Abstract
Background and Aim:
It is unclear whether blood pressure (BP) is associated with cognition after stroke. We examined associations between systolic and diastolic BP (SBP, DBP), pulse pressure (PP), mean arterial pressure (MAP), and cognition, each measured 90 days after stroke.
Methods:
Cross-sectional analysis of prospectively obtained data of 432 dementia-free subjects ≥45 (median age, 66; 45% female) with stroke (92% ischemic; median NIH stroke score, 3 [IQR, 2-6]) from the population-based Brain Attack Surveillance in Corpus Christi (BASIC) project in 2011-2013. Primary outcome: Modified Mini-Mental Status Examination (3MSE; range, 0-100). Secondary outcomes: Animal Fluency Test (AFT; range, 0-10) and Trail Making Tests A and B (number of correct items [range, 0-25]/completion time [Trails A: 0-180 seconds; Trails B: 0-300 second]). Linear or tobit regression adjusted associations for age, education, and race/ethnicity as well as variables significantly associated with BP and cognition.
Results:
Higher SBP, lower DBP, higher PP, and lower MAP each were associated with worse cognitive performance for all 4 tests (all P<0.001). After adjusting for patient factors, no BP measures were associated with any of the 4 tests (all P>0.05). Lower cognitive performance was associated with older age, less education, Mexican American ethnicity, diabetes, higher stroke severity, more depressive symptoms, and lower BMI. Among survivors with hypertension, anti-hypertensive medication use 90 days after stroke was significantly associated with higher AFT scores (P=0.02) but not other tests (P>0.15).
Conclusion:
Stroke survivors’ BP levels were not associated with cognitive performance at 90 days independent of sociodemographic and clinical factors.
Introduction
Incident stroke is associated with an acute decline in cognitive function and also accelerated and persistent cognitive decline over years.1, 2 Poststroke cognitive decline (PSCD) increases mortality3 and disability4. The identification of modifiable risk factors that lessen PSCD is critical. Based on some observational studies5-7 and the results from the SPRINT MIND randomized controlled trial8, high blood pressure (BP) might be a modifiable risk factor to reduce risk of PSCD.
Although it is known that high BP, particularly in mid-life, is associated with late-life cognitive impairment9, it is unknown whether BP levels are associated with PSCD. High BP is common in stroke survivors affecting most Mexican Americans and non-Hispanic whites.10 Control of high BP is sub-optimal in stroke survivors, especially for minorities.11, 12 Worse BP control and high BP levels might lead to higher rates of PSCD.13
We performed primary data collection to measure BP among survivors of acute stroke in the Brain Attack Surveillance in Corpus Christi (BASIC) project, a population-based stroke surveillance project of Mexican Americans and non-Hispanic whites. We combined these new clinical measures with anti-hypertensive medication use and cognition function to determine associations between systolic and diastolic BP (SBP and DBP) levels and anti-hypertensive medication use and cognitive function 90 days after stroke. We hypothesized that higher BP levels are associated with worse cognitive function after stroke whereas use of anti-hypertensive medication is associated with better cognitive function.
Methods
Study Population
The Brain Attack Surveillance in Corpus Christi (BASIC) project is a population-based stroke surveillance project of a non-immigrant community of Mexican Americans and non-Hispanic whites in Nueces County, Texas.14 Details are described elsewhere.14 Briefly, Nueces County is a predominantly urban location, where 95% of the population resides in the city of Corpus Christi on the Texas gulf coast.15 Corpus Christi is situated approximately 150 miles from potential referral centers in San Antonio and Houston.15 The geographic location and distance provide the opportunity for complete case capture of stroke in the county.15
Stroke cases presenting between March 2011 and December 2013 were ascertained Through active and passive surveillance, BASIC ascertains all cases of acute cerebrovascular disease presenting to the emergency department or directly admitted to any of the 7 hospitals in Nueces County. Trained abstractors verify stroke diagnoses based on rigorous criteria. Neurologists validated stroke cases using source documentation following international clinical criteria.16 At the time of their stroke hospitalization, patients or proxies for patients unable to participate completed an in-person, structured interview. Bilingual abstractors conducted the interview and cognitive testing in English (94%) or Spanish (6%) per patient preferences. It is plausible that a small percentage of individuals in the study sample requested cognitive testing in Spanish because most of the Mexican American participants are second- and third-generation Americans.15 Interview participation is similar by ethnicity. Patients with their first BASIC stroke (ischemic or hemorrhagic) were included. This project was approved by both the University of Michigan and Corpus Christi Health Systems’ Institutional Review Boards. All subjects or their proxies provided informed consent.
We required participants to have measurements of BP and cognition at the 90 day in-person outcome assessment. We excluded participants who were unable to complete the baseline interview. We also excluded participants with a history of dementia at the time of the index stroke defined as medical record documentation of dementia or Alzheimer’s disease or an Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) score ≥ 3.44 at baseline, this cut-point for prestroke dementia is based on previous research.17 The short form of the IQCODE is a validated instrument to assess pre-stroke cognitive status17, 18 has been validated in Spanish,19 and has been shown to be relatively unaffected by education level.18 It asks an informant to report on changes in functional and cognitive status over time and focuses on the pre-stroke time period.
