Abstract
The premise of this two-part theme issue is simple: the cognitive sciences should join the rest of the life sciences in how they approach the quarry within their research domain. Specifically, understanding how organisms on the lower branches of the phylogenetic tree become familiar with, value and exploit elements of an ecological niche while avoiding harm can be expected to aid understanding of how organisms that evolved later (including Homo sapiens) do the same or similar things. We call this approach basal cognition. In this introductory essay, we explain what the approach involves. Because no definition of cognition exists that reflects its biological basis, we advance a working definition that can be operationalized; introduce a behaviour-generating toolkit of capacities that comprise the function (e.g. sensing/perception, memory, valence, learning, decision making, communication), each element of which can be studied relatively independently; and identify a (necessarily incomplete) suite of common biophysical mechanisms found throughout the domains of life involved in implementing the toolkit. The articles in this collection illuminate different aspects of basal cognition across different forms of biological organization, from prokaryotes and single-celled eukaryotes—the focus of Part 1—to plants and finally to animals, without and with nervous systems, the focus of Part 2. By showcasing work in diverse, currently disconnected fields, we hope to sketch the outline of a new multidisciplinary approach for comprehending cognition, arguably the most fascinating and hard-to-fathom evolved function on this planet. Doing so has the potential to shed light on problems in a wide variety of research domains, including microbiology, immunology, zoology, biophysics, botany, developmental biology, neurobiology/science, regenerative medicine, computational biology, artificial life and synthetic bioengineering.
This article is part of the theme issue ‘Basal cognition: conceptual tools and the view from the single cell’.
Keywords: cognition, developmental biology, neuroscience, aneural cognition, prokaryotes, eukaryotes
1. Introduction
The premise of this two-part theme issue is fairly simple: the cognitive sciences should at last join the rest of the life sciences in the way they approach their quarry. This involves three essential steps. First, start with the smallest and simplest organisms that display the phenomenon of interest (the function, the mechanism). Second, in those organisms identify principles from observed and measured patterns of genetic, epigenetic and behavioural interactions. Third, scale up to more complex organisms and observe where the similarities and differences actually lie, not simply where we think they must lie. If the history of science tells us anything, it is that human intuition and tradition—which still too-often shape the study of cognition—rarely survive scientific scrutiny. The Earth is neither flat, nor immobile. The globe moves around the Sun (not the reverse) and is hurtling through space at an unimaginable speed that we intuit not at all. Further, the continents beneath our feet are not static and have travelled unimaginable distances over geological timescales.
These are facts that took decades to centuries to gain acceptance, but they make sense of continuing scientific observations and measurements, have successfully predicted novel findings and are now embedded in scientific theorizing. We believe that the study of cognition is on the cusp of a seismic shift similar to the Copernican and Wegnerian revolutions. If we truly recognize, in a biologically realistic fashion, the deep evolutionary inheritance of cognitive behaviour—individually and collectively, in both unicellular and multicellular organisms—a great deal of data that currently resist understanding will be more comprehensible and their implications less obscure. Or so we aim to demonstrate. Evidence that cognitive concepts such as ‘sensing’, ‘memory’, ‘learning’, ‘communication’ and ‘decision making’ can be applied non-metaphorically to the behaviour of bacteria (for example) is extensive and growing [1–8]. This suggests, first, that similar behaviour should be found in unicellular eukaryotes and, second, the molecular infrastructure for capacities typically associated with brains long predated the evolution of neurons, much less central nervous systems, which appears to be the case, as several contributions in this extended theme issue attest. (See, in particular, Part 2.) Organisms today are the beneficiaries of this inheritance.
Understanding behaviour in all its forms will require a dramatic shift in perspective. The result, however, should be a potentially productive cross-fertilization of the life sciences and the cognitive sciences that could help to solve major problems in both domains, which currently barely reference one another. Indeed, some of the most powerful advances in science have been unifications—discovering the invariant core that underlies phenomena that were previously thought to be distinct. Taking seriously modern evolutionary and cell biology arguably now requires recognition that the information-processing dynamics of ‘simpler’ forms of life are part of a continuum with human cognition. The commonalities are mechanistic, not metaphorical.
Why is this necessary now? In the past two decades, a mountain of new data has been generated in neurobiology and the neurosciences using ever-more sophisticated imaging techniques and methods for genetic manipulation and fluorescent labelling, enabling observation of physiological activity in individual behaving cells and organisms.1 CRISPR, the gene-editing tool for modifying the genomes of living organisms, is considerably accelerating this trend. We know more than we ever have, in granular detail, about what brains do. However, we are still very much in the dark about how brain activity generates behaviour. Connections among all 302 neurons of the tiny nematode Caenorhabditis elegans have been mapped, a stunning technical achievement from a half-century of detailed experimental effort, but we still don't know how the wiring diagram generates behaviour in a living worm [9]. Developing the tools so that we can understand how brains generate decision-making behaviour in real time is the mission of the US Brain Initiative and its international partners [10], but we need more than tools. We need a diversity of new models, as well as theories that apply not only to familiar ‘standard’ model species but to the entire option space of cognitive agents, which includes aneural organisms, somatic organs and novel bioengineered synthetic forms. Most of all, we need new theory. It is difficult to see where that will come from except from the study of simpler organisms.
2. Ion channels: a proof-of-concept case study
In 1980, Daniel E. Koshland argued in the Annual Review of Neuroscience that the burgeoning study of bacterial behaviour, and chemotaxis in particular, had the potential to provide relatively simple, manipulable models for investigating the properties, mechanisms and behaviour of neurons [11]. Despite Koshland's international influence as an innovative biochemist, the article met with a resounding silence. At the time, getting a handle on the mechanism involved in generating electrical action potentials across a neural membrane had been an active scientific goal for almost three decades [12]. However, another 15 years would pass before Roderick MacKinnon, a young biophysicist fascinated by ‘understanding the atomic basis of life's electrical system’, switched from studying Shaker K+ (potassium) ion channels in the fruit fly Drosophila melanogaster to studying KcsA potassium channels in the bacterium Streptomyces lividans [13].2 Years earlier, MacKinnon had co-discovered a method for locating the ion-trapping amino acids of the potassium channel protein in fruit flies using charybtoxin, a small protein in scorpion venom, but he had nearly despaired of producing enough of the ion channel protein to crystallize the structure.
Meanwhile, potassium ion channels increasingly were being discovered in widely differing organisms, including prokaryotes, and their genetic signatures were unexpectedly conserved. According to MacKinnon:
Despite its prokaryotic origin KcsA closely resembled the Shaker K+ channel's pore amino acid sequence, and even exhibited many of its pharmacological properties, including inhibition by scorpion toxins. This surprised us from an evolutionary standpoint, because why should a scorpion want to inhibit a bacterial K+ channel! But from the utilitarian point of view of protein biophysicists we knew exactly what the scorpion toxin sensitivity meant, that KcsA had to be very similar in structure to the Shaker K+ channel [13, p. 218].
Research on the structure and operation of cellular ion channels ultimately led to the 2003 Nobel Prize in Chemistry for MacKinnon, which he shared with physician Peter Agre, discoverer of aquaporin channels. Even then, however, no one knew what function these ion channels play in the existential economies of prokaryotes. That would take another dozen years after MacKinnon's Nobel and 35 years after Koshland's neglected manifesto. Thanks to research under the leadership of Gürol Süel on Bacillus subtilis, long a model organism for studying multicellular complexity in bacteria [14], Koshland's faith in bacterial models has been more than vindicated.
