ABSTRACT
Background:
Nutrition has a significant impact on the pathophysiology of periodontal disease. Both micro- and macronutrients have an impact on periodontal health. This study aimed at the evaluation of the effects of a diet low in carbohydrate and rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fiber on clinical outcomes in patients with gingival inflammation for four weeks.
Materials and Methods:
Overall, 54 systemically healthy subjects were enrolled in this study. The clinical trial consisted of two groups: Group A (test group) (n = 27) was instructed to consume a diet comprising low carbohydrates, rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fibers for the next four weeks; in Group B (control group) (n = 27), no alteration in dietary behavior was done, and these subjects were instructed to have their daily routine diet. Clinical parameters measured were plaque index (PI), gingival bleeding index (GI), probing depths (PD), clinical attachment level (CAL), and bleeding on probing (BOP) at one week without any dietary changes (baseline) for both the groups, followed by a one-week adaptation period; then, the parameters were checked on a weekly basis for the next four weeks.
Results:
Primary clinical outcome BOP and secondary outcome GI showed significant changes in the test group compared with the control group (P < 0.05). However, no significant changes were seen in the plaque scores in the test group (P > 0.05). The degree of diet compliance on the clinical parameters (PI, GI, and BOP) was assessed by using regression analysis.
Conclusion:
Dietary recommendations can be beneficial in managing gingival and periodontal inflammation. Nutritional interventional studies as monotherapy are required to evaluate the clinical significance of diet in periodontal therapy.
KEYWORDS: Antioxidants, diet, nonsurgical periodontal therapy, nutrition, omega-3 fatty acids, periodontitis
INTRODUCTION
Periodontitis is a chronic inflammatory disease in response to biofilm, causing dysregulated host inflammatory/immune response.[1] Various risk factors modify the host response, which can cause disease development and its further progression. These factors can be environmental (stress, bacterial load, and socioeconomic status [SES]), lifestyle (smoking, nutrition, and physical activity), and genetic. These risk factors can act alone or in consortium to modify the host response to microbial insult, resulting in reduced resolution of inflammation. Nutrition is an important aspect in any chronic disease regulation. Studies have shown micronutritional approaches, whereby systemically healthy volunteers are placed on a primitive diet to support the role of nutrition in periodontal inflammation.[2,3]
Chronic, nonresolving inflammation is destructive in nature and the impact of nutrition on such chronic diseases such as periodontitis can be attributed to oxidative stress. In health, a balance exists between oxidants and antioxidants; however, as inflammation sets in, excess production of oxidants and /or depletion of antioxidants leads to oxidative stress, which causes local tissue damage as seen in periodontitis. This imbalance causes direct tissue damage by altering molecules, such as proteins, lipids, and DNA, production of proinflammatory molecules or indirectly via alteration in periodontal microbiota.
The increase in oxidative stress can be regulated by complex systems of antioxidants and their supplementations can reduce the tissue damage. European workshop (2011) has suggested dietary recommendations for increasing the levels of diet-containing fruits, fibers, fish oils, vegetables and for reducing the levels of refined sugars as an adjunct to periodontal preventive/treatment.[4]
A diet low in carbohydrates is beneficial, as suggested by a Swiss study on 10 adults placed in a stone age environment for four weeks. In this study, diet (low simple sugar, high antioxidant micronutrients, fish oils, and fibers) showed a significant decrease in gingival bleeding and probing depths.[3] Kim et al.[5] showed in vitro promotion of apoptosis and inhibition of periodontal ligament cells proliferation due to high levels of glucose.
Studies on dietary supplementation with omega-3 fatty acids as an adjunctive to periodontal therapy showed a reduction in clinical parameters.[6,7] This indicates the essential role of omega-3 fatty acids in the resolution of inflammation. The benefits of the intake of vitamin C in periodontitis have shown a positive impact both clinically and in in vitro studies.[8]
Nishada et al.[9] suggested that dietary intake of vitamin C has a weak but statistically significant relation to periodontal disease. Vitamin C acts as an antioxidant and helps in reducing oxidative stress in inflammatory condition.[10] However, very few randomized controlled trials have been conducted to evaluate dietary effects on gingival health. The primary objective of this study was to determine the impact of a diet low in carbohydrate, rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fibers on PI, GI, PD, CAL, and BOP. The secondary objective was to determine the dietary compliance of the patient with diet modification.
MATERIALS AND METHODS
ETHICAL APPROVAL
This was a double-blind randomized intervention study approved by the institutional ethical committee (ref. No. HIDSAR/ethics/2019/138). This prospective study was conducted in the department of Periodontology at Haldia Institute of Dental Sciences and Research from March 2019 to February 2020. Written informed consent was taken from the patients.
Inclusion criteria were: subjects aged >18 and <60 years, patients with gingivitis (GI >0.5 and ≤3), and consuming a diet based primarily on carbohydrates as described by Frienman et al.[11]
Exclusion criteria were subjects with systemic diseases, pregnancy or lactating mother, history of smoking, intake of any antibiotics, anti-inflammatory drugs, vitamin supplementation within the past six months before the start of the study period, any condition requiring special diet intake (e.g., diabetes), intake of any medications such as oral contraceptive pills, and anticoagulants that might have a role in gingival inflammation.
