoactinomycin D (7AAD) staining. Correspondingly, Bc-NFκBdODN at 1 μM and higher concentrations induced cell apoptosis as detected by an elevated caspase-3 activity in both tested OCI-Ly3 and U2932 cells (Figure 3B). The cytotoxic effect of Bc-NFκBdODN seems to be mainly driven by the inhibition of NF-κB signaling with a rather minor role of TLR9 activation. As shown by our control experiments (Figure S3A), NF-κB dODNs conjugated to oligonucleotides comprising a GpC motif (not activating TLR9) induced a weaker cytotoxic effect in human lymphoma cells than did the Bc-NFκBdODN (with CpG motif activating TLR9), despite similar target cell uptake rate. We next verified activity of Bc-NFκBdODN in human U2932 lymphoma xenotransplants in mice. The established U2932 lymphomas were treated using three intratumoral injections of 10 mg/kg Bc-NFκBdODN, Bc-scrODN, or PBS only every day. We confirmed significant inhibition of NF-κB activity in nuclear extracts prepared from whole tumors treated using Bc-NFκBdODN, with moderate effect of Bc-scrODN (Figure 3C). Correspondingly, immunochemistry on tumor specimens showed that Bc-NFκBdODN also reduced nuclear localization of NF-κB/p65 compared to control treatments (Figure 3D), similar to earlier in vitro results (Figure 2A). Furthermore, the NF-κB inhibition found in lymphoma specimens was associated with the elevated activity of caspase-3 associated with the induction of cell death in Bc-NFκBdODN-treated but not in Bc-scrODN- or vehicle-treated lymphomas (Figure 3E).
Figure 3.
Targeting NF-κB Survival Signaling Using Bc-NFκBdODN Triggers B Cell Lymphoma Cell Death
(A and B) Bc-NFκBdODN treatment results in an increased cell death in human OCI-Ly3 and U2932 lymphoma cells. Cells were treated for 3 days using 5 μM Bc-NFκBdODN, control scrODN, or PBS before measuring cell viability using annexin V and 7AAD staining (A) or apoptosis using caspase-3 activation as determined by a Caspase-Glo 3/7 assay (B). Shown are representative results from one of two experiments in triplicates (means ± SD). (C–E) Local intratumoral injections of Bc-NFκBdODN reduce NF-κB activity and trigger cell death in human U2932 lymphoma in mice. Immunodeficient NSG mice were engrafted with 107 U2932 cells, and after tumors were established mice were injected intratumorally once daily during 3 days using 10 mg/kg Bc-NFκBdODN, Bc-scrODN, or PBS (n = 4–5 mice/group). NF-κB activity was detected using an EMSA assay in nuclear extracts isolated from single-cell suspensions isolated from whole tumors; n = 4 in each group. Means ± SEM are shown. Tumor tissues were collected and immunohistochemically stained for NF-κB/p65 (C) or activated caspase-3 (D).
Targeted NF-κB Inhibition Overcomes Radioresistance of Lymphoma Cells and Suppresses Regrowth of BCL Xenotransplants after Local Tumor Irradiation
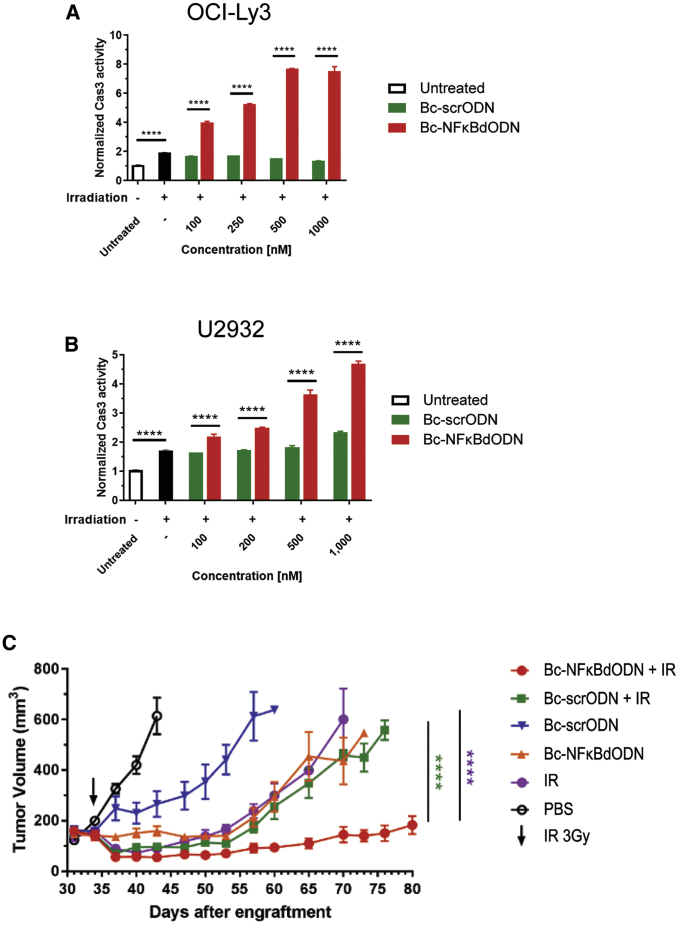
