Abstract
In marine ecosystems, fishing often targets predators, which can drive direct and indirect effects on entire food webs. Marine reserves can induce trophic cascades by increasing predator density and body size, thereby increasing predation pressure on populations of herbivores, such as sea urchins. In California's northern Channel Islands, two species of sea urchins are abundant: the red urchin Mesocentrotus franciscanus, which is targeted by an economically valuable fishery, and the virtually unfished purple urchin Strongylocentrotus purpuratus. We hypothesized that urchin populations inside marine reserves would be depressed by higher predation, but that red urchins would be less affected due to fishing outside reserves. Instead, our analyses revealed that purple urchin populations were unaffected by reserves, and red urchin biomass significantly increased in response to protection. Therefore, urchin biomass overall has increased inside reserves, and we found no evidence that giant kelp is positively affected by reserves. Our results reveal the overwhelming direct effect of protecting fished species in marine reserves over indirect effects that are often predicted but seldom clearly documented. Indirect effects due to marine reserves may eventually occur in some cases, but very effective predators, large reserves or extended time periods may be needed to induce them.
Keywords: trophic cascade, kelp forest, marine reserve, sea urchin
Significance statement
Trophic cascades are widely cited yet poorly documented ecological phenomena. In temperate coastal oceans, sea urchins can overgraze kelp forests, creating barren areas. Marine reserves are often framed as a solution: as protected urchin predators repopulate, they can control urchin populations directly or indirectly by inhibiting their grazing activity. In California's Channel Islands, however, urchins have actually increased inside reserves because one species is fished, and unfished urchins were unaffected by reserves. We found no evidence of trophic cascades influencing urchins and kelp. Marine reserves can effectively protect fished species and provide insight into the effects of fished species on ecosystems, but trophic cascades should not be assumed to occur in reserves and used as a primary justification for their establishment.
1. Introduction
Without fishing, there are more and bigger fish. This simple axiom has been confirmed by many studies of marine protected areas over the last 30 years [1–3], supporting the use of spatial reserves as a conservation tool. Beyond these direct effects on fished species, however, ecological theory and models predict deeper indirect ecological effects of marine reserves, particularly since fished species are often top predators within interacting communities and food webs [4]. Trophic cascades are a prominent example: top predators increase in reserves resulting in decreases in herbivore prey and in turn increases in primary producers, particularly foundational species like macroalgae and corals that characterize community states [5–7].
Trophic cascades and the relative importance of top-down (consumer-driven) and bottom-up (producer-driven) processes in the structuring of ecological communities have long captured the interest of ecologists [7,8]. Many early examples of trophic cascades came from freshwater and marine ecosystems, which may have stronger cascades than terrestrial ecosystems [9–11]. Marine reserves have been at the forefront of this research, as they represent large-scale manipulations of predator density, providing a milieu in which to investigate the effects of predation on marine ecosystems [12,13] at more realistic spatial and temporal scales than permitted by manipulative experiments [5]. Despite predictions of widespread top-down trophic cascades in marine reserves [14], however, evidence for their occurrence is equivocal [3,15–17] and controversial [18].
Although predicted by food web theory [19], there are many reasons why trophic cascades may not be apparent in natural ecosystems such as marine reserves. Detecting indirect effects may be hampered by study design, particularly spatial comparisons that rely on a few sites, which can be inevitable given that the number of reserves to work in is often limited to only one in a region [20]. Indirect effects of predators on the ecosystem can be confounded by stronger effects of environmental gradients, habitat, other impacts besides fishing and other factors [15,16]. The effect of reserves on predators may not be strong enough to induce a trophic cascade, due to small reserve sizes compared to predator home ranges [21], insufficient time since reserve establishment [1] or lack of enforcement of reserve protection. The pathways of indirect effects may not manifest as expected; for example, predators may prefer other prey than hypothesized or shift their feeding behaviour depending on the suite of available prey [22], or species interactions such as competition between fished and unfished species may obscure indirect effects [23]. Finally, the complexity of the food web and intrinsic characteristics of the species involved, such as body size ratios, life-history characteristics and productivity, may affect the strength of trophic cascades [9,11].
To tease apart the effects of direct versus indirect effects of reserves, it would be ideal to examine pairs of ecologically similar species that are fished and unfished, but both subject to predation by the same fished species. In southern California, we have a situation that approximates this, with two species of coexisting sea urchin, the red urchin Mesocentrotus franciscanus, the target of one of California's top five most valuable fisheries, and the virtually unfished purple urchin, Strongylocentrotus purpuratus. Both urchin species consume macroalgae, including the giant kelp Macrocystis pyrifera, and when densely populated can create so-called urchin barrens that are more or less devoid of macroalgae [24]. Both species are preyed on by sheephead fish, Semicossyphus pulcher, and California spiny lobster, Palinurus interruptus, which are important fisheries species themselves, as well as by the sea star Pycnopodia helianthoides. The sea otter Enhydra lutens, an iconic urchin predator [25], was historically present but has not successfully recolonized the region. Greater abundances and body sizes of sheephead and spiny lobster have been documented in marine reserves in the northern Channel Islands [26,27] and have been hypothesized to control urchin populations in the reserves [28,29]. Most studies, however, have focused on only one of the two urchin species, or all urchins in aggregate.
Channel Islands National Park (CINP) has maintained one of the longest-running time-series subtidal surveys in the eastern Pacific since 1982 [30]. In 2003, a network of marine protected areas, including eight no-take marine reserves, was established in the islands. Four of these reserves had been monitored since the early 1990s by CINP. Here, we use before-after control-impact (BACI) analysis of this exceptional ecological time-series dataset to examine how the establishment of marine reserves has affected populations, biomass and demography of the two dominant sea urchin species. We find that biomass of the fished red urchin has responded positively to reserve protection, while purple urchins have not responded to reserves. Overall, the biomass of urchins responded positively to reserve protection. Positive responses of urchin predators were strongest at the reserves with the largest increases in urchin biomass. We found no evidence of trophic cascades due to reserves.
2. Results
(a). Urchin biomass
There was a significant interaction (p = 0.0126) between treatment (control or reserve) and time (before or after the establishment of marine reserves) in the linear mixed-effects model of total urchin biomass. The interaction term for this full model was positive, indicating increases in urchin biomass in reserve sites over fished sites after 2004, and the BACI contrast (using estimated marginal means on the log scale) was significant for reserve sites (p = 0.0002), reflecting the significant increase in urchin biomass at reserve sites after protection began in 2004 (figure 2). The difference between the change in biomass at fished versus reserve sites was −55.00 g m−2 yr−1 with a standard error of 36.85 g m−2 yr−1. Thus, on average, urchin biomass increased nearly three times more in reserve sites than in fished sites after the network of marine reserves was established.
