Abstract
Research on the ‘ecology of fear’ posits that defensive prey responses to avoid predation can cause non-lethal effects across ecological scales. Parasites also elicit defensive responses in hosts with associated non-lethal effects, which raises the longstanding, yet unresolved question of how non-lethal effects of parasites compare with those of predators. We developed a framework for systematically answering this question for all types of predator–prey and host–parasite systems. Our framework reveals likely differences in non-lethal effects not only between predators and parasites, but also between different types of predators and parasites. Trait responses should be strongest towards predators, parasitoids and parasitic castrators, but more numerous and perhaps more frequent for parasites than for predators. In a case study of larval amphibians, whose trait responses to both predators and parasites have been relatively well studied, existing data indicate that individuals generally respond more strongly and proactively to short-term predation risks than to parasitism. Apart from studies using amphibians, there have been few direct comparisons of responses to predation and parasitism, and none have incorporated responses to micropredators, parasitoids or parasitic castrators, or examined their long-term consequences. Addressing these and other data gaps highlighted by our framework can advance the field towards understanding how non-lethal effects impact prey/host population dynamics and shape food webs that contain multiple predator and parasite species.
Keywords: natural enemies, sublethal effects, trait-mediated effects, community ecology, food webs, risk effects
1. Introduction
Whether we call it fear or good sense, efforts to avoid death lead animals to forgo foraging, reduce activity levels, seek refuges and exhibit other costly responses to predators [1,2]. Parasites can have similar influences. To reduce infection risk, hosts may avoid infected conspecifics [3–5], defend against infectious propagule attack [6] or avoid risky areas, such as faeces representing a hot spot of undetectable nematode eggs [7–9]. Basic emotions like ‘disgust’ [9–12] and the age-old cliché ‘avoid like the plague’ suggest that parasite avoidance is interwoven in our own history as much as is our fear of predators.
Fear exemplifies trait responses—adaptive morphological, physiological and behavioural changes—elicited by threats from predators and parasites. Trait responses to either predators or parasites can have trade-offs that trigger non-lethal effects, including reduced individual fitness and ‘trait-mediated indirect effects' [13], like trophic cascades [14–16] that shape communities [17]. Wolves, for example, frighten elk away from open foraging grounds into sheltered habitats with less nutritious vegetation, which then reduces elk birth rates [18] and alters vegetation structure [19]. By eliciting trait responses, predators and parasites impact prey/hosts, and wider communities, even without consuming them.
Trait responses to parasites, unlike most of those to predators, are not confined to proactive measures to reduce contact prior to attack. Because parasitism is not immediately lethal, hosts can respond after a successful attack by a parasite [20–22]. Immune responses are one of myriad host responses made after attack and during infections that can have non-lethal effects [23]. Further, post-infection trait responses can last a long time, leading some to hypothesize that parasites could cause stronger cumulative non-lethal effects than predators [16,24].
The question of how trait responses to parasites compare with those to predators lacks thorough testing, however, and in no small part owing to the lack of an adequate framework for drawing such comparisons. Frameworks for trait responses made in fear [13,14] and disgust [9,25] constrain the focus to trait responses to perceived risk of predation or parasitism, prior to being captured or infected. Yet, systematic examination of non-lethal effects must go beyond fear or disgust to consider the trait responses made throughout interactions. Until the research perspective on trait responses is broadened, the accumulation of non-lethal effects across different predator–prey and host–parasite interactions will remain incompletely characterized. As a result, efforts to manage ecosystems, for example, through species re-introductions and translocations, may have unanticipated consequences, as may pathogen emergence and spillover.
Here, we compare trait responses to predation and parasitism, considering how their strength, frequency and diversity (i.e. number of different types elicited) may drive differences in how non-lethal effects accrue in prey and hosts. Building on recent conceptual developments [22,25], our goal is to establish a quantitative foundation for estimating non-lethal effects in ecosystems containing multiple predator and parasite species. We draw from consumer–resource theory to construct a general framework for studying prey and host trait responses to all types of predatory and parasitic consumers, including predators, micropredators, parasitoids, parasitic castrators, typical parasites and pathogens [26]. We deconstruct interactions into sequential phases to consider trait responses before, during and after an attack, allowing us to compare and contrast the complete diversity of trait responses to predators and parasites. We use this framework to form specific hypotheses regarding how trait responses and associated non-lethal effects should manifest from interactions with different types of predators and parasites. We then use the framework to guide a systematic review of the comparative literature on trait responses that assessed the state of available information on the topic. We also analysed comparative data on larval amphibians, the most common animal group used by the reviewed studies, as a case study in quantitatively comparing trait responses to predators and parasites. We conclude by highlighting unresolved questions in the field and how to address them.
2. Definitions and terminology
We define prey/host trait responses broadly as changes in any trait (e.g. morphological, physiological (including immunological) or behavioural) to defend against predation or parasitism. We focus on prey/host adaptive, inducible responses. However, our framework (described below) can also consider maladaptive trait responses, such as those occurring from parasite manipulation [27], and constitutive defence adaptations [28]. We use ‘non-lethal effects’ as a general term to describe the direct and indirect consequences of prey/host trait responses to predators and parasites [13,29]. As we demonstrate below, terms developed for predator–prey systems, including ‘risk effects’ and ‘non-consumptive effects' [13], are insufficient for describing the diversity of trait responses relevant to consumer–resource interactions.
