Abstract
Purpose
Hypovitaminosis D has emerged as potential risk factor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in the general population with variable effects on the outcome of the coronavirus disease-19 (COVID-19). The aim of this retrospective single-center study was to investigate the impact of hypovitaminosis D and secondary hyperparathyroidism on respiratory outcomes of COVID-19.
Methods
Three-hundred-forty-eight consecutive patients hospitalized for COVID-19 at the IRCCS Humanitas Research Hospital, Rozzano, Milan (Italy) were evaluated for arterial partial pressure oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio, serum 25hydroxy-vitamin D [25(OH)D], parathyroid hormone (PTH) and inflammatory parameters at study entry and need of ventilation during the hospital stay.
Results
In the entire population, vitamin D deficiency (i.e., 25(OH)D values < 12 ng/mL) was significantly associated with acute hypoxemic respiratory failure at the study entry [adjusted odds ratio (OR) 2.48, 95% confidence interval 1.29–4.74; P = 0.006], independently of age and sex of subjects, serum calcium and inflammatory parameters. In patients evaluated for serum PTH (97 cases), secondary hyperparathyroidism combined with vitamin D deficiency was significantly associated with acute hypoxemic respiratory failure at study entry (P = 0.001) and need of ventilation during the hospital stay (P = 0.031).
Conclusion
This study provides evidence that vitamin D deficiency, when associated with secondary hyperparathyroidism, may negatively impact the clinical outcome of SARS-CoV-2-related pneumonia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40618-021-01535-2.
Keywords: PTH, Vitamin D, Hyperparathyroidism, Vitamin D deficiency, COVID-19, SARS-CoV-2 infection, Pneumonia
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped RNA beta-coronavirus responsible for the coronavirus disease-19 (COVID-19) ranging from asymptomatic cases to severe respiratory involvement [1]. The first clinical case of COVID-19 in Italy was diagnosed on February 20th 2020, then the infection has rapidly spread involving more than 2.0 million subjects at this time, configuring our country as one of the largest clusters of COVID-19 in the world.
Hypovitaminosis D is a pandemic clinical condition [2] involving mainly elderly subjects [3, 4], who are also those at higher risk of severe COVID-19 [5, 6]. Interestingly, hypovitaminosis D has emerged as potential risk factor of SARS-CoV-2 infection in the general population [7, 8], consistently with the hypothesis that deregulated innate and adaptive immunity may occur as effects of hypovitaminosis D [9]. Indeed, the interplay between vitamin D and viral infection is an area of growing interest and pre-COVID-19 studies reported an association between hypovitaminosis D and higher susceptibility to viral infections of the respiratory tract [10]. Moreover, vitamin D supplementation was revealed to be effective in preventing respiratory infections in patients with vitamin D deficiency [11].
A clinically relevant issue is to understand whether hypovitaminosis D may influence the outcome of COVID-19 [12]. Some studies provided evidence that subjects exposed to vitamin D deficiency may develop a more severe COVID-19 [13–16], whereas other studies failed to find such an association [17–19]. Moreover, the effects of vitamin D supplementation on clinical outcome of COVID-19 is still a matter of uncertainty [20–22]. Variability in clinical end-points may explain the heterogeneity of results of these studies evaluating the impact of hypovitaminosis D and its treatment on COVID-19 outcome. Moreover, the potential effects of low calcium [23–25] and high parathyroid hormone (PTH) [17, 25] may influence the impact of hypovitaminosis D on the outcome of COVID-19.
In this retrospective and single center study, we aimed at investigating the impact of hypovitaminosis D on respiratory outcomes in patients hospitalized for COVID-19, taking also into account hypocalcemia and secondary hyperparathyroidism that may occur as a consequence of vitamin D deficiency.
Materials and methods
This is a retrospective single-center study performed on 348 consecutive patients hospitalized for COVID-19 at the IRCCS Humanitas Research Hospital, Rozzano-Milan, Italy in the period between March 8th and November 15th 2020. The inclusion criteria were: (1) hospitalization for COVID-19 diagnosed by real-time reverse-transcriptase–polymerase-chain-reaction assay of nasal and pharyngeal swab specimens and or bronco-alveolar lavage fluid associated with clinical and radiological signs of pneumonia [1]; (2) at least one serum 25hydroxy-vitamin D [25(OH)D] measurement after COVID-19 diagnosis. Patients with requirement of intubation and mechanical respiratory support at the time of 25(OH)D evaluation were excluded from the study.
The first end-point of the study was the association between serum 25(OH)D values and respiratory insufficiency at study entry. The diagnostic criteria for acute hypoxemic respiratory failure were PaO2 < 60 mmHg on room air or arterial partial pressure oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio < 300 mmHG on oxygen, as measured by arterial blood gas analysis [26]. As secondary end-points we also explored the associations between (1) serum 25(OH)D values before SARS-CoV-2 infection and respiratory insufficiency at study entry, (2) serum 25(OH)D values at study entry and clinical outcome of COVID-19, (3) serum 25(OH)D values and comorbidities; (4) serum PTH and respiratory insufficiency and outcome during hospital stay. Moreover, the effects of vitamin D supplementation on respiratory end-points were also investigated.
These end-points were addressed by a retrospective review of laboratory findings and clinical charts of the patients. The following clinical and biochemical data were collected: the PaO2/FiO2 ratio, hypertension, coronary artery diseases (CAD), chronic kidney disease (CKD) (from stage 3A and worse, corresponding to eGFR < 60 mL/min/1.73 m2, calculated by CKD-EPI), obesity (defined as a Body Mass Index > 30 kg/m2), past or active cancer, chronic obstructive pulmonary disease (COPD), treatment with vitamin D before and during hospitalization, lymphocyte count (× 1000/mm3), serum 25(OH)D, total calcium (mmol/L), albumin (gr/dL), interleukin-6 (IL-6, pg/mL), D-Dimer (ng/mL), ferritin (ng/mL) and C reactive protein (CRP, mg/dL) at study entry. Serum PTH values at study entry and 25(OH)D values prior the SARS-CoV-2 infection (measured up to 6 months before hospitalization) were available in 97 and 28 patients, respectively.
