Abstract
Humans have a large impact on the distribution and abundance of animal species worldwide. The ecological effects of human-altered environments are being increasingly recognized and understood, but their effects on evolution are largely unknown. Enhanced cognitive abilities and the ability to innovate have been suggested as crucial traits for thriving in human-altered habitats. We tested if house mice (Mus musculus) subspecies have evolved enhanced innovative problem-solving abilities throughout their commensal lives with humans. The time that subspecies lived commensally with humans ranges between approximately 3000 years to more than 11 000 years, thus providing an excellent example of human–animal coexistence. In addition, we tested whether differences in problem-solving were mediated by differences in object and place exploration, motivation, persistence or inhibitory control. We found that populations of subspecies living commensally the longest excelled in problem-solving across seven food-extraction tasks over subspecies living commensally short or intermediate times. These differences were not mediated by exploration, motivation, persistence or inhibitory control suggesting that subspecies have evolved better cognitive abilities when living commensally in urban environments. This suggests that the ability to problem-solve may be an important trait promoting prosperity in human-altered environments.
Keywords: animal cognition, anthropogenic environmental change, inhibitory control, innovation, microevolution, Mus musculus
1. Backround
During the last centuries, humans have altered the planets' landscape severely, thereby also affecting the distribution and behaviour of animals. Animals need to adapt to new environmental challenges or are excluded from human-altered environments [1]. The behavioural repertoire of animals is crucial for coping with environmental challenges and growing evidence reports behavioural differences between populations living in close contact to humans over naturally occurring populations across taxa [2].
Behavioural flexibility is one of the most important behavioural traits allowing animals to prosper in the presence of humans [1]. For example, species that readily adopt novel foraging behaviours are more successful at establishing themselves in new environments [3]. In human-altered environments, animals may likely face novel situations and problems particularly often as these expose animals to all kinds of unnatural light, noise, disturbances and resources [3,4]. The ability to problem-solve, defined as inventing new behaviours or use of pre-existing ones in new contexts [5], has been suggested to be crucial for persistence in human-altered areas because it is an expression of behavioural flexibility and innovation propensity [6–9]. One of the most prominent examples of innovative problem-solving is the opening of milk bottles by great and blue tits observed in the UK about 70 years ago [10]. In the past decade, a series of studies focusing on various bird species found general support for enhanced problem-solving abilities of populations in urban over more natural areas (e.g. [11–14]). The underlying mechanisms of problem-solving differences are poorly understood. It is not yet known if the human environment acts as a filter in which individuals with certain behavioural types are more likely to settle in urban areas, if differential selection pressures lead to microevolution or if plastic changes in behaviour cause differences in problem-solving ability [3].
There is also little information regarding which behavioural traits promote successful problem-solving [15]. High levels of object and place exploration might increase the encounter rate of novel situations as well as the information gathered about a situation, thereby increasing the chance to solve [15,16]. In addition, an animal's performance might be influenced by persistence (i.e. the number of solving attempts or time spent manipulating the task set-up) [14]. Furthermore, the inability to perform a motor action or the ability to stop a non-successful action could cause differences in problem-solving performance [17,18]. Finally, differences in problem-solving ability might be caused by trial-and-error learning [19] or insight [20].
Here, we test problem-solving performance, object and place exploration and inhibitory control of three subspecies of wild house mice (Mus musculus) living in human-altered environments for approximately 3000 to more than 11 000 years [21]. Mice originated from six populations, derived from human dwellings and were kept and bred under standardized laboratory conditions for several generations prior to testing. Thereby, we ensured that all observed behavioural responses were the product of innate i.e. genetic, differences [3]. We hypothesized that problem-solving performance would be highest in mice that have established a commensal lifestyle earliest. In addition, we aimed to identify the mechanisms underlying differences in problem-solving performance focusing on the contribution of object and place exploration (neophilia), motivation to participate, persistence, motor diversity, associative learning and inhibitory control.