3. A general trait response framework
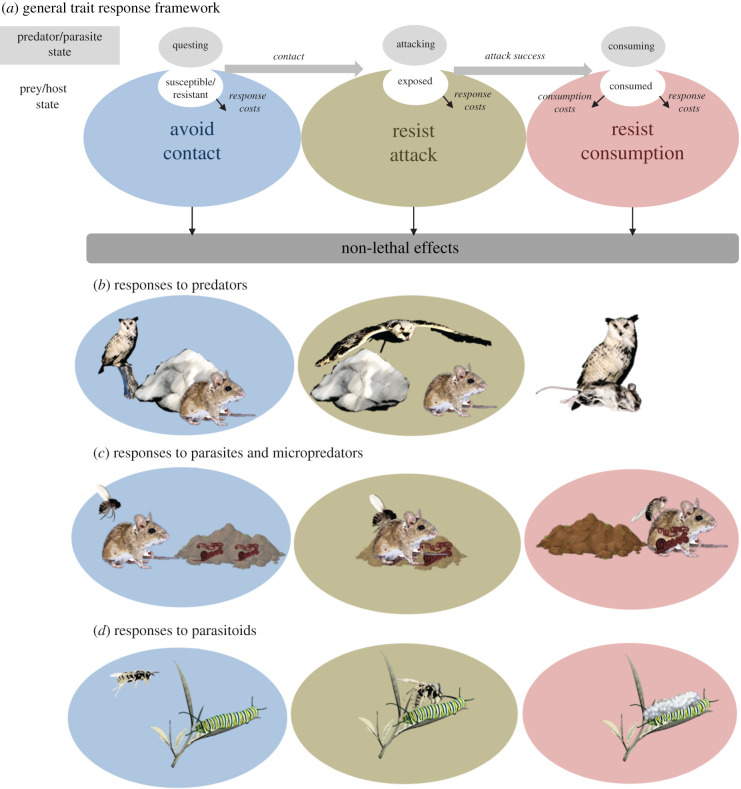
Our proposed framework captures the breadth of trait responses exhibited across different phases of predator–prey and host–parasite interactions (figure 1a). The framework is informed by a general consumer–resource model that views interactions as a sequence through which individuals transition among discrete sequential states [30] (electronic supplementary material, figure S1). Predators and parasites transition through three states—questing (pre-contact searching for prey/hosts), attacking (attempting to subdue prey/hosts) and consuming (actively ingesting prey/hosts; electronic supplementary material, figure S1). At the same time, prey/hosts transition through four states—susceptible (not contacting predator/parasite), exposed (being attacked), consumed (being eaten) and resistant (i.e. invulnerable to attack; electronic supplementary material, figure S1). Individuals transition between those states following sequential biological processes: contact, attack failure/success and feeding. The Lafferty et al. [30] model covers the dynamics of all types of host–parasite, predator–prey and other consumer–resource systems. Thus, although this review focuses on animals and their consumers, our framework applies to all resource taxa—e.g. plants, animals, fungi and bacteria—and their consumers. It can also include what might be considered to be ‘non-consumptive’ parasites, like brood parasites.
Figure 1.
A general trait response framework and predictions. (a) Prey/hosts can mount three sequential trait responses to consumers, each with distinct effects on the interaction: avoid contact, resist attack, resist consumption. Costs of responses may arise through trade-offs against other fitness-related activities (e.g. feeding and reproducing), and costs of consumption include energy drain, morbidity, tissue damage and other negative fitness effects of being eaten. The general framework can be tailored to specific types of consumer–resource interactions, such as interactions between field mice and (b) owl predators, (c) biting flies and infective nematodes (worms in faeces) or (d) between caterpillars and parasitoid wasps. The lack of a consumption stage in (b) illustrates that prey rarely respond during consumption by predators. (Online version in colour.)
Trait responses in our framework take three general forms based on their timing and function in defence—avoid contact, resist attack or resist consumption (figure 1). Susceptible prey/hosts may avoid contact with questing consumers before an attack. Effective avoidance reduces the rate that questing predators and parasites transition to attacking, and prey/hosts benefit from not transitioning from susceptible to exposed states (electronic supplementary material, table S3). Prey/hosts that become exposed may resist attack to increase the probability that attacks fail. Resisting attack includes ‘fight or flight’ responses, like hares sprinting to burrows when being chased by lynx, or tadpoles rapidly and erratically swimming when being attacked by trematode cercariae [6]. Finally, prey/hosts that are being consumed (i.e. being eaten or infected) and remain alive may resist consumption through resistance [20] and tolerance [21] mechanisms. Resistance mechanisms shorten or slow predator and parasite feeding rates, exemplified by behaviours like social grooming by primates [31] and adaptive immune responses to parasitism [23]. Tolerance mechanisms lessen the damage from feeding without affecting feeding rates. Increasing tissue repair and protecting high-risk areas of the body from parasite feeding, as tadpoles do in response to trematodes [6], exemplify tolerance mechanisms for resisting consumption.
4. Factors shaping the strength, duration and frequency of trait responses
With the full range of trait responses classified and integrated into a consumer–resource dynamics model, we can now consider the conditions that determine which responses predators and parasites are likely to elicit, and how strong and frequent they are likely to be. The extent to which prey/hosts avoid and resist consumers depends first and foremost on their basic physical and sensory abilities [32]. For instance, tadpoles cannot physically leave ponds when predators are present. They may, however, reduce activity to avoid contact [33]. Individuals must also be able to detect consumer threats to respond to them. Prey/hosts use both visual and non-visual cues to detect predation [34] and parasitism risk [35,36], making sensory limitations (e.g. sight, hearing and smell) potential constraints on trait responses. Impediments to either risk detection or the ability to act on detected risk should reduce the frequency of, or even preclude, induced trait responses, whether behavioural, morphological or physiological.