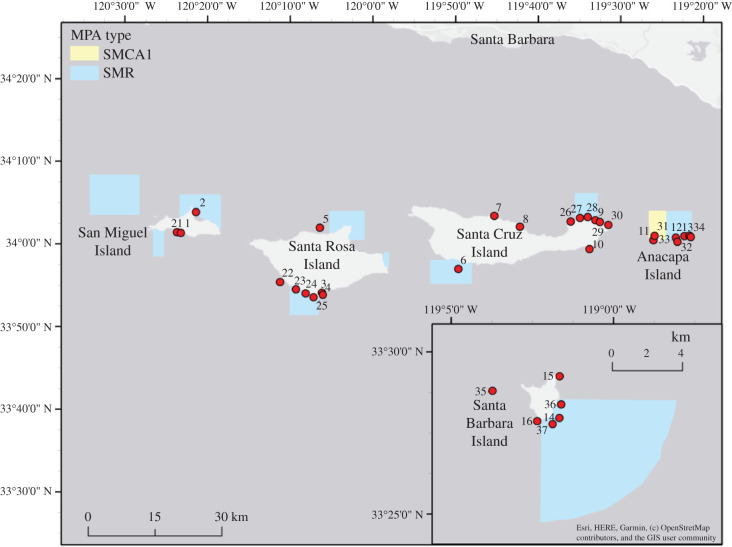
Figure 1.
Map of Kelp Forest Monitoring sites used in this analysis. Blue polygons represent the boundaries of State Marine Reserves (SMR) and the yellow polygon represents the boundaries of a State Marine Conservation Area (SMCA1). No fishing of any kind is allowed in marine reserves, while the conservation area allows for recreational take of spiny lobster and pelagic finfish and commercial take of spiny lobster. (Online version in colour.)
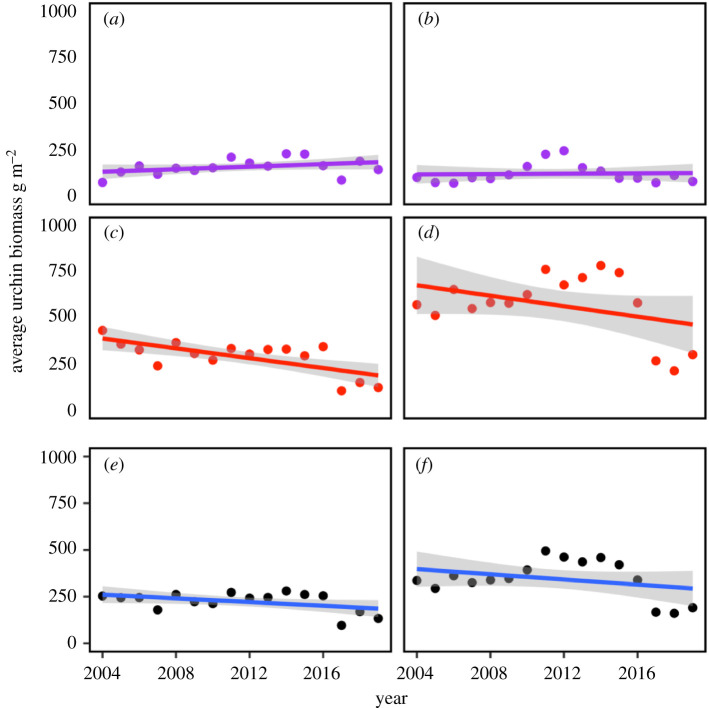
Figure 2.
Changes in average total biomass (g m−2) after 2004 for S. purpuratus in (a) all fished sites and (b) all reserve sites, and for M. francsicanus in (c) all fished sites and (d) all reserve sites. The bottom panes represent the average biomass for both species in (e) all fished sites and (f) all reserve sites. (Online version in colour.)
This change in urchin biomass was driven primarily by red urchins. A separate model for red urchins alone had a significant positive interaction term (p = 0.0004), meaning that control (fished) sites had a significantly smaller increase in red urchins than marine reserve sites after marine reserve establishment. On average, red urchin biomass density increased by 266 g m−2 at sites that became marine reserves, nearly quadrupling (397% increase) compared to the decrease of 89 g m−2 at fished sites. Two out of the four reserve sites, Gull Island and Hare Rock, had the largest increases in red urchin biomass, although in recent years red urchin biomass has declined at Hare Rock (figure 3). The BACI model for purple urchins had no significant terms, indicating that purple urchins did not change significantly between control and reserve sites or before and after 2004, nor was there an interaction between time and reserve status.
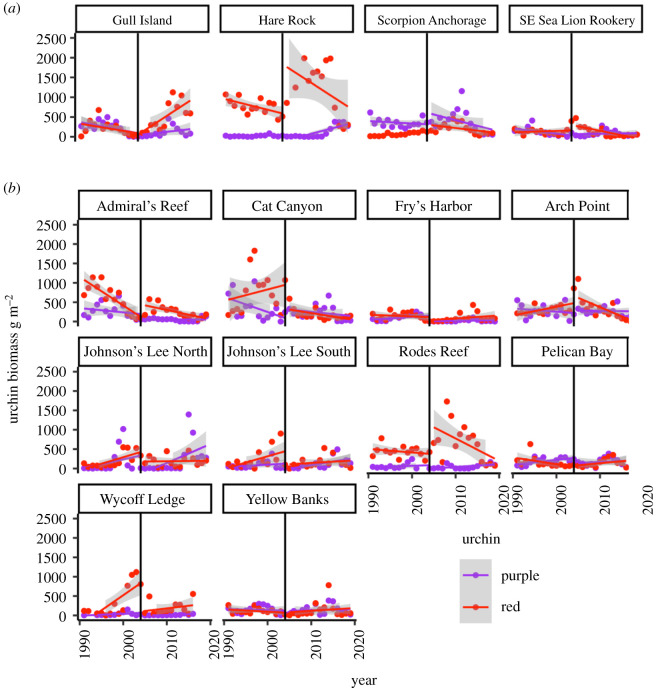
Figure 3.
Changes in urchin biomass over time with regression lines fit before and after the establishment of marine reserves in 2004 (vertical line) at reserve (a) and control (b) sites. Titles are site names. (Online version in colour.)
