Abstract
Concerns over the consequences of global climate change for biodiversity have spurred a renewed interest in organismal thermal physiology. However, temperature is only one of many environmental axes poised to change in the future. In particular, hydrologic regimes are also expected to shift concurrently with temperature in many regions, yet our understanding of how thermal and hydration physiology jointly affect performance and fitness is still limited for most taxonomic groups. Here, we investigated the relationship between functional performance, hydration state and temperature in three ecologically distinct amphibians, and compare how temperature and water loss can concurrently limit activity under current climate conditions. We found that performance was maintained across a broad range of hydration states in all three species, but then declines abruptly after a threshold of 20–30% mass loss. This rapid performance decline was accelerated when individuals were exposed to warmer temperatures. Combining our empirical hydrothermal performance curves with species-specific biophysical models, we estimated that dehydration can increase restrictions on species' activity by up to 60% compared to restriction by temperature alone. These results illustrate the importance of integrating species' hydration physiology into forecasts of climate vulnerability, as omitting this axis may significantly underestimate the effects of future climate change on Earth's biological diversity.
Keywords: dehydration, desiccation, ecophysiology, frogs, global change, thermal performance
1. Introduction
As climates continue to shift across the world, a preeminent goal in conservation science is to anticipate how Earth's biodiversity will respond in kind [1]. It is expected that species' extinction risk under future climate change will be shaped in part by ecological, demographic and physiological traits [2]. For ectotherms, thermal performance curves (TPCs) that link organisms' functional performance to body temperature have emerged as a major tool for estimating fitness under changing thermal regimes [3,4]. Estimates of ectotherm species' vulnerability to future climate change based on thermal physiology have been widely adopted [4–6], but one limitation of this approach is that it excludes other environmental axes that can also influence fitness [7].
Concurrent with global changes in temperature, both precipitation and water availability (surface water, water vapour, soil moisture) are also expected to shift considerably in the near future [8]. Many regions on Earth are predicted to experience both considerable warming and reduced water availability [8,9] a combination expected to exert considerable physiological challenges for many species [10]. Precipitation and water availability constitute major factors affecting species' distributions [11,12], population dynamics [13], local extinctions [14] and range shifts [12,15,16], but our understanding of how dehydration physiology ultimately affects ectotherm fitness is still limited compared to temperature.
Species' environmental physiology can vary considerably, with important consequences for performance and fitness under different environments [4,17]. In terms of thermal physiology, temperatures that exceed an ectotherm's critical thermal maximum (CTmax) or thermal optimum (Topt) can impair fitness, limit activity or induce mortality [5,6,18]. Temperatures exceeding thermal limits restrict activity by forcing organisms to seek microclimatic refugia [5,6], and estimates of hours of thermal restriction have reliably predicted climate-induced population loss in some ectotherms [5]. Dehydration can also have considerable performance consequences that can potentially restrict ectotherm activity and distributions [19–21]. Many studies have demonstrated that dehydration affects performance [22–25], but there is no generalized model of hydro-performance akin to the thermal performance curve [26]. The shape of this hydro-performance curve is an important foundation to understand whether performance declines gradually or abruptly as dehydration proceeds. The thermal and hydration (hydrothermal) state of an organism has been shown to synergistically shape performance [27–30]. It is possible that an organism's thermal state may also change the shape of the hydro-performance curve [22,31], with consequences for forecasting fitness across these physiological states. Evaporative water loss is dictated in part by the difference in water vapour density between an organism and the air, where water loss rates rise in tandem with the saturation vapour density, and vapour pressure deficit, at warmer temperatures [32,33]. Temperature and hydration state are therefore expected to be correlated physiological axes, with potential trade-offs to concurrently shape performance, fitness and activity.
As the most water-reliant tetrapod lineage [34], amphibians serve as an ideal model to understand how dehydration and temperature concurrently shape organismal performance and subsequently climate risk. Water availability strongly shapes the biogeographic distributions and ecology of amphibians [35,36] and is considered a more important driver for species' distributions than temperature [37]. There is substantial evidence suggesting that amphibians often exhibit a weak behavioural drive to thermoregulate compared to heliothermic reptiles [38,39]. Instead, amphibians often appear to select moist microhabitats over optimal thermal conditions [28,40–42]. In turn, studies have found that desiccation risk may be the primary determinant of amphibian activity and environmental restriction [19,43]. Collectively this suggests there are high fitness costs to dehydration in amphibians, and understanding these costs is imperative to estimating climate vulnerability for these species.
Here, we quantified how both thermal and hydration physiology may jointly limit performance and activity in three ecologically and evolutionarily diverse anuran species from the Pacific Northwest region of North America (figure 1). We combined empirical estimates of locomotory performance, assessed across hydration states and at variable body temperatures, with biophysical models to quantify how dehydration can further limit activity from what is imposed by temperature alone. Collectively, these data and analytical approaches advance our understanding of how multiple environmental axes and physiological limits interact to dictate species' current and future climate vulnerability.
