Abstract
Purpose
Researches revealed that probiotics maybe a potential strategy for COVID-19, whereas there is a lack of related evidence. This study aims to analyze the role of probiotics on severe COVID-19 patients.
Methods
In the current retrospective single-center study, we collected data of 311 consecutive severe patients with confirmed COVID-19 in Wuhan Union Hospital from Feb 3rd to Feb 20th, 2020. Epidemiological, clinical and medication characteristics were compared and analyzed between patients with or without probiotics.
Results
In total, 93 of the 123 patients (75.61%) who were treated with probiotics survived to hospital discharge with the median inpatient day of 32 days and mean virus clearance time of 23 days, which were significantly longer than those of patients without probiotics. There were no bias in laboratory parameters, except for IL-6 and ESR, which were significantly higher in patients treated probiotics. We tracked the dynamic changes of 8 selected laboratory parameters (IL-6, CRP, total T lymphocytes, NK cells, B lymphocyte, CD4 + T cells, CD8 + T cells and CD4/CD8 ratio) and found that probiotics could not reduce the increased IL-6 levels but possessed the ability to moderate the immunity and decreased the incidence of secondary infection in COVID-19 patients.
Conclusions
Probiotics could be an effective strategy for the treatment of COVID-19 patients to reduce the secondary infection and moderated the immunity.
Keywords: COVID-19, Probiotics, Immunity, Inflammatory
Abbreviations: CREA, creatinine; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; T-BIL, total bilirubin; HGB, hemoglobin; PLT, platelets; WBC, white blood cell; NE, neutrophil; EO, eosinophilic granulocyte; CRP, c-reactive protein; PCT, procalcitonin; IL-6, interleukin 6; FIB, Fibrinogen; ESR, erythrocyte sedimentation rate
1. Introduction
Because it is highly contagious and person-to-person transmission occurs through direct contact, droplets or fomites, COVID-19 has spread all over the world rapidly since December 2019, when the novel coronavirus (2019-nCoV) was isolated [1], [2]. As once the epicenter of COVID-19, there were 50,340 patients confirmed COVID-19 in Wuhan, approximately 7–10% of which were severe/critical cases [3] and transferred to the designated hospital for treatment.
At present, there is no proven regimen other than conventional medicine. Antiviruses (arbidol, alpha-interferon, ribavirin, lopinavir/ritonavir and resochin), anti-inflammatory agents (corticosteroids) and symptomatic supportive treatments (immunopotentiators, intestinal probiotics) are recommended for the treatment of severe COVID-19 patients in the Chinese management guidelines for COVID-19 (version 7.0) [4].
Probiotics, which are usually used to treat diarrhea, are live microorganisms or components of dead bacteria which could regulate the immunity through the route of the gut to exert the resistance against antibiotics, xenobiotics and pathogenicity or toxicity factors [5]. Interestingly, recent research found that probiotics could be used as co-adjuvants in treating metabolic disorders such as type 2 diabetes, obesity and metabolic syndrome [6]. At present, although the lesions are primarily located in the lung, later studies, expanded the distribution of COVID-19 to the GI system [7], which might explain the presence of diarrhea as one of the symptoms of COVID-19. Dysbiosis of the human gut microbiome is linked to respiratory tract infections (RTIs) through the gut-lung axis. Previous studies showed that various strains of Lactobacillus spp. and Bifidobacterium spp. through oral or intranasal administration have shown suppression of infection symptoms against viral infections [8], [9]. Other research found that SARS-CoV could bind angiotensin-converting enzyme 2 (ACE2) [10], which is expressed in endothelial cells of the vasculature, epithelia of the lungs and intestine [11]. ACE2 exerted protective effects in infection induced acute lung injury mediated through activation of the reninangiotensin system (RAS), and the expression of ACE2 was increased in patients treated with ACE inhibitors [12]. Meanwhile, several probiotics, particularly probiotic lactic acid bacteria, have been reported to be able to produce peptides with ACE inhibitory effects [13]. Thus, probiotics might improve RTIs in COVID-19 patients through the ACE2 pathway.
Moreover, the health effect of probiotics are benefit for the treatment of COVID-19, such as the ability to support intestinal integrity and maintain intestinal permeability, competition with pathogens for nutrients and attachment sites, and the modulation of immune cell activity against invading pathogens [14] the regulation of balancing proinflammatory and anti-inflammatory cytokines [15], which are grossly imbalanced in COVID-19 patients who suffer cytokine storms [16]. Patients with high concentrations of TNF-α, and IL-6 showed severe consequences of COVID-19. Probiotics have an immune regulation effect through local immunity (by maintaining gut wellbeing and gut wall integrity) and systemic immunity (by enhancing specific and nonspecific immune system) [17]. Probiotics improved the levels of NK cells, T and B lymphocytes, type I interferons and antigen-presenting cells (APCs) in the lung immune system [18]. Another study suggested that probiotics modulate the expression of interleukin-10 (IL-10) and decrease the expression of inflammatory cytokines, such as TNF-α, IL-1 and IL-8 [19]. And many studies have found that probiotics possessed a therapeutic role in viral respiratory infections [20]. Probiotic bifidobacteria could decrease the duration of respiratory, symptoms caused by the common cold coronavirus and the days with fever [21]. And clinical trial indicated that patients treated with probiotics containing Lactobacillus rhamnosus GG, live Bacillus subtilis, and Enterococcus faecalis showed significantly less ventilator-associated pneumonia compared to those without probiotics [22]. However, the therapeutic strength of probiotics in COVID-19 patients is still uncertain due to limitation of available clinical data [5].
