Abstract
Vascular plants are integrated into coherent bodies via plant-specific synaptic adhesion domains, action potentials (APs) and other means of long-distance signalling running throughout the plant bodies. Plant-specific synapses and APs are proposed to allow plants to generate their self identities having unique ways of sensing and acting as agents with their own goals guiding their future activities. Plants move their organs with a purpose and with obvious awareness of their surroundings and require APs to perform and control these movements. Self-identities allow vascular plants to act as individuals enjoying sociality via their self/non-self-recognition and kin recognition. Flowering plants emerge as cognitive and intelligent organisms when the major strategy is to attract and control their animal pollinators as well as seed dispersers by providing them with food enriched with nutritive and manipulative/addictive compounds. Their goal in interactions with animals is manipulation for reproduction, dispersal and defence.
This article is part of the theme issue ‘Basal cognition: multicellularity, neurons and the cognitive lens’.
Keywords: behaviour, cognition, individuality, plants, synapses, vascular systems
1. Introduction
Plants evolved from very small and relatively simple aquatic organisms up to most complex terrestrial vascular plants that can reach immense sizes, with aboveground shoot lengths of up to above 100 m in the case of sequoia trees, and attain unparalleled longevity of several thousand years when some Pinus longaeva trees are estimated to be older than 5000 years [1]. Nevertheless, higher plants enjoy several features that were originally considered specific only for animals, including sexuality, immunity, self/non-self and kin recognition, goal-directed behaviour based on plant-specific cognition and communication, as well as on intelligence and sociality. These surprising animal-like features of higher plants result from the convergent evolution of flowering plants and animals [2]. For example, mammals and flowering plants emerged some 180–130 MA ago and since then have been co-evolving at several levels. In the present paper, we are focusing on the individuality and self aspects of vascular plants, based on plant-specific synapses, action potentials (APs) and unique plant body organization, which allow them to act as communicative and social organisms both aboveground and underground.
2. Duality of plants: living both aboveground and underground
Plants represent unique multicellular organisms as they not only have both autotrophic and heterotrophic organs, tissues and cells, but they also live in two contrasting environments: an underground pedosphere and an aboveground atmosphere (we ignore here special situations such as aerial roots of some epiphytes or underground shoots of some clonal plants). This dichotomy implicates that plant organs live in two different environments: autotrophic shoots are aboveground organs exposed to day/night cycles whereas heterotrophic roots live underground in darkness. Obviously, roots live in a much more friendly and stable environment compared to shoots. Moreover, roots are much more active in their social aspects as they engage in several intracellular symbiotic relations, as we will discuss in more detail below, with bacteria and fungi. Colonization of the barren land was facilitated by these symbiotic interactions that allowed the evolving rhizoids and roots to better exploit the subterranean niche by transforming it into an active pedosphere [3]. This upper–lower duality (figure 1) is inherently connected to a duality of the mode of existence as the aboveground part is autotrophic whereas the underground root part is heterotrophic, resembling fungi and animals. Therefore, it is perhaps not surprising that the underground roots, more than the shoots, enter into intracellular symbiosis with fungi and bacteria.
Figure 1.
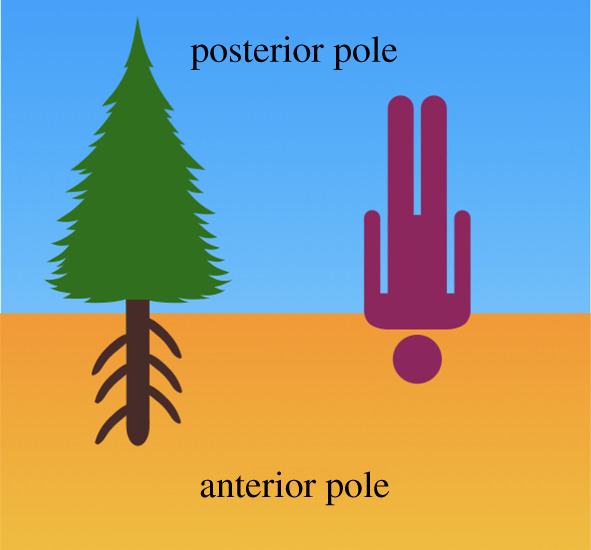
Darwin's view of the plant body. Charles Darwin proposed that root apices represent the anterior pole of the plant body, while shoot apices represent the posterior pole. Root apices are specialized for searching for nutrients and water, as well as identification and avoidance of toxic and dry soil areas. Shoot apices are specialized for the formation of lateral shoots (organs bearing leaves), and flowers (sexual organs). As with animal bodies, plant bodies are also polarized with two opposite poles: the cognitive anterior pole which is responsible for the uptake of nutrients and the posterior pole harbouring organs specialized for sexual reproduction and active movements of the whole body. In comparison with animals, plants are inverted upside-down. (Online version in colour.)
Importantly, a large part of the aboveground organs in mature plants are also heterotrophic as only a subset of the cells are photosynthetically active, primarily the mesophyll cells of leaves. In young plants and in fast-growing shoots of mature plants, the cortex (parenchymatic tissue between the endodermis and epidermis) is also photosynthetic. Most epidermal cells as well as all the cells of the vascular systems of leaves and stems, and many cells of flowers, are heterotrophic. Altogether, a significant amount of the cells in shoots are heterotrophic. The underground roots are completely heterotrophic. They provide plants with water, mineral nutrition and hormones that allow photosynthesis and growth in shoot cells. Vascular systems transport water and minerals (xylem) from roots to shoots, and sucrose-based photosynthates (phloem) from leaves to cells of all heterotrophic organs. Vascular systems are central for the integration of plant bodies into physiologically coherent individualities.
3. Duality of plants: outside cortex and epidermis versus inside vascular tissues
These inherent trophic environmental dualities are complemented by the outside–inside duality of plant tissues where both roots and shoots have internal stele tissues with their vascular systems, covered with endodermis in the roots, and the more peripheral cortex covered with the epidermis (figure 2). This outer–inner polarity of the plant body is defined not only anatomically but also physiologically [4,5]. In this conception, the outer pole is represented by epidermis and cortex tissues of young stems and roots and later by additional cortical tissues (cork, dilatation) as well as by leaf mesophyll; the inner pole of the plant body is represented by the stele including its vascular and parenchymatic tissues [4]. Interestingly, the root cap is part of the outer pole in this conception and it was reported that the root cap is the only part of the roots covered by the cuticle [6]. Again, this outer–inner duality is also inherently connected to the autotrophy/heterotrophy duality as photosynthesis is accomplished mostly in cortical mesophyll cells of leaves and many stem cortical tissues whereas other cells of the plant body are heterotrophic. Importantly, the number of heterotrophic cells of any mature plant body outnumbers those of photosynthetic cells.
Figure 2.
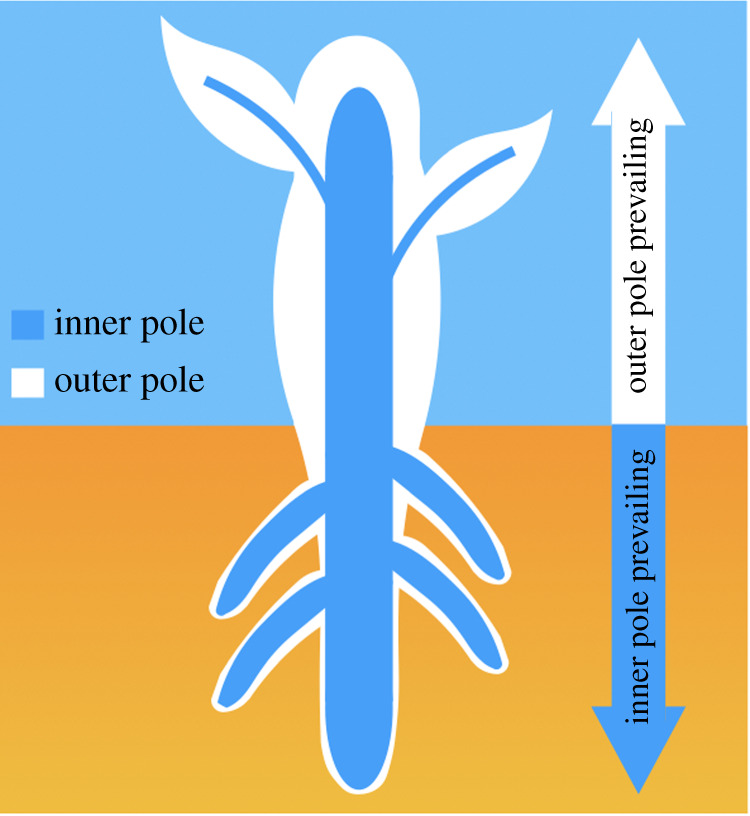
The inner–outer pole organization of the plant body. The inner pole of the plant body (shown in blue) is represented by the central cylinder (vascular systems and their parenchymatic cells) enclosed in roots by the endodermis (inner epidermis) and expanding through the aboveground shoot part in the form of vascular systems and their parenchymatic cells. The outer pole is covered by the epidermis with cuticle whereas the inner pole is covered by the endodermis. New roots are initiated at the endodermis/pericycle and new shoots at the epidermis. In other words, morphogenetic tissue supporting new organogenesis is epidermis in shoots and endodermis with pericycle (tissue layer under the endodermis) in roots. In older plants, root and shoot epidermis, as well as the cortex (parenchymatic tissue filling the space between the epidermis and endodermis), are often replaced by phellogen, which is initiated by phloem parenchyma in shoots and pericycle in roots (not shown). In trees, phellogen produces the bark of the tree trunks (not shown). (Online version in colour.)