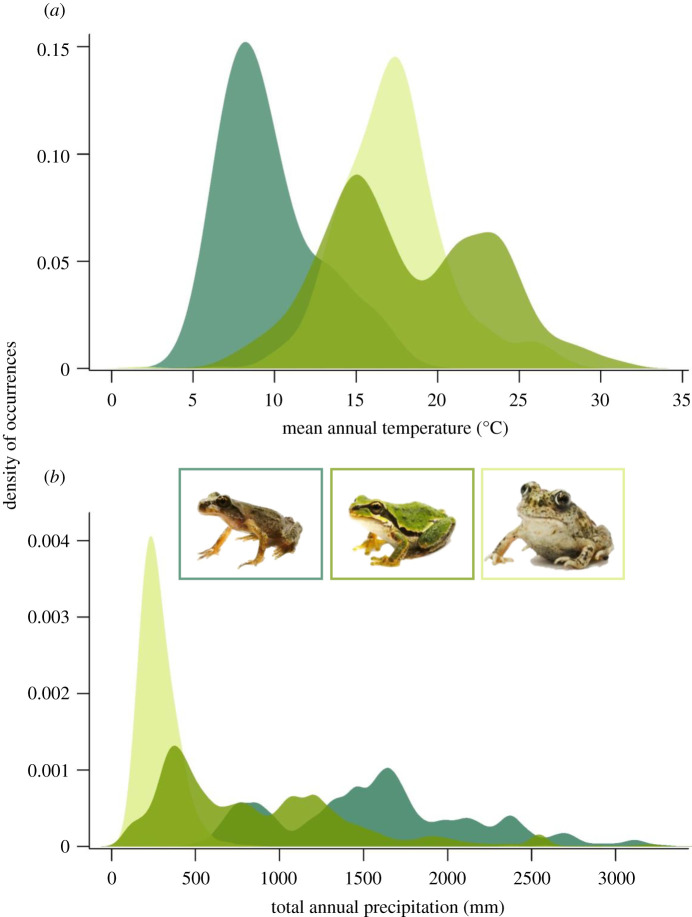
Figure 1.
Bioclimatic affiliations of each study species (insets, left to right: Ascaphus truei, Pseudacris regilla, Spea intermontana) based on sampling occurrence sites varying in (a) mean annual temperature and (b) total annual rainfall across their range. (Online version in colour.)
2. Methods
To understand how dehydration and temperature concurrently shape both performance and activity in amphibians, we conducted a series of experiments and integrated these empirical results into biophysical models for three anuran species. We assessed jump performance across a range of hydration states, and at different environmental temperatures, to determine both the generalized shape of the hydro-performance curve and how temperature may mediate that relationship. We integrated these empirical results with species-specific biophysical models to determine how both thermal and hydration physiology could restrict activity in these species. Ultimately, this allows us to illustrate how incorporating hydration physiology, in addition to thermal physiology, increases estimates of climate risk in amphibians and provides a path towards integrating dehydration into forecasts of future climate vulnerability.
(a). Study species
To assess variation in how dehydration affects performance among diverse anuran species, we studied three ecologically and evolutionarily divergent anurans that occur in the Pacific Northwest: the wet-adapted coastal tailed frogs, Ascaphus truei; the dry-adapted Great Basin spadefoot toads, Spea intermontana; and the climate and habitat generalist Pacific chorus frogs, Pseudacris regilla (figure 1). Details on animal collection and care are included in the electronic supplementary material.
(b). Hydrothermal performance
In the study of ectotherm ecophysiology, it is common to quantify functional performance over environmental gradients. Often, this is measured through locomotory performance curves in response to temperature [3,26], which in turn can be informative for estimating species' physiological and distributional limits [5,16]. We designed an equivalent experiment to estimate functional performance across a range of dehydration states and environmental temperatures in our three study species. We measured the maximum jump distance, from a set of six bouts, a widely used performance metric for amphibians that is relevant to fitness given the anaerobic ecology of anurans [22,26,27].
Using a repeated measures design, we tested individual jump performance at four levels of dehydration and at three or four different temperatures depending on the species (A. truei: 15, 20, 24°C; S. intermontana: 24, 27, 30°C; P. regilla: 20, 24, 27, 30°C; electronic supplementary material, table S1). All individuals (P. regilla, n = 14; S. intermontana, n = 8; A. truei, n = 10) were tested together at a given environmental temperature, with the order of temperatures randomized. Test temperatures were selected to span (±) each species's previously reported thermal optima [44]. Trials began with individuals immersed in a water bath set at the assigned test temperature for 3 h to ensure individuals were fully hydrated, a time period consistent with similar studies [24,28,29]. After this period, we immediately placed each individual on a planar surface and compelled them to repeatedly jump up to six times by probing their urostyle, marking their launching and landing points with ordered tape. We measured the Euclidian distance between bouts, at a precision of 0.1 cm, and used the maximum value as our measure of performance. Immediately after this first jumping trial, we patted down specimens with a dry paper towel and weighed them on an analytical balance (Ohaus Explorer 10640, Ohaus Corp.) with a precision of 0.0001 g. This initial weight and performance trial were considered to be at each individual's fully hydrated state. Subsequently, we serially dehydrated individuals in an environmental chamber (details in the electronic supplementary material), set at the same test temperature, by exposing specimens to a 3000 revolutions per minute (47.3 cubic feet min−1) oscillating fan (Thermaltake Technology Co., Ltd) at a distance of 10 cm. We monitored changes in body mass over time and retested individuals' jump performance at three subsequent levels of dehydration, measured as the proportion of initial mass lost, ranging from fully hydrated to near their sub-lethal dehydration limits (i.e. critical water loss maximum, CWLmax) based on righting response tests (see below). We ensured that each individual had at least 30 min of rest between jump trials to minimize fatigue. To test for potential fatigue, we randomly assigned a subset of individuals (n = 2–3) as controls (kept in sealed containers with full access to water) in each temperature treatment, and these individuals were similarly tested for jump performance. Upon completing their fourth jump trial, individuals were immediately returned to their acclimation water bath to recover for one hour. All individuals fully recovered and were subsequently retested at the remaining test temperatures after a minimum of 2 days of rest. We also tested for systematic performance changes in individuals over the course of the experiment, which is detailed in the electronic supplementary material (p. 9).
After testing individual jump performance across all temperature treatments, we estimated each individual's maximum tolerated water loss, CWLmax, after 3 days of rest. We measured CWLmax in an analogous way to the critical thermal maximum (CTmax [18]), using the sub-lethal righting response test. We randomly assigned each individual to undergo serial dehydration at one experimental temperature and tested their ability to right themselves after inversion every 2 min as signs of lethargy emerged. If an individual could not right itself in 10 s, they were immediately weighed and transferred to a recovery water bath (all subjects recovered from rehydration). We defined this dehydration limit CWLmax to be an individual's end weight, as a proportion to their initial fully hydrated weight.
