Abstract
Social bacteria display complex behaviours whereby thousands of cells collectively and dramatically change their form and function in response to nutrient availability and changing environmental conditions. In this review, we focus on Myxococcus xanthus motility, which supports spectacular transitions based on prey availability across its life cycle. A large body of work suggests that these behaviours require sensory capacity implemented at the single-cell level. Focusing on recent genetic work on a core cellular pathway required for single-cell directional decisions, we argue that signal integration, multi-modal sensing and memory are at the root of decision making leading to multicellular behaviours. Hence, Myxococcus may be a powerful biological system to elucidate how cellular building blocks cooperate to form sensory multicellular assemblages, a possible origin of cognitive mechanisms in biological systems.
This article is part of the theme issue ‘Basal cognition: conceptual tools and the view from the single cell’.
Keywords: Myxococcus xanthus, cognition, signal transduction, social bacteria
1. Myxococcus xanthus, a highly adaptive social microbe
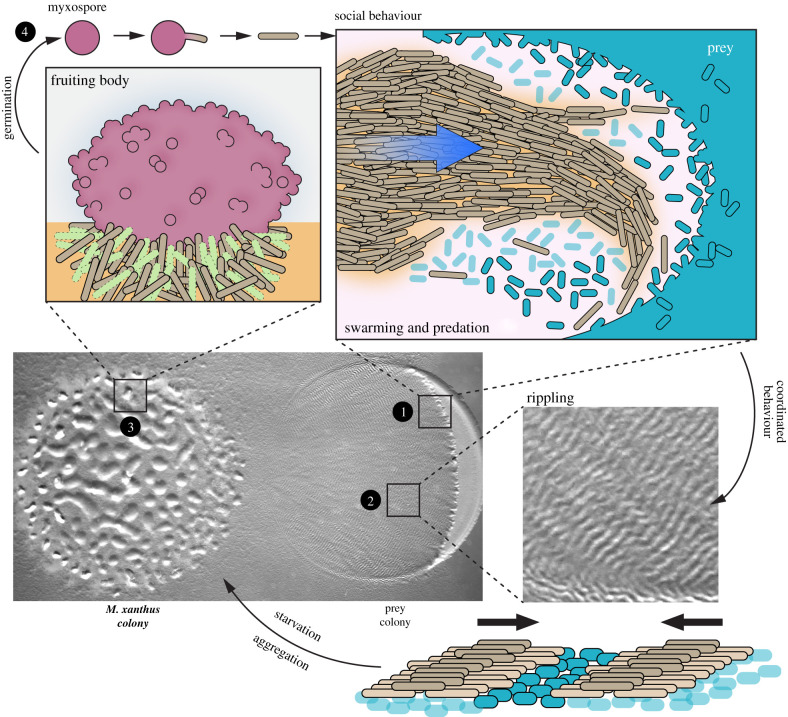
Myxobacteria are environmental bacteria that manifest a complex and fascinating social life cycle. In the soil, where they develop, species like Myxococcus xanthus (the organism that will be discussed in this review) derive their nutrients by collectively hunting and attacking other microorganisms—Gram-positive or negative bacteria, yeasts and even fungi—a behaviour that has been compared to a wolf-pack [1–4]. This life cycle is driven by highly cooperative behaviours where collective movements adapt the bacteria to the environment and manifest as striking multicellular transitions. A schematic view of the predatory Myxococcus cycle is shown in figure 1. Briefly, Myxococcus cells move by thousands to invade prey colonies and digest them using an arsenal of anti-microbial molecules. Prey invasion provokes a first striking multicellular transition, whereby swarming Myxococcus cells moving in coordinated groups transition into highly coordinated cellular waves (rippling) that scan the entire prey colonies. Following prey consumption and the onset of starvation, rippling dissipates and is followed by another massive transition where large groups move into aggregates that eventually mature into spore-filled fruiting bodies [3,4,6].
Figure 1.
Myxococcus xanthus social life cycle (adapted from [5]). Middle left: picture of a M. xanthus colony (left) preying on Escherichia coli (right) on agar. 1. Predation front: M. xanthus swarms towards the prey colony. Upper right: cartoon representation of an M. xanthus swarm (light brown cells) moving in a coordinated manner towards the prey colony (cyan cells). Lysed prey cells are in light blue. 2. Rippling patterns. Middle right: a zoomed-in picture of the macroscopic travelling waves (ripples) observed during predation and starvation. Bottom right: a cartoon representation of two M. xanthus rippling waves colliding. Myxococcus xanthus cells are in light brown, intact prey cells are in cyan and lysed prey cells in light blue. 3. In a nutrient-poor environment (i.e. absence of prey), starving M. xanthus cells aggregate and form macroscopic structures called fruiting bodies. Upper left: a fruiting body is a multicellular structure containing differentiated cells such as peripheral rods (in light brown), cells undergoing programmed cell death (in light green) and myxospores (in dark pink). 4. When conditions are favourable, myxospores (dark pink circle) can germinate. After germination, vegetative cells (in light brown) can form a new community.
Each of the above phases imply that thousands of Myxococcus cells must collectively change their behaviour very dramatically in response to prey availability. In the following paragraphs, we will describe the genetic basis for group movements, and develop an argument suggesting that these behaviours find their roots in each single cell's ability to make decisions based on potential cell–cell communication strategies.
