Abstract
Uncertainty has been shown to impact political evaluation, yet the exact mechanisms by which uncertainty affects the minds of citizens remain unclear. This experiment examines the neural underpinnings of uncertainty in political evaluation using functional magnetic resonance imaging (fMRI). During fMRI, participants completed an experimental task where they evaluated policy positions attributed to hypothetical political candidates. Policy positions were either congruent or incongruent with candidates' political party affiliation and presented with varying levels of certainty. Neural activity was modelled as a function of uncertainty and incongruence. Analyses suggest that neural activity in brain regions previously implicated in affective and evaluative processing (anterior cingulate cortex, insular cortex) differed as a function of the interaction between uncertainty and incongruence, such that activation in these areas was greatest when information was both certain and incongruent, and uncertainty influenced processing differently as a function of the valence of the attached information. These findings suggest that individuals are attuned to uncertainty in the stated issue positions of politicians, and that the neural processing of this uncertainty is dependent on congruence of these positions with expectations based on political party identification. Implications for the study of emotion and politics and political cognition are discussed.
This article is part of the theme issue ‘The political brain: neurocognitive and computational mechanisms’.
Keywords: evaluation, uncertainty, incongruence, fMRI, political cognition, political neuroscience
1. Introduction
Uncertainty is an inherent part of the political process, given that political issues are ever increasing in complexity and citizens often lack the motivation or ability to fully understand the details. Political scientists have long acknowledged uncertainty as part of the political process [1], and shown, for example, that people are less likely to use information to make decisions about political candidates when that information is subjectively uncertain [2] and that uncertainty can be associated with ambivalence and more negative evaluations of candidates [3]. Politicians may project uncertainty about positions they hold on particular issues intentionally or unintentionally. Some empirical work supports the popular assumption that ‘flip-flopping’, equivocating, or being vague has negative consequences for politicians because it portrays them as indecisive [4,5], whereas other work says it is inconsequential given partisan motivations [6,7] or that this indecision can even be viewed positively in the minds of some voters [8]. However, this work does not identify the effects of uncertainty, per se, and has revealed somewhat inconsistent findings.
Other political science work has examined the impact of emotions that are likely related to uncertainty, such as anxiety, suggesting that anxiety is likely to lead to a more open-minded cognitive style, whereby people consider new information and are more willing to engage in compromise [9–11]. Indeed, past work has shown that uncertainty can lead to increased political tolerance and willingness to compromise, but only under certain conditions [12,13]. Uncertainty is more likely to lead to positive outcomes in neutral or positive contexts, but more likely to produce the opposite when paired with negative affect or threat. What remains underexplored at this point is understanding why uncertainty has these effects on political attitudes and beliefs, and how the neural response to uncertainty may change as a function of affective context.
While some emotions or affective states tend to be clearly defined (e.g. fear or threat), uncertainty is a more diffuse affective state. Threat signals the direct potential for harm, whereas uncertainty is simply a signal that we lack information and requires context for interpretation. Responses to uncertainty are likely to differ based on what the uncertainty is attached to. In other words, we argue that effects of uncertainty are likely to be context-dependent [12,13]. From this perspective, it is important to examine how uncertainty affects political cognition differently when attached to different types of information.
Research in political neuroscience has begun to examine processes involved in political evaluation, such as political candidate perception and evaluation, but has not directly examined how uncertainty influences these processes. Much of this work has presented participants with names or faces of political candidates, and found activation in many of the same brain regions involved in evaluative processing more generally, including the amygdala, insula, anterior cingulate and orbitofrontal cortex (see e.g. [14–16]). For example, functional magnetic resonance imaging (fMRI) studies have shown activation in anterior cingulate cortex (ACC), insula and dorsolateral prefrontal cortex (dlPFC) when people are evaluating disliked or opposition political candidates [17,18]. However, other fMRI work has shown that some of these regions also respond to favoured candidates [19], suggesting that it may be premature to explain these effects purely on the basis of positive versus negative valence. Many of these brain regions appear to serve multiple functions, and there is ongoing debate about what the exact nature of brain functions in these regions might be. For example, the ACC has been implicated in cognitive control, conflict monitoring and exploring alternative courses of action [20–22] and is sensitive to uncertainty [23–25]. Insula responds to motivationally relevant information or salience and may aid in the process of integrating cognitive with emotional information [26,27] and has also been shown to be sensitive to uncertainty [28,29], although much of this work has been conducted in the context of decision making rather than politics. There is also some evidence that activation in these regions—insula and ACC—may be linked [30]. In prior work, we have demonstrated using fMRI that insula and ACC respond to incongruent (versus congruent) policy statements from hypothetical political candidates, and that this effect was more likely to occur when evaluating own party (versus out party) candidates and for participants who identified as politically liberal versus conservative [31]. However, we have yet to investigate the role of uncertainty in candidates' stated issue positions, which is of particular importance given the prevalence of uncertainty in contemporary electoral politics. In particular, we are interested here in whether people respond differently to political uncertainty as a function of affective context.
