Abstract
Introduction
Articles published in the literature report neurological manifestations or “complications” of SARS-CoV-2 infection and conclude that the different neurological manifestations are relatively similar, but with different frequencies. This study aimed to determine the frequency of neurological manifestations of COVID-19 and to identify which are associated with mortality.
Methods
We performed a retrospective study of all patients diagnosed with SARS-CoV-2 infection by RT-PCR at Hospital 1° de Octubre, in Mexico, from the beginning of the pandemic to 22 December 2020. A total of 561 patients were identified, 370 of whom presented neurological manifestations.
Results
The global mortality rate was 37.8% (140/370), increasing to 92.4% among intubated patients (135/146). Of the 370 patients included, approximately 20% of neurological symptoms (headache, neurological impairment, anosmia, ageusia) accounted for 80% of cases of neurological manifestations.
Conclusions
At our hospital, 80% of the patients with neurological manifestations of COVID-19 presented headache, neurological impairment, ageusia, and/or anosmia. Neurological impairment at admission or before arriving at hospital was identified as a risk factor for mortality.
Keywords: COVID-19, Coronavirus, SARS-CoV-2, Neurological manifestations, Prevalence, Mortality
Abstract
Introducción
Los artículos se han referido a manifestaciones neurológicas o «complicaciones» producidas por el SARS-CoV-2, concluyendo que las diferentes manifestaciones neurológicas han sido relativamente similares, pero con diferentes proporciones. Por ello, el objetivo de este estudio es cuantificar las manifestaciones neurológicas en COVID-19, e identificar cuál está asociada a la muerte.
Material y métodos
Se realizó un estudio retrospectivo con todos los pacientes del Hospital «1.° de Octubre» infectados por COVID-19, y detectados mediante RT-PCR, desde el inicio de la pandemia hasta el 22 de diciembre de 2020, donde se obtuvieron un total de 561 casos.
Resultados
La tasa de mortalidad global fue del 37,8% (140/370), mientras que para los pacientes intubados fue del 92,4% (135/146). De los 370 pacientes obtenidos, aproximadamente el 20% de las manifestaciones (cefalea, deterioro neurológico, anosmia, ageusia) ocurrieron con una frecuencia del 80% de manifestaciones neurológicas.
Conclusiones
Cefalea, afección neurológica, ageusia y anosmia representaron el 80% de las manifestaciones neurológicas en el Hospital «1.° de Octubre». El deterioro neurológico al ingreso al hospital o antes de llegar fue un factor de riesgo de muerte.
Palabras clave: COVID-19, Coronavirus, SARS-CoV-2, Manifestaciones neurológicas, Prevalencia, Mortalidad
The coronavirus is made up of a group of enveloped RNA viruses that cause the development of respiratory, enteric, gastrointestinal, liver and neurological diseases. SARS-CoV, identified in 2003 as a zoonotic infection in Guangdong province, China, in 2003 and MERS-CoV, identified in Jeddah, Saudi Arabia in the year 2012, belong to the beta coronavirus genus, where both agents are responsible for severe respiratory conditions. In late 2019, a group of patients with severe pneumonia was reported in Wuhan, China; identifying a coronavirus as the causal agent. This disease was named “2019-nCov” or “COVID-19”. Given its genetic similarity to the SARS species, the causal agent was named SARS-CoV-2.1, 2
In Mexico, the first case of COVID-19 was detected on February 27th, 2020 and as of January 1st, 2021 there are approximately 1.40 million cases, out of which 1 million have recovered and 127 thousand have resulted in death.3
Articles have referred to neurological manifestations or “complications” produced by SARS-CoV-2, concluding that the different neurological manifestations have been relatively similar, but with different ratios: Ling et al. mentioned symptoms in a retrospective study of 214 patients, where the manifestations were classified in 4 main groups: acute cerebrovascular disease, altered consciousness, peripheral nervous system affection and muscular manifestations.4, 5
Although much remains to be learned about the pathophysiology of COVID-19 in the central nervous system, in animal models (murine) it has been discovered that the coronavirus OC43 has a selective tropism for neurons and is capable of using the axonal transport system as a means of neuron-to-neuron propagation, and they are also capable of inducing both acute and persistent infection in neuronal lineages, oligodendrocytes and neuroglia in humans.5
In experimental studies with SARS-CoV-infected mice, it has been observed that this virus is able to enter the central nervous system through the olfactory nerves, following its path toward the thalamus and brainstem. Likewise, higher inoculum was found in the brainstem and brain compared to the amounts in lung tissue, which implies that the central nervous system is affected more frequently than the lung and it may have greater implications in terms of mortality rate.6, 7, 8, 9
Although some articles have concluded that the neurotropism of the coronavirus is common,10, 11 they do not always grant due relevance to neurological manifestations, given that they sometimes go unnoticed or because general, respiratory, renal, cardiovascular manifestations, among others, tend to receive more attention from the clinician in connection to the early mortality that they develop; however, paying attention to them could provide us with a benchmark to identify patients at risk of intubation or death. This article presents the main neurological manifestations exhibited by patients at the “1 de Octubre” Hospital.