In our institution, measurement of 25(OH)D was included in the routine biochemical evaluation of hospitalized patients with COVID-19 since March 2020, whereas serum PTH was measured only in the second wave of COVID-19, which started in October 2020. Serum 25(OH)D and PTH were measured by using chemiluminescent methods on the Beckman Coulter DxI 800 Access® immunoassay system. In our laboratory, patients were classified with vitamin D sufficiency when 25(OH)D values were above 30 ng/mL [27]. For the purposes of this study, patients were stratified in four groups according to the 25(OH)D cut-offs (<12 ng/mL, 12-20 ng/mL, 20-30 ng/mL and above 30 ng/mL) so far used to define vitamin D deficiency, insufficiency and sufficiency by different guidelines [27–29]. In our laboratory, the reference range of PTH was 12–88 pg/mL. Secondary hyperparathyroidism was defined by high PTH levels in the presence of either normal or low albumin‐corrected calcium levels.
According to the Guidelines of the Italian Society of Infectious Disease [30] and to the Institutional internal protocol, patients with COVID-19 at the admission started treated with standard therapy consisting of enoxaparin at either prophylactic or therapeutic doses according to the clinical judgement [31], antiviral agents (lopinavir/ritonavir, remdesivir), corticosteroids and antibiotics.
Some of the enrolled patients had been already involved in other COVID-19 studies evaluating endpoints which were different from the present study [31–35].
The retrospective study was approved by the Ethical Committee of IRCCS Humanitas Research Hospital and the patients gave their consent to use the clinical and biochemical data for research purposes.
Statistical analysis
Data were presented as median and absolute range, unless otherwise stated. Since most of variables were non-normally distributed as assessed by Kolmogorov–Smirnov test, non-parametric tests were used. The comparisons were performed by Mann–Whitney’s and Kruskal–Wallis’ tests, with post hoc Bonferroni’s correction when appropriate. Paired data were compared using the Wilcoxon’s test. Frequencies were compared by the Chi-Squared test, with Fisher correction when appropriate. A logistic regression analysis was performed and the odds ratio (OR) with 95% confidence interval (95% CI) were calculated to evaluate the determinants of acute hypoxemic respiratory failure at study entry and need of either invasive or non-invasive ventilation. All risk factors with a p value under 0.10 were then submitted to a backward multivariate logistic regression analysis. p value < 0.05 was considered as significant.
Results
Entire population (no. 348 cases)
At study entry, the median serum 25(OH)D values were 12.1 ng/mL (range: 1–67) and 161 patients (46.3%) had serum 25(OH)D values below 12 ng/mL, whereas only 36 patients (10.3%) had vitamin D sufficiency (i.e., 25(OH)D > 30 ng/mL) (Table 1). At the hospitalization, 28 patients (8.0%) were on treatment with cholecalciferol (median dose 1500 units per day, range: 800–2000) and 10 of them (35.7%) had serum 25(OH)D values above 30 ng/mL (Table 1). Subjects with serum 25(OH)D values below 12 ng/mL showed lower PaO2/FiO2 ratio (P = 0.017) and had more frequently acute hypoxemic respiratory failure at study entry (P = 0.001) as compared to subjects with 25(OH)D values ≥ 12 ng/mL, without significant differences between the other three 25(OH)D categories (i.e., 12–20 ng/mL, 20–30 ng/mL and > 30 ng/mL) (Table 1). Subjects with vitamin D sufficiency (i.e., 25(OH)D > 30 ng/mL) were more likely females (P = 0.005), were more frequently treated with vitamin D at study entry (P < 0.001) and had lower serum ferritin (P = 0.048) values than patients with serum 25(OH)D values < 30 ng/mL (Table 1).
Table 1.