2. Overview of present work
In the present work, we used fMRI to examine the impact of both uncertainty and incongruent information on political evaluation. Although work in political science has typically examined unidirectional effects of uncertainty on political evaluation, we expect the effects of uncertainty to depend on the context of evaluation—specifically, whether the information given with respect to a candidate is congruent with the candidate's party identification. We test this by leveraging instances of issue positions conflicting with candidates' party identification as an opportunity to study when context determines the effects of uncertainty on political evaluation. We focus our inquiry on brain regions previously implicated in evaluative processing, including the insula and anterior cingulate. Given past research in cognitive neuroscience, it is unclear whether some areas in the brain are just sensitive to uncertainty regardless of context, or if uncertainty is having effects on processing more indirectly. We think it is unlikely that there is an ‘uncertainty area’ of the brain, but rather uncertainty may change the motivational relevance of different types of information. Past research might suggest that either uncertainty or incongruence would activate regions such as insula or ACC, but we expect to find evidence for an interaction between uncertainty and incongruence.
3. Method
(a). Participants
The analyses reported here rely on the same dataset we used for analyses published in Haas et al. [31], but here we focus on uncertainty, which was not included in prior analyses. Fifty-eight healthy adults (34 female and 24 male; age range: 19–59, M = 25.4, s.d. = 9.2) participated in the experiment. Participants were politically diverse, with 32 identifying as liberal and 26 identifying as conservative. Participants were recruited from the University of Nebraska-Lincoln and surrounding community. All participants were right-handed, and had normal or corrected-to-normal vision, and no known history of neurological disorders. Participants were safety-screened to ensure eligibility for MRI and provided informed consent in accord with study approval by the institutional review board. They were compensated $30 US for their participation.
(b). Experimental design and stimuli
Full details of the experimental design are available in Haas et al. [31], but the relevant details are also summarized here for convenience. We did not examine uncertainty in the prior analyses, so that variable is explained in more detail here. Participants came to the MRI centre and participated in a blocked rapid event-related fMRI experiment where they evaluated the policy positions of hypothetical political candidates while undergoing MRI scanning. The experiment had a 2 uncertainty (certain/uncertain) × 2 incongruence (congruent/incongruent) × 2 block type (ingroup/outgroup) within-subjects design. The experimental paradigm was designed to manipulate both uncertainty and incongruence as a function of the candidates' issue positions and party identification. Prior to the start of each block, participants received information about the political candidate (Democrat or Republican) that they would be evaluating for that set of trials. All participants evaluated policy positions attributed to four different candidates (two Democrats and two Republicans) in a randomized order.
On each trial, participants received information about a specific policy position attributed to the candidate and information about both: (i) whether the candidate supports or opposes that issue and (ii) the certainty with which the candidate holds that position on the issue. Specifically, participants saw one of four cues on each trial: ‘may support,’ ‘may oppose,’ ‘definitely supports’ or ‘definitely opposes’. Next, a policy statement appeared, and participants were asked to evaluate how they felt about the candidate's position on that issue by selecting either good or bad using the response pad while in the scanner (1 = good, 2 = bad). Each trial consisted of presentation of a cue (750 ms) followed by a policy statement (4250 ms) and a jittered fixation cross (inter-stimulus interval, ISI: 2500, 5000, 7500, 10 000 or 12 500 ms). Policy statements were selected on the basis of pilot data and were selected based on clear categorization as issues that Republicans or Democrats tend to support and roughly equated on attitude extremity and importance (see [31]). Participants saw each statement twice over the course of the experiment, but never the same issue twice for the same candidate. A majority of the issue positions (66.6%) in each block were congruent with the candidate's political identification (as determined by behavioural pilot data), but a smaller subset were incongruent with his identification (33.3%) to allow for examination of both congruent and incongruent issue positions. Overall, half of the trials were uncertain (50%) and half were certain. But, uncertainty was not distributed evenly across congruent and incongruent trials. To increase external validity of the task, incongruent trials were more likely to be uncertain (62.5%) than congruent trials (43.75%). After the MRI portion of the study, participants completed an additional post-scan survey and responded to a series of demographic questions.
(c). MRI data acquisition
MRI data were acquired using a Siemens Skyra 3.0 T MRI with a 32-channel head coil. Prior to functional imaging, a high-resolution T1-weighted 3D anatomical image (MPRAGE; field of view (FoV) read = 256 mm, slice thickness = 1.0 × 1.0 × 1.0 mm, repetition time (TR) = 2400 ms, echo time (TE) = 3.37 ms, inversion time (TI) = 991 ms, prescan normalize on, PAT mode = GRAPPA) was collected for spatial normalization. fMRI data were acquired with acquisition parallel to the anterior commissure-posterior commissure (AC–PC) line (42 slices, FoV read = 220 mm, slice thickness = 3.0 × 3.0 × 3.0 mm, TR = 2500 ms, TE = 30 ms, flip angle = 80°, prescan normalize off). Participants completed four blocks of functional scanning, lasting approximately 8.5 min each. The first five volumes of each run were discarded to avoid variability due to pre-steady-state functional data.