On average, the biomass of both purple and red urchins was higher inside marine reserves than non-reserve sites after the network was established in 2004, although red urchins initially increased more rapidly than purple urchins, and only red urchins had a significant difference in biomass (p = 0.0020). Purple urchin biomass increased over time outside marine reserves, however, while red urchins decreased in biomass outside reserves (figure 2).
(b). Urchin size distributions
Since smaller urchins are typically more vulnerable to predation, the abundance of small urchins may be a more sensitive indicator of changes in predation pressure on urchin populations due to reserve protection of their predators. Nevertheless, we found a similar pattern in small urchins to that for urchin populations as a whole. The value for the interaction term in a BACI model for small red urchin biomass was significant (p = 0.0444) and positive (b = 0.1701), indicating that on average small red urchin biomass increased inside reserves compared to outside (electronic supplementary material, figure S1). There was no significant interaction term for small purple urchins, and when this term was dropped from the model, time period was significant (p = 0.0024) but reserve status was not (p = 0.6406). Biomass of small purple urchins was not significantly different between reserve and fished sites, but the negative estimate for time period (b = −0.3596) indicates a slight decline in small purple urchin biomass both inside and outside reserves after the reserves were established. The majority of red urchin biomass was distributed across large-size classes (electronic supplementary material, figure S7), while the majority of purple urchin biomass was distributed across small- and medium-size classes (electronic supplementary material, figure S8).
(c). Kelp density
No significant effect of reserve status (p = 0.0940) or time period (p = 0.4653) or the interaction between status and time (p = 0.1514) was evident for kelp stipe density (electronic supplementary material, figure S2). In the mixed-effects model with both urchin species grouped together, time period and average urchin biomass did not have a significant effect on mean stipe density. All the available data from KFM sites across all years showed no significant relationship between urchin densities and kelp stipe densities, and the correlation for red sea urchins was in fact weakly positive (electronic supplementary material, figure S9).
(d). Urchin predators
We planned to use BACI analyses to test whether marine reserves were effectively increasing predator densities. Because the densities of urchin predators were low, inconsistently measured, did not meet statistical assumptions for parametric tests and were not all sized to yield biomass estimates, these analyses were not possible. However, plots of these data show dramatic differences between sites that are not always consistent with reserve effects (electronic supplementary material, figures S4–S6). These data show that Gull Island and Hare Rock reserves, where urchins had the strongest positive response to reserve establishment, also had relatively high predator densities compared to the other two reserves (although similar to some fished sites). Hare Rock reserve, which showed the greatest urchin response, also had the highest densities of sheephead and sunflower stars among the four reserve sites (although data from the Scorpion Anchorage reserve was sparse).
(e). Anacapa Island urchin biomass
Anacapa Island was considered separately because it is uniquely old, and no data were available there prior to the establishment of the marine reserve in 1978. However, three sites there have been continuously monitored since 1981, two within and one outside the marine reserve. ANOVA revealed a significant difference in red urchin biomass between the reserves and the fished site (p = 2.45 × 10−7), and ANCOVA yielded a significant reserve (p = 2.15 × 10−8) and time effect (6.94 × 10−5) but not an interaction between the two. In general, red urchin biomass decreased at all Anacapa sites, but the highest rate of decrease and the lowest overall red urchin biomass was at the fished site. For purple urchins, there was a significant interaction between reserve status and time (p = 1.5 × 10−6); purple urchins had higher biomass at the fished site compared to the reserves in the 1990s but decreased sharply and by the early 2000s onward have been as low or lower at the fished site than the reserve sites (electronic supplementary material, figure S2).
3. Discussion
In temperate marine ecosystems, including the California Channel Islands, protection of top predators inside marine reserves has been widely predicted to cause trophic cascades—decreases in herbivore biomass, often sea urchins, and corresponding increases in kelp, along with increased biodiversity associated with kelp forests [31,32]. We found no evidence, however, of strengthened trophic cascades in response to 15 years of marine reserve protection in the Channel Islands. On the contrary, total sea urchin biomass density increased on average inside marine reserves. Rather than suppressing urchin populations, marine reserves have led to population recovery of fished red sea urchins (Mesocentrotus franciscanus), while populations of unfished purple urchins (Strongylocentrotus purpuratus) were unaffected. Two of the reserves we examined, Gull Island (at Santa Cruz Island) and Hare Rock (San Miguel Island), are in areas of historically high sea urchin catches [33] and showed particularly dramatic increases in red sea urchin biomass (figure 3), despite having some of the highest counts of urchin predators after reserve establishment (electronic supplementary material, figures S4–S6). This suggests that the benefits of release from fishing pressure far outweighed any effect of increases in predators on sea urchins inside the reserves.
Increases in predator density and biomass inside reserves do not necessarily translate to rapid effects on prey populations. For example, if small individuals are favoured by predators, as is the case with both purple and red sea urchins [27,29], the effects of increased predation would take time to propagate through the prey population as new recruits suffer higher mortality, but larger size classes remain unaffected. For this reason, we examined small urchin populations separately and found a similar pattern to overall population biomass—small red urchins increased inside reserves, while small purple urchins were unaffected. Therefore, the lack of a trophic cascade effect on urchin populations was apparently not due to a time lag caused by a holdover of large predation-resistant urchins pre-dating reserve establishment [6]. In fact, increases in red urchins in response to fishing protection were evident after only 6 years of marine reserve protection [34]. Reserve protection, moreover, appears to have increased local recruitment of fished red sea urchin populations, suggesting that retention of sea urchin larvae may be occurring on the scale of the reserves.
Another possible effect of increased predation inside reserves is altered behaviour of prey and consequent changes in their ecological impacts in a ‘landscape of fear’ [35]. In areas with abundant predators, herbivores may flee or take shelter and spend less time actively grazing [36]. Such a trait-mediated indirect interaction, rather than direct predation, can be the dominant cause of a trophic cascade [37]. Sea urchins may spend less time grazing in the presence of predators [38], and this pattern has been suggested to occur in the Channel Islands [34]. If marine reserves were causing such indirect effects, primary producers, in this case giant kelp, would be expected to increase in abundance. Yet the density of giant kelp, which is grazed by sea urchins, was unaffected by reserve protection in the Channel Islands. Our results demonstrate no evidence, therefore, that increases in predators inside Channel Islands marine protected areas are causing, either through direct or indirect effects, a trophic cascade leading to positive effects on kelp forests via decreased sea urchin biomass and grazing.