Demographical and clinical data of 348 COVID-19 patients, stratified for vitamin D status at study entry. Categorical data were presented as n/n or n (%), whereas continuous data were presented as median and absolute range
| Variables | Entire population | Subgroups by serum 25(OH)D values at the study entry | ||||
|---|---|---|---|---|---|---|
| < 12 ng/mL | 12–20 ng/mL | 20–30 ng/mL | > 30 ng/mL | P values | ||
| N | 348 | 161 | 98 | 53 | 36 | |
| Age (years) | 68.0 (26.0–95.0) | 69.0 (30.0–95.0) | 67.0 (26.0–94.0) | 67.0 (35.0–95.0) | 69.0 (45.0–87.0) | 0.795 |
| Sex (F/M) | 124/224 | 43/118 | 38/60 | 24/29 | 19/17 | 0.005 |
| BMI (kg/m2) | 26.0 (17.0–53.0) | 26.1 (18.0–53.0) | 25.0 (17.0–40.0) | 26.0 (18.0–38.0) | 28.0 (18.0–40.0) | 0.139 |
| Obesity | 83 (23.9) | 43 (27.2) | 21 (21.4) | 9 (17.0) | 10 (27.8) | 0.436 |
| Arterial hypertension | 163 (46.8) | 84 (52.2) | 44 (44.9) | 22 (41.5) | 13 (36.1) | 0.235 |
| Diabetes mellitus | 124 (35.6) | 54 (33.5) | 36 (36.7) | 15 (28.3) | 19 (52.8) | 0.102 |
| CAD | 76 (21.8) | 36 (22.4) | 23 (23.5) | 13 (24.5) | 4 (11.1) | 0.419 |
| Active or past cancer | 51 (14.7) | 24 (14.9) | 16 (16.3) | 10 (18.9) | 1 (2.8) | 0.169 |
| COPD | 48 (13.8) | 19 (11.8) | 15 (15.3) | 9 (17.0) | 5 (13.9) | 0.758 |
| CKD | 39 (11.2) | 20 (12.4) | 14 (14.3) | 1 91.9) | 4 (11.1) | 0.122 |
| ≥ 2 comorbidities | 106 (30.5) | 55 (34.2) | 31 (31.6) | 13 (24.5) | 7 (19.4) | 0.256 |
| Treatment with vitamin D3 | 28 (8.0) | 1 (0.6) | 9 (9.2) | 8 (15.1) | 10 (27.8) | < 0.001 |
| Lymphocyte (n/mm3) | 900 (100–5600) | 1000 (200–5600) | 800 (100–3300) | 900 (300–4000) | 900 (700–2700) | 0.389 |
| IL-6 (pg/mL) | 69 (3–1600) | 74.0 (4–1573) | 53.0 (3–1600) | 61.0 (3–1355) | 22.0 (8–451) | 0.388 |
| d-Dimer (ng/mL) | 530 (200–61,000) | 530.0 (200–61,000) | 554.0 (200–59,920) | 511 (200–35,589) | 524 (200–1950) | 0.944 |
| Ferritin (ng/mL) | 553 (15–7647) | 561.0 (15–3765) | 579 (38–7647) | 587 (78–3842) | 195.0 (32–930) | 0.048 |
| CRP (mg/dL) | 10 (0–333) | 10.5 (0–227) | 9.8 (1–329) | 8 (1–333) | 6 (0–21) | 0.528 |
| Corrected calcium (mmol/L) | 2.15 (1.64–2.78) | 2.15 (1.64–2.78) | 2.13 (1.65–2.55) | 2.14 (1.94–2.77) | 2.18 (1.85–2.41) | 0.757 |
| PaO2/FiO2 (mmHG) | 300 (46–561) | 290.0 (46–504) | 306.0 (54–561) | 314.0 (105–523) | 317.5 (110–480) | 0.017 |
| Acute hypoxemic respiratory failure at the study entry | 169 (48.6) | 97 (60.2) | 41 (41.8) | 18 (34.0) | 13 (36.1) | 0.001 |
BMI body mass index, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, CRP C-reactive protein, F females, IL-6 interleukin-6, M males, PaO2/FiO2 arterial partial pressure oxygen/fraction of inspired oxygen ratio, 25(OH)D 25 hydroxyvitamin D
At study entry, 169 patients (48.6%) had acute hypoxemic respiratory failure. These patients were older (P = 0.012) and had higher BMI (P = 0.034), serum IL-6 (P = 0.005), D-Dimer (P = 0.030), ferritin (P < 0.001), CRP (P < 0.001) and lower 25(OH)D (P < 0.001) values as compared to patients without acute hypoxemic respiratory failure (Supplemental Table 1). In the multivariate logistic regression analysis (including sex, age, BMI, IL-6, D-Dimer, CRP, ferritin and 25(OH)D < 12 ng/mL as covariates), acute hypoxemic respiratory failure maintained the significant and independent associations with 25(OH)D < 12 ng/mL (adjusted OR 2.48, 95% C.I. 1.29–4.74; P = 0.006) and serum ferritin values (adjusted OR 1.75, 95% C.I. 1.17–2.61 per 1-tertile increase; P = 0.007), whereas the associations with sex (P = 0.434), age (P = 0.194), BMI (P = 0.07), serum IL-6 (P = 0.123), D-Dimer (P = 0.376) and CRP (P = 0.308) values were lost.
Pre-COVID-19 serum 25(OH)D values were available in 28 patients. In these patients, the hospitalization for COVID-19 was accompanied by a significant decrease in serum 25(OH)D (from 20.8 ng/mL, range 8.0–52.0 to 12.0 ng/mL, range 4.0–36.0; P < 0.001). The decrease in serum 25(OH)D was significantly lower in patients who were on treatment with cholecalciferol as compared to those who did not take vitamin D supplements before hospitalization (− 23.4%, from − 53.9 to + 62.2 vs. − 55.6%, from − 84.9 to + 32.7%; P = 0.05). The decrease in serum 25(OH)D was not significantly associated with acute hypoxemic respiratory failure at study entry (OR 1.02, 95% C.I. 0.99–1.04; P = 0.23).
During hospital stay, 261 patients (75.0%) required oxygen support [160 with high-flow nasal or mask oxygen, 59 with continuous positive airways pressure (CPAP), 42 with invasive ventilation]. As compared to patients who were not ventilated, those requiring ventilation (either invasive or non-invasive) were younger (63 years, range 34–92 vs. 69 years, range 26–95; P = 0.04), were more frequently males (72.3% vs. 61.1%; P = 0.049), had at study entry lower PaO2/FiO2 ratio (233 mmHG, range: 46–561 vs. 319 mmHG, range: 72–523; P < 0.001), lymphocyte counts (800/mm3, range 200–5600 vs. 1000/mm3, range: 100–3600; P = 0.039), serum corrected calcium (2.05 mmol/L, range: 1.64–2.37 vs. 2.17 mmol/L, range: 1.70–2.78; P = 0.004) and higher BMI (28.0 kg/m2, range: 19.0–53.0 vs. 26.0 kg/m2, range: 17.0–42.0; P = 0.016), serum IL-6 (107 pg/mL, range: 7–1135 vs. 55 pg/mL, range: 3–1600; P < 0.001), ferritin (947 ng/mL, range: 15–6613 vs. 431 ng/mL, range: 26–7647; P < 0.001) and CRP (15 mg/dL, range: 0–333 vs. 8 mg/dL, range: 0–227; P < 0.001) values, without significant differences in 25(OH)D values (P = 0.290), diabetes (P = 0.803), hypertension (P = 0.267), COPD (P = 0.178), CAD (P = 0.148), CKD (P = 0.64) and serum D-Dimer (P = 0.140). In the multivariate logistic regression analysis, the need of ventilation maintained the significant and independent associations with age (OR 0.97, 95% CI 0.94–0.99; P = 0.010), PaO2\FiO2 ratio below 300 mmHG (OR 3.62, 95% CI 1.82–7.19; P < 0.001), serum corrected calcium (OR 0.10, 95% CI 0.02–0.33; P = 0.005) and ferritin (OR 1.65, 95% CI 1.04–2.60 per 1-tertile increase; P = 0.03).