2. Coordinating the movement of very large cell groups
Myxococcus cell groups exhibit remarkable group coordination (the so-called S or social-motility), similar to very large fish schools or bird flocks [4,7]. There is genetic evidence that sensory mechanisms regulate Myxococcus group movement because it can be deeply affected by the genetic disruption of signalling genes. First, S-motility is itself driven by sensory appendages known as Type-IV pili (Tfps) that, assembled at the bacterial cell pole, polymerize protein filaments that extend and pull cells forward as they retract [8] (figure 2). Tfps are thought to provide bacterial cells with a sense of touch, triggering cellular responses as they adhere to extracellular surfaces [9,10]. For example, in Myxococcus, Tfps activity is genetically linked to cellular secretion of an extracellular poly-saccharide (EPS) matrix, which creates EPS tracks across surfaces. These tracks are thought to form channels, funnelling cells into large streams and tightening cell–cell interactions, potentially providing spatial cues over long distances—a process known as stigmergy [4,11,12]. Interestingly, Tfps activate EPS secretion via a signalling pathway called Dif, which has typical features of bacterial chemotaxis pathways [13–16]. Chemotaxis allows cells to move in a directed manner towards a chemical source. In bacteria, these pathways allow single cells to precisely detect a wide range of nutrient gradients, owing to sophisticated properties of the receptors (so-called methyl-accepting proteins) allowing signal amplification and adaptation [17–20], which in animal cognition are typically referred to as sensitization and habituation. However, while the Dif system contains a predicted methyl-accepting protein, it is not currently understood how Tfps connect to Dif and how Dif further activates EPS secretion. Thus, while there is clearly a sensory potential in this pathway, we will not discuss it further in this review.
Figure 2.
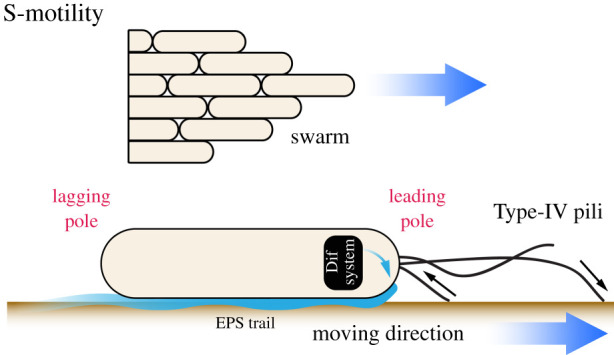
The S-motility system promotes coordinated group motility and involves the extension and retraction of a Type-IV pili at the leading pole that pulls cells forward. EPS secretion is activated by the Dif pathway, forming tracks across surfaces that are proposed to channel cells into larger streams. During reversal, cells switch direction by 180° owing to the inversion of their polarity and the re-assembly of Type-IV pili as the opposite cell pole.
In general, whether chemotaxis plays a role in Myxococcus life cycle remains unclear despite the large number of chemotaxis-like pathways encoded by the genome [3,13,16]. In particular, one pathway named Frz (see below), which will be at the core of this discussion, is required for cells to steer their motility via a process called directional reversals. These reversals involve cells switching direction by 180° by virtue of an inversion of polarity—the leading cell pole becomes the lagging pole and vice-versa—which is enabled by the intracellular relocation of the motility complexes to the opposite cell pole [7,8,21]. While it is tempting to imagine that reversal control can allow Myxococcus cells to detect chemical sources, this remains to be shown. Nevertheless, the control of cell reversals is at the heart of most of the Myxococcus S-motility transitions during the predation cycle, and frz mutants are deficient at multiple stages, including rippling, aggregation and fruiting bodies formation [22–24]. The evidence, therefore, indicates that cell motility reversals are at the core of social movements and their changes. Below, we discuss which sensory capacities might be conferred by Frz signalling and how such capacities might underlie the regulation of social movements.
3. Genetic circuits driving cell decisions
In order to clarify the potential sensory properties of Myxococcus cells, the genetic cell-autonomous circuit mentioned above must be described in detail. In recent years, intensive research from several laboratories has established that cellular reversals are controlled by a sophisticated protein complex, linking a chemosensory-like apparatus (Frz) to a polarity control system (Mgl) [7,8,21]. We first discuss the structure of each system and their interactions to identify key sensory properties in the global network.