STUDY PARTICIPANTS
Patients diagnosed with gingivitis visiting the Department of Periodontology were recruited in the study after written consent. In both the test and control groups, those with gingival index scores >0.5 and ≤3 were enrolled in this study with no evidence of clinical attachment loss.
Overall, 27 patients were enrolled in the control group, of whom 14 were women and 13 men. This group comprised those with a mean age of 35.2 ± 7.2 years (range 19 to 58 years). The test group enrolled 27 subjects, comprising 16 women and 11 men. This group comprised those with a mean age of 36.1 ± 8.3 years (range 21 to 59 years) [Table 1].
Table 1.
Demographic data of both the groups
| Group | Total number | Male/female | Age (years) | SES score (mean ± SD) | |
|---|---|---|---|---|---|
| (mean ± SD) | Range | ||||
| Control | 27 | 13/14 | 35.2 ± 7.2 | 19–58 | 21.6 ± 2.3 |
| Test | 27 | 11/16 | 36.1 ± 8.3 | 1–59 | 19.5 ± 2.7 |
SD = standard deviation
SAMPLE SIZE DETERMINATION
The sample size was based on a pilot study, which evaluated the effect of an optimal diet on patients with gingivitis.[12] Taking this study into consideration, a pilot study with 10 subjects in each group was undertaken, with a power = 80% and alpha = 5%; 22 subjects in each group were selected, taking a 20% attrition rate; and a sample of 27 subjects in each group was selected.
Randomization
Patients were randomly allocated to control or test group by using stratified randomization based on age, and a study was carried out by a dentist not directly involved in the study (P.S.). The patients were unaware of the randomisation and group allocation. The data on diet collection were taken by a dentist who was not involved in clinical parameter measurement (S.S.). Web-based randomization was done for the allotment of subjects using Prism 4.0 software package (GraphPad, La Jolla, CA, USA).
Blinding
The dentist who collected the measurement of primary and secondary outcomes (S.N.) was blinded to the group identity of the patients allocated in the test or control group. No questions on diet were asked to the patients during the clinical examination. The clinical measurement was done at baseline followed by a one-week adaptation period and then parameters were checked on a weekly basis for the next four weeks.
DIETARY INTERVENTIONS
Dietary recommendations were based on studies involving gingival/periodontal inflammation for reproducibility of the results.[12,13] Dietary recommendations in the test group included the following diet regimen:
-
–
Patients were advised to reduce intake of carbohydrates. A low-carb diet <130g/day intake was suggested. Subjects were instructed to have very minimal intake of fructose, flour-containing food (maida), rice, sweetened meals, beverages, and potatoes. No limitation was considered in terms of fruits and vegetables (polysaccharides).
-
–
Intake of omega-3 fatty acids (seeds such as two spoons of Chia seeds, flaxseeds, sea fish) was advised to the patients. They were also suggested to reduce intake of trans-fatty acids (such as hot chips, cakes, fried meals) and omega-6 fatty acids (oils such as safflower oil, sunflower oil, sesame oil) daily.
-
–
Intake of a source of ascorbic acid (citrus fruits such as orange, amla, lemon) daily.
-
–
Intake of antioxidants (such as green tea, coffee, fruits) daily.
-
–
Intake of fiber (fruits and vegetables) daily.
The dietary recommendations were instructed in local language (Bengali) to the patients verbally, and a leaflet with written instructions on diet was handed over. The patients were advised to maintain dietary activities in a diary and were instructed to bring it in at every subsequent appointment. After the first visit, patients were instructed to visit the hospital after one week for sharing their dietary experiences and any problems related to the diet.
CLINICAL MEASUREMENTS
All the clinical parameters were measured by a dentist masked to the intervention (S.N.). The clinical parameters assessed were GI (by Loe and Silness),[14] PI (by Silness and Loe),[15] BOP, and PD. All the measurements were taken by using a UNC 15 probe (Hufriedy). After initial recruitment, subjects were advised to maintain the same oral hygiene behavior throughout the study duration; a soft bristle toothbrush and toothpaste were distributed to all subjects, and they were instructed to not indulge in the use of interdental aids. Baseline measurements were taken in the first week and this week was considered as transition time for the patients to adapt the new dietary behavior in the test group. The patients were instructed to visit hospital on a weekly basis and GI, PI, and diet dairies were examined at each appointment. Patients in the control group were examined by similar protocol, except no dietary instructions were given to them. At the end of the four weeks, all the clinical parameters were again evaluated.
All the patients were asked questions related to SES, as per the modified Kuppaswamy scale.[16]
Primary clinical outcomes were bleeding on probing and probing depth. The secondary clinical outcomes were GI, clinical attachment loss, and PI.
STATISTICAL ANALYSIS
Statistical analyses were performed with statistical software (SPSS for Windows, Version 16.0, SPSS Inc., Chicago, IL). The descriptive analysis included mean and standard deviation. A mixed linear regression analysis was performed to test differences between both groups.