Immunonutrition, which includes feeding various pharmaconutrients, such as arginine, vitamins and probiotics, was used to modify inflammatory or immune responses. Many studies have shown that immunonutrients promote patient recovery by inhibiting inflammation or immune responses [23]. In fact, the gene expression and maturation and differentiation of immune cells are regulated by some nutrients and their metabolites [24]. Hence, immunonutrition, including functional food components, prebiotics and probiotics, may be a potential treatment for COVID-19 patients via microbiota modulation and immunoregulation [25]. However, all these assumptions were based on previous studies, and more clinical evidence is needed to support the use of probiotics in COVID-19 patients. Although many clinical trials on COVID-19 are ongoing, few studies the effect of intestinal probiotics on COVID-19.
Here, to confirm and further explore the possible mechanisms of probiotics in COVID-19 patients, we performed a single-center retrospective analysis at Union Hospital, Tongji Medical College, Huazhong University of Science and Technology. We collected the clinical characteristics, medication and outcomes of 311 severe COVID-19 patients and analyzed the effect of probiotics on immunity and inflammation. We hope this study may provide evidence for the treatment of probiotics in COVID-19.
2. Methods
2.1. Ethics statement
This study was approved by the Medical Ethical Committees of Wuhan Union Hospital (2020-0104). The requirement for written informed consent was waived because there was no intervention for treatment and potential risk to patients and the data were analyzed anonymously.
2.2. Study design and participants
This retrospective single-center study included severely ill adult inpatients (≥18 years old), who were admitted to Wuhan Union Hospital, the designated hospital for severe patients, from February 3 to February 20, 2020. All patients were diagnosed as COVID-19 positive according to WHO interim guidance, and the treatment was in line with the Chinese management guideline for COVID-19 (version 7.0). The clinical characteristics were followed up to March 15, 2020. Regarding complications of medication regimens, we excluded patients who died.
2.3. Medication
Probiotics were recommended to maintain intestinal microecological balance and prevent secondary bacterial infection in Chinese management guidelines for COVID-19 (version 7.0). According to whether probiotics were used, patients were divided into probiotic group and nonprobiotic group. Probiotics treatment included oral Combined Bifidobacterium, Lactobacillus, Enterococcus and Bacillus tablets (Bifidobacterium infantis [26], Lactobacillus acidophilus [27], Dung enterococcus, Bacillus cereus [28]) 1.5 g tid; Live combined Bifidobacterium and Lactobacillus tablets (Bifidobacterium longum [29] , Lactobacillus bulgaricus [30] , Streptococcus thermophiles [31]) 2 g tid; Live combined Bacillus Subtilis and Enterococcus Faecium Enteric-coated Capsules (Enterococcus faecium [32] , Bacillus subtilis [33]) 0.5 g tid. It was up to the attending physician to decide whether to give probiotics based on the patient's condition and the mean duration of probiotics was 12.94 days.
Additional medicines included the following. Chloroquine phosphate, 500 mg bid, the course of treatment should not exceed 10 days. Arbidol, 200 mg tid was administered, and the course of treatment should not exceed 10 days. Lopinavir/ritonavir, 200 mg/50 mg/grain, 2 grain a time, bid, the course of treatment should not exceed 10 days. Ribavirinb was recommended for use in combination with interferon alpha inhalation or lopinavir/ritonavir, 500 mg bid, and the course of treatment should not exceed 10 days. Interferon alpha inhalation, 5 million U bid. Antibiotics included quinolone, cephalosporin and carbapenems. Lianhua qingwen capsule, 4 grain a time, tid. Traditional Chinese medicine decoction, bid. The immune enhancer included thymopetin for injection (1 mg, qd), and thymalfasin for injection (1.6 mg, biw). Sedative hypnotic therapy included estazolam tablets (1 mg, qn). Corticosteroids (Solu Medrol) were given not more than 1–2 mg/kg/day (duration not more than 5 days). The utilization rates of these medications are showed in Table 3.
Table 3.
Treatment analysis of severe COVID-19 patients.
| No. (%) |
P Value | |||
|---|---|---|---|---|
| Total(n = 311) | Non-probioctics(n = 188) | Probiotics(n = 123) | ||
| Chloroquine phosphate | 41(13.18%) | 29(15.43%) | 12(9.76%) | 0.156 |
| Arbidol | 302(97.11%) | 182(96.81%) | 120(97.56%) | 0.699 |
| Lopinavir/ritonavir | 81(26.05%) | 31(16.49%) | 50(40.65%) | 0.001** |
| Ribavirinb | 99(31.83%) | 50(26.60%) | 49(39.84%) | 0.012* |
| Interferon alfa inhalation | 84(27.01%) | 36(19.15%) | 48(29.27%) | 0.001** |
| Antibiotic | 224(72.03%) | 136(72.34%) | 88(71.54%) | 0.879 |
| Traditional Chinese medicine | ||||
| Lianhua qingwen capsule | 161(51.77%) | 98(52.13%) | 63(51.22%) | 0.992 |
| Traditional Chinese medicine decoction | 298(95.82%) | 180(95.74%) | 118(62.77%) | 0.935 |
| Immune enhancer | 242(77.81%) | 142(75.53%) | 100(81.30%) | 0.231 |
| Sedative hypnotic therapy | 53(17.04%) | 30(15.96%) | 23(18.70%) | 0.543 |
| Corticosteroid | 68(21.86%) | 40(21.28%) | 28(22.76%) | 0.756 |
Data are presented as n (%).
P < 0.05.