Therapeutic effects of radiation therapy against NHL, especially in DLBCL patients, are often limited by considerable radioresistance of lymphoma cells.30 Thus, we assessed whether targeted inhibition of NF-κB using the decoy strategy will augment the cytotoxic effect of ionizing radiation. As shown in Figures 4A and 4B, the limited cytotoxic effect of the ionizing radiation alone was amplified when combined with Bc-NFκBdODN but not the control Bc-scrODN treatment. Cell death was significantly elevated in relatively radiosensitive OCI-Ly3 (Figure 4A; Figure S3B) as well as in radioresistant U2932 (Figure 4B) lymphoma cells even at low 0.1 μM concentrations of Bc-NFκBdODN, which suggested synergism between both treatment types.31 In contrast, the combination of Bc-NFκBdODN with bortezomib induced a significant but only additive cytotoxic effect against U2932 lymphoma (Figure S3C). We next assessed whether the Bc-NFκBdODN will sensitize BCL to the effect of local tumor irradiation in vivo. Mice with established subcutaneous OCI-Ly3 lymphomas were treated using daily intratumoral injections of 5 mg/kg Bc-NFκBdODN, Bc-scrODN, or PBS combined with or without local tumor irradiation using a single dose of 3 Gy. As shown in Figure 4C, injections of Bc-NFκBdODN alone delayed but did not suppress regrowth of lymphoma, similar to radiation alone, while the effect of Bc-scrODN was minimal. In contrast, the local tumor irradiation combined with targeted NF-κB inhibition (Bc-NFκBdODN) induced lymphoma regression and prevented tumor regrowth in most mice (Figure 4C). The combination of control Bc-scrODN with radiotherapy did not enhance therapeutic effects and was also followed by tumor recurrence within about 2 weeks. Altogether, our results confirm that NF-κB inhibition using Bc-NFκBdODN can augment cytotoxic effects of radiation therapy against BCL in vitro and in vivo.
Figure 4.
Targeted NF-κB Inhibition Sensitizes Lymphoma to Radiation Therapy
(A and B) OCI-Ly3 (A) and U2932 (B) cells treated using Bc-NFκBdODN or Bc-scrODN were irradiated using 2.5 or 10 Gy for radiosensitive OCI-Ly3 or radioresistant U2932 cells, respectively, before the assessment of cell death by measuring the caspase-3 activation. Shown are representative results from three independent experiments in triplicates (means ± SD). (C) Local administration of Bc-NFκBdODN augments antitumor efficacy of human lymphoma irradiation. Immunodeficient NSG mice with established subcutaneously engrafted OCI-Ly3 lymphomas were injected intratumorally with 10 mg/kg Bc-NFκBdODN, Bc-scrODN, or PBS alone daily, with a single 3-Gy dose of irradiation 2 days after the initial treatment. Tumor progression was analyzed using caliper measurements. Means ± SEM are shown (n = 5/group).
Targeted Disruption of NF-κB Signaling in Syngeneic BCL and in the Microenvironment Triggers Anti-tumor Immune Responses
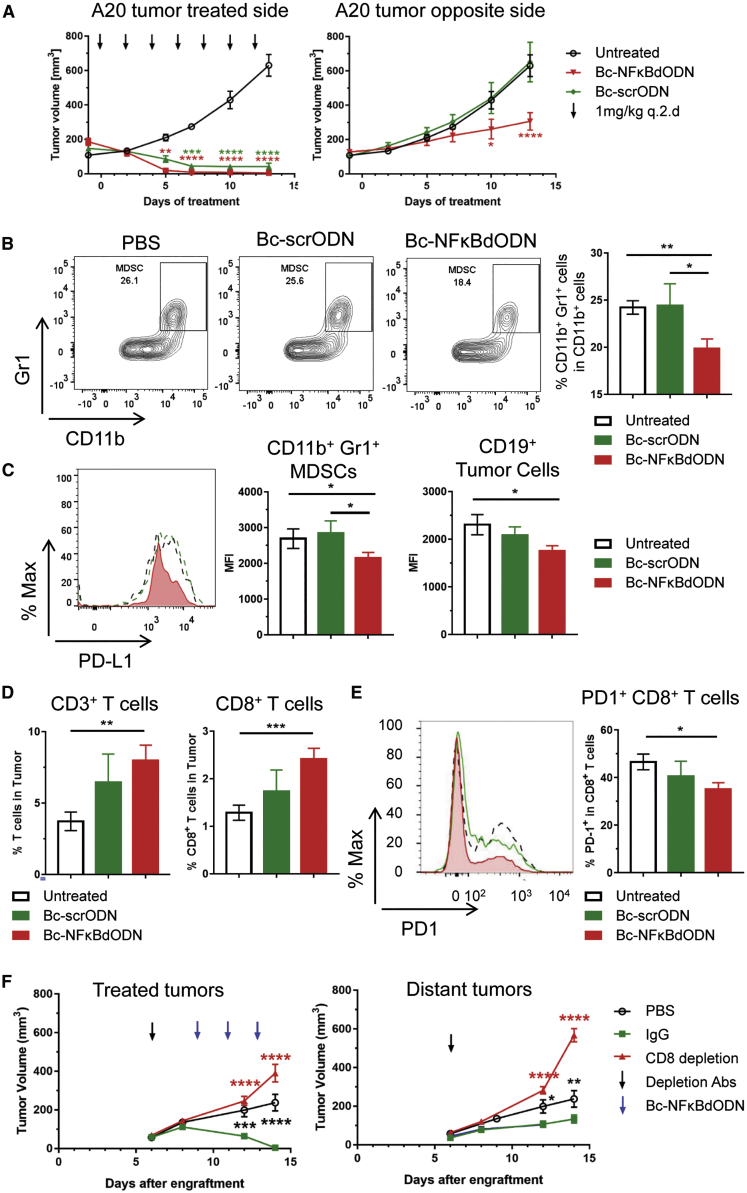
Beyond the NF-κB role in lymphoma cell survival, NF-κB signaling modulates activity of non-malignant immune cells, such as DCs or macrophages. In the tumor microenvironment, NF-κB acting together with STAT3 can promote chronic inflammation, leading to tolerogenic and proangiogenic effects.32 The abrogation of NF-κB signaling downstream of TLR9 can shift the balance of cytokine gene expression toward more T cell-promoting effects, as suggested by our in vitro data (Figure S2C). Thus, we next assessed whether targeting NF-κB in BCL cells as well as in tumor-associated immune cells will affect the outcome of Bc-NFκBdODN treatment. We used a dual tumor model of syngeneic