RESULTS
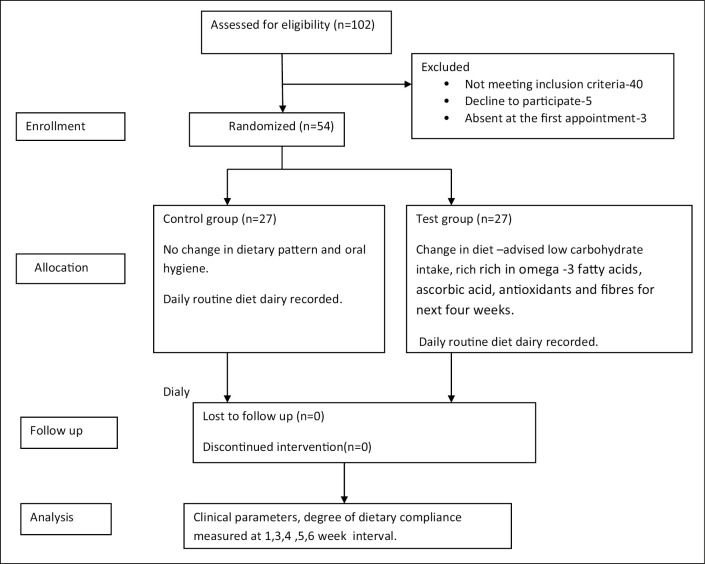
In total, 54 patients diagnosed as having gingivitis were enrolled in this study. Each group consisted of 27 patients who were randomly assigned in control or test group. A flow chart of the study procedure is presented in Figure 1. The baseline demographic details are presented in Table 1. Clinical parameters PI, GI, PD, CAL, BOP, and degree of compliance in relation to dietary recommendations are presented in Tables 2 and 3, respectively. In this study, the primary clinical outcome was BOP, which was significantly reduced from the baseline 53.88 ± 1.75 to 23.55 ± 1.79 after four weeks of follow-up (P < 0.05); the secondary outcome GI was significantly reduced from baseline 1.34 ± 0.03 to 0.80 ± 0.11 at four weeks (P < 0.05) in the test group as compared with the control group (P < 0.05) [Table 2]. Furthermore, plaque scores, PD, and CAL showed no significant changes compared with the control group (P > 0.05). All the subjects were diagnosed as having gingivitis, and any periodontitis case was excluded and referred for necessary periodontal therapy. After the completion of the study period, all the patients received oral prophylaxis.
Figure 1.
Study participants’ flowchart
Table 2.
Clinical parameters of test and control groups
| Clinical parameter | Group | Week 1, mean ± SD | Week 3, mean ± SD | Week 4, mean ± SD | Week 5, mean ± SD | Week 6, mean ± SD | P value |
|---|---|---|---|---|---|---|---|
| PI | Control Test | 0.76 ± 0.05 0.75 ± 0.09 |
0.87 ± 0.01 0.84 ± 0.01 |
0.93 ± 0.02 0.86 ± 0.02 |
0.92 ± 0.01 0.84 ± 0.01 |
0.91 ± 0.01 0.86 ± 0.02 |
>0.05 |
| GI | Control Test |
1.39 ± 0.18 1.34 ± 0.03 |
1.51 ± 0.12 1.39 ± 0.03 |
1.54 ± 0.06 1.34 ± 0.03 |
1.52 ± 0.05 1.13 ± 0.10 |
1.49 ± 0.03 0.80 ± 0.11 |
<0.05 |
| PD | Control Test |
– – |
2.22 ± 0.01 2.43 ± 0.07 |
– – |
– – |
2.33 ± 0.03 2.23 ± 0.01 |
>0.05 |
| CAL | Control Test |
– – |
2.49 ± 0.10 2.35 ± 0.06 |
– – |
– – |
2.79 ± 0.13 2.17 ± 0.05 |
>0.05 |
| BOP % | Control Test |
– – |
43.62 ± 2.49 53.88 ± 1.75 |
– – |
– – |
68.34 ± 0.88 23.55 ± 1.79 |
<0.05 |
PI = plaque index; GI = gingival index; PD = pocket depth; CAL = clinical attachment level; BOP = bleeding on probing, mean values with the standard deviations (SD), statistical significance P < 0.05
Table 3.
Dietary recommendations compliance
| Dietary factor | Group | Week 1, mean ± SD |
Week 3, mean ± SD |
Week 4, mean ± SD |
Week 5, mean ± SD |
Week 6, mean ± SD |
|---|---|---|---|---|---|---|
| Vitamin C | Control .Test | 0.45 ± 0.18 0.42 ± 0.07 |
0.44 ± 0.05 0.46 ± 0.15 |
0.45 ± 0.05 0.86 ± 0.13 |
0.34 ± 0.05 0.73 ± 0.11 |
0.36 ± 0.06 0.83 ± 0.12 |
| Omega-3 fatty acids | Control Test | 0.20 ± 0.13 0.06 ± 0.19 |
0.21 ± 0.16 0.08 ± 0.13 |
0.22 ± 0.14 0.24 ± 0.12 |
0.23 ± 0.14 0.27 ± 0.09 |
0.23 ± 0.12 0.26 ± 0.07 |
| Carbohydrate reduction | Control Test | 0.43 ± 0.06 0.22 ± 0.11 |
0.46 ± 0.07 0.19 ± 0.11 |
0.43 ± 0.07 0.76 ± 0.14 |
0.44 ± 0.08 0.82 ± 0.11 |
0.44 ± 0.10 0.92 ± 0.09 |
| Antioxidants | Control Test | 0.50 ± 0.08 0.76 ± 0.07 |
0.51 ± 0.06 0.80 ± 0.11 |
0.51 ± 0.55 0.81 ± 0.10 |
0.61 ± 0.04 0.83 ± 0.11 |
0.57 ± 0.04 0.88 ± 0.12 |
| Fibers | Control Test | 0.37 ± 0.08 0.26 ± 0.20 |
0.34 ± 0.08 0.36 ± 0.14 |
0.32 ± 0.13 0.89 ± 0.13 |
0.38 ± 0.18 0.92 ± 0.07 |
0.38 ± 0.12 0.94 ± 0.07 |
SD = standard deviations (0 = no compliance, 1 = consumption 100% as recommended)
Influence of the degree of compliance on the clinical parameters was performed for PI, GI, and BOP [Table 4] by using regression analysis. A score of 0 was given for no compliance and 1 when consumption was 100%. Regression analysis showed a significant negative association (P < 0.05) of PI with omega-3 fatty acids, antioxidants, and reduced carbohydrates and a positive association with fibers and vitamin C intake. GI was significantly positively associated (P < 0.05) with fibers and omega-3 fatty acid consumption. BOP was significantly negatively associated (P < 0.05) with antioxidants and positively associated with omega-3 fatty acids and fibers. Modified Kuppaswamy scale was used to determine the SES and in both the groups subjects with upper middle (II) class were enrolled for uniformity in the study.