The ancestral geographic range for Mus musculus was probably in present-day Transcaucasia or North India [21] where the three subspecies M. m. domesticus (MD), M. m. musculus (MM) and M .m. castaneus (MC) started to diverge approximately 350–500 kyr ago. They rapidly spread through Eurasia by establishing a commensal lifestyle with humans, following humans in migrations to different geographical areas [22]. The origin of MD synanthropization started around 11 000–13 000 years ago in the Near East and was followed by a dispersal towards Western Europe with maritime traffic [23,24]. The onset of MM synanthropization occurred around 8000 years ago as a consequence of the Neolithic cultivation process in the Balkans [23], while the expansion of MC into India, East Asia and Southeast Asia between 7600 and 3800 years ago was probably linked to the emergence of agricultural lifestyles in these areas [25]. House mice are known as one of the few ‘anthropodependent' species because of their specialization on anthropogenic resources [26]. They have evolved several morphological life history and behavioural adaptations such as changes in breeding cycles, territorial behaviour, diet and foraging behaviour (summarized in e.g. [27,28]). Frynta et al. [29], for example, found commensal MM populations to show increased exploration of elevated places over non-commensal populations. No study yet investigated evolutionary adaptations of cognitive traits to a commensal lifestyle.
2. Methods
(a). Animals and housing
We tested 148 individuals from three subspecies of wild house mice. Each subspecies was represented by two populations (24–25 adult individuals per population, males and females equally represented [30]; electronic supplementary material, S1 and S2). Offspring of wild-caught individuals were bred and maintained under laboratory conditions for more than five generations.
All subjects were raised in standard Type III cages, with woodchip bedding, shelter and varying enrichment under ad libitum food conditions (Altromin 1324) and at a 12 : 12 dark–light cycle. Animals were kept in same-sex sibling pairs.
During testing, pairs were transferred to two standard cages connected by a tube similarly equipped as standard housing cages (electronic supplementary material, S2). Experiments were conducted during the initial hours of the dark period (problem-solving, detour reaching) under low-light conditions (less than 20 lux) or in the early morning (neophilia, detour reaching).
Animals were habituated before being tested in a control foraging set-up and seven problem-solving apparatuses. Thereafter, we measured their neophilic behaviour towards an unknown environment and tested for inhibitory control behaviour. Pairs were separated before each experiment by gently directing one animal into one of the cages and inserting a wire separation in the connecting tube.
(b). Habituation
A mealworm was presented on a plastic plate (8 × 12.3 cm) for 40 min. The experimenter checked whether the mealworm had been consumed every 5 min. Animals consuming the mealworm in less than 5 min for three consecutive days advanced to the testing stage (n = 126).
(c). Problem-solving
Animals were presented with seven problem-solving set-ups, each equipped with a mealworm as a food reward. The first four set-ups could be opened by several strategies while the remaining three required to be opened by performing a specific motor action. A control set-up testing for the animals' willingness to participate was presented after the first three set-ups.
Animals were tested in one set-up per day. Each set-up was first presented ‘open' (i.e. the animal could freely access the food reward). Animals which consumed the food reward within 20 min were then presented with the closed set-up and given 10 min to solve. Animals that did not consume the mealworm from the open set-up were scored as not participating and were presented with the next set-up the following day. Tests were videorecorded for approach latency to open and closed set-ups, solving outcome and persistence (defined as time spent interacting with the set-up). To open the first set-up, the animal had to push or pull a metal lid (S1). Next, they had to pull over a plastic lid (S2), followed by extracting a ball of paper out of a tube (S3). Next, the same tube was covered with bedding material which animals had to dig through (C). Thereafter, animals had to open the window of a Lego brick house (S4), to pull a lid straight up by a metal rod (S5), to pull away a plastic lid (S6) and to open a half-sphere (S7). See electronic supplementary material, S2 for a detailed description of set-ups.
(d). Neophilia
An unknown environment consisting of a Type III cage equipped with bedding and three foreign objects was used to test the animals' propensity to explore unknown areas voluntarily. It was connected to the animals' home cage using the connection tube. Testing lasted for 1 h and was videotaped to analyse the latency to enter the unknown environment, the number of visits and time spent in the unknown environment.
(e). Inhibitory control
Detour tasks are commonly used to test for inhibitory control, the ability to inhibit counterproductive responses driven by a visual stimulus. We adapted a set-up developed for laboratory mice [31].