Even when prey/hosts have the physical and sensory capacity to mount responses, trade-offs may mediate the frequency and strength of trait responses [32]. Whether via reductions in foraging, reproduction or energy levels, fitness-related costs of exhibiting trait responses compete with the benefits of responses (i.e. the costs of not responding), making the frequency and strength with which individuals exhibit trait responses a matter of economics [32]. Perhaps less recognized is the possibility that trade-offs may change over different phases of a given interaction. The frequency and strength of trait responses will, interestingly, depend on their relative costs and benefits compared to available trait responses at other phases of the interaction. Exemplified by certain host–parasite interactions [37], avoiding contact may be more costly than becoming infected, potentially driving stronger and more frequent resistance responses after becoming infected. The relative costs and benefits of different trait responses can be accounted for in our framework through functions that link responses to phase-specific mortality and fecundity rates, which express response costs. Those costs are balanced by benefits of responses, expressed through response impacts on state transition rates, as described in the above section.
5. Hypothesized trait responses and non-lethal effects across different predators and parasites
The strength, frequency and diversity of trait responses in prey/hosts will also depend on traits of the predators and parasites. Predators and parasites have distinct consumer ‘strategies’ [26] that comprise traits for attacking, feeding on and impacting prey/hosts [26,30]. Strategies include: predators, micropredators, parasitoids, parasitic castrators, typical parasites and pathogens (see the electronic supplementary material for definitions) [26]. Incorporating differences in consumer strategies into our framework leads to hypotheses and predictions for how trait responses and their non-lethal effects vary among different types of interactions.
Hypothesis 1: the magnitude of trait responses scales with the fitness consequences of predator/parasite feeding. One key distinction among consumer strategies involves the fitness consequences of predation and parasitism on individual prey/hosts. Predators and most parasitoids eliminate future reproductive success of their prey/hosts by killing them, and parasitic castrators do so by blocking host reproduction. By contrast, feeding by most other types of parasites and micropredators is not so deadly and does not completely eliminate future fitness gains. Intuitively, prey/hosts should generally exhibit stronger trait responses against predators or parasites whose feeding imposes more severe damage to fitness. This leads to the prediction that consumers which eliminate prey/host fitness after successful attack—predators, parasitoids and parasitic castrators—should generally elicit the strongest trait responses of any consumers, resulting in strong non-lethal effects from a given response. Strong trait responses and non-lethal effects may also emerge with micropredators and parasites that do have strong negative fitness impacts, such as highly virulent pathogens, or the micropredators that vector them. However, trait responses to non-vectoring micropredators and less damaging parasites should be relatively weaker, with each response having weaker associated non-lethal effects. For example, we would expect amphibians to avoid breeding ponds containing fish predators and infective stages of highly virulent viruses (e.g. ranavirus) more than ponds containing pathogen-free leaches and infective stages of less virulent fungal parasites (e.g. Batrachochytrium dendrobatidis). These predictions highlight that differences in the fitness consequences of being consumed should drive variation in non-lethal effects not only between predators and parasites, but also between different types of parasites, with some parasites being more similar to predators than to other types of parasites.
Hypothesis 2: the frequency of trait responses scales with the frequency of interactions. While the magnitude of a trait response should scale with the consequence of consumption, how often prey/host exhibit responses should scale directly with how time is spent interacting with consumers. Parasites tend to be smaller bodied and more numerous than predators at the same trophic level [38], despite having comparable aggregated biomass or energy flux [38,39]. This pattern probably also applies to micropredators. If this pattern holds, prey/hosts should interact more frequently with parasites than with predators. For instance, many animals spend more time swatting at biting insects than hiding from predators [39]. Hence, parasites and micropredators may elicit high-frequency, low-magnitude responses that could have cumulative non-lethal effects as high or higher than those arising from low-frequency, strong responses.
Hypothesis 3: the timing of death determines the diversity of trait responses available to prey and hosts. Distinct consumer strategies also drive differences in the timing of prey and host death during interactions. Predators usually kill prey before or shortly after commencing to feed. This is not the case for micropredators and most parasites. In fact, keeping hosts alive during consumption can be critical to the persistence of certain parasites [40,41]. Even parasitoids and castrators can have a substantial amount of feeding time before hosts are killed or castrated, which allows hosts to resist consumption. Recognizing basic differences in the timing of prey and host mortality leads to two clear predictions for how non-lethal effects of predators and parasites differ. First, prey responses to predators will be constrained to avoiding contact and resisting attack (figure 1b). Second, parasites and micropredators will elicit defensive trait responses at all interaction phases (figure 1c,d), meaning that hosts generally have a broader toolkit for defending against parasites than prey have for defending against predators. This suggests non-lethal effects of parasites arise from more diverse types of responses than those arising from predators.
Integrating the above hypotheses leads to the predictions that: (i) predators, parasitic castrators and parasitoids should provoke strong individual responses that are concentrated in avoiding contact and resisting attack (because of their shared negation of prey/host fitness upon successful attack and consumption), and (ii) all types of parasites—including parasitoids and parasitic castrators—should generally provoke more frequent and diverse responses than do predators (because of their prolonged feeding stage on the live host). Exceptions probably exist, such as some parasitoids that paralyse hosts during initial attack, which can make hosts incapable of resisting consumption [42]. Also, as discussed above, constraints to detection may alter expected differences in how prey/hosts exhibit trait responses to different predators and parasites. For instance, to the extent that visual detection is important to elicit trait responses, animals should generally avoid contact with predators more than contact with micropredators and parasites if the generally larger size of predators makes them easier to see [26]. On average, however, our framework indicates that non-lethal effects will be strongest not from consumers that impose the highest risk of death, but rather from those that impose strong costs while keeping the consumed host or prey alive.