Cognitive Function Assessments
Trained BASIC interviewers administered cognitive function tests at the 90-day outcome assessment in-person. The cognitive tests were focused on stroke survivors without language dysfunction (e.g., aphasia). Each test was administered in the primary language (English or Spanish) of the subject or the preferred language of administration of bilingual subjects. All testing was conducted by bilingual/bicultural interviewers. The primary outcome was the Modified Mini-Mental Status Examination (3MSE). The 3MSE assesses global cognitive function (scores range, 0-100).20 Secondary outcomes were the Animal Fluency Test (AFT) and Trail Making Test, Parts A and B (Trails A and B). The AFT assesses executive function, with scores representing the number of animals generated in 30 seconds (scores range, 0-10).20, 21 The Trails A and B tests assess visuomotor tracking, information processing speed, divided attention, and cognitive flexibility. A large number of participants (n=44 for Trails A; n=78 for Trails B) could not complete the test within the allotted time limits (180 seconds for Trails A; 300 seconds for Trails B). So, we used derived Trails scores, rather than completion time, in order to improve measurement precision in light of the number of participants that could not complete the task within the allotted time limits. Derived scores reflected the number of correctly completed items (0-25 items) divided by completion time (0-180 seconds for Trails A and 0-300 seconds for Trails B which were the specified test discontinuation criteria).22 This approach to calculate a derived score (i.e., number of correctly completed items divided by completion time) is an accepted approach for use in elderly cognitively-impaired populations.23 For all cognitive tests, higher scores indicate better performance. These tests are used in the Vascular Cognitive Impairment Harmonization Standards24 and have been validated in Mexican Americans and whites.25 English and Spanish versions of the 3MSE and Trial Making Tests are valid and consistent26, 27 and have been used to study post-stroke cognitive decline.28
Measurement of Blood Pressure
Trained BASIC interviewers measured BP at the 90-day outcome assessment using a standard protocol (see Online Supplement). Following a 5-minute rest, BP was measured three times in the right arm of seated participants at 15-second intervals using an appropriately sized cuff and a standard automated BP measurement arm monitor (OmROn model 700 series; Omron, Mannheim, Germany), that has been validated by the Association for the Advancement of Medical Instrumentation to be accurate. The BP of record was the average of the 3 BP measurements. The primary independent variables were SBP and DBP. Secondary independent variables were mean arterial pressure (MAP) ([DBPx2)+SBP]/3) and pulse pressure (PP) (SBP-DBP).
Covariates
Variables measured at baseline (time of index stroke)
Participants self-reported age, gender, race/ethnicity (non-Hispanic White, Mexican American, Other), education (<high school, high school, >high school), and marital status (single/never married, married/living with someone, widowed, divorced/separated) in baseline interview at time of index stroke. Co-morbidities were abstracted from the medical record: dementia or Alzheimer’s disease, hypertension, history of stroke, diabetes, coronary artery disease, atrial fibrillation, high cholesterol, excessive alcohol use, cigarette smoking, heart failure, chronic lung disease, and chronic renal disease. National Institutes of Health Stroke Scale (NIHSS) score was also abstracted from the medical record. Body mass index was calculated from height and weight. Stroke type was validated by study neurologists (ischemic, hemorrhagic, other). The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) was assessed in an informant.
Variables measured at 90-day outcome assessment (90 days after index stroke)
Participants reported depressive symptoms the 8-item Patient Health Questionnaire (PHQ-8). Participants reported use of anti-hypertensive medication and BASIC interviewers confirmed participants’ reports by reviewing their medication bottles. Participants reported the frequency of missed doses of each prescribed medication in a typical week using a 5-point Likert scale (never, rarely, occasional, often, very often).29 We defined adherence to BP medication as never missing a dose of any BP medications in a typical week based on previous studies. 29, 30
Statistical Analysis
We followed a pre-specified analysis plan. We performed descriptive analyses of patient characteristics and cognitive measures and assessed bivariate associations between covariates, BP, and cognitive measures using visual plots, ANOVA and χ2 tests as appropriate. We transformed the left-skewed 3MSE outcome using –log(c – y), where y=3MSE and c=max(3MSE) + 1 so that lower scores indicated worse cognition. The NIHSS scores were not normally distributed and were transformed using the natural logarithm. We used single imputation for missing values of IQCODE at baseline (n=57) and depressive symptom scores (n=11) at 90 days after stroke.
We tested for associations between continuous BP measures and each continuous cognitive outcome using linear regression (3MSE, Trail Making Tests A and B) and Tobit regression (AFT) before and after adjusting for patient characteristics. Model 1 included SBP only. Model 2 included DBP only. Model 3 included both SBP and DBP. Model 4 included SBP, DBP, age, education, and race/ethnicity. Model 5 added body mass index, diabetes, NIHSS score at time of index stroke, and depressive symptoms at 90 days to Model 4. Other variables that did not reach statistical significance (defined as P<0.05) were not included in models; these were: stroke type, sex, marital status, IQCODE, history of stroke, coronary artery disease, atrial fibrillation, high cholesterol, alcohol use, cigarette smoking, heart failure, chronic renal disease, and chronic lung disease. We also performed analyses adding anti-hypertensive medication use to the fully adjusted Model 5. There was no evidence of non-linear associations between SBP or DBP and cognition. We examined residual plots to examine assumptions of models. We reran models replacing SBP and DBP with mean arterial pressure (MAP) ([DBPx2) + SBP]/3) and pulse pressure (PP) (SBP-DBP).