4. Plant individuality: epithelial and neuronal basis of plant body integration
The stele in roots is compact, forming a central cylinder, which is covered with the so-called inner epidermis epithelium-like endodermis which, together with root periphery epidermis, act as plant-specific epithelium-like border tissues [5,7,8]. The next epithelium-like tissue is represented by xylem parenchyma cells lining all xylem elements in roots, shoots and leaves. All epithelium-like tissues are characterised by the absence of intercellular spaces between metabolically active cells which actively control transport of water and solutes across the plant body and within the xylem elements throughout the plant body.
Notably, the anterior-like heterotrophic root part is specialized for active searching for water and mineral nutrients while the posterior-like shoot part accomplishes photosynthesis and sexual reproduction [9]. Despite their typical modular nature [10–15], vascular plants are unitary organisms integrated via their vascular systems which support the plant-specific but neural-like communication via chemical, electrical and hydraulic long-distance signalling [10,16–21].
Besides the vascular systems connecting all plant organs into integrated unitary plant organisms, other important integrating structures are the cell–cell adhesion domains which organize both the epithelial and neuronal synapses in animals [22]. As already mentioned, the epidermis is an epithelium-like tissue covering both the young shoots and roots. Intriguingly, the shoot epidermis together with underlying parenchymatic cells represent the morphogenic tissues initiating lateral shoots and leaf formation. In the roots, it is the inner skin known mostly as endodermis which joins the underlying pericycle in lateral root formation (figure 2). Another important difference between shoot and root organogenesis is that new shoots are initiated at the very shoot apex whereas lateral root primordia are initiated behind the primary root apex.
Yamada & Nelson [22] proposed that the cell–cell adhesion domains in animal epithels resemble neuronal synaptic domains via their tightly adhering adjacent cells which allows them to generate barriers. Similarly, in plant organs, epidermis, endodermis and vascular parenchyma cells form epithel-like tissues due to tightly adhering cells. The plant-specific F-actin/myosin VIII-based synapse-like cell–cell adhesion domains in root apices, where synaptic-like principles were first proposed and characterized some 15 years ago [23–26], are active in the unique root apex zone known as the root apex transition zone [27,28]. However, the plant-specific synaptic-like domains in the epidermis, endodermis and pericycle, as well as the vascular parenchyma lining vascular elements, support APs and integrate the whole plant body into a coherent unit (figures 1 and 2), enabling plant-specific cognition and sociality. Plant-specific immunological and symbiotic synapses allow plants to communicate with their endogenous symbionts such as Rhizobium bacteria and diverse fungi [23–26].
A strong example of the plant-specific sense of self is plant proprioception—perception and awareness of the individual physical self [29]. Proprioception is a term introduced by Sherrington [30] to explain the ability of animals and humans to actively sense their own body positions and movements. In animals and humans, proprioception depends on the communication of mechanosensory neurons distributed throughout their bodies and is considered to be their sixth sense [31]. All these features suggest that for a proper understanding of cognitive aspects of plants, we need to complement plant physiology with plant neurobiology. As plants evolved their multicellularity independently from animals, plant proprioception clearly represents not only an example of convergent evolution [2] but also strong support for the plant neurobiology view of plant body organization (figures 1 and 2). It is not surprising in this respect that roots interconnect clonal plants, having the same genome, into physiologically integrated higher-order individuality [32,33]; as well as symbiotic arbuscular mycorrhiza fungal hyphae connect to the root apices of different plant species to form huge integrated supraorganismal networks known as wood-wide-webs [3]. Moreover, it is known that supraorganismal root networks are also generated via natural root grafts [34].
5. Vascular systems integrate plant bodies into a coherent and unitary whole
In vascular plants having roots underground and shoots aboveground, supracellular systems integrating the whole plant body into a coherent unit are represented by vascular systems composed of vascular elements of xylem and phloem that are embedded within the vascular parenchyma. Although these vascular systems are studied mostly with respect to transport of solutes, minerals, hormones and other signals, as well as photosynthates, it is well known that vascular systems also allow rapid long-distance signalling and communication via electric, hydraulic and ROS/calcium waves [18–21,35–38]. The phloem is especially relevant in this respect as it is one huge and ramified cable-like compartment spanning the whole plant body, connecting all plant organs into one unified huge axon-like super-cell [39]. Intriguingly in this respect, phloem tubes provide a low-resistance medium allowing rapid spreading of plant-specific AP throughout the plant body [20,36–38,40]. This rapid electric signalling integrates the whole plant body into physiological and cognitive unity, allowing vascular plants to act as individualities having both plant-specific agency and cognition.
We are not discussing plasmodesmata here, due to the tight space limitation, but these plant-specific direct cell–cell channels are very relevant for integrating plant tissues and organs into coherent plant bodies. They contribute to the spreading of plant-specific APs through the whole plant body. In contrast with APs in animals and humans, plant APs are not based on sodium ions, which are toxic to the pectinic plant cell walls [41,42], but on calcium ions [43,44]. Nevertheless, APs in plants and animals have similar bioelectrical, cellular and communicative features and both evolved from very ancient membrane repair processes of early eukaryotic cells [45–48].
6. Invasive vascular systems and pollen tubes show neuronal features
A unique feature of plant vascular systems is that, besides roles in transport and long-distance signalling and communication, they also self-organize into networks and generate new wiring and connections during wound healing and plant grafting. They show very high flexibility and adaptability during organogenesis. For example, below developing new shoot and leaf primordia, firstly young procambial cells are generated, from which new vascular veins form docking into the main stem vasculature [10,16,49]. When vascular systems are cut during wounding, they regain vascular continuity via redifferetiation of parenchymatic cells into new xylem and phloem elements to repair the damage [10,16,17]. Auxin acts as the main player in this vascular self-organizing patterning and morphogenesis [10,16,49–51].
In contrast with restrictive compatibilities in grafting, parasitic plants can effectively overcome compatibility barriers, but the processes and factors behind the success of parasitic plants remain elusive [51]. Parasitic plants use their invasive organ, known as a haustorium, to locate and connect to host plant vascular systems. Intriguingly, vascular systems are also acting invasively when they invade nodules induced by symbiotic Rhizobium bacteria in roots or tumours induced by Agrobacteria in stems [51,52]. Moreover, there are several examples of intrusive plant cells, analogous to growing neurons [53,54]. For instance, pollen tubes grow invasively within the pistil tissues and their tip-growth not only resembles neuronal axons cytologically [55], but their growth is also controlled via neurotransmitter-like signalling when both GABA and glutamate are involved [56–59]. This neurotransmitter-based pollen tube guidance towards the ovules, which are protected by the embryo sac that is located deep within the maternal pistil tissues, is preceded by rapid electric signalling. Plant APs are initiated by pollen grains landing on the receptive stigma, alerting the embryo sac in advance about germinating pollen grains [60–62].
7. Fundamental differences between root and shoot organogenesis
Although auxin plays a central role in both shoot and root organogenesis, there are fundamental differences. Most prominently, while new leaves are initiated exogenously at the shoot periphery, new roots are initiated endogenously at the stele periphery. Moreover, while shoot apex morphogenesis is accomplished via a stereotypic and regular phyllotactic pattern starting at the very tip of the shoot apex, new root primordia form far from the root tip where cell growth in the primary root has already stopped [18]. Although founder cells for prospective lateral root primordia are already primed at the root apex transition/oscillatory zone [27,28], their fate depends largely on environmental factors, which allows fundamental flexibility and plasticity of root systems [63]. Similarly, the cambial meristem, forming the secondary vascular systems in dicotyledonous plants, is regulated not only via endogenous signals but also via environmental factors allowing flexible control of trees, especially their sizes and forms, according to the environmental context [64]. Importantly, all the meristems of plant bodies are integrated together into functional unities [65]. Nevertheless, some secondary meristems, especially the cambium and phellogen, often retain partial autonomy in small branches and branch tips.
