Main Text
Tumor-associated macrophages (TAMs) are effective promotors of cancer dissemination and resistance to chemotherapy and angiogenesis. Cancer cells, TAMs, and other stromal cells communicate with each other through the secretion of nanovesicles, carrying various molecules, including microRNAs (miRNAs). In a paper published in this issue of Molecular Therapy, Yang et al.1 explore the mechanism of this type of crosstalk between pancreatic ductal adenocarcinoma (PDAC) and endothelial cells. They show that macrophage-derived exosomes (MDEs) promote the proliferation of endothelial cells, thus increasing the vascular density of tumors. In particular, activated macrophages (M2) had significantly higher levels of miR-155-5p and miR-221-5p than their naive counterparts, and their transmission to endothelial cells promoted angiogenesis and PDAC growth in an E2F2-dependent manner. These findings further demonstrate the already established role that exosomes play in orchestrating cancer-stroma interactions in the tumor microenvironment and open a window for the development of new pharmacologic inhibitors of tumor angiogenesis (Figure 1).
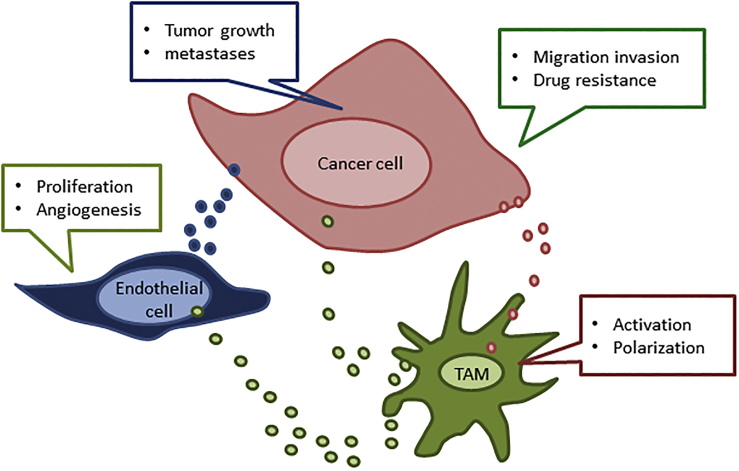
Figure 1.
Tumor-Associated Macrophages (TAMs) Orchestrate Cancer-Stroma Interactions in the Tumor Microenvironment.
Exosomes secreted from cancer cells initiate M2 macrophage activation whereby, in turn, M2 macrophages secrete exosomes, which participate in the reciprocal crosstalk between TAMs to endothelial cells to cancer cells. The exosomal cargo, which includes miRNAs, affects cellular processes and thereby promotes tumor progression and angiogenesis.
PDAC is an incurable disease that is a leading cause of cancer-related death. It is characterized by dense stromal cells, which is often greater than the number of cancer cells it contains. Stroma cells include fibroblasts, myofibroblasts, immunocytes, nerves, stellate cells, and endothelial cells, which are seen in the composition of the tumor or its metastases. These cells interact with each other via the transmission of exosomally delivered molecular cues.
Exosomes are nanosized vesicles consisting of a lipid membrane surrounding a cytosol compartment that contains various molecular constituents, including proteins and nucleic acid material. These have been shown to play a role in cancer biology and development as well as drug resistance coagulation, inflammation, and immunity.2 Analysis of the exosomal cargo secreted from TAMs reveals a significant diversity of RNA content, among which miRNAs are the most abundant. miRNA sequencing data has shown that macrophage activation toward the M2 type changed the miRNA profile of the exosomal cargo.3 Additional cargo of these exosomes includes specific molecules that could provide off-the-shelf fuel for the key metabolic cell pathways.
MDEs are differentially internalized by cancer, fibroblast, and endothelial cells. Using human PDAC specimens, Yang et al.1 revealed that TAM infiltration correlates with the microvessel density (MVD) of a given tumor. Using an in vivo PDAC flank model, they showed that, in comparison to the injection of naive macrophages and saline, the injection of M2 macrophages into tumors resulted in a higher microvessel count and enhancement of tumor growth.