A20 BCL injected subcutaneously (s.c.) into both flanks of the mice commonly used in preclinical testing of novel cancer immunotherapies. Only one tumor site was treated to compare local and systemic effects of the treatment. We verified in preliminary studies that locally (intratumorally [i.t.]) injected and fluorescently labeled oligonucleotides are not detectable in distant tumors using flow cytometry (Figure S4A). As expected due to the sensitivity of A20 lymphoma to a local administration of phosphorothioated oligonucleotides,33 both Bc-NFκBdODN and Bc-scrODN injections induced regression at the primary/treated site (Figure 5A, left). However, only Bc-NFκBdODN suppressed growth of distant tumors, which suggests generation of systemic antitumor immune responses (Figure 5A, right). This systemic antitumor effect depended not only on NF-κB inhibition but also CpG/TLR9 stimulation, since non-activating GpC-NFκBdODN conjugate suppressed lymphoma growth only locally (Figures S4B–S4D).
Figure 5.
Bc-NFκBdODN Induces Immune-Mediated Tumor Regression
A20 cells were injected subcutaneously in both flanks of BALB/c mice. After tumors were established (days 9–13), tumors on one side were treated every other day with 1 mg/kg Bc-NFκBdODN for 2 weeks while tumors on the other side were left untreated. (A) Tumor progression was analyzed using caliper measurements. Shown are combined data from two independent experiments (means ± SEM; n = 9 in each group). (B–E) The percentage of CD11b+Gr1+ immature myeloid cells (B) and PD-L1 expression (C), together with percentages of tumor-infiltrating CD3+ and CD8+ T cells (D) and PD-1 expression (E), were analyzed in cell suspensions from the untreated/distant tumors. Shown are representative flow cytometry histograms (means ± SEM; n = 6/group). (F) Mice were injected with 200 μg of CD8 depleting antibodies on day 6 after tumor engraftment. Three days later, mice were injected 1 mg/kg Bc-NFκBdODN intratumorally every other day three times. Means ± SEM are shown (n = 5/group).
The flow cytometric analysis of distant tumors revealed that Bc-NFκBdODN, but not control injections, significantly reduced the percentage of lymphoma-associated CD11b+/Gr1+ immature myeloid cells, likely representing myeloid-derived suppressor cells (MDSCs) (Figure 5B). In addition, Bc-NFκBdODN moderately decreased expression of PD-L1 immune checkpoint molecules on these potentially immunosuppressive myeloid cells and on lymphoma cells (Figure 5C). These changes in the composition of the tolerogenic lymphoma microenvironment were correlated with the significantly elevated percentage of lymphoma-infiltrating T cells, including CD8+ T cells (Figure 5D). The tumor-infiltrating CD8+ T cells showed lower levels of PD-1, suggesting reduced T cell exhaustion (Figure 5E). To validate the role of adaptive immune responses in the therapeutic efficacy of Bc-NFκBdODN against A20 lymphoma, we repeated these studies using antibody-mediated CD8 T cell depletion (Figure 5F; Figure S5). The neutralization of CD8 T cells abrogated the antitumor effect of Bc-NFκBdODN against both primary and distant tumors, confirming the key role of T cell-mediated immune responses to local and systemic tumor control.