Animals were tested on 3 consecutive days. The first day consisted of two open trials in which a mealworm was freely accessible on the plastic plate for 30 min each. On the second day, the mealworm was presented open in the first trial but an opaque, V-shaped barrier was placed outward between the animal and the food reward on the second trial. On the third day, the food reward was presented in an open trial followed by a transparent V-shaped barrier. We videotaped the latency to approach the barriers, number of interactions with the barrier, time spent interacting with or within 1 cm distance to the barrier and the latency to access the reward.
(f). Statistical analyses
All data were analysed using the free software R (v. 3.4.3.).
The problem-solving performance was analysed separately for set-ups allowing multiple solving strategies and set-ups requiring a specific motor action. Repeatability of problem-solving was calculated across all seven set-ups using 1000 bootstrappings to calculate CIs and 1000 permutations for p-value estimation (rptR package, details see electronic supplementary material, S5).
We used a binomial mixed model (package lme4), fitting problem-solving as response, subspecies, experimental set-up (four levels), interaction time and approach latency as fixed effects and animal ID as a random effect for set-ups with multiple solving strategies. For the second set of set-ups, we only scored the solving outcome, hence, subspecies was the sole fixed effect and animal ID was coded as a random effect. Both models were run twice, once with all animals included and once only with animals which actively participated. Since all models yielded similar results (electronic supplementary material, S4), we present the results of participating animals throughout.
Significance of fixed effects was assessed by likelihood ratio tests of nested models (lrtest, lmtest package). Significant categorical effects were further analysed by pairwise post hoc comparison (lsmeans package), applying a false discovery rate adjustment. We constructed two separate models to test for subspecies differences in approach latency and interaction time (persistence) assuming a Poisson distribution. Model fit was tested by visual inspection of residuals and Q-Q plots and by computing the coefficient of determination, R2 (package rsq).
To test whether subspecies differed in motivation to participate, we used a control set-up eliciting natural digging behaviour. Participation was analysed using a generalized linear model assuming a binomial distribution, with subspecies as a fixed effect. Full outputs of all models can be found in electronic supplementary material, S4. Figures present predicted model outputs.
To investigate subspecies and differences in the response to a novel environment, we used a permutation-based ANOVA with 1000 permutations (package RVAideMemoire). The population was coded nested within subspecies. We performed permutation-based pairwise t-tests with a false discovery rate p-value adjustment post hoc if a significant main effect of subspecies or population was found. Approach rate and solving success in the detour reaching task was analysed by proportion tests. Latency to approach, interaction time and number of interactions were analysed by permutation-based ANOVA.
3. Results
(a). Problem-solving
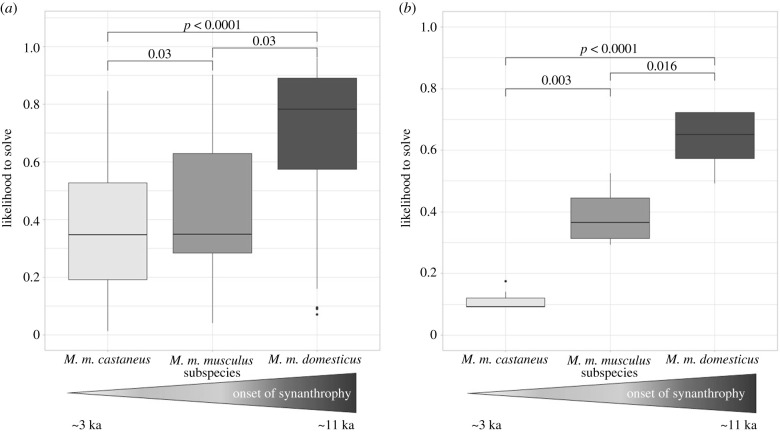
MD outperformed MM and MC populations in set-ups which could be solved by multiple different solving strategies (figure 1a) as well as in set-ups requiring a specific solving action (figure 1b). This result was consistent when animals that did not participate were excluded (multiple strategies: d.f. = 2, χ2 = 78.53, p < 0.001; specific strategy: d.f. = 2, χ2 = 49.26, p < 0.001). For participating animals, the latency to solve (from first approach to solving) across set-ups also differed between subspecies. MM needed longer to solve than MC (t = 2.6, p = 0.03) and longer than MD (t = 5.5, p < 0.001). MC and MD did not differ in latency to solve (t = 1.6, p = 0.18). While MD and MM did not differ in solving probability of set-ups with multiple versus single solving strategy, MC had a lower chance to solve set-ups with single solving strategies (t = 2.12, p < 0.001). Repeatability (R = 0.23, CI: 0.15–0.32, p = 0.001) of solving across all seven set-ups was significantly different from zero.