It turns out that potassium ion channels do the same thing in biofilms of B. subtilis that they do in neurons: long-distance communication across large numbers of cells [2]. In this case, nutrient-deprived cells in the interior of the bacterial community send bioelectrical distress signals to the cells on the periphery to stop feeding and growing, which allows nutrients at the periphery to diffuse to the interior. Süel's team found that collective oscillations formed between periods of peripheral growth and stasis, driven by a metabolic co-dependence that resolves an intrinsic conflict of interest between cells growing on the periphery, which also provide protection for the community, and cells in the interior, which benefit from that protection but may die [15]. Specifically, feeding by peripheral cells deprives the interior cells of glutamate, which the interior cells need to produce ammonium, a molecule necessary for continued biofilm expansion on the periphery [15]. When peripheral cells stop dividing, they become more vulnerable to external attack by predators in the soil or the immune system of a host, but to interior cells the deaths of their siblings release the glutamate they need to survive. B. subtilis biofilms are highly structured, with cellular division of labour at the bottom, middle and top layers, but they only form under conditions of declining nutrients [8]. The Süel group predicted and found that the minimum biofilm size required for the emergence of this kind of oscillatory behaviour grew smaller with increasing stress [16].
But there's more. As if this kind of altruistic cooperation under deteriorating conditions were not enough, Süel's group found that electrical signalling enables separate biofilms undergoing metabolic oscillations to synchronize their growth dynamics, which allows them to share scarce nutrients [17]. By coordinating behaviour to switch their oscillations from in-phase to anti-phase, two spatially distant communities take turns feeding, effectively resolving competition for nutrients. ‘Time-sharing enables biofilms to counterintuitively increase growth under reduced nutrient supply,’ the team observed [17, p.638].
But there's more. In a study of the interplay between metabolism and electrical signalling, the group found that metabolic stress is transmitted through a biofilm via a potassium wave, which regulates the membrane potential of B. subtilis cells in ways similar to neurons in the mammalian brain. The behaviour produced in the biofilm, the team noted, ‘is reminiscent of cortical spreading depression in the brain’, which is also ‘characterized by a wave of electrical activity mediated by potassium diffusion that has been linked to various neurological disorders’ [18]. Perhaps not surprisingly, they suggested future research into ‘the evolutionary link between the two phenomena’, electrical signalling in bacterial biofilms and information-processing in mammalian brains.
And still there's more. The Süel group investigated whether changes in cellular membrane potential might play a role in the formation of memory in B. subtilis cells as in neurons [3].3 Using light as a stimulus, not only did the team find that biofilm cells could form ion channel-mediated memories, but they found that complex memory patterns—in this case, a logo of the University of California at San Diego—could be encoded at the single-cell level. Across a spatially extended area of biofilm, light-exposed cells responded in an anti-phase manner relative to unexposed cells. The anti-phase response to the transient stimulus persisted for hours and was robust to external ionic perturbation (natural and induced), as predicted by a mathematical model proposed by Hodgkin and Huxley [12], who set the agenda for ion channel research in the 1950s. In summary, the findings suggested ‘a parallel between neurons and bacteria’ in the way changes in membrane potential encode memory.
Equally exciting are the implications for applications in biological computing, given the demonstration that light exposure not only reliably encodes information into living matter but also, through the anti-phase response in B. subtilis, enables production of ‘a clear signal that is either ‘on’ or ‘off’, as in traditional digital memory’. [3] The team concludes: ‘Overall, our work is likely to inspire new membrane potential-based approaches in synthetic biology and provide a bacterial paradigm for memory-capable biological systems’ [3].
Whether the stunning discoveries of the Süel group would have been possible significantly earlier is another matter. The point is that when MacKinnon was making his ground-breaking discoveries, the default assumption not only in neurobiology but also in microbiology was that whatever ion channels were doing in bacteria could not be anything like what they are doing in neurons. That was a mistake. More importantly, that mistake—compounded by the disciplinary silos in which microbiologists and neurobiologists work—delayed these kinds of experiments for 15 years. Who knows where the state of knowledge would be if this had not happened? Look how much has been accomplished in less than a decade. More to the point, who knows how much more we will miss if we don't shift perspective now? The shift is not so radical, in actual fact.
3. Reviving a dormant Darwinian program
In On the Origin of Species, Charles Darwin [19] provided a scientific basis for developing a cognitive theory based on close observation of and experiment with different forms of life [20,21].4 Darwin proposed that the abundant diversity of organisms on Earth, along with their ‘mental’ faculties, evolved from much simpler forms, ultimately from single cells. Of the ‘far more important researches' that Darwin saw arising in the future from this view of nature was ‘Psychology…based on a new foundation, that of the necessary acquirement of each mental power and capacity by gradation’ [19, pp. 488–489]. Inspired by Darwin's thinking, nineteenth-century scientists—first in Germany [22] and France [23], then in North America [24]—began studying the behaviour of microbes. In a monograph written at the beginning of his career, psychologist Alfred Binet (of IQ test fame) claimed (perhaps prematurely): ‘We could, if it were necessary, take every single one of the psychical faculties' then attributed solely to animals ‘and show that the greater part of those faculties belonged equally to Micro-organisms’ [23, p. v]. In the first four decades of the twentieth century, the leading US textbook on comparative psychology, The Animal Mind, began with a chapter on amoeba and moved upward (in terms of complexity) to mammals, including humans [25]. This strand of research arguably ended owing to the confluence of four unrelated factors: the methodological limitations of available imaging technologies, the First World War, the rise of behaviourism and the debate in biology over vitalism, which remained active at least into the 1970s (see, for example, [26]).
By contrast, the so-called Cognitive Revolution of the latter twentieth century was slow to embrace a Darwinian, or even a biological, perspective [21,27–29], and the belief that cognition requires a nervous system remains the default view. Nevertheless, the twenty-first century has already forced radical reassessments of much of what we thought we knew, including about cognition and the central nervous system (CNS), thanks to the completion of the Human Genome Project, the advent of comparative genomics and the development of increasingly sensitive tools for imaging behaving unicellular organisms going about their business. In the first comparative approach to the origin and evolution of the CNS, it was perhaps not so surprising (even in 2003) that planaria harboured 116 nucleotide sequences highly similar to genes known to be related to the nervous system [30]. A bit more astonishing was the finding that more than 95% of these nervous system-related genes, including some involved in neural and brain morphogenesis, were commonly shared with Drosophila, C. elegans and Homo sapiens. The unexpected finding was that
[approximately] 30% of planarian nervous system-related genes had homologous sequences in [the plant] Arabidopsis and yeast, which do not possess a nervous system. This implies that the origin of nervous system-related genes greatly predated the emergence of the nervous system, and that these genes might have been recruited toward the nervous system. [30, p. 7666]
Today, as several articles in Part 2 of this collection show [31–33], this supposition is now well-established and the focus of increasingly interesting research. For some, this ongoing reassessment has been destabilizing. For others, it opens up opportunities for expanding the kinds of organisms in which cognitive processes can be studied. What this extended theme issue hopes to show is that this is a good thing. There is so much we don't know, especially about the evolution of cognition. At the same time, interesting behaviour is being discovered in a wide variety of aneural organisms, simple neural organisms and even in aneural tissue, for example, the formation of blood vessels (see [34] in Part 2). New findings appear almost weekly in a wide variety of disparate journals that until now have had a limited impact beyond their individual fields of study, for example, microbiology, zoology, botany, biophysics, developmental biology, neurobiology, regenerative medicine, computational biology, immunology, artificial life, synthetic bioengineering, animal behaviour and cognitive science. Absent a connecting framework, this research will continue to be produced in specialist bubbles where discussion remains mostly parochial and evolutionary development often obscure. Developing a connecting framework is the aim of basal cognition research.
