Recently, Aung et al. reported that distribution frequency of angiotensin converting enzyme (ACE) insertion/insertion (II) genotype had a significant impact on coronavirus disease 2019 (COVID-19) mortality.1 Dyslipidemia is one of the most comorbidities among COVID-19 patients, however, the conclusions from published articles on the relationship between dyslipidemia and COVID-19 mortality are still controversial. For instance, several studies found that there was a significant relationship between dyslipidemia and an increased risk for mortality among COVID-19 patients,2, 3, 4 while other studies reported that dyslipidemia was not significantly associated with COVID-19 mortality.5 , 6 Therefore, there is an urgent need to address the relationship between dyslipidemia and COVID-19 mortality by a quantitative meta-analysis. It has been reported that demographical characteristics (age and gender) and certain comorbidities (diabetes mellitus, cardiovascular disease, hypertension, chronic kidney disease and autoimmune diseases, etc.) are well-known modulators that affect the clinical outcomes of COVID-19 patients,7, 8, 9 suggesting that these factors might modulate the relationship between dyslipidemia and COVID-19 mortality. Thus, in this current meta-analysis, risk factors-adjusted effect estimates rather than crude effect estimates were utilized to calculate the pooled effect sizes.
We did this systematic meta-analysis in accordance with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). All potentially eligible articles published between January 1, 2020 and February 26, 2021 were identify in the online databases (PubMed, Web of Science and EMBASE) with the following keywords: “SARS-CoV-2” or “severe acute respiratory syndrome coronavirus 2” or “COVID-19” or “coronavirus disease 2019” or “2019-nCoV” or "2019 novel coronavirus" and “dyslipidemia” or “hyperlipidemia” or “low-density lipoprotein” or “high-density lipoprotein” or “triglycerides” or “total cholesterol”. Reference lists of eligible articles were also searched to look for additional studies. The exposure group was defined as COVID-19 patients with dyslipidemia and the control group was defined as COVID-19 patients without dyslipidemia. The outcome of interest was mortality, which was defined as mortality, death, died, non-survivor, fatality or deceased. All peer-reviewed articles published in English language reporting the risk factors-adjusted effect estimate on the relationship between dyslipidemia and COVID-19 mortality were eligibly selected. Accordingly, we excluded preprints, case reports, review papers, corrections, comments, animal studies and in vitro studies, studies reporting crude effect estimate, studies without sufficient data and studies reporting clinical outcomes as severe/critical illness, intensive care unit admission, invasive mechanical ventilation/intubation or composite outcomes rather than mortality. Essential information including first author, number of COVID-19 patients, gender distribution, age (mean and standard deviation (SD) or median and interquartile range (IQR)), study design, region/country, clinical outcomes, adjusted effect estimates and adjusted variables was extracted from each included study (Table 1 ).
Table 1.
Characteristics of the included studies.
