Abstract
In cooperatively breeding species where rearing effort is shared among multiple group members, increases in group size typically reduce average per capita contributions to offspring care by all group members (load-lightening) but it is not known how changes in group size affect the distribution of workload among group members. The socioeconomic collective action theory suggests that, in larger groups, the incentives for free riding are stronger, leading to greater inequalities in work division among group members. Here, we use the Gini index to measure inequality at the group level in the contributions of helpers to three different cooperative behaviours (babysitting, pup-provisioning and raised guarding) in groups of varying size in wild Kalahari meerkats (Suricata suricatta). In larger groups, inequality in helpers' contributions to cooperative activities and the frequency of free riding both increased. Elevated levels of inequality were generated partly as a result of increased differences in contributions to cooperative activities between helpers in different sex and age categories in larger groups. After controlling for the positive effect of group size on total provisioning, increasing levels of inequality in contributions were associated with reductions in total pup-provisioning conducted by the group. Reductions in total pup-provisioning were, in turn, associated with reductions in the growth and survival of pups (but pup growth and survival were not directly affected by inequality in provisioning). Our results support the prediction of collective action theory described above and show how the Gini index can be used to investigate the distribution of cooperative behaviour within the group.
Keywords: group size, cooperative breeding, free riding, inequality, meerkat, animal cooperation
1. Introduction
Selection favouring ‘free riding' represents a pervasive threat to the maintenance of cooperation in animal and human societies, and its incidence is predicted to rise with increasing group size according to the collective action theory [1,2]. In many cooperatively breeding species where offspring rearing is shared among multiple group members, increases in group size reduce average per capita contributions to offspring care in all group members—a process known as ‘load-lightening' (e.g. [3,4–9]). However, it is unclear whether load-lightening affects the equality of work division within groups. Some studies of cooperative breeders show that increases in group size accentuate disparities in workload between age and sex categories of group members [6,10,11]. For example, in white-winged choughs (Corcorax melanorhamphos) young helpers contribute less to incubation than adults in large but not in small groups [11]. However, comparisons between categories of animals cannot determine whether individual variation and inequality in workloads increase in larger groups: what is needed is a single estimate of inequality for each group, calculated using records of the workload from all group members allowing comparisons of inequality across groups of different size.
As group size increases, at least three different processes may elevate inequalities in cooperative contributions among group members, but their relative importance is unclear. First, increases in group size and intragroup competition for resources [12–14] may depress the growth of weaker individuals [15], increasing variance in size and condition among group members and affecting their capacity to provide assistance to other individuals [16,17]. Second, as group size increases and the ratio between helpers and offspring rises, the benefits of additional units of assistance to dependent juveniles will fall, and individuals with the lowest benefit-cost ratios of helping are likely to reduce their contributions most or, in some cases, to cease contributing altogether, as collective action theory [1] suggests. If individuals with the lowest benefit-cost ratios reduce their workloads disproportionately, individuals who have higher benefit-cost ratios may increase their own contributions disproportionately to achieve the collective good (‘exploitation of the great by the small' ([1, p. 35]), further enhancing inequality in workload. Third, assuming that social enforcement reduces the incidence of free riding, a decline in the capacity of dominant individuals to enforce cooperation in larger groups [1,18,19], may lead to increased inequality in contributions among group members. Variation in group members' contributions may also be associated with variation in the total amount of food given to dependant juveniles with consequences for their growth and survival. For example, increased inequalities in workload may reduce total investment in juveniles if individuals do not fully compensate for the presence of free riders [20,21] or if they respond by adjusting their own contributions downwards (inequity aversion; [22,23]) so that the average contributions of individuals decline and the total amount of food received by juveniles is reduced.
Here, we investigate the relationships between group size and variation in the contributions of individual helpers to cooperative activities within groups in wild meerkats (Suricata suricatta). Our analyses use data from a long-term study of meerkats in the southern Kalahari that provides extensive records of cooperative behaviour and body weight in large numbers of recognizable individuals of known age across different group sizes [24]. To measure the extent of inequality in contributions among the helpers within groups, we used the Gini index, which quantifies the extent to which the distribution of a given value deviates from a state of complete equality [25]. We also examined some other indices of inequality to validate our results, as recommended by Kokko [26], but the Gini index was our primary index as it provides a straightforward measure, which is the most widely used one for inequality (particularly in economics [25]) and also has a simple correction for small sample size bias [27] that was useful for us (see Methods). Our aim was to determine: (i) whether inequality in cooperative contributions and the frequency of free riders (defined below) is higher in larger groups, (ii) whether sex and age-related differences in individual contributions increase with group size; and (iii) whether increased inequality in workload among group members is associated either with reductions in the total amount of care provided to dependent juveniles or with reductions in their growth and survival.
Group size in our meerkat study population has ranged from 2 to 50 (mean ± s.d.: 12.7 ± 6.2) and reproduction is largely monopolized by a single dominant pair in each group who produce over 80% of the surviving young born in the group [28]. In addition to the dominant pair, groups include varying numbers of subordinate helpers of both sexes that are typically animals born in the group to the current or previous dominant females and usually remain in the group for 2–3 years [24]. All group members contribute to three principal cooperative activities (babysitting, pup-provisioning and raised guarding, see Methods for details) though the extent of their contributions varies with helper sex, age and condition but not with their kinship to the pups they are rearing [29]. Dominant meerkats of both sexes contribute less than helpers to babysitting and provisioning [6], while female helpers babysit and provision pups more than males [30] and young helpers contribute less to babysitting and guarding than adults [31]. The combined contributions of helpers are substantially greater than those of breeders [6] and pup growth and survival are reduced in small groups [32] as well as in groups of more than twenty-five individuals [33], probably as a result of increased competition for food resources [15]. Previous studies have shown that cooperative activities have substantial energetic costs to helpers and breeders [16,17] and that the workload of individual group members falls as group size increases [6].
