Abstract
This article is part of the theme issue ‘Basal cognition: multicellularity, neurons and the cognitive lens'.
Keywords: cognition, developmental biology, neuroscience, non-neural cognition
1. Introduction
What are the essential features of brains that make them the canonical instantiators of cognition? Obviously, human beings and many other animals have them, but how many neurons, how many neuronal cell types and how much structure are necessary for a proper brain, non-metaphorically speaking? And why should we think that one is necessary, in order for an organism to become familiar with, value and explore, exploit and evade features of an environment to keep itself alive, growing and (with luck) reproducing? Many have wrestled with such questions, which remain important knowledge gaps. One very useful contribution of the emerging field of basal cognition, we believe, is the potential to identify capacities for knowing and navigating that can be found in organisms that do not have brains, or even nervous systems.
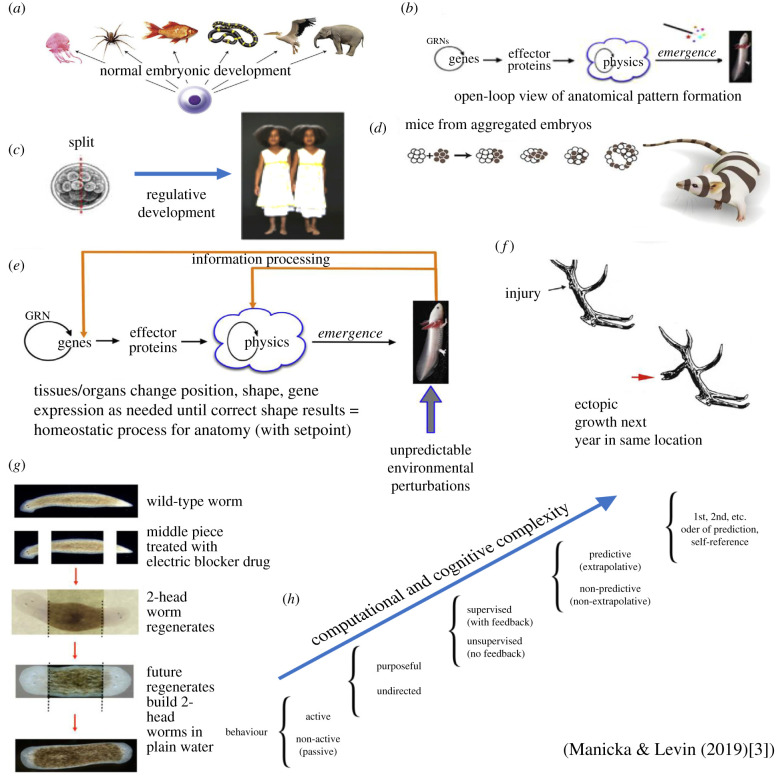
Evidence that characteristics frequently cited for the cognitive indispensability of brains can be crossed off the list comes from two basic streams of work, one functional, the other mechanistic. First, functional data on aneural systems show that the cognitive operations we usually ascribe to brains—sensing, information processing, memory, valence, decision making, learning, anticipation, problem solving, generalization and goal directedness—are all observed in living forms that don't have brains or even neurons [1,2] (figure 1a–g). Indeed, the great variety of such systems suggests not a binary dichotomy of cognitive versus mechanical but, rather, a continuum of cognition from modest to complex (figure 1h). Second, phylogenetic data reveal that the molecular machinery we associate with traditional brain-based cognition—ion channels, neurotransmitters, synaptic proteins, networks and circuits, oscillatory activity—are present not only in aneural animals but also in our closest unicellular relatives, and many are also found in a wide variety of extant unicellular organisms and in plants.
Figure 1.