Methodology
A retrospective study was carried out with all the patients from the “1 de Octubre” Hospital who were infected with COVID-19, and detected through RT-PCR, from the beginning of the pandemic until December 22nd, 2020, where a total of 561 cases were obtained. Electronic files were reviewed, and the cases included in the study were only those who exhibited at least one neurological manifestation/comorbidity (SAH or DM2) during the course of the disease, where a total of 370 patients were obtained. Patients with psychiatric comorbidities were ruled out.
Statistical analysis
The 370 patients were used to take the count in order to fill out in each table, where only the mean and standard deviation were used for the age since it is the only continuous variable, and contingency tables were used for categorical variables. In Fig. 1 , a Pareto chart was made using Excel. In Table 1 , only Chi-square was used to differentiate demographic groups (Sex, Age, Comorbidities, etc.), which was compared against VMA and death. In Table 2 , a binary logistic regression was performed for the p values and the odds ratio with their corresponding 95% confidence intervals (Table 3 ), where the statistical software used was “R” through the RStudio IDE version 4.0.3.
Figure 1.
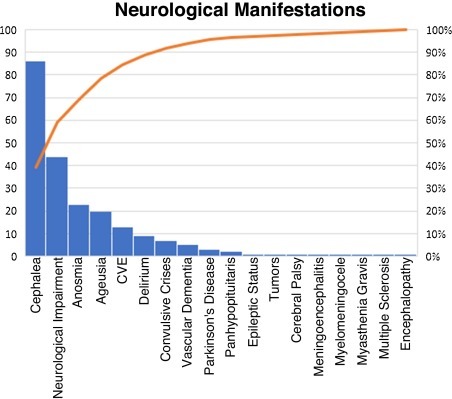
Pareto diagram of neurological manifestations.
Table 1.
Demographic characteristics of NM.
| NM | Age | χ2 | |
|---|---|---|---|
| >60 years | <60 years | ||
| 70 (8) | 41 (11) | ||
| VMA | 103 | 43 | <0.001 |
| Death | 101 | 39 | <0.001 |
| Gender (frequency) | Males | Females | |
| Total | 216 | 154 | 0.001 |
| VMA | 92 | 55 | 0.001 |
| Death | 85 | 55 | <0.05 |
| Comorbidity (frequency) | HAS | DM2 | |
| Total | 240 | 191 | <0.05 |
| VMA | 94 | 79 | 0.25 |
| Death | 92 | 76 | 0.21 |
NM = neurological manifestations.
VMA = Ventilatory Mechanical Assistance.
= Chi-squared. SD = standard deviation.
Table 2.
Neurological manifestations and mortality.