(d). MRI data preprocessing and analysis
MRI data were preprocessed as reported in Haas et al. [31] using the fMRI expert analysis tool (FEAT) in the FMRIB Software Library (FSL; [32,33]) on macOS. The high-resolution 3D anatomical image (MPRAGE) was skull-stripped using FSL's brain extraction function (BET; [34]). Data from functional runs were subjected to normalization, registration to both MPRAGE and standard space (MNI152), spatial smoothing at FWHM (full width at half maximum) of 5 mm, slice timing correction (to correct for interleaved data acquisition), and motion correction using MCFLIRT [35].
Statistical analyses were conducted using the general linear model (GLM) as implemented in FSL. Time-series data were modelled at the first level (the trial level) using FMRIB's improved linear model (FILM), and then, higher-level analysis (across sessions first, and then across subjects) was carried out using FMRIB's local analysis of mixed effects (FLAME; see [33]). First, the blood oxygen level-dependent (BOLD) signal was modelled at the trial level for each run as a function of uncertainty (certain/uncertain), incongruence (congruent/incongruent) and their interaction. Data from each run were then averaged across subjects using a fixed effects model.1 The subject-level analyses were then combined into group-level region of interest (ROI) analyses using FSL FLAME1. ROI analyses on left amygdala, right amygdala, bilateral insula, and ACC were masked prior to analysis (using anatomical masks from the Harvard–Oxford Cortical/Subcortical Atlases provided with FSL) and cluster corrected for multiple comparisons. In FSL, a Z-statistic > 2.0 was used to define contiguous clusters, and then cluster probabilities were compared with the (corrected) cluster significance threshold of p < 0.05 using Gaussian random field theory [36].2
In order to plot the BOLD activation to decompose interaction effects, cluster masks were created using fslmaths in FSL for each significant cluster of activation, and mean activation to each of the four conditions (certain congruent, certain incongruent, uncertain congruent, uncertain incongruent) was extracted using these cluster masks in FEATQuery. Post hoc tests were conducted in R, and this extracted data was used to plot MRI interaction effects (MRI summary data and syntax are available at https://osf.io/hpv8m/).
4. Results
(a). Behavioural task data
A within-subject ANOVA was used to examine response latency as a function of uncertainty (certain/uncertain), incongruence (congruent/incongruent) and evaluative response (good/bad). Overall, response latency was slightly faster on certain trials (M = 2567, s.d. = 982) compared with uncertain trials (M = 2591, s.d. = 961; F1,51 = 3.162, p = 0.0813). This effect was moderated by incongruence (F1,54 = 4.027, p = 0.0498), such that participants were faster to respond on both certain congruent trials (M = 2542, s.d. = 800) compared with certain incongruent trials (M = 2625, s.d. = 765; F1,57 = 24.86, p < 0.001), as well as uncertain congruent trials (M = 2567, s.d. = 797) compared with uncertain incongruent trials (M = 2643, s.d. = 801; F1,57 = 12.72, p < 0.001) trials, but the size of the difference was a bit larger for certain relative to uncertain trials (figure 1). The three-way interaction of uncertainty × incongruence × response was not statistically significant (F1,57 = 1.69, p = 0.199), but there was a significant two-way interaction of uncertainty × response (F1,50 = 5.741, p = 0.0204). When participants responded good, they were faster to respond on certain trials (M = 2521, s.d. = 819) relative to uncertain trials (M = 2560, s.d. = 789; F1,57 = 4.268, p = 0.0434). When participants responded bad, the difference between certain trials (M = 2611, s.d. = 782) and uncertain trials (M = 2624, s.d. = 778; F1,57 = 0.434, p = 0.513) was not significant.3 Raw data and syntax for behavioural task analyses in R are available at https://osf.io/hpv8m/.
Figure 1.
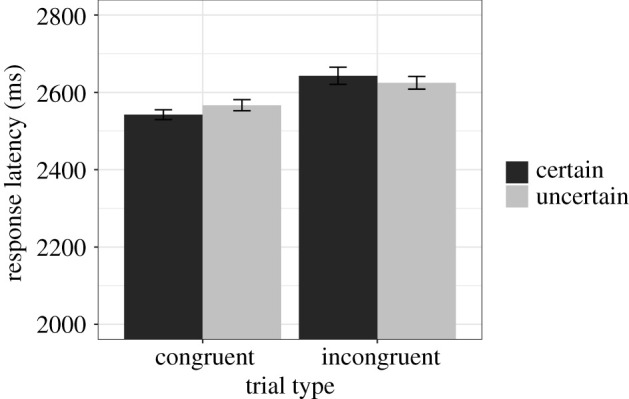
Raw response latency (in milliseconds) as a function of uncertainty (certain/uncertain) and incongruence (congruent/incongruent). Error bars represent standard errors.
(b). fMRI data
BOLD signal was modelled as a function of uncertainty (certain/uncertain) and incongruence (congruent/incongruent).4 ROI analyses revealed significant clusters of activation in our primary ROI—insular cortex and ACC—which will be detailed below (see table 1 for full list of significant clusters).5 Overall, we find no significant clusters of amygdala activation, which is consistent with our prior work [31].