We assessed the general shape of the anuran hydro-performance curve by comparing the fit of several models representing different hypotheses for how hydration state may influence performance. We fitted four models of hydro-performance to the jump data (electronic supplementary material, table S3, and further details in the supplemental material): where performance declines linearly with dehydration (e.g. linear model), where performance is maintained with dehydration but declines past a threshold (e.g. exponential model) or where performance rises and falls with dehydration (e.g. quadratic and cubic models). Given that our data were repeated measures on individuals, all models included a random intercept for performance (αi) for each individual i. For visualization, but not analysis, we transformed the raw data to individual relative performance (see electronic supplementary material, figure S2 for absolute performance) by scaling jump distance by each individual's maximum performance among trials. In addition to our measures of jump performance, we also included our estimates of individual CWLmax as anchor points where performance was set to zero. We assessed the relative fit of each of these models by calculating Akaike Information Criterion (AICc) fit to all temperature treatments combined. After determining the best-supported hydro-performance curve shape for each species, we then included temperature as a covariate, as having either an additive or interacting effect with dehydration (electronic supplementary material, table S4). We used a similar model selection approach to test whether temperature interacts with dehydration to affect performance. Models were fitted using maximum likelihood, with all covariates scaled by 1 standard deviation and centred, using either the lme or nlme functions in the R package nlme [45]. All analyses were conducted in R v. 3.4.4.
(c). Biophysical models and environmental restriction
To determine how dehydration in our test species restricts activity beyond what is imposed by thermal limits alone [5], we combined species-specific biophysical models with estimates of microclimate conditions to simulate the body temperature and evaporative water loss an exposed frog would experience over a typical day during its active season.
We used a modified version of the dynamic biophysical ectotherm model developed by Rubalcaba et al. [46], which implements the energy balance model for a wet-skinned ectotherm proposed by Tracy [32,47], to estimate the heat balance (Qmet) and evaporative water loss (me) for a given model organism (equations S1–S3 in the electronic supplementary material). The dynamics of energy exchange between an ectotherm and its environment will be influenced by various physical characteristics including mass, body shape and cutaneous resistance to water loss [32,47]. We used species-specific estimates of skin resistance, surface area and body mass drawn from the literature (electronic supplementary material, table S2), with three body size estimates for each species based on the minimum, midpoint and maximum snout–vent length of adults. These parameters dictate the heat flux, and corresponding changes to body temperature, of an organism in response to its microclimate through the absorption of solar radiation, emission of long-wave radiation, and convective, conductive and evaporative heat loss [32]. In turn, changes to body temperature alter the vapour density of an organism's skin surface relative to the temperature-dependent vapour saturation density of the surrounding air, which ultimately drives evaporative water loss [33,47].
We explored how both body temperature and evaporative water loss may unfold give typical microclimate exposures at 100 randomly sampled occurrence sites spanning each species's entire latitudinal distribution (see details on site selection in the electronic supplementary material, p. 10). We used estimated microclimate data from NicheMapR [48], which uses long-term monthly averages (covering the years 1960–1991) from a global weather dataset [49] to infer microclimate conditions on an hourly basis for an average day in each month at 1 cm from the surface for a given location. At each location, we extracted hourly estimates of air temperature, soil temperature, solar radiation (assuming 50% shade), air relative humidity and wind speed for an average day in July (see example schematic electronic supplementary material, figure S3) to parameterize the microclimate exposure in the ectotherm energy balance biophysical model [32,46]. From this biophysical model, we could then estimate time-series of an exposed individual's body temperature and cumulative water loss over the course of a 24 h period at different sites.
From our hydrothermal performance experiment, and previous studies on these species' thermal performance [44], we estimated physiological limits on activity based on either temperature or dehydration. We used estimated body temperatures at each location to calculate the hours in a day that exceeded each species's upper 80% thermal performance breadth [44]. The remaining hours in the day were assumed to be available for activity, but this activity may still incur performance costs due to dehydration. For each minute of the day that was not thermally restricted, we determined the time-series of body temperature and cumulative water loss if a frog emerged at each timepoint (e.g. minute). We used each species's best-fit hydrothermal performance models to predict the time-series of performance for an active frog over this remaining period based on its modelled body temperature and water loss, and then scaled this performance to between 0 and 1 based on the maximum potential performance. We used the same upper 80% performance limit for dehydration to quantify the additional hours of the day where dehydration would then exceed this boundary (see example in electronic supplementary material, figure S3). We calculated the geometric mean of hydrothermal hours of restriction from the range of estimates produced by different emergence times. Our analysis compared total hours of restriction from thermal and hydrothermal physiological limits, and across modelled body size classes, for each species using ordinary least-squares regressions.