4. What do we mean by ‘cognition’?
The short answer is we don't know because we can't agree, and unambiguous, biologically grounded proposals are effectively non-existent. Cognition—and where it is found in the natural world—has been an inexhaustably meaty bone of contention since (in ‘western’ culture) Aristotle (384–322 BCE), for whom animals marked a singular transition [35], and (in ‘eastern’ culture) the Rig Veda (1700–1000 BCE) and associated texts, many of which admitted cognition in plants [36]. Not so long ago, it was suggested that an adequate definition would be possible when we have sufficient data [37], but this is not how it has worked out. The data available even 20 years ago ‘almost inhibited meaning’ [38]. Today the data are oceanic and meaning seems impossible without machine learning to detect relevant patterns. Defining cognition can even seem unnecessary, leaving its articulation a matter of scientific progress [39,40]. However, we do not have that luxury in this case. We cannot claim that basal cognition is a worthy research pursuit if we fail to provide at least a provisional working definition of the critical construct.
In her seminal Cognition, evolution, and behavior [41], comparative psychologist Sara Shettleworth proposed a definition of cognition that probably is the most cited in the life and behavioural sciences and remains unchanged in the second (2010) edition: ‘Cognition refers to the mechanisms by which animals acquire, process, store, and act on information from the environment. These include perception, learning, memory, and decision-making’ [41, p. 5]. Shettleworth's target is animal cognition, but the capacities she lists are now known to be evident in bacteria, to say nothing of single-celled eukaryotes, plants, fungi and simple animals without nervous systems.
From our perspective, however, the definition is not ideal [21].5 Importantly, it fails to specify the adaptive value these mechanisms have in the functional economy of the organism: what cognition thus defined does for the organism. Additionally, ‘information’ does all the conceptual heavy lifting yet remains uncharacterized. This is not unusual, far from it, but the emphasis on information specifically from the environment is misleading. Information from the external milieu only ever makes sense in relation to the state of the cognizing system: under-nourished, starving, sated, reproductive, dormant, acting alone, acting with others, experienced, naïve, threatened, secure and so on. Moreover, the cognizing system is never a passive recipient of input from the environment, but is ever and always endogenously active [42]. Taking Shettleworth as the jumping-off point, we offer the following working definition:
Cognition comprises the sensory and other information-processing mechanisms an organism has for becoming familiar with, valuing, and interacting productively with features of its environment [exploring, exploiting, evading] in order to meet existential needs, the most basic of which are survival/persistence, growth/thriving, and reproduction. [21, p. 416]
This phyletically neutral definition specifies the adaptive value of cognition for an organism and has the additional virtue of differentiating cognition from metabolic functions, such as respiration, which arguably also employ mechanisms for acquiring, processing and acting on information to meet existential goals but does not involve familiarization, valuing, exploring, exploiting and evading [21]. Information remains uncharacterized but can be explicated in the biological context, as follows:
A state of affairs is information for an organism if it triggers a change in physiology or behaviour relative to that state of affairs. Whatever state of affairs induces a change in physiology or interactive potential in an organism is information for that organism. (adapted from [43, pp. 17, 21])
This definition allows for regulatory stochasticity in the absence of a detectable change in environmental conditions.
A fairly straightforward entailment of this approach is that cognition is a function necessary for any autonomous biological system's survival, wellbeing and reproduction, which is an uncomfortable proposition for many. We say that whether one wishes to concede cognition to prokaryotes (for example) remains a matter of personal choice. This is not the issue before us. The issue before us is whether proceeding as though this were the case, in a biologically realistic fashion, is productive. The answer to that question, we believe, is yes.
5. What basal cognition is: approach, toolkit, mechanisms
Basal cognition includes the fundamental processes and mechanisms that enabled organisms to track some environmental states and act appropriately to ensure survival (finding food, avoiding danger) and reproduction long before nervous systems, much less central nervous systems, evolved.6 Tracking existentially important states of affairs is inseparable from generating behaviour, by which we mean actions that change circumstances such that the products of actions enter the stream of stimuli being tracked. These products may act as signals to others, which means that tracking often involves sending, receiving and, in some cases, manipulating. For example, bacteria produce molecules (autoinducers) that they secrete into the environment. When a threshold density of these molecules is reached, which requires large numbers of other bacteria doing the same thing, the population as a whole coordinate metabolically costly changes in genetic expression that would be unproductive in just a few individuals, a phenomenon called quorum sensing [44]. Similarly, molecules of cAMP accumulate in colonies of Escherichia coli, which makes it a marker for adventurous cells to (re)join a population. However, owing to this role the slow-moving bacterial predator Myxococcus xanthus has been shown to secrete cAMP to lure the much swifter E. coli into killing range [45].
Basal cognition, like uncaveated cognition, importantly involves learning, including via epigenetic and genetic embedding in the course of evolutionary change, what neuroscientist Antonio Damasio elegantly calls ‘holding know-how in dispositions’ [46]. Basal cognition thus implies a degree of tacit familiarity or acquaintance concerning correlations between environmental states. A gradient of one chemical, for example, may indicate the presence of a concentrated patch of food from which it diffused, which means the organism must track it even if the chemical itself may not be interesting or useable. Tracking in this situation is based on implicit knowledge that the organism has about how the world works, and what that bodes for its own functioning. Cues often covary or occur independently in nontrivial ways, however. In open water, for example, increasing light not only means ‘up’, which means less pressure and higher temperature, but also more ultraviolet light (and UV-induced DNA damage) and more predators. The organism living in such an environment needs to optimize along all these axes, which requires often nontrivial decisions based on the integration of information from many sources. Life at every level of development demands tough choices. This is what basal cognition is for. When nervous systems first evolved, this is also what they evolved for.
As it is used here, basal cognition also describes a toolkit of biological capacities involved in becoming familiar with, valuing and exploring, exploiting or evading environmental features in the furtherance of existential goals. The cognitive toolkit set out in table 1 is ‘basal’ because each of these capacities, defined in minimal terms, has been observed and described in prokaryotes [1,4,48], the lowest branch on the evolutionary tree of life, to say nothing of other aneural organisms.