6. Applying the trait response framework
We used the above framework to carry out: (i) a systematic review of the literature to compare trait responses to predation and parasitism, and (ii) a case study of trait responses to predators and parasites in larval amphibians (tadpoles). The two exercises serve two purposes. First, they provide a demonstration of how our framework can be applied to gain more thorough understanding of trait responses and their non-lethal effects. Second, they identify the limits of comparative data on the topic and, within those limits, offer a preliminary synthesis of general trait response patterns across different predator and parasite strategies.
(a). Studies to measure trait responses to predation and parasitism
We compiled studies that directly compared trait responses to both predation and parasitism in a single prey/host species (see the electronic supplementary material for details of literature search). Almost all such studies measured trait responses in larval frogs (i.e. ‘tadpoles’, n = 106 entries across 13 studies; electronic supplementary material, table S1), perhaps because they are very tractable experimentally. Behavioural traits were most common, with activity level being the most reported trait (electronic supplementary material, tables S1 and S2). We did not find studies that measured immunological trait responses, probably because this is distinct to host–parasite systems. The studies spanned the following consumer strategies: solitary predators, trophically transmitted parasites, typical parasites and pathogens (electronic supplementary material, table S1); in no cases were responses to parasitoids, parasitic castrators, micropredators or social predators considered. Predator-induced trait responses were only measured during questing predator states, representing avoidance of contact, whereas measurements of parasite-induced responses included all three states: questing (n = 9), attacking (n = 5) and consuming (n = 24). The limited data we found constrained our ability to make statistical comparisons, although some general patterns did emerge.
(b). A case study in tadpoles
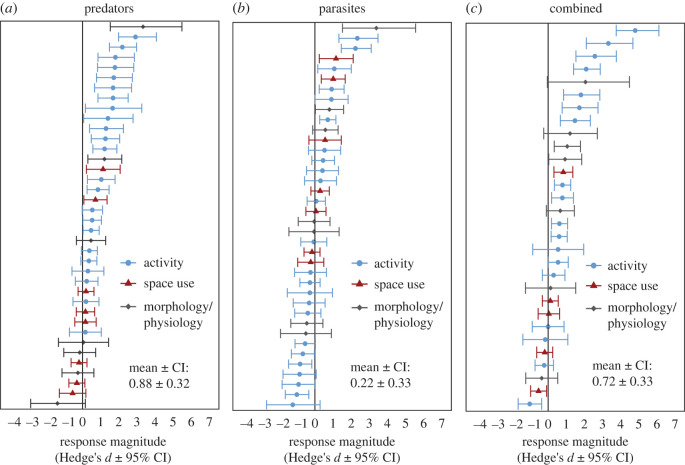
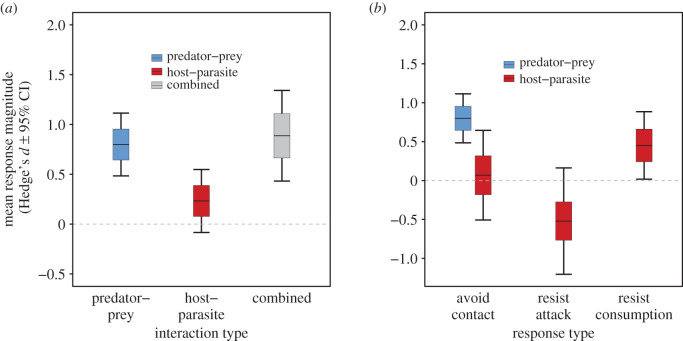
Despite the limited comparative work on trait responses to predators and parasites, there were sufficient data on predator- and parasite-induced trait responses in tadpoles, the most well-studied animal group in the reviewed studies, to perform a provisional quantitative comparison of trait responses to predators and parasites. Analysis of the data (see the detailed methods in the electronic supplementary material) showed that the magnitude and direction of tadpole responses to predators (figure 2a), parasites (figure 2b) and their combination (figure 2c) varied from study to study. However, on average and across all consumer and prey/host states, predator-induced trait responses were stronger in magnitude than parasite-induced trait responses (figure 3a; electronic supplementary material, table S4). These patterns were evident after controlling for consumer state (comparing questing predators to questing parasites) (figure 3b), though they were weaker (electronic supplementary material, table S4), probably owing to lower power of the data subset. Distinguishing between trait response types (avoid contact, resist attack, resist consumption) revealed that tadpoles did respond to parasites, but only by resisting parasites after infection and at lower magnitudes than their responses to avoid predator contact (figure 3b; electronic supplementary material, table S4). Tadpoles also responded to the simultaneous presence of predators and parasites on average, and at similar magnitudes to their responses to predators alone (figure 3a). The strong tadpole responses to predators primarily represented reductions in activity levels (electronic supplementary material, figure S7a and table S4), and they were most evident when measuring group-level responses as opposed to individual-level responses (electronic supplementary material, figure S7b and table S4). Across the host–parasite interactions studied, responses did not differ between pathogens and trophically transmitted parasites, the two parasite strategies investigated (electronic supplementary material, table S3). None of the studies measured response frequency in the wild and few considered long-term effects.
Figure 2.