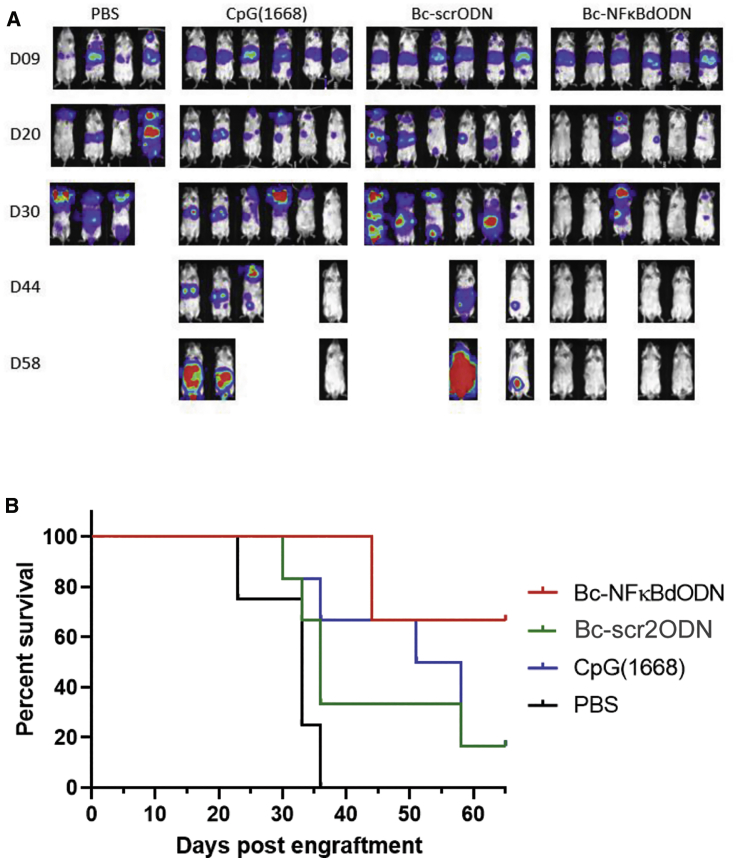
The Bc-NFκBdODN showed sufficient stability both intracellularly after uptake (Figure S6) as well as in human serum (t½ [half-time] of ∼12 h; Figure S7) to enable testing using a more broadly applicable delivery route using intravenous (i.v.) injections. Thus, we next tested the feasibility of using Bc-NFκBdODN against disseminated BCL. As shown in Figure 6, the i.v. injected Bc-NFκBdODN resulted in remarkable antitumor effects against A20 lymphoma and resulted in complete regression of tumors in most treated mice. No bioluminescence imaging (BLI) signal was detectable at day 62 in all Bc-NFκBdODN-treated surviving mice. The control Bc-scrODN or CpG ODN treatments delayed tumor progression only weakly, likely due to inefficient TLR9-mediated immune activation.
Figure 6.
Systemic Administration of Bc-NFκBdODN Induces Lymphoma Regression and Extends Survival of Mice
(A and B) Mice with established, disseminated A20 lymphoma (expressing luciferase) were treated from day 9 using i.v. injections of 1 mg/kg Bc-NFκBdODN, Bc-scrODN (new version), or an equivalent molar amount of CpG ODN every other day for 4 weeks. Shown are representative bioluminescence images (A) and a Kaplan-Meier survival curve (B) for all treatment groups (n = 6/group except for n = 4 in the PBS group). No bioluminescence imaging (BLI) signal was detectable at day 62 in all Bc-NFκBdODN-treated surviving mice.
Importantly, we did not observe myeloablative or lymphopenic effects of Bc-NFκBdODN after more than 2 weeks of local/i.t. or systemic/i.v. administration in vivo (Figure S8). This is consistent with the lack of dependence on NF-κB survival signaling in non-malignant immune cells in adult mice. Local or i.v. injections of Bc-NFκBdODN, similar to control Bc-scrODN conjugates or CpG alone, did not reduce DCs, myeloid cells, B cells, or T cells in the spleen and in the bone marrow. As expected, we observed some expansion of B cell and T cell lineages due to known effects of TLR9-induced IL-6, especially after i.v. injections of all tested oligonucleotides.
Discussion
Treatment of relapsed/refractory DLBCL remains a challenge even for novel immunotherapies, such as CD19 chimeric antigen receptor (CAR) T cell transfer.34, 35, 36 In this study, we demonstrate the potential of using a decoy-based oligonucleotide strategy for eliminating NF-κB signaling specifically in B cell and Burkitt lymphoma to augment cancer cell sensitivity to radiation therapy and to antitumor immune responses. The Bc-NFκB dODN prevented the nuclear translocation, transcriptional activity of NF-κB, and the expression of its downstream target genes. The NF-κB inhibition increased sensitivity of lymphoma cells to cell death induced by ionizing radiation in vitro and in vivo. Local administration of Bc-NFκBdODN augmented the efficacy of radiotherapy against two types of xenotransplanted human lymphoma and prevented tumor recurrence. In immunocompetent mice, local Bc-NFκBdODN treatments alone were sufficient to induce systemic antitumor effects against distant syngeneic lymphoma. The suppression of NF-κB in lymphoma cells and in the tumor microenvironment concurrently reduced tolerogenic MDSC population, together with expression of PD-L1, while stimulating CD8+ T cell-dependent antitumor immune responses.