| Author | Country | Cases (n) | Male (%) | Age (years)§ | Study design | Adjusted-effect (95% CI) | Adjusted variables | Clinical outcomes |
|---|---|---|---|---|---|---|---|---|
| Hashemi et al. (PMID: 32585065) | USA | 363 | 55.4% | 63.4 ± 16.5 | Retrospective study | OR: 0.91 (0.46–1.81) | Chronic liver disease, age, obesity, male, cardiac diseases, hypertension, diabetes, pulmonary disorders | Death |
| Pettit et al. (PMID: 32589784) | USA | 238 | 47.5% | 58.5 ± 17 | Retrospective study | OR: 1.7 (0.4–7.0) | Obesity, age, gender, hypertension, diabetes, pulmonary disease, cardiovascular disease, kidney disease, cancer, stroke, venous thromboembolism | Mortality |
| Grasselli et al. (PMID: 32667669) | Italy | 3988 | 79.9% | 63 (56–69) | Retrospective study | HR: 1.25 (1.02–1.52) | Age, men, respiratory support, hypertension, heart disease, type 2 diabetes, malignancy, chronic obstructive pulmonary disease, angiotensin-converting enzyme inhibitor therapy, angiotensin receptor blocker therapy, statin, diuretic, positive end-expiratory pressure at admission, fraction of inspired oxygen at admission, arterial partial pressure of oxygen/fraction of inspired oxygen at admission | Mortality |
| Tartof et al. (PMID: 32783686) | USA | 6916 | 45% | 49.1 ± 16.6 | Retrospective study | RR: 1.47 (1.02–2.11) | Body mass index, age, sex, race/ethnicity, smoking, metastatic tumor/cancer, myocardial infarction, other immune condition, organ transplant, congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, renal disease, hypertension, asthma, diabetes mellitus status, time | Death |
| Czernichow et al. (PMID: 32815621) | France | 5795 | 65.4% | 59±14 | Retrospective study | OR: 0.97 (0.74–1.27) | Body mass index, age, diabetes, hypertension, sleep apnea, chronic kidney disease, heart failure, malignancies, history of smoking, sex | Mortality |
| Nimkar et al. (PMID: 32838205) | USA | 327 | 55.7% | 71 (59–82) | Retrospective study | OR: 1.4 (0.8–2.2) | Race, chronic kidney disease in addition to six essential covariates (age, sex, race, hypertension, diabetes, cardiac disease) | Mortality |
| Giorgi-Rossi et al. (PMID: 32853230) | Italy | 2653 | 50.1% | 72±24 | Prospective study | HR: 1.4 (0.9–2.2) | Age, sex | Death |
| Esme et al. (PMID: 32871002) | Turkey | 16,942 | 49% | 71.2 ± 8.5 | Retrospective study | OR: 0.77 (0.64–0.93) | Gender, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, atrial fibrillation, chronic kidney disease, dementia, depression, malnutrition | Mortality |
| Yan et al. (PMID: 32949175) | China | 1103 | 48.6% | 63 (51–71) | Retrospective study | HR: 1.91 (0.46–7.99) | Age, male, diabetes, hypertension, chronic obstructive pulmonary disease, chronic heart diseases, chronic kidney diseases, chronic liver diseases, cerebrovascular diseases, tumor, C-reactive protein, d-dimer | Mortality |
| Ioannou et al. (PMID: 32965502) | USA | 10,131 | 91% | 63.6 ± 16.2 | Longitudinal corhot study | HR: 0.96 (0.83–1.11) | All sociodemographic characteristics, comorbid conditions, symptoms | Mortality |
| Ghany et al. (PMID: 33024960) | USA | 400 | 40% | 72±8 | Retrospective study | RH: 0.99 (0.99–1.00) | Age, gender, charlson score | Death |