| Manifestations | Frequency | Gender | Age | SAH | MD2 | VMA | Dead | Dead | p-value |
|---|---|---|---|---|---|---|---|---|---|
| with NM | w/out NM | BLR | |||||||
| Cephalea | 86 | M (53) F (33) | 58 (16) | 47 | 37 | 36 | 34 | 106 | 0.71 |
| Neurological impairment | 44 | M (26) F (18) | 65 (17) | 25 | 19 | 34 | 33 | 107 | <0.001 |
| Anosmia | 23 | M (16) F (7) | 52 (17) | 4 | 9 | 9 | 9 | 131 | 0.70 |
| Ageusia | 20 | M (13) F (7) | 55 (19) | 3 | 8 | 8 | 8 | 132 | 0.60 |
| CVE | 13 | M (8) F (5) | 73 (8) | 10 | 8 | 7 | 7 | 133 | 0.17 |
| Delirium | 9 | M (5) F (4) | 63 (15) | 6 | 5 | 5 | 3 | 137 | 0.97 |
| Convulsive crises | 7 | M (5) F (2) | 38 (21) | 3 | 3 | 2 | 2 | 138 | 0.37 |
| Vascular dementia | 5 | M (3) F (2) | 74 (16) | 3 | 3 | 3 | 3 | 137 | 0.30 |
| Parkinson's disease | 3 | M (2) F (1) | 77 (12) | 2 | 2 | 2 | 2 | 138 | 0.75 |
| Panhypopituitarism | 2 | M (2) F (0) | 43 (22) | 2 | 0 | 2 | 0 | – | – |
| Epileptic status | 1 | M (1) F (0) | 29 | 0 | 0 | 0 | 0 | – | – |
| Tumors | 1 | M (1) F (0) | 62 | 1 | 0 | 1 | 1 | – | – |
| Cerebral palsy | 1 | M (1) F (0) | 51 | 0 | 0 | 1 | 1 | – | – |
| Meningoencephalitis | 1 | M (0) F (1) | 18 | 0 | 0 | 1 | 1 | – | – |
| Myelomeningocele | 1 | M (0) F (1) | 29 | 0 | 0 | 1 | 1 | – | – |
| Myasthenia gravis | 1 | M (0) F (1) | 65 | 0 | 0 | 0 | 0 | – | – |
| Multiple sclerosis | 1 | M (0) F (1) | 18 | 0 | 0 | 1 | 1 | – | – |
| Encephalopathy | 1 | M (0) F (1) | 64 | 0 | 0 | 0 | 0 | – | – |
SAH = systemic arterial hypertension, MD2 = Mellitus Diabetes 2, VMA = Ventilatory Mechanical Assistance, NM = neurological manifestations, = mean, SD = standard deviation, χ2 = Chi-squared, BLR = binary logistic regression.
Table 3.
Risk ratio of neurological deterioration and death.
| Manifestation | Frequency | p-value | OR | 95% CI |
|
|---|---|---|---|---|---|
| BLR | Death | Inf | Sup | ||
| Neurological impairment | 44 | <0.001 | 6.41 | 2.45 | 16.74 |
Ethical aspects
The current study regarding the patient's confidentiality is adjusted to the General Health Law Regulations of Mexico, promulgated in 1986, and to the Declaration of Helsinki in 1975 also modified in 1988.
Results
The overall mortality rate was 37.8% (140/370), while for the intubated patients it was 92.4% (135/146).
Out of the 370 patients obtained, approximately 20% of the manifestations (cephalea, neurological deterioration, anosmia, ageusia) occurred with an 80% frequency of neurological manifestations, thus conforming to the Pareto principle (Fig. 1).
Neurological deficit was understood as synonymous with loss of consciousness, according to the medical record annotations, where said deficit was first experienced before arriving at the hospital or at the emergency room.
The reported cephalea was holocranial-type, hyposmia was not reported, only anosmia and ageusia. CVEs were ischemic except for a multi-infarct/hemorrhagic dementia case, and in general, they arrived due to CVE and were later infected with COVID-19 at the hospital. Delirium and acute confusional syndrome were treated as synonymous, where each delirium diagnosis was corroborated through psychiatry, patients with vascular dementia were admitted to the hospital due to dysfunction of said pathology, becoming infected with COVID-19 during their stay at the hospital, patients with Parkinson's disease were admitted due to tremors or bradykinesia, becoming infected during their hospital stay, the same happened with patients with panhypopituitarism who had symptoms consistent with their pathology, the patient with epileptic status was a neurosurgery resident who had been suffering seizures for the last 6 yeas, but not epileptic status, which was exhibited at the hospital and coincided with the COVID-19 infection, the tumor case was a left temporal glioblastoma, it became infected during the hospital stay, the same happened to the patients with the rest of the diseases, where the encephalopathy was diagnosed as “multifactorial”.
Although manifestation frequencies are interesting, it is important to put into context the gender, age, most common comorbidities, at least in general, and if there was Ventilatory Mechanical Assistance (VMA) or if it resulted in death (Table 1).
On the other hand, it was also deemed important to observe the specific context of neurological manifestations and the frequencies/averages of demographic characteristics, thus manifestations were compared and risk measures were correspondingly obtained (Table 2, Table 3).