(a). The Frz chemotaxis-like system
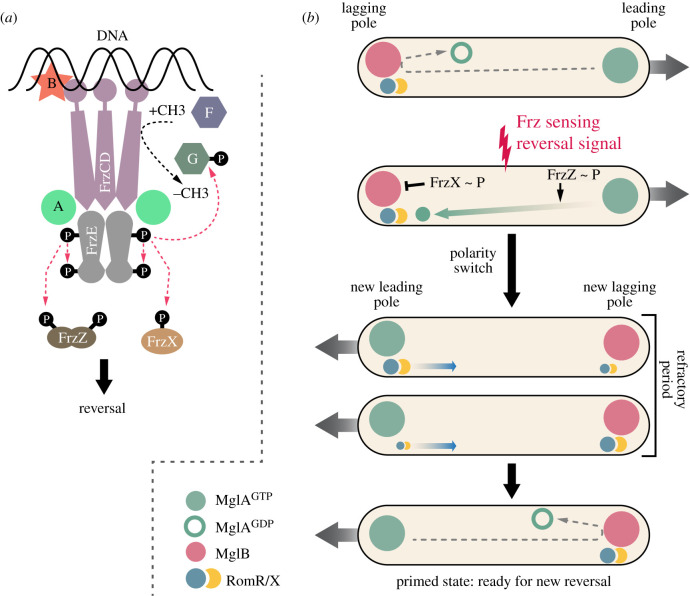
As early as 1985, Blackhart & Zusman [25] discovered that cellular reversals are under the genetic control of the so-called frz locus, which was later discovered to carry chemotaxis-like genes [26]. Genetically, loss of function mutations in frz genes were found to abolish cell reversals, while gain-of-function mutations led to cells reversing at high frequency, demonstrating that these genes regulate the frequency of cellular reversal, that is, the number of reversals per time unit for a given cell [25]. The structure of the Frz complex is shown in figure 3a. While not a classical chemotaxis system relying on Che proteins, the Frz complex has many features of such systems. The signalling apparatus consists of a receptor complex that includes a modifiable methyl-accepting protein (FrzCD) and a full adaptation complex: a methyl-transferase (FrzF) and a methyl-esterase (FrzG). Downstream, this complex interacts with two proteins (FrzA and FrzB) similar to CheW, the protein in canonical chemotaxis systems that links environmental information received by methyl-accepting proteins to proteins that regulate a response. In this case, FrzA and FrzB link FrzCD to FrzE, a hybrid CheA-like kinase fused in tandem with a response regulator CheY-like domain, and two soluble response regulator proteins (FrzZ and FrzX) [7,8,13,22,28–31].
Figure 3.
(a) Schematic of M. xanthus Frz pathway. At the surface of the nucleoid, the methyl-accepting protein FrzCD associates with the FrzE histidine kinase via the coupling protein FrzA. Methylation and demethylation of FrzCD is controlled by FrzF and FrzG, respectively. FrzE phosphorylates two distinct response regulator domains: FrzX and FrzZ. The accessory protein FrzB is important for the formation of multiple nucleoid Frz arrays (adapted from [27]). (b) Myxococcus xanthus cell polarity switch is controlled by a gated-relaxation oscillator. Prior to a reversal, MglA-GTP (in green) is at the leading cell pole, MglB (in red) and RomRX (in blue and yellow) are at the lagging cell pole. The cell cannot reverse because the GATE is closed, MglB converting MglA into the GDP-bound inactive state (open green circle). When a reversal signal is perceived by the Frz system, phosphorylated FrzX localizes at the lagging pole and opens the GATE by inactivating MglB. MglA is then recruited by the RomRX complex to the new leading pole, leading to a reversal. After the reversal, RomRX slowly detaches from the pole to accumulate at the opposite pole and interacts with MglB. This process defines the relaxation step for the system and introduces a refractory period during which no new reversal can happen. Phosphorylated FrzZ acts to limit the duration of the refractory period set by RomRX (adapted from [8]).
Unlike paradigmatic Che receptors, FrzCD does not assemble as a receptor protein array in the bacterial membrane, but rather uniquely forms signalling complexes by directly associating with the bacterial chromosome [27,32]. This unusual signalling complex-chromosomal association is mediated by a N-terminal DNA-binding domain in FrzCD and direct interaction with the coupling protein FrzB, which organizes potential signalling arrays directly at the surface of the chromosome (figure 3a). How the receptor system is activated intracellularly is still an enigma. However, as in other chemotaxis systems, its activation leads to the auto-phosphorylation of the FrzE kinase from ATP and phosphotransfer of up to three downstream receiver domains: its own receiver domain, FrzZ and FrzX (figure 3a). How these transfers affect reversals is discussed in detail below.
(b). The Mgl polarity complex
During a reversal, the activity of the motility complexes switches from one cell pole to the other [7,8,21]. While the Frz complex catalyses the switch, it is not itself required for implementing the underlying change in cell polarity. The master polarity controller is a protein named mutual gliding protein A (MglA). At the biochemical level, MglA acts like a molecular switch with features similar to the Ras protein family of small GTPases: it is active when bound to the nucleotide guanosine triphosphate (GTP) and is inactive when bound to guanosine diphosphate (GDP). The function of MglA is, therefore, to recruit critical activators of the motility machinery at the (leading) pole when it is bound to GTP [8,21,28,29,33–38] (figure 3b).
The mechanism of MglA polar localization is now established in molecular detail. At the leading cell pole, MglA is recruited by the so-called required for motility protein R and X (RomRX) complex, which promotes the loading of GTP by MglA at the leading pole [28,29]. RomRX is not itself responsible for the unipolar distribution of MglA, which is contributed by yet another factor, the MglB protein, localized at the opposite (lagging) cell pole. The MglB protein interacts directly with MglA and catalyses the transition from MglA-GTP hydrolysis, which inactivates MglA spatially and thereby prevents its accumulation at the lagging cell pole [35,36]. Thus, a four-protein system—MglA, MglB and RomRX—defines a cell polarity axis that directs the movement of the cell. Inversion of this polarity axis by the signalling activity of Frz provokes a reversal [8,21,28,29].