During hospital stay, 116 patients (33.3%) were treated with cholecalciferol; 28 patients continued the previous supplements at maintenance doses (median dose 1500 U/day; range: 800–3000) which were adapted to the 25(OH)D values at study entry, whereas the remaining 88 patients started supplementation with loading doses of cholecalciferol (from 50.000 to 300.000 units). Treatment with vitamin D with either regimen was not significantly associated with need of ventilation during hospital stay (OR 1.67, 95% C.I. 0.95–2.86; P = 0.08).
During hospital stay, 64 patients (18.4%) died due to COVID-19. The mortality was not significantly associated with serum 25OHD values below 12 ng/mL at the study entry (OR 1.03, 95% C.I. 0.60–1.78; P = 0.914) and treatment with vitamin D3 (OR 0.81, 95% C.I. 0.45–1.47; P = 0.494).
Subjects evaluated for serum PTH values (no. 97 cases)
Ninety-seven subjects were evaluated for serum PTH values at the study entry. These subjects had higher BMI (P = 0.043), less frequently hypertension (P < 0.001), CAD (P = 0.008), multiple comorbidites and 25(OH)D < 12 ng/mL (P < 0.001), had more frequently diabetes (P = 0.035) and COPD (P = 0.008) and were more frequently on treatment with cholecalciferol at study entry (P = 0.003) than subjects who were not evaluated by serum PTH measurement, without significant differences in age (P = 0.851), sex (P = 0.391), obesity (P = 0.808), history of cancer (P = 0.277), CKD (P = 0.142), serum corrected calcium (P = 0.643), PaO2/FiO2 ratio (P = 0.075), acute hypoxemic respiratory failure (P = 0.326) and mortality (P = 0.796) (Table 2).
Table 2.
Demographical and clinical data of 348 patients with COVID-19 stratified for serum parathyroid hormone (PTH) measurement. Categorical data were presented as n/n or n (%), whereas continuous data were presented as median and range
| Measurement of PTH values | P values | ||
|---|---|---|---|
| No | Yes | ||
| N | 251 | 97 | |
| Age (years) | 68.0 (27.0–95.0) | 69.0 (26.0–93.0) | 0.851 |
| Sex (F/M) | 86/165 | 38/59 | 0.391 |
| BMI (kg/m2) | 25.9 (17.0–46.0) | 27.0 (19.0–53.0) | 0.043 |
| Obesity | 59 (23.5) | 24 (24.7) | 0.808 |
| Arterial hypertension | 136 (54.2) | 27 (27.8) | < 0.001 |
| Diabetes mellitus | 81 (32.3) | 43 (44.3) | 0.035 |
| CAD | 64 (25.5) | 12 (12.4) | 0.008 |
| Active or past cancer | 40 (15.9) | 11 (11.3) | 0.277 |
| COPD | 27 (10.8) | 21 (21.6) | 0.008 |
| CKD | 32 (12.7) | 7 (7.2) | 0.142 |
| ≥ 2 Comorbidities | 91 (36.3) | 15 (15.5) | < 0.001 |
| 25(OH)D (ng/mL) | 10.0 (1.0–39.0) | 21.0 (4.0–67.0) | < 0.001 |
| 25(OH)D < 12 ng/mL | 138 (55.0) | 23 (23.7) | < 0.001 |
| Treatment with vitamin D | 13 (5.2) | 15 (15.5) | 0.003 |
| Corrected calcium (mmol/L) | 2.15 (1.64–2.78) | 2.15 (1.85–2.41) | 0.643 |
| PaO2/FiO2 (mmHG) | 299.0 (46.0–561.0) | 309 (110.0–480.0) | 0.075 |
| Acute hypoxemic respiratory failure at the study entry | 126 (50.2) | 43 (44.3) | 0.326 |
| In-hospital mortality | 47 (18.7) | 17 (17.5) | 0.796 |
BMI body mass index, CAD coronary artery disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, F females, M males, PTH parathyroid hormone, 25(OH)D 25hydroxyvitamin D
Secondary hyperparathyroidism was found in 42 of patients (43.3%) who were evaluated for serum PTH values. Patients with secondary hyperparathyroidism had more frequently clinical history of cancer (21.4% vs. 3.6%; P = 0.006) and lower PaO2\FiO2 ratio (233 mmHg, range: 110–342 vs. 324 mmHG, range: 172–480; P < 0.001) and serum 25(OH)D values (19.0 ng/mL, range: 4–39 vs. 22.0 ng/mL, range: 4–67; P < 0.001) and required more frequently either invasive or non-invasive ventilation (35.3% vs. 14.5%; P = 0.029) as compared to subjects with normal serum PTH values, without significant differences in age (P = 0.137), sex (P = 0.542), BMI (P = 0.159), prevalence of obesity (P = 0.087), hypertension (P = 0.888), diabetes (P = 0.569), CAD (P = 0.510), COPD (P = 0.963), CKD (P = 0.119), serum corrected calcium values (P = 0.320) and mortality (P = 0.161).