Among BASIC stroke survivors with a diagnosis of hypertension (n=348), we created a 3-level anti-hypertensive medication adherence variable (adherent to anti-hypertensive medication, non-adherent to anti-hypertensive medication [missing 1 or more doses of any BP medication in a typical week], and not using anti-hypertensive medication) and we examined the effect of medication adherence on 90-day cognition. We included those not prescribed anti-hypertensive medication in the medication adherence variable so we could compare results of the final model (Model 5) before and after adding the medication adherence variable in the full sample of stroke survivors with hypertension.
Sensitivity Analysis
Trails A and B are written tests requiring use of one’s hand. When individuals were not able to write with their dominant hand due to weakness, they completed this test with their non-dominant hand. So we examined the association between writing hand weakness (i.e., either use of dominant, weak hand or use of non-dominant hand due to dominant hand weakness) and completion of the Trails tests, and we also repeated analyses excluding individuals with writing hand weakness. Some participants completed the 3MSE but not the Trails tests so we examine adjusted associations between patient factors and completion of the Trails A test among patients who completed the 3MSE using logistic regression models that included covariates in Model 5. We repeated separate analyses after a) excluding individuals with Trails A and B tests exceeding the specified test discontinuation criteria; b) excluding those with any residual point over 4/n (where n is the number of sample in the analysis); and C) those with a Cook’s D more than 3 times the mean.
Statistical significance for all analyses was set at P<0.05 (2-sided). We performed all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
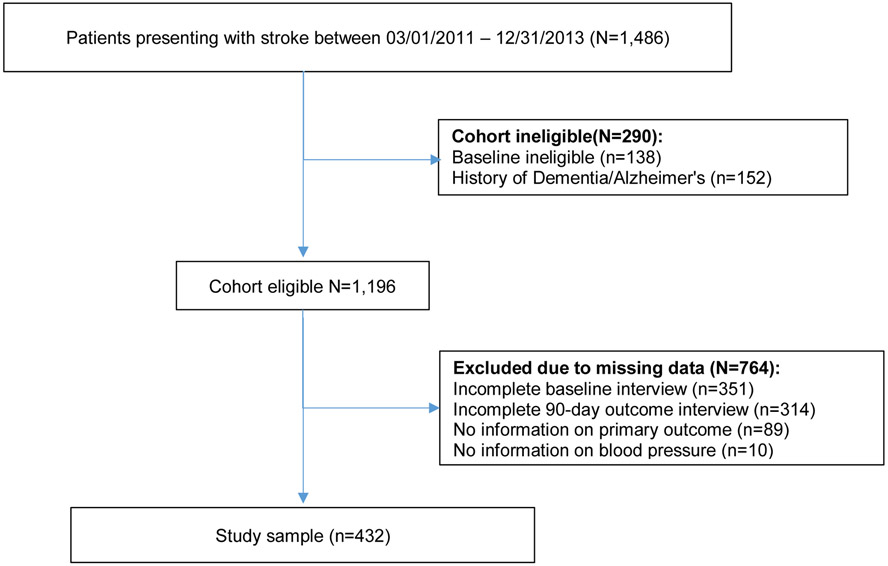
The study sample include 432 participants. Figure 1 presents the derivation of the cohort. Table 1 presents patient characteristics. Median age was 66 (IQR, 58-76) and 45% were female. Most strokes were ischemic (92%) and median NIHSS score was 3 (IQR, 2-6). At 90 days after stroke, median SBP was 132 (118-150) and median DBP was 79 (72-88).