DNA damage and genotoxic stress induced by conventional NHL therapies, such as chemotherapy or radiation therapy, are likely to trigger activation of innate immune receptors, including TLR9, thereby activating NF-κB.37 As we showed previously in solid tumor models in mice, radiation-induced cell death stimulates TLR9/NF-κB signaling in myeloid cells and the release of proangiogenic cytokines/growth factors, which promote tumor survival and recurrence.38 Due to common although heterogeneous expression of TLR9 in NHL cells, it is likely that NF-κB supports similar tumorigenic responses in BCL after low-dose radiotherapy. While the outcome of TLR9 activation is defined by a coactivation of multiple downstream pathways, the immunogenic effects of JNK, p38 mitogen-activated protein (MAP) kinases (MAPKs), and IFN regulatory factor (IRF) TF signaling can be offset by the NF-κB-mediated Jak/STAT3 activation.39 In fact, TLR9 signaling and specifically NF-κB signaling has been shown to upregulate a number of immune checkpoint regulators such as PD-L1, IDO, or CEACAM1, likely as an element of the negative feedback regulation of immune response.40,41 Chronic NF-κB activity in BCL cells and myeloid cells limits their immunogenicity and antigen presentation. These effects result from NF-κB-dependent production of cytokines such as IL-6 and IL-10, which in turn trigger activity of STAT3, a master regulator of immunosuppression.13,42 In fact, NF-κB and STAT3 collaborate in many cancer cells on the transcriptional upregulation of tolerogenic genes.32 Both NF-κB and STAT3 directly regulate expression of the PD-L1 immune checkpoint in many cancer cells and stromal cells, including BCL.14,15,43 Correspondingly, Bc-NFκBdODN treatment reduced PD-L1 expression on both lymphoma cells and on the tumor-associated MDSCs, which may partly promote antitumor immunity.44 The inhibition of NF-κB using Bc-NFκBdODN has the potential to correct the aberrant signaling downstream of TLR9 in BCL cells and the tumor-associated myeloid suppressor cells. The elimination of survival promoting and tolerogenic NF-κB can unleash immunostimulatory TLR9 signaling via MAPKs and IRFs, and release of IL-12, as mentioned earlier. There is a growing number of small molecules targeting various elements of NF-κB signaling such as NEDD8-activating enzyme,45 Hsp90 inhibitors,46 and new BTK inhibitors47 with promising activity in preclinical and initial clinical studies. However, the cell-selective NF-κB inhibition within the immune cell network can be critical for the overall antitumor efficacy and persistence of the therapeutic effect. The preferential uptake of Bc-NFκBdODN by B cell and myeloid cell lineages, but not T cells, reduces the risk of the oligonucleotide interfering with T cell functions. Since NF-κB plays the key role of in T cell receptor (TCR) signaling, NF-κB inhibition in T cells could compromise adaptive immune responses to cancer and infections.11,48 Our myeloid and B cell-targeted NF-κB inhibitor can maximize long-term antitumor therapeutic effects while limiting potential toxicities in contrast to non-selective small molecule inhibitors.10,17 The combination of radiotherapy with i.t. injections of TLR9 ligands had already showed promise in indolent lymphomas.49,50 However, such such combination of radiotherapy with TLR9 agonists would be less likely to succeed against recurrent DLBCL, especially ABC-DLBCL, that are addicted to high constitutive levels of NF-κB signaling.13,37 Overall, our results demonstrate that cell-selective blocking of NF-κB signaling using a decoy oligonucleotide strategy has potential to sensitize BCLs to radiotherapy and to stimulate immune-mediated antitumor responses. With the ongoing translation of the first TLR9-targeted oligonucleotide STAT3 inhibitor to treatment of recurrent/relapsed BCL, we anticipate that further development of Bc-NFκBdODN will provide novel therapeutic strategies for the most aggressive forms of B cell malignancies.
Materials and Methods
Cells
Human ABC-DLBCL OCI-Ly3, Ly10, and U2932 cells were acquired from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), while HBL-1 cells were kindly provided by Dr. G. Inghirami (Weill Cornell Medicine, New York, NY, USA). Human Burkitt lymphoma cells (RaJi), MCL cells (JeKo-1), and mouse BCL A20 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and RAWBlue cells were from InvivoGen (San Diego, CA, USA). We are grateful to Dr. Iain Fraser (NIAID, Rockville, MD, USA) for sharing RAW264.7 cells expressing p65-GFP fusion protein. Cells were cultured in RPMI 1640 with 10%–20% fetal bovine serum (FBS) with antibiotic/antimycotic supplements. All cell lines were authenticated by the Integrative Genomics Core at City of Hope and regularly tested for mycoplasma contamination using LookOut mycoplasma PCR detection kit (Sigma-Aldrich, St. Louis, MO, USA).
Animal Studies
All animal experiments followed established institutional guidance and approved protocols from the Institutional Animal Care and Use Committee (City of Hope [CoH]). BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA, USA); NOD/SCID/IL-2RgKO (NSG) mice and NOD-scid IL2Rgnull-3/GM/SF (NSG-SGM3) mice were from Jackson Laboratory (Bar Harbor, ME, USA). Mice were injected subcutaneously using 107 of various lymphoma cells, and tumor progression was monitored using caliper measurements. Established subcutaneous tumors were irradiated using a single collimated dose of radiation from a Cs-137 source using a MARK-I irradiator (J.L. Shepherd) under xylazine/ketamine anesthesia with or without prior treatment using intratumoral injections of Bc-NFκBdODN. To deplete CD8+ T cells, mice were injected intraperitoneally using 200 μg of CD8-specific neutralizing antibodies or the matched isotype control. The successful cell depletion was verified by flow cytometry. For the immunohistochemical staining on tumor sections, antibodies specific to NF-κB (E379, Abcam) or activated caspase-3 (ASP175, Cell Signaling) were used.
Oligonucleotide Design
All oligonucleotides were synthesized in the DNA/RNA Synthesis Core (CoH) by linking type B CpG to the modified sequence of NF-κB dODN (NFκBdODN) in a manner similar to that previously described.27 The resulting conjugates are as follows (o indicates single C3 unit; asterisks indicate phosphorothioate sites): Bc-NFκBdODN, 5′-T∗C∗C∗A∗T∗G∗A∗C∗G∗T∗T∗C∗C∗T∗G∗A∗T∗G∗C∗T-ooooo-C∗C∗T∗TGAAGGGATTTCCCT CC-oooo-GGAGGGAAATCCCTTCAA∗G∗G∗-ooooo-3′; Bc-scrODN, 5′-T∗C∗C∗A∗T∗G∗A∗C∗G∗T∗T∗C∗C∗T∗G∗A∗T∗G∗C∗T-ooooo-A∗C∗T∗CTTGCCAATTAC-oooo-GTAATTGGCAAGA∗G∗T∗-ooooo-3′; Bc-scr2ODN, 5′-T∗C∗C∗A∗T∗G∗A∗C∗G∗T∗T∗C∗C∗T∗G∗A∗T∗G∗C∗T-ooooo-C∗G∗T∗CTAGGGTATATCCCTCC-oooo-GGAGGGATATACCCTAG∗A∗C∗G-ooooo-3′; and NF-κBdODN, 5′-ooooo-C∗C∗T∗TGAAGGGATTTCCCTCC-oooo-GGAGGGAAATCCCTTCAA∗G∗G-ooooo-3′. For internalization studies, oligonucleotides were 3′ labeled using Cy3 fluorochrome.