Although data on the density of urchin predators were not available for all sites and years, there is clearly a high degree of variability in predator populations across space and time (electronic supplementary material, figures S4–S6). This variability, combined with the lack of data on predator body size and biomass, limits the conclusions we can draw about the effect of the reserves on predators. However, other studies have shown positive effects of Channel Islands reserves on population density and body size of urchin predators including sheephead [27] and lobster [26]. The unfished sea star Pycnopodia helianthoides is a voracious predator of S. purpuratus in particular, so even a few individuals could have a large impact on urchin populations [39]. Fluctuations in its density due to disease and other factors may have affected some of the site-specific temporal patterns in urchin abundance. In addition, urchin populations that are abundant due to release from predation or other factors may be reduced by density-dependent disease outbreaks [40]. Any effect of these processes on urchin populations, however, did not appear to vary with reserve status.
Why have increased predator populations inside reserves apparently not affected urchin populations and led to trophic cascades? There are two obvious possibilities. First, predator populations and sizes have not yet, after 15 years, rebounded to the extent necessary for cascades to occur. Second, purported urchin predators do not rely on urchins as prey with enough frequency to significantly affect their populations. These two explanations could be related, in that predator populations might be expected to exploit all prey once a density-dependent threshold is reached. In the following paragraphs, we discuss the evidence for each of these two possible explanations.
The main predators of sea urchins in our region at present are considered to be sheephead fish (Semicossyphus pulcher), spiny lobster (Palinurus interruptus) and the sunflower star (Pycnopodia helianthoides), which is unfished and not considered further here. A decade after reserve establishment, Hamilton & Caselle [27] found positive effects on both density and biomass of sheephead, with the strongest effects on densities of large individuals and total biomass at Santa Cruz and Santa Rosa Islands. Mean total length of sheephead inside reserves at all five islands was larger than the approximately 25 cm that Selden et al. [29] observed as the size at which sheephead become effective predators of small urchins, and was much larger at some islands—the average total length in Santa Rosa reserves was greater than 40 cm. Densities Hamilton and Caselle reported were comparable, albeit somewhat lower, to those in an unfished population at San Nicholas Island [41], and large individuals were likely more abundant in the historic populations [27]. On transects across the northern Channel Islands in reserve and non-reserve sites, Hamilton & Caselle [27] found a negative relationship between sheephead biomass and urchin density (both species combined). However, no overall effects of reserve status on urchin biomass or kelp abundance were found.
Spiny lobster exhibited a very strong positive response to reserve protection in the Channel Islands, increasing 4–6 times in density at the reserves in this study after only 6 years of protection [26]. Since then, surveys in two of the reserves, Gull Island and Scorpion Anchorage, showed that 15 years after reserve protection lobsters have continued to increase, reaching approximately 20 times the biomass of pre-reserve populations [42]. Spiny lobsters presented with relatively unpalatable prey, the sea hare Aplysia californica, only attacked them inside reserves throughout southern California, suggesting food limitation due to higher densities [22].
Based on a comparative analysis of monitoring data, Babcock et al. [43] estimated that indirect effects should take longer to detect than direct effects (13.1 years versus 5.13 years, respectively). Overall, the evidence suggests that, after 15 years of protection from fishing, predator populations have rebounded in the reserves to an extent that would be expected to affect urchin prey populations. Although it is still possible that urchin numbers will be affected as predator populations continue to grow in the reserves, this suggests that the presumed predator species are not heavily reliant on sea urchins for prey. Studies of sheephead diet have shown that it is highly variable, often including urchins but to varying degrees [44]. Larger fish tend to rely more on sea urchins as food [27,45] and are more effective sea urchin predators [29]. In situ observations showed highly variable feeding by sheephead on urchins, but also demonstrated that sheephead could cause high urchin mortality at small scales [46]. Although aquarium experiments have shown that lobster will consume urchins [47], evidence from nature on the importance of urchins in spiny lobster diet is sparse. A study of spiny lobster gut contents in Baja California showed that gastropods were most important, and no echinoderms were detected [48]. Off San Diego, lobster gut contents were mostly made up of mollusks and crustaceans, although urchins were also frequently consumed, particularly in deeper rocky habitats [49]. More information on spiny lobster feeding habits and their reliance on sea urchins is needed.
Complex food webs have been thought to weaken the strength of top-down control in terrestrial ecosystems, potentially explaining why many examples of trophic cascades are aquatic [9,10]. Like many marine ecosystems, however, giant kelp forests are highly diverse [50], with greater than 8700 trophic links identified in a California giant kelp forest food web, not including parasites [51]. Byrnes et al. identified 39 and 25 kelp forest species that serve as prey for sheephead and spiny lobster, respectively, and a more recent food web found that sheephead had the widest diet breadth of any kelp forest species, consuming 129 genera [51]. Increased predator diversity can also dampen the impact of predation on herbivores, as different predator species feed on each other and on shared prey species, weakening potential cascades [52,53]. Post-settlement movement of mobile predators outside reserves could also act to dilute their impact, allowing increases of both predators and fished prey [54].
Around the world, but particularly along the west coast of North America, top-down control and resulting trophic cascades have often been cited as maintaining kelp forests that would otherwise collapse under pressure of sea urchin grazing [55,56], although this view has not been universal [57,58]. Urchin grazing, however, is affected by multiple factors, including recruitment, disease and physical disturbance, and the spatial scale of its impact varies widely [59]. In the northwest Atlantic, an early paradigm that lobster predation on urchins drove top-down control of urchin barrens has not been supported by empirical or experimental evidence [60], although historically high abundances of groundfish may have driven trophic cascades in the past [61]. Trophic cascades have been documented in northeastern New Zealand, but their strength depends on environmental context [16], and other factors are more important in structuring kelp forests across much of New Zealand [18]. In Tasmania, increasing recruitment of urchins due to changes in oceanographic patterns and warming have interacted with lower predation due to lobster overfishing to reduce the resiliency of kelp forests to urchin grazing [62]. In other regions, kelp forest ecosystems are mainly structured by non-trophic factors. In the northeast Atlantic, ocean climatic patterns drive kelp forest community structure, and urchin grazing plays a minor role [63,64]. In Chile, South Africa and southwestern Australia, although sea urchin densities vary greatly, they depend on drift algae as food (as California urchins often do) and have not been associated with large-scale kelp deforestation [65–67]. Overall, this suggests that the widespread depiction of kelp forests and urchin barrens as alternative stable states controlled by predation is an unwarranted paradigm.