We investigated the effect of variation in the number of helpers per group on the distribution of helpers’ contributions to the three different cooperative breeding activities using data collected over a period of 20 years, involving roughly 450 breeding attempts and 1540 helpers (that are above six months of age, see Methods). For each activity, we quantified inequality (Gini index) in the contributions of group members and tested whether the degree of inequality and the frequency of free riders (individuals that contributed less than 10% of the average contribution in their group, see Methods) rose with helper number. Because changes in average contributions to pup-provisioning are likely to be more closely related to helper–pup ratios than to helper number [34] while average contributions to babysitting appear to be independent of pup number ([17]; see Results), we also investigated the relationships between both inequality and free riding in pup-provisioning and helper–pup ratios. We further tested whether sex and age-related differences in the contributions of helpers to cooperative behaviour increased with helper number (or helper–pup ratios in the case of pup-provisioning). To determine whether changes in helping contributions with increasing helper number might be caused by increased differences in body weight or in daily weight gain between helpers, we tested whether variation in body weight or helper weight gain (both corrected for age) increased with helper number. Finally, we investigated whether increased inequalities in contributions to cooperative behaviour were associated with reductions in overall pup-provisioning and guarding performed by the group and with reductions in pup growth or survival.
2. Methods
(a). The study system and data collection
The study was based on long-term observational data collected from a meerkat population in the southern Kalahari Desert between 1995 and 2016 (26°58′ S, 21°49′ E, [24]) and all procedures were approved by the Animal Ethics Committee of the University of Pretoria, South Africa (no. EC010-13) and by the Northern Cape Department of Environment and Nature Conservation, South Africa (FAUNA 1020/2016). Animals were habituated to close observation and were individually recognizable by dye marks in their fur [24]. This allowed us to record individual contributions to three cooperative activities during pup-rearing periods: (i) babysitting during the first 3–4 weeks of the life of litters when at least one adult stays with the pups at the birth burrow throughout the day while the rest of the group forages [24]. During the babysitting period (from birth until the pups started joining to the group's foraging forays) groups were visited once or twice a day (morning and afternoon) and babysitting group members were recorded; (ii) pup-provisioning, when pups begin to forage with the group but are not yet nutritionally independent (roughly 4–12 weeks after birth); and (iii) guarding or ‘sentinel duty', where an individual provides sustained vigilance for potential dangers from a raised position while the group members are foraging. Groups were observed 3–5 times per week, for 3 h in the morning from the time the group left the burrow and for an hour prior to the group returning to the burrow in the evening. During group observations, the observer\s recorded: feeding events, guarding time (start and end times of guarding bouts) and individual presence in the group (see [29], for more details), providing extensive observational data. Additionally, comprehensive weight data were available as most of the individuals were trained to climb on electronic scales and were weighed three times a day (dawn; after 3 h of foraging; and dusk) on approximately 2–3 days a week [6].
(b). Data processing and filtering
Individual contributions to babysitting were quantified as total half-days spent babysitting a litter (each morning/afternoon record is considered a half-day babysitting). This was performed on a half-day basis as individuals acting as babysitters often changed between morning and afternoon, particularly if the group returned to the burrow in the middle of the day [35]. We included only group babysitting periods for which more than 50% of the half-days were recorded (resulting in 6228 individual periods of babysitting on 491 litters from 1664 individuals at 41 groups (group identity was carried forward even when all original group members had been replaced by descendants or immigrants)). Pup-provisioning data were restricted to the main provisioning period—the first 45 days after the pups first joined the group's foraging forays (around the age of four weeks). We calculated the total number of provisioning events per helper per provisioning period. Guarding time was calculated as the total time (minutes) the helper was on guard per pup-provisioning period. Guarding behaviour was examined just during the pup-provisioning period to concentrate on the cooperative pup-defence motive of guarding [36] over the selfish ones (see [37]). To enhance data reliability, we included only provisioning periods for which we had more than 1400 min of observations on the group, resulting in 5582 individual periods of provisioning/guarding of 453 litters by 1540 individuals at 38 groups. In all analyses, we included only helpers that were present in their groups for more than 50% of the observed pup-care period (provisioning or babysitting), and juvenile helpers below the age of six months were not included as they contribute very little to cooperative activities [31]. From here onwards, every mention of ‘helper' or ‘helpers' refers only to the helpers that were included in our analyses (see above).
We calculated the helper weight gain over the period of pup-provisioning by subtracting its average weight in the week after this period from its average weight the week before this period (for 18% of the records these data were not available as individuals could not always be weighed). In order to control for the effects of age on weight gain [31], we used the helper residual weight gain based on a linear regression of weight gain as a function of age (first- and second-order effects of age). Helpers' residual weight gains were also examined over the babysitting period (in the same way), but results did not change qualitatively and are not reported.
The accumulated rainfall in the three months preceding each pup-care session was extracted from the National Oceanic and Atmospheric Administration project [38], see the electronic supplementary material S1 for details.
(c). Gini index of inequality
We used the Gini index to quantify inequality among the group's helpers in cooperative behaviours and weight gain (or loss) over the pup-rearing period. The Gini index measures the extent to which the distribution of the observed values among individuals deviates from a perfectly equal distribution [27,39]. It is bound between 0 and 1 where 1 represents maximum inequality. The Gini index can be calculated from the mean absolute difference between all pairs of individuals divided by two times the mean [27]:
n—number of individuals.
We added a correction for small sample bias of the Gini index [27] and an adjustment to allow negative values [25], which is needed to examine inequality in weight gains (that can be negative), resulting in the revised formula:
where , and and are the sum of the positive and negative values, respectively.
Using Monte Carlo simulations, George Deltas [27] has shown that the Gini index decreases with sample size, and provided a correction for small sample bias, which we used above. Nevertheless, because potential biases of this kind could be of crucial concern to our study, it was essential to verify that there was no inherent link between the corrected Gini index and group size owing to computational rather than biological reasons. To tackle this concern, we tested whether in randomized data there was an effect of group size on the corrected Gini index. Using computational permutations, we randomly distributed 330 provisioning events (which is the average total events per pup-provisioning period in our data) among individuals in varying group sizes of 5,10,15,20 (1000 permutations per group size) and tested for the effect of group size on inequality calculated per group. To further assess the reliability of our findings and following the recommendation of Kokko et al. [26], we also examined other inequality indices including the Thiel index (with jack-knife correction for small sample size), Hoover index, 20 : 20 ratio index and coefficient of variance ([26,40], see the electronic supplementary material S2 for details).
(d). Data analysis
Inequality in cooperation within groups was measured with the Gini index per each pup-provisioning or babysitting period. We tested inequality among helpers in pup-provisioning, babysitting, guarding time and residual weight gain. We included in the inequality analyses only groups with 3–25 helpers. The Gini index cannot be calculated for fewer than three individuals, and for very large groups of above 25 helpers, data are scarce (2% of the data) and less accurate since the individual contribution to pup-provisioning is more difficult to record.