EMSA
EMSAs to detect DNA-binding NF-κB activity were performed as described previously.26 Briefly, 10 μg of each nuclear extract was incubated with 32P-labeled oligonucleotide probes specific to NF-κB.28 Antibodies specific to p65 (F6), p50 (D4P4D), or c-Rel (B6) were used for supershift controls to identify proteins (Santa Cruz Biotechnology, Dallas, TX, USA).
Confocal Microscopy
Imaging was performed using an LSM 880 with Airyscan confocal microscope (Zeiss, Oberkochen, Germany). For uptake assays, cells were incubated with Cy3-labeled CpG-NFκBdODN or NF-κB dODN and then fixed using 2% paraformaldehyde (Fisher Scientific, NH, USA). For NF-κB nuclear translocation assays, RAW264.7 cells expressing p65-GFP fusion protein were seeded on 12-well plates and treated with 100 ng/mL LPS for 30 min before imaging. The PLAs were performed using Cy3- and NF-κB-specific antibodies following the manufacturer’s protocol, as reported before.40 Slides mounted in Vectashield HardSet medium (#H-1400, Vector Laboratories, Burlingame, CA, USA) were visualized on an LSM 510-Axiovert inverted confocal microscope (Zeiss) and analyzed using LSM ImageBrowser (version 4.2.0.121; Zeiss).
Cell Apoptosis Assays
Cell death was detected using annexin V and 7AAD staining as described before.25,51 Caspase-3 activity in apoptotic cells was examined using a Caspase-Glo 3/7 assay (Promega, WI, USA), and the readings were normalized to total number of viable cells detected using a CellTiter Glo assay (Promega) as described before.52
Statistical Analysis
An unpaired t test was used to calculate the two-tailed p value to estimate statistical significance of differences between two experimental groups. A one-way ANOVA plus a Bonferroni post-test were applied to assess the statistical significance of differences between multiple treatment groups. The relationship between two groups was assessed using correlation and linear regression. The p values are indicated in the figures as follows: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001. Data were analyzed using Prism v.6.03 software (GraphPad).
Acknowledgments
We are grateful to the staff at Analytical Cytometry, DNA/RNA Synthesis, Pathology, Animal Resource Cores (CoH). This work was supported in part by the National Cancer Institute/National Institutes of Health award nos. R01CA213131 (to M.K.), P50CA107399 (to S.F.), and P30CA033572 (to CoH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions
Conceptualization, M.K. and Z.Z.; Methodology, X.Z., Y.-L.S., D.M., and Z.Z.; Investigation, Z.Z., X.Z., and D.W.; Writing – Original Draft, Z.Z., M.A., and M.K.; Writing – Review & Editing, Z.Z. and M.K.; Funding Acquisition, M.K. and S.F.; Resources, P.S., J.W., S.H., M.K. and L.K.; Supervision, M.K.
Declaration of Interests
M.K. and P.S. are on the patent application submitted by CoH that covers the design of oligonucleotides presented in this report. The remaining authors declare no competing interests.
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.ymthe.2020.11.026.
Supplemental Information
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Klyuchnikov E., Bacher U., Kroll T., Shea T.C., Lazarus H.M., Bredeson C., Fenske T.S. Allogeneic hematopoietic cell transplantation for diffuse large B cell lymphoma: who, when and how? Bone Marrow Transplant. 2014;49:1–7. doi: 10.1038/bmt.2013.72. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute . DLBCL; 2020. Cancer stat facts: NHL—diffuse large B-cell lymphoma.https://seer.cancer.gov/statfacts/html/dlbcl.html [Google Scholar]
- 4.Lenz G., Davis R.E., Ngo V.N., Lam L., George T.C., Wright G.W., Dave S.S., Zhao H., Xu W., Rosenwald A. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 5.Davis R.E., Ngo V.N., Lenz G., Tolar P., Young R.M., Romesser P.B., Kohlhammer H., Lamy L., Zhao H., Yang Y. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young R.M., Staudt L.M. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat. Rev. Drug Discov. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao Q., Jiang Y., Li G. Inhibition of the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced apoptosis in human Burkitt’s lymphoma. J. Pharmacol. Sci. 2013;121:247–256. doi: 10.1254/jphs.12149fp. [DOI] [PubMed] [Google Scholar]
- 8.Ruan J., Martin P., Furman R.R., Lee S.M., Cheung K., Vose J.M., Lacasce A., Morrison J., Elstrom R., Ely S. Bortezomib plus CHOP-rituximab for previously untreated diffuse large B-cell lymphoma and mantle cell lymphoma. J. Clin. Oncol. 2011;29:690–697. doi: 10.1200/JCO.2010.31.1142. [DOI] [PubMed] [Google Scholar]