3. Results
(a). Shape of the hydro-performance curve
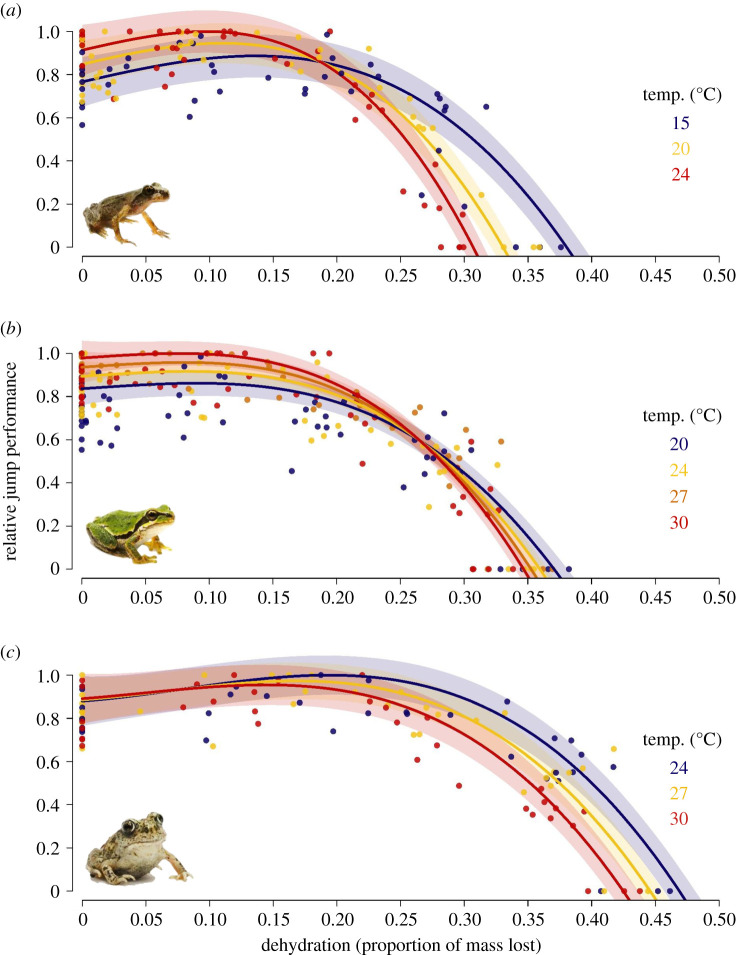
Performance across all three species of anurans was maintained at a broad range of dehydration, before declining nonlinearly towards the maximum water loss limit (figure 2). Critical water loss levels (CWLmax) varied significantly between species (F2,30 = 37.3, p < 0.001), with the arid specialist, S. intermontana, having a significantly higher CWLmax (42.9% of body mass, 95% CI = 44.9–41.0%) than the wet specialist A. truei (32.9%, 95% CI = 31.2–34.7%) or generalist P. regilla (34.2%, 95% CI = 32.8–35.6%). The threshold at which water loss reduces performance to 80% of its maximum (WL80) also varied significantly across species (A. truei, WL80 = 21.3%, 95% CI = 17.8–24.0%; P. regilla, WL80 = 21.2%, 95% CI = 19.2–22.9%; S. intermontana, WL80 = 28.0%, 95% CI = 24.6–30.8%). This suggests that the overall shape of the hydro-performance response appears to be highly conserved, but that the critical levels where performance declines have some degree of evolutionary lability.
Figure 2.
Relative jump performance (scaled to maximum individual performance) as a function of both dehydration (proportion of initial mass lost) and environmental temperature for (a) Ascaphus truei, (b) Pseudacris regilla and (c) Spea intermontana. Absolute performance curves are available in electronic supplementary material, figure S2. (Online version in colour.)
(b). Hydrothermal performance
Building off the best-fit nonlinear relationship between jump performance and dehydration, including an interaction between environmental temperature and dehydration substantially improved the model fit for all species (LRA. truei = 63.8, LRP. regilla = 23.4, LRS. intermontana = 33.5, all p < 0.001; electronic supplementary material, table S4). The interaction between temperature and dehydration, rather than an additive effect of temperature, was much better supported for all species (LRA. truei = 63.7, LRP. regilla = 14.7, LRS. intermontana = 13.2, all p < 0.001). This suggests that dehydration and temperature act synergistically to shape performance, whereby warmer temperatures exacerbate the decline of performance with dehydration and with the CWLmax subsequently occurring at lower levels of dehydration (figure 2).
4. Thermal and dehydration limits on activity
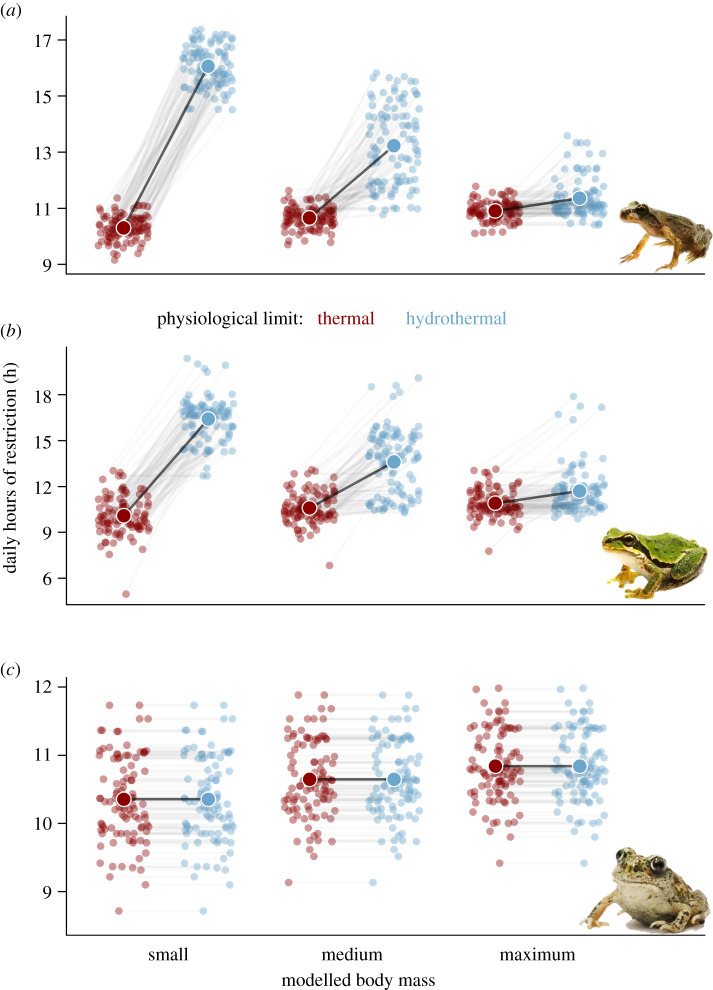
By coupling our hydrothermal models of performance with species-specific biophysical models, we found that estimated hours of restriction varied considerably across species identity and individual size, and when comparing thermal and dehydration limits (figure 3). Water loss substantially increased the hours of restriction over thermal limits for both A. truei (p < 0.001) and P. regilla (p < 0.001), but was unlikely to further restrict the arid adapted S. intermontana (p = 1). Body size was crucial for influencing dehydration and subsequently activity restriction. Dehydration increased the hours of restriction by +55.9% for the smallest adults of A. truei (p < 0.001), by +24.1% for the median body size (p < 0.001), but only by +4.2% for the maximum adult size. We found similar results for the generalist P. regilla: we estimated that the smallest adults had +62.8% additional hours of restriction (p < 0.001), +28.4% for the median body size (p < 0.001) and +7.3% for the maximum body size, when considering dehydration. For S. intermontana, however, there was no additional restriction from dehydration at any body size.