Cognitive processes in embryogenesis and regeneration. (a) An egg will reliably give rise to a species-specific anatomical outcome. (b) This process is usually described as a feed-forward system where the activity of gene-regulatory networks (GRNs) within cells results in the expression of effector proteins that, via structural properties of proteins and physical forces, will result in the emergence of complex shape. This class of models (bottom-up process driven by self-organization and parallel activity of large numbers of local agents) is difficult to apply to several biological phenomena. Regulative development can alter subsequent steps to reach the correct anatomical goal state despite drastic deviations of the starting state. (c) For example, mammalian embryos can be divided in half, giving rise to perfectly normal monozygotic twins each of which has regenerated the missing cell mass. (d) Mammalian embryos can also be combined, giving rise to a normal embryo in which no parts are duplicated. (e) Such capabilities suggest that pattern control is fundamentally a homeostatic process—a closed-loop system using feedback to minimize the error (distance) between a current shape and a target morphology. Although these kinds of decision-making models are commonplace in engineering, they are only recently beginning to be employed in biology…. This kind of pattern-homeostatic process must store a setpoint that serves as a stop condition; however, as with most types of memory, it can be specifically modified by experience. (f) In the phenomenon of trophic memory, damage created at a specific point on the branched structure of deer antlers is recalled as ectopic branch points in subsequent years’ antler regeneration. This reveals the ability of cells at the scalp to remember the spatial location of specific damage events and alter cell behaviour to adjust the resulting pattern appropriately—a pattern memory that stretches across months of time and considerable spatial distance and is able to modify low-level (cellular) growth rules to construct a pre-determined stored pattern that differs from the genome-default for this species. (g) A similar capability was recently shown in a molecularly tractable model system…, in which genetically normal planarian flatworms were bioelectrically reprogrammed to regenerate two-headed animals when cut in subsequent rounds of asexual reproduction in plain water. (h) The decision making revealed by the cells, tissues and organs in these examples of dynamic remodelling toward specific target states could be implemented by cybernetic processes at various positions along a scale of proto-cognitive complexity…. See [3] for full legend of figure 1.
In the first part of this basal cognition theme issue, we concentrated on unicellular organisms. Here, we build on this foundation to cover critical issues in the scaling of cognition [4] from humble unicellulars to complex animals, focusing on evolutionary changes in molecular mechanisms, architecture and function that have accompanied the evolution of the nervous system. Together, we will approach crucial questions regarding the yet poorly understood and unsolved link between the evolutionary emergence of the nervous system and the concomitant rise in cognitive complexity. What was the structure that henceforth deserved to be called a nervous system once it was in place? What function did it enable that was not possible before and how does this relate to cognition?
Strikingly, the narrative revealed by recent work, ranging from plant biology to developmental genetics, highlights the value of neuroscience to understanding the evolution of cognition that goes well beyond a particular class of cells (neurons) to a variety of important fundamental concepts that are proving their capacity to enlighten diverse fields, such as (but not limited to) regenerative biology (see Bentley group's article on angiogenesis, this issue [5]). A key philosophical idea, borrowed from computer science, is substrate independence: as long as the components of a living system can carry out appropriate, clearly specified cognitive functions, deep ideas can be readily ported from neuroscience and applied to diverse—including, as articles in this issue demonstrate, non-neural—implementations [6–8]. Indeed, ‘multiple realizability’ (in its biological forms) is the single tenet of computational functionalism, the philosophy that dominated cognitive science in the latter half of the twentieth century, which appears to have survived the fusillade of critique in the past two decades from even its strongest advocates (e.g. [9,10]).
The developments described in these two issues not only raise critical questions about what's so special about a brain, but also about what constitutes the defining characteristics of neurons, other than their speed-optimizing properties. Bioelectric computation is carried out by all cell networks, including bacterial cell networks [11]. The molecular phylogeny of ‘neuronal’ proteins extends to aneural animals (see Moroz et al. [12]) and unicellular organisms (see Göhde et al. [13]). Furthermore, most of the properties of neurons can be found in cell types such as skin, bone, pancreas and even tumours [14].
Similarly, basal cognition can be expected to open new ways of investigating what nervous systems and brains do, the better to articulate how relevant functions are implemented. The basal cognition approach can be expected to uncover the mechanistic details of a substantial range of biological phenomena to help provide the in-depth understanding of how complex brains work that is needed to develop therapeutic interventions in a wide variety of areas. For example, networks and circuits have been proposed as the new fundamental unit of brain function that will be key to understanding how neural activity generates animal behaviour in real time [15]. Research into how large, connected arrays of chemotaxis proteins function as information processing networks and circuits in the generation of (perhaps surprisingly) bacterial behaviour holds considerable promise for understanding general principles underlying these mechanisms, both descriptively as existentially critical biological phenomena and as models for computational approaches (see, for example, [16,17]).
The challenge for the field of basal cognition—accepted by the contributors to this theme issue, who address key innovations along the evolutionary continuum of cognition—is to reveal the gradualism of cellular properties underwriting this critical biological function to leverage an understanding of the clear phase transitions observed in cognitive capacities across the web of life, of which the origin and development of nervous systems is (so far) the most dramatic example.