Table 1.
| capacity | function |
|---|---|
| orienting response | Ability to selectively attend to a state of affairs to the exclusion of others. Biological basis of attention. |
| sensing/perception | The capacity to sense and recognize (re-cognize) existentially salient features of the external and internal milieux. |
| discrimination | The ability to determine that a state of affairs affords an existential opportunity or presents a challenge requiring a change in internal state or relation to the external environment. |
| memory | The capacity to retain information about the immediate (and possibly distant) past, and to calibrate the sensorium to take account of this information, at a minimum via habituation or sensitization. |
| valence | An organism's capacity to assign a value (advantage/good, harm/bad) to a particular stimulus or the summary of information about its surroundings relative to its own current state. The ‘building block’ of affect [47]. |
| decision making | The capacity to combine information from multiple sources and act, typically in furtherance of an implicit or explicit goal. |
| behaviour | An organism's capacity to adapt to changes in its internal or external milieux by changing its own structure and function (internal) and/or its spatial and interactive relations (external). |
| problem solving | Behaviour selection in circumstances with multiple (possibly conflicting) parameters and high degrees of uncertainty. |
| error detection | The capacity to determine whether a behaviour has succeeded or failed. Together with homeostasis/allostasis, this is the basis of biological normativity. |
| motivation | Implicit goals arising from existential conditions. Teleonomic striving. The inclination of a thing to continue to exist and enhance itself. |
| learning | The capacity to adapt behaviour to salient stimuli according to past experience by altering the threshold for or the nature of a response. |
| anticipation | The capacity to predict what is likely to happen next based on an early stimulus. |
| communication | The capacity to interact productively with other organisms via forms of signalling, notably (but not limited to) conspecifics, including initiating collective action, which may or may not include an explicit means of differentiating ‘like me’ (us) from ‘not-like me’ (them). |
The phyla covered in this two-part theme issue range from bacteria to slime moulds and other single-celled eukaryotes to plants, aneural animals (placozoa, sponges) and simple neural animals (Hydra and other cnidaria, planaria, ctenophores) to animals with complex nervous systems capable of what Ginsburg and Jablonka call unlimited associative learning ([49]; figure 1). Most contributions relate to one or more of the capacities in the toolkit, often explicitly in terms of one or more of the common mechanisms involved in implementing the toolkit (table 2).
Figure 1.
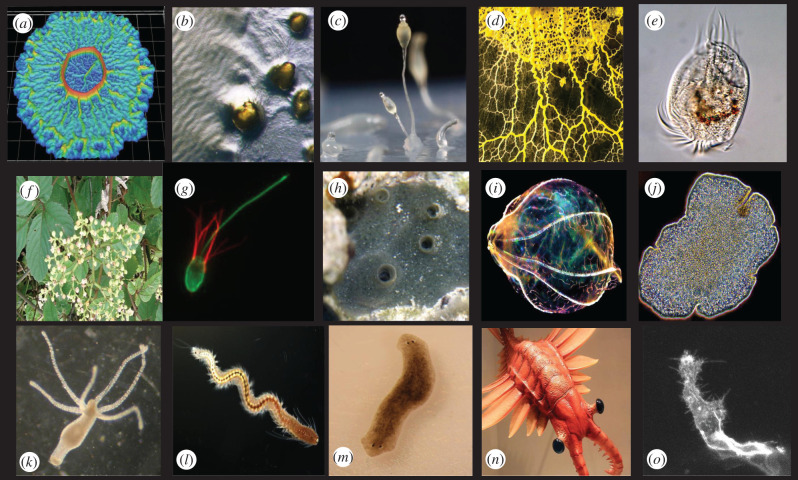
Organisms represented in basal cognition theme issues. Note: Phyla discussed primarily in the second issue have citations. Those discussed primarily in this issue do not. (a) A digital scanned model of a Bacillus subtilis biofilm suggests how the bacterial community develops and changes structure in three dimensions. (Image: Scott Chimileski and Roberto Kolter. https://www.wired.com/story/seeing-the-beautiful-intelligence-of-microbes/.) (b) Myxococcus xanthus has a complex multicellular lifestyle that includes ‘rippling’ behaviour in the presence of prey. (Image: Zalman Vaksman and Heidi Kaplan, University of Texas Medical School, Houston, USA. https://doi.org/10.1371/journal.pcbi.1002715) (c) The social amoeba Dictyostelium discoideum is a unicellular model for studying the evolution of multicellularity. (Image: Usman Bashir, Queller/Strassman laboratory. CC BY-SA. https://commons.wikimedia.org/wiki/File:Dictyostelium_discoideum_02.jpg). (d) The acellular slime mould Physarum polycephalum, by contrast, is a single giant, expanding cell that coordinates behaviour via an oscillating, vascular plasma-transport network. (Image: David Villa SCIENCEIMAGE CBI CNRS.) (e) Predatory ciliate Euplotes exhibits behavioural innovations enabled by the origin of the excitable eukaryotic cell. (Image: Hannah Laeverenz Schlogelhofer and Kirsty Wan, EvoMotion Lab, Living Systems Institute, Exeter, UK.) (f) Tendrils of the plant Cayratia japonica have been found to display self-recognition [50]. (Image: KENPEI, own photo. CC BY-SA 3.0. https://commons.wikimedia.org/w/index.php?curid=2461105). (g) Comparative genomic analysis of the colonial choanoflagellate Salpingoeca rosetta reveals that animals' closest unicellular relative possesses almost as much of the molecular infrastructure for producing neurosecretory vesicles as (h) the sponge Amphimedon queenslandica (Porifera), a closer multicellular relative [33]. (Image of S. rosetta: Burkhardt Group, Sars International Centre for Marine Molecular Biology, University of Bergen, Norway. Image of A. queenslandica: M Adamska et al., Centre for Marine Science, University of Queensland, Brisbane, Australia. CC BY 2.5. https://commons.wikimedia.org/w/index.php?curid=9950094) (i) Beroe sp. (with just-swallowed Bolinopsis inside), a comb jelly found off the Florida Keys, is within the early-branching phylum of eumetazoa (Ctenophora) thought to have independently evolved a nervous system [32]. (Image: L.L. Moroz and G. Pauley.) (j) The aneural placozoan Trichoplax adhaerens possesses a surprising number of signalling peptides that resemble those used in nervous systems [32]. (Image: L.L. Moroz, Whitney Laboratory for Marine Bioscience, Florida, USA.) (k) Hydra vulgaris (Cnidaria), the recent go-to model for imaging whole-organism neural activity, provides additional unexpected insights. (Image: Corvana, own work. CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=6349873.) (l) Platynereis dumerilii, an annelid polychaete worm and model organism for studying the origin and evolution of the nervous system [51] (Image: Martin Gühmann, own work. CC BY-SA 4.0, https://commons.wikimedia.org/w/index.php?curid=61283244). (m) Research with an experimentally produced 2-headed Planarian (Platyhelminthes) suggests bioelectricity plays a key role in organism regeneration [52]. (n) Artist's impression of Lararapax unguispinus, a large marine predator from the Cambrian Era, when Ginsburg and Jablonka [49] propose that ‘unlimited associative learning’ originated. (Image: Yinan Chen. https://commons.wikimedia.org/wiki/File:Gfp-anomalocaris-predator.jpg). (o) A blood vessel sprout grows in the zebrafish (Danio rerio) embryo, where long, thin projections (filopodia) speed up collective cell decisions based on ‘active perception’—sensorimotor feedback as they move [34]. (Image: Cellular Adaptive Behaviour Lab, Francis Crick Institute, London, UK.)
Table 2.