Root branching plasticity allows roots to accomplish foraging behaviour which is critical in their difficult task of finding water and mineral nutrition [65–67]. In the root foraging behaviour, root apices continually evaluate, in a context-dependent manner, the amount of nutrients and integrate this information in dependency of their neighbouring plants [68]. In other words, root apices make their decisions [69] in order to secure relevant resources in soils that have patchy distributions of nutrients [70]. This is the main reason that root apex organogenesis, in contrast with shoot organogenesis, is developmentally postponed and depends more on the local environmental situation [68]. Importantly, root foraging behaviour is affected by damage to shoot organs [67], implying shoot–root communication via the vascular systems is involved in both root organogenesis and root apex foraging decisions.
8. The root apex as a brain-like command centre
Charles and Francis Darwin were the first scientists to appreciate the crucial role of the root apices in the life of plants [71]. Their book The Power of Movements in Plants ends with a final paragraph in which they conclude that the root apex acts as the brain of lower animals. Using precise microsurgery experiments, they reported that the intact root cap is essential for diverse root apex tropisms but not for root growth per se [27,28,71]. Importantly, several aspects and features of cells in the root apex transition zone correspond to the Darwinian brain-like organ. These include synaptic-like cell–cell communication based on plant-specific F-actin/myosin VIII-based vesicle recycling, high electric/spiking activities, a peak in the oxygen consumption related to synaptic-like activities, and oscillatory features in the plasma membrane transport and gene expression activities [23,27,28]. The root apex transition zone also decides about root tropisms; a differential release of cells from the transition zone into the rapid elongation region is responsible for the first root apex bending zone [27,28]. Root apex organogenesis is based on the oscillatory processes and patterning [72,73], which resemble somite segment formation in vertebrates [73]. The root apex brain-like decision-making centre also integrates environmental signals into root system plasticity [74] and dormancy decisions [75].
9. Self-incompatibility in sexual reproduction, immunity and symbiosis
Besides the already mentioned need for compatibility during plant grafting and parasitic interactions [51], there are several other examples of plant self-recognition either during sexual reproduction or during pathogen defence via plant immunity. In sexual self-incompatibility, plants reject self-pollen to promote outcrossing and to prevent or reduce inbreeding [76,77]. The sporophytic self/non-self pollen grain recognition is typically accomplished via the stigma papilla cells which represent the first entry point of germinating pollen tubes into maternal tissues of the pistil. Pollen self/non-self recognition is accomplished via integrated signalling pathways based on diverse peptides, receptors, cytoskeletal systems, calcium and ROS signalling [76,77]. Besides these early self-incompatibility events at the stigma papilla, there are also late-acting mechanisms of plant reproductive barriers which are not well understood yet. Invasion of pistil tissues via pollen tubes resembles fungal invasion and it is perhaps relevant in this respect that sexual self-incompatibility might have evolutionary origins in fungal invasions of ancient plants [78]. Intriguingly in this respect, MILDEW RESISTANCE LOCUS O (MLO) controls both AM fungal invasion of roots and pollen tube reception in delivery of sperm cells to the embryo sac [79,80]. Moreover, MLO is recruited to and polarized towards the fungal penetration sites [81].
The best example of plant awareness of their biotic environment via self-sense is the recognition of intrusive growth, for instance, of fibres as self [54], as well as in the ability of plants to rapidly detect and effectively fight viral, microbial and fungal invaders and pathogens via their plant-specific innate immunity [82,83]. Surprisingly, the plant innate immune systems rely on similar receptor proteins as those of the animal and human innate immune systems [26,84,85]. However, the damaged-self recognition has an ancient cellular basis as animal and plant cells are continuously monitoring both their structural and functional integrities and mount immediate responses if problems are detected [84]. Interestingly, receptors involved in plant innate immunity resemble those that are relevant for symbiosis [86]. This reflects the probable evolutionary origin of symbiosis from infections at the organismal evolution level [24], as is similarly the case at the cellular level [87].
The aboveground young plant body integrity is also supported via the outermost non-cellular layer known as the cuticle. This protective layer is composed of the lipid polymer cutine and of diverse waxes, forming a complex hydrophobic polymer mixture [88] protecting shoots from water loss, UV irradiation and pathogen attacks [89,90]. Although roots are considered to be devoid of cuticle, a recent study discovered that the Arabidopsis root cap is covered by a cuticle layer [6]. Moreover, the Arabidopsis embryo is also covered by a cuticle, starting with the torpedo stage of embryo development [91,92].
10. Self/non-self recognition, kin recognition and mimicry
Plants apply their plant-specific self-sense also at the higher organ and organismal levels. Although it is still a mystery how the above-discussed cellular levels of self generate the higher levels of self also in animals and humans, plants obviously are capable of this feature too. It can be speculated that the vascular systems discussed above, with their neuronal-like features, are relevant in this respect. It is well known that plants integrate information about the biotic and abiotic location in which they grow, with a particular interest in the identity of their neighbours [93]. The first reports on self/non-self recognition in plants were published by Ariel Novoplansky and his co-workers working on roots of Pisum sativum [94] and Buchloe dactyloide [95]. Later, they confirmed their results also for roots of Trifolium repens [96]. Interestingly, self-sense of roots is based on their root apex exudation of a specific chemical finger-prints [95] which allows them to effectively recognize neighbour roots, if they belong to the same plant, another plant of the same species, or another plant of a different species, as well as other organisms or even objects in their vicinity. Plants live in a very competitive environment when mineral nutrition is present in limited and not easily reached soil patches, forcing roots into fierce plant–plant competition [97–99]. The presence of belowground competitors also alerts their aboveground organs [100–103]. Importantly, plant self-recognition is relevant for both plant communication and defence [104].
Self/non-self recognition is also typical of and well characterized in shoot tendrils, which are associated with the climbing habit of many vascular plants [104–106]. The climbing habit evolved several times in the evolutionary history of flowering plants and is supported via several different organs, including tendrils performing helical movements, which had already attracted the interest of Charles Darwin who published The Movements and Habits of Climbing Plants in 1875 [107]. For example, tendrils of the perennial vine Cayratia japonica exhibit self-recognition that allows them to coordinate their coiling responses [108]. Later, this self/non-self recognition in tendrils was also reported for other plants [109]. As is the case with root apices, the shoot tendrils also use their chemical sense for this self-discrimination [110]. Tendrils of the vine C. japonica were reported to be able to recognize and to avoid host plants when these were plagued with spider mites [111]. Interestingly, there are very intriguing similarities between root and tendril behaviour where both roots and tendrils possess two coordinating bending zones allowing them much more flexible movements than other plant organs. In maize roots, this is recognizable as root apex crawling behaviour [112–115], while tendrils show helical coiling-based growth allowing them to twist around host plants [104,107]. Intriguingly, auxin controls both root crawling and tendril coiling [112,114,116].
Besides the self/non-self recognition, socially and cognitively active plants also enjoy kin recognition [117,118]. Interestingly, plant-specific kin recognition can also be mediated via root exudation [119]. Kin recognition controls new root allocation within root systems in correlation with the distribution and acquisition of nutrients [120,121]. More recently, plant kin recognition was reported in rice and it was shown to have relevance for crop production and grain yields. Non-kin neighbour rice plants invest more photosynthates to their root systems and less in shoots and grains [112,122]. Plant kin recognition is emerging as an important phenomenon for commercial agriculture to control crop productivity.
Plants can also recognize kin plants via shoots using photoreceptors [123,124]. Intriguingly, kin recognition also allows plant to control attraction of pollinators to their flowers [125]. Whether this is accomplished via root exudates or light-sensing photoreceptors is not known. But there is one example where kin recognition in roots is related to plant sexual organs. In Populus cathayana trees, it was shown that the recognition of the sexual identity of plant neighbours is mediated via their root systems [126]. As with the root-mediated and exudate-based plant kin recognition, the shoot-mediated and photoreceptor-based plant recognition also plays roles in productivity as those plants which interact with their kin also produce more seeds [123,124].