Table 4.
Regression analysis regarding clinical parameters and degree of compliance
| Clinical parameter | Dietary factor | Coefficient (beta value) | SE | P-value | 95% confidence interval |
|---|---|---|---|---|---|
| PI | Vitamin C | 0.13 | 0.04 | 0.124 | [−0.58; 0.42] |
| Omega-3 fatty acids | −0.23 | 0.12 | 0.031 | [−0.46; −0.05] | |
| Carbohydrate reduction | −0.12 | 0.23 | 0.257 | [−0.46; 0.16] | |
| Antioxidants | −0.06 | 0.19 | 0.571 | [−0.34; 0.16] | |
| Fibers | 0.34 | 0.16 | 0.039 | [0.04; 0.78] | |
| GI | Vitamin C | −0.05 | 0.17 | 0.402 | [−0.36; 0.15] |
| Omega-3 fatty acids | 0.49 | 0.16 | 0.036 | [−0.61; −0.19] | |
| Carbohydrate reduction | −0.52 | 0.17 | 0.002 | [−0.81; −0.28] | |
| Antioxidants | −0.23 | 0.16 | 0.230 | [−0.40; 0.06] | |
| Fibers | 0.06 | 0.25 | 0.970 | [−0.14; 0.43] | |
| BOP | Vitamin C | −0.04 | 0.23 | 0.555 | [−0.39; 0.15] |
| Omega-3 fatty acid | 0.02 | 0.08 | 0.78 | [−0.27; 0.15] | |
| Carbohydrate reduction | −0.41 | 0.35 | 0.02 | [−0.64; −0.26] | |
| Antioxidants | −0.12 | 0.07 | 0.08 | [−0.35; 0.02] | |
| Fibers | 0.32 | 0.24 | 0.22 | [−0.03; −0.41] |
PI = plaque index; GI = gingival index; BOP = bleeding on probing; SE = standard error; P = probability value, statistical significance P < 0.05
DISCUSSION
In the current study, we hypothesized that changes in dietary recommendations can lead to an improvement in periodontal clinical parameters. However, the study does not neglect the importance of nonsurgical periodontal therapy (NSPT), but it focuses on the possible adjunct role of dietary modification in periodontal health. The rationale for such hypothesis is based on literature substantiating the possible link between nutrition, inflammation, and periodontitis; the role of nutrients in reducing oxidative stress and their homeostatic role in balancing reactive oxygen species and the antioxidant defense system.[17]
The current study was a part of an ongoing study evaluating the effects of diet modification as an adjunct to NSPT on periodontal health.
The role of diet in oral health has been explored since ancient times. In their study, Hunter et al. and Adler et al. explored the role of ancestors’ oral health in relation to diet and found an association between oral health and dietary intake.[18,19]
The results of the current study showed that a diet low in carbohydrate and high in antioxidants, ascorbic acid, antioxidants, and fibers can significantly reduce gingival inflammation, which might be of clinical significance. This result is similar to a study by Woelber et al., where they found a positive association of plaque scores in a changed dietary pattern.[12] Several studies have shown an association of high glycemic carbohydrate intake and caries.[20,21] A recent study on athletes’ dietary pattern and its effect on oral microbiome suggested that the consumption of a ketogenic low-carbohydrate high-fat diet leads to a reduction in the quantity of Haemophilus, Neisseria, and Prevotella and an increase in the number of Streptococcus species.[22]
Moreover, a consensus report on the interaction of lifestyle, systemic disease with caries and periodontal disease identified processed carbohydrate as a shared risk factor for dental caries and periodontitis.[23] A study on the influence of the amount of ingested sugars on the microbial profile in dental plaque and saliva suggested the possibility of marginal influence on a high-carbohydrate diet on microorganisms, causing tissue destruction. Also, some caries-causing bacteria were less prevalent in subjects with low sugar consumption.[24] Another study by Edlund et al. on the metabolome analysis of the oral microbiome in in vitro sugar metabolism suggested the possible role of sugar metabolism in the oral microbiome.[25] All these studies suggest the possible role of nutrients intake and microbial association in various systemic diseases and oral health. In the current study, patients were advised to take a low-carbohydrate diet to reduce the microbial load, thereby reducing periodontal inflammation.