Figure 3.
Hours of restriction estimated for an average day in July across 100 sampled occurrence sites from species-specific biophysical models for (a) Ascaphus truei, (b) Pseudacris regilla and (c) Spea intermontana. Hours of restriction are estimated at different body sizes for each species (x-axis), and for different physiological limits based on just temperature (red) or both temperature and dehydration (blue). Each point represents an average estimate of physiological restriction at a site, with lines to illustrate the per-site effect of incorporating dehydration limits, the large points and lines represent the average effect across sites for each model organism size. (Online version in colour.)
5. Discussion
These results demonstrate that hydration state is an important driver of anuran performance, behaviour and subsequently climate risk. Across three anuran species with widely divergent evolutionary histories and climatic affiliations, we show that there are both commonalities in the performance effects of dehydration, but also considerable differences in how this environmental pressure is likely to have shaped each species's ecology and distribution. Across all species the performance consequences of dehydration were considerable past a given threshold (WL80), underscoring the importance of maintaining hydration state for fitness. Hydration state in amphibians is often maintained at very high levels in nature [28,50], as individuals must balance activity with the costs of ongoing water loss [51]. Although our study suggests that burst jump performance can be maintained across moderate levels of dehydration in all species, the relatively sharp decline in performance after the WL80 threshold suggests that there is a potentially lethal risk in approaching this threshold if opportunities for rehydration are scarce. Though temperature regimes may not elicit a strong behavioural drive in many amphibians [38,39], it clearly plays an important role in how these species experience and respond to water loss.
Environmental temperature has two important effects on dehydration in anurans: it both mediates rates of evaporative water loss by changing the water vapour pressure deficit [32,33], and it also appears to affect the hydro-performance relationship. Warmer temperatures resulted in a faster decay in performance with dehydration and a lower critical water loss threshold in all three species. This suggests a consistent physiological mechanism across species, whereby the stress of high temperatures and dehydration interact to reduce functional performance and is consistent with studies from other amphibian species [22,29,30]. When individuals are fully hydrated, these warmer temperatures were generally beneficial for performance—consistent with our understanding of amphibian thermobiology [38,39]. This warm and wet state will be ephemeral, however, as evaporative water loss is accelerated at warmer temperatures with higher vapour pressure deficits and can potentially incur greater fitness costs on the dehydration axis [32,51]. This illustrates the fundamental trade-off between thermal and hydration physiology that shapes amphibian behaviour and ecology. When we quantified water loss at different temperatures in P. regilla (electronic supplementary material, figure S4a), we found that individuals rapidly adopted water-conserving postures when exposed to warmer temperatures (electronic supplementary material, figure S4b). This underscores both the behavioural drive towards water conservation in amphibians [32,39], and that the fitness consequences of dehydration are considerable and can outweigh the performance benefits of being at a higher body temperature [28,41,42]. The consequences of the coupling between temperature and dehydration are probably considerable for amphibian autecology across environments.
In thermobiology, it has long been recognized that individuals appear to select thermal environments that are well below their theoretical thermal optimum [52]. Though the ‘optimum is suboptimal' phenomenon seems paradoxical, it can be explained by the fact that organisms are imperfect thermoregulators and thermal environments in nature are variable [53]. In turn, an individual that maintains a body temperature near its optimum in nature risks experiencing temperatures above the optimum that may impair fitness [53]. An additional element to this pattern is that the optimal temperature for performance comes at the cost of a faster rate of water loss compared to cooler temperatures [32,54]. If individuals manage both temperature and dehydration performance concurrently, then it is likely that thermoregulatory behaviour will also be mediated by desiccation rate [42,55]. This would predict that species that are particularly sensitive to dehydration, as is the case for most amphibians [34], are likely to maintain a body temperature further away from their thermal optimum in order to limit water loss. This hypothesis would then predict that individuals should prefer warmer temperatures when evaporative water loss can be alleviated [55,56]. Supporting this prediction, several thermal preference studies in amphibians have found that individuals prefer higher temperatures when the substrate is moist rather than dry [40,41]. This trade-off between performance and water loss across temperatures may be crucial to explain why some species appear to exhibit limited thermoregulatory behaviour [28,38,39,54]. Given the considerable performance costs of dehydration that we demonstrate here, water loss may drive the behaviour of many ectotherm species.
Body size influences evaporative water loss and therefore has the potential to be a key trait dictating fitness for amphibians under changing environmental landscapes. Both our experiments and biophysical modelling approach indicate that larger individuals should be much less restricted by dehydration than smaller individuals, a result consistent with previous theoretical and empirical works [21,32,46]. Other traits, including body shape and skin resistance, can similarly influence evaporative water loss and subsequently shape how selection may act upon body size in a desiccating environment [36,46]. Reductions in body size due to climate warming have been widely reported across a number of ectotherms [57], which in turn sets up a compounding environmental challenge for ectotherms under global change: smaller individuals in warmer environments will be intrinsically more susceptible to evaporative water loss in addition to the heightened desiccation risk from warming itself. Further, the warming and drying of aquatic larval habitats can result in smaller post-metamorphic juveniles in amphibians [58], and these individuals will then have to navigate a drier terrestrial environment with the added disadvantage of being smaller [59]. If conditions are sufficiently harsh in the terrestrial landscape, then this could result in a collapse of recruitment in addition to the persistent challenges a warmer, drier climate will pose for adult demographic fitness [13]. We may therefore expect climate warming to exert dual pressures of developmental shrinking and accelerated dehydration that could magnify climate-induced population declines and losses into the future.