Because the Gini index is bounded between 0 and 1, it was examined with beta regression [41] using generalized linear mixed models (GLMM) with beta error distribution and logit link function using the glmmTMB package [42]. All inequality analyses included group identity (ID) as random factor and the following fixed factors: (i) helper number—number of helpers above six months of age that were present in the group for more than 50% of focal pup-care period (following visual examination of the data, a quadratic term was also tested); (ii) pup number; (iii) total observation time per pup-care period (not included in the weight gain inequality analysis); (iv) rainfall, which strongly affects food availability and cooperative behaviour in our study system [43,44]; (v) three metrics of group composition to control for potential positive effects of heterogeneity in group composition on inequality in group members' contributions: the proportion of female helpers, the proportion of adult helpers (greater than 2 years), and the coefficient of variation (CV) of helpers' age in the group as an index of age heterogeneity; and (vi) helper–pup ratio, which provides an index for the pup-provisioning workload in the group, and we used the log of this ratio that improves the linearity of the effect. For each inequality analysis, we reported the best model based on the all-subset model selection using Akaike's information criterion (AIC) (using ‘dredge' R function [45]), and in cases where the focal parameter helper number is not part of the best model we provide its coefficients based on the full model. Helper–pup ratio was not included together with helper and pup numbers in the same subset model, only in competing models, as a helper–pup ratio and helper number were correlated (see Results).
As inequality in pup-provisioning was more strongly affected by helper–pup ratios than by helper number in contrast with inequalities in babysitting and guarding (see Results), helper–pup ratio was used for plotting trends in provisioning inequality, as well as in follow-up analyses on provisioning differences between categories of individuals (see below), and for the examination of free riding in provisioning.
The proportion of free-riding helpers in the group was examined per pup-provisioning or babysitting period. Free-riding helpers were defined as the ones that contributed less than 10% of the average contribution in the group—namely, less than 10% of what they would be expected to contribute under a hypothetical state of equal work division. The free riders contributed very little to pup-care: on average they provisioned 0.4 food items per 10 foraging hours, guarded 0.5 min per 10 foraging hours and babysat 0.02 half-days during the whole babysitting period (of approximately 20 days), versus 6.4 food items, 13.3 min guarding and 6.5 half-days babysitting contributed by non-free riders, respectively. The proportions of free-riding helpers per group per pup-care period per cooperative activity were examined using binomial GLMMs with a zero-inflation factor with the predictors: helper number, pup number (or helper–pup ratio for pup-provisioning), rainfall and total observation time and with group ID as a random factor. Full models are reported throughout, and for the analysis of free riding in provisioning, we used AIC comparison to decide whether to use helper and pup numbers or helper–pup ratios.
To better understand the origins of the inequality trends, we tested how contributions of individual helpers of different sex and age interact with helper number (or helper–pup ratio in the case of pup-provisioning). For this, (i) individual total pup-provisioning events per pup-provisioning period and (ii) individual total guarding time per pup-provisioning period were examined (separately) using GLMMs with negative binomial error distribution and log link function, where the log of the total observation time per provisioning period was included as an offset in the models; and (iii) individual contributions to babysitting per period were modelled using a GLMM with a beta-binomial error distribution and a logit link function with the proportion of half-days babysat out of the total half-days observed as the response variable. Beta-binomial was chosen over binomial distribution to account for overdispersion and a zero-inflation parameter was added to the model (applied across all observations, zi = ∼1). The GLMMs of helpers' contributions to the provisioning / guarding / babysitting included the factors: sex and age and their interaction with helper number (or helper–pup ratio in the case of pup-provisioning), pup number (except for provision models in which helper-pup ratio was included), residual weight (standardized against first- and second-order effects of age), rainfall and the random factors: individual, group and litter IDs. All models of individual cooperative behaviour having GLMM as model type in the Result section follow these specifications.
We tested for an association between inequality in pup-provisioning and the total provisioning by the group and the effects of both on pup survival and pup weight gain. In all these analyses, we also accounted for the following predictors: helper number, pup number and rainfall. In addition, we also included the random factor group ID, and in the analyses of pup survival and weight gain, also litter ID. Similarly, we tested for an association between inequality in guarding time and the total guarding by the group and their effects on pup survival and weight gain, controlling for the additional predictors and random factors listed above. We did not conduct a similar examination on the total amount of babysitting conducted by the group as totals did not vary with group size [16]. Pup survival was examined as a binary variable indicating whether the pup survived from the age of 40 days, shortly after starting to forage with the group (start foraging age ± s.d.: 29 ± 4 days), until the age of 90 days, and pup weight gain was the weight increase over this period.
All continuous predictors in all analyses were scaled to two s.d. from the mean to allow for relative effect size comparison [46]. Collinearity for all fixed effects was tested using variance inflation factors with a threshold value of three [47]. Residuals' fit to the expected distribution and absence of heteroscedasticity were assessed with the DHARMa package [48]. Data were processed in Matlab [49] and statistically analysed in Matlab and in R3.6 [45] using the glmmTMB package [42].
3. Results
Helper number was highly correlated with group size (the average number of non-pup group members; r = 0.96, p < 0.001, n [number of breeding sessions] = 429) and was significantly correlated with helper–pup ratio (r = 0.66, p < 0.001, n = 429), but not with pup number (r = −0.01, p = 0.76, n = 429).