- 9.Paul J., Soujon M., Wengner A.M., Zitzmann-Kolbe S., Sturz A., Haike K., Keng Magdalene K.H., Tan S.H., Lange M., Tan S.Y. Simultaneous inhibition of PI3Kδ and PI3Kα induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-κB and AKT. Cancer Cell. 2017;31:64–78. doi: 10.1016/j.ccell.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat. Rev. Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 11.Liu T., Zhang L., Joo D., Sun S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 13.Ngo V.N., Young R.M., Schmitz R., Jhavar S., Xiao W., Lim K.-H., Kohlhammer H., Xu W., Yang Y., Zhao H. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen H., Li C., Peng X., Zhou Z., Weinstein J.N., Liang H., Cancer Genome Atlas Research Network A pan-cancer analysis of enhancer expression in nearly 9000 patient samples. Cell. 2018;173:386–399.e12. doi: 10.1016/j.cell.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godfrey J., Tumuluru S., Bao R., Leukam M., Venkataraman G., Phillip J., Fitzpatrick C., McElherne J., MacNabb B.W., Orlowski R. PD-L1 gene alterations identify a subset of diffuse large B-cell lymphoma harboring a T-cell-inflamed phenotype. Blood. 2019;133:2279–2290. doi: 10.1182/blood-2018-10-879015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jost P.J., Ruland J. Aberrant NF-κB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 17.Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakanishi C., Toi M. Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 19.Bennett J., Capece D., Begalli F., Verzella D., D’Andrea D., Tornatore L., Franzoso G. NF-κB in the crosshairs: rethinking an old riddle. Int. J. Biochem. Cell Biol. 2018;95:108–112. doi: 10.1016/j.biocel.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prescott J.A., Cook S.J. Targeting IKKβ in cancer: challenges and opportunities for the therapeutic utilisation of IKKβ inhibitors. Cells. 2018;7:115. doi: 10.3390/cells7090115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manasanch E.E., Orlowski R.Z. Proteasome inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 2017;14:417–433. doi: 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunleavy K., Pittaluga S., Czuczman M.S., Dave S.S., Wright G., Grant N., Shovlin M., Jaffe E.S., Janik J.E., Staudt L.M., Wilson W.H. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–6076. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desmet C., Gosset P., Pajak B., Cataldo D., Bentires-Alj M., Lekeux P., Bureau F. Selective blockade of NF-κB activity in airway immune cells inhibits the effector phase of experimental asthma. J. Immunol. 2004;173:5766–5775. doi: 10.4049/jimmunol.173.9.5766. [DOI] [PubMed] [Google Scholar]
- 24.Fabre S., Apparailly F. Gene therapy for rheumatoid arthritis: current status and future prospects. BioDrugs. 2011;25:381–391. doi: 10.2165/11595490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q., Hossain D.M.S., Nechaev S., Kozlowska A., Zhang W., Liu Y., Kowolik C.M., Swiderski P., Rossi J.J., Forman S. TLR9-mediated siRNA delivery for targeting of normal and malignant human hematopoietic cells in vivo. Blood. 2013;121:1304–1315. doi: 10.1182/blood-2012-07-442590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X., Zhang Z., Moreira D., Su Y.-L., Won H., Adamus T., Dong Z., Liang Y., Yin H.H., Swiderski P. B cell lymphoma immunotherapy using TLR9-targeted oligonucleotide STAT3 inhibitors. Mol. Ther. 2018;26:695–707. doi: 10.1016/j.ymthe.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kortylewski M., Swiderski P., Herrmann A., Wang L., Kowolik C., Kujawski M., Lee H., Scuto A., Liu Y., Yang C. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat. Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan F., Lenardo M.J. Specification of DNA binding activity of NF-κB proteins. Cold Spring Harb. Perspect. Biol. 2009;1:a000067. doi: 10.1101/cshperspect.a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernal-Mizrachi L., Lovly C.M., Ratner L. The role of NF-κB-1 and NF-κB-2-mediated resistance to apoptosis in lymphomas. Proc. Natl. Acad. Sci. USA. 2006;103:9220–9225. doi: 10.1073/pnas.0507809103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elstrom R.L., Ruan J., Christos P.J., Martin P., Lebovic D., Osborne J., Goldsmith S., Greenberg J., Furman R.R., Avram A. Phase 1 study of radiosensitization using bortezomib in patients with relapsed non-Hodgkin lymphoma receiving radioimmunotherapy with 131I-tositumomab. Leuk. Lymphoma. 2015;56:342–346. doi: 10.3109/10428194.2014.914195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rødland G.E., Melhus K., Generalov R., Gilani S., Bertoni F., Dahle J., Syljuåsen R.G., Patzke S. The dual cell cycle kinase inhibitor JNJ-7706621 reverses resistance to CD37-targeted radioimmunotherapy in activated B cell like diffuse large B cell lymphoma cell lines. Front. Oncol. 2019;9:1301. doi: 10.3389/fonc.2019.01301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H., Deng J., Xin H., Liu Y., Pardoll D., Yu H. A requirement of STAT3 DNA binding precludes Th-1 immunostimulatory gene expression by NF-κB in tumors. Cancer Res. 2011;71:3772–3780. doi: 10.1158/0008-5472.CAN-10-3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qi X.-F., Zheng L., Kim C.-S., Lee K.-J., Kim D.-H., Cai D.-Q., Qin J.W., Yu Y.H., Wu Z., Kim S.K. CpG oligodeoxynucleotide induces apoptosis and cell cycle arrest in A20 lymphoma cells via TLR9-mediated pathways. Mol. Immunol. 