2. Obligate multicellularity and the signals that turn societies into individuals
While there are many different instances of multicellular behaviour among unicellulars, only a few evolutionary lineages led to obligatory forms of highly differentiated and integrated multicellularity [18,19]. This section contains three papers that focus on two of these rare lineages: plants and animals. In both cases, large numbers of differentiated cells, each playing a wide variety of roles within the collective, are physically and organizationally tied together into units that constitute a new kind of individual. Many of these multicellular individuals have evolved into complex macroscopic structures, orders of magnitude larger than their cellular building blocks. Externally, these new individuals may look just like oversized unicellular individuals, but internally they consist of complex and new self-constructed environments. The constitutive cells must both create and maintain this environment and, in general, act in ways that benefit this whole. Both the global orchestration of the activity of these inner multitudes—involving what we can call the organizing bureaucracy—as well as the activities of the ‘inbodied’ individual cells provide major targets for basal cognition [20]. The papers in this section focus on the global level and the signalling that transform these multicellulars into such new individuals.
For the record, the fact that we do not have a paper dedicated to fungi, the third lineage exhibiting highly differentiated and integrated multicellularity, does not mean that this group falls outside the scope of basal cognition. On the contrary, there are many examples of phenomena that fit the domain of basal cognition [21]. The tips of fungal hyphae, the tubular filaments that are the basic growth form of a fungus, invade substrates and show directional growth that is sensitive to various environmental circumstances [22]. Fungal mycelia, the network constituted by these hyphae, exhibit sensing, decision making and intercellular communication systems to forage for scarce resources—which can include carnivorous behaviour [23,24]—and to distribute these resources across the mycelium [25,26]. There are also symbiotic relations with plant roots, which involve specific forms of communication and even specialized interface structures [27]. In all this, electricity acts as a key factor to shape growth and development [28,29], and evidence for action potential-like spikes goes back a long time [30,31]. When it comes to basal cognition, it is plausible that fungi are at least on a par with plants, even when focused research on such phenomena has not yet been pursued equally vigorously in the fungal domain [21], something that may now be changing rapidly [32].
That plants exhibit a broad range of features that fit the cognitive toolkit has been argued for quite some time and is now increasingly accepted, a change in which František Baluška and Stefano Mancuso [33] played a prominent role [34–36]. Here they discuss the ways in which plants can act as individuals by the use of synaptic-like adhesion domains, as well as action potentials and other forms of long-distance signalling. Plants face specific challenges when it comes to multicellular individuality, most notably living in two very different environments at once—above and below ground—and the need to integrate the parts inhabiting these environments. They are also sessile and physically extend far into their environments, which provide a range of specific self/non-self issues that they must deal with and do so in various ways.
The next two papers develop new ideas concerning the potential precursor system of animal nervous systems. Both posit a key role for signal diffusion through an extracellular fluid (volume transmission) to effect changes in nearby cells (paracrine signalling) to thus enable complex, integrated and potentially large multicellular bodies.
Gáspár Jékely [37] develops his chemical brain hypothesis for the origin of nervous systems by exploring a scenario where chemically organized cellular networks appeared before synapses and nervous systems emerged in evolution. The idea builds on the widespread use of signalling using peptides, small chains of amino acids, in all animals except sponges (but see also [38]). He observes that nervous systems have two modes of transmission to propagate activity between cells: synapses that require close contact between sender and receiver, and volume transmission that uses diffusible chemical signals, which can travel substantial distances between sender and receiver. Small peptides are ideal messengers in such chemical networks because of their unlimited diversity, high diffusivity and ease of production. Jékely argues that an initial chemical network would become slow and inefficient in larger bodies, a problem that could have been overcome when peptidergic cells evolved projections and became synaptically connected networks. The evolution of circulatory systems would also have helped to facilitate chemical signalling by diffusion, leading to the use of both systems in extant animals.
Moroz et al. [12] also discuss the role that transmitter signalling might have played in an early stage of animal and nervous system evolution via alternative integrative systems. They suggest that ‘transmitters made the nervous system by integrating ancestral populations of secretory cells for behavioural coordination without synapses’. This idea is supported and further developed while discussing an interesting sequence of different but closely related topics. A bird's eye view of the list gives a good impression of the range of the paper. The authors start by accenting the organizational differences between the nervous systems of the three most basal lineages possessing them (ctenophores, cnidaria and simple bilaterians), and discuss ‘the battle of spikes and soups', more prosaically, a controversy over electrical versus chemical signalling. They question why so many different transmitters exist, and stress the evolutionary role of injury/regeneration signalling. Finally, they elaborate on the parallel recruitment of different non-neural transmitters to neuronal functions in lineage-specific ways and suggest that modern-day Placozoa are the closest living representative of the primordial organization they propose.