Forest plots of effect sizes used in the meta-analysis. The distribution of effect sizes for responses elicited by (a) predators, (b) parasites, and (c) their combined presence resulting from prey/host adjustments in activity, space use and morphological/physiological traits. Error bars denote the 95% confidence intervals (CIs). (Online version in colour.)
Figure 3.
Relative magnitude of responses to predation versus parasitism. (a) The estimated mean magnitude of trait responses to predation, parasitism and both. (b) Mean trait responses to predators and parasites when distinguishing the type of trait responses, as defined in our framework. Only avoidance responses to questing predators were found in our literature review, probably owing to the low probability of surviving attack or consumption by predators. Responses to the combined presence of predators and parasites are not shown in (b) because only one study with this treatment had predators and parasites in the same state. Lines denote the mean response magnitudes, boxes denote the standard error of the mean and error bars denote the 95% confidence intervals. (Online version in colour.)
7. Discussion
Our framework predicts that non-lethal effects from predation and parasitism are a function of diverse and potentially interacting defensive responses by prey/hosts, exhibited at different phases of interactions. However, current comparative data comprise brief snapshots of interactions that do not fully capture the temporal dynamics of trait responses, which precludes reliable comparisons of non-lethal effects. Similarly, the growing literature on ‘non-consumptive effects' [22,43] focuses on contact avoidance, yet this misses several other ways that hosts can respond to predation and parasitism. Tracking individuals through all interaction phases could provide insight into how non-lethal effects accrue from multiple trait responses and may even detect interactive effects between trait responses (box 1; electronic supplementary material, figure S5). For example, hosts that invest heavily in immune defences may exhibit weaker avoidance of contact with parasites, particularly if avoidance conflicts with feeding, reproducing and other fitness-related activities. Longitudinal trait response data could test for prioritization of trait responses to inform estimates of the severity of non-lethal effects.
Box 1. Future directions for research of non-lethal effects.
Comparative data on trait responses to predators and parasites are limited. Multiple research avenues can address these limitations to produce more comprehensive estimates of non-lethal effects in multi-trophic ecosystems.
1. Comparative experiments of trait responses that cover a broader range of consumer–resource systems can determine how generalizable the patterns are across systems, and opens avenues to consider factors like consumer and resource phylogenies on response magnitudes. Incorporating treatments of multiple consumer threats minimizes between-study biases that arise through inherent discrepancies in environmental conditions and protocol execution of independent studies. The studies used in our case study provide examples for comparative experimental designs that future research can apply.
2. Comparative studies that include responses to parasitic castrators, parasitoids and micropredators are needed to test the mechanisms, such as detection and fitness consequences, driving trait responses to predator and parasite threats (see Discussion). Comparisons of trait responses to different consumer types are currently limited to typical and trophically transmitted parasites, pathogens and solitary predators—only three of the 10 consumer strategies found in natural ecosystems [26,30].
3. Immunological responses to parasites, although one of the most common forms of anti-parasite defence, were not factored into the comparative literature and probably obscured the results of our tadpole case study. Future research may factor in immunological responses of hosts when assessing how the magnitude of non-lethal effects arising from parasitism compares with those of predation.
4. Longitudinal data on prey and host responses are needed to determine both the frequency and range of trait responses that individuals may exhibit throughout the course of interactions with consumers. For example, because hosts can resist parasitism during the consumption phase, an open question is whether individual hosts exhibit both avoidance and resistance responses when encountering parasites or if they choose one or the other. The strength of parasite-based non-lethal effects is a function of the cumulative strength of responses made to all consumer states, but the studies reviewed here provided only snapshots of responses to single consumer states. With the types of potential trait responses now identified in our framework, future research can determine the range and frequency of responses exhibited by resources during non-lethal interactions to develop more comprehensive estimates of non-lethal effects.
5. Our focus on trait responses lays a foundation for scaling non-lethal effects to individual fitness and community function. Future work can extend the length of prey and host monitoring to link trait responses to individual fitness. Mesocosm and field experiments mirroring the experimental designs of the studies reviewed here can introduce primary producers and other species in food webs into the picture, allowing associations between response magnitudes to predators and parasites and trophic flows and cascades to be quantified.
Trait response data on tadpoles, although limited, underscore the importance of distinguishing the timing of trait responses. Pooling all trait response measurements suggested that parasites generally did not elicit changes in tadpole activity levels, while predators did. However, accounting for response timing revealed substantial changes in activity levels that were confined to post-infection periods of interaction. Adult amphibians show similar tendencies to resist infections rather than avoid them [37]. Resisting consumption appears to a be primary form of trait response to parasitism in this group of animals, whose role in non-lethal effects can most fully be estimated by treating the responses as separate from those made earlier in interactions.
The post-infection responses to resist consumption in tadpoles are particularly notable considering that immune responses were not factored in. Immune responses are a very common form of resisting parasitism that can be exhibited for prolonged periods. The combined non-lethal effects of tadpole parasites were, therefore, probably much stronger than the analysed data suggest. Additionally, non-lethal effects of parasitism could have occurred from host phenotypic changes caused by parasite manipulation [27,44], and even directly from parasite feeding independent of defensive responses. For instance, general energy drain or direct tissue damage caused by parasite infection can substantially impact host performance [45,46]. Together, the numerous unmeasured non-lethal effects of parasites might rival predator avoidance in tadpoles. Accounting for distinct predator and parasite strategies led to predictions that did not align with the predator versus parasite dichotomy. Pinpointing the sources of variation in non-lethal effects, therefore, demands a functional approach rather than a taxonomic approach to distinguishing predators and parasites [26]. Parasitoids and parasitic castrators in particular, could offer rewarding insights because they share different functional characteristics with either predators or other parasites (box 1). Avoidance and resistance responses to parasitoids are well documented [42], but how their frequency and strength compare with responses to predators and less debilitating parasites is unknown. The latter can also be said for parasitic castrators. Given the high fitness consequences of infection from parasitoids and castrators, together with infections that are not immediately lethal, their combined non-lethal effects may very well be the strongest of all predator and parasite functional groups.