Table 1.
Significant clusters of BOLD activation in insula and ACC for main effects and interactions of uncertainty (certain/uncertain) and incongruence (congruent/incongruent). X, Y, Z coordinates are in MNI space. Cluster information for main effects is based on directional contrasts (t-tests) and cluster information for interactions is based on non-directional contrasts (F-tests).
| contrast | anatomical label(s) | side | cluster size | p-value | peak activation (Z-score) | X | Y | Z |
|---|---|---|---|---|---|---|---|---|
| certain > uncertain | insular cortex; lateral orbitofrontal cortex | left | 720 | 0.000271 | 4.37 | −32 | 20 | −16 |
| certain > uncertain | insular cortex; lateral orbitofrontal cortex | right | 479 | 0.00277 | 3.95 | 32 | 28 | −2 |
| certain > uncertain | paracingulate gyrus | — | 1392 | <0.001 | 4.14 | −4 | 24 | 42 |
| incongruent > congruent | insular cortex; lateral orbitofrontal cortex | right | 409 | 0.00863 | 3.89 | 36 | 24 | −2 |
| incongruent > congruent | cingulate gyrus, anterior division; paracingulate gyrus | — | 1644 | <0.001 | 4.15 | −6 | 16 | 34 |
| uncertainty × incongruence | insular cortex; lateral orbitofrontal cortex | left | 919 | <0.001 | 4.44 | −32 | 24 | 2 |
| uncertainty × incongruence | insular cortex; lateral orbitofrontal cortex | right | 638 | 0.000467 | 3.33 | 42 | 20 | 2 |
| uncertainty × incongruence | paracingulate gyrus; cingulate gyrus, anterior division | — | 2548 | <0.001 | 4.85 | 0 | 20 | 42 |
(c). Main effects of uncertainty and incongruence
First, we examined main effects of uncertainty and incongruence by examining directional (t) contrasts designed to compare differences in BOLD activation for uncertain > certain trials (and for incongruent > congruent trials). Here we examine results from ROI analyses in ACC, insula and amygdala. These analyses revealed significant clusters of activation in bilateral insula (extending into lateral orbitofrontal cortex) and ACC that showed greater activation to certain > uncertain trials (figure 2). The insula analysis revealed significant clusters of activation for certain > uncertain trials on both the left (720 voxels, Z-max = 4.37, p = 0.000271; MNI coordinates: X = −32, Y = 20, Z = −16) and right (479 voxels, Z-max = 3.95, p = 0.00277; MNI coordinates: X = 32, Y = 28, Z = −2). The activation in ACC for certain > uncertain trials was centred in paracingulate gyrus (1392 voxels, Z-max = 4.14, p < 0.001; MNI coordinates: X = −4, Y = 24, Z = 42). Consistent with the analyses on incongruence reported in our prior work [31], we also find significant clusters of activation in right insula (409 voxels, Z-max = 3.89, p = 0.00863; MNI coordinates: X = 36, Y = 24, Z = −2) and ACC (1644 voxels, Z-max = 4.15, p < 0.001; MNI coordinates: X = −6, Y = 16, Z = 34) for incongruent > congruent trials (figure 2). We found no significant clusters of activation in insula or ACC for the reverse contrasts (congruent > incongruent or uncertain > certain). Masked analyses for left and right amygdala showed no significant clusters of activation for any of these four directional contrasts.
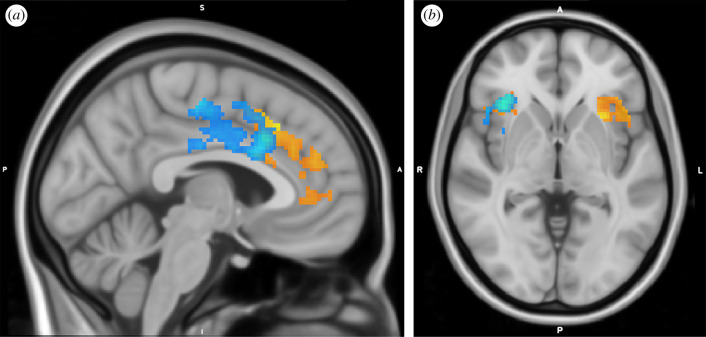
Figure 2.
BOLD activation in (a) ACC and (b) bilateral insula in response to certain > uncertain trials (red–yellow) and incongruent > congruent trials (blue–light blue). Images were created by overlaying the thresholded Z-statistic images on a standard space template (MNI152) and are centred on the peak voxel for (a) the certain > uncertain contrast in ACC and (b) the certain > uncertain contrast in right insula from the ROI analyses.
(d). Interaction of uncertainty and incongruence
Next, we examined the uncertainty by incongruence interaction in the same ROI using non-directional (F) contrasts. As expected, the above main effects for uncertainty and incongruence were qualified by significant clusters of activation for the interaction in both bilateral insula and ACC (figure 3). As above, we find no significant clusters of activation for the interaction effect in left or right amygdala.