Both papers address the origin(s) of nervous systems, but the perspectives provided do not centre on nervous systems as exceptional forms of organization. Rather, they position and help to reinterpret nervous systems as a variation of more basic biological signalling systems. These papers provide an antidote for the still-widespread assumption that brain-based nervous systems stand out and apart from the body.
3. Origins and evolution of nervous systems
Regardless whether it happened once, twice or even several times in independent lineages, the origin of the nervous system can be regarded as the game changer in the evolution of cognition. Yet, despite more than 150 years of comparative research on animals with simple nervous systems, such as cnidarians and ctenophores, and on animals that appear not to possess a nervous system, such as sponges and the placozoans, it is not clear what exactly happened during this step, both structurally and functionally. What did this early system look like and what was its function?
This section contains four papers that approach the non-neural-to-neural transition from different angles. Each of them discusses an important novelty that came with the nervous system at Precambrian times and enabled the subsequent explosion of body plan and behavioural complexity in the Cambrian. Topics range from the assembly of the synaptic machinery, a review of the 150 years of debate on nervous system origins, the relevance of reafference—self-induced sensing—for the evolution of the neural organization, and, finally the boost in learning capacities via habituation and sensitization as a key to improving cognitive capacities.
The first article in this section addresses the evolutionary origin of the nervous system from a strictly molecular point of view, focusing on the assembly of the synaptic machinery as a prerequisite for the non-neural-to-neural transition. Overcoming the diffusion limit of volume transmission, the synapse allowed more targeted and much more rapid communication between cells (which is essential for the nerve net, see below), and its plasticity provided a new form of intercellular memory specific to these connections (which is the prerequisite for learning and reafference, see below). In an original research contribution, Burkhardt and collaborators [13] address the evolutionary origin of the synapse, focusing on synaptic vesicles. Comparing the core vesicle proteome between metazoan and non-metazoan species, they find that the choanoflagellates have a rather complete complement of vesicle proteins, including important components such as the vesicular membrane-associated SNARE protein synaptobrevin. Localization of this protein via immunocytochemistry reveals a polarized distribution of different kinds of vesicles, some of which are secreted apically and others basally. This is reminiscent of the targeted localization of vesicles in the synapse. Based on these similarities, the authors develop an evolutionary model of how synaptic targeting of vesicles in the first nervous systems may have evolved from the situation in unicellular eukaryotes.
To this day, animals exist that have never acquired a nervous system; others show non-centralized, simple nervous systems in the form of diffuse nerve nets. Their existence has always inspired comparative neurobiologists to put forward hypotheses about the evolutionary transition from the non-neural to neural. Starting more than 150 years ago, this led to the notion that the first manifestation of the nervous system was a simple vertical sensory-effector arc—most prominently in Parker's ‘elementary nervous system’ [39]. In a comprehensive history of thought in this domain, Arendt [40] surveys the long path from these initial ideas to modern hypotheses on nervous system origins, culminating in the consensus that the early nervous system was indeed an elementary nerve net. These nets acted as endogenous pacemakers and integrated sensory input for the coordinated control of entire downstream effector tissues—which may have been contractile or bearing motile cilia. This leads to two alternative views of how nerve nets might have emerged: following the contractile network hypothesis, the elementary nerve net originated by the division of labour from a network of multipolar conductive and contractile cells; or, following the neurosecretory network hypothesis, it may have formed from overlapping lateral processes of sensory-neurosecretory cells (as put forward by Jékely [37]). The paper ends with the exciting conclusion that modern comparative molecular and single-cell data support both hypotheses, which lends support to the notion that nerve nets may have indeed evolved more than once, in parallel, consecutively, or even in independent evolutionary lineages.
Jékely et al. [41] also focus on the functionality of early nervous systems but address a different aspect. Their paper addresses the role of reafference, which consists of the systemic influences on the senses of an organism by the active movement or deformation generated by the organism itself. Reafference is a basic feature of self-moving animals, providing a mechanism to anticipate sensory changes and create useful signals, such as in the optic flow produced by forward motion. In a way, reafference reverses the standard causal path from sensors to effectors, enabling useful options for external feedback control. The authors distinguish between reafference relating to body deformations, and changes that involve translocations or other movement with respect to an external medium or field. They survey basic and often-neglected forms of reafference including gravity sensing, flow sensing and proprioception. Reafference is linked to a basic form of self, which here is called the body-self, a particular organization capable of motility and sensing tied together through reafference.