M2 macrophages and their exosomes were enriched with miR-155-5p and miR-221-5p, which were transported to endothelial cells via MDEs. This, in turn, increased tumor angiogenesis and growth. Interestingly, both miR-155-5p and miR-221-5p were shown to be a common target of the E2F2 transcription factor, which inhibits endothelial angiogenesis. Accordingly, delivery of these miRNAs to endothelial cells inhibits E2F2 expression, a finding that is consistent with previous evidence regarding the role of E2F2 as a suppressor of endothelial cell proliferation.
Several papers have indicated that angiogenesis can be induced by TAMs and that their infiltration correlates with poor prognosis.4 The mechanism for the crosstalk between TAMs and endothelial cells suggests a potential target to inhibit angiogenesis or to overcome resistance to vascular endothelial growth factor (VEGF) inhibitors in cancer patients.
The study highlights the specific effect of miRNAs on endothelial cells. However, there are several other miRNAs that are transmitted by MDEs, which also directly affect the migration and proliferation of PDAC cells. Furthermore, other MDE molecular cargos can also promote tumor growth in hostile environments. MDEs can modify the physiology of the stromal compartment (including infiltrated immune cells), with fibroblast and vascular cells both contributing functional and structural support to the tumor.
Constant crosstalk occurs between stromal and tumor cells, and a large part of this communication is mediated by extracellular vesicles. For example, the transmission of miR-21-5p and miR-155-5p through MDEs promotes invasion and migration in colorectal cancer cells.5 The evidence presented by Yang et al.1 supports the notion that exosomes play a detrimental role in many cancers. This is the rationale for the development of drugs that will inhibit the effect of nanovesicles.6 Potential strategies that can be used to overcome the effects of exosomes include: (1) inhibition of their biogenesis or secretions, (2) depleting them from the plasma, and (3) targeting potential miRNAs in their cargo.
Notably, while several studies support the pro-tumorigenic role of exosomes, there are papers that suggest a role for exosomes in drug sensitization and anti-tumor immune response. Therefore, the benefits and risks of exosome depletion should be weighed for each type of cancer.
The efforts of Yang et al.1 and other scientists to decipher the molecular mechanism involving exosome biogenesis, secretion, and crosstalk between cancer and stromal cells is beginning to bear fruit. This knowledge can be harnessed and refined, eventually allowing us to interfere with the effects of exosomes on tumor dissemination and even use them as a drug delivery platform. Initiatives should be launched to develop technologies to produce inhibitors that will interfere with exosomal biomachinery and/or to block specific molecules in their cargo.
Acknowledgments
Declaration of Interest
The authors declare no competing interests.
References
- 1.Yang Y., Guo Z., Chen W., Wang X., Cao M., Han X., Zhang K., Teng B., Cao J., Wu W. M2 macrophage-derived exosomes promote angiogenesis and growth of pancreatic, ductal adenocarcinoma by targeting E2F2. Mol. Ther. 2021;29:1226–1238. doi: 10.1016/j.ymthe.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milman N., Ginini L., Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist. Updat. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Binenbaum Y., Fridman E., Yaari Z., Milman N., Schroeder A., Ben David G., Shlomi T., Gil Z. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018;78:5287–5299. doi: 10.1158/0008-5472.CAN-18-0124. [DOI] [PubMed] [Google Scholar]
- 4.Guo L., Akahori H., Harari E., Smith S.L., Polavarapu R., Karmali V., Otsuka F., Gannon R.L., Braumann R.E., Dickinson M.H. CD163+ macrophages promote angiogenesis and vascular permeability accompanied by inflammation in atherosclerosis. J. Clin. Invest. 2018;128:1106–1124. doi: 10.1172/JCI93025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan J., Sun L., Xu F., Liu Lu, Hu F., Song D., Hou Z., Wu W., Luo X., Wang J. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. 2019;79:146–158. doi: 10.1158/0008-5472.CAN-18-0014. [DOI] [PubMed] [Google Scholar]
- 6.Guo W., Li Y., Pang W., Shen H. Exosomes: A Potential Therapeutic Tool Targeting Communications between Tumor Cells and Macrophages. Mol. Ther. 2020;28:1953–1964. doi: 10.1016/j.ymthe.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]