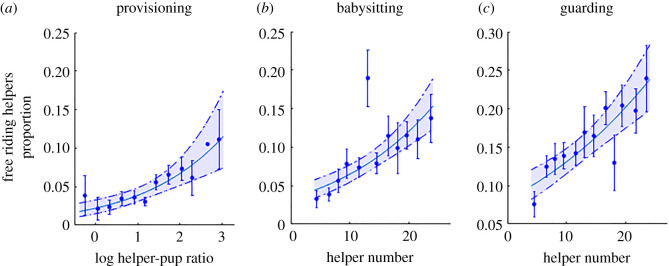
Within-group inequality (Gini index) in helpers' contributions to all three cooperative activities increased with helper number (for babysitting this relationship was quadratic; figure 1a–c and table 1; electronic supplementary material, table S1). In randomized data (see Methods), there was no effect of helper number on the Gini index (linear regression; β = 0.0003 ± 0.003, t3998 = 0.1, p = 0.92), indicating that there was no computationally driven link between them that could underlie our findings, and the above trends persisted when we examined other indices of inequality (Thiel, Hoover, 20 : 20 ratio and CV; electronic supplementary material, figure S1, examined just for the provisioning behaviour). Inequality in pup-provisioning increased more closely with helper–pup ratios than with helper number (table 1; electronic supplementary material, table S1), whereas inequalities in babysitting and guarding, that were not affected by pup number, increased only with helper number (table 1; electronic supplementary material, table S1). Inequality in cooperative behaviours was also affected by several additional factors: inequality in pup-provisioning was affected by the proportion of adults in the group; inequality in babysitting was affected by rainfall, age variation, proportion of females and observation time; and inequality in guarding was affected by rainfall, age variation and proportion of adults (see the electronic supplementary material, table S1, for full details). Like inequalities in workload, the frequency of ‘free riders' also rose with helper number or helper–pup ratios (figure 2; electronic supplementary material, table S2). There was no indication that these changes were a consequence of increasing variation in helper weight gain or body condition in larger groups: inequality in the weight gains of group members over the pup-provisioning period (standardized for age) did not change with helper number or helper–pup ratios (figure 1d), it was only affected by rainfall (table 1; electronic supplementary material, table S1), and the CV of helpers' residual body weight (corrected for age) was also not affected by helper number (β = 0.14 ± 0.26, p = 0.60; linear mixed model (LMM); electronic supplementary material, table S3).
Figure 1.
Inequality (Gini index) in cooperative behaviours and in weight gain among the group's helpers. Gini index was corrected for small sample bias (see Methods). The dots and bars mark the mean ± s.e. of the raw data along 20 equal intervals. Lines and shaded areas mark the values predicted by the models ± 95% confidence interval (CI). Asterisk (*) in the upper left denotes significant trends (p < 0.001, statistical details in table 1 and in the electronic supplementary material, table S1). The plots display inequality in: (a) pup-provisioning, (b) babysitting, (c) guarding time and (d) residual weight gains (after controlling for age) of helpers over the pup-provisioning period. In pup-provisioning only, inequality was affected more strongly by helper–pup ratio than by helper number (electronic supplementary material, tables S1 and S10) and therefore it was used in plot (a). (Online version in colour.)
Table 1.
helper number effects on inequalities in cooperative behaviours. (The table summarizes the effects of helper number or helper pup ratio on inequality based on the best model (AIC comparisons) for each of the inequality analyses (electronic supplementary material, table S1), with the exception of the ‘helper weight gain' inequality analysis, in which helper number was not part of the best model and its parametrization is based on the full model (electronic supplementary material, table S13). The response variable is the Gini index (corrected for small sample bias, see Methods) calculated per group per pup-care period, and modelled using a GLMM with beta error distribution and logit link function. Other predictors also had significant effects on inequality (see the electronic supplementary material, table S1 for details). All predictors were scaled to two s.d. from the mean. * p ≤ 0.001.)
| response variable - inequality in: | predictors | β | s.e. | z | p |
|---|---|---|---|---|---|
| pup-provisioning | (log) helper–pup ratioa | 0.25 | 0.05 | 5.48 | * |
| babysitting | helper number | 0.46 | 0.06 | 7.93 | * |
| helper number^2 | −0.32 | 0.09 | −3.66 | * | |
| guarding | helper number | 0.28 | 0.06 | 4.65 | * |
| helper weight gain | helper numberb | −0.10 | 0.09 | −1.10 | 0.27 |
aHelper number and pup number had both significant effects on inequality in provisioning when replacing ‘log helper–pup ratio' in the model (helper number: β ± s.e. = 0.15 ± 0.05, z = 3.16, p = 0.002; pup number: β ± s.e. = −0.18 ± 0.05, z = −3.86, p < 0.001), other than that pup number had no significant effects on inequality in babysitting, guarding and weight gain (electronic supplementary material, tables S11–13).
bNot included in the best model.
Figure 2.
Proportion of free riding helpers versus helper number (or helper–pup ratio). Free riding helpers are ones that contribute less than 10% of the average helper contribution in the group, namely, less than 10% of what they would be expected to contribute under a hypothetical state of equal work division. The dots and bars mark the mean ± s.e. of the raw data. Lines and shaded areas mark the values predicted by the models ± 95% CI. All the effects are significant (p ≤ 0.006, GLMMs, see the electronic supplementary material, table S2 for statistical details). (Online version in colour.)
Contrasts in contributions between different categories of helpers also increased with helper number or helper–pup ratios. Older helpers contributed progressively less than younger ones to pup-provisioning as helper–pup ratio increased, but sex differences in provisioning did not vary with helper–pup ratios (figure 3a,b). Sex and age-related differences in babysitting increased with helper number as males and younger helpers contributed relatively less in larger groups (figure 3c,d). Sex differences in guarding behaviour did not change with helper number while age-related differences increased as younger helpers contributed progressively less as helper number rose (figure 3e,f; electronic supplementary material, table S4).
Figure 3.
Interaction of sex- and age-related differences in cooperative behaviours with helper number. The y-axis displays a ratio between the absolute contribution to the cooperative activity divided by the expected contribution if all group members were to contribute equally (dashed line at y = 1 marks a state of observed equals expected contribution). Mean ± s.e. are plotted at 10 equal intervals spanning the central 95% of the data range (2.5%–97.5% CI's). The left and right columns display sex and age differences, respectively. Age classes (months): 6 < subadults < 12; 12 ≤ yearlings < 24; adults ≥ 24. Asterisks mark significant interaction of the displayed category (sex/age) with the x-axis variable (helper number or helper–pup ratio; *p = 0.004, **p < 0.001; see the electronic supplementary material, table S4 for statistical details). (Online version in colour.)