Regardless of how individuals respond to predators and parasites alone, risks of predation and parasitism in real ecosystems rarely occur in isolation. Future research could apply our framework to investigate the additive and interactive non-lethal effects of simultaneous exposure to predators and parasites (box 1; electronic supplementary material, figure S5). Our analysis of the tadpole data suggests that responses to simultaneous exposure are non-additive, perhaps owing to the prioritization of responses to the more severe threat (electronic supplementary material, figure S5). Although not a focus of this review, evidence for increasing predation of parasitized prey [47–49], and increasing parasitism in predator-rich environments [50], provide further indication that predators and parasites interact to impose non-lethal effects. Fewer studies have considered single responses that defend against both predators and parasites. Nevertheless, there were several cases where tadpole responses to predators and parasites were in the same direction (i.e. a reduction in the trait expression). Trait responses that effectively deter both predators and parasites may mitigate the non-lethal effects incurred from the essential task of defending oneself against being eaten.
8. Conclusion
Whether through fear or through infection, predatory and parasitic consumers elicit defensive trait responses in prey/hosts that give rise to non-lethal effects on individuals and their ecosystems. A general consumer–resource model helped us to develop a framework for systematically comparing trait responses to various types of predators and parasites. Different types of predators and parasites should elicit different trait responses and, therefore, have different non-lethal effects, given differences in consumer strategies that influence when and how strongly they impact prey and hosts. However, many predator and parasite strategies have not yet been tested comparatively. Expanding research of non-lethal effects to regularly consider different predator and parasite strategies sets the foundation for exploring how non-lethal effects manifest in the multi-dimensional food webs found in real ecosystems.
Supplementary Material
Acknowledgements
We thank Gordon Research Conferences and Andy Sih, chair of the 2016 meeting ‘Predator–Prey Interactions', for providing the forum that inspired this work; the EGLIDE group (Amy Pedersen, Amy Sweeny, Saudamini Venkatesan, Dishon Muloi, Alexandra Morris, Shaun Keegan and Kayleigh Gallagher), the EEGID group at the University of Liverpool (Mike Begon, Greg Hurst, David Montagnes, Mark Viney, Steve Parratt), the Garner laboratory at the Zoological Society of London (Trent Garner, Chris Owen, Lola Brooks, Stephen Price, Goncalo Rosa, Bryony Allan) and Chris Carbone for their comments on earlier versions of the review; and Daniel Noble, Raj Whitlock and Wolfgang Viechtbauer for advice on the meta-analysis. Any use of trade, product or firm names in this publication is for descriptive purposes only and does not imply endorsement by the US Government.
Data accessibility
The data and R code supporting the review and tadpole analysis can be downloaded from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fxpnvx0qf [51].
Authors' contributions
This review was broadly conceived during a 2016 Gordon Conference group meeting on predator–prey interactions. D.R.D. and E.M. organized initial planning and idea development. D.R.D. performed the systematic review and analysis with input from the other authors. D.R.D., K.D.L., R.F.H. and A.F. constructed the framework and drafted the manuscript and its revisions. All authors contributed to the writing and ideas expressed in the final piece.
Competing interests
We declare we have no competing interests.
Funding
This manuscript benefited from NSF Ecology of Infectious Diseases grant (OCE-1115965) and a grant from the Natural Environment Research Council UK (NE/N009800/1).
References
- 1.Lima SL, Dill LM. 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619-640. ( 10.1139/z90-092) [DOI] [Google Scholar]
- 2.Dröge E, Creel S, Becker MS, M'soka J. 2017. Risky times and risky places interact to affect prey behaviour. Nat. Ecol. Evol. 1, 1123-1128. ( 10.1038/s41559-017-0220-9) [DOI] [PubMed] [Google Scholar]
- 3.Milinski M, Bakker TCM. 1990. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344, 330. ( 10.1038/344330a0) [DOI] [Google Scholar]
- 4.Kavaliers M, Colwell DD, Braun WJ, Choleris E. 2003. Brief exposure to the odour of a parasitized male alters the subsequent mate odour responses of female mice. Anim. Behav. 65, 59-68. ( 10.1006/anbe.2002.2043) [DOI] [Google Scholar]
- 5.Behringer DC, Butler MJ, Shields JD. 2006. Avoidance of disease by social lobsters. Nature 441, 421. ( 10.1038/441421a) [DOI] [PubMed] [Google Scholar]