Woundenberg et al. used the adapted dietary inflammatory index to associate with carbohydrate intake and they concluded that a high total caloric intake leads to systemic proinflammatory effects.[26] Lula et al. suggested that high glycemic food consumption can increase gingival and periodontal inflammation (NHANES III data).[27] However, in the current study, regression analysis showed no significant association between GI, PI, BOP index, and low-carbohydrate intake.
In their study on high-fiber, low-fat-diet consumption for eight weeks, Kondo et al. showed improved periodontal disease markers and metabolic profiles.[28] Keeping this in view, in the current study the patients were encouraged to increase fiber content in their diet and we got similar results as in bleeding on probing and GI was significantly associated with high fiber diet intake.
Fatty acids such as omega-6 fatty acids have proinflammatory potential and can promote periodontal inflammation.[29] In contrast, omega-3 fatty acids lower the systemic inflammation; several studies have suggested that the supplementation of omega-3 fatty acids can reduce periodontal inflammation and cytokines level.[30,31] The current study also showed a significant positive association between GI in relation to omega-3 fatty acids intake.
Antioxidants exhibit strong defense action by acting on the free radicals, neutralizing the primary radicals, forming chelates with transition metals, and maintaining homeostasis. Application of antioxidants as an adjunct to periodontal therapy has favorable results in improving the clinical parameters, lowering levels of local and systemic ROS.[32] Antioxidants are present in various micro- and macronutrients. A cross-sectional study conducted in the Japanese elderly population on the frequency of fruit and vegetable intake and its effect on oral health-related quality of life showed a strong positive association between the frequency of diet consumption and quality of oral health.[33] In an interventional study, Dodington et al. advised patients to have an intake consisting of a high amount of fruits and vegetables, β-carotene, vitamin C, α-tocopherol, EPA, and DHA; they observed that nonsurgical treatment with dietary modification was beneficial in nonsmokers in terms of pocket depth reduction.[34]
A systematic review on the role of antioxidant agents in periodontal therapy concluded that the administration of antioxidants and its outcome on oxidative stress does not follow a definite pattern and significant changes depend on various factors such as the quantity of antioxidants, their duration of administration, study design, and measurement methods.[35] This review also suggests that the evaluation of the effect of nutrition on periodontal outcomes depends on various factors, and generalization of its effect by a particular study design cannot warrant the success of treatment. A study assessed antioxidant status in chronic periodontitis by a customized dietary intervention (aiming at increasing the consumption of fruits, vegetables, and whole grains) and found that dietary advice can increase healthy dietary intake and improve periodontal status at six months of follow-up.[36] In the current study, no significant association was seen between clinical parameters and antioxidants.
Adequate literature reviews support that vitamin C supplementation reduces periodontal inflammation; vitamin C taken as daily dietary supplements or in synthetic form acts as a potential antioxidant and reduces oxidative stress.[37] Vitamin C has a synergistic effect with other antioxidants[38] and so subjects were encouraged in the current study to also take antioxidants in the form of green tea, coffee, fruits, and ascorbic acid. Wonzniewicz et al. suggested that consumption of cranberry improved plaque and GI in patients with gingivitis, and the compliance was high.[39] Compliance was observed based on patients’ diaries, and an empty bottle of cranberry was collected at follow-up visits. Similar results were obtained in the current study; we found high compliance toward dietary changes in patients with gingivitis, and a significant positive association in PI was seen in relation to ascorbic acid.
Widen et al. showed a reduction in cytokine levels of interleukin-1 beta, interleukin-6, and vascular endothelial factor after administration of bilberries 500g/day in patients with gingivitis. Also, bleeding on probing was reduced and similar results were observed in the current study.[40]
Periodontal disease is a complex chronic disease; microbial factor, host tissue response, and various risk factors play a vital role in disease progression and treatment planning. Jenzsch et al.'s study on nutritional intervention in patients with periodontal disease suggested that nutrition may influence oral microbiological ecology.[41] This leads us to a common risk factor approach that stresses that nutritional dysregulation can lead to dental caries, periodontal disease, systemic diseases such as diabetes, and cardiovascular disease.
Symbiotic supplementation of probiotics and fructo-oligosaccharides as an adjunct to NSPT in the treatment of chronic periodontitis with type 2 diabetes mellitus yielded a significant decrease in the oxidative stress markers and clinical parameters such as PI and CAL.[42] This study gives us further insight into the beneficial use of nutritional supplements in chronic periodontitis with systemic involvement.
Diet modification can be also done by advising a fixed dose of commercially available dietary supplements, which can help in the quantification of the amount of supplements taken. In the current study, no such fixed dose was given to the patients and the patients were encouraged to take natural available resources of antioxidants, vitamin C, fibers, and omega-3 fatty acids as it is more economical. The objective of this study was also to note their dietary compliance toward this method of nutrient consumption. All these diet-microbial studies emphasize the potential role of diet in altering host bacterial response, thereby reducing and resolving inflammation.
The therapeutic measures to reduce plaque reduction by various mechanical and chemical approaches should also focus on the possible etiology of a lack of certain, macro- or micronutrients, leading to periodontal inflammation due to overconsumption of proinflammatory nutrients and the possibility of nutritional intervention along with conventional periodontal therapy as a therapeutic strategy.