Plant mimicry is typically explained via classical plant physiology and ecology concepts [127], not involving active sensory manipulation of interacting partners via plant cognition. But there are examples where this approach is unable to explain all the complexities and nuances of these phenomena. For example, the woody vine Boquila trifoliata, from the rainforest of South America, is capable of mimicking shapes, size, colours and texture of up to three different host plants [128]. We have proposed that the ocelli concept of Gottlieb Haberlandt would be applicable here as it is almost impossible to explain mimicking of so many parameters without some kind of vision [129,130]. It should be not so surprising to have vision-supporting ocelli in vascular plants as eye-like ocelloids are involved in rudimentary vision of unicellular algae [131,132]. Otherwise, delicate chemical sensing should be involved.
11. Sociality of vascular plants via roots and arbuscular mycorhizal fungal networks
According to the Darwinian view of the plant body organization, the root apices are acting as the nutritional and cognitive pole whereas the shoot apices are responsible for the generation of sexual (flowers) and support (photosynthetic leaves) plant organs (figure 1, [71]). Numerous discoveries and findings support the Darwinian view of roots as acting in a social and cognitive manner [9,94–99,133–136] controlling numerous microorganisms in the rhizosphere via chemical inter-kingdom communication and entering into symbioses with fungi and bacteria [3,137–140]. By contrast, shoots are not enjoying such a symbiotic social life based on direct intracellular organism–organism connections [23–25], although the phylosphere is also abundant with plant-supporting fungi and microorganisms. Vascular plants form root–fungal networks [141] not only for nutrition and water [142,143] but also for information exchange [144–147]). Moreover, clonal plants are integrated via their root systems too, which allows them to act in an intelligent manner [33,34]. Besides the symbiotic networks with arbuscular mycorrhiza fungi, plant roots are also often interconnected via natural root grafts [34]. Finally, it should not be surprising to have sociality in plants and trees as unicellular amoebae also have a rich social life [148].
12. Outlook
Although many still consider plants as semi-living automata [149,150], evidence that vascular plants are cognitive, communicative and intelligent organisms is accumulating and new data are overwhelming [3,75,151–156].
For example, consider the classical model object Arabidopsis thaliana. For more than 50 years, young seedlings of this plant have been cultivated in our laboratories within transparent Petri dishes where roots, which outside in nature grow underground in darkness, are exposed to light [157,158]. Arabidopsis roots grow fast when illuminated, and this feature is evaluated as a good growth condition. However, the plant neurobiology perspective, which considers plants as cognitive organisms, implicates that such light-exposed roots of young seedlings are stressed and they try to avoid exposure to light via light-escape tropism [159,160]. This is a clear example that the plant neurobiology view is both relevant and important for our understanding of living plants [3,18] in their whole complexity.
In contrast with animals and humans, plants are not only inverted (figures 1 and 2) but also rely on distributed control with from a single to several thousands of root apices accomplishing collective decision-making [3], a feature which allows them to act very robustly and to survive even though the plant cannot move itself as a whole [3,9–15,161–163].
Importantly, plants are sensitive to anaesthetics and they fail to move under anaesthesia [164–167]. This sensitivity to anaesthetics is very strong evidence that plants are enjoying similar life to animals and humans. There are numerous examples where plants manipulate animals for their own needs [168–172], suggesting that social aspects of vascular plants are largely underestimated.
Finally, considering plants as cognitive organisms following their own plant-specific aims, goals, objectives and interests, it is also necessary to re-consider our human relationships with plants. The classical interpretation of plant domestication still is: an intentional process driven by our human needs. However, plant domestication instead emerges in certain cases as the coevolution between two partners profiting in their own ways from this partnership [173–175]. Ancient humans teamed-up with their crop plants and entered into the sedentary lifestyle which allowed them to organize large religious societies implementing private property and moralistic gods [176–179]. Cultivation of crop plants in dense monocultures resulted in larger grains via plant-specific kin recognition [122–124,180]. The weed plants growing within the population of crop plants mimic these crop plants via Vavilovian mimicry [127,169]. This baffling ability of weed plants to mimic crop plants seems to suggest that these weed plants are somehow aware of advantages that the crop plants get from having humans as their seed dispersers. The question recently posed by Daniel Chamovitz What a Plant Knows? [181] is given a new dimension in this respect. As our survival on planet Earth is completely dependent on plants [3], we should change our attitude to plants and start to study them as cognitive and intelligent organisms [182] endowed with lots of behavioural and cognitive competencies.
Acknowledgements
We are thankful to Fred Keijzer and Simcha Lev-Yadun for their numerous constructive and helpful suggestions and comments.
Data accessibility
This article has no additional data.
Authors' contributions
Both authors wrote the text.
Competing interests
We declare we have no competing interests.
Funding
F.B. acknowledges funding obtained via the Stiftung Zukunft jetzt! (Munich, Germany).
References
- 1.Munné-Bosch S. 2018. Limits to the tree growth and longevity. Trends Plant Sci. 23, 985-993. ( 10.1016/j.tplants.2018.08.001) [DOI] [PubMed] [Google Scholar]
- 2.Baluška F, Mancuso S. 2009. Plants and animals: convergent evolution in action? In Plant-environment interactions (ed. Baluška F.), pp. 285-301. Berlin, Germany: Springer. [Google Scholar]
- 3.Baluška F, Mancuso S. 2020. Plants, climate and humans: plant intelligence changes everything. EMBO Rep. 21, e50109. ( 10.15252/embr.202050109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S-J, Tillberg E. 1984. Sensitivity to phytohormones determined by outer-inner polarity of higher plants: an overall model for phytohormone action. Plant Cell Environ. 7, 75-80. ( 10.1111/j.1365-3040.1984.tb01559.x) [DOI] [Google Scholar]
- 5.Alassimone J, et al. 2012. The endodermis—development and differentiation of the plant's inner skin. Protoplasma 249, 433-443. ( 10.1007/s00709-011-0302-5) [DOI] [PubMed] [Google Scholar]
- 6.Berhin A, et al. 2019. The root cap cuticle: a cell wall structure for seedling establishment and lateral root formation. Cell 176, 1367-1378. ( 10.1016/j.cell.2019.01.005) [DOI] [PubMed] [Google Scholar]
- 7.Baluška F. 2012. Rethinking origins of multicellularity: convergent evolution of epithelia in plants. Bioessays 34, 1085. ( 10.1002/bies.201200134) [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishna P, Barberon M. 2019. Polarized transport across root epithelia. Curr. Opin. Plant Biol. 52, 23-29. ( 10.1016/j.pbi.2019.05.010) [DOI] [PubMed] [Google Scholar]
- 9.Baluška F, et al. 2006. Neurobiological view of plants and their body plan. In Communication in plants—neuronal aspects of plant life (eds Baluška F, et al.), pp. 19-35. Berlin, Germany: Springer. [Google Scholar]
- 10.Dostal R. 1967. On integration in plants. Cambridge, MA: Harvard University Press. [Google Scholar]
- 11.White J. 1979. The plant as a metapopulation. Annu. Rev. Ecol. Syst. 10, 109-145. ( 10.1146/annurev.es.10.110179.000545) [DOI] [Google Scholar]
- 12.Hallé F. 1986. Modular growth in seed plants. Phil. Trans R. Soc. Lond. B 313, 77-87. ( 10.1098/rstb.1986.0026) [DOI] [Google Scholar]