- 6.Sears BF, Snyder PW, Rohr JR. 2013. Infection deflection: hosts control parasite location with behaviour to improve tolerance. Proc. R. Soc. B 280, 20130759. ( 10.1098/rspb.2013.0759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart BL. 1994. Behavioural defense against parasites: interaction with parasite invasiveness. Parasitology 109, S139-S151. ( 10.1017/S0031182000085140) [DOI] [PubMed] [Google Scholar]
- 8.Curtis VA. 2014. Infection-avoidance behaviour in humans and other animals. Trends Immunol. 35, 457-464. ( 10.1016/j.it.2014.08.006) [DOI] [PubMed] [Google Scholar]
- 9.Weinstein SB, Buck JC, Young HS. 2018. A landscape of disgust. Science 359, 1213-1214. ( 10.1126/science.aas8694) [DOI] [PubMed] [Google Scholar]
- 10.Curtis V, de Barra M.. 2018. The structure and function of pathogen disgust. Phil. Trans. R. Soc. B 373, 20170208. ( 10.1098/rstb.2017.0208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dougherty ER, Seidel DP, Carlson CJ, Spiegel O, Getz WM. 2018. Going through the motions: incorporating movement analyses into disease research. Ecol. Lett. 21, 588-604. ( 10.1111/ele.12917) [DOI] [PubMed] [Google Scholar]
- 12.Tybur JM, Çınar Ç, Karinen AK, Perone P. 2018. Why do people vary in disgust? Phil. Trans. R. Soc. B 373, 20170204. ( 10.1098/rstb.2017.0204) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peacor SD, Barton BT, Kimbro DL, Sih A, Sheriff MJ. 2020. A framework and standardized terminology to facilitate the study of predation-risk effects. Ecology 101, e03152. ( 10.1002/ecy.3152) [DOI] [PubMed] [Google Scholar]
- 14.Brown JS. 1998. The ecology of fear: optimal foraging, game theory, and trophic interactions. IFAC Proc. 31, 31. ( 10.1016/S1474-6670(17)38332-5) [DOI] [Google Scholar]
- 15.Ritchie EG, Johnson CN. 2009. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 12, 982-998. ( 10.1111/j.1461-0248.2009.01347.x) [DOI] [PubMed] [Google Scholar]
- 16.Buck JC, Ripple WJ. 2017. Infectious agents trigger trophic cascades. Trends Ecol. Evol. 32, 681-694. ( 10.1016/j.tree.2017.06.009) [DOI] [PubMed] [Google Scholar]
- 17.Pringle RM, et al. 2019. Predator-induced collapse of niche structure and species coexistence. Nature 570, 58-64. ( 10.1038/s41586-019-1264-6) [DOI] [PubMed] [Google Scholar]
- 18.Creel S, Christianson D, Liley S, Winnie JA. 2007. Predation risk affects reproductive physiology and demography of elk. Science 315, 960. ( 10.1126/science.1135918) [DOI] [PubMed] [Google Scholar]
- 19.Fortin D, Beyer HL, Boyce MS, Smith DW, Duchesne T, Mao JS. 2005. Wolves influence elk movements: behavior shapes a trophic cascade in Yellowstone National Park. Ecology 86, 1320-1330. ( 10.1890/04-0953) [DOI] [Google Scholar]
- 20.Rigby MC, Hechinger RF, Stevens L. 2002. Why should parasite resistance be costly? Trends Parasitol. 18, 116-120. ( 10.1016/S1471-4922(01)02203-6) [DOI] [PubMed] [Google Scholar]
- 21.Raberg L, Graham AL, Read AF. 2009. Decomposing health: tolerance and resistance to parasites in animals. Phil. Trans. R. Soc. B 364, 37-49. ( 10.1098/rstb.2008.0184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buck JC. 2019. Indirect effects explain the role of parasites in ecosystems. Trends Parasitol. 35, 835-847. ( 10.1016/j.pt.2019.07.007) [DOI] [PubMed] [Google Scholar]
- 23.Hawley DM, Altizer SM. 2011. Disease ecology meets ecological immunology: understanding the links between organismal immunity and infection dynamics in natural populations: disease ecology meets ecological immunology. Funct. Ecol. 25, 48-60. ( 10.1111/j.1365-2435.2010.01753.x) [DOI] [Google Scholar]
- 24.Rohr JR, Swan A, Raffel TR, Hudson PJ. 2009. Parasites, info-disruption, and the ecology of fear. Oecologia 159, 447-454. ( 10.1007/s00442-008-1208-6) [DOI] [PubMed] [Google Scholar]
- 25.Doherty J-F, Ruehle B. 2020. An integrated landscape of fear and disgust: the evolution of avoidance behaviors amidst a myriad of natural enemies. Front. Ecol. Evol. 8, 564343. ( 10.3389/fevo.2020.564343) [DOI] [Google Scholar]
- 26.Lafferty KD, Kuris AM. 2002. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 17, 507-513. ( 10.1016/S0169-5347(02)02615-0) [DOI] [Google Scholar]
- 27.Lafferty KD, Shaw JC. 2013. Comparing mechanisms of host manipulation across host and parasite taxa. J. Exp. Biol. 216, 56-66. ( 10.1242/jeb.073668) [DOI] [PubMed] [Google Scholar]
- 28.Westra ER, et al. 2015. Parasite exposure drives selective evolution of constitutive versus inducible defense. Curr. Biol. 25, 1043-1049. ( 10.1016/j.cub.2015.01.065) [DOI] [PubMed] [Google Scholar]
- 29.Abrams P. 2007. Defining and measuring the impact of dynamic traits on interspecific interactions. Ecology 88, 2555-2562. ( 10.1890/06-1381.1) [DOI] [PubMed] [Google Scholar]
- 30.Lafferty KD, DeLeo G, Briggs CJ, Dobson AP, Gross T, Kuris AM. 2015. A general consumer-resource population model. Science 349, 854-857. ( 10.1126/science.aaa6224) [DOI] [PubMed] [Google Scholar]