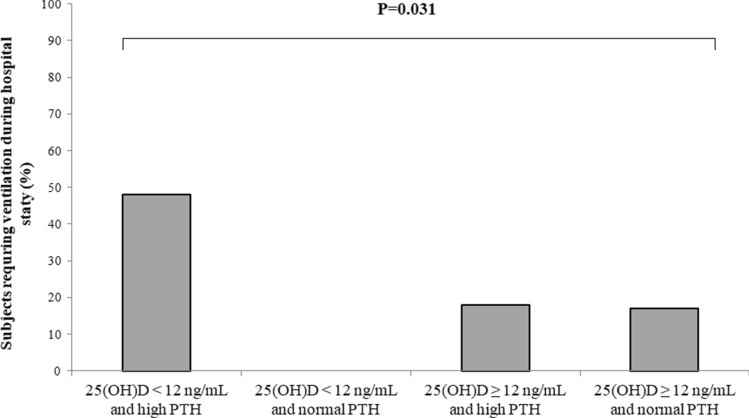
Stratifying the subjects for serum 25(OH)D and PTH values, those with secondary hyperparathyroidism had more frequently acute hypoxemic respiratory failure as compared to those with normal PTH values regardless of serum 25(OH)D values (Fig. 1). Moreover, ventilation was more frequently needed in subjects with coexistent 25(OH)D values < 12 ng/mL and secondary hyperparathyroidism (Fig. 2).
Fig. 1.
Prevalence of acute hypoxemic respiratory failure at study entry in 97 patients with COVID-19 stratified for serum 25hydroxy-vitamin D [25(OH)D] and parathyroid hormone (PTH) values
Fig. 2.
Number of cases (%) requiring either invasive or non-invasive ventilation during the hospital stay in 97 patients with COVID-19 stratified for serum 25hydroxy-vitamin D [25(OH)D] and parathyroid hormone (PTH) values
Discussion
This retrospective study reported an association between vitamin D deficiency and acute hypoxemic respiratory failure in hospitalized patients with COVID-19. Moreover, patients with coexistent vitamin D deficiency and secondary hyperparathyroidism were shown to be at higher risk to undertake either invasive or non-invasive ventilation during hospital stay. However, supplementation with cholecalciferol did not significantly modify the outcome of lung infection in patients with COVID-19.
In our study, more than 90% of subjects hospitalized for COVID-19 were found with hypovitaminosis D and about one-half of them had vitamin D deficiency, as defined by serum 25(OH)D values < 12 ng/mL [29]. The reasons of this finding were not clarified by our study, but some hypotheses could be made. One could argue that such a high prevalence of vitamin D deficiency may reflect the older age and frailty of hospitalized subjects with SARS-CoV-2 infection [3, 4]. However, in our series no significant differences in age and comorbidities were found between subjects with vitamin D deficiency and those with serum 25(OH)D values in the range of sufficiency, suggesting that hypovitaminosis D was not a casual finding but it might be really associated with SARS-CoV-2 infection and COVID-19. Previous studies provided evidence that hypovitaminosis D may predispose subjects to SARS-CoV-2 infection [7, 8] consistently with the several actions of vitamin D on innate and adaptive immune system [9]. Conversely, we cannot exclude that low vitamin D values in our hospitalized patients with COVID-19 might be consequent to the effects of disease-related inflammation on vitamin D metabolism [36–38]. In fact, in our patients who were longitudinally evaluated for vitamin D status, SARS-CoV-2 infection was accompanied by a significant decrease in serum 25(OH)D values.
In our patients, vitamin D deficiency was shown to be associated with acute hypoxemic respiratory failure. Interestingly, this association was independent of inflammatory status, suggesting a possible direct effect of vitamin D deficiency on lung parenchyma. In experimental models, vitamin D deficiency resulted to cause impairment of lung function and overexpression of profibrotic factors via activation of renin–angiotensin system [39]. It is possible that this is further exacerbated by SARS-CoV-2 infection, in which viral binding with cellular entry receptor angiotensin-converting enzyme 2 leads to dysregulation of the renin-angiotensin-system [9, 40]. However, the association between vitamin D deficiency and acute hypoxemic respiratory failure may also reflect a reverse causality possibly related to the effects of critical illness on vitamin D metabolism and transport [38, 41].
In previous studies, hospitalized patients with COVID-19 receiving vitamin D3 [20, 21] or calcifediol [42] showed a less severe outcome of disease as compared to those not receiving vitamin D supplements. Moreover, a high-dose oral vitamin D supplementation helped to achieve SARS-CoV-2 RNA negativity in greater proportion of asymptomatic vitamin D-deficient individuals with SARS-CoV-2 infection along with a significant decrease in inflammatory markers [43]. These favorable effects of vitamin D supplementation were not confirmed in other real-life experiences [22] and in our study, vitamin D supplementation was not shown to influence the outcome of SARS-CoV-2-related pneumonia in terms of preventing acute hypoxemic respiratory failure and need of either invasive or non-invasive ventilation. Differences in therapeutic protocols and variability in biochemical response to vitamin D supplementation in terms of changes in serum 25(OH)D, calcium and PTH may explain the discrepancy between our results and previous observations reporting clinically relevant benefits of vitamin D supplementation in COVID-19 patients.