Figure 1.
Pairwise comparison of subspecies in problem-solving performance. (a) Set-ups allowing multiple solving options, ; ; (b) set-ups requiring a specific solving action, ; .
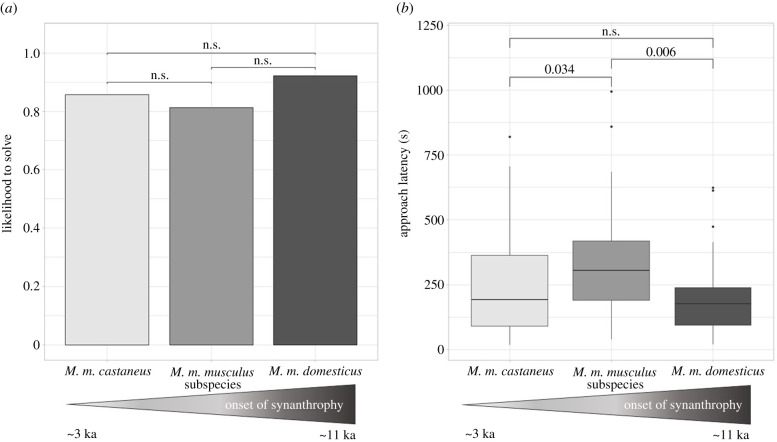
Particularly set-up S3 was more difficult to solve than the remaining set-ups (all pairwise comparisons p < 0.001, see electronic supplementary material, S3). However, even when excluding this set-up, subspecies differences in solving success were consistent (d.f. = 2, χ2 = 25.7, p < 0.001). Subspecies performed equally well in the control set-up with more than 80% of all animals participating (figure 2a).
Figure 2.
(a) Pairwise comparison of subspecies' problem-solving performance in the control set-up, ; ; (b) pairwise comparison approach latency for the first four set-ups, ; .
Animals approaching the test set-ups faster were better at problem-solving (d.f. = 1, χ2 = 10.07, p < 0.001). Subspecies showed a significant difference in approach latencies (d.f.=2, χ2 = 9.50, p < 0.001) with both MD (z = 3.09, p < 0.001) and MC (z = 2.27, p = 0.03) approaching faster than MM (figure 2b). Although MC approached faster than MM, it did not outperform it in problem-solving. Interaction times were not associated with problem-solving.
(b). Neophilia
Neither subspecies, nor any of the populations differed in the latency to enter a novel environment (subspecies: F = 1.07, perm. p = 0.36, corresponding to an R2 = 0.07) or in the number of exploration bouts (subspecies: F = 0.14, perm. p = 0.88, R2 = 0.05). Subspecies differed in the amount of time spent in the novel environment (F = 6.16, perm. p = 0.005, R2 = 0.15). MD spent approximately 40% (554 ± 50 s) more time in the novel environment than MM (pairwise p = 0.02; 283 ± 48 s) and MC (pairwise p = 0.03; 271 ± 47 s).
(c). Inhibitory control
Fewer MM (73%) interacted with the transparent barrier compared to MD (96%) and MC (85%, p = 0.04) although the same percentage of animals per subspecies interacted with the barrier in the opaque control situation (p = 0.22). Out of approaching animals, also the solving success of MM was on average 30% lower than that of the other two subspecies (p = 0.007). The latency to solve and the time actively interacting with the barrier did not differ between subspecies but MM went back to their shelter and approached the barrier anew more often than the other two subspecies (F = 5.11, perm. p = 0.02).
4. Discussion
House mouse subspecies having evolved a commensal lifestyle earlier show enhanced problem-solving performances. MD populations had the highest likelihood to solve, while MC had the lowest likelihood. While MM showed intermediate solving probability, they needed longer to solve. These results cannot be explained by differences in neophilia, higher motivation or longer interaction with test set-ups, nor by better inhibitory control but rather mice appear to have evolved enhanced cognitive abilities to cope with the challenges of human-altered habitats.