Some mechanisms involved in implementing the toolkit.
| mechanism | function/means |
|---|---|
| signal transduction (intracellular) | Sensing and memory Post-translational protein modification DNA modification Small RNAs |
| cell–cell signalling (intercellular) | Autocrine—cell secretes and receives signal → alters own behaviour Paracrine—cell secretes signal → alters behaviour of nearby cells Endocrine—feedback-regulated secretion → alters behaviour of distant cells Neuroendocrine—signals secreted by neurons → alters behaviour of local & distant organs Juxtacrine—requires cell contact or close proximity |
| oscillations (intra- and intercellular) | Information processing Information distribution Set-point maintenance (behaviour and physiology) |
| networks + circuits (intra- and intercellular) | Signal integration Decision making Behavioural sequencing |
| bioelectricity (intercellular) | Signalling/communication (including in stress responses) ‘Memory’ template for regenerative patterning Developmental patterning |
| cell–cell adhesion (intercellular) | Proximity for signalling Proximity for transmitting molecules across membranes Structural stability (obligate multicellularity) |
| local, regional + systemic regulatory responses (intra- and intercellular) | Nutrient use/growth Stressors Reproduction Sporulation Recovery (from injury, germination) |
A major implication of basal cognition is not only the plausibility but also the usefulness of viewing other fundamental problems in biology through a ‘cognitive lens’, that is, ‘a strategy using well-established concepts from cognitive and computer science’ in a non-metaphorical sense ‘to complement mechanistic investigation in biology’ [53]. Needless to say, informatic terminology has been used productively in biology for the past half-century. Until now, however, it has been on the general understanding that this terminology implies nothing about cognitive processes. The cognitive lens approach suggests that given evolution—not simply conceptual utility—it makes sense that information-dependent developmental processes in multicellular organisms (e.g. embryogenesis; tissue growth, maintenance and repair; regeneration; immunity) might share fitness-enhancing processes derived from the cognitive endowments of their unicellular ancestors. As a field, then, basal cognition has the potential to bring into productive contact heretofore disparate sectors of the life sciences in order to develop the conceptual and theoretical tools necessary for answering outstanding fundamental questions in a variety of areas of biology, not simply the cognitive sciences. For example, if the tools of cognitive science can be extended to apply to unconventional embodiments, novel applications (e.g. training or motivating tissues for regenerative medicine contexts) become feasible [54].
6. The structure of the two issues
The collection of 16 articles, in addition to this introduction, which comprise the basal cognition theme issue is organized into five groups with similar themes: (i) conceptual tools and organizing principles that appear to apply across the domains of life; (ii) the view from single cells, prokaryotic and eukaryotic, together with a review article that explains why eukaryogenesis marked a critical behavioural transition as well as a morphological one; (iii) the challenge of integrating and coordinating (often larger) bodies in constitutively multicellular organisms, which focuses on plants and (proto-)neural signalling in animals; (iv) the origin and evolution of essential molecular infrastructure for nervous systems, the likely function of the first nervous systems, how a sense of ‘self’ emerged in neural animals, and the major transitions in learning that nervous systems afforded; and (v) how the basal cognition approach illuminates phenomena traditionally well outside the cognitive sciences, including the bioelectric basis of pattern memory in regenerating planaria and active perception by endothelial cells in the formation of blood vessels.
This issue (Part 1) features the articles listed in the preceding paragraph, under themes (i) and (ii).
(a). Conceptual tools and organizing principles
The collection opens with a bold theoretical proposal by William Bechtel and Leonardo Bich about the biomolecular origins of cognition [55]. The argument focuses on two classes of physiological mechanism that function in a particular type of organization. Production mechanisms ‘extract and use energy, break down and synthesize materials, move organisms through space, enable division or replication of the organism, etc.’. Control mechanisms regulate the activity of production mechanisms, taking account of changing internal and external conditions and making decisions about the application of the production mechanisms for which they are controllers in the face of higher and lower degrees of uncertainty. Control mechanisms, they argue in impressive detail, are the basis of cognition. Their operation is organized for the most part heterarchically, that is, the constituent components are unranked or have the potential to assume different ranks [56], a more distributed, flexible and robust structure compared to the hierarchical structures classically postulated. Examples range from allosteric control of glucose metabolism in mammals to protein synthesis, chemotactic motility, quorum sensing and circadian rhythmicity in prokaryotes.
All organisms must be capable of determining what behaviour works and what doesn't, what promotes survival, growth and/or reproduction and what threatens it. The second contribution in this section, by Pamela Lyon and Franz Kuchling, concerns how organisms value what they encounter as a necessary adjunct to marshalling behaviour [57]. In emotion research, reinforcement and aversion are aspects of valence, an organism's response to perceived advantage and harm. The paper provides a historical excavation of the valence concept, shows how it figures in contemporary affective science and makes a biological case for its application beyond the realm of animals with complex nervous systems using a bacterial case study. A preliminary model for computing the role of valence in basal cognition is sketched, expanding the range of existing machine learning models that currently subsume value under homeostasis. The paper argues that homeostatic regulation could not have evolved to the degree of complexity it currently displays, even in the simplest organisms, without valence.
Hydra, a family of tiny tentacled freshwater cnidaria, has long been a model organism for regeneration. In the past 5 years, Hydra vulgaris has assumed an important new role as a model for imaging an entire nervous system in a behaving organism [58]. The epicentre of this work is Rafael Yuste's laboratory at Columbia University, where Alison Hanson, who contributed the third contribution in this section, is a postdoctoral fellow. The focus of Hanson's article is not the activity of Hydra's nerve net per se but, rather, spontaneous electrical low-frequency oscillations (SELFOs) detected in the organism by researchers many decades ago and confirmed again relatively recently [59]. Hanson describes what currently is known about SELFOs in Hydra, draws parallels with the mammalian default mode network, provides evidence of their presence in a wide range of diverse phyla (including unicellular organisms) and hypothesizes that SELFOs might constitute bioelectrical ‘organism organizers’ by serving as system-wide electrical information integrators. Because of this wider biological focus, the article is included in this issue rather than the next one, which focuses on multicellularity.
(b). The view from the single cell
Single-celled organisms figure significantly in this essay and the theme issue generally because unicellulars not only are the earliest forms of life to appear on Earth but also dominated the planet for 2.5 billion years. Fossil evidence in stromatolites of colonial living prokaryotes, believed to be photosynthetic cyanobacteria, has been dated to approximately 3.5 billion years ago, while the eukaryotic cell is estimated to have emerged approximately 1.4–1.9 billion years later. Needless to say, no extant unicellular organism is the ancestor of any living multicellular organism, and contemporary examples are often, but not always, more complex than their distant ancestors would have been. However, single-celled organisms provide a window into the diverse and surprisingly complex behaviours that can be generated, singly or socially, in an organizational setup orders of magnitude smaller than the cells of the human body, at least in the case of prokaryotes. In that sense, they are as basal as it gets.