The grand finale of this section on the origin of nervous systems is the contribution of Ginsburg & Jablonka [42]. They envisage a unifying theme for all major steps of nervous system evolution, from aneural to neural, and from nerve net to centralized nervous systems with the brain. Each step entails a key transition in learning and memory: first, the acquisition of the nerve net enabled habituation and sensitization on an unprecedented level; second, the evolution of a central nervous system and brain brought about within-lifespan associative learning. Regarding the first transition, key innovations of the incipient nerve net were the targeted axonal connections, the action potentials as a new currency in information transfer, electrical transmission and, most important, synaptic plasticity as a new means of information storage. This plasticity allowed activity-dependent strengthening and weakening of the synapse, as a prerequisite for habituation and sensitization in the context of now-possible complex coordinated activities. Finally, Ginsburg & Jablonka [42] describe how the centralization of the nervous system into nerve cord and brain not only initiated a revolution in associative learning, but also set the stage for an extended perception of self as a kick-start for the immensely successful evolutionary radiation of the bilaterians. From then onwards, organisms were able to distinguish between ‘body-self’ and ‘world-self’.
4. The cognitive lens
The typical context for cognition is the processing of information to guide an organism in three-dimensional space. However, this function is the result of a clever pivot by the evolution of a much more ancient system: the guidance of body structure in morphospace [43]. All cells cooperate together via electrical and chemical networks to coordinate their activity toward anatomical goals at the tissue and organ levels [4]. The recognition that concepts from traditional cognitive science naturally transfer to other aspects of biology besides brain-driven behaviours is powerful.
First, it allows the application of Marr's levels of analysis [44] to cells, tissues and organs—it gives us explanatory power at levels beyond the molecular and allows access to reasons, as well as causes, of cell- and tissue-level decision making. Neuroscience is comfortable with the need for multi-scale explanations of bioelectrical activity and its semantics—memories, plans, comparisons, goals, representations, visual illusions, etc., are as important as molecular details of synapses. Developmental biology however has, heretofore, largely been focused on one level—molecules—despite warnings that there is no philosophically privileged level of causation [45] and recent progress in information theory that shows this level not to always be the one with maximal causal power [46]. The emerging field of basal cognition suggests a rich toolkit, such as psychophysics, active inference and models of representation and encoding [47,48], which can be applied to understand the remarkable goal directedness of regulative embryogenesis and regeneration (figure 1).
The paper by Levin and colleagues in this issue [49] shows an example of this approach, using the concepts of perceptual bistability to understand an extremely puzzling regenerative phenotype. Planarian flatworms, which normally regenerate after amputations with 100% fidelity, can be driven into a permanently destabilized state by transient modulation of their body-wide bioelectric circuit. This alteration of the bioelectrically encoded setpoint of their anatomical homeostatic machinery causes planarian fragments to randomly regenerate with 1 or 2 heads after amputation (despite their wild-type genetics) [50]. It illustrates a number of aspects relevant to cognition and implemented by bioelectric circuits: the ability to store a memory (anatomical, not behavioural), the ability to store new information on the same ‘hardware’ (re-writing the target morphology without genetically re-wiring the cells) and the ability to represent counterfactual states, in this case, a two-headed pattern stored in a one-headed body, corresponding not to the current anatomy but to the anatomy that they will build if they get injured in the future. Moreover, unlike permanently two-headed worms [51], these worms exist in a bistable state that can readily (and stochastically) flip between two representations of what anatomy to build following damage—a model that suggests numerous additional experiments driven by the considerable knowledge of perceptual theory.
In addition to explaining the data of cell and developmental biology, an important aspect of a successful conceptual approach is the degree of control it confers on the system in question. Increased control, for example, top-down induction of specific anatomical outcomes, would be a very welcome addition to regenerative medicine, which is facing a complexity crisis. Even when all the problems of stem cell biology and genomic editing are solved, restoring a human hand or eye will be little closer to attainment because of the immense complexity. Controlling such systems from the bottom-up is very challenging, but cognitive approaches suggest new strategies. For example, exploiting triggers of modular responses, re-writing goal states without rewiring cellular hardware and motivating tissue decision making instead of micromanagement [52].
One of the key components of this field is learning to control the collective decisions cells make during morphogenesis. In this issue, a team from the Bentley laboratory at the Crick Institute (UK) shows the application of techniques from computational neuroscience to analyse cell-level behaviour as a form of active perception [6]. Specifically, they shed new light on the process by which zebrafish endothelial cells control their filopodia (exploratory protrusions of their cell membrane) to establish ‘leaders’ and migration patterns that enable them to navigate the in vivo environment during blood vessel formation. They establish angiogenesis as a novel, highly tractable model for basal cognition research that should have myriad implications for biomedical strategies aimed at influencing blood vessel formation by affecting the decision making of individual cells as well as of the collective. They conclude with a rigorous discussion considering the tools and payoffs associated with cognitive models versus alternative mainstream perspectives — a critical task for ensuring that the appropriateness of the application of cognitive concepts is decided on an empirical, case-by-case basis.