| Graziani et al. (PMID: 33053774) | Spain | 14,339 | 49% | 66±15 | Retrospective study | OR: 1.03 (0.81–1.31) | Chronic obstructive pulmonary disease, sex, age, heart failure, high blood pressure, stroke, arrythmia, ischemic heart disease, diabetes, sleep apnea, pulmonary thromboembolism, smoking | Death |
| An et al. (PMID: 33127965) | Korea | 10,237 | 39.9% | 44.97±19.79 | Nationwide cohort study | HR: 0.89 (0.66–1.20) | Age, sex, income level, residence, household type, disability, symptom, infection route | Death |
| Zhang et al. (PMID: 33122929) | China | 98 | 59.2% | 63.9 ± 1.4 | Retrospective study | OR: 2.94 (1.22–7.12) | Age, gender, lymphocyte count, glycated hemoglobin, hypersensitive C-reactive protein, N-terminal brain natriuretic propeptide, creatinine | Mortality |
| Shah et al. (PMID: 33169090) | USA | 487 | 56.1% | 68±17 | Retrospective study | OR: 1.36 (0.83–2.21) | Age, gender, patient admitted from home, hypertension, cardiomyopathy, atrial fibrillation, chronic obstructive pulmonary disease, cerebrovascular accident, diabetes mellitus, acute kidney injury, initial chest x-ray/computed tomography findings, dyspnea in emergency department noted as positive | Mortality |
| Tomasoni et al. (PMID: 33179839) | Italy | 692 | 69.5% | 67.4 ± 13.2 | Retrospective study | HR: 0.82 (0.47–1.44) | Age, sex, heart failure, hypertension, atrial fibrillation, coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease, oxygen saturation, arterial partial pressure of oxygen/fraction of inspired oxygen, hemoglobin, lymphocytes count, estimated glomerular filtration rate, C-reactive protein on admission, troponin | Death |
| Loffi et al. (PMID: 33229434) | Italy | 1252 | 63.7% | 64.7 ± 15.5 | Retrospective study | HR: 0.94 (0.63–1.41) | Sex, left ventricular ejection fraction < 35%, cerebrovascular disease, atrial fibrillation, diabetes mellitus, hypertension, coronary artery disease, chronic kidney disease, age | Death |
| Rossi et al. (PMID: 33222020) | Italy | 590 | 67.6% | 76.2 (68.2–82.6) | Retrospective study | HR: 1.108 (0.859–1.431) | Age, gender, temperature, arterial partial pressure of oxygen / fraction of inspired oxygen, lactate dehydrogenase, C-reactive protein, white blood cell count, lymphocytes rate, cardiovascular disease, diabetes, atrial fibrillation, chronic obstructive pulmonary disease, chronic kidney disease, stroke, malignancy, 3 or more comorbidities, angiotensin-converting enzyme inhibitor, angiotensin receptor blockers, calcium-channel blockers, alpha blockers, diuretics, beta blockers | Mortality |
| Rosenthal et al. (PMID: 33301018) | USA | 35,302 | 53.4% | 63.6 ± 17.7 | Retrospective study | OR: 1.11 (1.03–1.19) | Age, sex, race, payer type, admission point of origin, hospital region, hospital beds, hospital teaching status, statin, vitamin C, zinc, angiotensin-converting enzyme inhibitor, b blocker, calcium channel blocker, hydroxychloroquine and azithromycin use, sepsis, acute kidney failure, hypokalemia, hyperkalemia, hyponatremia, acidosis, acute liver damage, neurological disorder, myocardial infarction, congestive heart failure, cerebrovascular disease, chronic pulmonary disease, dementia, diabetes, any malignant neoplasm, metastatic solid tumor, hemiplegia, acquired immunodeficiency syndrome, hypertension | Mortality |