(c). The mechanism of reversals
Figure 3b depicts the temporal cascade of events that lead to cell reversal. Following a reversal, the RomRX complex is fully associated with MglA at the leading pole, while MglB localizes at the lagging pole. The RomRX complex then relocalizes to the lagging pole, while MglA remains at the leading pole, probably via interaction with its motility effectors [8,28,29,39]. Relocation of the RomRX complex prepares the cell for the next reversal event, but it is not sufficient to provoke it and thus polarity remains stable with MglA localizing at the leading pole and MglB and RomRX localizing at the lagging pole.
Switching of this axis and resetting the cycle require activation (again by unknown signals) of nucleoid-bound Frz-kinase receptor complexes, which phosphorylate the two diffusible response regulators, FrzX and FrzZ [8,29]. The molecular modes of action of phosphorylated FrzX (FrzX ∼ P) and phosphorylated FrzZ (FrzZ ∼ P) remain elusive. However, they each interact at distinct cell poles to trigger the reversal. When provoked, the following sequence of events occurs. In the first stage, the cell pauses as MglA detaches from the pole and accumulates at the lagging cell pole. Two complementary mechanisms operate during this first step. Detachment of MglA is facilitated both by itself, because increase of the MglA-GDP pool facilitates detachment of polar bound MglA-GTP [40], and FrzZ ∼ P, which may accelerate this release as it binds to the leading pole [29]. By contrast, FrzX ∼ P is essential for MglA-GTP accumulation, which may block MglB action at the lagging cell pole [29]. In the second stage, MglB detaches fully (perhaps displaced by the RomRX complex) and relocalizes to the opposite cell pole [8,28,29,38,41]. The cell then resumes movement in the opposite direction. The entire sequence unfolds on average within a minute. Although this molecular design is yet partially defined and could be more complex, we argue below that its structure confers important sensory properties to single motile cells.
(d). Signal dependence of the Frz-Mgl genetic circuit
Despite the overwhelming genetic evidence that reversal control is vital to the Myxococcus life cycle, the exact biological cues that provoke cell reversals remain unidentified. In this context, most of the properties discussed below were derived from in vitro studies where single cells are artificially stimulated by (non-physiological) addition of branched alcohols—iso-amyl alcohol (IAA)—which stimulates the FrzCD receptor specifically [30,42]. We nevertheless will assume that these properties also apply in native multicellular contexts, which is suggested by analyses of mutant behaviours [30].
Because IAA modulates the levels of Frz activation in a dose-dependent manner, several properties of the signalling pathway become apparent.
-
(i)
The FrzE response regulator domain prevents low-activation states of the kinase from activating FrzX and FrzZ, as the FrzE phosphorylated form becomes titrated by its own receiver domain. This mechanism prevents noisy activation of the reversal complex and, mutants that lack this inhibition display aberrant multicellular phenotypes.
-
(ii)
Higher levels of activation (perhaps physiological) alleviate this inhibition and allow phosphorylation of FrzX and FrzZ to occur. As we will see below, the reversal frequency will be tuned into reversal modes as a function of signal intensity.
The following properties have led to the suggestion that the Mgl switch functions as a so-called gated-relaxation oscillator, where two molecular events must be combined for cells to reverse. First, a sufficient amount of RomRX must be present at the lagging pole to recruit a critical concentration of MglA at the lagging pole. Second, FrzX ∼ P must be present at sufficient concentrations at the lagging pole to open the ‘gate’ [8,29]. Thus, cells can adopt distinct behaviours depending on local signal concentration. At low signal levels, the gate is mostly closed; the RomRX complex is armed at the lagging pole, ready to fire. The cell moves persistently in one direction but will reverse as soon as the kinase is turned on. This defines a so-called excitable state [8,29]. At high stimulation levels, the gate is permanently open but the slow accumulation of RomRX at the lagging pole defines a limiting step because the cell will not reverse until a critical concentration is reached at the lagging pole. In this state, oscillatory reversals occur as the cycle resets, and as RomRX dissociates again. In theory, the constant and slow RomRX dissociation rates would prevent fast reversal periods. However, this limitation is circumvented by the action of FrzZ ∼ P, which bypasses the RomRX threshold by directly promoting MglA dissociation (suggested by simulations) [29], and thus allows reversal periods that are faster than RomRX relocalization dynamics—approximately 2 min as measured at the maximum level.
In short, levels of FrzX ∼ P and FrzZ ∼ P as a function of FrzE activity can switch cell motility from directional to oscillatory. We argue below that these properties are essential for social transitions in the Myxococcus life cycle.
4. Are Myxococcus cells cognitive?
In her bacterial cognitive toolkit, Lyon proposed that bacteria display a number of counterparts to well-known cognitive processes [43]. In Myxococcus, sensory adaptation to environmental changes is strongly suggested by the large genomic repertoire of signalling systems [44]. It is not the focus of this review to discuss how these systems interact with each other to promote integrated responses; in fact, much of this knowledge remains elusive. Here, discussing the details of the core genetic system driving motility decisions, we wish to highlight potential features that would be eligible as cognitive as defined by Lyon [43]. A difficulty for this demonstration lies in the unproven sensing capacity of the Frz system, given that the actual biological signals remain unknown. Nevertheless, activation of the Frz receptor complex by extracellular addition of an artificial signal (IAA) shows that it can be sensory, although the exact relationship of nucleoid-bound complexes with the extracellular milieu remains enigmatic.