Trophic cascades are sometimes considered dominant forces in ecosystems, controlling their entire structure [68]. Cascades are considered more common in marine ecosystems [9,10], where most examples involve echinoderms, particularly sea urchins, as key herbivores [6]. Our results demonstrate, however, that trophic cascades are not always prevalent in marine ecosystems, and that fishing bans in marine reserves will not necessarily result in clear trophic cascades. In part, this reflects the fact that humans are often the ultimate predator, and direct effects of protection from us dominate community patterns [69] in conjunction with pervasive environmental effects [70]. In addition, only certain marine predators may be effective enough to drive cascades, and many ecosystems may lack such species due to biogeography or past extirpations. In southern California, kelp forest trophic cascades may never be realized until sea otters return in significant numbers, if that ever occurs. Until then, we have much to learn about the true ecological effects of marine reserves and their value for testing hypotheses about ecological interactions on large spatial and temporal scales. Our study demonstrates the value of studying marine reserves even as the results fail to support the paradigmatic view that marine reserves will result in lush, resilient kelp forests through predator-mediated trophic cascades.
4. Material and Methods
(a). Diver surveys
The National Parks Service's Kelp Forest Monitoring Program (KFM) has been annually collecting data at 33 sites throughout the Channel Islands, with 16 original sites established between 1981 and 1986. Divers collected data annually on the density and sizes of sea urchins—along with metrics on many other species—on a 100 m permanent transect line at each site. Beginning with an initial random sampling point between 0 and 7 m, a pair of 1 m2 quadrats were laid out on either side of the transect line at 8.33 m intervals. A total of 12 quadrat pairs were sampled for counts of 25 target species, including M. franciscanus and S. purpuratus. The first 200 urchins of each species, or all individuals if less than 200, were sampled along the transect line and test diameter measured to the nearest millimetre using calipers. In this analysis, we also used data collected by KFM on Semicossyphus pulcher (California sheephead), Pycnopodia helianthoides (sunflower star) and Panulirus interruptus (California spiny lobster). Sunflower stars and spiny lobsters were counted along twelve 3 × 20 m (60 m2) transects. Lobsters were found infrequently and size information was not collected. For California sheephead, divers surveyed fish communities visually on four 3 × 2 × 50 m transects. Starting in 2007, estimated total length of fish was also collected to the nearest 5 cm for large fish (greater than 15 cm) and 1 cm for small fish. Giant kelp (Macrocystis pyrifera) was surveyed along the 100 m permanent transect lines by counting the number of kelp stipes at 1 m above the bottom in 40 quadrats of 5 m2 that covered the entire span of the transect line on each side.
(b). Before–after control-impact analyses
All data analysis was performed in R [71]. For the BACI analysis, we only considered the continuous dataset for KFM sites 1–11 and 14–16 from 1991 to 2019. These sites were the first set of sites to be surveyed, whereas newer sites were not surveyed until after the establishment of marine reserves in 2004. Sites 12 and 13 were excluded because they were located in the Anacapa Marine Reserve, which is a much older reserve (established 1978). Urchin test diameters were converted to wet mass (g) with the following formulae [72]:
Total species-specific biomass in grams per square metre was calculated by multiplying the average wet mass of the species at a site by the average density of individuals at that site.
All analyses were performed in R following methods from Underwood [73] and Schwarz [74]. Sites 2 (San Miguel Island), 6 (Santa Cruz Island, South), 9 (Santa Cruz Island, North) and 14 (Santa Barbara Island) transitioned from non-reserve to reserve sites in 2004. These sites were considered impact sites, whereas the other 10 sites were control sites.
The linear mixed-effects model to evaluate effects of marine reserve protection on sea urchin populations was formulated as follows:
where μ is the mean urchin biomass (g m−2), x1 and x2 are categorical reserve status variables [x1: 0 indicates before the impact (before 2004) and 1 indicates after impact, x2: 0 indicates control site (not a marine reserve) and 1 indicates an impact site (marine reserve)], (β1β2)ij is the interaction between time period (before or after) and location (reserve or non-reserve), βk characterizes the fixed effects, bk,j[i] characterizes the group-level effects, j[i] denotes the group (site ID) of observation i, and ei is the error term. Since ACF (autocorrelation function) and pACF (partial autocorrelation function) plots indicated short lags in the data, we added an AR(1) correlation term to account for temporal autocorrelation. Models were fit using the lme4 package in R [75]. After model diagnostics (residuals plots and QQ plots) and selection via AICc (Akaike information criterion with correction for small sample sizes), we used a two-way repeated measures ANOVA to compare results. The BACI contrast (effect size) was estimated using the emmeans package in R [76].
We used separate BACI models to evaluate reserve effects on total urchin biomass, on the two sea urchin species separately and on densities of kelp stipes. In the total urchin biomass model, we used urchin species as a grouping factor in the error structure to account for the heterogeneity of variance. The response variable (urchin biomass) was log transformed to normalize residuals in all models. For each species, we also grouped urchins into size classes following Selden et al. [29] using test diameters (small less than 35 mm, medium 35–50 mm, large 50–70 mm, very large 70+ mm) to estimate the biomass occupied by each class and construct BACI models on small urchins only. Due to low densities of urchin predators in the surveys, there were not enough data to perform a statistically rigorous BACI analysis and these values were presented graphically.
Supplementary Material
Acknowledgements
Thank you to Joshua Sprague and David Kushner of the US National Park Service's Kelp Forest Monitoring Program for collecting and providing the datasets for this analysis. Thank you also to Peter Kalvass of California Department of Fish and Wildlife for providing sea urchin catch data and Li Kui of UC Santa Barbara for assisting in supplying and managing data. Rhiannon Rognstad and Stephen Schroeder provided valuable comments and discussion.
Data accessibility
Data on all organism counts can be found via SBC MBON [77]. All the data for this paper including size information are also publicly available through the Channel Islands National Park repository via the National Parks Service. Code for this project can be accessed online at github.com/kmalakhoff/urchin-baci/.
Authors' contributions
K.D.M. and R.J.M. designed research; K.D.M. and R.J.M. performed research; K.D.M. analysed data; K.D.M and R.J.M. wrote the paper.
Competing interests
The authors have no competing interests to declare.