Assessing extinction risk under future climate change requires a thorough understanding of the interactions between species' physiology, behaviour and their environment [3,21]. Even if temperature changes are expected to remain well within the thermal safety margins of ectotherms [6], there may still be fitness consequences related to dehydration [43]. Warmer temperatures increase rates of body water loss [32], and in turn we can expect this to reduce the activity time an individual can sustain until it suffers dehydration-induced performance loss [19]. Given that water economy must be carefully managed for many amphibian species [28,50], even slight changes to thermal landscapes could have substantial effects on performance and fitness through their effects on dehydration. Our biophysical models represent a simplified scenario of the microclimates that an individual would experience in nature, as they omit both hydroregulatory behaviour and the availability of hydrothermal refugia on the landscape. Behaviour can substantially alter the sorts of environmental conditions that an individual would experience [3,18,19], and therefore the ability to find and exploit suitable microhabitats is key to understanding the effects of climate change on species [43]. However, our knowledge of how anurans, or any species, shift their behaviour in response to dehydration and water availability is still very limited [19,43]. Further complicating the assessment of risk is that humans are modifying ecosystems through multiple pathways beyond just climate, as habitat modification of natural landscapes is likely to exacerbate climate effects by removing the microhabitats that function as moist, thermal refugia [60]. Incorporating water loss and its concurrent effects on performance, physiology, behaviour and activity into mechanistic models is therefore likely to provide more integrative and accurate estimates of extinction risk for terrestrial ectotherms under future climate change.
Supplementary Material
Acknowledgements
The authors are grateful to many people for their assistance with this project. For animal care and logistics support: B. Leighton, R. Murray and S. Riviere. For field and laboratory assistance: M. Arbeider, S. Carncross, F. Faroon, M. Gao, A. Gentile, K. Hall, V. Han, M. Shakeri, C. Shi, G. Lertzman-Lepofsky, R. Murray, L. Perry and R. Walls. For experimental design and feedback: N. Dulvy, R. Huey, A. Mooers, R. Murray, C. Navas and members of the Earth-to-Ocean and Fab*Lab research groups. Thanks to two anonymous reviewers for their constructive feedback on the manuscript.
Data availability
Supporting data and code to replicate this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.98sf7m0h1 [61].
Authors' contributions
D.A.G. and W.J.P. conceived of the study and experimental design. D.A.G. collected and analysed the data. D.A.G. led the manuscript writing with editorial support from W.J.P. Both authors contributed critically to the drafts and gave final approval for publication.
Competing interests
I/We declare I/we have no competing interests.
Funding
This project was supported by Natural Sciences and Engineering Research Council of Canada through a graduate scholarship to D.A.G. and a Discovery grant to W.J.P.
References
- 1.Schwartz MW, Iverson LR, Prasad AM, Matthews SN, O'Connor RJ. 2006. Predicting extinctions as a result of climate change. Ecology 87, 1611-1615. ( 10.1890/0012-9658(2006)87[1611:PEAARO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 2.Bozinovic F, Pörtner H-O. 2015. Physiological ecology meets climate change. Ecol. Evol. 5, 1025-1030. ( 10.1002/ece3.1403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huey RB, Kearney MR, Krockenberger A, Holtum JAM, Jess M, Williams SE. 2012. Predicting organismal vulnerability to climate warming: roles of behaviour, physiology and adaptation. Phil. Trans. R. Soc. B 367, 1665-1679. ( 10.1098/rstb.2012.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinclair BJ, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372-1385. ( 10.1111/ele.12686) [DOI] [PubMed] [Google Scholar]
- 5.Sinervo B, et al. 2010. Erosion of lizard diversity by climate change and altered thermal niches. Science 328, 894-899. ( 10.1126/science.1184695) [DOI] [PubMed] [Google Scholar]
- 6.Sunday JM, Bates AE, Kearney MR, Colwell RK, Dulvy NK, Longino JT, Huey RB. 2014. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610-5615. ( 10.1073/pnas.1316145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chown SL, Sorensen JG, Terblanche JS. 2011. Water loss in insects: an environmental change perspective. J. Insect. Physiol. 57, 1070-1084. ( 10.1016/j.jinsphys.2011.05.004) [DOI] [PubMed] [Google Scholar]
- 8.Schewe J, et al. 2014. Multimodel assessment of water scarcity under climate change. Proc. Natl Acad. Sci. USA 111, 3245-3250. ( 10.1073/pnas.1222460110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai A. 2013. Increasing drought under global warming in observations and models. Nat. Clim. Change 3, 52-58. ( 10.1038/nclimate1633) [DOI] [Google Scholar]
- 10.Albright TP, Mutiibwa D, Gerson AR, Smith EK, Talbot WA, O'Neill JJ, McKechnie AE, Wolf BO. 2017. Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proc. Natl Acad. Sci. USA 114, 2283-2288. ( 10.1073/pnas.1613625114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCain CM, Colwell RK. 2011. Assessing the threat to montane biodiversity from discordant shifts in temperature and precipitation in a changing climate. Ecol. Lett. 14, 1236-1245. ( 10.1111/j.1461-0248.2011.01695.x) [DOI] [PubMed] [Google Scholar]