- 13.Barlow PW. 1989. Meristems, metamers and modules and the development of shoot and root systems. Bot. J. Linn. Soc. 100, 255-279. ( 10.1111/j.1095-8339.1989.tb01721.x) [DOI] [Google Scholar]
- 14.Sachs T, Novoplansky A, Cohen D. 1993. Plants as competing populations of redundant organs. Plant Cell Environ. 16, 765-770. ( 10.1111/j.1365-3040.1993.tb00498.x) [DOI] [Google Scholar]
- 15.Oborny B. 2019. The plant body as a network of semi-autonomous agents: a review. Phil. Trans. R. Soc. B 374, 20180371. ( 10.1098/rstb.2018.0371) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sachs T. 1991. Pattern formation in plant tissues. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Sachs T. 2004. Self-organization of tree form: a model for complex social systems. J. Theor. Biol. 230, 197-202. ( 10.1016/j.jtbi.2004.05.006) [DOI] [PubMed] [Google Scholar]
- 18.Baluška F, Mancuso S, Volkmann D (eds). 2006. Communication in plants—neuronal aspects of plant life. Berlin, Germany: Springer. [Google Scholar]
- 19.Gilroy S, et al. 2016. ROS, calcium and electric signals: key mediators of rapid systemic signaling in plants. Plant Physiol. 171, 1606-1615. ( 10.1104/pp.16.00434) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hedrich R, Salvador-Recatalà V, Dreyer I. 2016. Electrical wiring and long-distance plant communication. Trends Plant Sci. 21, 376-387. ( 10.1016/j.tplants.2016.01.016) [DOI] [PubMed] [Google Scholar]
- 21.Baluška F, Mancuso S. 2019. Actin cytoskeleton and action potentials: forgotten connections. In The cytoskeleton. Plant cell monographs 24 (eds Baluška F, Snahi VP), pp. 63-83. Berlin, Germany: Springer. [Google Scholar]
- 22.Yamada S, Nelson WJ. 2007. Synapses: sites of cell recognition, adhesion, and functional specification. Annu. Rev. Biochem. 76, 267-294. ( 10.1146/annurev.biochem.75.103004.142811) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baluška F, Volkmann D, Menzel D. 2005. Plant synapses: actin-based domains for cell-to-cell communication. Trends Plant Sci. 10, 106-111. ( 10.1016/j.tplants.2005.01.002) [DOI] [PubMed] [Google Scholar]
- 24.Lima PT, et al. 2009. Plant-microbe symbioses: new insights into common roots. Bioessays 31, 1233-1244. ( 10.1002/bies.200800177) [DOI] [PubMed] [Google Scholar]
- 25.Baluška F, Mancuso S. 2013. Microorganism and filamentous fungi drive evolution of plant synapses. Front. Cell Infect. Microbiol. 3, 44. ( 10.3389/fcimb.2013.00044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwon C, Panstruga R, Schulze-Lefert P. 2008. Les liaisons dangereuses: immunological synapse formation in animals and plants. Trends Immunol. 29, 159-166. ( 10.1016/j.it.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 27.Baluška F, et al. 2010. Root apex transition zone: a signalling-response nexus in the root. Trends Plant Sci. 15, 402-408. ( 10.1016/j.tplants.2010.04.007) [DOI] [PubMed] [Google Scholar]
- 28.Baluška F, Mancuso S. 2013. Root apex transition zone as oscillatory zone. Front. Plant Sci. 4, 354. ( 10.3389/fpls.2013.00354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moulia B, et al. 2019. Posture control in land plants: growth, position sensing proprioception, balance, and elasticity. J. Exp. Bot. 70, 3467-3494. ( 10.1093/jxb/erz278) [DOI] [PubMed] [Google Scholar]
- 30.Sherrington CS. 1906. The integrative action of the nervous system. New York, NY: Scribner's Sons. [Google Scholar]
- 31.Tuthill JV, Azim E. 2018. Proprioception. Curr. Biol. 28, R187-R203. ( 10.1016/j.cub.2018.02.033) [DOI] [PubMed] [Google Scholar]
- 32.Adonsou E, Desrochers A, Tremblay F. 2016. Physiological integration of connected balsam poplar ramets. Tree Physiol. 36, 797-806. ( 10.1093/treephys/tpv142) [DOI] [PubMed] [Google Scholar]
- 33.Latzel V, et al. 2016. Epigenetic memory as a basis for intelligent behavior in clonal plants. Front. Plant Sci. 7, 1354. ( 10.3389/fpls.2016.01354) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lev-Yadun S. 2011. Why should trees have natural root grafts? Tree Physiol. 31, 575-578. ( 10.1093/treephys/tpr061) [DOI] [PubMed] [Google Scholar]
- 35.Mancuso S, Mugnai S. 2006. Long-distance signal transmission in trees. In Communication in plants—neuronal aspects of plant life (eds Baluška F, et al.), p. 335. Berlin, Germany: Springer. [Google Scholar]
- 36.Fromm J, et al. 2013. Electrical signaling along the phloem and its physiological responses in the maize leaf. Front. Plant Sci. 4, 239. ( 10.3389/fpls.2013.00239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Bel AJ, et al. 2014. Spread the news: systemic dissemination and local impact of Ca2+ signals along the phloem pathway. J. Exp. Bot. 65, 1761-1787. ( 10.1093/jxb/ert425) [DOI] [PubMed] [Google Scholar]
- 38.Zimmermann MR, et al. 2019. Measurement of electropotential waves in intact phloem sieve elements using microelectrodes. Methods Mol. Biol. 2014, 439-447. ( 10.1007/978-1-4939-9562-2_34) [DOI] [PubMed] [Google Scholar]
- 39.van Bell AJ. 2003. The phloem, a miracle of ingenuity. Plant Cell Environ. 26, 125-149. ( 10.1046/j.1365-3040.2003.00963.x) [DOI] [Google Scholar]
- 40.Canales J, Henriquez-Valencia C, Brauchi S. 2018. The integration of electrical signals originating in the root of vascular plants. Front. Plant Sci. 8, 2173. ( 10.3389/fpls.2017.02173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng W, et al. 2018. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 28, 666-675. ( 10.1016/j.cub.2018.01.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verger S, Hamant O. 2018. FERONIA defends the cell walls against corrosion. Curr. Biol. 28, R215-R217. ( 10.1016/j.cub.2018.01.043) [DOI] [PubMed] [Google Scholar]
- 43.Beilby MJ. 2007. Action potential in charophytes. Int. Rev. Cytol. 257, 43-82. ( 10.1016/S0074-7696(07)57002-6) [DOI] [PubMed] [Google Scholar]
- 44.Beilby MJ, Al Khazaaly S.. 2016. Re-modeling Chara action potential. I. From Thiel model of Ca2+ transient to action potential form. AIMS Biophys. 3, 431-449. ( 10.3934/biophy.2016.3.431) [DOI] [Google Scholar]
- 45.Goldsworthy A. 1983. The evolution of plant action potentials . J. Theor. Biol. 103, 645-648. ( 10.1016/0022-5193(83)90287-4) [DOI] [Google Scholar]
- 46.Steinhardt RA, Bi G, Alderton JM. 1994. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science 263, 390-394. ( 10.1126/science.7904084) [DOI] [PubMed] [Google Scholar]
- 47.Schapire AL, Valpuesta V, Botella MA. 2009. Plasma membrane repair in plants. Trends Plant Sci. 14, 645-652. ( 10.1016/j.tplants.2009.09.004) [DOI] [PubMed] [Google Scholar]
- 48.Brunet T, Arendt D. 2016. From damage response to action potentials: early evolution of neural and contractile modules in stem eukaryotes. Phil. Trans. R. Soc. B 371, 20150043. ( 10.1098/rstb.2015.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aloni R, et al. 2003. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 216, 841-853. ( 10.1007/s00425-002-0937-8) [DOI] [PubMed] [Google Scholar]
- 50.Verna C, et al. 2015. Control of vein network topology by auxin transport. BMC Biol. 13, 94. ( 10.1186/s12915-015-0208-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melnyk CW. 2017. Connecting the plant vasculature to friend or foe. New Phytol. 213, 1611-1617. ( 10.1111/nph.14218) [DOI] [PubMed] [Google Scholar]
- 52.Aloni R, Pradel KS, Ullrich CI. 1995. The three-dimensional structure of vascular tissues in Agrobacterium tumefaciens-induced crown galls and in the host stems of Ricinus communis L. Planta 196, 597-605. ( 10.1007/BF00203661) [DOI] [Google Scholar]
- 53.Baluška F. 2010. Recent surprising similarities between plant cells and neurons. Plant Signal. Behav. 5, 87-89. ( 10.4161/psb.5.2.11237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lev-Yadun S. 2001. Intrusive growth—the plant analog of dendrite and axon growth in animals. New Phytol. 150, 508-512. ( 10.1046/j.1469-8137.2001.00143.x) [DOI] [Google Scholar]
- 55.Palanivelu R, Preuss D. 2000. Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol. 10, 517-524. ( 10.1016/S0962-8924(00)01849-3) [DOI] [PubMed] [Google Scholar]