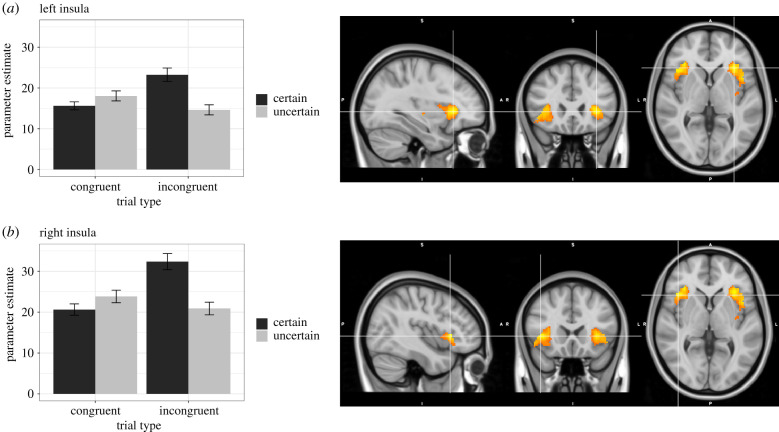
Figure 3.
BOLD activation in bilateral insula in response to the interaction of uncertainty and incongruence. Images are centred on peak voxel for each cluster from the ROI analysis: (a) left insula (MNI coordinates: X = −32, Y = 24, Z = 2) and (b) right insula (MNI coordinates: X = 42, Y = 20, Z = 2). Plots represent mean parameter estimates for each of the four trial types (certain congruent, uncertain congruent, certain incongruent, uncertain incongruent), extracted from functional cluster masks and separated by cluster. Error bars on the bar graphs represent within-subject standard errors.
The larger insula cluster was centred on the left (see figure 3a; 919 voxels, Z-max = 4.44, p < 0.001; MNI coordinates: X = −32, Y = 24, Z = 2). There was also a sizeable cluster of activation centred in right insula (see figure 3b; 638 voxels, Z-max = 3.33, p = 0.000467; MNI coordinates: X = 42, Y = 20, Z = 2). Decomposing the interaction effect in bilateral insula reveals a similar pattern of activation on both sides (figure 3). The greatest increase in activation was observed for certain incongruent trials, relative to the other three conditions, suggesting that the insula response was strongest when candidates expressed definite support for issues incongruent with their party affiliation. In left insula, activation in response to certain incongruent trials (M = 23.26, s.d. = 12.50) was greater than to uncertain incongruent trials (M = 14.64, s.d. = 9.41; F1,57 = 15.68, p < 0.001), but for congruent trials activation, was greater to uncertain (M = 18.05, s.d. = 9.35) than certain (M = 15.62, s.d. = 7.45; F1,57 = 2.802, p = 0.0996) trials. In right insula, activation in response to certain incongruent trials (M = 32.38, s.d. = 15.03) was greater than to uncertain incongruent trials (M = 20.89, s.d. = 11.82; F1,57 = 19.56, p < 0.001), but for congruent trials activation was greater for uncertain (M = 23.85, s.d. = 11.71) than certain (M = 20.64, s.d. = 10.57; F1,57 = 2.784, p = 0.101) trials.
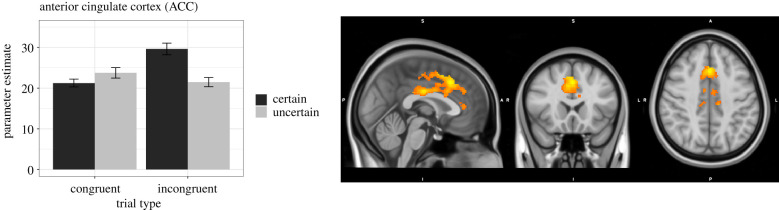
In the ACC, there was one large cluster of activation in response to the interaction of uncertainty and incongruence (figure 4). The cluster was centred on ACC/paracingulate gyrus (2548 voxels, Z-max = 4.85, p < 0.001; MNI coordinates: X = 0, Y = 20, Z = 42). Decomposing the interaction effect in ACC shows a pattern of activation that looks similar to what was observed in bilateral insula—the greatest increase in activation occurred in response to certain incongruent trials, relative to the other three trial types. Activation in response to certain incongruent trials (M = 29.64, s.d. = 10.92) was greater than to uncertain incongruent trials (M = 21.49, s.d. = 8.59; F1,57 = 19.73, p < 0.001), but for congruent trials activation was greater for uncertain (M = 23.76, s.d. = 9.76) than certain (M = 21.26, s.d. = 7.28; F1,57 = 2.837, p = 0.0976) trials.
Figure 4.
BOLD activation in ACC in response to the interaction of uncertainty and incongruence. Images are centred on peak voxel of activation from the ROI analysis (MNI coordinates: X = 0, Y = 20, Z = 42). Plots represent mean parameter estimates for each of the four trial types (certain congruent, uncertain congruent, certain incongruent, uncertain incongruent), extracted from the functional cluster mask. Error bars on the bar graphs represent within-subject standard errors.