| Ozyilmaz et al. (PMID: 33322097) | Turkey | 105 | 72.4% | 45 (20–87) | Retrospective study | OR: 4.060 (0.011–1555.792) | Troponin I, C-reactive protein, lymphocyte count, shortness of breath, hypertension, diabetes mellitus, coronary artery disease | Mortality |
| Lohia et al. (PMID: 33453090) | USA | 1871 | 51.6% | 66 (54–75) | Retrospective study | OR: 0.97 (0.76–1.23) | Age, sex, race, smoking, body mass index, insurance and comorbidities which include coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, asthma, chronic kidney disease, end-stage renal disease on dialysis, any malignancy, any liver disease, history of previous stroke, hypertension, diabetes | Mortality |
| Gupta et al. (PMID: 33461499) | USA | 473 | 45.5% | 70 (61–80) | Retrospective study | OR: 1.28 (0.75–2.19) | Race, age, sex, coronary artery disease, diabetes, hypertension, chronic obstructive pulmonary disease/asthma, autoimmune diseases history of cancer, immunocompromised, congestive heart failure, chronic kidney disease with dialysis, chronic kidney disease without dialysis, end-stage renal disease with dialysis | Mortality |
| Mayer et al. (PMID: 33496668) | Spain | 23,844 | 42.3% | 49.93±19.4 | Retrospective study | OR: 1.19 (1.03–1.39) | Age, sex | Death |
| Muhammad et al. (PMID: 33538998) | USA | 200 | 60.5% | 58.9 ± 15.1 | Retrospective study | OR: 2.12 (0.94–4.77) | Age, hypertension, coronary artery disease, chronic kidney disease, history of stroke, oxygen saturation, creatinine, blood urea nitrogen, creatine phosphokinase, troponin, procalcitonin, lactic acid, lactate dehydrogenase, C-reactive protein, initial d-dimer, ferritin, highest d-dimer | Mortality |
| Yoshida et al. (PMID: 33546750) | USA | 776 | 47.3% | 60.5 ± 16.1 | Retrospective study | OR: 0.95 (0.53–1.71) | Age, sex, hospital site, the charlson comorbidity index | Death |
| Girardin et al. (PMID: 33550849) | USA | 4446 | 58.1% | 62±18 | Retrospective study | HR: 0.92 (0.79–1.06) | Age, ethnic minority, male sex, low income, smoking, obesity, chronic obstructive pulmonary disease, asthma, sleep apnea, hypertension, diabetes, peripheral artery disease, coronary artery disease, autoimmune disease, cancer | Mortality |
| Wargny et al. (PMID: 33599800) | France | 2796 | 63.7% | 67.9 ± 13.2 | Retrospective study | OR: 1.15 (0.95–1.40) | Age | Death |
Note:
The values are presented as mean ± standard deviation or median (interquartile range, IQR); USA, the United States of America; CI, confidence interval; OR, odds ratio; RR, risk ratio; RH, relative hazard; HR, hazard ratio.
We utilized Stata (version 12.1) for all statistical analyses. The pooled effect estimate and its 95% confidence interval (CI) were computed using a random-effects model. Inter-study heterogeneity was investigated using the cochrane Q test and I 2 statistic, P < 0.1 or I 2 > 50% shows a statistically significant heterogeneity. The statistical stability of the overall effects was assessed using leave-one-out sensitivity analysis. The risk of publication bias was evaluated using Begg's test. Subgroup analyses were carried out by sample size, age, male percentage, study design and effect estimate. Two-tailed P-value < 0.05 was considered statistically significant.