- 12.Smith AB. 2013. The relative influence of temperature, moisture and their interaction on range limits of mammals over the past century. Global Ecol. Biogeogr. 22, 334-343. ( 10.1111/j.1466-8238.2012.00785.x) [DOI] [Google Scholar]
- 13.Kissel AM, Palen WJ, Ryan ME, Adams MJ. 2019. Compounding effects of climate change reduce population viability of a montane amphibian. Ecol. Appl. 29, e01832. ( 10.1002/eap.1832) [DOI] [PubMed] [Google Scholar]
- 14.Iknayan KJ, Beissinger SR. 2018. Collapse of a desert bird community over the past century driven by climate change. Proc. Natl Acad. Sci. USA 115, 8597-8602. ( 10.1073/pnas.1805123115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR. 2011. Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331, 324-327. ( 10.1126/SCIENCE.1199040) [DOI] [PubMed] [Google Scholar]
- 16.Kearney M, Phillips BL, Tracy CR, Christian KA, Betts G, Porter WP. 2008. Modelling species distributions without using species distributions: the cane toad in Australia under current and future climates. Ecography 31, 423-434. ( 10.1111/j.0906-7590.2008.05457.x) [DOI] [Google Scholar]
- 17.Huey RB, Berrigan D. 2001. Temperature, demography, and ectotherm fitness. Am. Nat. 158, 204-210. ( 10.1086/321314) [DOI] [PubMed] [Google Scholar]
- 18.Cowles RB, Bogert CM. 1944. A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Museum Nat. Hist. 83, 261-296. [Google Scholar]
- 19.Feder ME, Londos PL. 1984. Hydric constraints upon foraging in a terrestrial salamander, Desmognathus ochrophaeus (Amphibia: Plethodontidae). Oecologia 64, 413-418. ( 10.1007/BF00379141) [DOI] [PubMed] [Google Scholar]
- 20.Kearney MR, Munns SL, Moore D, Malishev M, Bull CM. 2018. Field tests of a general ectotherm niche model show how water can limit lizard activity and distribution. Ecol. Monogr. 88, 672-693. ( 10.1002/ecm.1326) [DOI] [Google Scholar]
- 21.Riddell EA, Odom JP, Damm JD, Sears MW. 2018. Plasticity reveals hidden resistance to extinction under climate change in the global hotspot of salamander diversity. Sci. Adv. 4, eaar5471. ( 10.1126/sciadv.aar5471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beuchat CA, Pough FH, Stewart MM. 1984. Response to simultaneous dehydration and thermal stress in three species of Puerto Rican frogs. J. Comp. Physiol. B 154, 579-584. ( 10.1007/bf00684411) [DOI] [Google Scholar]
- 23.Moore FR, Gatten RE Jr. 1989. Locomotor performance of hydrated, dehydrated and osmotically stressed anuran amphibians. Herpetologica 45, 101-110. [Google Scholar]
- 24.Prates I, Angilleta MJ, Wilson RS, Niehaus AC, Navas CA. 2013. Dehydration hardly slows hopping toads (Rhinella granulosa) from xeric and mesic environments. Physiol. Biochem. Zool. 86, 451-457. ( 10.1086/671191) [DOI] [PubMed] [Google Scholar]
- 25.Titon B, Navas CA, Jim J, Gomes FR. 2010. Water balance and locomotor performance in three species of neotropical toads that differ in geographical distribution. Comp. Biochem. Physiol. Mol. Integr. Physiol. 156, 129-135. ( 10.1016/j.cbpa.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 26.Huey RB, Stevenson RD. 1979. Integrating thermal physiology and ecology of ectotherms: a discussion of approaches. Am. Zool. 19, 357-366. ( 10.1093/icb/19.1.357) [DOI] [Google Scholar]
- 27.Walvoord ME. 2003. Cricket frogs maintain body hydration and temperature near levels allowing maximum jump performance. Physiol. Biochem. Zool. 76, 825-835. ( 10.1086/378912) [DOI] [PubMed] [Google Scholar]
- 28.Mitchell A, Bergmann PJ. 2016. Thermal and moisture habitat preferences do not maximize jumping performance in frogs. Funct. Ecol. 30, 733-742. ( 10.1111/1365-2435.12535) [DOI] [Google Scholar]
- 29.Anderson RCO, Andrade DV. 2017. Trading heat and hops for water: dehydration effects on locomotor performance, thermal limits, and thermoregulatory behavior of a terrestrial toad. Ecol. Evol. 7, 9066-9075. ( 10.1002/ece3.3219) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosmala G, Christian K, Brown G, Shine R. 2017. Locomotor performance of cane toads differs between native-range and invasive populations. R. Soc. Open Sci. 4, 170517. ( 10.1098/rsos.170517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozen-Rechels D, Dupoué A, Lourdais O, Chamaillé-Jammes S, Meylan S, Clobert J, Le Galliard JF. 2019. When water interacts with temperature: ecological and evolutionary implications of thermo-hydroregulation in terrestrial ectotherms. Ecol. Evol. 9, 10 029-10 043. ( 10.1002/ece3.5440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tracy CR. 1975. Water and energy relations of terrestrial amphibians: Insights from mechanistic modeling. In Perspectives of biophysical ecology (eds DM Gates, RB Schmerl), pp. 325-346. Berlin, Germany: Springer. ( 10.1007/978-3-642-87810-7_19) [DOI] [Google Scholar]
- 33.Anderson DB. 1936. Relative humidity or vapor pressure deficit. Ecology 17, 277-282. ( 10.2307/1931468) [DOI] [Google Scholar]
- 34.Lillywhite HB. 2006. Water relations of tetrapod integument. J. Exp. Biol. 209, 202-226. ( 10.1242/JEB.02007) [DOI] [PubMed] [Google Scholar]
- 35.Buckley LB, Jetz W. 2007. Environmental and historical constraints on global patterns of amphibian richness. Proc. R. Soc. B 274, 1167-1173. ( 10.1098/rspb.2006.0436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gouveia SF, Bovo RP, Rubalcaba JG, Da Silva FR, Maciel NM, Andrade D V, Martinez PA. 2019. Biophysical modeling of water economy can explain geographic gradient of body size in anurans. Am. Nat. 193, 51-58. ( 10.1086/700833) [DOI] [PubMed] [Google Scholar]