Bearing these limitations in mind, the cognitive features of the Myxococcus sensory apparatus become apparent. First, the FrzCD receptor's dose-dependent response curve to IAA has a sigmoidal shape, suggesting signal amplification [30,32]. Cooperativity depends on the formation of receptor complexes on the nucleoid [32], which suggests that, similar to membrane-bound chemoreceptors, Frz receptor organization promotes signal integration and nonlinear responses to stimulation. This property leads to sharp transitions from slow reversals to fast reversals triggered by relatively narrow signal fluctuations. Second, in canonical chemotaxis systems, receptor methylation promotes adaptation and thereby a memory of signal availability [18–20]. While a methyl-transferase/methyl-esterase pair (FrzF and FrzG) is present in the Frz system, its importance in FrzCD adaptation is unclear. Knocking out the methyl-esterase does not create major phenotypes (except during rippling [22,45]). Nevertheless, at the level of the Mgl switch, RomRX dissociation dynamics determine a time window during which the cell is insensitive to further reversal activation. Such a refractory period provides a memory of the last reversal event. Remarkably, the length of this refractory period is further tuned as a function of receptor activation via FrzZ ∼ P, which, therefore, is responsible for the amplitude of the sigmoid [29].
A consequence of these properties suggests that single-cell motility can switch dramatically from non-reversing to fast oscillatory-like reversals based on narrow signal changes. Thus, in theory, the Frz-Mgl circuit allows a number of possible servo-type mechanisms—formally speaking, navigation error-correction by negative feedback—which, depending on the biological context, could allow cells to navigate within highly heterogeneous spatial environments, as expected in ever-rearranging cell groups. It is theoretically possible, for example, that transitions from slow to fast reversals could increase dwell times in contact with prey cells. In such groups, cell–cell contacts rather than diffusible molecules could function as signals. This is speculation. However, such regulation currently is strongly favoured to explain the formation of the rippling patterns. During rippling, synchronized cellular waves move in opposite directions and appear to traverse each other when they collide. In fact, cell tracking experiments reveal that wave formation is an optical effect of the cells reversing synchronously when cellular waves collide and therefore adopting ‘accordion-like’ movements with the appearance of travelling waves [6,23,29,46–48]. Mathematical models suggest that signal transmission following head-to-head cell contact in colliding waves could induce reversal and explain the observed pattern [48]. Remarkably, the model also requires a built-in refractory period (possibly provided by RomRX), necessary for the cells to effectively move away from the collision sites.
The polar determinant that would induce rippling still awaits identification. The enigmatic C-signal was originally proposed to be a possible surface ligand with a contact-dependent reversal-inducing capacity, but this is now controversial [6,49]. Nevertheless, contact-dependent signalling has been characterized in Myxococcus. One clear example is the recently discovered Tra system. In this case, homotypic interactions between TraA surface proteins on two Myxococcus interacting cells promotes transient fusion of the bacterial outer-membrane (OM), thereby allowing the efficient exchange of OM proteins between cells, for example, toxins for kin recognition [50–53]. Although there is no evidence that OM protein exchange connects to Frz regulation, the example reveals sensory capacities that Myxococus cells have evolved to interact with their siblings and exchange information. Analogous mechanisms could, therefore, also operate to elicit reversals and synchronize cells, as was initially speculated for the C-signal.
5. Conclusion
In Myxococcus, developmental transitions rely on a multi-network sensory repertoire coupling morphogenetic movements to differential genetic programmes. Indeed, there is a large body of work describing the transcriptional regulatory cascades and master regulators (MrpC, FruA, etc.) that govern progression through the various developmental stages [54]. Our aim was not to discuss these developmental processes in detail, but to propose a hypothesis that autonomous decisions by single cells are at the core of large-scale multicellular transitions based on the current (yet partial) understanding of the central motility regulation system. This suggestion is backed by several research studies using high-resolution cell tracking, which make strong arguments that fruiting body formation, which might be viewed as a cellular phase transition [55], is controlled at the single-cell level via various mechanisms involving biased random walks towards aggregates, decreased cell motility in aggregates and high cellular alignment [56]. Given that all these behaviours are deeply perturbed in frz-mgl mutants, we propose that changes in the Frz signalling states described above are at the core of the observed motility changes. A challenge for the future will be to test this hypothesis, which will require the identification of the signals and interaction inputs into the Frz complex.
From a general perspective, understanding how single cells sense and interact to give rise to cellular coordination at very large scales may prove central to elucidating the building blocks of biological cognition. This is a challenging problem because new properties can also emerge from the interactions of large numbers of single cells that individually lack these properties [57]. Therefore, such non-intuitive phenomena must be studied by a combination of experimental and theoretical approaches. In this respect, social microbes are ideal model systems because their entire life cycle can be captured in a single experiment, the signalling pathways can be genetically perturbed, and single-cell behaviours can be tracked at high spatio-temporal resolution allowing quantitative studies. Arguably, studies in social amoeba have uncovered conserved mechanisms of chemotaxis and its role in multicellular aggregation [58]. Thus, studies with Myxococcus have the potential to reveal genetic architectures at the core of sensory behaviours, which might well prove universal in important respects. The structure of the Frz-Mgl pathway, for example, combines two conserved genetic modules, a prototypical bacterial Che-system and eukaryotic-like G-protein regulators.