Figure 1:
Derivation of Analytic Cohort: BASIC, 2011-2013
Table 1:
Characteristics of Participants in the Study Sample (n=432): BASIC 2011 to 2013
| Variables measured at baseline (time of index stroke) | N(%) or Median(IQR) |
|---|---|
| Age | 66 (58-76) |
| Stroke type | |
| Ischemic | 399 (92) |
| Hemorrhagic | 32 (7) |
| Other | 1 (0.2) |
| National Institutes of Health Stroke Scale score | 3 (2-6) |
| Women | 196 (45) |
| Race/ethnicity | |
| Non-Hispanic White | 150 (35) |
| Mexican American | 246 (57) |
| Other | 36 (8) |
| Education | |
| <high school | 140 (33) |
| High school | 131 (30) |
| >high school | 161 (37) |
| Marital status (n=431) | |
| Single/Never married | 27 (6) |
| Married/Living with someone | 220 (51) |
| Widowed | 82 (19) |
| Divorced/Separated | 102 (24) |
| IQCODE score (n=375) | 3.0 (3.0-3.3) |
| Hypertension | 348 (81) |
| Body mass index | 29 (25-34) |
| History of stroke | 108 (25) |
| Diabetes | 204 (47) |
| Coronary artery disease | 131 (30) |
| Atrial fibrillation | 44 (10) |
| High cholesterol | 225 (52) |
| Excessive alcohol use | 51 (12) |
| Cigarette smoking | 180(42) |
| Heart failure | 25 (6) |
| Chronic lung disease | 48 (11) |
| Chronic renal disease | 14 (3) |
| Variables measured at 90 days after stroke | |
| Depressive symptoms (n= 420) | 14 (11-20) |
| Systolic BP | 132 (118-150) |
| Diastolic BP | 79 (72-88) |
| Anti-hypertensive medication use | 365 (84) |
| 3MSE score | 89 (78-95) |
| AFT score (n=431) | 8 (6-10) |
| Trails A score* (n=335) | 0.34 (0.22-0.47) |
| Trails B score* (n=283) | 0.13 (0.09-0.20) |
Abbreviations: IQR is interquartile range. BASIC= Brain Attack Surveillance in Corpus Christi. IQCODE=Informant Questionnaire on Cognitive Decline in the Elderly and measured prestroke cognitive decline. BP=blood pressure. 3MSE= Modified Mini-Mental Status Examination. AFT=Animal Fluency Test. Trails=Trail Making Test.
scores reflected task accuracy [0-25 correct items] divided by completion time [0-180 seconds for Trails A and 0-300 seconds for Trails B which were the specified test discontinuation criteria]).
Depressive symptoms were measured with the 8-item Patient Health Questionnaire (PHQ).
BP and Global Cognition
SBP was not significantly associated with 3MSE (Model 1, Table 2) unless DBP was included in the model (Model 2, Table 2). Higher SBP and lower DBP each were significantly associated with lower 3MSE scores (difference per 10 mmHg increase in SBP: −0.10 points [95% CI, −0.15, −0.05]; P<0.001; difference per 10 mmHg increase in DBP: 0.28 points [95% CI, 0.19, 0.37]; P<0.001) (Model 3, Table 2). After adjusting for age, education, and race/ethnicity, DBP remained significantly associated with 3MSE scores (difference per 10 mmHg increase: 0.11 points [95% CI, 0.02, 0.20]; P=0.02) but SBP did not (Model 4, Table 2). With further adjustment for clinical factors (body mass index, diabetes, baseline NIHSS, depressive symptoms at 90 days), DBP did not remain independently associated with 3MSE scores (difference per 10 mmHg increase: 0.06 points [95% CI, −0.03, 0.15]; P=0.17) (Model 5, Table 2).
Table 2:
Association between Blood Pressure and Cognition 90 days after Stroke: Brain Attack Surveillance in Corpus Christi Project, 2011-2013
| Estimates (95% Confidence Intervals) | |||||
|---|---|---|---|---|---|
| Coefficient | Model 1 with SBP only |
Model 2 with DBP only |
Model 3 with SBP and DBP |
Model 4 with SBP, DBP, and Socio- demographics |
Model 5 with SBP, DBP, and Socio- demographics , and Clinical Factors |
| Outcome: 3MSE (n=432) | |||||
| Difference per 10 mmHg increase in SBP | .01 (−.02, .05) P=.44 |
NA | −.10* (−.15, −.05) P<.001 |
−.01 (−.06, .03) P=.57 |
−.01 (−.05, .04) P=.74 |
| Difference per 10 mmHg increase in DBP | NA | .16* (.09, .22) P<.001 |
.28* (.19, .37) P<.001 |
.11* (.02, .20) P=.02 |
.06 (−.03, .15) P=.17 |
| Outcome: Animal Fluency Test (n=431) | |||||
| Difference per 10 mmHg increase in SBP | .014 (−.11, .14) P=.83 |
NA | −.20* (−.38, −.02) P=.03 |
−.03 (−.22, .15) P=.73 |
−.03 (−.22, .15) P=.72 |
| Difference per 10 mmHg increase in DBP | NA | .29* (.06, .53) P=.01 |
.56* (.23, .89) P=.001 |
.22 (−.16, .59) P=.25 |
.13 (−.24, .51) P=.48 |
| Outcome: Trails A (n=335) | |||||
| Difference per 10 mmHg increase in SBP | .0002 (−.01, .01) P=.96 |
NA | −.02* (−.04, −.01) P<.001 |
−.004 (−.02, .009) P=.54 |
−.003 (−.02, .01) P=.68 |
| Difference per 10 mmHg increase in DBP | NA | .04* (.02, .06) P<.001 |
.07* (.04, .09) P<.001 |
.02 (−.006, .05) P=.13 |
.01 (−.02, .04) P=.46 |
| Outcome: Trails B (n=283) | |||||
| Difference per 10 mmHg increase in SBP | −.002 (−.01, .003) P=0.37 |
NA | −.01* (−.02, −.008) P<.001 |
−.006 (−.01, .0001) P=.05 |
−.005 (−.01, .001) P=.10 |
| Difference per 10 mmHg increase in DBP | NA | .015* (.01, .024) P=.001 |
.03* (.02, .04) P<.001 |
.02* (.004, .03) P=.01 |
.01* (.0001, .03) P=.05 |
Abbreviations: NA=not applicable. SBP=systolic blood pressure. DBP=diastolic blood pressure.