Although it makes evolutionary sense to start with prokaryotes, we begin this section instead with a highly original review by Kirsty Wan and Gáspár Jékely [60] on the origins of eukaryotic excitability and the behavioural revolution enabled by the emergence of this form of cellular organization. With the exception of certain behaviours such as phagocytosis, analysis of eukaryogenesis typically is conducted relative to morphology, how eukaryotic cells differ structurally from their prokaryotic forebears, and genomic innovations, which provided greater flexibility in gene regulation and expression as well as scope for larger-scale change. This is the first attempt, to our knowledge, to map the considerable behavioural differences, particularly in relation to motility, enabled by the innovations found in eukaryotes. While the mechanisms that implement the cognitive toolkit (table 2) are found on either side of the prokaryote–eukaryote division and the phrase ‘once considered exclusive to eukaryotes' is now a cliché for describing discoveries in prokaryotes, Wan and Jékely make an impressive case that the divide is quite real in terms of the complexity and flexibility of the molecular machinery driving behaviour, and the high degree of coordination required to make it function properly, which sets the stage for further evolutionary innovation.
Having this review first also throws into sharp relief the extraordinary capacities of the golden, rod-shaped prokaryote Myxococcus xanthus, given the lesser mechanistic endowment with which it must work. M. xanthus is a predatory proteobacterium that lives in soil, probably the most complex biotic environment on Earth [61], and is relentlessly social in almost every facet of life: foraging for and feeding on prey, responding to nutrient stress through the construction of complex multicellular structures, sporulation and germination. Fundamental to these behaviours is a kind of collective motility called ‘swarming’ (S-motility), which involves periodic reversals in the direction of individual cells, whereby the leading pole of each cell becomes its lagging pole and vice versa, facilitated by the rhythmic intracellular migration of the protein complex driving the reversals. In their review, members of Tâm Mignot's [62] laboratory (CNRS-Aix-Marseille University, FR) describe in detail several swarming-enabled social transitions in M. xanthus, linking single-cell decisions to collective behaviours in this remarkable microbe. All of these multicellular behaviours require environmental sensing, signal integration, memory and decision-making, as well as solving problems faced by all flocking, shoaling and swarming animals, such as how to avoid interfering with another individual's progress [63]. Strikingly, they demonstrate that complex social Myxococcus behaviours may be explained by interactions and cooperation between individual cells, which themselves are equipped with sensory circuits, allowing them to make memory-based decisions that are propagated over very large spatio-temporal scales. Thus, M. xanthus appears to be ‘ideally suited’ for investigating ‘cognitive-like behaviours that emerge from the collective interactions of thousands of single sensory cells'.
While a vast evolutionary distance exists between Myxococcus and the eukaryote Dictyostelium discoideum, both independently evolved a life cycle that involves the aggregation and multicellular coordination of single-celled individuals, processes that share some common mechanisms, even a few molecular components. However, the Dictyostelid social amoeba are the largest group of eukaryotes known to aggregate into and behave as multicellular units, first as motile, light- and temperature-sensing slugs then as multi-part fruiting bodies, the development of which may involve up to five different cell types. These traits have long made D. discoideum a model organism for studying cell–cell communication, cell differentiation and programmed cell death (apoptosis), as well as kin selection, the evolution of cooperation and ‘cheating’.
In her fascinating review article on cognitive evolution in Dictyostelid social amoebas, the third contribution in this section, Pauline Schaap [64] builds a case, step by step, for the transformation of a ‘mundane stimulus-response pathway’—environmental sensing associated with nutrient depletion—into a complex, tightly coordinated yet flexible behavioural sequence the goal of which is survival of a large population, or most of it. This was achieved, she suggests, via the merger of at least two excitable signalling networks exhibiting positive and negative feedback—one of which was chemotactic—which ‘enabled a collection of stressed amoebas to self-organize into a motile multicellular structure, capable of stimulus-driven decision making’. Schaap focuses on the molecule cAMP (cyclic adenosine monophosphate), a regulator of diverse cellular functions in all domains of life and a common second messenger in mammals, in which it regulates aspects of metabolism, physiology, development and memory. In D. discoideum, cAMP controls all multicellular development through its interconnected regulation of cell motility and cell differentiation.
The section ends with a potentially game-changing multidisciplinary collaboration on the role of oscillations in learning in Physarum polycephalum [65], the acellular slime mould and current global celebrity of eukaryotic unicellular behaviour. Members of the animal cognition laboratory of Audrey Dussutour (CNRS-Toulouse, France) and the biological physics laboratory of Hans-Günther Döbereiner (University of Bremen, Germany) provide a basal mechanistic-behavioural starting point for answering ‘one of the most fundamental questions in cognitive science’: how information from an organism's interactions with its environment can be ‘encoded in physical/chemical changes' in that organism and then decoded sometime later for generating adaptive behaviour. The article details an option space for investigating the role in slime mould learning of coordination among relatively simple oscillatory processes, at the cell and molecular levels. Studies of learning in single-celled eukaryotes are few, but habituation—the simplest form of learning by which prior experience of a stimulus alters the threshold for a response on subsequent exposure—has been demonstrated in ciliates (30 years ago) and also recently in P. polycephalum. The article introduces the slime mould as a potentially ‘ideal model system for relating basal cognitive functions to biological mechanisms’, describes what is known about its cognitive capacities in general, the habituation experiments in particular, and the several known oscillatory processes that govern the life of the organism. Guided by what is known about the role of oscillatory processes in neural learning, the authors draw on historical as well as up-to-date models and methods for studying an alternative architecture on a continuum of learning mechanisms which, as Ginsburg and Jablonka [49] show in the next issue, undergo multiple evolutionary transitions once nervous systems develop.
7. Opening the future
Findings in a variety of fields point to a historical moment where ‘connecting the dots’ in the evolution of cognition is crucial and (at last) possible. Arguably, we will never fully understand the human mind until we understand its origins and evolution in the cognitive biology that Homo sapiens shares with others in the living world. This includes identifying continuities and discontinuities in functions and mechanisms from prokaryotes to eukaryotes, single-celled organisms to facultatively multicellular organisms and obligately multicellular organisms (plant and animal) to animals with nervous systems. At the same time, advances in synthetic biology and machine learning have clearly identified capability gaps that can only be overcome by significant conceptual advances.
Insights from the highly diverse cognitive processes found across the tree of life are essential to advancing research in several fields, such as understanding decision making by cells during regeneration, embryogenesis and cancer, or the computational processes that allow transcriptional and physiological networks to exhibit adaptive plasticity and memory when confronted with novel stresses. Recent advances in molecular genetics have enabled unprecedented control over protein function, but progress in many fields (e.g. synthetic biology, artificial intelligence and regenerative medicine) is stymied by a rudimentary understanding of information processing and decision making performed by living matter at all levels of the organization. While evolutionary studies demonstrate that neurons evolved by speed-optimizing functions that existed in pre-neural cell types, this fact is rarely appreciated or exploited.
The motivation for the basal cognition themed issue was to catalyse a wave of (ideally) innovative, transdisciplinary work among groups and communities that currently do not interact. Erasing artificial boundaries between disciplines on questions of mutual interest should allow insights in one field to benefit work in others. The original research in these two issues—empirical and theoretical, across multiple scientific disciplines—was brought together for a single purpose: to demonstrate the feasibility of ‘connecting the dots’ in the evolution of cognition from prokaryotes to metazoans with nervous systems and everything in between. We believe the collection demonstrates how this diverse work potentially constitutes a coherent, conceptually unified research domain with significant implications for understanding cognition not only in more complex animals, such as ourselves, but also in places yet to be discovered.