- 56.Palanivelu R, Brass L, Edlund AF, Preuss D. 2003. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114, 47-59. ( 10.1016/S0092-8674(03)00479-3) [DOI] [PubMed] [Google Scholar]
- 57.Michard E, et al. 2011. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science 332, 434-437. ( 10.1126/science.1201101) [DOI] [PubMed] [Google Scholar]
- 58.Yu GH, et al. 2014. Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J. Exp. Bot. 65, 3235-3248. ( 10.1093/jxb/eru171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wudick MM, et al. 2018. CORNICHON sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science 360, 533-536. ( 10.1126/science.aar6464) [DOI] [PubMed] [Google Scholar]
- 60.Sinyukhin AM, Britikov EA. 1967. Action potentials in the reproductive system of plants. Nature 215, 1278-1280. ( 10.1038/2151278a0) [DOI] [Google Scholar]
- 61.Spanjers AW. 1981. Bioelectric potential changes in the style of Lilium longiflorum Thumb. after self- and cross-pollination of the stigma. Planta 153, 1-5. ( 10.1007/BF00385310) [DOI] [PubMed] [Google Scholar]
- 62.Wedzony M, Filek M. 1998. Changes of electric potential in pistils of Petunia hybrida Hort. and Brassica napus L. during pollination. Acta Physiol. Plant. 20, 291-297. ( 10.1007/s11738-998-0061-x) [DOI] [Google Scholar]
- 63.Laskowski M, Ten Tusscher KH. 2017. Periodic lateral root priming: what makes it tick? Plant Cell 29, 432-444. ( 10.1105/tpc.16.00638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Begum S, et al. 2013. Regulation of cambial activity in relation to environmental conditions: understanding the role of temperature in wood formation of trees. Physiol. Plant. 147, 46-54. ( 10.1111/j.1399-3054.2012.01663.x) [DOI] [PubMed] [Google Scholar]
- 65.Oldroyd GED, Leyser O. 2020. A plant's diet, surviving in a variable nutrient environment. Science 368, eaba0196. ( 10.1126/science.aba0196) [DOI] [PubMed] [Google Scholar]
- 66.McNickle GG, St Claire CC, Cahill JF. 2009. Focusing the metaphor: plant root foraging behaviour. Trends Ecol. Evol. 24, 419-426. ( 10.1016/j.tree.2009.03.004) [DOI] [PubMed] [Google Scholar]
- 67.Yamawo A, Ohsaki H, Cahill JF Jr. 2019. Damage to leaf veins suppresses root foraging precision. Am. J. Bot. 106, 1126-1130. ( 10.1002/ajb2.1338) [DOI] [PubMed] [Google Scholar]
- 68.Karst JD, et al. 2012. Context dependence in foraging behaviour of Achillea millefolium. Oecologia 170, 925-933. ( 10.1007/s00442-012-2358-0) [DOI] [PubMed] [Google Scholar]
- 69.Hodge A. 2009. Root decisions. Plant Cell Environ. 32, 628-640. ( 10.1111/j.1365-3040.2008.01891.x) [DOI] [PubMed] [Google Scholar]
- 70.Forde BG, Walch-Liu P. 2009. Nitrate and glutamate as environmental cues for behavioural responses in plant roots. Plant Cell Environ. 32, 682-693. ( 10.1111/j.1365-3040.2008.01927.x) [DOI] [PubMed] [Google Scholar]
- 71.Darwin CR 1880. The power of movements in plants. London, UK: John Murray. [Google Scholar]
- 72.Moreno-Risueno MA, et al. 2010. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science 329, 1306-1311. ( 10.1126/science.1191937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Traas J, Vernoux T. 2010. Oscillating roots. Science 329, 1290-1291. ( 10.1126/science.1195572) [DOI] [PubMed] [Google Scholar]
- 74.Topham AT, et al. 2017. Temperature variability is integrated by a spatially embedded decision-making center to break dormancy in Arabidopsis seeds. Proc. Natl Acad. Sci. USA 114, 6629-6634. ( 10.1073/pnas.1704745114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fromm H. 2019. Root plasticity in the pursuit of water. Plants 8, 236. ( 10.3390/plants8070236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takayama S, Isogai A. 2005. Self-incompatibility in plants. Annu. Rev. Plant Biol. 56, 467-489. ( 10.1146/annurev.arplant.56.032604.144249) [DOI] [PubMed] [Google Scholar]
- 77.Wilkins KA, Poulter NS, Franklin-Tong VE. 2014. Taking one for the team: self-recognition and cell suicide in pollen. J. Exp. Bot. 65, 1331-1342. ( 10.1093/jxb/ert468) [DOI] [PubMed] [Google Scholar]
- 78.Elleman CJ, Dickinson HG. 1999. Commonalities between pollen/stigma and host/pathogen interactions: calcium accumulation during stigmatic penetration by Brassica oleracea pollen tubes. Sex. Plant Reprod. 12, 194-202. ( 10.1007/s004970050192) [DOI] [Google Scholar]
- 79.Jones DS, et al. 2017. MILDEW RESISTANCE LOCUS O function in pollen tube reception is linked to its oligomerization and subcellular distribution. Plant Physiol. 175, 172-185. ( 10.1104/pp.17.00523) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacott CN, et al. 2020. Mildew Locus O facilitates colonization by arbuscular mycorrhizal fungi in angiosperms. New Phytol. 227, 343-351. ( 10.1111/nph.16465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Panstruga R. 2005. Serpentine plant MLO proteins as entry portals for powdery mildew fungi. Biochem. Soc. Trans. 33, 389-392. ( 10.1042/BST0330389) [DOI] [PubMed] [Google Scholar]
- 82.Dodds PN, Rathjen JP. 2010. Plant immunity: towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 11, 539-548. ( 10.1038/nrg2812) [DOI] [PubMed] [Google Scholar]
- 83.Sanabria NM, Huang JC, Dubery IA. 2010. Self/nonself perception in plants in innate immunity and defense. Self Nonself 1, 40-54. ( 10.4161/self.1.1.10442) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heil M, Land WG. 2014. Danger signals—damaged-self recognition across the tree of life. Front. Plant Sci. 5, 578. ( 10.3389/fpls.2014.00578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bentham A, Burdett H, Anderson PA, Williams SJ, Kobe B. 2017. Animal NLRs provide structural insights into plant NLR function. Ann. Bot. 119, 689-702. ( 10.1093/aob/mcw171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Girardin A, et al. 2019. LCO receptors involved in arbuscular mycorrhiza are functional for rhizobia perception in legumes. Curr. Biol. 29, 4249-4259. ( 10.1016/j.cub.2019.11.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baluška F. 2009. Cell-cell channels, viruses, and evolution: via infection, parasitism, and symbiosis toward higher levels of biological complexity. Ann. NY Acad. Sci. 1178, 106-119. ( 10.1111/j.1749-6632.2009.04995.x) [DOI] [PubMed] [Google Scholar]
- 88.Bernard A, Joubès J. 2013. Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Prog. Lipid Res. 52, 110-129. ( 10.1016/j.plipres.2012.10.002) [DOI] [PubMed] [Google Scholar]
- 89.Bessire M, et al. 2007. A permeable cuticle in Arabidopsis leads to a strong resistance to Botrytis cinerea. EMBO J. 26, 2158-2168. ( 10.1038/sj.emboj.7601658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serrano M, Coluccia F, Torres M, L'Haridon F, Metraux JP. 2014. The cuticle and plant defense to pathogens. Front. Plant Sci. 5, 274. ( 10.3389/fpls.2014.00274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moussu S, et al. 2013. Embryonic cuticle establishment: the great (apoplastic) divide. Plant Signal. Behav. 8, e27491. ( 10.4161/psb.27491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Creff A, et al. 2019. A stress-response-related inter-compartmental signalling pathway regulates embryonic cuticle integrity in Arabidopsis. PLoS Genet. 15, e1007847. ( 10.1371/journal.pgen.1007847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cahill JF Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, St Clair CC. 2010. Plants integrate information about nutrients and neighbors. Science 328, 1657. ( 10.1126/science.1189736) [DOI] [PubMed] [Google Scholar]
- 94.Falik O, et al. 2003. Self/non-self discrimination in roots. J. Ecol. 91, 525-531. ( 10.1046/j.1365-2745.2003.00795.x) [DOI] [Google Scholar]
- 95.Gruntman M, Novoplansky A. 2004. Physiologically mediated self/non-self discrimination in roots. Proc. Natl Acad. Sci. USA 101, 3863-3867. ( 10.1073/pnas.0306604101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Falik O, de Kroon H, Novoplansky A. 2006. Physiologically-mediated self/nonself root discrimination in Trifolium repens has mixed effects on plant performance. Plant Signal. Behav. 1, 116-121. ( 10.4161/psb.1.3.2639) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Casper BB, Jackson RB. 1997. Plant competition underground. Annu. Rev. Ecol. Syst. 28, 545-570. ( 10.1146/annurev.ecolsys.28.1.545) [DOI] [Google Scholar]