Initial search yielded 2608 articles. After screening eligible articles according to inclusion and exclusion criteria, a total of twenty-seven studies composing of 146,364 cases were enrolled into this quantitative meta-analysis. Among the included studies, twenty-four studies were retrospective, one was prospective, one was longitudinal cohort study and one was nationwide cohort study. The sample sizes across the eligible studies ranged from 98 to 35,302. There were sixteen odds ratio (OR)-reported studies, nine hazard ratio (HR)-reported studies, one risk ratio (RR)-reported study and one relative hazard (RH)-reported study.
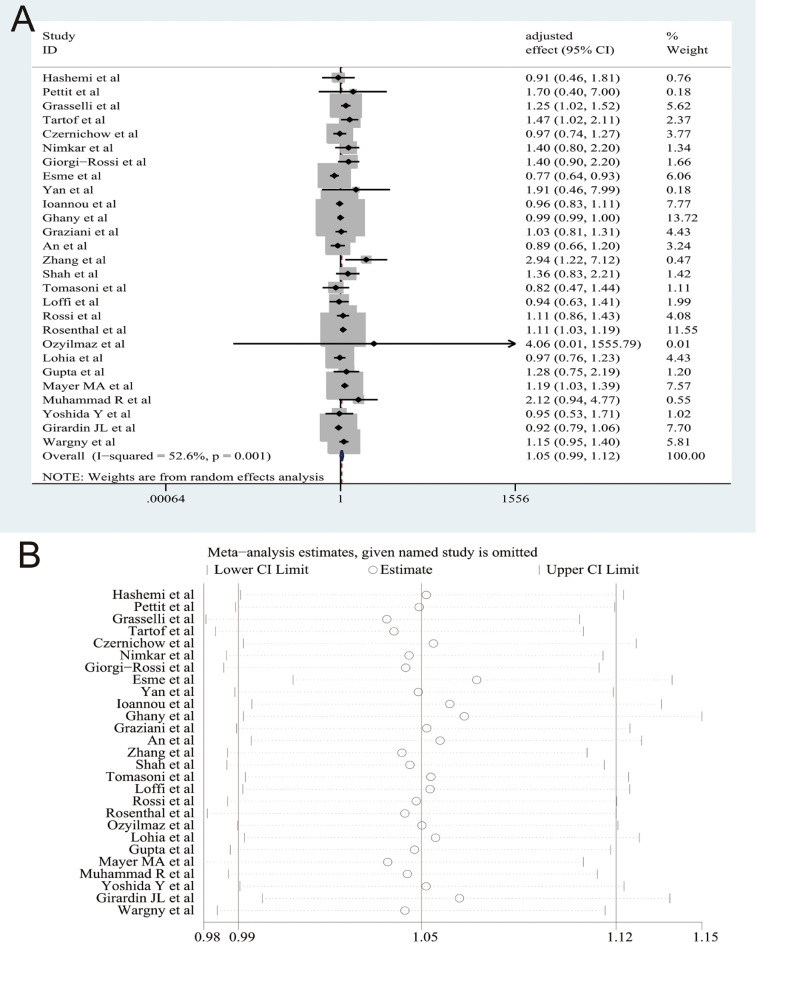
The results of our pooled analysis are presented in Fig. 1 A, which indicates that there was no significant relationship between dyslipidemia and COVID-19 morality (pooled effect size = 1.05, 95% CI [0.99–1.12], P = 0.100; I 2 = 52.6%, random-effects model). Sensitivity analysis by deleting each study one by one demonstrated that our results were stable (Fig. 1B). When we limited dyslipidemia to hyperlipidemia, there was no significant relationship between hyperlipidemia and COVID-19 mortality (pooled effect size = 1.03, 95% CI [0.95–1.12]). We still observed no significant relationship between dyslipidemia and COVID-19 mortality in the subgroup analyses by age (pooled effect size = 1.08, 95% CI [0.99–1.18] for < 65 years old and pooled effect size = 1.02, 95% CI [0.93–1.12] for ≥ 65 years old), male percentage (pooled effect size = 1.04, 95% CI [0.96–1.13] for < 55% and pooled effect size = 1.08, 95% CI [0.97–1.20] for ≥ 55%), study design (pooled effect size = 1.06, 95% CI [0.99–1.14] for retrospective study), sample size (pooled effect size = 1.09, 95% CI [0.96–1.24] for < 1500 cases and pooled effect size = 1.04, 95% CI [0.96–1.14] for ≥ 1500 cases), and effect estimates (OR = 1.08, 95% CI [0.98–1.20] and HR = 1.02, 95% CI [0.92–1.13]). Begg's test indicated that there was no obvious publication bias (P = 0.505).
Fig. 1.
(A) The forest plots demonstrated that dyslipedemia was not significantly associated with the risk of mortality among patients with coronavirus disease 2019 (COVID-19) on the basis of twenty-seven studies with 146,364 cases; (B) Sensitivity analysis by omitting individual study one by one indicated that the results were stable and robust.
This meta-analysis has several limitations that need to be mentioned:1 most of the included studies were from USA, which limits its wider applicability of the present findings;2 the majority of studies were retrospective, thus further well-designed studies with more prospective researches are required to verify our results;3 although the pooled effect estimate was calculated on the basis of adjusted effects, the adjusted variables are not completely consistent across the included studies;4 only one included study explicitly states the specific type of dyslipidemia as total cholesterol, additional studies does not explicitly states the specific type of dyslipidemia such as abnormal levels of low-density lipoprotein, high-density lipoprotein, triglycerides and total cholesterol. Further studies should focus on the relationship between specific type of dyslipidemia and COVID-19 mortality when more data are available;5 the detailed information on medications for patients with pre-existing dyslipidemia is not available presently, thus we could not address the effects of medications on the relationship between dyslipidemia and COVID-19 mortality.