The 95% confidence intervals do not contain zero.
The Modified Mini-Mental Status Examination (3MSE) assesses global cognitive function (scores range, 0-100). The 3MSE outcome was transformed using the natural logarithm and reverse coded to have participants with worse cognition having lower scores. The Animal Fluency Test assesses executive function (complex cognitive processing), with scores representing the number of animals generated in 30 seconds (scores range 0-10). The Trailmaking Tests A and B assess visuomotor tracking, information processing speed, divided attention, and cognitive flexibility (scores reflected task accuracy [0-25 correct items] divided by completion time [0-180 seconds for Trails A and 0-300 seconds for Trails B]. For all cognitive tests, higher scores indicate better performance.
Linear regression used for Modified Mini-Mental Status Examination and Trailmaking Tests A and B. Tobit regression used for Animal Fluency Test.
Model 1 included SBP. Model 2 included DBP. Model 3 included SBP and DBP. Model 4 included SBP, DBP, age, education and race/ethnicity. Model 5 added body mass index, diabetes, NIH stroke severity score at time of index stroke, and depressive symptoms at 90 days to Model 4.
BP and Secondary Cognitive Outcomes
Similar to the 3MSE, SBP was not significantly associated with the AFT, Trails A, and Trails B (Model 1, Table 2) unless DBP was included in the model (Model 2, Table 2). Higher SBP and lower DBP each were significantly associated with worse cognitive performance for all 3 tests (Model 3, Table 2). After adjusting for age, education, and race/ethnicity, neither SBP nor DBP remained significantly associated with AFT or Trails A. Lower DBP remained significantly associated with worse Trails B scores (difference per 10 mmHg increase: 0.02 points [95% CI, 0.004, 0.03]; P=0.01) but SBP did not (Model 4, Table 2). With further adjustment for clinical factors, the association between DBP and Trails B scores was attenuated (difference per 10 mmHg increase: 0.01 points [95% CI, 0.0001, 0.03]; P=0.05) (Model 5, Table 2).
Factors Associated with Poststroke Cognition
Lower cognitive performance 90 days after stroke was significantly associated with older age (P<.01 for all 4 tests), less education (P≤.03 for all tests), Mexican American ethnicity (P≤.05 for all tests), diabetes (P≤.01 for all tests except AFT), higher stroke severity (P≤.01 for all tests except Trails B), more depressive symptoms (P=.03 for 3MSE), and lower BMI (P<.02 for 3MSE and Trails B) (see Supplemental Table 1).
Antihypertensive Medication Use and Poststroke Cognition
Most stroke survivors with hypertension were adherent to anti-hypertensive medication (247/348; 71%) whereas fewer survivors were non-adherent to anti-hypertensive medication (67/348; 19%) and not prescribed medication (36/348; 10%). Results of the effect of SBP and DBP on cognitive outcomes were similar in models that included the 3-level anti-hypertensive medication adherence variable in the subgroup of stroke survivors with hypertension (see Supplemental Table 2). Nonadherence to antihypertensive medication was not significantly associated with cognition scores. However, among hypertensive stroke survivors, those who did not use antihypertensive medication had significantly lower animal fluency test scores than those who adhered to prescribed antihypertensive medication (adjusted difference, −1.21 points [95% CI, −2.24 to −.19]; P=.02) although this association was not observed for the 3MSE or Trails tests.
Sensitivity Analysis
Results were similar in analyses excluding 34 of 335 (10.1%) individuals with writing hand weakness from the Trails A analysis and 24 of 283 (8.5%) individuals from Trails B analysis. Multivariable logistic regression showed that completion of the Trails A test was significantly associated with lower NIHSS scores (P=.002) but not SBP (P=.15) and DBP (P=.83). Completion of the Trails B test was significantly associated with higher education (P<.001), lower NIHSS scores (P<.001), and lower age (P<0.001) but not SBP (P=.80) and DBP (P=.29). Results were similar in analyses excluding outliers.
In analyses replacing SBP and DBP with PP and MAP, higher PP and lower MAP each were associated with worse cognitive performance for all 4 tests (Model 3, Table 3). After adjusting for age, education, and race/ethnicity, MAP remained significantly associated with 3MSE (P=.003) and Trails B (P=.03); whereas, PP remained significantly associated with Trails B (P=.02) but not the other tests (Model 4, Table 3). With further adjustment for clinical factors, MAP did not remain independently associated with cognition (all P>.08) and PP was non-significantly associated with Trails B (P=.05) (Model 5, Table 3).