The nutritional-based approach in the maintenance of periodontal health requires a diet that is balanced in macro- and micronutrients. The dietary recommendations in the current study were in accordance with adopting the key food approach based on the Indian Food Composition Table (IFCT), and this was recommended to all the subjects.[43] Though the dietary instructions were given and reinforcement was done on a weekly basis, still India has diverse food patterns; to minimize such bias associated with food consumption, particularly in the West Bengal region where fish and rice is a staple food, subjects with similar dietary habits and socioeconomic status (semiurban) were taken into consideration. Also, due to diversity, the results cannot be generalized to an entire population. A multicenter study comparison might give us a better insight on dietary compliance and the effect of diet on clinical parameters. Modified Kuppaswamy scale was used to determine the SES, and scores were given; only subjects with an SES score between 16 and 25 were taken in the study. Family members were not considered in the study design, as randomization may lead to the distribution of members in the test or control group, which might result in contamination among the sample population.
STRENGTHS AND LIMITATIONS
The strength of the study was the evaluation of the role of diet in clinical outcomes in gingival inflammation. Very few studies in literature have examined a dietary combination of low carbohydrate, rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fibers in gingivitis. In the current study, we also evaluated the dietary compliance among patients, which can help further in diet modification that is beneficial to periodontal therapy.
The limitations of the study are self-diet diary maintenance and no diet counselor being available for counseling. Also, the quantitative assessment of the consumption frequency of foods was based on the participant's memory. The food list did not cover all the foods consumed and the recommendation was to include a diet rich in omega-3 fatty acids, ascorbic acid, antioxidants, and fibers; however, a fixed quantity was not assigned for individuals. These can cause underreporting. It was surprisingly difficult to recruit participants who were ready to consume a low-carbohydrate diet; this might be attributed to the local food habits in the study region. In general, potato, rice, and fish was the staple food of all the recruited participants. Both potato and rice are a rich source of carbohydrate and so reducing the quantity of daily diet consumption of carbohydrate was challenging.
The duration of the study was only four weeks: A longer study duration would be desirable if concrete results are to be established, and also a quantifiable diet intake might give us an idea about which nutrient is more beneficial in reducing gingival inflammation. Another limitation encountered was during the initial pilot study with sample size n = 10; after the first week of clinical parameters measurement and dietary instructions, two weeks were assigned for diet adaption and then the patients were followed for four more weeks. However, the two-week adaption period led to lost follow-up and so the adaptation period was reduced to a one-week duration only. The reason for withdrawal as per telephonic conversation was that mostly subjects felt less motivated to continue the study, as they did not visit the hospital in these two weeks and found difficulty in time management.
A pilot study was conducted for sample size calculation, feasibility of the study and to determine the confounding variables in the study. The potential confounding variable in this study was based on the literature review and previous studies on nutrition and periodontal disease. The confounding variables in the current study were participant characteristics such as age, sex, marital status, physical activity, urban or rural location, oral habits, systemic health, and menopausal status. SES, general health status, BMI, medications, family history of disease, and diet-related variables were the other factors.
The study design was conducted after the matching of participants’ characteristics such as age, SES, semiurban location, oral habits, and overall general health.
Moreover, additional variables such as the amount of daily vegetables and fruits intake were difficult to control, as only a low-carbohydrate diet was the restriction made and other dietary regimens were the inclusion of diet-containing ascorbic acid, antioxidants, omega-3 fatty acids, and fibers. This was the potential limitation of this randomized trial and there always lies the possibility of residual confounding factors that are unmeasured or unrecorded.
CONCLUSION
The current study showed that a dietary pattern with low carbohydrate, rich in omega-3 fatty acids, vitamin C, antioxidants, and fibers can significantly reduce gingival inflammation. Furthermore, interventional studies, particularly having groups as monotherapy (nutritional supplementation alone), are necessary for proper evaluation of nutritional impact on periodontal status.
FINANCIAL SUPPORT AND SPONSORSHIP
Nil.
CONFLICTS OF INTEREST
There are no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Not applicable.
ETHICAL POLICY AND INSTITUTIONAL REVIEW BOARD STATEMENT
Not applicable.
PATIENT DECLARATION OF CONSENT
Not applicable.
DATA AVAILABILITY STATEMENT
Not applicable.
ACKNOWLEDGMENT
Not applicable.