After controlling for the positive effects of helper and pup numbers on total group provisioning rates (electronic supplementary material, figure S2 and table S5), increased inequality in pup-provisioning was associated with reduced total provisioning rate by the group (figure 4; electronic supplementary material, table S5). Lower group provisioning rates were, in turn, associated with a reduced weight gain of pups (β = 11.14 ± 3.91, p < 0.001, LMM; electronic supplementary material, table S6) and with reduced pup survival from 40 days to three months of age (β = 0.84 ± 0.29, p = 0.004, GLMM; electronic supplementary material, table S7), but the degree of inequality in provisioning was not directly associated with weight gain and survival of pups (p > 0.09; electronic supplementary material, tables S6–7). When the predictors helper and pup numbers in the models reported above in electronic supplementary material, tables S5–7 were replaced with a helper–pup ratio, the results of the other predictors—inequality in provisioning and total provisioning, did not change qualitatively (not reported). After controlling for the positive effect of helper number (electronic supplementary material, figure S2, table S5), increased inequality in guarding time was associated with reduced total guarding by the group (β = −0.30 ± 0.06, p < 0.001, GLMM; electronic supplementary material, table S5), but both had no effect on pup growth (p ≥ 0.16, LMM; electronic supplementary material, table S8) or survival (p ≥ 0.53, GLMM; electronic supplementary material, table S9).
Figure 4.

Inequality and total provisioning rate. Total provisioning rate by: all group members (blue, solid line), members that contribute above the median (green, dash-dotted line) and below the median (red, dashed line), displayed against the group inequality in pup-provisioning. Increased inequality was associated with reduced total provisioning (p < 0.001) as a result of reduced provisioning by the lower half members (p < 0.001) with no compensation for that by the upper half ones (p = 0.53, GLMMs; see the electronic supplementary material, table S5 for statistical details of these three analyses). (Online version in colour.)
4. Discussion
Across all three cooperative behaviours, increases in helper number were associated with increased inequality in helpers' contributions within groups as well as with higher frequencies of free riding. The degree of inequality in pup-provisioning behaviour was more closely related to helper–pup ratios than to helper numbers per se, whereas inequalities in babysitting and guarding contributions were related to helper number and not to pup number, probably because the need for contributions to these activities does not depend on the number of pups present [17]. Increased inequality in workload in large groups was generated in part by increases in sex- and age-related differences in helpers' contributions in larger groups, and previous studies of other cooperative breeders have shown similar effects of group size elevating contribution differences between animals of different age and social rank [6,11,50].
Cooperative breeding activities generate indirect fitness benefits for meerkat helpers who are usually raising the offspring of close kin [29]. They can also provide direct benefits to helpers resulting from the benefits of increased group size [51], such as reduced per capita workload [6] and the capacity to displace neighbouring groups [52]. However, they also have substantial energetic costs [16,17]. We found that male helpers, which have less to gain from recruits to the group than females as they disperse from their natal groups to breed, reduced their contributions to babysitting more than females as the group's helper number increased. In addition, young, subadult helpers (6–12 months) contributed progressively less than other helpers to babysitting and guarding as helper number increased. This was consistent with previous findings in white-winged choughs [11] and was probably a consequence of the greater helping costs to subadults that are still investing in growth and possibly have inferior foraging skills [11,31,53,54]. Surprisingly, subadults did not contribute less than others to pup feeding as helper number rose possibly, because they spent less time on guard than others in larger groups and thus could spend slightly more time foraging. Lastly, we found that older helpers (older than 2 years) contributed relatively less than others to pup feeding as helper number rose. A possible explanation for this intriguing pattern is that older non-breeders that are about to face the challenges of competing for dominance and/or dispersal have to conserve their body condition more than others, so donating food might be more costly for them. In line with this, adult male meerkats who conduct prospecting forays that usually precede dispersal showed reductions in both pup-provisioning behaviour and body weight [55].
Our comparisons indicated that there was a clearer division of tasks between age classes in larger groups, with subadults contributing relatively less to babysitting and guarding and older helpers contributing relatively less to pup feeding. This augmented task division could increase the survival of pups because subadults are less capable of opposing intrusions to the babysitting burrow by predators or rival group members and are less experienced and less reliable as guards [56]. Among vertebrates, task specialization is rare (but see [57]), but our results suggest that it might emerge in unusually large groups. In social insects, which commonly form much larger groups than vertebrates, task specialization is more common and is positively related to interspecific differences in group size [58,59].
As we describe in the Introduction, three different mechanisms might contribute to increases in the inequality of workloads in larger groups: (i) increased variation in condition or growth among group members in larger groups; (ii) reductions in the net benefits of additional care to dependent juveniles in larger groups that include many helpers, causing individuals with the lower benefit-cost ratios to reduce their cooperative contributions disproportionately; and (iii) reductions in the ability of dominant individuals to enforce cooperation on subordinates in larger groups. Of these three processes, it seems most likely that the second is responsible for the increase in inequalities of workload in larger meerkat groups for individual differences in age-corrected body weights and daily weight gains were not greater in larger groups than in smaller ones and there is little evidence that helping is enforced among meerkats [24]. As would be expected if increased inequality in workload was a consequence of reductions in the net benefits of helping, as group size increased categories of helpers that either were likely to suffer greater costs (like younger individuals) or were likely to gain smaller benefits from helping (like males, who normally disperse when they reach breeding age) showed stronger reductions in cooperative behaviour. A previous field experiment that manipulated the workforce in groups of cooperatively breeding carrion crows (Corvus corone corone) by temporary handicapping one helper came to a similar conclusion [8]. This study showed that when the group workforce was larger, the ‘laziest’ helper of the group contributed significantly less than others compared to times when the workforce was smaller, leading to increases in individual differences within groups. Baglione et al. [8] suggest that the lazy non-breeders may voluntarily increase their contribution if they perceive a sudden reduction of group chick provisioning (for example, through detection of changes in chick begging), because in this circumstance their help is most likely to increase the reproductive success of the group, and it is therefore more beneficial in terms of personal indirect fitness gains. A similar pattern may occur in meerkats, where hungry pups give begging calls at higher rates, stimulating helpers to feed them [60]. It is important to note that investigating the adaptive aspects of inequality and free riding to helpers was beyond the scope of this paper and further analyses are needed to truly comprehend the potential fitness drivers of our findings.
Despite higher levels of inequality and free riding, increased group size generally enhances cooperative breeding in meerkats. As group size increases, both helpers and breeders contribute less to cooperative activities owing to load-lightening [6], breeders reproduce more frequently [32], the total rate of pup-provisioning by the group increases asymptotically (electronic supplementary material, figure S2 and table S5) and pup growth and survival both increase, especially under harsh climate conditions [61]. This indicates that generally, the impact of higher free-riding frequency in larger groups of meerkats does not exceed the positive effects of helper number. It also supports the suggestion that, as group size increases, helpers would have a higher incentive for free riding as it would have less influence on the breeding success of the group.