P < 0.01.
2.4. Procedures
The epidemiological and clinical characteristics, medication, and laboratory parameters of confirmed severe cases of COVID-19 were collected from electronic medical records by a standardized case report form. All data were checked by two experienced individuals independently. The illness severity of COVID-19 was defined according to the Chinese management guideline for COVID-19 (version 7.0) [4].
The method used for laboratory confirmation of COVID-19 is to perform real-time reverse-transcriptase polymerase-chain-reaction (RT-PCR) assay tests using throat swab specimens that were obtained from upper respiratory tracts every other day after clinical remission of symptoms, including fever, cough, and dyspnea. These laboratory confirmations were performed at Wuhan Union Hospital following the standard protocol. Routine blood examinations, coagulation profiles, serum biochemical tests, plasma levels of inflammatory factors (including interleukin-6 (IL-6)), C-reactive protein, and procalcitonin were collected as long as these tests were performed. The frequency of tests was determined by the treating physician according to the conditions of the patients. Medication regimen were divided into antiviral drugs, antimicrobial drugs, traditional Chinese medicine, immunopotentiators, and probiotics.
The criteria for discharge were absence of fever for at least 3 days, substantial improvement in both lungs on chest CT, clinical remission of respiratory symptoms, and two throat-swab samples negative for the COVID-19 test obtained at least 24 h apart [4].
2.5. Statistical analysis
Categorical variables are described as frequency rates and percentages. Proportions for categorical variables were compared using the χ2 test, although Fisher’s exact test was used when the data were limited. Means for continuous variables were compared using independent group t tests when the data were normally distributed and described using the mean ± standard deviation; otherwise, the Mann-Whitney test was used and were described using mean, median, and interquartile range (IQR) values. Logistic regression was used to select independent risk factors that affect outcomes. All statistical analyses were performed using SPSS version 20.0 software. P < 0.05 was considered statistically significant.
3. Results
3.1. Outcome
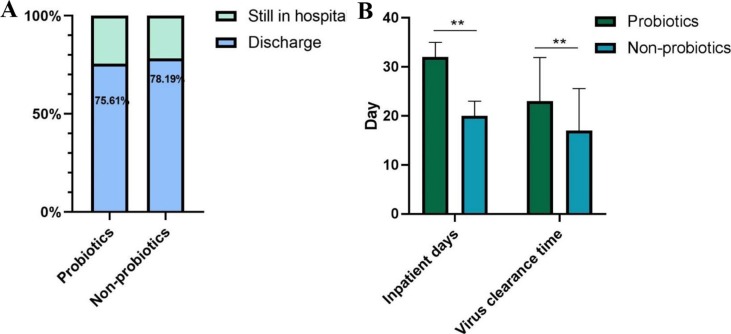
The current study described 311 severe patients with COVID-19 who admitted in Wuhan Union hospital from Feb 3, 2020 to Feb 15, 2020. Outcomes were assessed as discharged, remaining in hospital till Mar 15, 2020. Fig. 1 illustrated the outcome of patients confirmed with COVID-19. In total, 93 of the 123 patients (75.61%) who were treated with probiotics survived to hospital discharge with the median inpatient day of 32 days and mean virus clearance time of 23 days, and 147 of the 188 (78.19%) patients without the treatment of probiotics discharged with the median inpatient day of 20 days and mean virus clearance time of 17 days. Significant differences existed across the treatment of probiotics with regards to inpatient days (P < 0.001) and virus clearance time (P < 0.001).
Fig 1.
The outcomes between patients treated with or without probiotics. (A), the discharge rate of patients in different probiotics treated groups; (B), inpatient days and virus clearance time of patients in different probiotics treated groups.
4. Demographic and epidemiologic characteristics
Table 1 shows the epidemiologic characteristics of 311 severe patients infected with COVID-19. Of 311 patients, the mean time from illness onset to hospital admission (P = 0.323) was 13 days. A total of 184 patients (59.16%, P = 0.712) had comorbid diseases, such as chronic bronchitis (4.18%, P = 0.069), hypertension (35.05%, P = 0.482), diabetes (19.29%, P = 0.497), heart disease (15.43%, P = 0.110), renal failure (3.22%, P = 0.199), liver failure (2.57%, P = 0.394) and tumors (7.72%, P = 0.499). On admission, 254 patients had fever (81.67%, P = 0.182) as the initial symptom, and 226 patients had cough (72.67%, P = 0.719). Other symptoms were cough phlegm (36.98%, P = 0.042), shortness of breath (45.33%, P = 0.774) and diarrhea (21.54%, P = 0.323). Among them, only cough phlegm (P < 0.05) showed a significant difference between patients with or without probiotics.
Table 1.
Demographic and Epidemiologic Features of severe COVID-19 Patients on admission.