5. Conclusion
The papers of this extended theme issue set the stage for an entirely new view on cognition, which did not—as commonly assumed—start with the nervous system, but evolved with it, in many steps and elaborations on a theme. Fascinating links have emerged between the birth and step-wise complexification of the nervous system, from nerve nets to centralized nervous systems and brains, and transitions in cognition, from simple forms of epigenetic learning to habituation and sensitization and finally associative learning. Although the establishment of new eukaryote and animal models and the forthcoming work in synthetic bioengineering are poised to produce numerous model systems for experimental study of novel, primitive bodies and their basal cognition, so far, these links remain mostly hypothetical owing to the absence of hard experimental data. This extended theme issue is meant to be a call to actively change this state of affairs and turns an incipient new discipline into an experimental field. Indeed, a flurry of new molecular techniques, such as single-cell genomics and imaging, metabolomics and proteomics, coupled with an increasing ease of transfer of neurobiological and behavioural studies to new systems, opens up the possibility of developing new experimental models, from unicellular prokaryotes and eukaryotes to plants, fungi, choanoflagellates, sponges, ctenophores, cnidarians and flatworms. The nuts and bolts of organismal cognition reveal themselves, as we embrace the diversity of both experimental model systems and the conceptual approaches used to understand them.
Contributor Information
Michael Levin, Email: michael.levin@tufts.edu.
Detlev Arendt, Email: arendt@embl.de.
Data accessibility
This article has no additional data.
Authors' contributions
All authors contributed to writing this article.
Competing interests
We declare we have no competing interests.
Funding
The guest editors and many of the contributors to this theme issue are indebted to the Konrad Lorenz Institute for Evolution and Cognition Research (KLI) for hosting the 'The Groundfloor of Cognition' workshop at Klosterneuburg (AT) in June 2018, which gave birth to this work, and to Eva Jablonka for priceless help and guidance in getting the workshop off the ground. Thanks also to Helen Eaton for her unflappable calm, professionalism and assistance throughout the publication process. Pamela Lyon warmly thanks Richard Bradshaw, whose support makes her work possible. Michael Levin gratefully acknowledges support of the Barton Family Foundation, the Elisabeth Giauque Trust and the Templeton World Charity Foundation.
References
- 1.Baluška F, Levin M. 2016. On having no head: cognition throughout biological systems. Front. Psychol. 7, 902. ( 10.3389/fpsyg.2016.00902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyon P. 2015. The cognitive cell: bacterial behaviour reconsidered. Front. Microbiol. 6, 264. ( 10.3389/fmicb.2015.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manicka s, Levin M. 2019. The Cognitive Lens: a primer on conceptual tools for analysing information processing in developmental and regenerative morphogenesis. Phil. Trans. of R. Soc. B 374, 20180369. ( 10.1098/rstb.2018.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levin M. 2019. The computational boundary of a ‘Self’: developmental bioelectricity drives multicellularity and scale-free cognition. Front. Psychol. 10, 2688. ( 10.3389/fpsyg.2019.02688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zakirov B, Charalambous G, Thuret R, Aspalter IM, Van-Vuuren K, Mead T, Harrington K, Regan ER, Herbert SP, Bentley K. 2021. Active perception during angiogenesis: filopodia speed up Notch selection of tip cells in silico and in vivo. Phil. Trans. R. Soc. B 376, 20190753. ( 10.1098/rstb.2019.0753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchland PS, Sejnowski TJ. 2017. The computational brain, 25th Anniversary edition. Cambridge, MA: The MIT Press. [Google Scholar]
- 7.Bronfman ZZ, Ginsburg S, Jablonka E. 2016. The transition to minimal consciousness through the evolution of associative learning. Front. Psychol. 7, 1954. ( 10.3389/fpsyg.2016.01954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beer RD. 2014. The cognitive domain of a glider in the game of life. Artif. Life 20, 183-206. ( 10.1162/ARTL_a_00125) [DOI] [PubMed] [Google Scholar]