2013;54:327–337. doi: 10.1016/j.molimm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gisselbrecht C., Glass B., Mounier N., Singh Gill D., Linch D.C., Trneny M., Bosly A., Ketterer N., Shpilberg O., Hagberg H. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J. Clin. Oncol. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crump M., Neelapu S.S., Farooq U., Van Den Neste E., Kuruvilla J., Westin J., Link B.K., Hay A., Cerhan J.R., Zhu L. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu L., Li L., Medeiros L.J., Young K.H. NF-κB signaling pathway and its potential as a target for therapy in lymphoid neoplasms. Blood Rev. 2017;31:77–92. doi: 10.1016/j.blre.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao C., Kozlowska A., Nechaev S., Li H., Zhang Q., Hossain D.M.S., Kowolik C.M., Chu P., Swiderski P., Diamond D.J. TLR9 signaling in the tumor microenvironment initiates cancer recurrence after radiotherapy. Cancer Res. 2013;73:7211–7221. doi: 10.1158/0008-5472.CAN-13-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bai L., Chen W., Chen J., Li W., Zhou L., Niu C., Han W., Cui J. Heterogeneity of Toll-like receptor 9 signaling in B cell malignancies and its potential therapeutic application. J. Transl. Med. 2017;15:51. doi: 10.1186/s12967-017-1152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang W., Chapman N.M., Zhang B., Li M., Fan M., Laribee R.N., Zaidi M.R., Pfeffer L.M., Chi H., Wu Z.H. Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-κB contributes to UV radiation-induced immune suppression. Cancer Res. 2019;79:2909–2922. doi: 10.1158/0008-5472.CAN-18-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D., Jiang W., Zhu F., Mao X., Agrawal S. Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int. J. Oncol. 2018;53:1193–1203. doi: 10.3892/ijo.2018.4456. [DOI] [PubMed] [Google Scholar]
- 42.Cheng F., Wang H.-W., Cuenca A., Huang M., Ghansah T., Brayer J., Kerr W.G., Takeda K., Akira S., Schoenberger S.P. A critical role for Stat3 signaling in immune tolerance. Immunity. 2003;19:425–436. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 43.Marzec M., Zhang Q., Goradia A., Raghunath P.N., Liu X., Paessler M., Wang H.Y., Wysocka M., Cheng M., Ruggeri B.A., Wasik M.A. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc. Natl. Acad. Sci. USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paterson A.M., Lovitch S.B., Sage P.T., Juneja V.R., Lee Y., Trombley J.D., Arancibia-Cárcamo C.V., Sobel R.A., Rudensky A.Y., Kuchroo V.K. Deletion of CTLA-4 on regulatory T cells during adulthood leads to resistance to autoimmunity. J. Exp. Med. 2015;212:1603–1621. doi: 10.1084/jem.20141030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milhollen M.A., Traore T., Adams-Duffy J., Thomas M.P., Berger A.J., Dang L., Dick L.R., Garnsey J.J., Koenig E., Langston S.P. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-κB-dependent lymphoma. Blood. 2010;116:1515–1523. doi: 10.1182/blood-2010-03-272567. [DOI] [PubMed] [Google Scholar]
- 46.Rajan A., Kelly R.J., Trepel J.B., Kim Y.S., Alarcon S.V., Kummar S., Gutierrez M., Crandon S., Zein W.M., Jain L. A phase I study of PF-04929113 (SNX-5422), an orally bioavailable heat shock protein 90 inhibitor, in patients with refractory solid tumor malignancies and lymphomas. Clin. Cancer Res. 2011;17:6831–6839. doi: 10.1158/1078-0432.CCR-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byrd J.C., Harrington B., O’Brien S., Jones J.A., Schuh A., Devereux S., Chaves J., Wierda W.G., Awan F.T., Brown J.R. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnes S.E., Wang Y., Chen L., Molinero L.L., Gajewski T.F., Evaristo C., Alegre M.L. T cell-NF-κB activation is required for tumor control in vivo. J. Immunother. Cancer. 2015;3:1. doi: 10.1186/s40425-014-0045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frank M.J., Reagan P.M., Bartlett N.L., Gordon L.I., Friedberg J.W., Czerwinski D.K., Long S.R., Hoppe R.T., Janssen R., Candia A.F. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov. 2018;8:1258–1269. doi: 10.1158/2159-8290.CD-18-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y.H., Gratzinger D., Harrison C., Brody J.D., Czerwinski D.K., Ai W.Z., Morales A., Abdulla F., Xing L., Navi D. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood. 2012;119:355–363. doi: 10.1182/blood-2011-05-355222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qaqish A., Huang D., Chen C.Y., Zhang Z., Wang R., Li S., Yang E., Lu Y., Larsen M.H., Jacobs W.R., Jr. Adoptive transfer of phosphoantigen-specific γδ T cell subset attenuates Mycobacterium tuberculosis infection in nonhuman primates. J. Immunol. 2017;198:4753–4763. doi: 10.4049/jimmunol.1602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z., Yang E., Hu C., Cheng H., Chen C.Y., Huang D., Wang R., Zhao Y., Rong L., Vignuzzi M. Cell-based high-throughput screening assay identifies 2′,2′-difluoro-2′-deoxycytidine gemcitabine as a potential antipoliovirus agent. ACS Infect. Dis. 2017;3:45–53. doi: 10.1021/acsinfecdis.6b00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.