| No. (%) |
P Value | |||
|---|---|---|---|---|
| Total(n = 311) | Nonprobioctics(n = 188) | Probiotics(n = 123) | ||
| Age | 60.10 ± 12.37 | 60.20 ± 12.67 | 62.019 ± 10.88 | 0.196 |
| Sex (Male) | 150(48.23%) | 83(44.15%) | 67(54.47%) | 0.075 |
| Comorbidities | 184(59.16%) | 114 (60.64%) | 72 (58.54%) | 0.712 |
| Chronic bronchitis | 13(4.18%) | 11(5.85%) | 2(1.63%) | 0.069 |
| Hypertension | 109(35.05%) | 63(33.51%) | 46(37.40%) | 0.482 |
| Diabetes | 60(19.29%) | 34(18.09%) | 26(21.14%) | 0.497 |
| Heart disease | 48(15.43%) | 34(18.09%) | 14(11.38%) | 0.110 |
| Renal failure | 10(3.22%) | 8(4.26%) | 2(1.63%) | 0.199 |
| Liver failure | 8(2.57%) | 6(3.19%) | 2(1.63%) | 0.394 |
| Tumor | 24(7.72%) | 13(6.91%) | 11(8.94%) | 0.499 |
| Time from onset of illness to inpatients | 13.00 ± 6.13 | 12.3 ± 5.88 | 13.1 ± 6.41 | 0.323 |
| Fever | 254(81.67%) | 158 (84.04%) | 96(78.05%) | 0.182 |
| Cough | 226(72.67%) | 138(73.40%) | 88(71.54%) | 0.719 |
| Cough phlegm | 115(36.98%) | 78(41.49%) | 37(30.08%) | 0.042* |
| Shortness of breath | 141(45.33%) | 84(44.68%) | 57(46.34%) | 0.774 |
| Diarrhea | 67(21.54%) | 37(19.68%) | 30(24.39%) | 0.323 |
Data are presented as mean ± SD, n (%).
Significant at P < 0.05.
4.1. Laboratory findings
Table 2 shows the laboratory findings of severe COVID-19 patients on admission.
Table 2.
Laboratory findings of severe COVID-19 patients on admission.
| Normal range | Median (IQR) |
P Value | ||
|---|---|---|---|---|
| Nonprobioctics(n = 188) | Probiotics(n = 123) | |||
| Blood biochemistry | ||||
| CREA, μmol/L | 57–111 | 63.8(53.8–76.4) | 68.0(53.8–72.1) | 0.088 |
| BUN, mmol/L | 2.9–8.2 | 4.0(1.0–4.5) | 4.0(3.4–5.6) | 0.183 |
| AST, U/L | 8–40 | 41.0(35.5–51.0) | 23.0(15.0–32.0) | 0.692 |
| ALT, U/L | 5–40 | 69.0(53.0–91.0) | 19.0(13.0–41.5) | 0.595 |
| LDH, U/L | 109–245 | 194.0(166.5–249.5) | 211.0(188.0–239.5) | 0.122 |
| T-BIL, umol/L | 3–20 | 12.5 ± 7.19 | 12.4 ± 5.72 | 0.847 |
| Blood routine | ||||
| Lymphocytes, G/L | 1.1–3.2 | 1.1 ± 0.49 | 1.0 ± 0.46 | 0.635 |
| HGB, g/L | 40–50 | 122.8 ± 21.47 | 125.4 ± 16.30 | 0.254 |
| PLT, G/L | 125–350 | 248.8 ± 92.4 | 233.4 ± 92.69 | 0.159 |
| WBC, G/L | 3.5–9.5 | 5.9 ± 2.43 | 5.9 ± 2.43 | 0.858 |
| NE, G/L | 1.8–6.3 | 4.4 ± 2.42 | 4.3 ± 2.35 | 0.923 |
| EO, G/L | 0.01–0.52 | 0.07 ± 0.13 | 0.07 ± 0.30 | 0.820 |
| Infection-related biomarkers | ||||
| CRP, mg/L | 0–8 | 2.5(1.3–2.7) | 11.7(2.7–73.2) | 0.077 |
| PCT, ng/ml | ≤0.05 | 0.05(0.03–0.05) | 0.05(0.03–0.12) | 0.476 |
| ESR, mm/h | 0–20 | 49.0 ± 30.59 | 63.4 ± 36.56 | 0.049* |
| IL-6 | 0.1–5 | 4.1(3.6–5.4) | 8.1(5.2–12.6) | 0.001** |
| Coagulation function | ||||
| D-Dimer, mg/L | 0–0.5 | 1.3(0.7–1.4) | 0.4(0.3–1.0) | 0.697 |
| FIB, G/L | 2.0–4.0 | 4.4 ± 1.19 | 4.3 ± 1.31 | 0.476 |
| Immunity | ||||
| Total T lymphocyte | 58.17–84.22 | 71.8 ± 9.88 | 71.9 ± 8.53 | 0.965 |
| CD4 | 25.34–51.37 | 42.8 ± 8.66 | 43.4 ± 9.01 | 0.750 |
| CD8 | 14.23–38.95 | 24.95 ± 9.79 | 26.01 ± 8.6 | 0.669 |
| B lymphocyte | 4.1–18.31 | 13.77 ± 9.75 | 12.44 ± 8.33 | 0.462 |
| NK cell | 3.33–30.47 | 8.5 ± 5.24 | 9.8 ± 6.11 | 0.280 |
| CD4/CD8 ratio | 0.41–2.72 | 2.18 ± 1.23 | 2.07 ± 1.36 | 0.651 |
Data are presented as medians (interquartile ranges, IQR), n (%) and mean ± SD; *P < 0.05, **P < 0.01.
CREA, creatinine; BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; T-BIL, total bilirubin; HGB, hemoglobin; PLT, platelets; WBC, white blood cell; NE, neutrophil; EO, eosinophilic granulocyte; CRP, c-reactive protein; PCT, procalcitonin; IL-6, interleukin 6; FIB, Fibrinogen.