5. Discussion
This experiment provides evidence that uncertainty impacts neural processing differently as a function of affective context. Both insula and ACC showed the greatest activation in response to policy positions that were both incongruent with candidates' party affiliation and presented with certainty. On congruent trials, the pattern was reversed but weaker, such that activation was greater to uncertain congruent versus certain congruent trials. This suggests that regions involved in evaluative processing may be especially sensitive to information that is known to be incongruent with a political candidate's stated position, perhaps so that the information can be encoded and help inform decision making later down the line.
There is an extant literature in political science on how individuals process political information and assess political candidates, yet an understanding of the psychological and neural mechanisms by which people engage in political evaluative processing is far from complete, and an understanding of the neural mechanisms underlying political evaluations is in its infancy. The primary implication of the findings presented here are that (i) people attend to uncertainty in stated issue positions for political candidates and (ii) the effects of uncertainty on how people process political information are context-dependent. It is not uncommon that issue positions are incongruent with party identification, or that voters may be uncertain about where candidates truly stand on the issues, owing to both lack of political knowledge and a lack of clarity from politicians. The present work suggests that evaluations of such incongruent issue positions depend on the level of certainty with which the incongruence exists. In the present work, we were agnostic as to the source of the uncertainty—participants were simply told positions were uncertain without attribution as to the cause of the uncertainty. In future work, it may be interesting to examine different types of uncertainty—for example, whether the uncertainty comes from candidates' having not made up their minds on an issue versus strategically obfuscating their position.
Relatedly, future work may want to consider variation in voters’ expectations of expressed uncertainty by candidates. Voters may vary in terms of how much they expect candidates to express clarity in their issue positions. Some work suggests attitude consistency is one of the most valued qualities in a political candidate [37,38], but other work in this special issue shows a nontrivial number of people just want to ‘watch the world burn’ and may prefer some degree of uncertainty [39]. Studies of individual variation in neural responses to uncertainty may improve our understanding of the electoral consequences for politicians who hold uncertain issue positions.
Future work may also want to consider voter knowledge on the issues, as it is possible those who are highly knowledgeable (and/or highly identified) may be more sensitive to these policy deviations, especially on issues with high partisan ownership (e.g. Republicans on taxes; [40,41]). Relatedly, voters are often argued to be ‘issue publics’—driven predominantly by interest in a relatively small subset of issues [42,43], and so another interesting avenue for future work may be to consider the role of issue importance at the individual level. Inconsistency or ambiguity on policy stances should matter more to voters who care deeply about particular issues, and those voters may even be willing to overlook inconsistencies on less important issues if a candidate has a clear stance on the issue(s) they care about most.
This work is consistent with the view that the psychological effects of uncertainty on political cognition and behaviour are context-dependent [12,13]—uncertainty seems to affect neural processing differently when attached to congruent versus incongruent information, at least in the neural regions examined here. This has important implications for the study of emotion and politics and political cognition more generally. Affective states such as uncertainty may sometimes be viewed as negative or attached to negative information, but can also be positively valenced. This means that we need to consider not only how individual emotions or affective states may affect processing, but how interactions of multiple affective states influence both neural processing and political behaviour.
Acknowledgments
The UNL Political Attitudes and Cognition Lab and MRI Users Group provided useful feedback on this work. Additional thanks to undergraduate research assistants Allison Haindfield, Grace Stallworth, and Sarah Sweeney and MRI Technologist Joanne Murray for assistance with data collection.
Endnotes
One contribution of 18 to a theme issue ‘The political brain: neurocognitive and computational mechanisms’.
At the subject level, we also modelled the effect of block type—whether the political candidate in each block shared the participant's political identification (ingroup candidate) or not (outgroup candidate), although this was not a primary focus of the analyses reported in this manuscript and is reported in the supplementary material.
We also ran whole brain analyses at a more stringent threshold (cluster correction with Z > 3.0 and p < 0.001) and those results are reported in the supplementary material.
We also modelled the effect of block type (ingroup versus outgroup block) to examine possible interactions with uncertainty, but none of those interactions was statistically significant so we do not focus further on block type in this paper. As reported in Haas et al. [31], we find significant main effects of incongruence and response, a significant interaction of incongruence × block type, and a marginal interaction of incongruence × response.
Analyses including block type (ingroup versus outgroup) are reported in the electronic supplementary material.
Whole brain analyses are reported in the electronic supplementary material.
Ethics
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Nebraska-Lincoln Institutional Review Board (IRB Approval no. 20141014467FB) and with the Declaration of Helsinki. All participants provided informed consent.
Data accessibility
Data, code and materials are available on Open Science Framework at: https://osf.io/hpv8m/. This includes the experimental script (E-Prime), raw data and syntax for behavioural task analyses (in R), and MRI summary data and syntax (in R).