Hypocalcemia has been reported in several patients with COVID-19 and it has been associated with poor outcome of disease, defined by the need of hospitalization, the need for mechanical ventilation, intensive care unit admission or death of any cause after hospitalization [23, 24]. In our subjects, lower serum corrected calcium levels did not correlate with acute hypoxemic respiratory failure at the hospital admission, but it was associated with more severe outcome of lung infection as defined by the need of ventilation during hospital stay. Vitamin D status was not likely the only determinant of calcium levels in our hospitalized patients, since the association between lower calcium values and unfavorable pneumonia outcome was independent of vitamin D deficiency. In this context, low calcium levels may be also caused by an interaction of calcium with unsaturated fatty acids [44] released during the disease [45] and potentially involved in the modulation of systemic inflammation induced by SARS-CoV-2 infection [46].
A novel finding of this study was the associations between secondary hyperparathyroidism and clinical presentation and outcome of SARS-CoV-2-related pneumonia. One could argue that high PTH values may reflect a more severe hypovitaminosis D [29]. However, in our patients, the association between secondary hyperparathyroidism and clinical end-points of COVID-19 resulted to be independent of 25(OH)D and calcium levels, suggesting that high PTH values were likely more important than vitamin D deficiency and hypocalcemia in predisposing patients with COVID-19 to develop acute hypoxemic respiratory failure. A possible triggering effect of secondary hyperparathyroidism on systemic inflammation may be hypothesized [47], although we cannot exclude also possible effects of secondary hyperparathyroidism on cardio-pulmonary performance of patients [48, 49].
This study has some limitations, partly connected with its retrospective nature which may have caused selection biases. The lack of a control group of non-hospitalized subjects with SARS-CoV-2 infection did not allow to define the true impact of vitamin D deficiency in determining the severity of COVID-19. Since measurement of PTH values was performed only in the second wave of COVID-19, one could argue that this selection may have influenced the final results due to possible different clinical features of patients in the two periods of COVID-19 outbreak [50]. As a matter of fact, subjects included in the analysis of PTH appeared to have a better vitamin D status as compared to the subjects of the first wave of COVID-19, likely reflecting the different period of year (Autumn vs. Spring) and a more frequent treatment with cholecalciferol. Nevertheless, the better vitamin D status allowed us to better investigate the independent effects of secondary hyperparathyroidism on clinical presentation and outcome of COVID-19, although the small number of subjects evaluated for PTH did not permit to perform a multivariate analysis with all the possible confounding factors. Finally, treatment with vitamin D supplements was not randomized and biochemical parameters were not monitored during treatment, precluding a reliable investigation of the possible favorable effects of vitamin D supplementation in individuals with vitamin D deficiency and COVID-19.
In conclusion, this retrospective study provides a first evidence that secondary hyperparathyroidism and, at lesser extent, vitamin D deficiency may be associated with unfavorable outcome of SARS-CoV-2-related pneumonia. These results encourage to perform comprehensive evaluation of vitamin D status in hospitalized individuals with COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Compliance with ethical standards
Conflict of interest
All the authors do not have conflict of interest that is relevant to the subject matter or materials included in this work.
Ethics approval
All the procedures performed in the study were in accordance with the ethical standards of the Ethics Committee of IRCCS Humanitas Research Hospital, Rozzano-Milan, Italy, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all the individual partecipants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
G. Mazziotti and E. Lavezzi equally contributed to this work.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 3.Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporosis Int. 2003;14(7):577–582. doi: 10.1007/s00198-003-1390-7. [DOI] [PubMed] [Google Scholar]
- 4.Romagnoli E, Caravella P, Scarnecchia L, Martinez P, Minisola S. Hypovitaminosis D in an Italian population of healthy subjects and hospitalized patients. Br J Nutr. 1999;81(2):133–137. doi: 10.1017/S0007114599000264. [DOI] [PubMed] [Google Scholar]
- 5.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, Cereda D, Coluccello A, Foti G, Fumagalli R, Iotti G, Latronico N, Lorini L, Merler S, Natalini G, Piatti A, Ranieri MV, Scandroglio AM, Storti E, Cecconi M, Pesenti A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeng F, Deng G, Cui Y, Zhang Y, Dai M, Chen L, Han D, Li W, Guo K, Chen X, Shen M, Pan P. A predictive model for the severity of COVID-19 in elderly patients. Aging. 2020;12(21):20982–20996. doi: 10.18632/aging.103980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M (2020) 25-Hydroxyvitamin D concentrations are lower in patients with positive PCR for SARS-CoV-2. Nutrients 12(5). 10.3390/nu12051359 [DOI] [PMC free article] [PubMed]