- 98.Gersani M, et al. 2001. Tragedy of the commons as a result of root competition. J. Ecol. 89, 660-669. ( 10.1046/j.0022-0477.2001.00609.x) [DOI] [Google Scholar]
- 99.Schenk HJ. 2006. Root competition: beyond resource depletion. J. Ecol. 94, 725-739. ( 10.1111/j.1365-2745.2006.01124.x) [DOI] [Google Scholar]
- 100.Fragoso V, et al. 2014. Root jasmonic acid synthesis and perception regulate folivore-induced shoot metabolites and increase Nicotiana attenuata resistance. New Phytol. 202, 1325-1345. ( 10.1111/nph.12747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen BJW, et al. 2019. Presence of belowground neighbors activates defense pathways at the expense of growth in tobacco plants. Front. Plant Sci. 10, 751. ( 10.3389/fpls.2019.00751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ninkovic V, et al. 2019. Who is my neighbor? Volatile cues in plant interactions. Plant Signal. Behav. 14, 1634993. ( 10.1080/15592324.2019.1634993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Karban R, Shiojiri K. 2009. Self-recognition affects plant communication and defense. Ecol. Lett. 12, 502-506. ( 10.1111/j.1461-0248.2009.01313.x) [DOI] [PubMed] [Google Scholar]
- 104.Jaffe MJ, Galston AW. 1968. The physiology of tendrils. Annu. Rev. Plant Physiol. 19, 417-434. ( 10.1146/annurev.pp.19.060168.002221) [DOI] [Google Scholar]
- 105.Riehl TE, Jaffe MJ. 1982. Physiological studies on pea tendrils. XIII. Respiration is necessary for contact coiling. Physiol. Plant. 55, 192-196. ( 10.1111/j.1399-3054.1982.tb02286.x) [DOI] [Google Scholar]
- 106.Gianoli E. 2004. Evolution of a climbing habit promotes diversification in flowering plants. Proc. R. Soc. Lond. B 271, 2011-2015. ( 10.1098/rspb.2004.2827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Darwin C. 1875. The movements and habits of climbing plants. London, UK: John Murray. [Google Scholar]
- 108.Fukano Y, Yamawo A.. 2015. Self-discrimination in the tendrils of the vine Cayratia japonica is mediated by physiological connection. Proc. R. Soc. B 282, 20151379. ( 10.1098/rspb.2015.1379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sato M, et al. 2018. Self-discrimination in vine tendrils of different plant families. Plant Signal. Behav. 13, e1451710. ( 10.1080/15592324.2018.1451710) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fukano Y. 2017. Vine tendrils use contact chemoreception to avoid conspecific leaves. Proc. R. Soc. B 284, 20162650. ( 10.1098/rspb.2016.2650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakai T, Yano S. 2019. Vines avoid coiling around neighbouring plants infested by polyphagous mites. Sci. Rep. 9, 6589. ( 10.1038/s41598-019-43101-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baluška F, et al. 2009. Intracellular domains and polarity in root apices: from synaptic domains to plant neurobiology. Nova Acta Leopold. 96, 103-122. [Google Scholar]
- 113.Burbach C, et al. 2012. Photophobic behavior of maize roots. Plant Signal. Behav. 7, 874-878. ( 10.4161/psb.21012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suzuki H, et al. 2016. Root cap-dependent gravitropic U-turn of maize root requires light-induced auxin biosynthesis via the YUC pathway in the root apex. J. Exp. Bot. 67, 4581-4591. ( 10.1093/jxb/erw232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yokawa K, Baluška F. 2018. Sense of space: tactile sense for exploratory behavior of roots. Commun. Integr. Biol. 11, 1-5. ( 10.1080/19420889.2018.1440881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaffe MJ. 1975. The role of auxin in the early events of the contact coiling of tendrils. Plant Sci. Lett. 5, 217-225. ( 10.1016/0304-4211(75)90015-2) [DOI] [Google Scholar]
- 117.Dudley SA, File AL. 2007. Kin recognition in an annual plant. Biol. Lett. 3, 435-438. ( 10.1098/rsbl.2007.0232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pennisi E, 2019. Do plants favor their kin? Science 363, 15-16. ( 10.1126/science.363.6422.15) [DOI] [PubMed] [Google Scholar]
- 119.Biedrzycki ML, et al. 2010. Root exudates mediate kin recognition in plants. Commun. Integr. Biol. 3, 28-35. ( 10.4161/cib.3.1.10118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bhatt MV, Khandelwal A, Dudley SA. 2011. Kin recognition, not competitive interactions, predicts root allocation in young Cakile edentula seedling pairs. New Phytol. 189, 1135-1142. ( 10.1111/j.1469-8137.2010.03548.x) [DOI] [PubMed] [Google Scholar]
- 121.Palmer AG, et al. 2016. Kin recognition is a nutrient-dependent inducible phenomenon. Plant Signal. Behav. 11, e1224045. ( 10.1080/15592324.2016.1224045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bais HP. 2018. We are family: kin recognition in crop plants. New Phytol. 220, 357-359. ( 10.1111/nph.15399) [DOI] [PubMed] [Google Scholar]
- 123.Crepy MA, Casal JJ. 2015. Photoreceptor-mediated kin recognition in plants. New Phytol. 205, 329-338. ( 10.1111/nph.13040) [DOI] [PubMed] [Google Scholar]
- 124.Bais HP. 2015. Shedding light on kin recognition response in plants. New Phytol. 205, 4-6. ( 10.1111/nph.13155) [DOI] [PubMed] [Google Scholar]
- 125.Torices R, Gómez JM, Pannell JR. 2018. Kin discrimination allows plants to modify investment towards pollinator attraction. Nat. Commun. 9, 2018. ( 10.1038/s41467-018-04378-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dong T, et al. 2017. Root-mediated sex recognition in trees. Sci. Rep. 7, 801. ( 10.1038/s41598-017-00894-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pannell JR, Farmer EF. 2016. Mimicry in plants. Curr. Biol. 26, R779-R793. ( 10.1016/j.cub.2016.04.005) [DOI] [PubMed] [Google Scholar]
- 128.Gianoli E, Carrasco-Urra F. 2014. Leaf mimicry in a climbing plant protects against herbivory. Curr. Biol. 24, 984-987. ( 10.1016/j.cub.2014.03.010) [DOI] [PubMed] [Google Scholar]
- 129.Baluška F, Mancuso S. 2016. Vision in plants via plant-specific ocelli? Trends Plant Sci. 21, 727-730. ( 10.1016/j.tplants.2016.07.008) [DOI] [PubMed] [Google Scholar]
- 130.Mancuso S, Baluška F. 2017. Plant ocelli for visually guided plant behavior. Trends Plant Sci. 22, 5-6. ( 10.1016/j.tplants.2016.11.009) [DOI] [PubMed] [Google Scholar]
- 131.Hayakawa S, et al. 2015. Function and evolutionary origin of unicellular camera-type eye structure. PLoS ONE 10, e0118415. ( 10.1371/journal.pone.0118415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gavelis GS, et al. 2015. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature 523, 204-207. ( 10.1038/nature14593) [DOI] [PubMed] [Google Scholar]
- 133.Barlow PW. 2008. Reflections on plant neurobiology. Biosystems 92, 132-147. ( 10.1016/j.biosystems.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 134.Barlow P. 2010. Plant roots: autopoietic and cognitive constructions. Plant Root 4, 40-52. ( 10.3117/plantroot.4.40) [DOI] [Google Scholar]
- 135.Barlow PW. 2010. Plastic, inquisitive roots and intelligent plants in the light of some new vistas in plant biology. Plant Biosyst. 144, 396-407. ( 10.1080/11263501003718570) [DOI] [Google Scholar]
- 136.Chaffey N, Volkmann D, Baluška F. 2019. The botanical multiverse of Peter Barlow. Commun. Integr. Biol. 12, 14-30. ( 10.1080/19420889.2019.1575788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Barlow PW, Fisahn J. 2013. Swarms, swarming and entanglements of fungal hyphae and of plant roots. Commun. Integr. Biol. 6, e25299. ( 10.4161/cib.25299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mommer L, Kirkegaard J, van Ruijven J.. 2016. Root-root interactions: towards a rhizosphere framework. Trends Plant Sci. 21, 209-217. ( 10.1016/j.tplants.2016.01.009) [DOI] [PubMed] [Google Scholar]
- 139.Witzany G, Baluška F (eds). 2016. Biocommunication of plants. Berlin, Germany: Springer. [Google Scholar]
- 140.Sasse J, Martinoia E, Northen T. 2018. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 23, 25-41. ( 10.1016/j.tplants.2017.09.003) [DOI] [PubMed] [Google Scholar]