After the effects of helper number have been controlled for, increased inequality in pup-provisioning was associated with a decrease in total provisioning by the group, where total provisioning was correlated with pup growth and survival. Groups with high inequality in provisioning were characterized by low contributions by some helpers with little or no compensation by others (figure 4), which is in line with previous studies of birds and fishes showing that experimental reductions in contributions of particular individuals to brood care are often not fully compensated for by other carers [20,21]. The negative relationship between inequality and total cooperation corresponds to the idea emphasized by the collective action theory that free riding poses a problem to maintaining cooperation [1].
The study of cooperative breeding behaviour may suffer from ‘the tyranny of the golden mean' [62], i.e. mostly comparing individual averages [63] and neglecting individual variation in contributions within groups. Our study concentrated on within-group inequality in cooperative behaviour, and by examining the distribution of contributions it unveiled patterns that the common analyses of average individual contributions could not detect. Similar studies in other species are now needed to assess the generality of the relationship between group size and inequality that we have found in meerkats. The Gini index has rarely been used before in animal studies [64] and we believe that this is its first application to vertebrate cooperative behaviour, although it has previously been used to quantify inequality in foraging in bumblebees [65]. The Gini index provides an intuitive metric of inequality which offers a useful way of quantifying the extent of overall individual differences in the group because it is standardized ([0–1]) and simple to calculate [25]. In future, it may be useful in investigations of the social and ecological factors that affect workload inequality within groups and for comparing inequality in cooperation across species and behaviours.
Supplementary Material
Acknowledgements
We are grateful to the many volunteers and field managers of the Kalahari Meerkat Project (KMP) for their contribution to data collection and to Marta Manser for her contribution to the organisation of the KMP. We thank the Kalahari Research Trust for permission to work at the Kuruman River Reserve and the neighbouring farmers for the use of their land. We would also like to thank all the members of the Large Animal Research Group for helpful discussions and in particular Chris Duncan, Jack Thorley, Ramona Rauber and Penny Roth. Finally, we thank the Mammal Research Institute at the University of Pretoria, South Africa, for logistic support and the Northern Cape Department of Environment and Nature Conservation for permission to conduct the research.
Ethics
The research was approved by the Animal Ethics Committee of the University of Pretoria, South Africa (no. EC010-13) and by the Northern Cape Department of Environment and Nature Conservation, South Africa (FAUNA 1020/2016).
Data accessibility
The data supporting the analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.x69p8czgt [66].
Authors' contributions
S.R. and T.C.-B. conceived of the study; S.R. analysed the data and wrote the first draft and T.C.-B. contributed to the manuscript and approved the final version
Competing interests
We declare we have no competing interests
Funding
We thank the Blavatnik fellowship and the Rothschild fellowship granted to S.R. This paper has relied on research by the Kalahari Meerkat Project which has been supported by grants from: the European Research Council Advanced Grant (nos 742808 and 294494) to T.C.-B., the Human Frontier Science Program (funding reference RGP0051/2017), the University of Zurich to Marta Manser, the MAVA foundation and the Swiss National Science Foundation.
References
- 1.Olson M. 1965. The logic of collective action. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Nunn CL. 2000. Collective benefits, free-riders, and male extra-group conflict. In Primate males: causes and consequences of variation in group composition (ed. Kappeler PM), pp. 192-204. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Houston AI, Davies NB. 1985. The evolution of cooperation and life history in the dunnock, Prunella modularis. In Behavioural ecology (eds Sibly RM, Smith RH), pp. 471-487. Oxford, UK: Blackwell Scientific. [Google Scholar]
- 4.Hatchwell BJ. 1999. Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 154, 205-219. ( 10.1086/303227) [DOI] [PubMed] [Google Scholar]
- 5.Johnstone RA. 2011. Load lightening and negotiation over offspring care in cooperative breeders. Behav. Ecol. 22, 436-444. ( 10.1093/beheco/arq190) [DOI] [Google Scholar]
- 6.Clutton-Brock TH, Russell AF, Sharpe LL. 2004. Behavioural tactics of breeders in cooperative meerkats. Anim. Behav. 68, 1029-1040. ( 10.1016/j.anbehav.2003.10.024) [DOI] [Google Scholar]
- 7.Pike KN, Ashton BJ, Morgan KV, Ridley AR. 2019. Social and individual factors influence variation in offspring care in the cooperatively breeding Western Australian magpie. Front. Ecol. Evol. 7, 92. [Google Scholar]
- 8.Baglione V, Canestrari D, Chiarati E, Vera R, Marcos JM. 2010. Lazy group members are substitute helpers in carrion crows. Proc. R. Soc. B 277, 3275-3282. ( 10.1098/rspb.2010.0745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinsohn RG. 2004. Parental care, load-lightening, and costs. In Ecology and evolution of cooperative breeding in birds (eds Koenig WD, Dickinson JL), pp. 228-238. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 10.Baglione V, Canestrari D. 2016. Carrion crows: family living and helping in a flexible social system. In Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior (eds Dickinson JL, Koenig WD), pp. 97-114. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 11.Heinsohn R, Cockburn A. 1994. Helping is costly to young birds in cooperatively breeding white-winged choughs. Proc. R. Soc. Lond. B 256, 293-298. ( 10.1098/rspb.1994.0083) [DOI] [Google Scholar]
- 12.Jones B, Knapp L, Hardie S, Majolo B, Koyama N, Ventura R, Schino G. 2009. Analysing the effects of group size and food competition on Japanese macaque social relationships. Behaviour 146, 113-137. ( 10.1163/156853908X390959) [DOI] [Google Scholar]
- 13.Pride E. 2005. Optimal group size and seasonal stress in ring-tailed lemurs (Lemur catta). Behav. Ecol. 16, 550-560. ( 10.1093/beheco/ari025) [DOI] [Google Scholar]
- 14.Zöttl M, Thorley J, Gaynor D, Bennett NC, Clutton-Brock T. 2016. Variation in growth of Damaraland mole-rats is explained by competition rather than by functional specialization for different tasks. Biol. Lett. 12, 20160820. ( 10.1098/rsbl.2016.0820) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozgul A, Bateman AW, English S, Coulson T, Clutton-Brock TH. 2014. Linking body mass and group dynamics in an obligate cooperative breeder. J. Anim. Ecol. 83, 1357-1366. ( 10.1111/1365-2656.12239) [DOI] [PubMed] [Google Scholar]