- 9.Churchland PM. 2005. Functionalism at forty: a critical retrospective. J. Phil. CII 102, 33-50. ( 10.5840/jphil2005102136) [DOI] [Google Scholar]
- 10.Fodor J. 2000. The mind doesn't work that Way, The scope and limits of computational psychology. Cambridge, MA: MIT Press/Bradford. [Google Scholar]
- 11.Lyon P, Keijzer F, Arendt D, Levin M. 2021. Reframing cognition: getting down to biological basics. Phil. Trans. R. Soc. B 376, 20190750. ( 10.1098/rstb.2019.0750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moroz LL, Romanova DY, Kohn AB. 2021. Neural versus alternative integrative systems: molecular insights into origins of neurotransmitters. Phil. Trans. R. Soc. B 376, 20190762. ( 10.1098/rstb.2019.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Göhde R, Naumann B, Laundon D, Imig C, McDonald K, Cooper BH, Varoqueaux F, Fasshauer D, Burkhardt P. 2021. Choanoflagellates and the ancestry of neurosecretory vesicles. Phil. Trans. R. Soc. B 376, 20190759. ( 10.1098/rstb.2019.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arendt D. 2020. The evolutionary assembly of neuronal machinery. Curr. Biol. 30, R603-R616. ( 10.1016/j.cub.2020.04.008) [DOI] [PubMed] [Google Scholar]
- 15.Yuste R. 2015. From the neuron doctrine to neural networks. Nat. Rev. Neurosci. 16, 487-497. ( 10.1038/nrn3962) [DOI] [PubMed] [Google Scholar]
- 16.Sourjik V, Armitage JP. 2010. Spatial organization in bacterial chemotaxis. EMBO J. 29, 2724-2733. ( 10.1038/emboj.2010.178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni B, Colin R, Link H, Endres RG, Sourjik V. 2020. Growth-rate dependent resource investment in bacterial motile behavior quantitatively follows potential benefit of chemotaxis. Proc. Natl Acad. Sci. USA 117, 595-601. ( 10.1073/pnas.1910849117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoll AH. 2011. The multiple origins of complex multicellularity. Annu. Rev. Earth Planet. Sci. 39, 217-239. ( 10.1146/annurev.earth.031208.100209) [DOI] [Google Scholar]
- 19.Niklas KJ, Newman SA. 2013. The origins of multicellular organisms. Evol. Dev. 15, 41-52. ( 10.1111/ede.12013) [DOI] [PubMed] [Google Scholar]
- 20.Keijzer F. 2020. Demarcating cognition: the cognitive life sciences. Synthese ( 10.1007/s11229-020-02797-8) [DOI] [Google Scholar]
- 21.Keijzer FA. 2017. Evolutionary convergence and biologically embodied cognition. Interface Focus 7, 20160123. ( 10.1098/rsfs.2016.0123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Etxebeste O, Espeso EA. 2016. Neurons show the path: tip-to-nucleus communication in filamentous fungal development and pathogenesis. FEMS Microbiol. Rev. 40, 610-624. ( 10.1093/femsre/fuw021) [DOI] [PubMed] [Google Scholar]
- 23.Thorn RG, Barron GL. 1984. Carnivorous mushrooms. Science 224, 76-78. ( 10.1126/science.224.4644.76) [DOI] [PubMed] [Google Scholar]
- 24.Barron GL. 1977. The nematode-destroying fungi. Guelph, Ontario: Canadian Biological Publications Ltd. [Google Scholar]
- 25.Heaton L, Obara B, Grau V, Jones N, Nakagaki T, Boddy L, Fricker MD. 2012. Analysis of fungal networks. Fungal Biol. Rev. 26, 12-29. ( 10.1016/j.fbr.2012.02.001) [DOI] [Google Scholar]
- 26.Ugalde U, Rodriguez-Urra AB. 2014. The Mycelium Blueprint: insights into the cues that shape the filamentous fungal colony. Appl. Microbiol. Biotechnol. 98, 8809-8819. ( 10.1007/s00253-014-6019-6) [DOI] [PubMed] [Google Scholar]
- 27.Parniske M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat. Rev. Microbiol. 6, 763-775. ( 10.1038/nrmicro1987) [DOI] [PubMed] [Google Scholar]
- 28.Adamatzky A. 2018. On spiking behaviour of oyster fungi Pleurotus djamor. Sci. Rep. 8, 1-7. ( 10.1038/s41598-018-26007-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gow NAR. 2013. Polarity and branching in fungi induced by electrical fields [1987]. In AGRIS, pp. 25-41. Rome, Italy: Food and Agriculture Organization of the United Nations. [Google Scholar]
- 30.Slayman CL, Scott Long W, Gradmann D. 1976. ‘Action potentials’ in NEUROSPORA CRASSA, a mycelial fungus. Biochim. Biophys. Acta 426, 732-744. ( 10.1016/0005-2736(76)90138-3) [DOI] [PubMed] [Google Scholar]
- 31.Olsson S, Hansson BS. 1995. Action potential-like activity found in fungal mycelia is sensitive to stimulation. Naturwissenschaften 82, 30-31. [Google Scholar]