Acknowledegments
The authors would like to thank Sofiène Seef for the gift of the picture presented in figure 1. C.D. is supported by funding from the Centuri—Turing Centre for Living Systems.
Contributor Information
Julien Herrou, Email: jherrou@imm.cnrs.fr.
Tâm Mignot, Email: tmignot@imm.cnrs.fr.
Data accessibility
This article does not contain any additional data.
Authors' contributions
C.D., A.M., J.H. and T.M. wrote the manuscript. All authors reviewed the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Berleman JE, Kirby JR. 2009. Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol. Rev. 33, 942-957. ( 10.1111/j.1574-6976.2009.00185.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall RC, Whitworth DE. 2019. Is ‘wolf-pack’ predation by antimicrobial bacteria cooperative? Cell behaviour and predatory mechanisms indicate profound selfishness, even when working alongside kin. Bioessays 41, 1800247. ( 10.1002/bies.201800247) [DOI] [PubMed] [Google Scholar]
- 3.Thiery S, Kaimer C. 2020. The predation strategy of Myxococcus xanthus. Front. Microbiol. 11, 1-7. ( 10.3389/fmicb.2020.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muñoz-Dorado J, Marcos-Torres FJ, García-Bravo E, Moraleda-Muñoz A, Pérez J. 2016. Myxobacteria: moving, killing, feeding, and surviving together. Front. Microbiol. 7, 1-18. ( 10.3389/fmicb.2016.00781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claessen D, Rozen DE, Kuipers OP, Søgaard-Andersen L, van Wezel GP. 2014. Bacterial solutions to multicellularity: a tale of biofilms, filaments and fruiting bodies. Nat. Rev. Microbiol. 12, 115-124. ( 10.1038/nrmicro3178) [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Ducret A, Shaevitz J, Mignot T. 2012. From individual cell motility to collective behaviors: insights from a prokaryote, Myxococcus xanthus. FEMS Microbiol. Rev. 36, 149-164. ( 10.1111/j.1574-6976.2011.00307.x) [DOI] [PubMed] [Google Scholar]
- 7.Mercier R, Mignot T. 2016. Regulations governing the multicellular lifestyle of Myxococcus xanthus. Curr. Opin. Microbiol. 34, 104-110. ( 10.1016/j.mib.2016.08.009) [DOI] [PubMed] [Google Scholar]
- 8.Herrou J, Mignot T. 2020. Dynamic polarity control by a tunable protein oscillator in bacteria. Curr. Opin. Cell Biol. 62, 54-60. ( 10.1016/j.ceb.2019.09.001) [DOI] [PubMed] [Google Scholar]
- 9.Burrows LL. 2012. Pseudomonas aeruginosa twitching motility: type IV pili in action. Annu. Rev. Microbiol. 66, 493-520. ( 10.1146/annurev-micro-092611-150055) [DOI] [PubMed] [Google Scholar]
- 10.Ellison CK, et al. 2017. Obstruction of pilus retraction stimulates bacterial surface sensing. Science 358, 535-538. ( 10.1126/science.aan5706) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Igoshin OA, Welch R, Kaiser D, Oster G. 2004. Waves and aggregation patterns in myxobacteria. Proc. Natl Acad. Sci. USA 101, 4256-4261. ( 10.1073/pnas.0400704101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balagam R, Igoshin OA. 2015. Mechanism for collective cell alignment in Myxococcus xanthus bacteria. PLoS Comput. Biol. 11, 1-20. ( 10.1371/journal.pcbi.1004474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zusman DR, Scott AE, Yang Z, Kirby JR. 2007. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 5, 862-872. ( 10.1038/nrmicro1770) [DOI] [PubMed] [Google Scholar]
- 14.Black WP, Xu Q, Yang Z. 2006. Type IV pili function upstream of the Dif chemotaxis pathway in Myxococcus xanthus EPS regulation. Mol. Microbiol. 61, 447-456. ( 10.1111/j.1365-2958.2006.05230.x) [DOI] [PubMed] [Google Scholar]
- 15.Xu Q, Black WP, Cadieux CL, Yang Z. 2008. Independence and interdependence of Dif and Frz chemosensory pathways in Myxococcus xanthus chemotaxis. Mol. Microbiol. 69, 714-723. ( 10.1111/j.1365-2958.2008.06322.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moine A, Agrebi R, Espinosa L, Kirby JR, Zusman DR, Mignot T, Mauriello EMF. 2014. Functional organization of a multimodular bacterial chemosensory apparatus. PLoS Genet. 10, 1-16. ( 10.1371/journal.pgen.1004164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szurmant H, Ordal GW. 2004. Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol. Mol. Biol. Rev. 68, 301-319. ( 10.1128/mmbr.68.2.301-319.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wadhams GH, Armitage JP. 2004. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 12, 1024-1037. ( 10.1038/nrm1524) [DOI] [PubMed] [Google Scholar]
- 19.Sourjik V, Wingreen NS. 2012. Responding to chemical gradients: bacterial chemotaxis. Curr. Opin. Cell Biol. 24, 262-268. ( 10.1016/j.ceb.2011.11.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vladimirov N, Sourjik V. 2009. Chemotaxis: how bacteria use memory. Biol. Chem. 390, 1097-1104. ( 10.1515/BC.2009.130) [DOI] [PubMed] [Google Scholar]
