Abstract
One molluscan autapomorphy is the radula, the organ used for feeding. Here, for the first time, the performance and failure of taenioglossan radular teeth were tested in a biomechanical experiment which in turn allowed building hypotheses about tooth functionalities. Shear load was applied to tooth cusps with a force transducer until structural failure occurred, the broken area was measured, and finally breaking stress was calculated. These experiments were carried out under dry and wet conditions. Our results show that certain tooth types can resist higher stresses and are rather specialised to loosen food items from a surface, whereas other teeth can only gather food particles. The experiments additionally illustrate the high influence of the water content on the resulting breaking stress. When wet teeth were tested, their ductility and ability to avoid being fractured by an obstacle increased. Their flexibility also allowed them support from teeth of adjacent tooth rows, which made the whole system less prone to failure. Our results were compared with the previous data on the mechanical properties and feeding simulations. This study provides a keystone for further comparative studies aiming at connecting diversity of radulae with their possible adaptations to the ingesta.
Keywords: breaking stress, taenioglossan radula, performance, structural failure, water content, biomechanics
1. Introduction
Mollusca as the second largest animal phylum (e.g. [1]) exhibit a high biodiversity (e.g. [2]) which includes feeding on a variety of food types and items that are gathered and mechanically processed by one molluscan autapomorphy, the radula.
The radula is a set of teeth embedded in a flexible, chitinous membrane [3,4], supported by odontophoral cartilages, and moved by numerous muscles during feeding [5], resulting in the interaction of teeth with the ingesta (food and the substrate which the food is attached to). This interaction naturally leads to tooth wear (e.g. [6–10]) and probably also structural failure, but through a continuous growth from posterior to anterior the radular membrane and its teeth are always replaced (e.g. [11–15]). Despite of this mechanism, sometimes a substantial amount of iron is incorporated in the chitinous tooth material which results in harder and stiffer teeth and presumably makes the teeth more wear resistant (e.g. [16–20]).
As teeth can be considered an interface between the organism and its environment, they tightly link the molluscs with their ingesta. Approaches aiming at relating the gastropod's feeding ecology with the function of its radular teeth (e.g. scratching, loosening food items, collecting and gathering particles) had been undertaken [7,21–29], but in most previous studies hypotheses are primarily based on the consideration of the tooth shape and only few include the mechanical properties (usually elasticity modulus = Young's modulus, or hardness) of teeth. Additionally, the individual components of the radula interact during foraging [30,31]. Thus, functionality of teeth and their acting on the ingesta probably depends on the interlocking of teeth from adjacent rows, presumably leading to a force transmission and stress distribution from one tooth to another (see also [32–35]).
The detection of material property gradients (in hardness and Young’ modulus) in the taenioglossan radular teeth of the African Paludomidae Spekia zonata [29] which were, together with the three-dimensional tooth shape, included in numerical simulations on the stress and strain distribution of its teeth [36] strengthen such hypotheses about the capability of teeth to interact and interlock. Additionally, hypotheses about tooth function could be proposed: central and lateral teeth are rather capable of loosening ingesta, whereas marginal teeth gather the particles afterwards. However, these simulations of the proposed mechanical behaviour are based on the analysis of individual teeth and not of teeth in combination; additionally, the material property data, referenced above, underlying these models was achieved by testing dry and embedded tooth samples. The past hypotheses on tooth functionality inferred from simulations and dry-tested hardness and elasticity values are not unproblematic, because the native water content has a high influence on the mechanical behaviour of biological materials (see Discussion and e.g. [37]). Usually, hardness and stiffness of wet materials is lower than of same dry materials, whereas dry materials have lower fracture toughness than wet ones. Certainly, material properties influence the mechanics of structures, e.g. the ability of a structure to transfer force can be linked with Young's modulus (e.g. [38–41]) and some authors additionally correlate this with mechanical behaviours while puncturing and in direct turn with the resistance of structures to failure (e.g. [42]). However, determining Young's modulus and hardness of native (wet) radular teeth would be important, but due to the smallness of these teeth and their three-dimensional morphology is highly problematic. To deeply understand radular tooth function, it is thus utterly necessary to develop experimental setups that allow testing under dry as well as wet (native) condition including the direct observation of the tooth's mechanical behaviour (e.g. twisting, relying on each other, bending) when exposed to a force.