Funding
This work was supported by the National Science Foundation's funding of the Santa Barbara Coastal Long Term Ecological Research programme, by the National Aeronautics and Space Administration, Biodiversity and Ecological Forecasting Program (grant no. NNX14AR62A); the Bureau of Ocean Energy Management, Environmental Studies Program (BOEM Agreement MC15AC00006) and the National Oceanic and Atmospheric Administration in support of the Santa Barbara Channel Marine Biodiversity Observation Network.
References
- 1.Molloy PP, McLean IB, Cote IM. 2009. Effects of marine reserve age on fish populations: a global meta-analysis. J. Appl. Ecol. 46, 743-751. ( 10.1111/j.1365-2664.2009.01662.x) [DOI] [Google Scholar]
- 2.Lester S, Halpern BS, Grorud-Colvert K, Lubchenco J, Ruttenberg BI, Gaines SD, Airamé S, Warner RR. 2009. Biological effects within no-take marine reserves: a global synthesis. Mar. Ecol. Prog. Ser. 384, 33-46. ( 10.3354/meps08029) [DOI] [Google Scholar]
- 3.Sciberras M, Jenkins SR, Kaiser MJ, Hawkins SJ, Pullin AS. 2013. Evaluating the biological effectiveness of fully and partially protected marine areas. Environ. Evidence 2, 4. ( 10.1186/2047-2382-2-4) [DOI] [Google Scholar]
- 4.Baskett ML, Micheli F, Levin SA. 2007. Designing marine reserves for interacting species: insights from theory. Biol. Conserv. 137, 163-179. ( 10.1016/j.biocon.2007.02.013) [DOI] [Google Scholar]
- 5.Shears NT, Babcock RC. 2002. Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132, 131-142. ( 10.1007/s00442-002-0920-x) [DOI] [PubMed] [Google Scholar]
- 6.Salomon AK, Gaichas S, Shears N, Smith J, Madin E, Gaines S. 2009. Key features and context-dependence of fishery-induced trophic cascades. Conserv. Biol. 24, 382-394. ( 10.1111/j.1523-1739.2009.01436.x) [DOI] [PubMed] [Google Scholar]
- 7.Ripple WJ, et al. 2016. What is a trophic cascade? Trends Ecol. Evol. 31, 842-849. ( 10.1016/j.tree.2016.08.010) [DOI] [PubMed] [Google Scholar]
- 8.Hairston NG, Smith FE, Slobodkin LB. 1960. Community structure, population control, and competition. Am. Nat. 94, 421-425. ( 10.1086/282146) [DOI] [Google Scholar]
- 9.Strong DR. 1992. Are trophic cascades all wet-differentiation and donor-control in speciose ecosystems? Ecology 73, 747-754. ( 10.2307/1940154) [DOI] [Google Scholar]
- 10.Shurin JB. 2002. A cross-ecosystem comparison of the strength of trophic cascades. Ecol. Lett. 5, 785-791. ( 10.1046/j.1461-0248.2002.00381.x) [DOI] [Google Scholar]
- 11.Shurin JB, Seabloom EW. 2005. The strength of trophic cascades across ecosystems: predictions from allometry and energetics. J. Anim. Ecol. 74, 1029-1038. ( 10.1111/j.1365-2656.2005.00999.x) [DOI] [Google Scholar]
- 12.Madin EM, Dill LM, Ridlon AD, Heithaus MR, Warner RR. 2016. Human activities change marine ecosystems by altering predation risk. Glob. Change Biol. 22, 44-60. ( 10.1111/gcb.13083) [DOI] [PubMed] [Google Scholar]
- 13.Palumbi SR, Gaines SD, Leslie H, Warner RR. 2003. New wave: high-tech tools to help marine reserve research. Front. Ecol. Environ. 1, 73-79. ( 10.1890/1540-9295(2003)001[073:NWHTTT]2.0.CO;2) [DOI] [Google Scholar]
- 14.Pinnegar J, et al. 2000. Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179-200. ( 10.1017/S0376892900000205) [DOI] [Google Scholar]
- 15.Sala E, Boudouresque CF, Harmelin-Vivien M. 1998. Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82, 425-439. ( 10.2307/3546364) [DOI] [Google Scholar]
- 16.Shears NT, Babcock RC, Salomon AK. 2008. Context-dependent effects of fishing: variation in trophic cascades across environmental gradients. Ecol. Appl. 18, 1860-1873. ( 10.1890/07-1776.1) [DOI] [PubMed] [Google Scholar]
- 17.Bruno JF, Côté IM, Toth LT. 2019. Climate change, coral loss, and the curious case of the parrotfish paradigm: why don't marine protected areas improve reef resilience? Annu. Rev. Mar. Sci. 11, 307-334. ( 10.1146/annurev-marine-010318-095300) [DOI] [PubMed] [Google Scholar]
- 18.Schiel DR. 2013. The other 93%: trophic cascades, stressors and managing coastlines in non-marine protected areas. New Zealand J. Mar. Freshw. Res. 47, 374-391. ( 10.1080/00288330.2013.810161) [DOI] [Google Scholar]
- 19.Barbier M, Loreau M. 2018. Pyramids and cascades: a synthesis of food chain functioning and stability. Ecol. Lett. 22, 405-419. ( 10.1111/ele.13196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sale PF, et al. 2005. Critical science gaps impede use of no-take fishery reserves. Trends Ecol. Evol. 20, 74-80. ( 10.1016/j.tree.2004.11.007) [DOI] [PubMed] [Google Scholar]
- 21.Parnell PE, Dayton PK, Lennert-Cody CE, Rasmussen LL, Leichter JJ. 2006. Marine reserve design: optimal size, habitats, species affinities, diversity, and ocean microclimate. Ecol. Appl. 16, 945-962. ( 10.1890/1051-0761(2006)016[0945:MRDOSH]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 22.Berriman JS, Kay MC, Reed DC, Rassweiler A, Goldstein DA, Wright WG. 2015. Shifts in attack behavior of an important kelp forest predator within marine reserves. Mar. Ecol. Prog. Ser. 52, 193-201. ( 10.3354/meps11157) [DOI] [Google Scholar]
- 23.Shears N, Kushner D, Katz S, Gaines S. 2012. Reconciling conflict between the direct and indirect effects of marine reserve protection. Environ. Conserv. 39, 225-236. ( 10.1017/S0376892912000082) [DOI] [Google Scholar]
- 24.Graham MH. 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7, 341-357. ( 10.1007/s10021-003-0245-6) [DOI] [Google Scholar]
- 25.Estes JA, Palmisano JF. 1974. Sea otters: their role in structuring nearshore communities. Science 185, 1058-1060. ( 10.1126/science.185.4156.1058) [DOI] [PubMed] [Google Scholar]