Discussion
Similarly to our results, Guan et al. in a retrospective study with 1099 patients reported cephalea as one of the main neurological manifestations in patients with COVID-19, with 150 (13.6%) patients. Another coincidence were CVEs with 15 (1.4%) patients, unexpectedly only 25 (2.3%) patients needed VMA and 15 (1.4%) deaths were reported.12
Huang et al. in a cohort study of 49 patients only reported cephalea in 3 (8%) of the patients without reporting other neurological manifestations, with fever as the most frequent, where 6 (15%) out of 41 patients died.13
On the other hand, Yanan Li et al. reported in a retrospective study of 219 patients with COVID-19 from a single center, a frequency of 10 (4.6%) patients with acute CVEs, 9 ischemic cases and 1 hemorrhagic stroke, whose data is similar to our study, where no other manifestations were reported, and the mortality rate was 54.5%.14
In addition, a case15 and a series of cases16 were reported in connection to COVID-19 and Guillian-Barré where manifestations such as tetraplegia, diplegia/facial weakness and ataxia were reported, but none of this was found in our patients.
As far as neurodegenerative diseases, patients with Parkinson's disease, Alzheimer's and multiple sclerosis with coronavirus in the brain have been found, but also in healthy ones,17 in fact, it has been shown that some coronaviruses such as OC43-CoV can induce degeneration and cause cell death.18, 19, 20 Despite this, the prevalence of neurodegenerative diseases appears to be low in our hospital. In the same way, it is worth noting that some authors consider that SARS-CoV-2 can chronically affect the CNS and this inflammatory response can accelerate the subclinical mechanisms of neurodegenerative diseases,21 which may be the reason why, in the case of our patients, the inflammation caused by the comorbidities of systemic arterial hypertension and diabetes coincided with Parkinson's disease patients who exhibited such diseases.
According to Kuroda Naoto (2020), there is no association between convulsive crises/epilepsy and COVID-19, and perhaps our data support this evidence due to the low frequency we had in this regard.22
As far as manifestations of anosmia/hyposmia and ageusia/hypogeusia, in a prospective study of 355 patients it was determined that exhibiting hyposmia and cough was associated with a 5.46 odds ratio of getting a positive RT-PCR. Lastly, it was observed that a total of 138 cases (64.1%) and 114 cases (53%) had subjective hyposmia and hypogeusia, respectively. 85.4% of these patients recovered olfactory function within the first 14 days of the onset of symptoms.23 In contrast to our study, the frequency of anosmia and ageusia was much lower and closer to the first COVID-19 studies.24
After analyzing the distinctive demographic characteristics by gender, there is a higher prevalence of neurological manifestations in men. 92% (85/92) of male patients requiring VMA died; in contrast, 100% of women who needed VMA died, which does not fully coincide with Viveiros et al., 2020 where they concluded that men are more likely to have a greater severity of COVID-19 compared to women, despite the fact that 100% of intubated patients died compared to 92%, although this does not seem to be statistically significant, it should be noted that women died less frequently (although there could be some confusion due to the lower frequency of women in the study in relation to men), and this may be possibly due to the innate immune system's participation, such as the classical renin-angiotensin system, where it seems that women develop a rapid and aggressive innate immunity while men have an attenuated antiviral response, which confers a greater susceptibility to serious disease.25
On the other hand, it is important to point out that the frequency of neurological deterioration has a high mortality (77%), if we recall that these patients arrive unconscious or suffer from said deficit in the emergency room, it may lead to believe think that they were COVID-19 patients who exhibited symptoms for a long time and who did not want to go to the hospital until the last moment, although this may be mistaken for a severe complication that was not reported due to the lack of information from the unconscious patient and from family members who were more concerned at that time with the relative's survival than giving information to the clinician.
On a final note, when comparing MD2 patients with VMA and Death (Table 2), it seems that most of them coincide, perhaps there is a pro-inflammatory component here that affects the health of COVID-19 patients.
Conclusion
The mortality rate was 37.8% (140/370), where 92.4% (135/146) of intubated patients died. Of the 571 patients with COVID-19, 370 exhibited at least 1 neurological manifestation/comorbidity out of which Cephalea, Neurological Impairment, Ageusia and Anosmia represented 80% of the neurological manifestations at the “1 de Octubre” Hospital. Neurological deterioration upon admission to the hospital or before arriving at the hospital was a risk factor for death.
Funding
None declared.
Conflict of interest
The authors declare there is no conflict of interest.
Acknowledgement
Dr Carlos Castillo Rangel, Dr José Manuel Vivero Utrera and Dr Jaime Ordoñez Granja for all your support in my medical training. The authors express their solidarity to the victims of COVID-19.