We included 6 blood biochemistry parameters (CREA (P = 0.088), BUN (P = 0.183), AST (P = 0.692), ALT (P = 0.0.595), LDH (P = 0.122) and T-BIL (P = 0.847), 6 routine blood parameters (lymphocytes (P = 0.635), HGB (P = 0.254), PLT (P = 0.159), WBC (P = 0.858), NE (P = 0.923) and EO (P = 0.820)), 4 inflammatory-related biomarkers (CRP (P = 0.077), PCT (P = 0.476), ESR (P = 0.049) and IL-6 (P = 0.001)), 2 coagulation function biomarkers (D-Dimer (P = 0.697) and FIB (P = 0.476)), and 6 immune-related biomarkers (Total T lymphocyte (P = 0.965), CD4 (P = 0.750), CD8 (P = 0.669), B lymphocyte (P = 0.462), NK cell (P = 0.280) and CD4/CD8 ratio (P = 0.651)). Among these biochemistry parameters, only IL-6 (P = 0.001) and ESR (P = 0.049) were at significantly higher levels in patients treated with probiotics.
4.2. Treatment analysis
Patients confirmed with COVID-19 were treated with supportive care and empiric medication. As Table 3 shows, almost all patients received one or more antiviral treatments, such as chloroquine (13.18%, P = 0.156), arbidol (97.11%, P = 0.699), lopinavir and ritonavir (26.05%, P = 0.001), ribavirin (31.83%, P = 0.012) and interferon (27.01%, P = 0.001). The use of lopinavir/ritonavir (P = 0.001), ribavirinb (P = 0.012) and interferon alpha inhalation (P = 0.001) was significantly higher in patients treated with probiotics. Other treatments, including antibiotics (72.03%, P = 0.879), Lianhua qingwen capsules (51.77%, P = 0.992), traditional Chinese medicine decoctions (95.82%, P = 0.935), immune enhancement (77.81%, P = 0.231), sedative hypnotic therapy (17.04%, P = 0.543) and corticosteroids (21.86%, P = 0.756) were also used in COVID-19 patients and showed no significant differences between patients treated with probiotics and those treated without probiotics.
4.3. Effect of probiotics on immune and inflammation
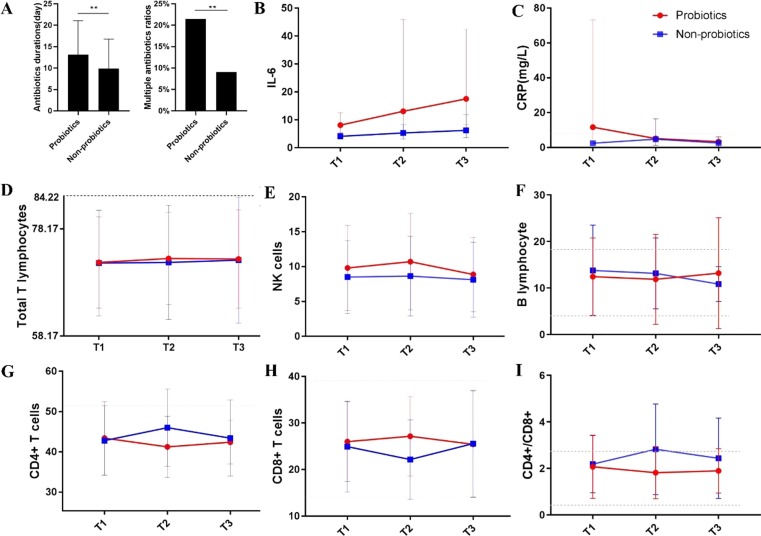
To determine the potential effects of probiotics on immunity and inflammation, we tracked the dynamic changes in 8 selected laboratory parameters (IL-6, CRP, total T lymphocytes, NK cells, B lymphocytes, CD4 + T cells, CD8 + T cells and CD4/CD8 ratio) during the course of COVID-19. T1 refers to the first test after hospital admission, T2 refers to the midpoint test during the whole hospital stay, and T3 refers to the last test before discharge. As Fig. 2 A shows, patients treated with probiotics had higher multiple antibiotic ratios (P = 0.001) and longer antibiotic durations (P = 0.001). However, there were partial superpositions between the curve of CRP for patients who were treated with or without probiotics (Fig. 2C). For IL-6 (Fig. 2B), the curve separated clearly (P greater than 0.05) and showed an increasing trend in probiotic-treated patients. As Fig. 2D-2I shows, immune parameters between patients with probiotics and without probiotics revealed opposite trends. Total T lymphocytes, NK cells and B lymphocytes were upregulated in probiotic-treated patients. The CD4+/CD8 + ratio in patients without probiotics increased beyond the normal range, whereas this parameter in probiotic-treated patients remained within the normal range.
Fig 2.
The dynamic changes in selected inflammatory and immunological parameters during the course of COVID-19. (A), the antibiotic treatment of patients in different probiotics treated groups; (B), the dynamic change of IL-6 in patients in different probiotics treated groups; (C), the dynamic change of CRP level in patients in different probiotics treated groups; (D), the dynamic change of total T lymphocytes in patients in different probiotics treated groups; (E), the dynamic change of NK cells in patients in different probiotics treated groups; (F), the dynamic change of B lymphocytes in patients in different probiotics treated groups; (G), the dynamic change of CD4 + T cells in patients in different probiotics treated groups; (H), the dynamic change of CD8 + T cells in patients in different probiotics treated groups; (I), the dynamic change of CD4/CD8 ratio in patients in different probiotics treated groups.
5. Discussion
At present, there are no approved specific antiviral agents targeting the novel coronavirus [34], and supportive therapies are the main treatments. Many researchers believe probiotics may be a potential therapy for COVID-19 [35], [36], [37]. However, to our knowledge, there is a lack of research evidence about the effect of probiotics on COVID-19. In the current study, we conducted a single center retrospective analysis of 311 severe COVID-19 patients, providing clinical evidence that probiotics could enhance immune function and reduce secondary infection in severe COVID-19 patients.