Acknowledgements
The guest editors and many of the contributors to this theme issue are indebted to the Konrad Lorenz Institute for Evolution and Cognition Research (KLI) for hosting the ‘The Groundfloor of Cognition’ workshop at Klosterneuburg (Austria) in June 2018, which gave birth to this work, and to Eva Jablonka for priceless help and guidance in getting the workshop off the ground. Thanks also to Rafael Yuste and Pauline Schaap for helping the editorial team get to the starting gate for this issue; to Gürol Süel for his review of the section in this article relating to his laboratory's work; and to the incomparable Helen Eaton for her unflappable calm, professionalism and good humour throughout the publication process. Pamela Lyon warmly thanks Richard Bradshaw, without whose unflagging support her work would not be possible. Michael Levin gratefully acknowledges the support of the Barton Family Foundation, the Elisabeth Giauque Trust and the Templeton World Charity Foundation.
Endnotes
The material in this paragraph is adapted from the text of a letter of support for the basal cognition theme issue from Rafael Yuste.
All references to MacKinnon's work mentioned here were derived from his Nobel Prize speech of 8 December 2004 [13].
Everything in this paragraph comes from [3].
The material in this paragraph is adapted from [21].
All of the information in this and the remaining paragraphs of this section were adapted from this source, except where otherwise noted [21].
We are grateful to Gáspár Jékely for ideas in the first two paragraphs of this section.
Contributor Information
Pamela Lyon, Email: pamela.lyon64@gmail.com.
Michael Levin, Email: michael.levin@tufts.edu.
Data accessibility
This article has no additional data.
Authors' contributions
P.L., F.K., D.A. and M.L. all contributed text to the paper. P.L. synthesized the material and finalized the draft.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Lyon P. 2015. The cognitive cell: bacterial behaviour reconsidered. Front. Microbiol. 6, 264. ( 10.3389/fmicb.2015.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prindle A, Liu J, Asally M, Ly S, Garcia-Ojalvo J, Suel GM. 2015. Ion channels enable electrical communication in bacterial communities. Nature 527, 59-63. ( 10.1038/nature15709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang C-Y, Bialecka-Fornal M, Weatherwax C, Prindle A, Liu J, Garcia-Ojalvo J, Süel GM. 2020. Encoding membrane-potential-based memory within a microbial community. Cell Syst. 10, 417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapiro JA. 2020. All living cells are cognitive. Biochem. Biophys. Res. Commun. ( 10.1016/j.bbrc.2020.08.120) [DOI] [PubMed] [Google Scholar]
- 5.Goo E, et al. 2012. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc. Natl Acad. Sci. USA 109, 19 775-19 780. ( 10.1073/pnas.1218092109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagage V, Uphoff S. 2020. Pulses and delays, anticipation and memory: seeing bacterial stress responses from a single-cell perspective. FEMS Microbiol. Rev. 44, 565-571. ( 10.1093/femsre/fuaa022) [DOI] [PubMed] [Google Scholar]
- 7.Tagkopoulos I, Liu Y-C, Tavazoie S. 2008. Predictive behavior within microbial genetic networks. Science 320, 1313-1317. ( 10.1126/science.1154456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Losick RM. 2020. Bacillus subtilis: a bacterium for all seasons. Curr. Biol. 30, R1146-R1150. ( 10.1016/j.cub.2020.06.083) [DOI] [PubMed] [Google Scholar]
- 9.Bargmann CI, Marder E. 2013. From the connectome to brain function. Nat. Methods 10, 483-490. ( 10.1038/nmeth.2451) [DOI] [PubMed] [Google Scholar]
- 10.Yuste R. 2017. The origins of the BRAIN initiative: a personal journey. Cell 171, 726-735. ( 10.1016/j.cell.2017.10.026) [DOI] [PubMed] [Google Scholar]
- 11.Koshland DE. 1980. Bacterial chemotaxis in relation to neurobiology. Annu. Rev. Neurosci. 3, 43-75. ( 10.1146/annurev.ne.03.030180.000355) [DOI] [PubMed] [Google Scholar]
- 12.Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKinnon R. 2004. Potassium channels and the atomic basis of selective ion conduction. In The Nobel Prizes 2003 (ed. Frängsmyr T), pp. 214–235. Stockholm, Sweden: Nobel Foundation. See https://www.nobelprize.org/prizes/chemistry/2003/mackinnon/lecture/. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar C, Vlamakis H, Losick R, Kolter R. 2007. Thinking about Bacillus subtilis as a multicellular organism. Curr. Opin. Microbiol. 10, 638-643. ( 10.1016/j.mib.2007.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, et al. 2015. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550-554. ( 10.1038/nature14660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Corral R, Liu J, Süel GM, Garcia-Ojalvo J. 2018. Bistable emergence of oscillations in growing Bacillus subtilis biofilms. Proc. Natl Acad. Sci. USA 115, E8333-E8340. ( 10.1073/pnas.1805004115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Martinez-Corral R, Prindle A, Lee D-YD, Larkin J, Gabalda-Sagarra M, Garcia-Ojalvo J, Süel GM. 2017. Coupling between distant biofilms and emergence of nutrient time-sharing. Science 356, 638-642. ( 10.1126/science.aah4204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Corral R, Liu J, Prindle A, Süel GM, Garcia-Ojalvo J. 2019. Metabolic basis of brain-like electrical signalling in bacterial communities. Phil. Trans. R. Soc. B 374, 20180382. ( 10.1098/rstb.2018.0382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darwin CR. 1860. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 2nd edn. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 20.de Waal FBM. 2002. Evolutionary psychology: the wheat and the chaff. Curr. Dir. Psychol. Sci. 11, 187-191. ( 10.1111/1467-8721.00197) [DOI] [Google Scholar]
- 21.Lyon P. 2020. Of what is ‘minimal cognition’ the half-baked version? Adapt. Behav. 28, 407-424. ( 10.1177/1059712319871360) [DOI] [Google Scholar]
- 22.Verworn M. 1889. Psycho-Physiologische protisten-studien: experimentelle Untersuchungen. Jena, Germany: Verlag von Gustav Fischer. [Google Scholar]
- 23.Binet A. 1890. The psychic life of micro-organisms: a study in experimental psychology. by Alfred Binet. Translated from the French by Thomas McCormack. Chicago, 1889. J. Ment. Sci. 36, 89-91. ( 10.1192/bjp.36.152.89) [DOI] [Google Scholar]
- 24.Jennings HS. 1904. Contributions to the study of the behavior of the lower organisms. Washington, DC: Carnegie Institution of Washington. [Google Scholar]
- 25.Washburn MF. 1936. The animal mind: A text-book of comparative psychology. New York, NY: The MacMillan Company. [Google Scholar]
- 26.Monod J. 1972. Chance and necessity: An essay on the natural philosophy of modern biology. New York, NY: Vintage Books. [Google Scholar]
- 27.Beer RD. 2020. Some historical context for minimal cognition. Adapt. Behav. 105971232093159. ( 10.1177/1059712320931595) [DOI] [Google Scholar]
- 28.Beer RD. 1990. Intelligence as adaptive behavior: An experiment in computational neuroethology. San Diego, CA: Academic Press. [Google Scholar]