Our results are in line with findings in various bird species showing that populations living in human-altered areas show enhanced problem-solving performances [3,13,32], suggesting that increased cognitive abilities are a general adaptation in populations thriving in urban habitats. The 'cognitive buffer hypothesis’ states that animals which have to cope with a high degree of environmental change or instability such as frequently found in human environments, would develop elevated cognitive abilities to enable flexible use of environmental information and the production of novel behavioural responses [8,33]. The ability to produce innovative behaviours in changing or challenging environments has indeed been shown to have a critical effect on fitness [34,35]. Despite the growing body of research demonstrating behavioural adaptations to urban living in wild animals, whether these modifications represent evolutionary adaptations or are the results of phenotypic plasticity is often unknown [1,3]. Given that the animals in our study had been kept in standardized laboratory conditions for several generations, differences between subspecies imply an evolutionary adaptation of cognitive ability. The significant non-zero repeatability in addition indicates a heritable component of problem-solving capacities, a pre-requisite for evolution. Other factors such as the geographical area, specifics about the population history or the degree of urbanization may in addition also influence cognitive abilities. In striped file mice (Apodemus agrarius), populations living in a big city outperform more rural conspecifics [36]. Here, although all our population descended from animals in small or medium human settlements, the two MM populations differed in problem-solving performance while populations of MC and MD performed on similar levels. In summary, we here show that problem-solving, representing flexibility and general cognitive ability, is an important trait evolving rapidly, presumably to facilitate success in human-altered environments.
To fully understand how the ability to problem-solve provides adaptive advantages for animals, we need to disentangle the mechanisms supporting this ability. Recent studies have highlighted several behavioural traits potentially contributing to problem-solving success. Animals can differ in the rate with which they encounter novel stimuli [37], for example, because they differ in neophobia or neophilia (fearfulness of versus attraction to novel objects or places). Commensal rats initially avoid novel objects while non-commensal rats explore them readily [38]. Studies so far report mixed results on the influence of neophobia and neophilia on problem-solving performance [15,39,40]. Here, we found no evidence for an influence of object or place neophilia on problem-solving. While MD investigated a novel place on average 40% longer than the other two subspecies, MM and MC did not differ in exploration although they differed in problem-solving success. Likewise, the latency to approach the test set-ups was consistently higher for MM but did not differ for the other two subspecies. Furthermore, there were no differences between subspecies in motivation to approach and interact with the control test set-up. The absence of an influence of neophilia might in part be explained by our extensive habituation process which was designed to equalize subspecies reactions to the experimenter and the test set-ups [41].
Further, we tested if traits such as the inability to perform a motoric action, inhibitory control, persistence or the association of action and consequences influenced solving success, as these traits were shown to correlate with problem-solving performances in multiple species [15,42]. An animal might not be able to solve a given problem either because it is not physically able to perform the motor action required or because it does not express the necessary motor action within a short amount of time. To test these two possibilities, we presented five non-solvers per population with the set-up overnight for all set-ups. All animals that interacted with the set-up were able to problem-solve, showing that a general inability to perform a motor action was not causing the difference in solving outcome. In addition, we tested if inhibitory control differs between subspecies. In general, the diversity of motoric actions shown within a limited time has a positive effect on problem-solving [42,43]. We found that while MM performed significantly worse than MD and MC in the inhibitory control task, there was no difference between MD and MC although these two subspecies showed most pronounced differences in problem-solving success, indicating no effect of inhibitory control on successful problem-solving.
Since complex problems are unlikely to be solved on the first attempt, it is necessary to be persistent in order to solve them. Several studies in the past indicated that more persistent individuals are more likely to successfully problem-solve [42–44]. Contrary to these results, we found no influence of persistence on problem-solving performance. A recent study in striped field mice, however, indicated that individuals interacting longer with open set-ups (as opposed to interaction with closed set-ups as measured here) were more likely to successfully problem-solve, suggesting that longer interaction provides the individual with more information, thus enabling it to later perform an appropriate solving action [36].
Several of our set-ups were designed in a way that an action had to be repeated several times to lead to a solving success. In order to solve, the animal either had to learn that a particular action leads to solving (association of action and consequence [19]), or it had to understand how the set-up works (insight [20]). A difference in the association of action with consequence seems unlikely to have caused the differences in problem-solving success since we found similar subspecies differences in problem-solving success across set-ups which were specifically designed to be opened by a single-motor action.