REFERENCES
- 1.Milward MR, Chapple ILC. The role of diet in periodontal disease. Dental Health. 2013;52:18–21. [Google Scholar]
- 2.Vander Velden U, Kuzmanova D, Chapple ILC. Micronutritional approaches to periodontal therapy. J Clin Periodontol. 2011;38:142–58. doi: 10.1111/j.1600-051X.2010.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner S, Imfeld T, Schicht O, Rath C, Persson RE, Persson GR. The impact of the Stone Age diet on gingival conditions in the absence of oral hygiene. J Periodontol. 2009;80:759–68. doi: 10.1902/jop.2009.080376. [DOI] [PubMed] [Google Scholar]
- 4.Tonetti MS, Chapple ILC. Biological approaches to the development of novel periodontal therapies – Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38:114–8. doi: 10.1111/j.1600-051X.2010.01675.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim HS, Park JW, Yeo SI, Choi BJ, Suh JY. Effects of high glucose on cellular activity of periodontal ligament cells in vitro. Diabetes Res Clin Pract. 2006;74:41–7. doi: 10.1016/j.diabres.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 6.El-Sharkawy H, Aboelsaad N, Eliwa M, Darweesh M, Alshahat M, Kantarci A, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. J Periodontol. 2010;81:1635–43. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- 7.Elkhouli AM. The efficacy of host response modulation therapy (omega-3plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study) J Periodont Res. 2011;46:261–8. doi: 10.1111/j.1600-0765.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 8.Merchant AT. Plasma vitamin C is inversely associated with periodontitis. J Evid Based Dent Pract. 2008;8:103–4. doi: 10.1016/j.jebdp.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Nishida M, Grossi SG, Dunford RG, Ho AW, Trevisan M, Genco RJ. Dietaryvitamin C and the risk for periodontal disease. J Periodontol. 2000;71:1215–23. doi: 10.1902/jop.2000.71.8.1215. [DOI] [PubMed] [Google Scholar]
- 10.Amaliya A, Timmerman MF, Abbas F, Loos BG, Van der Weijden GA, Van Winkelhoff AJ, et al. Java project on periodontal diseases: the relationship between vitamin C and the severity of periodontitis. J Clin Periodontol. 2007;34:299–304. doi: 10.1111/j.1600-051X.2007.01053.x. [DOI] [PubMed] [Google Scholar]
- 11.Feinman RD, Pogozelski WK, Astrup A, Bernstein RK, Fine EJ, Westman EC, et al. Dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base. Nutrition. 2015;31:1–13. doi: 10.1016/j.nut.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Woelber JP, Bremer K, Vach K, Konig D, Hellwig E, Ratka-Kruger P, et al. An oral health optimized diet can reduce gingival and periodontal inflammation inhumans—a randomized controlled pilot study. BMC Oral Health. 2017;17:28. doi: 10.1186/s12903-016-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauner H, Bechthold A, Boeing H, Brönstrup A, Buyken A, Leschik-Bonnet E, et al. Evidence-based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition-related diseases. Ann Nutr Metab. 2012; 60:1–58. doi: 10.1159/000335326. [DOI] [PubMed] [Google Scholar]
- 14.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 15.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 16.Wani RT. Socioeconomic status scales—modified Kuppuswamy and Udai Pareekh's scale updated for 2019. J Family Med Prim Care. 2019;8:1846–9. doi: 10.4103/jfmpc.jfmpc_288_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chapple ILC, Matthews JB. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontology. 2000 2007;43:160–232. doi: 10.1111/j.1600-0757.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Hunter P. Pulling teeth from history: DNA from ancient teeth can help to yield information about our ancestors’ health, diet and diseases. EMBO Rep. 2014;15:923–5. doi: 10.15252/embr.201439353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler CJ, Dobney K, Weyrich LS, Kaidonis J, Walker AW, Haak W, et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat Genet. 2013;45:450–5. doi: 10.1038/ng.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hujoel PP, Lingström P. Nutrition, dental caries and periodontal disease: A narrative review. J Clin Periodontol. 2017;44(Suppl. 18):S79–84. doi: 10.1111/jcpe.12672. [DOI] [PubMed] [Google Scholar]
- 21.Merchant AT, Pitiphat W, Franz M, Joshipura KJ. Whole-grain and fiber intakes and periodontitis risk in men. Am J ClinNutr. 2006;83:1395–400. doi: 10.1093/ajcn/83.6.1395. [DOI] [PubMed] [Google Scholar]
- 22.Murtaza N, Burke LM, Vlahovich N, Charlesson B, O’Neill HM, Ross ML. Analysis of the effects of dietary pattern on the oral microbiome of elite endurance athletes. Nutrients. 2019;11:614. doi: 10.3390/nu11030614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chapple IL, Bouchard P, Cagetti MG, Campus G, Carra MC, Cocco F, et al. Interaction of lifestyle, behaviour or systemic diseases with dental caries and periodontal diseases: Consensus report of group 2 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin Periodontol. 2017;44:S39–51. doi: 10.1111/jcpe.12685. [DOI] [PubMed] [Google Scholar]