- 16.Clutton-Brock TH, et al. 1998. Costs of cooperative behaviour in suricates (Suricata suricatta). Proc. R. Soc. Lond. B 265, 185-190. ( 10.1098/rspb.1998.0281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell AF, Sharpe LL, Brotherton PNM, Clutton-Brock TH. 2003. Cost minimization by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA 100, 3333-3338. ( 10.1073/pnas.0636503100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer S, Zottl M, Groenewoud F, Taborsky B. 2014. Group-size-dependent punishment of idle subordinates in a cooperative breeder where helpers pay to stay. Proc. R. Soc. B 281, 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang W, Liu W, Viña A, Tuanmu MN, He G, Dietz T, Liu J. 2013. Nonlinear effects of group size on collective action and resource outcomes. Proc. Natl Acad. Sci. USA 110, 10 916-10 921. ( 10.1073/pnas.1301733110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison F, Barta Z, Cuthill I, Szekely T. 2009. How is sexual conflict over parental care resolved? A meta-analysis. J. Evol. Biol. 22, 1800-1812. ( 10.1111/j.1420-9101.2009.01792.x) [DOI] [PubMed] [Google Scholar]
- 21.Zottl M, Fischer S, Taborsky M. 2013. Partial brood care compensation by female breeders in response to experimental manipulation of alloparental care. Anim. Behav. 85, 1471-1478. ( 10.1016/j.anbehav.2013.03.045) [DOI] [Google Scholar]
- 22.Brosnan SF. 2006. Nonhuman species' reactions to inequity and their implications for fairness. Soc. Justice Res. 19, 153-185. ( 10.1007/s11211-006-0002-z) [DOI] [Google Scholar]
- 23.Range F, Horn L, Viranyi Z, Huber L. 2009. The absence of reward induces inequity aversion in dogs. Proc. Natl Acad. Sci. USA 106, 340-345. ( 10.1073/pnas.0810957105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huchard E, English S, Bell MBV, Thavarajah N, Clutton-Brock T. 2016. Competitive growth in a cooperative mammal. Nature 533, 532-534. ( 10.1038/nature17986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffinetti E, Siletti E, Vernizzi A. 2017. Analyzing the effects of negative and non-negative values on income inequality: evidence from the survey of household income and wealth of the Bank of Italy (2012). Soc. Indic. Res. 133, 185-207. ( 10.1007/s11205-016-1354-x) [DOI] [Google Scholar]
- 26.Kokko H, Mackenzie A, Reynolds JD, Lindström J, Sutherland WJ. et al. 1999. Measures of inequality are not equal. Am. Nat. 154, 358-382. ( 10.1086/303235) [DOI] [PubMed] [Google Scholar]
- 27.Deltas G. 2003. The small-sample bias of the Gini coefficient: results and implications for empirical research. Rev. Econ. Stat. 85, 226-234. ( 10.1162/rest.2003.85.1.226) [DOI] [Google Scholar]
- 28.Spong GF, Hodge SJ, Young AJ, Clutton-Brock TH. 2008. Factors affecting the reproductive success of dominant male meerkats. Mol. Ecol. 17, 2287-2299. ( 10.1111/j.1365-294X.2008.03734.x) [DOI] [PubMed] [Google Scholar]
- 29.Duncan C, Gaynor D, Clutton-Brock T, Dyble M. 2019. The evolution of indiscriminate altruism in a cooperatively breeding mammal. Am. Nat. 193, 841-851. ( 10.1086/703113) [DOI] [PubMed] [Google Scholar]
- 30.Clutton-Brock TH, Russell AF, Sharpe LL, Young AJ, Balmforth Z, McIlrath GM. 2002. Evolution and development of sex differences in cooperative behavior in meerkats. Science 297, 253-256. ( 10.1126/science.1071412) [DOI] [PubMed] [Google Scholar]
- 31.Clutton-Brock TH, Russell AF, Sharpe LL. 2003. Meerkat helpers do not specialize in particular activities. Anim. Behav. 66, 531-540. ( 10.1006/anbe.2003.2209) [DOI] [Google Scholar]
- 32.Russell AF, Brotherton PNM, McIlrath GM, Sharpe LL, Clutton-Brock TH. 2003. Breeding success in cooperative meerkats: effects of helper number and maternal state. Behav. Ecol. 14, 486-492. ( 10.1093/beheco/arg022) [DOI] [Google Scholar]
- 33.Bateman AW, Ozgul A, Coulson T, Clutton-Brock TH. 2012. Density dependence in group dynamics of a highly social mongoose, Suricata suricatta. J. Anim. Ecol. 81, 628-639. ( 10.1111/j.1365-2656.2011.01934.x) [DOI] [PubMed] [Google Scholar]
- 34.Russell AF, Clutton-Brock TH, Brotherton PN, Sharpe L, Mcilrath G, Dalerum F, Cameron EZ, Barnard JA. et al. 2002. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 71, 700-709. ( 10.1046/j.1365-2656.2002.00636.x) [DOI] [Google Scholar]
- 35.English S, Nakagawa S, Clutton-Brock TH. 2010. Consistent individual differences in cooperative behaviour in meerkats (Suricata suricatta). J. Evol. Biol. 23, 1597-1604. ( 10.1111/j.1420-9101.2010.02025.x) [DOI] [PubMed] [Google Scholar]
- 36.Santema P, Clutton-Brock T. 2013. Meerkat helpers increase sentinel behaviour and bipedal vigilance in the presence of pups. Anim. Behav. 85, 655-661. ( 10.1016/j.anbehav.2012.12.029) [DOI] [Google Scholar]
- 37.Clutton-Brock TH, O'riain MJ, Brotherton PN, Gaynor D, Kansky R, Griffin AS, Manser M. 1999. Selfish sentinels in cooperative mammals. Science 284, 1640-1644. ( 10.1126/science.284.5420.1640) [DOI] [PubMed] [Google Scholar]
- 38.Adler RF, et al. 2018. The global precipitation climatology project (GPCP) monthly analysis (New Version 2.3) and a review of 2017 global precipitation. Atmosphere 9, 138. ( 10.3390/atmos9040138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ceriani L, Verme P. 2012. The origins of the Gini index: extracts from VariabilitA e MutabilitA (1912) by Corrado Gini. J. Econ. Inequal. 10, 421-443. ( 10.1007/s10888-011-9188-x) [DOI] [Google Scholar]
- 40.Breunig R, Hutchinson DLA. 2008. Small sample bias corrections for inequality indices. In New econometric modeling research (ed. Toggins WN), pp. 61-83. New York, NY: Nova Science Publishers. [Google Scholar]
- 41.Ferrari SLP, Cribari-Neto F. 2004. Beta regression for modelling rates and proportions. J. Appl. Stat. 31, 799-815. ( 10.1080/0266476042000214501) [DOI] [Google Scholar]
- 42.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielson A, Skaug HJ, Maechler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modelling. The R J. 9, 378-400. See https://journal.r-project.org/archive/2017/RJ-2017-066/index.html. [Google Scholar]
- 43.Rauber R, Clutton-Brock TH, Manser MB. 2019. Drought decreases cooperative sentinel behavior and affects vocal coordination in meerkats. Behav. Ecol. 30, 558-566. [Google Scholar]
- 44.Paniw M, Maag N, Cozzi G, Clutton-Brock T, Ozgul A. 2019. Life history responses of meerkats to seasonal changes in extreme environments. Science 363, 633-635. ( 10.1126/science.aau5905) [DOI] [PubMed] [Google Scholar]
- 45.R Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 46.Gelman A. 2008. Scaling regression inputs by dividing by two standard deviations. Stat. Med. 27, 2865-2873. ( 10.1002/sim.3107) [DOI] [PubMed] [Google Scholar]
- 47.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer. [Google Scholar]
- 48.Hartig F. 2019. DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. (R package version 0.2.4.). See https://CRAN.R-project.org/package=DHARMa.