In conclusion, our results show that house mice evolved enhanced cognitive abilities during their commensal life with humans. Cognitive abilities and especially innovative problem-solving may thus be key factors for animals to thrive in human-altered habitats. With urban environments increasing rapidly, how wild animals adjust to the challenges of living in close proximity to humans becomes increasingly more relevant, and an understanding of the traits that adapt and their underlying mechanisms will be key.
Supplementary Material
Acknowledgements
We thank our caretaker Milan Jovičić for his help in animal maintenance and the team of the institute's workshop for helping to build the test set-ups.
Ethics
Animal facilities were approved for breeding and keeping animals by the local German government authority. All animals could voluntarily choose to take part in experiments, thus, no further permits were required according to national guidelines.
Data accessibility
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vt4b8gtqw [45]. The R code used for data analysis and the full statistical output have also been uploaded as part of the electronic supplementary material (electronic supplementary material, S4).
Authors' contributions
L.V. and A.G. designed the study; L.V. conducted and analysed problem-solving tests; V.M. conducted and analysed exploration and inhibitory control tests; L.V. and A.G. contributed equally to writing.
Competing interests
We received no funding for this study.
Funding
Open Access funding provided by the Max Planck Society.
References
- 1.Lowry H, Lill A, Wong BBM. 2013. Behavioural responses of wildlife to urban environments. Biol. Rev. 88, 537-549. ( 10.1111/brv.12012) [DOI] [PubMed] [Google Scholar]
- 2.Miranda AC. 2017. Mechanisms of behavioural change in urban animals: the role of microevolution and phenotypic plasticity. In Ecology and conservation of birds in urban environments (eds Murgui E, Hedblom M), pp. 113-132. Berlin, Germany: Springer International Publishing AG. ( 10.1007/978-3-319-43314-1_7) [DOI] [Google Scholar]
- 3.Sol D, Lapiedra O, González-Lagos C. 2013. Behavioural adjustments for a life in the city. Anim. Behav. 85, 1101-1112. ( 10.1016/j.anbehav.2013.01.023) [DOI] [Google Scholar]
- 4.Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM. 2008. Global change and the ecology of cities. Science 319, 756-760. ( 10.1126/science.1150195) [DOI] [PubMed] [Google Scholar]
- 5.Reader S. 2003. Innovation and social learning: individual variation and brain evolution. Anim. Biol. 53, 147-158. ( 10.1163/157075603769700340) [DOI] [Google Scholar]
- 6.Ducatez S, Sol D, Sayol F, Lefebvre L. 2020. Behavioural plasticity is associated with reduced extinction risk in birds. Nat. Ecol. Evol. 4, 788-793. ( 10.1038/s41559-020-1168-8) [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre L, Reader SM, Sol D. 2004. Brains, innovations and evolution in birds and primates. Brain Behav. Evol. 63, 233-246. ( 10.1159/000076784) [DOI] [PubMed] [Google Scholar]
- 8.Sol D. 2009. Sol, Daniel. ‘The cognitive-buffer hypothesis for the evolution of large brains. In Cognitive ecology II (eds Dukas R, Ratcliffe JM), pp. 111-136. Chicago, IL: University of Chicago Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sih A. 2013. Understanding variation in behavioural responses to human-induced rapid environmental change: a conceptual overview. Anim. Behav. 85, 1077-1088. ( 10.1016/j.anbehav.2013.02.017) [DOI] [Google Scholar]
- 10.Fisher J, Hinde R. 1949. The opening of milk bottles by birds. Br. Birds 42, 347-357. [Google Scholar]
- 11.Bókony V, et al. 2014. Necessity or capacity? Physiological state predicts problem-solving performance in house sparrows. Behav. Ecol. 25, 124-135. ( 10.1093/beheco/art094) [DOI] [Google Scholar]