- 32.Sheldrake M. 2020. Entangled life: how fungi make our worlds, change our minds, and shape our futures. London, UK: Bodley Head. [Google Scholar]
- 33.Baluška F, Mancuso S. 2021. Individuality, self and sociality of vascular plants. Phil. Trans. R. Soc. B 376, 20190760. ( 10.1098/rstb.2019.0760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baluška F, Mancuso S, Volkmann D, Barlow PW. 2010. Root apex transition zone: a signalling–response nexus in the root. Trends Plant Sci. 15, 402-408. ( 10.1016/j.tplants.2010.04.007) [DOI] [PubMed] [Google Scholar]
- 35.Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluška F, Van Volkenburgh E. 2006. Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci. 11, 413-419. ( 10.1016/j.tplants.2006.06.009) [DOI] [PubMed] [Google Scholar]
- 36.Mancuso S, Viola A. 2015. Brilliant green: the surprising history and science of plant intelligence. Washington, DC: Island Press. [Google Scholar]
- 37.Jékely G. 2021. The chemical brain hypothesis for the origin of nervous systems. Phil. Trans. R. Soc. B 376, 20190761. ( 10.1098/rstb.2019.0761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leys SP, Mah JL, McGill PR, Hamonic L, De Leo FC, Kahn AS. 2019. Sponge behavior and the chemical basis of responses: a post-genomic view. Integr. Comp. Biol. 59, 751-764. ( 10.1093/icb/icz122) [DOI] [PubMed] [Google Scholar]
- 39.Parker GH. 1919. The elementary nervous system. Philadelphia, PA: JB Lippincott. [Google Scholar]
- 40.Arendt D. 2021. Elementary nervous systems. Phil. Trans. R. Soc. B 376, 20200347. ( 10.1098/rstb.2020.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jékely G, Godfrey-Smith P, Keijzer F. 2021. Reafference and the origin of the self in early nervous system evolution. Phil. Trans. R. Soc. B 376, 20190764. ( 10.1098/rstb.2019.0764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ginsburg S, Jablonka E. 2021. Evolutionary transitions in learning and cognition. Phil. Trans. R. Soc. B 376, 20190766. ( 10.1098/rstb.2019.0766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fields C, Bischof J, Levin M. 2020. Morphological coordination: a common ancestral function unifying neural and non-neural signaling. Physiology 35, 16-30. ( 10.1152/physiol.00027.2019) [DOI] [PubMed] [Google Scholar]
- 44.Bechtel W, Shagrir O. 2015. The non-redundant contributions of Marr's three levels of analysis for explaining information-processing mechanisms. Topics Cogn. Sci. 7, 312-322. ( 10.1111/tops.12141) [DOI] [PubMed] [Google Scholar]
- 45.Noble D. 2012. A theory of biological relativity: no privileged level of causation. Interface Focus 2, 55-64. ( 10.1098/rsfs.2011.0067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoel EP, Albantakis L, Tononi G. 2013. Quantifying causal emergence shows that macro can beat micro. Proc. Natl Acad. Sci. USA 110, 19 790-19 795. ( 10.1073/pnas.1314922110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Friston K, Levin M, Sengupta B, Pezzulo G. 2015. Knowing one's place: a free-energy approach to pattern regulation. Interface Focus 12, 20141383. ( 10.1098/rsif.2014.1383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossberg S. 1978. Communication, memory and development. In Progress in theoretical biology (eds Rosen RJ, Snell FM), pp. 183-232. New York, NY: Academic Press. [Google Scholar]
- 49.Pezzulo G, LaPalme J, Durant F, Levin M. 2021. Bistability of somatic pattern memories: stochastic outcomes in bioelectric circuits underlying regeneration. Phil. Trans. R. Soc. B 376, 20190765. ( 10.1098/rstb.2019.0765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durant F, Morokuma J, Fields C, Williams K, Adams DS, Levin M. 2017. Long-term, stochastic editing of regenerative anatomy via targeting endogenous bioelectric gradients. Biophys. J. 112, 2231-2243. ( 10.1016/j.bpj.2017.04.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn J-M, Hwang JS, Gojobori T, Levin M. 2010. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev. Biol. 339, 188-199. ( 10.1016/j.ydbio.2009.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathews J, Levin M. 2018. The body electric 2.0: recent advances in developmental bioelectricity for regenerative and synthetic bioengineering. Curr. Opin Biotechnol. 52, 134-144. ( 10.1016/j.copbio.2018.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.