Therefore, to provide a keystone for further studies on the vast diversity of radulae, we here propose the first experiments that provide insight into the mechanical limitations and the performance of the wet feeding organ. For experiments we chose the species Spekia zonata [43] belonging to the African Paludomidae: Caenogastropoda and foraging on algae covering rocks in Lake Tanganyika (e.g. [44,45]). This species was targeted because its tooth's dry mechanical properties are known [29], and mechanical behaviour simulations had been performed [36]. The amount of breaking stress (the force over cross sectional area) that is needed to break structures was determined by exerting force onto the tooth cusps, the actual ingesta-interface, and measuring it until structural failure occurred. Experiments were carried out under dry and wet condition allowing hypotheses about the effects of water on the mechanical behaviour of teeth.
The following hypotheses were tested: Do the tooth types (central, lateral, marginal teeth) show distinct mechanical behaviours? Are some teeth capable of resisting to higher forces? Is the mechanical behaviour of native (wet) teeth different from the mechanical behaviour of dry ones? Do our results accord with previous hypotheses about tooth functionality in Spekia—do stress and strain simulations correspond to the native mechanical behaviour?
The forces that teeth can resist to are not only of high interest for biomechanics, but also for species ecology, because mechanical limitations of teeth can result in a limitation of resources (e.g. food). Determining the performance of radular teeth and their constraints can further help understanding ecological adaptations (trophic specializations) and also precise function of different tooth types.
2. Material and methods
Overall, six adult specimens were analysed in breaking stress experiments. They are inventoried at the Zoologisches Museum Hamburg (ZMH 154652/999), preserved in 70% EtOH, and were collected by Heinz Büscher in Lake Tanganyika at Zambia, Kalambo, in 2018. Specimens were dissected, buccal masses extracted, and radulae manually freed from surrounding tissue. For experiments in dry condition three radulae (ZMH 154652/999-16, -17, -18) were mounted on a microscope glass slide (Carl Roth, Karlsruhe, Germany) with double-sided adhesive tape after being manually unwound and teeth being carefully stroked into proposed feeding position. The exact position and interaction of teeth is unknown since the foraging of living Spekia has not been documented yet. However, as observed from dissection, the outermost, mature part of Spekia's radula is maintained in spanned position by the alary processus, a chitinous plate attached to either side of the radular membrane and also to the buccal mass. The radula is thus not folded as it had been documented for other gastropod species and the central and lateral teeth are almost exposed and positioned as in the SEM images, only the long marginal teeth cover them. For experiments, marginal teeth were stroked from the cusps of the central and lateral teeth and positioned adjacently (figure 1a). For experiments under wet condition three radulae (ZMH 154652/999-19, -20, -21) were fixed to the glass slide using epoxy (RECKLI Epoxy WST, RECKLI GmbH, Herne, Germany). First radulae were unwound, teeth stroked into feeding position, the sample positioned on the glass slide, and epoxy was dribbled to both sides of the radula without applying it onto the teeth. We have chosen this specific epoxy because from previous studies [29,46] we know that it does not infiltrate the teeth and does hence not influence mechanical behaviour of the material under consideration. After hardening of the epoxy, radulae were rehydrated with filtered water by dribbling it onto the teeth with a pipette. Larger water drops, attached to the teeth, were absorbed with paper and thus removed. When radulae became too dry during experiments the procedure was repeated.