Table 3:
Association between Mean Arterial Pressure, Pulse Pressure and Cognition 90 days after Stroke: Brain Attack Surveillance in Corpus Christi Project, 2011-2013
| Estimates (95% Confidence Intervals) | |||||
|---|---|---|---|---|---|
| Coefficient | Model 1 with MAP only |
Model 2 with PP only |
Model 3 with MAP and PP |
Model 4 with MAP, PP, and Socio- demographics |
Model 5 with MAP, PP, and Socio- demographics, and Clinical Factors |
| Outcome: 3MSE (n=432) | |||||
| Difference per 10 mmHg increase in MAP | .09* (.03, .14) P=.003 |
NA | .19* (.12, .25) P<.001 |
.10* (.03, .16) P=.003 |
.05 (−.01, .12) P=.09 |
| Difference per 10 mmHg increase in PP | NA | −.06* (−.11, −.01) P=.01 |
−.16* (−.22, −.10) P<.001 |
−.05 (−.10, .01) P=.12 |
−.03 (−.08, .03) P=.37 |
| Outcome: Animal Fluency Test (n=431) | |||||
| Difference per 10 mmHg increase in MAP | .15 (−.05, .35) P=.14 |
NA | .36* (.12, .60) P=.004 |
.18 (−.08, .45) P=.17 |
.10 (−.16, .37) P=.45 |
| Difference per 10 mmHg increase in PP | NA | −.14 (−.31, −.04) P=.13 |
−.32* (−.53, −.11) P=.003 |
−.09 (−.33, .14) P=.43 |
−.07 (−.30, .17) P=.57 |
| Outcome: Trails A (n=335) | |||||
| Difference per 10 mmHg increase in MAP | .02* (.002, .03) P=.02 |
NA | .04* (.02, .06) P<.001 |
.02 (−.003, .03) P=.09 |
.01 (−.01, .03) P=.45 |
| Difference per 10 mmHg increase in PP | NA | −.02* (−.03, −.01) P=.005 |
−.04* (−.05, −.02) P<.001 |
−.01 (−.03, .01) P=.26 |
−.01 (−.02, .01) P=.53 |
| Outcome: Trails B (n=283) | |||||
| Difference per 10 mmHg increase in MAP | .01 (−.002, .01) P=.13 |
NA | .02* (.01, .03) P<.001 |
.01* (.001, .02) P=.03 |
.01 (−.002, .02) P=.11 |
| Difference per 10 mmHg increase in PP | NA | −.01* (−.02, −.006) P<.001 |
−.02* (−.03, −.01) P<.001 |
−.01* (−.02, −.002) P=.02 |
−.01 (−.02, .0001) P=.05 |
Abbreviations: NA=not applicable. MAP=mean arterial pressure. PP=pulse pressure.
The 95% confidence intervals do not contain zero.
The Modified Mini-Mental Status Examination (3MSE) assesses global cognitive function (scores range, 0-100). The 3MSE outcome was transformed using the natural logarithm and reverse coded to have participants with worse cognition having lower scores. The Animal Fluency Test assesses executive function (complex cognitive processing), with scores representing the number of animals generated in 30 seconds (scores range 0-10). The Trailmaking Tests A and B assess visuomotor tracking, information processing speed, divided attention, and cognitive flexibility (scores reflected task accuracy [0-25 correct items] divided by completion time [0-180 seconds for Trails A and 0-300 seconds for Trails B]. For all cognitive tests, higher scores indicate better performance.
Linear regression used for Modified Mini-Mental Status Examination and Trailmaking Tests A and B. Tobit regression used for Animal Fluency Test. Model 1 included MAP. Model 2 included PP. Model 3 included MAP and PP. Model 4 included MAP, PP, age, education and race/ethnicity. Model 5 added body mass index, diabetes, NIH stroke severity score at time of index stroke, and depressive symptoms at 90 days to Model 4.
Discussion
In this population-based cohort of Mexican Americans and non-Hispanic white Americans 45 years or older with stroke, worse cognitive performance in global cognition, executive function, and visuomotor tracking/processing speed 90 days after stroke was associated with higher SBP, lower DBP, higher PP, and lower MAP, however, socio-demographic and clinical factors appeared to explain these associations. Lower cognitive performance after stroke was observed among stroke survivors who had older age, less education, Mexican American ethnicity, diabetes, higher stroke severity, more depressive symptoms, and lower BMI. There was no evidence that nonadherence to antihypertensive medication was significantly associated with worse cognition scores. However, among hypertensive stroke survivors, those who did not use antihypertensive medication had significantly lower executive function scores than those who adhered to prescribed antihypertensive medication although this association was not observed for the 3MSE or Trails tests.
Our data suggest that patients with higher SBP and PP have worse cognition 90 days after stroke but the association is largely explained by patients with higher SBP and higher PP being more likely to have older age, less education, Mexican American ethnicity, diabetes, higher stroke severity, greater depressive symptoms, and lower BMI. Our data are consistent with a previous meta-analysis31 suggesting that older age, less education, non-white race/ethnicity, diabetes, and higher stroke severity are independent predictors of PSCD. Cognitive status might have bi-directional relationships with mood and BMI so our findings that greater depressive symptoms and lower BMI are associated with poststroke cognition warrant cautious interpretation and confirmation.