- 29.Brooks R. 1991. Intelligence without representation. Artif. Intell. 47, 139-159. [Google Scholar]
- 30.Mineta K, Nakazawa M, Cebria F, Ikeo K, Agata K, Gojobori T. 2003. Origin and evolutionary process of the CNS elucidated by comparative genomics analysis of planarian ESTs. Proc. Natl Acad. Sci. USA 100, 7666-7671. ( 10.1073/pnas.1332513100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jékely G. 2021. The chemical brain hypothesis for the origin of nervous systems. Phil. Trans. R. Soc. B 376, 20190761. ( 10.1098/rstb.2019.0761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moroz L, Romanova D, Kohn A. 2021. Neural vs. alternative integrative systems: molecular insights into origins of neurotransmitters. Phil. Trans. R. Soc. B 376, 20190762. ( 10.1098/rstb.2019.0762) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Göhde R, Naumann B, Laundon D, Imig C, McDonald K, Cooper B, Varoqueaux F, Fasshauer D, Burkhardt P. 2021. Choanoflagellates and the ancestry of neurosecretory vesicles. Phil. Trans. R. Soc. B 376, 20190759. ( 10.1098/rstb.2019.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakirov B, Charalambous G, Thuret R, Aspalter I, Van-Vuuren K, Mead T, Harrington K, Regan E, Herbert S, Bentley K. 2021. Active perception during angiogenesis: filopodia speed up Notch selection of tip cells in silico and in vivo. Phil. Trans. R. Soc. B 376, 20190753. ( 10.1098/rstb.2019.0753) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aristotle. 2001. De Anima. In The basic works of Aristotle (ed. McKeon R), pp. 533-603. New York, NY: Modern Library Classics/Random House. [Google Scholar]
- 36.Hall M. 2011. Plants as persons: A philosophical botany. Albany, NY: SUNY Press. [Google Scholar]
- 37.Sterelny K, Griffiths PE. 1999 Sex and Death: An Introduction to the Philosophy of Biology. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 38.Rose S. 1998. The rise of neurogenetic determinism. In Consciousness and human identity (ed. Cornwell J), pp. 86-100. Oxford, NY: Oxford University Press. [Google Scholar]
- 39.Allen C. 2017. On (not) defining cognition. Synthese 194, 4233-4249. ( 10.1007/s11229-017-1454-4) [DOI] [Google Scholar]
- 40.Keijzer F. 2020. Demarcating cognition: the cognitive life sciences. Synthese. ( 10.1007/s11229-020-02797-8) [DOI] [Google Scholar]
- 41.Shettleworth SJ. 1998. Cognition, evolution and behavior. Oxford, UK: Oxford University Press. [Google Scholar]
- 42.Bechtel W. 2013. The endogenously active brain: the need for an alternative cognitive architecture. Phil. Sci. 17, 3-30. ( 10.4000/philosophiascientiae.846) [DOI] [Google Scholar]
- 43.Lyon P. 2006. The biogenic approach to cognition. Cog. Proc. 7, 11-29. ( 10.1007/s10339-005-0016-8) [DOI] [PubMed] [Google Scholar]
- 44.Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165-199. ( 10.1146/annurev.micro.55.1.165) [DOI] [PubMed] [Google Scholar]
- 45.Shi W, Zusman DR. 1993. Fatal attraction. Nature 366, 414-415. ( 10.1038/366414a0) [DOI] [PubMed] [Google Scholar]
- 46.Damasio AR. 1999. The feeling of what happens: body and emotion in the making of conscousness. New York, NY: Harcourt Brace & Company. [Google Scholar]
- 47.Barrett LF. 2006. Valence is a basic building block of emotional life. J. Res. Pers. 40, 35-55. ( 10.1016/j.jrp.2005.08.006) [DOI] [Google Scholar]
- 48.Koshland DE Jr. 1980. Bacterial chemotaxis as a model behavioral system. New York, NY: Raven Press. [Google Scholar]
- 49.Ginsburg S, Jablonka E. 2021. Evolutionary transitions in Learning and cognition. Phil. Trans. R. Soc. B 376, 20190766. ( 10.1098/rstb.2019.0766) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baluska F, Mancuso S. 2021. Individuality, self and sociality of vascular plants. Phil. Trans. R. Soc. B 376, 20190760. ( 10.1098/rstb.2019.0760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arendt D. 2021. The elementary nervous system: expanded local reflex arc or whole-body integrative system? Phil. Trans. R. Soc. B 376, 20200347. ( 10.1098/rstb.2020.0347) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pezzulo G, LaPalme J, Durant F, Levin M. 2021. Bistability of somatic pattern memories: stochastic outcomes in bioelectric circuits underlying regeneration. Phil. Trans. R. Soc. B 376, 20190765. ( 10.1098/rstb.2019.0765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manicka S, Levin M. 2019. The cognitive lens: a primer on conceptual tools for analysing information processing in developmental and regenerative morphogenesis. Phil. Trans. R. Soc. B 374, 20180369. ( 10.1098/rstb.2018.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pezzulo G, Levin M. 2015. Re-membering the body: applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr. Biol. 7, 1487-1517. ( 10.1039/c5ib00221d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bechtel W, Bich L. 2021. Grounding cognition: heterarchical control mechanisms in biology. Phil. Trans. R. Soc. B 376, 20190751. ( 10.1098/rstb.2019.0751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crumley CL. 2008. Heterarchy and the analysis of complex societies. Archeol. Papers Am. Anthropol. Assoc. 6, 1-5. ( 10.1525/ap3a.1995.6.1.1) [DOI] [Google Scholar]
- 57.Lyon P, Kuchling F. 2021. Valuing what happens: a biogenic approach to valence and (potentially) affect. Phil. Trans. R. Soc. B 376, 20190752. ( 10.1098/rstb.2019.0752) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dupre C, Yuste R. 2017. Non-overlapping neural networks in Hydra vulgaris. Curr. Biol. 27, 1085-1097. ( 10.1016/j.cub.2017.02.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hanson A. 2021. Spontaneous electrical low-frequency oscillations: a possible role in Hydra and all living systems. Phil. Trans. R. Soc. B 376, 20190763. ( 10.1098/rstb.2019.0763) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wan KY, Jékely G. 2021. Origins of eukaryotic excitability. Phil. Trans. R. Soc. B 376, 20190758. ( 10.1098/rstb.2019.0758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barrios E. 2007. Soil biota, ecosystem services and land productivity. Ecol. Econ. 64, 269-285. ( 10.1016/j.ecolecon.2007.03.004) [DOI] [Google Scholar]
- 62.Dinet C, Michelot A, Herrou J, Mignot T. 2021. Linking single cell decisions to collective behaviours in social bacteria. Phil. Trans. R. Soc. B 376, 20190755. ( 10.1098/rstb.2019.0755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Y, Kaiser AD, Jiang Y, Alber MS. 2009. Periodic reversal of direction allows Myxobacteria to swarm. Proc. Natl Acad. Sci. USA 106, 1222-1227. ( 10.1073/pnas.0811662106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaap P. 2021. From environmental sensing to developmental control: cognitive evolution in dictyostelid social amoebas. Phil. Trans. R. Soc. B 376, 20190756. ( 10.1098/rstb.2019.0756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boussard A, Fessel A, Oettmeier C, Briard L, Döbereiner H-G, Dussutour A. 2021. Adaptive behaviour and learning in slime moulds: the role of oscillations. Phil. Trans. R. Soc. B 376, 20190757. ( 10.1098/rstb.2019.0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.