- 24.Keller KM, Kressirer AC, Belstrom D, Twetman S, Anne CR, Tanner CRA. Oral microbial profiles of individuals with different levels of sugar intake. J Oral Microbiol. 2017;9:1–7. doi: 10.1080/20002297.2017.1355207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edlund A, Yang Y, Yooseph S, Hall AP, Nguyen DD, Dorrestein PC, et al. Meta-omics uncover temporal regulation of pathways across oral microbiome genera during in vitro sugar metabolism. ISME J. 2015;9:2605–19. doi: 10.1038/ismej.2015.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Woudenbergh GJ, Theofylaktopoulou D, Kuijsten A, Ferreira I, Van Greevenbroek MM, Van der Kallen CJ, et al. Adapted dietary inflammatory index and its association with a summary score for low-grade inflammation and markers of glucose metabolism: the Cohortstudy on Diabetes and Atherosclerosis Maastricht (CODAM) and the Hoorn study. Am J Clin Nutr. 2013;98:1533–42. doi: 10.3945/ajcn.112.056333. [DOI] [PubMed] [Google Scholar]
- 27.Lula EC, Ribeiro CC, Hugo FN, Alves CM, Silva AA. Added sugars and periodontal disease in young adults: an analysis of NHANES III data. Am J ClinNutr. 2014;100:1182–7. doi: 10.3945/ajcn.114.089656. [DOI] [PubMed] [Google Scholar]
- 28.Kondo K, Ishikado A, Morino K, Nishio Y, Ugi S, Kajiwara S, et al. A high-fiber, low-fat diet improves periodontal disease markers in high-risk subjects: A pilot study. Nutr Res. 2014;34:491–8. doi: 10.1016/j.nutres.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Iwasaki M, Manz MC, Moynihan P, Yoshihara A, Muramatsu K, Watanabe R, et al. Relationship between saturated fatty acids and periodontal disease. J Dent Res. 2011;90:861–7. doi: 10.1177/0022034511405384. [DOI] [PubMed] [Google Scholar]
- 30.Chee B, Park B, Fitzsimmons T, Coates AM, Bartold PM. Omega-3 fatty acids as an adjunct for periodontal therapy—a review. Clin Oral Investig. 2016;20:879–94. doi: 10.1007/s00784-016-1750-2. [DOI] [PubMed] [Google Scholar]
- 31.Umrania VV, Rao Deepika PC, Kulkarni M. Evaluation of dietary supplementation of omega-3 polyunsaturated fatty acids as an adjunct to scaling and root planing on salivary interleukin-1β levels in patients with chronic periodontitis: A clinico-immunological study. J Indian Soc Periodontol. 2017;21:386–90. doi: 10.4103/jisp.jisp_16_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapple ILC, Milward MR, Ling-Mountford N, Weston P, Carter K, Askey K, et al. Adjunctive daily supplementation withencapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double-blind RCT. J Clin Periodontol. 2012;39:62–72. doi: 10.1111/j.1600-051X.2011.01793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanri H, Yamada Y, Itoi A, Yamagata E, Watanabe Y, Yoshida T, et al. Frequency of fruit and vegetable consumption and the oral health-related quality of life among Japanese elderly: A cross-sectional study from the Kyoto-Kameoka Study. Nutrient. 2017;9:e1362. doi: 10.3390/nu9121362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodington DW, Fritz PC, Sullivan PJ, Ward WE. Higher intakes of fruits and vegetables, β-carotene, vitamin C, α-tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J Nutr. 2015;145:2512–9. doi: 10.3945/jn.115.211524. [DOI] [PubMed] [Google Scholar]
- 35.Muniz FW, Nogueira SB, Mendes FLV, Rösing CK, Moreira MMSM, de Andrade GM, et al. The impact of antioxidant agents complimentary to periodontal therapy on oxidative stress and periodontal outcomes: A systematic review. Arch Oral Biol. 2015;60:1203–14. doi: 10.1016/j.archoralbio.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Zare Javid A, Seal CJ, Heasman P, Moynihan PJ. Impact of a customized dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J Hum Nutr Diet. 2014;27:523–32. doi: 10.1111/jhn.12184. [DOI] [PubMed] [Google Scholar]
- 37.Abou Sulaiman AE, Shehadeh RM. Assessment of total antioxidant capacity and the use of vitamin C in the treatment of non-smokers with chronic periodontitis. J Periodontol. 2010;81:1547–54. doi: 10.1902/jop.2010.100173. [DOI] [PubMed] [Google Scholar]
- 38.Staudte H, Sigusch BW, Glockmann E. Grapefruit consumption improves vitamin C status in periodontitis patients. Br Dent J. 2005;199:213–7. doi: 10.1038/sj.bdj.4812613. [DOI] [PubMed] [Google Scholar]
- 39.Woźniewicz M, Nowaczyk PM, Kurhańska-Flisykowska A, Wyganowska-Świątkowska M, Lasik-Kurdyś M, Walkowiak J, et al. Consumption of cranberry functional beverage reduces gingival index and plaque index in patients with gingivitis. Nutr Res. 2018;58:36–45. doi: 10.1016/j.nutres.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Widen C, Coleman M, Cariten S, Karlgren-Andersson P, Renvert S, Persson GR. Consumption of bilberries controls gingival inflammation. Int J Mol Sci. 2015;16:10665–73. doi: 10.3390/ijms160510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenzsch A, Eick S, Rassoul F, Purschwitz R, Jentsch H. Nutritional intervention in patients with periodontal disease: clinical, immunological and microbiological variables during 12 months. Br J Nutr. 2009;101:879–85. doi: 10.1017/S0007114508047776. [DOI] [PubMed] [Google Scholar]
- 42.Bazyar H, Norouzabad ML, Yarahmadi M, Gholinezhad H, Moradi L, Salehi P. The impacts of synbiotic supplementation on periodontal indices and biomarkers of oxidative stress in type 2 diabetes mellitus patients with chronic periodontitis under non-surgical periodontal therapy. A double-blind, placebo-controlled trial. Diabetes Metab Syndr Obes Targets Ther. 2020;13:19–29. doi: 10.2147/DMSO.S230060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Longvah T, Ananthan R, Bhaskarachary K, Venkaiah K. Indian Food Composition Tables. Hyderabad (India): National Institute of Nutrition, Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Government of India; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.