The epidemiological data for COVID-19 showed no probiotic bias except for the significant reduction in cough phlegm in probiotic-treated patients. However, there are no studies of cough phlegm in COVID-19 outcomes. Regarding clinical characteristics, probiotic-treated patients showed a significantly higher level of IL-6 on admission (Table 2). Many studies have found that increased IL-6 levels are in turn significantly associated with adverse clinical outcomes, and inhibition of IL-6 may be a target for therapeutics for the management of dysregulated host responses in patients with COVID-19 [38], [39]. Therefore, to some extent, this study showed that probiotics could not attenuate the increased IL-6 levels, which maybe the cause of the significantly longer inpatient day of probiotic-treated patients (Fig. 1). At present, a clinical trial of lopinavir/ritonavir has shown that no benefit was observed with lopinavir-ritonavir treatment beyond standard care in severe COVID-19 patients [40]. In our study, patients treated with probiotics showed significantly higher rates of in lopinavir-ritonavir, ribavirinb and interferon alfa inhalation (Table 3). However, the virus clearance time was significantly longer in probiotic-treated patients (Fig. 1). These results indicated that lopinavir-ritonavir, ribavirinb and interferon alpha may not have beneficial effects in severe COVID-19 patients, which is consistent with previous studies. Although probiotics might have the ability to protect against SARS-CoV through ACE2, the effect of probiotic-treatment on SARS-CoV was limited in this study.
Lymphocytopenia was observed in many COVID-19 patients [26], [41]. Lymphocyte subsets were regarded as immunological biomarkers for host immune system. Studies have found that the total number of B cells, T cells and NK cells were significantly decreased in patients with COVID-19 and much lower in severe cases [42]. Lower levels of CD4 + T cells and CD8 + T cells also found in severe COVID-19 patients and these characteristics were important for predicting the state of the illness changes from mild to severe and the disease outcomes [43]. Consistent with previous study that probiotics supplementation increased the number of neutrophils and CD4 and CD8 T lymphocytes [44], our studies found that patients treated with probiotics have higher levels of total T cells, CD8 + T cells compared to patients who not used probiotics (Fig. 2), which indicated that probiotics could moderate the reduction immunity in severe COVID-19 patients.
During treatment in the hospital, antibiotics were used to treat and prevent infection in the early days of the outbreak, which may lead to the intestinal dysbacteriosis, bacterial resistance and secondary infection. Probiotics supplementation were found to significantly remodel the microbiome of an individual recovering from antibiotic therapy [45] and, therefore, were recommended for the prevention of secondary infection. In our study, patients treated with probiotics showed significantly longer antibiotic durations and higher multiple antibiotic ratios (Fig. 2A), which meant a higher rate of secondary infection in these patients. We analyzed dynamic changes of CRP level, which is a parameter of infection [46]. The results showed that the CRP level of probiotics treated patients in T2 and T3 were almost the same as those of patients without probiotics, which indicated that the secondary infection in probiotic-treated patients was not higher than that in patients without probiotics. Moreover, recent studies have reported a consistent association between CRP and disease severity and outcomes [47], [48]. Higher levels of CRP were observed in severe patients compared to non-severe patients and more likely to get bad outcomes. Lu et al. [49] developed a simple death risk index, which consisted of age and CRP, to predict the short-term mortality of COVID-19 patients. Therefore, CRP is recognized as a certain inflammatory parameter to predict the progression and outcome of COVID-19. In our study, CRP was higher in probiotic-treated patients at T1 and reduced to the level of T2, which was close to the level of non-probiotic-treated patients at T2. Finally, the CRP levels decreased with the same trend as nonprobiotic-treated patients. All these results indicated that probiotics could reduce the inflammatory factor CRP and then prevent the progression of COVID-19.
This study has some limitations. First, this is a single-center retrospective study, and large-scale research is needed to provide high-quality evidence. Second, fatal cases of COVID-19 were excluded, and selection bias might have occurred. Third, pathological findings were not available. Therefore, additional studies are needed to investigate the outcomes of severe patients confirmed with COVID-19.
In summary, we reported the probiotic-specific differences in the epidemiology, medication and dynamic changes in inflammatory and immunological parameters in severe COVID-19 patients and provided evidence that probiotics could reduce secondary infection and moderate the immunity in severe COVID-19 cases, which is consistent with the findings of other studies. Further studies on the mechanism of probiotics on COVID-19 are warranted. It is our hope that these findings may serve as a guide to the clinical therapy of COVID-19.
CRediT authorship contribution statement
Qiang Li: Data curation, Writing - original draft, Visualization. Fang Cheng: Data curation, Formal analysis, Writing - review & editing. Qiling Xu: Data curation, Software, Validation. Yuyong Su: Data curation, Investigation. Xuefeng Cai: Data curation, Supervision. Fang Zeng: Conceptualization, Methodology. Yu Zhang: Conceptualization, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
This work was supported by National Key R&D Program of China (2017YFC0909900).
Data availability
The datasets are available from the corresponding author on reasonable request.