- 26.Kay MC, Lenihan HS, Guenther CM, Wilson JR, Miller CJ, Shrout SW. 2012. Collaborative assessment of California spiny lobster population and fishery responses to a marine reserve network. Ecol. Appl. 22, 322-335. ( 10.1890/11-0155.1) [DOI] [PubMed] [Google Scholar]
- 27.Hamilton SL, Caselle JE. 2015. Exploitation and recovery of a sea urchin predator has implications for the resilience of southern California kelp forests. Proc. R. Soc. B 282, 20141817. ( 10.1098/rspb.2014.1817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eurich J, Selden R, Warner RR. 2014. California spiny lobster preference for urchins from kelp forests: implications for urchin barren persistence. MEPS 498, 217-225. ( 10.3354/meps10643) [DOI] [Google Scholar]
- 29.Selden R, Gaines SD, Hamilton SL, Warner RR. 2017. Protection of large predators in a marine reserve alters size-dependent prey mortality. Proc. R. Soc. B 284, 20161936. ( 10.1098/rspb.2016.1936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushner DJ, Rassweiler A, McLaughlin JP, Lafferty KD. 2013. A multi-decide time series of kelp forest community structure at the California Channel Islands. Ecology 94, 2655. ( 10.1890/13-0562R.1) [DOI] [Google Scholar]
- 31.Byrnes J, Stachowicz JJ, Hultgren KM, Randall Hughes A, Olyarnik SV, Thornber CS. 2006. Predator diversity strengthens trophic cascades in kelp forests by modifying herbivore behaviour. Ecol. Lett. 9, 61-71. [DOI] [PubMed] [Google Scholar]
- 32.Halpern BS, Cottenie K, Broitman BR. 2006. Strong top-down control in southern California kelp forest ecosystems. Science 312, 1230-1232. ( 10.1126/science.1128613) [DOI] [PubMed] [Google Scholar]
- 33.Kalvass PE, Hendrix JM. 1997. The California red sea urchin, Strongylocentrotus fanciscanus, fishery: catch, effort, and management trends. Mar. Fish. Rev. 59, 1-17. [Google Scholar]
- 34.Teck SJ, Lorda J, Shears NT, Bell TW, Cornejo-Donoso J, Caselle JE, Hamilton SL, Gaines SD. 2017. Disentangling the effects of fishing and environmental forcing on demographic variation in an exploited species. Biol. Cons. 209, 488-498. ( 10.1016/j.biocon.2017.03.014) [DOI] [Google Scholar]
- 35.Laundré JW, Hernández L, Ripple WJ. 2010. The landscape of fear: ecological implications of being afraid. Open Ecol. J. 3, 1-7. ( 10.2174/1874213001003030001) [DOI] [Google Scholar]
- 36.Lima SL. 1998. Nonlethal effects in the ecology of predator-prey interactions: what are the ecological effects of anti-predator decision-making? BioScience 48, 25-34. ( 10.2307/1313225) [DOI] [Google Scholar]
- 37.Schmitz OJ, Krivan V, Ovadia O. 2004. Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol. Lett. 7, 153-163. ( 10.1111/j.1461-0248.2003.00560.x) [DOI] [Google Scholar]
- 38.Freeman A. 2005. Size-dependent trait-mediated indirect interactions among sea urchin herbivores. Behav. Ecol. 17, 182-187. ( 10.1093/beheco/arj014) [DOI] [Google Scholar]
- 39.Herrlinger TJ. 1983. The diet and predator–prey relationships of the sea star Pycnopodia helianthoides from a central California kelp forest. MS thesis, San Jose State University, Moss Landing, San Jose, CA. [Google Scholar]
- 40.Lafferty KD. 2004. Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. App. 14, 1566-1573. ( 10.1890/03-5088) [DOI] [Google Scholar]
- 41.Cowen RK. 1983. The effects of sheephead (Semicossyphus pulcher) predation on red sea urchin (Strongylocentrotus franciscanus) populations: an experimental analysis. Oecologia 58, 249-255. ( 10.1007/BF00399225) [DOI] [PubMed] [Google Scholar]
- 42.Fitzgerald SP. 2019. Collaborative research and data-limited assessment of small-scale trap fisheries in the Santa Barbara Channel. PhD thesis, University of California, Santa Barbara, CA. [Google Scholar]
- 43.Babcock EA, Shears NT, Alcala AC, Barrett NS, Edgar GJ, Lafferty KD, Mcclanahan TR, Russ GR. 2010. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl Acad. Sci. USA 107, 18 256-18 261. ( 10.1073/pnas.0908012107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowen RK. 1986. Site-specific differences in the feeding ecology of the California sheephead, Semicossyphus pulcher (Labridae). Environ. Biol. Fish 16, 193-203. ( 10.1007/BF00005171) [DOI] [Google Scholar]
- 45.Hamilton SL, Newsome SD, Caselle JE. 2014. Dietary niche expansion of a kelp forest predator recovering from intense commercial exploitation. Ecology 95, 164-172. ( 10.1890/13-0014.1) [DOI] [PubMed] [Google Scholar]
- 46.Dunn RP, Hovel KA. 2019. Experiments reveal limited top-down control of key herbivores in southern California kelp forests. Ecology 100, e02625. ( 10.1002/ecy.2625) [DOI] [PubMed] [Google Scholar]
- 47.Tegner MJ, Levin LA. 1983. Spiny lobsters and sea urchins: analysis of a predator–prey interaction. J. Exp. Mar. Biol. Ecol. 73, 125-150. ( 10.1016/0022-0981(83)90079-5) [DOI] [Google Scholar]
- 48.Diaz-Arredondo MA, Guzmán-de-Próo SA. 1995. Feeding habits of the spiny lobster (Panulirus interruptus Randall, 1840) in Bahia Tortugas, Baja California Sur. Ciencias Marinas 21, 439-462. ( 10.7773/cm.v21i4.1000) [DOI] [Google Scholar]
- 49.Winget RR. 1968. Trophic relationships and metabolic energy budget of the California spiny lobster, Panulirus interruptus (Randall). PhD thesis, San Diego State College, San Diego, CA. [Google Scholar]
- 50.Schiel DR, Foster MS. 2015. The biology and ecology of giant kelp forests. Berkeley, CA: University of California Press. [Google Scholar]
- 51.Morton DN. 2020. The effects of parasites on the kelp-forest food web. PhD thesis, University of California, Santa Barbara, CA. [Google Scholar]
- 52.Hart DR. 2002. Intraguild predation, invertebrate predators, and trophic cascades in lake food webs. J. Theor. Biol. 218, 111-128. ( 10.1006/jtbi.2002.3053) [DOI] [PubMed] [Google Scholar]