While hypertension is a major risk factor for stroke, it is unclear whether BP is associated with PSCD independent of the index stroke or recurrent stroke. Several potential explanations might explain this discordance. One possibility is that BP has an indirect effect on post-stroke cognition through a pathway mediated by stroke but BP does not have a direct effect on post-stroke cognition independent of stroke. This hypothesis seems implausible given the results of both observational studies13 and clinical trials8 showing that high BP is associated with worse cognitive function. Another possibility is that BP has a modest effect on post-stroke cognition so larger sample sizes of stroke survivors and cognitive measures sensitive to detect vascular cognitive impairment are needed. It is plausible that we did not detect a significant association between BP and cognition after stroke after adjusting for patient socio-demographics and clinical factors because the sample size was small and/or the cognitive measures were not sensitive to detect vascular cognitive impairment. One study of selected stroke survivors (i.e., those with NIHSS ≥ 4, arm and leg weakness, and hypertension) in a Chinese registry found that mean SBP within 7 days of stroke onset had a U-shaped association with post-stroke cognitive impairment at 90 days after stroke; however, the association was modest accounting for multiple comparisons and BP was not associated with cognitive impairment at 4 other time points after stroke.7 Our results might differ because we measured BP at 90 days after stroke, we used different cognitive measures, and we studied a Mexican Americans and non-Hispanic white Americans.
Our study has limitations. Results are generalizable to stroke survivors not represented by a proxy (e.g., those without aphasia). A large number of stroke patients were excluded due to incomplete data which might bias results toward or away from the null if missingness was not at random. Most strokes were ischemic and mild severity. We were unable to account for stroke features (e.g., site of lesion, lesion volume, and laterality) or structural brain features (cerebral atrophy) associated with poststroke cognition.31 BP was measured only at 90 days after stroke. The cognitive outcomes were assessed only at 90 days after stroke. The IQCODE might have inadequately captured prestroke cognitive decline. We did not have cognitive measures during the hyperacute period because many acute stroke patients were unable to complete cognitive testing during the index stroke hospitalization. While PSCD might be underestimated because stroke survivors with worse cognition at baseline or after stroke die, drop out, or require a proxy, research suggests that selection attrition does not change results.1 This cross-sectional analysis of BP and cognition 90-days poststroke does not allow us to assess the directionality of the relationship. Ethnic differences in cognitive performance might be due to cultural factors influencing test performance.32 Relatively small sample size might reduce ability to detect significant associations between BP and cognition after adjusting for potential confounders.
Our study has clinical and policy implications. Some observational studies, but not all33, have suggested that BP lowering is associated with lower risk of PSCD in specific patient subgroups such as patients with recurrent stroke5, and patients with ischemic stroke without atrial fibrillation6. Although results of the SPRINT MIND trial suggest that aggressive lowering of SBP reduces cognitive decline in older adults at high cardiovascular risk8, the trial excluded patients with stroke. Another trial showed that anti-hypertensive medication use improves executive function in older hypertensive adults.34 Our results raise the hypothesis that anti-hypertensive medication use might modestly improve executive function in stroke survivors with hypertension.
Summary and Conclusion
Patients with higher SBP and PP are more likely to have worse cognitive performance after stroke but sociodemographic and clinical factors might explain these associations. Among stroke survivors with hypertension, anti-hypertensive medication use is significantly associated with higher executive function.
Supplementary Material
References
- 1.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314:41–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levine DA, Wadley VG, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Risk factors for poststroke cognitive decline: The regards study (reasons for geographic and racial differences in stroke). Stroke. 2018;49:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatemichi TK, Paik M, Bagiella E, Desmond DW, Pirro M, Hanzawa LK. Dementia after stroke is a predictor of long-term survival. Stroke. 1994;25:1915–1919 [DOI] [PubMed] [Google Scholar]
- 4.Patel MD, Coshall C, Rudd AG, Wolfe CD. Cognitive impairment after stroke: Clinical determinants and its associations with long-term stroke outcomes. J Am Geriatr Soc. 2002;50:700–706 [DOI] [PubMed] [Google Scholar]
- 5.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075 [DOI] [PubMed] [Google Scholar]
- 6.Douiri A, McKevitt C, Emmett ES, Rudd AG, Wolfe CD. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation. 2013;128:1341–1348 [DOI] [PubMed] [Google Scholar]
- 7.He M, Wang J, Liu N, Xiao X, Geng S, Meng P, et al. Effects of blood pressure in the early phase of ischemic stroke and stroke subtype on poststroke cognitive impairment. Stroke. 2018;49:1610–1617 [DOI] [PubMed] [Google Scholar]