- 21.Schumacher D, Søgaard-Andersen L. 2017. Regulation of cell polarity in motility and cell division in Myxococcus xanthus. Annu. Rev. Microbiol. 71, 61-78. ( 10.1146/annurev-micro-102215-095415) [DOI] [PubMed] [Google Scholar]
- 22.Berleman JE, Scott J, Chumley T, Kirby JR. 2008. Predataxis behavior in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 105, 17 127-17 132. ( 10.1073/pnas.0804387105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igoshin OA, Goldbeter A, Kaiser D, Oster G. 2004. A biochemical oscillator explains several aspects of Myxococcus xanthus behavior during development. Proc. Natl Acad. Sci. USA 101, 15 760-15 765. ( 10.1073/pnas.0407111101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wireman JW, Dworkin M. 1977. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J. Bacteriol. 129, 796-802. ( 10.1128/jb.129.2.798-802.1977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blackhart BD, Zusman DR. 1985. ‘Frizzy genes of Myxococcus xanthus are involved in control of frequency of reversal of gliding motility. Proc. Natl Acad. Sci. USA 82, 8767-8770. ( 10.1073/pnas.82.24.8767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McBride MJ, Weinberg RA, Zusman DR. 1989. ‘Frizzy’ aggregation genes of the gliding bacterium Myxococcus xanthus show sequence similarities to the chemotaxis genes of enteric bacteria. Proc. Natl Acad. Sci. USA 86, 424-428. ( 10.1073/pnas.86.2.424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mauriello EMF. 2020. How an unusual chemosensory system forms arrays on the bacterial nucleoid. Biochem. Soc. Trans. 48, 347-356. ( 10.1042/bst20180450) [DOI] [PubMed] [Google Scholar]
- 28.Szadkowski D, Harms A, Carreira LAM, Wigbers M, Potapova A, Wuichet K, Keilberg D, Gerland U, Sogaard-Andersen L. 2019. Spatial control of the GTPase MglA by localized RomR-RomX GEF and MglB GAP activities enables Myxococcus xanthus motility. Nat. Microbiol. 4, 1344-1355. ( 10.1038/s41564-019-0451-4) [DOI] [PubMed] [Google Scholar]
- 29.Guzzo M, et al. 2018. A gated relaxation oscillator mediated by FrzX controls morphogenetic movements in Myxococcus xanthus. Nat. Microbiol. 3, 948-959. ( 10.1038/s41564-018-0203-x) [DOI] [PubMed] [Google Scholar]
- 30.Guzzo M, Agrebi R, Espinosa L, Baronian G, Molle V, Mauriello EMF, Brochier-Armanet C, Mignot T. 2015. Evolution and design governing signal precision and amplification in a bacterial chemosensory pathway. PLoS Genet. 11, 1-26. ( 10.1371/journal.pgen.1005460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inclán YF, Vlamakis HC, Zusman DR. 2007. FrzZ, a dual CheY-like response regulator, functions as an output for the Frz chemosensory pathway of Myxococcus xanthus. Mol. Microbiol. 65, 90-102. ( 10.1111/j.1365-2958.2007.05774.x) [DOI] [PubMed] [Google Scholar]
- 32.Moine A, et al. 2017. The nucleoid as a scaffold for the assembly of bacterial signaling complexes. PLoS Genet. 13, 1-20. ( 10.1371/journal.pgen.1007103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercier R, Bautista S, Delannoy M, Gibert M, Guiseppi A, Herrou J, Mauriello EMF, Mignot T. 2020. The polar Ras-like GTPase MglA activates type IV pilus via SgmX to enable twitching motility in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 117, 28 366-28 373. ( 10.1073/pnas.2002783117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potapova A, Menezes Carreira LM, Søgaard-Andersen L. 2020. The small GTPase MglA together with the TPR domain protein SgmX stimulates type IV pili formation in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 117, 23 859-23 868. ( 10.1073/pnas.2004722117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Franco M, Ducret A, Mignot T. 2010. A bacterial Ras-like small GTP-binding protein and its cognate GAP establish a dynamic spatial polarity axis to control directed motility. PLoS Biol. 8, 1-12. ( 10.1371/journal.pbio.1000430) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leonardy S, Miertzschke M, Bulyha I, Sperling E, Wittinghofer A, Søgaard-Andersen L. 2010. Regulation of dynamic polarity switching in bacteria by a Ras-like G-protein and its cognate GAP. EMBO J. 29, 2276-2289. ( 10.1038/emboj.2010.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Guzzo M, Ducret A, Li YZ, Mignot T. 2012. A dynamic response regulator protein modulates G-protein-dependent polarity in the bacterium Myxococcus xanthus. PLoS Genet. 8, 1-11. ( 10.1371/journal.pgen.1002872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keilberg D, Wuichet K, Drescher F, Søgaard-Andersen L. 2012. A response regulator interfaces between the Frz chemosensory system and the MglA/MglB GTPase/GAP module to regulate polarity in Myxococcus xanthus. PLoS Genet. 