- 31.Hart BL, Hart LA. 2018. How mammals stay healthy in nature: the evolution of behaviours to avoid parasites and pathogens. Phil. Trans. R. Soc. B 373, 20170205. ( 10.1098/rstb.2017.0205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ydenberg RC, Dill LM. 1986. The economics of fleeing from predators. Adv. Study Behav. 16, 229-249. ( 10.1016/S0065-3454(08)60192-8) [DOI] [Google Scholar]
- 33.Hossie T, Landolt K, Murray DL. 2017. Determinants and co-expression of anti-predator responses in amphibian tadpoles: a meta-analysis. Oikos 126, 173-184. ( 10.1111/oik.03305) [DOI] [Google Scholar]
- 34.Kats LB, Dill LM. 1998. The scent of death: chemosensory assessment of predation risk by prey animals. Écoscience 5, 361-394. ( 10.1080/11956860.1998.11682468) [DOI] [Google Scholar]
- 35.Behringer DC, Karvonen A, Bojko J. 2018. Parasite avoidance behaviours in aquatic environments. Phil. Trans. R. Soc. B 373, 20170202. ( 10.1098/rstb.2017.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephenson JF, Perkins SE, Cable J. 2018. Transmission risk predicts avoidance of infected conspecifics in Trinidadian guppies. J. Anim. Ecol. 87, 1525-1533. ( 10.1111/1365-2656.12885) [DOI] [PubMed] [Google Scholar]
- 37.Daversa DR, Manica A, Bosch J, Jolles JW, Garner TWJ. 2018. Routine habitat switching alters the likelihood and persistence of infection with a pathogenic parasite. Funct. Ecol. 32, 1262-1270. ( 10.1111/1365-2435.13038) [DOI] [Google Scholar]
- 38.Hechinger RF, Lafferty KD, Dobson AP, Brown JH, Kuris AM. 2011. A common scaling rule for abundance, energetics, and production of parasitic and free-living species. Science 333, 445-448. ( 10.1126/science.1204337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuris AM, et al. 2008. Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454, 515-518. ( 10.1038/nature06970) [DOI] [PubMed] [Google Scholar]
- 40.Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Proc. R. Soc. Lond. B 291, 452-491. [Google Scholar]
- 41.Jensen KH, Little T, Skorping A, Ebert D. 2006. Empirical support for optimal virulence in a castrating parasite. PLoS Biol. 4, e197. ( 10.1371/journal.pbio.0040197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abram PK, Brodeur J, Urbaneja A, Tena A. 2019. Nonreproductive effects of insect parasitoids on their hosts. Annu. Rev. Entomol. 64, 259-276. ( 10.1146/annurev-ento-011118-111753) [DOI] [PubMed] [Google Scholar]
- 43.Buck JC, Weinstein SB. 2020. The ecological consequences of a pandemic. Biol. Lett. 16, 20200641. ( 10.1098/rsbl.2020.0641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poulin R, Brodeur J, Moore J. 1994. Parasite manipulation of host behaviour: should hosts always lose? Oikos 70, 479-484. ( 10.2307/3545788) [DOI] [Google Scholar]
- 45.Munger JC, Karasov WH. 1989. Sublethal parasites and host energy budgets: tapeworm infection in white-footed mice. Ecology 70, 904-921. ( 10.2307/1941358) [DOI] [Google Scholar]
- 46.Delahay RJ, Speakman JR, Moss R. 1995. The energetic consequences of parasitism: effects of a developing infection of Trichostrongylus tenuis (Nematoda) on red grouse (Lagopus lagopus scoticus) energy balance, body weight and condition. Parasitology 110, 473. ( 10.1017/S0031182000064817) [DOI] [Google Scholar]
- 47.Lafferty KD, Morris AK. 1996. Altered behavior of parasitized killifish increases susceptibility to predation by bird final hosts. Ecology 77, 1390-1397. ( 10.2307/2265536) [DOI] [Google Scholar]
- 48.Johnson PTJ, Stanton DE, Preu ER, Forshay KJ, Carpenter SR. 2006. Dining on disease: how interactions between infection and environment affect predation risk. Ecology 87, 1973-1980. ( 10.1890/0012-9658(2006)87[1973:DODHIB\2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 49.Rae J, Murray D. 2019. Pathogen vs. predator: ranavirus exposure dampens tadpole responses to perceived predation risk. Oecologia 191, 325-334. ( 10.1007/s00442-019-04501-1) [DOI] [PubMed] [Google Scholar]
- 50.Stephenson JF, Van Oosterhout C, Mohammed RS, Cable J.. 2015. Parasites of Trinidadian guppies: evidence for sex-and age-specific trait-mediated indirect effects of predators. Ecology 96, 489-498. ( 10.1890/14-0495.1) [DOI] [PubMed] [Google Scholar]
- 51.Daversa DR, Hechinger RF, Madin E, Fenton A, Dell AI, Ritchie EG, Rohr J, Rudolf VHW, Lafferty KD. 2021. Data from: Broadening the ecology of fear: non-lethal effects arise from diverse responses to predation and parasitism. Dryad Digital Repository. ( 10.5061/dryad.fxpnvx0qf) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Daversa DR, Hechinger RF, Madin E, Fenton A, Dell AI, Ritchie EG, Rohr J, Rudolf VHW, Lafferty KD. 2021. Data from: Broadening the ecology of fear: non-lethal effects arise from diverse responses to predation and parasitism. Dryad Digital Repository. ( 10.5061/dryad.fxpnvx0qf) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data and R code supporting the review and tadpole analysis can be downloaded from the Dryad Digital Repository: https://doi.org/10.5061/dryad.fxpnvx0qf [51].