- 12.Cook MO, Weaver MJ, Hutton P, McGraw KJ. 2017. The effects of urbanization and human disturbance on problem solving in juvenile house finches (Haemorhous mexicanus). Behav. Ecol. Sociobiol. 71, 1-10. ( 10.1007/s00265-017-2304-6) [DOI] [Google Scholar]
- 13.Papp S, Vincze E, Preiszner B, Liker A, Bókony V. 2015. A comparison of problem-solving success between urban and rural house sparrows. Behav. Ecol. Sociobiol. 69, 471-480. ( 10.1007/s00265-014-1859-8) [DOI] [Google Scholar]
- 14.Prasher S, Evans JC, Thompson MJ, Morand-Ferron J. 2019. Characterizing innovators: ecological and individual predictors of problem-solving performance. PLoS ONE 14, 1-19. ( 10.1371/journal.pone.0217464) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin AS, Guez D. 2014. Innovation and problem solving: a review of common mechanisms. Behav. Process. 109, 121-134. ( 10.1016/j.beproc.2014.08.027) [DOI] [PubMed] [Google Scholar]
- 16.Overington SE, Cauchard L, Côté KA, Lefebvre L. 2011. Innovative foraging behaviour in birds: what characterizes an innovator? Behav. Process. 87, 274-285. ( 10.1016/j.beproc.2011.06.002) [DOI] [PubMed] [Google Scholar]
- 17.Diquelou MC, Griffin AS, Sol D. 2016. The role of motor diversity in foraging innovations: a cross-species comparison in urban birds. Behav. Ecol. 27, 584-591. [Google Scholar]
- 18.Kabadayi C, Bobrowicz K, Osvath M. 2018. The detour paradigm in animal cognition. Anim. Cogn. 21, 21-35. ( 10.1007/s10071-017-1152-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Horik JO, Madden JR. 2016. A problem with problem solving: motivational traits, but not cognition, predict success on novel operant foraging tasks. Anim. Behav. 114, 189-198. ( 10.1016/j.anbehav.2016.02.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seed A. 2017. Problem solving. In Handbook of comparative psychology: perception, learning, and cognition), pp. 601-625. Washington, DC: American Psychological Association. [Google Scholar]
- 21.Macholán M, Baird SJ, Munclinger P, Piálek J. 2012. Evolution of the house mouse. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 22.Bonhomme F, Searle JB. 2012. House mouse phylogeography. In Evolution of the house mouse (eds Macholán M, Baird SJE, Munclinger P, Piálek J), pp. 278-296. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Cucchi T, Auffray J, Vigne J. 2012. History of house mouse synanthropy and dispersal in the Near East and Europe: zooarcheological review and perspectives. In Evolution of the house mouse (eds Macholán M, Baird SJ, Munclinger P, Piálek J), pp. 65-93. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Cucchi T, et al. 2013. On the trail of Neolithic mice and men towards Transcaucasia: Zooarchaeological clues from Nakhchivan (Azerbaijan). Biol. J. Linn. Soc. 108, 917-928. ( 10.1111/bij.12004) [DOI] [Google Scholar]
- 25.Suzuki H, et al. 2013. Evolutionary and dispersal history of Eurasian house mice Mus musculus clarified by more extensive geographic sampling of mitochondrial DNA. Heredity (Edinb) 111, 375-390. ( 10.1038/hdy.2013.60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulme-Beaman A, Dobney K, Cucchi T, Searle JB. 2016. An ecological and evolutionary framework for commensalism in anthropogenic environments. Trends Ecol. Evol. 31, 633-645. ( 10.1016/j.tree.2016.05.001) [DOI] [PubMed] [Google Scholar]
- 27.Berry RJ. 1981. Biology of the house mouse. Oxford, UK: Academic Press. [Google Scholar]
- 28.Pockock MJO, Hauffe HC, Searle JB. 2005. Dispersal in house mice. Biol. J. Linn. Soc. 84, 565-583. [Google Scholar]
- 29.Frynta D, Kaftanová-Eliášová B, Žampachová B, Voráčková P, Sádlová J, Landová E. 2018. Behavioural strategies of three wild-derived populations of the house mouse (Mus m. musculus and M. m. domesticus) in five standard tests of exploration and boldness: searching for differences attributable to subspecies and commensalism. Behav. Process. 157, 133-141. ( 10.1016/j.beproc.2018.09.008) [DOI] [PubMed] [Google Scholar]