8, 1-13. ( 10.1371/journal.pgen.1002951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baranwal J, Lhospice S, Kanade M, Chakraborty S, Gade PR, Harne S, Herrou J, Mignot T, Gayathri P. 2019. Allosteric regulation of a prokaryotic small Ras-like GTPase contributes to cell polarity oscillations in bacterial motility. PLoS Biol. 17, 1-30. ( 10.1371/journal.pbio.3000459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galicia C, Lhospice S, Varela PF, Trapani S, Zhang W, Navaza J, Herrou J, Mignot T, Cherfils J. 2019. MglA functions as a three-state GTPase to control movement reversals of Myxococcus xanthus. Nat. Commun. 10, 1-12. ( 10.1038/s41467-019-13274-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carreira LAM, Tostevin F, Gerland U, Søgaard-Andersen L. 2020. Protein-protein interaction network controlling establishment and maintenance of switchable cell polarity. PLoS Genet. 16, 1-30. ( 10.1371/journal.pgen.1008877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McBride MJ, Köhler T, Zusman DR. 1992. Methylation of FrzCD, a methyl-accepting taxis protein of Myxococcus xanthus, is correlated with factors affecting cell behavior. J. Bacteriol. 174, 4246-4257. ( 10.1128/jb.174.13.4246-4257.1992) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyon P. 2015. The cognitive cell: bacterial behaviour reconsidered. Front. Microbiol. 6, 1-18. ( 10.3389/fmicb.2015.00264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldman BS, et al. 2006. Evolution of sensory complexity recorded in a myxobacterial genome. Proc. Natl Acad. Sci. USA 103, 15 200-15 205. ( 10.1073/pnas.0607335103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bustamante VH, Martínez-Flores I, Vlamakis HC, Zusman DR. 2004. Analysis of the Frz signal transduction system of Myxococcus xanthus shows the importance of the conserved C-terminal region of the cytoplasmic chemoreceptor FrzCD in sensing signals. Mol. Microbiol. 53, 1501-1513. ( 10.1111/j.1365-2958.2004.04221) [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Vaksman Z, Litwin DB, Shi P, Kaplan HB, Igoshin OA. 2012. The mechanistic basis of Myxococcus xanthus rippling behavior and its physiological role during predation. PLoS Comput. Biol. 8, 1-13. ( 10.1371/journal.pcbi.1002715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igoshin OA, Mogilner A, Welch RD, Kaiser D, Oster G. 2001. Pattern formation and traveling waves in myxobacteria: theory and modeling. Proc. Natl Acad. Sci. USA 98, 14 913-14 918. ( 10.1073/pnas.221579598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sliusarenko O, Neu J, Zusman DR, Oster G. 2006. Accordion waves in Myxococcus xanthus. Proc. Natl Acad. Sci. USA 103, 1534-1539. ( 10.1073/pnas.0507720103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boynton TO, Shimkets LJ. 2015. Myxococcus CsgA, Drosophila Sniffer, and human HSD10 are cardiolipin phospholipases. Genes Dev. 29, 1903-1914. ( 10.1101/gad.268482.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nudleman E. 2005. Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science 309, 125-127. ( 10.1126/science.1112440) [DOI] [PubMed] [Google Scholar]
- 51.Cao P, Wall D. 2019. Direct visualization of a molecular handshake that governs kin recognition and tissue formation in myxobacteria. Nat. Commun. 10, 1-10. ( 10.1038/s41467-019-11108-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pathak DT, Wei X, Bucuvalas A, Haft DH, Gerloff DL, Wall D. 2012. Cell contact–dependent outer membrane exchange in myxobacteria: genetic determinants and mechanism. PLoS Genet. 8, 1-12. ( 10.1371/journal.pgen.1002626) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vassallo CN, Cao P, Conklin A, Finkelstein H, Hayes CS, Wall D. 2017. Infectious polymorphic toxins delivered by outer membrane exchange discriminate kin in myxobacteria. Elife 6, 1-24. ( 10.7554/eLife.29397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kroos L. 2017. Highly signal-responsive gene regulatory network governing Myxococcus development. Trends Genet. 33, 3-15. ( 10.1016/j.tig.2016.10.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu G, Patch A, Bahar F, Yllanes D, Welch RD, Marchetti MC, Thutupalli S, Shaevitz JW. 2019. Self-driven phase transitions drive Myxococcus xanthus fruiting body formation. Phys. Rev. Lett. 122, 248102. ( 10.1103/PhysRevLett.122.248102) [DOI] [PubMed] [Google Scholar]
- 56.Cotter CR, Schüttler HB, Igoshin OA, Shimkets LJ. 2017. Data-driven modeling reveals cell behaviors controlling self-organization during Myxococcus xanthus development. Proc. Natl Acad. Sci. USA 114, 4592-4601. ( 10.1073/pnas.1620981114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor RG, Welch RD. 2008. Chemotaxis as an emergent property of a swarm. J. Bacteriol. 190, 6811-6816. ( 10.1128/JB.00662-08) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregor T, Fujimoto K, Masaki N, Sawai S. 2010. The onset of collective behavior in social amoebae. Science 328, 1021-1025. ( 10.1126/science.1183415) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article does not contain any additional data.