Contributor Information
Nerosurgical Group:
Cortez Saldias Wilmar, Garza Montalvo Luis Jesús, Deoca Mora Thania, Marín González Victor, Morales Sánchez Juan Manuel, Riley Moguel Ámbar Elizabeth, Urcid Garcia Luis Alberto, Rojo Noriega Fernando Arturo, Zúñiga Córdova Jonathan Samuel, Ferrufino Mejia Bill Roy, Juárez Jiménez Gustavo Alan, Mejía Frías Alberto Antonio, Luna Álvarez Guillermo, and Landeta Amezcua Carlos
Appendix A. Neurosurgical Group
Cortez Saldias Wilmar, Garza Montalvo Luis Jesús, De oca Mora Thania, Marín González Victor, Morales Sánchez Juan Manuel, Riley Moguel Ámbar Elizabeth, Urcid Garcia Luis Alberto, Rojo Noriega Fernando Arturo, Zúñiga Córdova Jonathan Samuel, Ferrufino Mejia Bill Roy, Juárez Jiménez Gustavo Alan, Mejía Frías Alberto Antonio, Luna Álvarez Guillermo, Landeta Amezcua Carlos
References
- 1.Chafekar A., Fielding B.C. MERS-CoV: understanding the latest human coronavirus threat. Viruses. 2018;10:93. doi: 10.3390/v10020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. Epub 2020 Feb 7. PMID: 32035028; PMCID: PMC7154514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carod-Artal F.J. Complicaciones neurológicas por coronavirus y COVID-19. Rev Neurol. 2020;70:311–322. doi: 10.33588/rn.7009.2020179. [DOI] [PubMed] [Google Scholar]
- 6.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.To K.F., Lo A.W. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2) J Pathol. 2004;203:740–743. doi: 10.1002/path.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumino L., Álvarez H.J.M., Arturi J., Ciarrocchi N.M., Díaz M.F., Domeniconi G. COVID-19: Fisiopatología y manifestaciones neurológicas Revisión narrativa. RATI. 2020;37:24–28. [Google Scholar]
- 12.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G. Guillain–Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020;76:5. doi: 10.1016/j.jocn.2020.04.062. S0967586820308821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matías-Guiu J., Gomez-Pinedo U., Montero-Escribano P., Gomez-Iglesias P., Porta-Etessam J., Matias-Guiu J.A. ¿Es esperable que haya cuadros neurológicos por la pandemia por SARS-CoV-2? Neurología. 2020;35:170–175. doi: 10.1016/j.nrl.2020.03.001. S0213485320300657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favreau D.J., Desforges M., St-Jean J.R., Talbot P.J. A human coronavirus OC43 variant harboring persistence-associated mutations in the S glycoprotein differentially induces the unfolded protein response in human neurons as compared to wild-type virus. Virology. 2009;395:255–267. doi: 10.1016/j.virol.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favreau D.J., Meessen-Pinard M., Desforges M., Talbot P.J. Human coronavirus-induced neuronal programmed cell death is cyclophilin D dependent and potentially caspase dispensable. J Virol. 2012;86:81–93. doi: 10.1128/JVI.06062-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meessen-Pinard M., le Coupanec A., Desforges M., Talbot P.J. Pivotal role of receptor-interacting protein kinase 1 and mixed lineage kinase domain-like in neuronal cell death induced by the human neuroinvasive coronavirus OC43. J Virol. 2016;91 doi: 10.1128/JVI.01513-16. e01513–16, 10.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres L.F.A., Taype K.R.P. Manifestaciones neurológicas de COVID-19: Una revisión de la literatura. Neurol Argentina. 2020;12:271–274. doi: 10.1016/j.yebeh.2020.107122. [DOI] [Google Scholar]
- 22.Kuroda Naoto . vol. 108. 2020. Epilepsia y COVID-19: asociaciones y consideraciones importantes; p. 107122. (Epilepsia y comportamiento: E&B). [DOI] [Google Scholar]
- 23.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eduardo M.-S., Riestra J., Yebra L., Larran A., Mancino F., Yanes-Diaz J. Estudio prospectivo en 355 pacientes con sospecha de infección por COVID-19: valor de la tos, la hiposmia subjetiva y la hipogeusia. El laringoscopio. 2020;130:2674–2679. doi: 10.1002/lary.28999. [DOI] [Google Scholar]
- 25.Viveiros A., Rasmuson J., Vu J., Mulvagh S.L., Yip C.Y.Y., Norris C.M. Sex differences in COVID-19: candidate pathways, genetics of ACE2, and sex hormones. Am J Physiol Heart Circ Physiol. 2021;320:H296–H304. doi: 10.1152/ajpheart.00755.2020. Epub ahead of print. PMID: 33275517. [DOI] [PMC free article] [PubMed] [Google Scholar]