- 37.Cunningham HR, Rissler LJ, Buckley LB, Urban MC. 2015. Abiotic and biotic constraints across reptile and amphibian ranges. Ecography 39, 1-8. ( 10.1111/ecog.01369) [DOI] [Google Scholar]
- 38.Navas CA, Gomes FR, Carvalho JE. 2008. Thermal relationships and exercise physiology in anuran amphibians: integration and evolutionary implications. Comp. Biochem. Physiol. Mol. Integr. Physiol. 151, 344-362. ( 10.1016/j.cbpa.2007.07.003) [DOI] [PubMed] [Google Scholar]
- 39.Bovo R, et al. 2018. Ecophysiology of amphibians: information for best mechanistic models. Diversity 10, 118. ( 10.3390/d10040118) [DOI] [Google Scholar]
- 40.O'Connor MP, Tracy CR. 1992. Thermoregulation by juvenile toads of Bufo woodhousei in the field and in the laboratory. Copeia 1992, 865-876. ( 10.2307/1446164) [DOI] [Google Scholar]
- 41.Köhler A, Sadowska J, Olszewska J, Trzeciak P, Berger-Tal O, Tracy CR. 2011. Staying warm or moist? Operative temperature and thermal preferences of common frogs (Rana temporaria), and effects on locomotion. Herpetol. J. 21, 17-26. [Google Scholar]
- 42.Tracy CR, Christian KA, O'Connor MP, Tracy CR. 1993. Behavioral thermoregulation by Bufo americanus: the importance of the hydric environment. Herpetologica 49, 375-382. [Google Scholar]
- 43.Lertzman-Lepofsky GF, Kissel AM, Sinervo B, Palen WJ. 2020. Water loss and temperature interact to compound amphibian vulnerability to climate change. Glob. Change Biol. 26, 4868-4879. ( 10.1111/gcb.15231) [DOI] [PubMed] [Google Scholar]
- 44.Gerick AA, Munshaw RG, Palen WJ, Combes SA, O'Regan SM. 2014. Thermal physiology and species distribution models reveal climate vulnerability of temperate amphibians. J. Biogeogr. 41, 713-723. ( 10.1111/jbi.12261) [DOI] [Google Scholar]
- 45.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. 2017. nlme: Linear and nonlinear mixed effects models. R package version 3.1-131. [Google Scholar]
- 46.Rubalcaba JG, Gouveia SF, Olalla-Tárraga MA. 2019. A mechanistic model to scale up biophysical processes into geographical size gradients in ectotherms. Global Ecol. Biogeogr. 28, 793-803. ( 10.1111/geb.12893) [DOI] [Google Scholar]
- 47.Tracy CR. 1976. A model of the dynamic exchanges of water and energy between a terrestrial amphibian and its environment. Ecol. Monogr. 46, 293-326. ( 10.2307/1942256) [DOI] [Google Scholar]
- 48.Kearney MR, Porter WP. 2017. NicheMapR—an R package for biophysical modelling: the microclimate model. Ecography 40, 664-674. ( 10.1111/ecog.02360) [DOI] [Google Scholar]
- 49.New M, Lister D, Hulme M, Makin I. 2002. A high-resolution data set of surface climate over global land areas. Clim. Res. 21, 1-25. ( 10.3354/cr021001) [DOI] [Google Scholar]
- 50.Tracy CR, Tixier T, Le Nöene C, Christian KA. 2014. Field hydration state varies among tropical frog species with different habitat use. Physiol. Biochem. Zool. 87, 197-202. ( 10.1086/674537) [DOI] [PubMed] [Google Scholar]
- 51.Heatwole H, Torres F, Blasini De Austin S, Heatwole A. 1969. Studies on anuran water balance—I. Dynamics of evaporative water loss by the coquí, Eleutherodactylus portoricensis. Comp. Biochem. Physiol. 28, 245-269. ( 10.1016/0010-406X(69)91342-5) [DOI] [PubMed] [Google Scholar]
- 52.Huey RB, Bennett AF. 1987. Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41, 1098-1115. ( 10.2307/2409194) [DOI] [PubMed] [Google Scholar]
- 53.Martin TL, Huey RB. 2008. Why ‘suboptimal’ is optimal: Jensen's inequality and ectotherm thermal preferences. Am. Nat. 171, E102-E118. ( 10.1086/527502) [DOI] [PubMed] [Google Scholar]
- 54.Brattstrom BH. 1963. A preliminary review of the thermal requirements of amphibians. Ecology 44, 238-255. ( 10.2307/1932171) [DOI] [Google Scholar]
- 55.Tracy CR, Christian KA. 2005. Preferred temperature correlates with evaporative water loss in hylid frogs from northern Australia. Physiol. Biochem. Zool. 78, 839-846. ( 10.1086/432151) [DOI] [PubMed] [Google Scholar]
- 56.Malvin GM, Wood SC. 1991. Behavioral thermoregulation of the toad, Bufo marinus: effects of air humidity. J. Exp. Zool. 258, 322-326. ( 10.1002/jez.1402580307) [DOI] [PubMed] [Google Scholar]
- 57.Sheridan JA, Bickford D. 2011. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401-406. ( 10.1038/nclimate1259) [DOI] [Google Scholar]
- 58.O'Regan SM, Palen WJ, Anderson SC. 2014. Climate warming mediates negative impacts of rapid pond drying for three amphibian species. Ecology 95, 845-855. ( 10.1890/13-0916.1) [DOI] [PubMed] [Google Scholar]
- 59.Lee S-Y, Ryan ME, Hamlet AF, Palen WJ, Lawler JJ, Halabisky M. 2015. Projecting the hydrologic impacts of climate change on montane wetlands. PLoS ONE 10, e0136385. ( 10.1371/journal.pone.0136385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.González Del Pliego P, et al. 2016. Thermally buffered microhabitats recovery in tropical secondary forests following land abandonment. Biol. Conserv. 201, 385-395. ( 10.1016/j.biocon.2016.07.038) [DOI] [Google Scholar]
- 61.Greenberg DA, Palen WJ. 2021. Data from: Hydrothermal physiology and climate vulnerability in amphibians. Dryad Digital Repository. ( 10.5061/dryad.98sf7m0h1) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting data and code to replicate this study are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.98sf7m0h1 [61].