References
- 1.Phelan A.L., Katz R., Gostin L.O. The Novel Coronavirus Originating in Wuhan, China Challenges for Global Health Governance. JAMA-J. Am. Med. Assoc. 2020;323(8):709–710. doi: 10.1001/jama.2020.1097. [DOI] [PubMed] [Google Scholar]
- 2.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., V. Coronaviridae Study Group of the International Committee on Taxonomy of The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nature Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan K.W., Wong V.T., Tang S.C.W. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am. J. Chin. Med. 2020:1–26. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 4.New coronavirus pneumonial prevention and control program (version 7.0) (in Chinese), 2020. http://www.nhc.gov.cn/xcs/zhengcwj/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 5.Din A.U., Mazhar M., Waseem M., Ahmad W., Bibi A., Hassan A., Ali N., Gang W., Qian G., Ullah R., Shah T., Ullah M., Khan I., Nisar M.F., Wu J. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hur K.Y., Lee M.-S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015;39(3):198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.F. Wu, A. Xiao, J. Zhang, X. Gu, W.L. Lee, K. Kauffman, W. Hanage, M. Matus, N. Ghaeli, N. Endo, C. Duvallet, K. Moniz, T. Erickson, P. Chai, J. Thompson, E. Alm, SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases, 2020, 2020.04.05.20051540. [DOI] [PMC free article] [PubMed]
- 8.Zolnikova O., Komkova I., Potskherashvili N., Trukhmanov A., Ivashkin V. Application of probiotics for acute respiratory tract infections. Italian J. Med. 2018;12(1):32–38. [Google Scholar]
- 9.Kawahara T., Takahashi T., Oishi K., Tanaka H., Masuda M., Takahashi S., Takano M., Kawakami T., Fukushima K., Kanazawa H., Suzuki T. Consecutive oral administration of Bifidobacterium longum MM-2 improves the defense system against influenza virus infection by enhancing natural killer cell activity in a murine model. Microbiol. Immunol. 2015;59(1):1–12. doi: 10.1111/1348-0421.12210. [DOI] [PubMed] [Google Scholar]
- 10.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai Y., Kuba K., Ohto-Nakanishi T., Penninger J.M. Angiotensin-converting enzyme 2 (ACE2) in disease pathogenesis. Circ. J.: Official J. Jpn. Circ. Soc. 2010;74(3):405–410. doi: 10.1253/circj.cj-10-0045. [DOI] [PubMed] [Google Scholar]
- 12.Cole-Jeffrey C.T., Liu M., Katovich M.J., Raizada M.K., Shenoy V. ACE2 and Microbiota: Emerging Targets for Cardiopulmonary Disease Therapy. J. Cardiovasc. Pharmacol. 2015;66(6):540–550. doi: 10.1097/FJC.0000000000000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ettinger G., MacDonald K., Reid G., Burton J.P. The influence of the human microbiome and probiotics on cardiovascular health. Gut Microbes. 2014;5(6):719–728. doi: 10.4161/19490976.2014.983775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu D., Lewis E.D., Pae M., Meydani S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018;9:3160. doi: 10.3389/fimmu.2018.03160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isolauri E., Sütas Y., Kankaanpää P., Arvilommi H., Salminen S. Probiotics: effects on immunity. The American journal of clinical nutrition. 2001;73(2 Suppl):444s–450s. doi: 10.1093/ajcn/73.2.444s. [DOI] [PubMed] [Google Scholar]
- 16.Aziz M., Fatima R., Assaly R. Elevated Interleukin-6 and Severe COVID-19: A Meta-Analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018;24(6):710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baud D., Dimopoulou Agri V., Gibson G.R., Reid G., Giannoni E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva S., Di Nardo G., Ferrari F., Mallardo S., Rossi P., Patrizi G., Cucchiara S., Stronati L. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment. Pharmacol. Ther. 2012;35(3):327–334. doi: 10.1111/j.1365-2036.2011.04939.x. [DOI] [PubMed] [Google Scholar]
- 20.Hu J., Zhang L., Lin W., Tang W., Chan F.K.L., Ng S.C. Review article: Probiotics, prebiotics and dietary approaches during COVID-19 pandemic. Trends Food Sci. Technol. 2021;108:187–196. doi: 10.1016/j.tifs.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozkurt H.S., Quigley E.M. The probiotic Bifidobacterium in the management of Coronavirus: A theoretical basis. Int.. J. Immunopathol. Pharmacol. 2020;34 doi: 10.1177/2058738420961304. 2058738420961304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng J., Wang C.T., Zhang F.S., Qi F., Wang S.F., Ma S., Wu T.J., Tian H., Tian Z.T., Zhang S.L., Qu Y., Liu L.Y., Li Y.Z., Cui S., Zhao H.L., Du Q.S., Ma Z., Li C.H., Li Y., Si M., Chu Y.F., Meng M., Ren H.S., Zhang J.C., Jiang J.J., Ding M., Wang Y.P. Effect of probiotics on the incidence of ventilator-associated pneumonia in critically ill patients: a randomized controlled multicenter trial. Intensive Care Med. 2016;42(6):1018–1028. doi: 10.1007/s00134-016-4303-x. [DOI] [PubMed] [Google Scholar]
- 23.Di Renzo L., Gualtieri P., Pivari F., Soldati L., Attinà A., Leggeri C., Cinelli G., Tarsitano M.G., Caparello G., Carrano E., Merra G., Pujia A.M., Danieli R., De Lorenzo A. COVID-19: Is there a role for immunonutrition in obese patient? J. Transl. Med. 2020;18(1):415. doi: 10.1186/s12967-020-02594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.N. EFSA Panel on Dietetic Products, Allergies, Guidance on the scientific requirements for health claims related to the immune system, the gastrointestinal tract and defence against pathogenic microorganisms, 14(1) (2016) 4369.