- 49.Matlab . 2016. Matlab, versions 9.0.0.341360. Natick, MA: The Mathworks Inc.
- 50.Houslay TM, Vullioud P, Zöttl M, Clutton-Brock TH. 2020. Benefits of cooperation in captive Damaraland mole-rats. Behav. Ecol. 31, 711-718. ( 10.1093/beheco/araa015) [DOI] [Google Scholar]
- 51.Kingma SA, Santema P, Taborsky M, Komdeur J. 2014. Group augmentation and the evolution of cooperation. Trends Ecol. Evol. 29, 476-484. ( 10.1016/j.tree.2014.05.013) [DOI] [PubMed] [Google Scholar]
- 52.Dyble M, Houslay TM, Manser MB, Clutton-Brock T. 2019. Intergroup aggression in meerkats. Proc. R. Soc. B 286, 20191993. ( 10.1098/rspb.2019.1993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woxvold IA, Mulder RA, Magrath MJL. 2006. Contributions to care vary with age, sex, breeding status and group size in the cooperatively breeding apostlebird. Anim. Behav. 72, 63-73. ( 10.1016/j.anbehav.2005.08.016) [DOI] [Google Scholar]
- 54.Nichols HJ, Amos W, Bell MB, Mwanguhya F, Kyabulima S, Cant MA. 2012. Food availability shapes patterns of helping effort in a cooperative mongoose. Anim. Behav. 83, 1377-1385. ( 10.1016/j.anbehav.2012.03.005) [DOI] [Google Scholar]
- 55.Young AJ, Carlson AA, Clutton-Brock T. 2005. Trade-offs between extraterritorial prospecting and helping in a cooperative mammal. Anim. Behav. 70, 829-837. ( 10.1016/j.anbehav.2005.01.019) [DOI] [Google Scholar]
- 56.Rauber R, Manser MB. 2018. Experience of the signaller explains the use of social versus personal information in the context of sentinel behaviour in meerkats. Sci. Rep. 8, 1-7. ( 10.1038/s41598-018-29678-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruintjes R, Taborsky M. 2011. Size-dependent task specialization in a cooperative cichlid in response to experimental variation of demand. Anim. Behav. 81, 387-394. ( 10.1016/j.anbehav.2010.10.004) [DOI] [Google Scholar]
- 58.Anderson C, McShea DW. 2001. Individual versus social complexity, with particular reference to ant colonies. Biol. Rev. 76, 211-237. ( 10.1017/S1464793101005656) [DOI] [PubMed] [Google Scholar]
- 59.Holbrook CT, Barden PM, Fewell JH. 2011. Division of labor increases with colony size in the harvester ant Pogonomyrmex californicus. Behav. Ecol. 22, 960-966. ( 10.1093/beheco/arr075) [DOI] [Google Scholar]
- 60.Manser MB, Avey G. 2000. The effect of pup vocalisations on food allocation in a cooperative mammal, the meerkat (Suricata suricatta). Behav. Ecol. Sociobiol. 48, 429-437. ( 10.1007/s002650000248) [DOI] [Google Scholar]
- 61.Groenewoud F, Clutton-Brock T. In press. Meerkat helpers buffer the detrimental effects of adverse environmental conditions on fecundity, growth and survival. J. Anim. Ecol. [DOI] [PubMed] [Google Scholar]
- 62.Bennett AF. 1987. Interindividual variability: an underutilized resource. In New directions in ecological physiology (eds Feder ME, Bennett AF, Burggren WW, Huey RB), pp. 147-169. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 63.Koenig WD, Dickinson JL. 2016. Cooperative breeding in vertebrates: studies of ecology, evolution, and behavior. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 64.Gorelick R, Bertram SM. 2007. Quantifying division of labor: borrowing tools from sociology, sociobiology, information theory, landscape ecology, and biogeography. Insect. Soc. 54, 105-112. ( 10.1007/s00040-007-0923-z) [DOI] [Google Scholar]
- 65.Crall JD, Gravish N, Mountcastle AM, Kocher SD, Oppenheimer RL, Pierce NE, Combes SA. 2018. Spatial fidelity of workers predicts collective response to disturbance in a social insect. Nat. Commun. 9, 1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rotics S, Clutton-Brock T. 2021. Data from: Group size increases inequality in cooperative behaviour. Dryad Digital Repository. ( 10.5061/dryad.x69p8czgt) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Rotics S, Clutton-Brock T. 2021. Data from: Group size increases inequality in cooperative behaviour. Dryad Digital Repository. ( 10.5061/dryad.x69p8czgt) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting the analyses are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.x69p8czgt [66].