- 30.Harr B, et al. 2016. Genomic resources for wild populations of the house mouse, Mus musculus and its close relative Mus spretus. Sci. Data 3, 1-14. ( 10.1038/sdata.2016.75) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juszczak GR, Miller M. 2016. Detour behavior of mice trained with transparent, semitransparent and opaque barriers. PLoS ONE 11, 1-23. ( 10.1371/journal.pone.0162018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audet JN, Ducatez S, Lefebvre L. 2016. The town bird and the country bird: problem solving and immunocompetence vary with urbanization. Behav. Ecol. 27, 637-644. ( 10.1093/beheco/arv201) [DOI] [Google Scholar]
- 33.Reader SM, MacDonald K. 2003. Environmental variability and primate behavioural flexibility. In Animal innovation (eds Reader SM, Laland KN), pp. 83-116. Oxford, UK: Oxford University Press. [Google Scholar]
- 34.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. 2012. Cognitive ability influences reproductive life history variation in the wild. Curr. Biol. 22, 1808-1812. ( 10.1016/j.cub.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 35.Preiszner B, Papp S, Pipoly I, Seress G, Vincze E, Liker A, Bókony V. 2017. Problem-solving performance and reproductive success of great tits in urban and forest habitats. Anim. Cogn. 20, 53-63. ( 10.1007/s10071-016-1008-z) [DOI] [PubMed] [Google Scholar]
- 36.Mazza V, Guenther A. 2021 City mice and country mice: innovative problem-solving in rural and urban non-commensal rodents. Anim. Behav. 172, 197-210. [Google Scholar]
- 37.Greenberg R. 2003. The role of neophobia and neophilia in the development of innovative behaviour of birds. In Animal innovation (eds SM Reader, KN Laland), pp. 175–196. Oxford, UK: Oxford University Press. [Google Scholar]
- 38.Cowan PE. 1977. Neophobia and neophilia: new-object and new-place reactions of three Rattus species. J. Comp. Physiol. Psychol. 91, 63-71. ( 10.1037/h0077297) [DOI] [Google Scholar]
- 39.Aplin LM, Sheldon BC, Morand-Ferron J. 2013. Milk bottles revisited: social learning and individual variation in the blue tit, Cyanistes caeruleus. Anim. Behav. 85, 1225-1232. ( 10.1016/j.anbehav.2013.03.009) [DOI] [Google Scholar]
- 40.Guenther A, Brust V. 2017. Individual consistency in multiple cognitive performance: behavioural versus cognitive syndromes. Anim. Behav. 130, 119-131. ( 10.1016/j.anbehav.2017.06.011) [DOI] [Google Scholar]
- 41.Myers RE, Hyman J. 2016. Differences in measures of boldness even when underlying behavioral syndromes are present in two populations of the song sparrow (Melospiza melodia). J. Ethol. 34, 197-206. ( 10.1007/s10164-016-0465-9) [DOI] [Google Scholar]
- 42.Griffin AS, Diquelou MC. 2015. Innovative problem solving in birds: a cross-species comparison of two highly successful passerines. Anim. Behav. 100, 84-94. ( 10.1016/j.anbehav.2014.11.012) [DOI] [Google Scholar]
- 43.Benson-Amram S, Holekamp KE. 2012. Innovative problem solving by wild spotted hyenas. Proc. Biol. Sci. 279, 4087-4095. ( 10.1098/rspb.2012.1450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morand-Ferron J, Cole EF, Rawles JEC, Quinn JL. 2011. Who are the innovators? A field experiment with 2 passerine species. Behav. Ecol. 22, 1241-1248. ( 10.1093/beheco/arr120) [DOI] [Google Scholar]
- 45.Vrbanec L, Matijević V, Guenther A. 2021. Data from: Enhanced problem-solving ability as an adaptation to urban environments in house mice. Dryad Digital Repository. ( 10.5061/dryad.vt4b8gtqw) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Vrbanec L, Matijević V, Guenther A. 2021. Data from: Enhanced problem-solving ability as an adaptation to urban environments in house mice. Dryad Digital Repository. ( 10.5061/dryad.vt4b8gtqw) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.vt4b8gtqw [45]. The R code used for data analysis and the full statistical output have also been uploaded as part of the electronic supplementary material (electronic supplementary material, S4).