- 25.Gombart A.F., Pierre A., Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. 2020;12(1) doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheng K., He S., Sun M., Zhang G., Kong X., Wang J., Wang Y. Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. 2020;11(5):3964–3974. doi: 10.1039/d0fo00518e. [DOI] [PubMed] [Google Scholar]
- 27.Taghizadeh Moghaddam S., Javadi A., Matin A.A. Reduction of bisphenol A by Lactobacillus acidophilus and Lactobacillus plantarum in yoghurt. Int. J. Dairy Technol. 2020;73(4):737–742. [Google Scholar]
- 28.Liu X.Y., Hu Q., Xu F., Ding S.Y., Zhu K. Characterization of Bacillus cereus in Dairy Products in China. Toxins. 2020;12(7) doi: 10.3390/toxins12070454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C., Yu Z., Zhao J., Zhang H., Zhai Q., Chen W. Colonization and probiotic function of Bifidobacterium longum. J. Funct. Foods. 2019;53:157–165. [Google Scholar]
- 30.M. Mohtashami, M. Mohamadi, M. Azimi-Nezhad, J. Saeidi, F.F. Nia, A. Ghasemi, Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors, Biotechnol. Appl. Biochem. n/a(n/a). [DOI] [PubMed]
- 31.Cui Y., Jiang X., Hao M., Qu X., Hu T. New advances in exopolysaccharides production of Streptococcus thermophilus. Arch. Microbiol. 2017;199(6):799–809. doi: 10.1007/s00203-017-1366-1. [DOI] [PubMed] [Google Scholar]
- 32.Gao W., Howden B.P., Stinear T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2018;41:76–82. doi: 10.1016/j.mib.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Losick R.M. Bacillus subtilis: a bacterium for all seasons. Curr. Biol.: CB. 2020;30(19):R1146–R1150. doi: 10.1016/j.cub.2020.06.083. [DOI] [PubMed] [Google Scholar]
- 34.Lu H.Z. Drug treatment options for the 2019-new coronavirus (2019-nCoV) BioSci. Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 35.Rozga M., Cheng F.W., Handu D. Effects of Probiotics in Conditions or Infections Similar to COVID-19 on Health Outcomes: An Evidence Analysis Center Scoping Review. J. Acad. Nutr. Diet. 2020 doi: 10.1016/j.jand.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahooti M., Miri S.M., Abdolalipour E., Ghaemi A. The immunomodulatory effects of probiotics on respiratory viral infections: A hint for COVID-19 treatment? Microb. Pathog. 2020;148 doi: 10.1016/j.micpath.2020.104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.A. Sundararaman, M. Ray, P.V. Ravindra, P.M. Halami, Role of probiotics to combat viral infections with emphasis on COVID-19, Appl. Microbiol. Biotechnol. 16. [DOI] [PMC free article] [PubMed]
- 38.E.A. Coomes, H. Haghbayan, Interleukin-6 in Covid-19: A systematic review andmeta-analysis, Rev. Med. Virol. 9. [DOI] [PMC free article] [PubMed]
- 39.T. Liu, J. Zhang, Y. Yang, L. Zhang, J.J.S.E.J. Yi, The Potential Role of IL-6 in Monitoring Coronavirus Disease 2019, (2020).
- 40.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. New Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao J., Li C., Zhang K., Kang H., Chen W., Gu B. Comparative analysis of laboratory indexes of severe and non-severe patients infected with COVID-19. Clin. Chim. Acta. 2020;509:180–194. doi: 10.1016/j.cca.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.B. Diao, C. Wang, Y. Tan, X. Chen, Y. Liu, L. Ning, L. Chen, M. Li, Y.-P. Liu, G. Wang, Z. Yuan, Z. Feng, Y. Wu, Y. Chen, Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19), 2020. [DOI] [PMC free article] [PubMed]
- 44.Cox A.J., Pyne D.B., Saunders P.U., Fricker P.A. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br. J. Sports Med. 2010;44(4):222–226. doi: 10.1136/bjsm.2007.044628. [DOI] [PubMed] [Google Scholar]
- 45.Wan M.L.Y., Forsythe S.J., El-Nezami H. Probiotics interaction with foodborne pathogens: a potential alternative to antibiotics and future challenges. Crit. Rev. Food Sci. Nutr. 2019;59(20):3320–3333. doi: 10.1080/10408398.2018.1490885. [DOI] [PubMed] [Google Scholar]
- 46.Ling W. C-reactive protein levels in the early stage of COVID-19. Med. Maladies Infectieuses. 2020 doi: 10.1016/j.medmal.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo X., Zhou W., Yan X., Guo T., Wang B., Xia H., Ye L., Xiong J., Jiang Z., Liu Y., Zhang B., Yang W. Prognostic value of C-reactive protein in patients with COVID-19. Clin. Infect. Diseas.: An Official Publication of the Infectious Diseases Society of America. 2020 doi: 10.1093/cid/ciaa641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou Z., Li W., Qian J., Lin B., Nan Y., Lu F., Wan L., Zhao X., Luo A., Liao X., Ren Y., Jin H., Zomaya A. Predicting the Risk of Clinical Deterioration in Patients with Severe COVID-19 Infection Using Machine Learning. SSRN Electron. J. 2020 [Google Scholar]
- 49.Lu J., Hu S., Fan R., Liu Z., Yin X., Wang Q., Lv Q., Cai Z., Li H., Hu Y., Han Y., Hu H., Gao W., Feng S., Liu Q., Li H., Sun J., Peng J., Yi X., Zhou Z., Guo Y., Hou J. ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv. 2020 2020.02.20.20025510. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.