- 53.Finke DL, Denno RF. 2004. Predator diversity dampens trophic cascades. Nature 429, 407-410. ( 10.1038/nature02554) [DOI] [PubMed] [Google Scholar]
- 54.Jiao J, Pilyugin SS, Osenberg CW. 2016. Random movement of predators can eliminate trophic cascades in marine protected areas. Ecosphere 7, e01421. ( 10.1002/ecs2.1421) [DOI] [Google Scholar]
- 55.Estes JA, Duggins DO, Rathbun GB. 1989. The ecology of extinctions in kelp forest communities. Conserv. Biol. 3, 252-264. ( 10.1111/j.1523-1739.1989.tb00085.x) [DOI] [Google Scholar]
- 56.Jackson JB, et al. 2001. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629-637. ( 10.1126/science.1059199) [DOI] [PubMed] [Google Scholar]
- 57.Foster MS, Schiel DR. 2010. Loss of predators and the collapse of southern California kelp forests (?): alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 393, 59-70. ( 10.1016/j.jembe.2010.07.002) [DOI] [Google Scholar]
- 58.Foster MS. 1990. Organization of macroalgal assemblages in the Northeast Pacific: the assumption of homogeneity and the illusion of generality. Hydrobiologia 192, 21-33. ( 10.1007/BF00006225) [DOI] [Google Scholar]
- 59.Filbee-Dexter K, Scheibling RE. 2014. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar. Ecol. Prog. Ser. 495, 1-25. ( 10.3354/meps10573) [DOI] [Google Scholar]
- 60.Scheibling RE. 1996. The role of predation in regulating sea urchin populations in eastern Canada. Oceanol. Acta 19, 421-430. [Google Scholar]
- 61.Steneck R, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ. 2002. Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ. Conserv. 29, 436-459. ( 10.1017/S0376892902000322) [DOI] [Google Scholar]
- 62.Ling SD, Johnson CR, Frusher SD, Ridgway KR. 2009a. Overfishing reduces resilience of kelp beds to climate-driven catastrophic phase shift. Proc. Natl Acad. Sci. USA 106, 22 341-22 345. ( 10.1073/pnas.0907529106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smale DA, Burrows MT, Moore P, O'Connor N, Hawkins SJ. 2013. Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecol. Evol. 3, 4016-4038. ( 10.1002/ece3.774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smale DA, Moore PJ. 2017. Variability in kelp forest structure along a latitudinal gradient in ocean temperature. J. Exp. Mar. Biol. Ecol. 486, 255-264. ( 10.1016/j.jembe.2016.10.023) [DOI] [Google Scholar]
- 65.Castilla JC, Moreno CA. 1982. Sea urchins and Macrocystis pyrifera: experimental test of their ecological relations in southern Chile. In Proc. of the International Echinoderm Conf., 1982 June, Tampa Bay, Florida, USA (ed. Lawrence JM), pp. 257-263. [Google Scholar]
- 66.Santelices B, Ojeda FP. 1984. Population dynamics of coastal forests Macrocystis pyrifera in Puerto Toro, Isla Navarino, Southern Chile. Mar. Ecol. Prog. Ser. 14, 175-183. ( 10.3354/meps014175) [DOI] [Google Scholar]
- 67.Vanderklift MA, Kendrick GA. 2005. Contrasting influence of sea urchins on attached and drift macroalgae. Mar. Ecol. Prog. Ser. 299, 101-110. ( 10.3354/meps299101) [DOI] [Google Scholar]
- 68.Terborgh J, Estes JA. 2010. Trophic cascades: predators, prey, and the changing dynamics of nature. Washington, DC: Island Press. [Google Scholar]
- 69.Trites AW, Christensen V, Pauly D. 2006. Effects of fisheries on ecosystems: just another top predator? In Top predators in marine ecosystems, pp. 11-27. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 70.Lamy T, Koenigs C, Holbrook SJ, Miller RJ, Stier AC, Reed DC. 2020. Foundation species promote community stability by increasing diversity in a giant kelp forest. Ecology 101, e02987. ( 10.1002/ecy.2987) [DOI] [PubMed] [Google Scholar]
- 71.R Core Team. 2018. R: a language and environment for statistical computing, version 3.5.1. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- 72.Reed DC, Nelson JC, Harrer SL, Miller RJ. 2016. Estimating biomass of benthic kelp forest invertebrates from body size and percent cover data. Mar. Biol. 163, 101. ( 10.1007/s00227-016-2879-x) [DOI] [Google Scholar]
- 73.Underwood AJ. 1993. The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. Austral. J. Ecol. 18, 99-116. ( 10.1111/j.1442-9993.1993.tb00437.x) [DOI] [Google Scholar]
- 74.Schwarz CJ. 2015. Analysis of BACI experiments. Course notes for beginning and intermediate statistics. See http://www.stat.sfu.ca/~cschwarz/CourseNotes. Retrieved, pp. 08–20.
- 75.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1-48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 76.Lenth R. 2020. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.4.5. https://CRAN.R-project.org/package=emmeans.
- 77.SCB Marine Biodiversity Observation Network, Miller R, Rassweiler A, Caselle J, Kushner D, Reed D, Lafferty K, Kui L, O'Brien M. 2021. Santa Barbara Channel Marine BON: Nearshore kelp forest integrated quad and swath cover, 1980-ongoing ver 3. Environmental Data Initiative. ( 10.6073/pasta/85a8f46dabe413973195c8530911088a) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- SCB Marine Biodiversity Observation Network, Miller R, Rassweiler A, Caselle J, Kushner D, Reed D, Lafferty K, Kui L, O'Brien M. 2021. Santa Barbara Channel Marine BON: Nearshore kelp forest integrated quad and swath cover, 1980-ongoing ver 3. Environmental Data Initiative. ( 10.6073/pasta/85a8f46dabe413973195c8530911088a) [DOI]
Supplementary Materials
Data Availability Statement
Data on all organism counts can be found via SBC MBON [77]. All the data for this paper including size information are also publicly available through the Channel Islands National Park repository via the National Parks Service. Code for this project can be accessed online at github.com/kmalakhoff/urchin-baci/.