- 8.Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9):e2019722. doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, Madhavan MV, Nair N, Babalyan V, Hutchings N, Napoli N, Accili D, Binkley N, Landry DW, Giustina A. Mechanisms in endocrinology: vitamin D and COVID-19. Eur J Endocrinol. 2020;183(5):R133–r147. doi: 10.1530/eje-20-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabetta JR, DePetrillo P, Cipriani RJ, Smardin J, Burns LA, Landry ML. Serum 25-hydroxyvitamin d and the incidence of acute viral respiratory tract infections in healthy adults. PLoS ONE. 2010;5(6):e11088. doi: 10.1371/journal.pone.0011088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S, Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA., Jr Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ (Clin Res Ed) 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman P. The link between vitamin D and COVID-19: distinguishing facts from fiction. J Intern Med. 2020 doi: 10.1111/joim.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo X, Liao Q, Shen Y, Li H, Cheng L. Vitamin D deficiency is inversely associated with COVID-19 incidence and disease severity in Chinese people. J Nutr. 2020 doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Smet D, De Smet K, Herroelen P, Gryspeerdt S, Martens GA. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2020 doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martín Giménez VM, Inserra F, Ferder L, García J, Manucha W (2020) Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2. J Hum Hypertens 1–3. 10.1038/s41371-020-00398-z [DOI] [PMC free article] [PubMed]
- 16.Carpagnano GE, Di Lecce V, Quaranta VN, Zito A, Buonamico E, Capozza E, Palumbo A, Di Gioia G, Valerio VN, Resta O (2020) Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Investig 1–7. 10.1007/s40618-020-01370-x [DOI] [PMC free article] [PubMed]
- 17.Pizzini A, Aichner M, Sahanic S, Böhm A, Egger A, Hoermann G, Kurz K, Widmann G, Bellmann-Weiler R, Weiss G, Tancevski I, Sonnweber T, Löffler-Ragg J (2020) Impact of vitamin D deficiency on COVID-19-a prospective analysis from the CovILD Registry. Nutrients 12(9). 10.3390/nu12092775 [DOI] [PMC free article] [PubMed]
- 18.Cereda E, Bogliolo L, Klersy C, Lobascio F, Masi S, Crotti S, De Stefano L, Bruno R, Corsico AG, Di Sabatino A, Perlini S, Montecucco C, Caccialanza R (2020) Vitamin D 25OH deficiency in COVID-19 patients admitted to a tertiary referral hospital. Clin Nutr (Edinburgh, Scotland). 10.1016/j.clnu.2020.10.055 [DOI] [PMC free article] [PubMed]
- 19.Hernández JL, Nan D, Fernandez-Ayala M, García-Unzueta M, Hernández-Hernández MA, López-Hoyos M, Muñoz-Cacho P, Olmos JM, Gutiérrez-Cuadra M, Ruiz-Cubillán JJ, Crespo J, Martínez-Taboada VM. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2020 doi: 10.1210/clinem/dgaa733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Annweiler G, Corvaisier M, Gautier J, Dubée V, Legrand E, Sacco G, Annweiler C (2020) Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients 12(11). 10.3390/nu12113377 [DOI] [PMC free article] [PubMed]
- 21.Ling SF, Broad E, Murphy R, Pappachan JM, Pardesi-Newton S, Kong MF, Jude EB (2020) High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: a cross-sectional multi-centre observational study. Nutrients 12(12). 10.3390/nu12123799 [DOI] [PMC free article] [PubMed]
- 22.Cereda E, Bogliolo L, Lobascio F, Barichella M, Zecchinelli AL, Pezzoli G, Caccialanza R (2020) Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition (Burbank, Los Angeles County, Calif) 111055. 10.1016/j.nut.2020.111055 [DOI] [PMC free article] [PubMed]
- 23.Liu J, Han P, Wu J, Gong J, Tian D. Prevalence and predictive value of hypocalcemia in severe COVID-19 patients. J Infect Public Health. 2020;13(9):1224–1228. doi: 10.1016/j.jiph.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Filippo L, Formenti AM, Rovere-Querini P, Carlucci M, Conte C, Ciceri F, Zangrillo A, Giustina A. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68(3):475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun JK, Zhang WH, Zou L, Liu Y, Li JJ, Kan XH, Dai L, Shi QK, Yuan ST, Yu WK, Xu HY, Gu W, Qi JW. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging. 2020;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/nejm200005043421806. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 28.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, Giustina A, Binkley N (2018) Vitamin D assays and the definition of hypovitaminosis D: results from the first international conference on controversies in vitamin D. Br J Clin Pharmacol 84(10):2194–2207. 10.1111/bcp.13652 [DOI] [PMC free article] [PubMed]
- 30.Lombardy Section Italian Society I, Tropical D (2020) Vademecum for the treatment of people with COVID-19. Edition 2.0, 13 March 2020. Le Infezioni in Med 28 (2):143–152 [PubMed]
- 31.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, Fernández J, Prati D, Baselli G, Asselta R, Grimsrud MM, Milani C, Aziz F, Kässens J, May S, Wendorff M, Wienbrandt L, Uellendahl-Werth F, Zheng T, Yi X, de Pablo R, Chercoles AG, Palom A, Garcia-Fernandez AE, Rodriguez-Frias F, Zanella A, Bandera A, Protti A, Aghemo A, Lleo A, Biondi A, Caballero-Garralda A, Gori A, Tanck A, Carreras Nolla A, Latiano A, Fracanzani AL, Peschuck A, Julià A, Pesenti A, Voza A, Jiménez D, Mateos B, Nafria Jimenez B, Quereda C, Paccapelo C, Gassner C, Angelini C, Cea C, Solier A, Pestaña D, Muñiz-Diaz E, Sandoval E, Paraboschi EM, Navas E, García Sánchez F, Ceriotti F, Martinelli-Boneschi F, Peyvandi F, Blasi F, Téllez L, Blanco-Grau A, Hemmrich-Stanisak G, Grasselli G, Costantino G, Cardamone G, Foti G, Aneli S, Kurihara H, ElAbd H, My I, Galván-Femenia I, Martín