- 141.Bonfante P, Genre A. 2015. Arbuscular mycorrhizal dialogues: do you speak ‘plantish’ or ‘fungish’? Trends Plant Sci. 20, 150-154. ( 10.1016/j.tplants.2014.12.002) [DOI] [PubMed] [Google Scholar]
- 142.Bader MKF, Leuzinger S. 2019. Hydraulic coupling of a leafless kauri tree remnant to conspecific hosts. iScience 19, 1238-1247. ( 10.1016/j.isci.2019.05.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Singh D, et al. 2020. Deep-rooted pigeon pea promotes the water relations and survival of shallow-rooted finger millet during drought despite strong competitive interactions at ambient water availability. PLoS ONE 15, e0228993. ( 10.1371/journal.pone.0228993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Song YY, et al. 2010. Interplant communication of tomato plants through underground common mycorrhizal networks. PLoS ONE 5, e13324. ( 10.1371/journal.pone.0013324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barto EK, et al. 2012. Fungal superhighways: do common mycorrhizal networks enhance below ground communication? Trends Plant Sci. 17, 633-637. ( 10.1016/j.tplants.2012.06.007) [DOI] [PubMed] [Google Scholar]
- 146.Gorzelak MA, Asay AK, Pickles BJ, Simard SW. 2015. Inter-plant communication through mycorrhizal networks mediates complex adaptive behaviour in plant communities. AoB Plants 7, plv050. ( 10.1093/aobpla/plv050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simard SW. 2018. Mycorrhizal networks facilitate tree communication, learning and memory. In Memory and learning in plants (eds Baluška F, et al.), pp. 191-213. Berlin, Germany: Springer. [Google Scholar]
- 148.Bonner JT. 2009. The social amoebae. Princeton, NJ: Princeton University Press. [Google Scholar]
- 149.Alpi A, et al. 2007. Plant neurobiology: no brain, no gain? Trends Plant Sci. 12, 135-136. ( 10.1016/j.tplants.2007.03.002) [DOI] [PubMed] [Google Scholar]
- 150.Robinson DG, Draguhn A, Taiz L. 2020. Plant ‘intelligence’ changes nothing. EMBO Rep. 21, e50395. ( 10.15252/embr.202050395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Trewavas A. 2003. Aspects of plant intelligence. Ann. Bot. 92, 1-20. ( 10.1093/aob/mcg101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trewavas TAJ. 2017. The foundations of plant intelligence. Interface Focus 7, 20160098. ( 10.1098/rsfs.2016.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karban R. 2015. Plant sensing and communication. Chicago, IL: University of Chicago Press. [Google Scholar]
- 154.Calvo P, et al. 2020. Plants are intelligent, here's how. Ann. Bot. 125, 11-28. ( 10.1093/aob/mcz155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Baluška F, Levin M. 2016. On having no head: cognition throughout biological systems. Front. Psychol. 7, 902. ( 10.3389/fpsyg.2016.00902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Novoplansky A. 2019. What plant roots know? Semin. Cell Dev. Biol. 92, 126-133. ( 10.1016/j.semcdb.2019.03.009) [DOI] [PubMed] [Google Scholar]
- 157.Yokawa K, et al. 2011. llumination of Arabidopsis roots induces immediate burst of ROS production. Plant Signal. Behav. 6, 1460-1464. ( 10.4161/psb.6.10.18165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yokawa K, Kagenishi T, Baluška F. 2013. Root photomorphogenesis in laboratory-maintained Arabidopsis seedlings. Trends Plant Sci. 18, 117-119. ( 10.1016/j.tplants.2013.01.002) [DOI] [PubMed] [Google Scholar]
- 159.Yokawa K, et al. 2014. Light as stress factor to plant roots—case of root halotropism. Front. Plant Sci. 5, 718. ( 10.3389/fpls.2014.00718) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wan Y, Yokawa K, Baluška F. 2019. Arabidopsis roots and light: complex interactions. Mol. Plant 12, 1428-1430. ( 10.1016/j.molp.2019.10.001) [DOI] [PubMed] [Google Scholar]
- 161.Bassel GW. 2018. Information processing and distributed computation in plant organs. Trends Plant Sci. 23, 994-1005. ( 10.1016/j.tplants.2018.08.006) [DOI] [PubMed] [Google Scholar]
- 162.Duran-Nebreda S, Bassel GW. 2019. Plant behaviour in response to the environment: information processing in the solid state. Phil. Trans. R. Soc. B 374, 20180370. ( 10.1098/rstb.2018.0370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Debono MW, Souza GM. 2019. Plants as electromic plastic interfaces: a mesological approach. Prog. Biophys. Mol. Biol. 146, 123-133. ( 10.1016/j.pbiomolbio.2019.02.007) [DOI] [PubMed] [Google Scholar]
- 164.Grémiaux A, et al. 2014. Plant anesthesia supports similarities between animals and plants: Claude Bernard's forgotten studies. Plant Signal. Behav. 9, e27886. ( 10.4161/psb.27886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yokawa K, et al. 2018. Anaesthetics stop diverse plant organ movements, affect endocytic vesicle recycling and ROS homeostasis, and block action potentials in Venus flytraps. Ann. Bot. 122, 747-756. ( 10.1093/aob/mcx155) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yokawa K, Kagenishi T, Baluška F. 2019. Anesthetics, anesthesia, and plants. Trends Plant Sci. 24, 12-14. ( 10.1016/j.tplants.2018.10.006) [DOI] [PubMed] [Google Scholar]
- 167.Pavlovič A, et al. 2020. Anaesthesia with diethyl ether impairs jasmonate signalling in the carnivorous plant Venus flytrap (Dionaea muscipula). Ann. Bot. 125, 173-183. ( 10.1093/aob/mcz177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schiestl FP. 2005. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92, 255-264. ( 10.1007/s00114-005-0636-y) [DOI] [PubMed] [Google Scholar]
- 169.McElroy JS. 2014. Vavilovian mimicry: Nikolai Vavilov and his little-known impact on weed science. Weed Sci. 62, 207-216. ( 10.1614/WS-D-13-00122.1) [DOI] [Google Scholar]
- 170.Grasso DA, et al. 2015. Extrafloral-nectar-based partner manipulation in plant–ant relationships. AoB Plants 7, plv002. ( 10.1093/aobpla/plv002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Orrock J, Connolly B, Kitchen A. 2017. Induced defences in plants reduce herbivory by increasing cannibalism. Nat. Ecol. Evol. 1, 1205-1207. ( 10.1038/s41559-017-0231-6) [DOI] [PubMed] [Google Scholar]
- 172.Nepi M, Grasso DA, Mancuso S. 2018. Nectar in plant–insect mutualistic relationships: from food reward to partner manipulation. Front. Plant Sci. 9, 1063. ( 10.3389/fpls.2018.01063) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pollan M. 2001. The botany of desire: a plant's-eye view of the world. New York, NY: Random House. [Google Scholar]
- 174.Bowles S. 2011. Cultivation of cereals by the first farmers was not more productive than foraging. Proc. Natl Acad. Sci. USA 108, 4760-4765. ( 10.1073/pnas.1010733108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spengler RN III, Mueller NG. 2019. Grazing animals drove domestication of grain crops. Nat. Plants 5, 656-662. ( 10.1038/s41477-019-0470-4) [DOI] [PubMed] [Google Scholar]
- 176.Spengler RN III. 2020. Anthropogenic seed dispersal: rethinking the origins of plant domestication. Trends Plant Sci. 25, 340-348. ( 10.1016/j.tplants.2020.01.005) [DOI] [PubMed] [Google Scholar]
- 177.Bowles S, Choi J-K. 2019. The Neolithic agricultural revolution and the origins of private property. J. Polit. Econ. 127, 2186-2228. ( 10.1086/701789) [DOI] [Google Scholar]
- 178.Norenzayan A, Shariff AF. 2008. The origin and evolution of religious prosociality. Science 322, 58-62. ( 10.1126/science.1158757) [DOI] [PubMed] [Google Scholar]
- 179.Lang M, et al. 2019. Moralizing gods, impartiality and religious parochialism across 15 societies. Proc. R. Soc. B 286, 20190202. ( 10.1098/rspb.2019.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yang XF, et al. 2018. Kin recognition in rice (Oryza sativa) lines. New Phytol. 220, 567-578. ( 10.1111/nph.15296) [DOI] [PubMed] [Google Scholar]
- 181.Chamovitz D. 2012. What a plant knows: a field guide to the senses. New York, NY: Farrar Straus & Giroux. [Google Scholar]
- 182.Baluška F, Mancuso S. 2020. Plants are alive: with all behavioural and cognitive consequences. EMBO Rep. 21, e50495. ( 10.15252/embr.202050495) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.