Authors' contributions
I.J.H. designed the experiment, assisted with data collection, analysed the data and drafted the manuscript; M.N.B. provided input on experimental design, assisted with data collection and provided critical revisions on the manuscript; F.J.G. provided input on experimental design, assisted with data collection and provided critical revisions on the manuscript. All authors gave final approval for publication and agree to be held accountable for the work performed herein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the University of Nebraska-Lincoln Office for Research and Economic Development, College of Arts and Sciences, Center for Brain, Biology, and Behaviour, and Department of Political Science.
References
- 1.Downs A. 1957. An economic theory of political action in a democracy. J. Polit. Econ. 65, 135-150. ( 10.1086/257897) [DOI] [Google Scholar]
- 2.Alvarez RM, Franklin C. 1994. Uncertainty and political perceptions. J. Politics 56, 671-688. ( 10.2307/2132187) [DOI] [Google Scholar]
- 3.McGraw KM, Hasecke E, Conger K. 2003. Ambivalence, uncertainty, and processes of candidate evaluation. Polit. Psychol. 24, 421-448. ( 10.1111/0162-895X.00335) [DOI] [Google Scholar]
- 4.Fearon JD. 1994. Domestic political audiences and the escalation of international disputes. Am. Polit. Sci. Rev. 88, 577-592. ( 10.2307/2944796) [DOI] [Google Scholar]
- 5.Tomz M. 2007. Domestic audience costs in international relations: an experimental approach. Int. Org. 61, 821-840. ( 10.1017/S0020818307070282) [DOI] [Google Scholar]
- 6.Croco SE. 2016. The flipside of flip-flopping: leader inconsistency, citizen preferences, and the war in Iraq. Foreign Policy Anal. 12, 237-257. ( 10.1093/fpa/orw006) [DOI] [Google Scholar]
- 7.McDonald J, Croco SE, Turitto C. 2019. Teflon Don or politics as usual? An examination of foreign policy flip-flops in the age of Trump. J. Polit. 81, 757-766. ( 10.1086/702234) [DOI] [Google Scholar]
- 8.Tomz M, Van Houweling RP. 2009. The electoral implications of candidate ambiguity. Am. Polit. Sci. Rev. 103, 83. ( 10.1017/S0003055409090066) [DOI] [Google Scholar]
- 9.MacKuen M, Marcus GE, Neuman WR, Keele L. 2007. The third way: the theory of affective intelligence and American democracy. In The affect effect: dynamics of emotion in political thinking and behavior (eds Neuman WR, Marcus GE, Crigler AN, MacKuen M), pp. 124-151. Chicago, IL: University of Chicago Press. [Google Scholar]
- 10.MacKuen M, Wolak J, Keele L, Marcus GE. 2010. Civic engagements: resolute partisanship or reflective deliberation. Am. J. Polit. Sci. 54, 440-458. ( 10.1111/j.1540-5907.2010.00440.x) [DOI] [Google Scholar]
- 11.Marcus GE, Neuman WR, MacKuen M. 2000. Affective intelligence and political judgment. Chicago, IL: University of Chicago Press. [Google Scholar]
- 12.Haas IJ, Cunningham WA. 2014. The uncertainty paradox: perceived threat moderates the effect of uncertainty on political tolerance. Polit. Psychol. 35, 291-302. ( 10.1111/pops.12035) [DOI] [Google Scholar]
- 13.Haas IJ. 2016. The impact of uncertainty, threat, and political identity on support for political compromise. Basic Appl. Soc. Psychol. 38, 137-152. ( 10.1080/01973533.2016.1169181) [DOI] [Google Scholar]
- 14.Cunningham WA, Haas IJ, Jahn A. 2011. Attitudes. In The Oxford handbook of social neuroscience (eds Decety J, Cacioppo JT), pp. 212-226. New York, NY: Oxford University Press. [Google Scholar]
- 15.Cunningham WA, Zelazo PD. 2007. Attitudes and evaluations: a social cognitive neuroscience perspective. Trends Cogn. Sci. 11, 97-104. ( 10.1016/j.tics.2006.12.005) [DOI] [PubMed] [Google Scholar]
- 16.Cunningham WA, Zelazo PD, Packer DJ, Van Bavel JJ. 2007. The iterative reprocessing model: a multilevel framework for attitudes and evaluation. Social Cogn. 25, 736-760. ( 10.1521/soco.2007.25.5.736) [DOI] [Google Scholar]
- 17.Kaplan JT, Freedman J, Iacoboni M. 2007. Us versus them: political attitudes and party affiliation influence neural responses to faces of presidential candidates. Neuropsychologia 45, 55-64. ( 10.1016/j.neuropsychologia.2006.04.024) [DOI] [PubMed] [Google Scholar]
- 18.Spezio ML, et al. 2008. A neural basis for the effect of candidate appearance on election outcomes. Social Cogn. Affect. Neurosci. 3, 344-352. ( 10.1093/scan/nsn040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusche A, Kahnt T, Wisniewski D, Haynes JD. 2013. Automatic processing of political preferences in the human brain. Neuroimage 72, 174-182. ( 10.1016/j.neuroimage.2013.01.020) [DOI] [PubMed] [Google Scholar]