In conclusion, our current study based on adjusted effect sizes demonstrated that dyslipidemia was not significantly associated with COVID-19 mortality. Further well-designed studies with large sample sizes are warranted to confirm our findings.
Declaration of Competing Interest
The authors declare that they have no any potential conflict of interest regarding this submitted manuscript.
Acknowledgments
Acknowledgments
We would like to thank Li Shi, Ying Wang, Jian Wu, Peihua Zhang, Yang Li and Wenwei Xiao (All are from Department of Epidemiology, School of Public Health, Zhengzhou University) for their kind help in searching articles and collecting data, and valuable suggestions for data analysis.
Author contributions
Haiyan Yang and Yadong Wang designed the study. Hongjie Hou and Jie Xu performed literature search. Hongjie Hou and Haiyan Yang performed data extraction. Xuan Liang, Haiyan Yang, Hongjie Hou and Jie Xu performed statistical analyses. Haiyan Yang, Hongjie Hou and Yadong Wang wrote and reviewed the manuscript. All the authors approved the final version of the manuscript.
Funding
This study was supported by grants from National Natural Science Foundation of China (grant number 81973105), Key Scientific Research Project of Henan Institution of Higher Education (grant number 21A330008) and Joint Construction Project of Henan Medical Science and Technology Research Plan (grant number LHGJ20190679). The funders have no role in the data collection, data analysis, preparation of manuscript and decision to submission.
References
- 1.Aung A.K., Aitken T., Teh B.M., Yu C., Ofori-Asenso R., Chin K.L., et al. Angiotensin converting enzyme genotypes and mortality from COVID-19: an ecological study. J. Infect. 2020;81(6):961–965. doi: 10.1016/j.jinf.2020.11.012. PubMed PMID: 33197472. Pubmed Central PMCID: 7666537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern. Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. PubMed PMID: 32667669. Pubmed Central PMCID: 7364371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer M.A., Vidal-Alaball J., Puigdellivol-Sanchez A., Marin Gomez F.X., Leis A., Mendioroz Pena J. Clinical characterization of patients with COVID-19 in primary care in catalonia: retrospective observational study. JMIR Public Health Surveill. 2021;7(2):e25452. doi: 10.2196/25452. PubMed PMID: 33496668. Pubmed Central PMCID: 7871981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw. Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.29058. PubMed PMID: 33301018. Pubmed Central PMCID: 7729428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An C., Lim H., Kim D.W., Chang J.H., Choi Y.J., Kim S.W. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci. Rep. 2020;10(1):18716. doi: 10.1038/s41598-020-75767-2. PubMed PMID: 33127965. Pubmed Central PMCID: 7599238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graziani D., Soriano J.B., Del Rio-Bermudez C., Morena D., Diaz T., Castillo M., et al. Characteristics and prognosis of COVID-19 in patients with COPD. J. Clin. Med. 2020;9(10) doi: 10.3390/jcm9103259. PubMed PMID: 33053774. Pubmed Central PMCID: 7600734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020:1–12. doi: 10.1159/000512592. PubMed PMID: 33296901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang X., Shi L., Wang Y., Xiao W., Duan G., Yang H., et al. The association of hypertension with the severity and mortality of COVID-19 patients: evidence based on adjusted effect estimates. J. Infect. 2020;81(3):e44–ee7. doi: 10.1016/j.jinf.2020.06.060. PubMed PMID: 32593655. Pubmed Central PMCID: 7315979 conflicts of interest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang H., Xu J., Liang X., Shi L., Wang Y. Autoimmune diseases are independently associated with COVID-19 severity: evidence based on adjusted effect estimates. J. Infect. 2020 doi: 10.1016/j.jinf.2020.12.025. PubMed PMID: 33383087. [DOI] [PMC free article] [PubMed] [Google Scholar]