- 8.Group SMIftSR, Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, et al. Effect of intensive vs standard blood pressure control on probable dementia: A randomized clinical trial. JAMA. 2019;321:553–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function. The honolulu-asia aging study. JAMA. 1995;274:1846–1851 [PubMed] [Google Scholar]
- 10.Levine DA, Neidecker MV, Kiefe CI, Karve S, Williams LS, Allison JJ. Racial/ethnic disparities in access to physician care and medications among us stroke survivors. Neurology. 2011;76:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner DA, Zweifler RM, Gomez CR, Kissela BM, Levine D, Howard G, et al. Awareness, treatment, and control of vascular risk factors among stroke survivors. J Stroke Cerebrovasc Dis. 2010;19:311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roumie CL, Ofner S, Ross JS, Arling G, Williams LS, Ordin DL, et al. Prevalence of inadequate blood pressure control among veterans after acute ischemic stroke hospitalization: A retrospective cohort. Circ Cardiovasc Qual Outcomes. 2011;4:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Blood pressure and cognitive decline over 8 years in middle-aged and older black and white americans. Hypertension. 2019;73:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MA, Risser JM, Moye LA, Garcia N, Akiwumi O, Uchino K, et al. Designing multi-ethnic stroke studies: The brain attack surveillance in corpus christi (basic) project. Ethn Dis. 2004;14:520–526 [PubMed] [Google Scholar]
- 15.Morgenstern LB, Smith MA, Sanchez BN, Brown DL, Zahuranec DB, Garcia N, et al. Persistent ischemic stroke disparities despite declining incidence in mexican americans. Ann Neurol. 2013;74:778–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asplund K, Tuomilehto J, Stegmayr B, Wester PO, Tunstall-Pedoe H. Diagnostic criteria and quality control of the registration of stroke events in the monica project. Acta Med Scand Suppl. 1988;728:26–39 [DOI] [PubMed] [Google Scholar]
- 17.Murao K, Bodenant M, Cordonnier C, Bombois S, Henon H, Pasquier F, et al. Does pre-existing cognitive impairment no-dementia influence the outcome of patients treated by intravenous thrombolysis for cerebral ischaemia? Journal of neurology, neurosurgery, and psychiatry. 2013;84:1412–1414 [DOI] [PubMed] [Google Scholar]
- 18.Jorm AF. The informant questionnaire on cognitive decline in the elderly (iqcode): A review. Int Psychogeriatr. 2004;16:275–293 [DOI] [PubMed] [Google Scholar]
- 19.Morales JM, Bermejo F, Romero M, Del-Ser T. Screening of dementia in community-dwelling elderly through informant report. Int J Geriatr Psychiatry. 1997;12:808–816 [PubMed] [Google Scholar]
- 20.Tombaugh TN. Test-retest reliable coefficients and 5-year change scores for the mmse and 3ms. Arch Clin Neuropsychol. 2005;20:485–503 [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez HM, Mungas D, Haan MN. A semantic verbal fluency test for english- and spanish-speaking older mexican-americans. Arch Clin Neuropsychol. 2005;20:199–208 [DOI] [PubMed] [Google Scholar]
- 22.Ashendorf L, Jefferson AL, O'Connor MK, Chaisson C, Green RC, Stern RA. Trail making test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol. 2008;23:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub S, Besser L, Dodge HH, Teylan M, Ferris S, Goldstein FC, et al. Version 3 of the alzheimer disease centers' neuropsychological test battery in the uniform data set (uds). Alzheimer Dis Assoc Disord. 2018;32:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241 [DOI] [PubMed] [Google Scholar]
- 25.Gavett BE, Stypulkowski K, Johnson L, Hall J, O'Bryant SE. Factor structure and measurement invariance of a neuropsychological test battery designed for assessment of cognitive functioning in older mexican americans. Alzheimers Dement (Amst). 2018;10:536–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for english- and spanish-speaking older people. J Int Neuropsych Soc. 2001;7:544–555 [DOI] [PubMed] [Google Scholar]
- 27.Arango-Lasprilla JC, Rivera D, Aguayo A, Rodriguez W, Garza MT, Saracho CP, et al. Trail making test: Normative data for the latin american spanish speaking adult population. NeuroRehabilitation. 2015;37:639–661 [DOI] [PubMed] [Google Scholar]
- 28.Levine DA, Haan MN, Langa KM, Morgenstern LB, Neuhaus J, Lee A, et al. Impact of gender and blood pressure on poststroke cognitive decline among older latinos. J Stroke Cerebrovasc Dis. 2013;22:1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older americans in the health and retirement study. Arch Intern Med. 2007;167:1853–1860 [DOI] [PubMed] [Google Scholar]
- 30.Lank RJ, Lisabeth LD, Levine DA, Zahuranec DB, Kerber KA, Shafie-Khorassani F, et al. Ethnic differences in 90-day poststroke medication adherence. Stroke. 2019;50:1519–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: A systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018 [DOI] [PubMed] [Google Scholar]
- 32.Brickman AM, Cabo R, Manly JJ. Ethical issues in cross-cultural neuropsychology. Appl Neuropsychol. 2006;13:91–100 [DOI] [PubMed] [Google Scholar]
- 33.Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: A secondary analysis from the sps3 randomised trial. Lancet Neurol. 2014;13:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajjar I, Hart M, Chen YL, Mack W, Milberg W, Chui H, et al. Effect of antihypertensive therapy on cognitive function in early executive cognitive impairment: A double-blind randomized clinical trial. Arch Intern Med. 2012;172:442–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.