- 20.Kolling N, Behrens T, Wittmann MK, Rushworth M. 2016. Multiple signals in anterior cingulate cortex. Curr. Opin. Neurobiol. 37, 36-43. ( 10.1016/j.conb.2015.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botvinick MM. 2007. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cogn. Affect. Behav. Neurosci. 7, 356-366. ( 10.3758/CABN.7.4.356) [DOI] [PubMed] [Google Scholar]
- 22.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. 1998. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 280, 747-749. ( 10.1126/science.280.5364.747) [DOI] [PubMed] [Google Scholar]
- 23.Rushworth MF, Behrens TE. 2008. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 11, 389-397. ( 10.1038/nn2066) [DOI] [PubMed] [Google Scholar]
- 24.Yu R, Zhou W, Zhou X. 2011. Rapid processing of both reward probability and reward uncertainty in the human anterior cingulate cortex. PLoS ONE 6, e29633. ( 10.1371/journal.pone.0029633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris S, Sheth SA, Cohen MS. 2008. Functional neuroimaging of belief, disbelief, and uncertainty. Ann. Neurol. 63, 141-147. ( 10.1002/ana.21301) [DOI] [PubMed] [Google Scholar]
- 26.Uddin LQ. 2015. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55-61. ( 10.1038/nrn3857) [DOI] [PubMed] [Google Scholar]
- 27.Gu X, Liu X, Van Dam NT, Hof PR, Fan J.. 2013. Cognition–emotion integration in the anterior insular cortex. Cereb. Cortex 23, 20-27. ( 10.1093/cercor/bhr367) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singer T, Critchley HD, Preuschoff K. 2009. A common role of insula in feelings, empathy and uncertainty. Trends Cogn. Sci. 13, 334-340. ( 10.1016/j.tics.2009.05.001) [DOI] [PubMed] [Google Scholar]
- 29.Grinband J, Hirsch J, Ferrera VP. 2006. A neural representation of categorization uncertainty in the human brain. Neuron 49, 757-763. ( 10.1016/j.neuron.2006.01.032) [DOI] [PubMed] [Google Scholar]
- 30.Medford N, Critchley HD. 2010. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 214, 535-549. ( 10.1007/s00429-010-0265-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haas IJ, Baker MN, Gonzalez FJ. 2017. Who can deviate from the party line? Political ideology moderates evaluation of incongruent policy positions in insula and anterior cingulate cortex. Soc. Justice Res. 30, 355-380. ( 10.1007/s11211-017-0295-0) [DOI] [Google Scholar]
- 32.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. 2012. FSL. Neuroimage 62, 782-790. ( 10.1016/j.neuroimage.2011.09.015) [DOI] [PubMed] [Google Scholar]
- 33.Smith SM, et al. 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208-SS19. ( 10.1016/j.neuroimage.2004.07.051) [DOI] [PubMed] [Google Scholar]
- 34.Smith SM. 2002. Fast robust automated brain extraction. Hum. Brain Mapp. 17, 143-155. ( 10.1002/hbm.10062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825-841. ( 10.1006/nimg.2002.1132) [DOI] [PubMed] [Google Scholar]
- 36.Worsley KJ. 2001. Statistical analysis of activation images. In Functional MRI: an introduction to methods (eds Jezzard P, Matthews PM, Smith SM), ch. 14. Oxford, UK: Oxford University Press. [Google Scholar]
- 37.Kinder DR, Peters MD, Abelson RP, Fiske ST. 1980. Presidential prototypes. Polit. Behav. 2, 315-337. ( 10.1007/BF00990172) [DOI] [Google Scholar]
- 38.McGraw KM, Fischle M, Stenner K, Lodge M. 1996. What's in a word? Polit. Behav. 18, 263-287. ( 10.1007/BF01498602) [DOI] [Google Scholar]
- 39.Arceneaux K, Gravelle TB, Osmundsen M, Peterson MB, Reifler J, Scotto TJ. 2021. Some people just want to watch the world burn: the prevalence, psychology and politics of the ‘Need for Chaos’. Phil. Trans. R. Soc. B 376, 20200147. ( 10.1098/rstb.2020.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budge I, Farlie D. 1983. Explaining and predicting elections: issue effects and party strategies in twenty-three democracies. London, UK: George Allen & Unwin. [Google Scholar]
- 41.Stokes DE. 1963. Spatial models of party competition. Am. Polit. Sci. Rev. 57, 368-377. ( 10.2307/1952828) [DOI] [Google Scholar]
- 42.Converse PE. 1964. The nature of belief systems in mass publics. In Ideology and discontent (ed. Apter DE), pp. 206-261. New York, NY: Free Press. [Google Scholar]
- 43.Krosnick JA. 1990. Government policy and citizen passion: a study of issue publics in contemporary America. Polit. Behav. 12, 59-92. ( 10.1007/BF00992332) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, code and materials are available on Open Science Framework at: https://osf.io/hpv8m/. This includes the experimental script (E-Prime), raw data and syntax for behavioural task analyses (in R), and MRI summary data and syntax (in R).