J, Erdmann J, Ferrusquía-Acosta J, Garcia-Etxebarria K, Izquierdo-Sanchez L, Bettini LR, Sumoy L, Terranova L, Moreira L, Santoro L, Scudeller L, Mesonero F, Roade L, Rühlemann MC, Schaefer M, Carrabba M, Riveiro-Barciela M, Figuera Basso ME, Valsecchi MG, Hernandez-Tejero M, Acosta-Herrera M, D'Angiò M, Baldini M, Cazzaniga M, Schulzky M, Cecconi M, Wittig M, Ciccarelli M, Rodríguez-Gandía M, Bocciolone M, Miozzo M, Montano N, Braun N, Sacchi N, Martínez N, Özer O, Palmieri O, Faverio P, Preatoni P, Bonfanti P, Omodei P, Tentorio P, Castro P, Rodrigues PM, Blandino Ortiz A, de Cid R, Ferrer R, Gualtierotti R, Nieto R, Goerg S, Badalamenti S, Marsal S, Matullo G, Pelusi S, Juzenas S, Aliberti S, Monzani V, Moreno V, Wesse T, Lenz TL, Pumarola T, Rimoldi V, Bosari S, Albrecht W, Peter W, Romero-Gómez M, D'Amato M, Duga S, Banales JM, Hov JR, Folseraas T, Valenti L, Franke A, Karlsen TH. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020;383(16):1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–387. doi: 10.1530/eje-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, Mazziotti G, Lania AG. Impact of comorbidities and glycemia at admission and dipeptidyl peptidase 4 inhibitors in patients with type 2 diabetes with COVID-19: a case series from an academic hospital in Lombardy. Italy Diabetes care. 2020;43(12):3042–3049. doi: 10.2337/dc20-1340. [DOI] [PubMed] [Google Scholar]
- 35.Bifulco M, Ciccarelli M, Bruzzese D, Dipasquale A, Lania AG, Mazziotti G, Gazzerro P (2020) The benefit of statins in SARS-CoV-2 patients: further metabolic and prospective clinical studies are needed. Endocrine 1–3. 10.1007/s12020-020-02550-8 [DOI] [PMC free article] [PubMed]
- 36.Reijven PLM, Soeters PB. Vitamin D: a magic bullet or a myth? Clin Nutr (Edinburgh, Scotland) 2020;39(9):2663–2674. doi: 10.1016/j.clnu.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 37.Isaia G, Medico E. Associations between hypovitaminosis D and COVID-19: a narrative review. Aging Clin Exp Res. 2020;32(9):1879–1881. doi: 10.1007/s40520-020-01650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain SK, Parsanathan R, Achari AE, Kanikarla-Marie P, Bocchini JA., Jr Glutathione stimulates vitamin D regulatory and glucose-metabolism genes, lowers oxidative stress and inflammation, and increases 25-hydroxy-vitamin D levels in blood: a novel approach to treat 25-hydroxyvitamin D deficiency. Antioxid Redox Signal. 2018;29(17):1792–1807. doi: 10.1089/ars.2017.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Liu T, Yao L, Xing Y, Zhao X, Fu J, Xue X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci Rep. 2017;7(1):3312. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128(1):119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amrein K, Venkatesh B. Vitamin D and the critically ill patient. Curr Opin Clin Nutr Metab Care. 2012;15(2):188–193. doi: 10.1097/MCO.0b013e32834f0027. [DOI] [PubMed] [Google Scholar]
- 42.Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203:105751. doi: 10.1016/j.jsbmb.2020.105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rastogi A, Bhansali A, Khare N, Suri V, Yaddanapudi N, Sachdeva N, Puri GD, Malhotra P. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study) Postgrad Med J. 2020 doi: 10.1136/postgradmedj-2020-139065. [DOI] [PubMed] [Google Scholar]
- 44.Singh VP, Khatua B, El-Kurdi B, Rood C. Mechanistic basis and therapeutic relevance of hypocalcemia during severe COVID-19 infection. Endocrine. 2020;70(3):461–462. doi: 10.1007/s12020-020-02530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, Hudson KE, Zimring JC, Hansen KC, Hod EA, Spitalnik SL, D'Alessandro A (2020) COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 5(14). 10.1172/jci.insight.140327 [DOI] [PMC free article] [PubMed]
- 46.de Oliveira C, Khatua B, Noel P, Kostenko S, Bag A, Balakrishnan B, Patel KS, Guerra AA, Martinez MN, Trivedi S, McCullough A, Lam-Himlin DM, Navina S, Faigel DO, Fukami N, Pannala R, Phillips AE, Papachristou GI, Kershaw EE, Lowe ME, Singh VP. Pancreatic triglyceride lipase mediates lipotoxic systemic inflammation. J Clin Investig. 2020;130(4):1931–1947. doi: 10.1172/jci132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarty MF. Secondary hyperparathyroidism promotes the acute phase response—A rationale for supplemental vitamin D in prevention of vascular events in the elderly. Med Hypotheses. 2005;64(5):1022–1026. doi: 10.1016/j.mehy.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 48.Cellini M, Piccini S, Ferrante G, Carrone F, Olivetti R, Cicorella N, Aroldi M, Pini D, Centanni M, Lania AG, Mazziotti G. Secondary hyperparathyroidism and thoracic vertebral fractures in heart failure middle-aged patients: a 3-year prospective study. J Endocrinol Invest. 2020;43(11):1561–1569. doi: 10.1007/s40618-020-01237-1. [DOI] [PubMed] [Google Scholar]
- 49.Palermo A, Sanesi L, Colaianni G, Tabacco G, Naciu AM, Cesareo R, Pedone C, Lelli D, Brunetti G, Mori G, Colucci S, Manfrini S, Napoli N, Grano M. A novel interplay between irisin and PTH: from basic studies to clinical evidence in hyperparathyroidism. J Clin Endocrinol Metab. 2019;104(8):3088–3096. doi: 10.1210/jc.2018-02216. [DOI] [PubMed] [Google Scholar]
- 50.Palmieri L, Palmer K, Lo Noce C, Meli P, Giuliano M, Floridia M, Tamburo de Bella M, Piccioli A, Brusaferro S, Onder G (2020) Differences in the clinical characteristics of COVID-19 patients who died in hospital during different phases of the pandemic: national data from Italy. Aging Clin Exp Res 1–7. 10.1007/s40520-020-01764-0 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.