Figure 1.
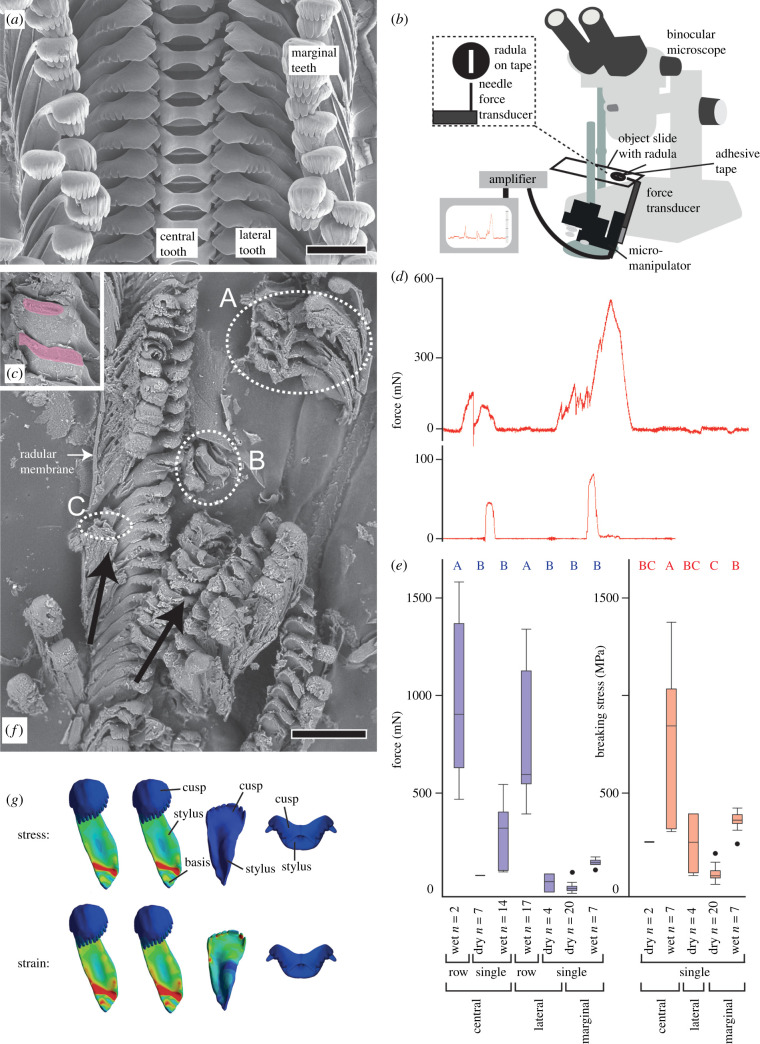
(a) Unbroken radula of Spekia zonata ZMH 150008/999-2 in proposed feeding position. (b) Experimental set-up depicting the taped radula, the force transducer with adjusted needle under the binocular microscope. (c) Exemplary lateral teeth with painted broken area. (d) Representative force measurement curves, above = central tooth row with membrane failure under wet condition, below: individual marginal tooth failure under dry condition. (e) Force, mN, needed to break structures (blue letters are connecting letters from Tukey–Kramer test and represent homogeneous groups for force) and calculated breaking stress, MPa, (red letters are connecting letters from Tukey–Kramer test and represent homogeneous groups for stress) for each tooth type, single or row failure, and condition (dry and wet), n = quantity of experiments. (f) Exemplary broken radular teeth (A,B: broken central tooth row = radular membrane failure; C: broken marginal teeth at tooth styli) under wet condition (ZMH 150008/999-20), black arrows resembles the direction of force applied through the needle causing the failure. (g) Results of finite element analysis (adapted from [36]). Red areas depict the region where high stress (above) and high deformation (below) is expected under modelled feeding simulations. Green colour, intermediate stress and strain values; blue colour, low stress and strain values. Scale bars: a = 100 µm, f = 400 µm. (Online version in colour.)
For measuring the force that is needed to break teeth, the glass slide with radula was positioned by stand and clamps so that interaction and failure of teeth could be observed by binocular microscope (figure 1b). A rounded steel needle (diameter of 0.4 mm) was firmly mounted onto a force transducer FORT-1000 (World Precision Instruments, Sarasota, FL, USA) connected to an amplifier (Biopac System Inc., CA, USA) and computer-based data acquisition and processing system (Acq Knowledge, Biopac Systems, v.: 3.7.0.0, World Precision Instruments, Sarasota, FL, USA). This equipment was already used for other type of experiments (e.g. [46,47]). The force transducer with the needle was mounted onto a remote-controlled micromanipulator (DC 3001R, World Precision Instruments) so that fine movements in all directions were possible. The needle tip was positioned on the concave part of the tooth cusps at 30° to the horizontal plane and moved onto the cusps. By employing the micromanipulator, the needle was further dragged onto the tooth until structural failure occurred. The force corresponding to the tooth/membrane failure was documented with the software Acq Knowledge (Biopac Systems, v.: 3.7.0.0) (figure 1d).
Afterwards radulae were analysed (figure 1f) with scanning electron microscope (SEM) TM4000 Plus (Hitachi, Tokyo, Japan). From SEM images the part of structural failure for each tooth type (central, lateral, marginal tooth) was identified (e.g. at the denticles of the cusps, the stylus, the basis, the radular membrane). When structural failure occurred on a single tooth (not on a row of teeth, because row failure = membrane failure) the area, where the failure occurred, could be measured and averaged. First, the broken teeth were individually visualized via SEM; subsequently, for determining the area, SEM images were transferred into Adobe Illustrator CS 6 (Adobe, San José, USA) and the broken part was painted (figure 1c). By using the scale bar from SEM as reference a square area (in µm2) was also computed. Images were imported into Adobe Photoshop CS 6 (Adobe), here the quantity of pixels was read out for the square and for painted part of tooth failure. By accounting pixel quantity of the square with the pixel quantities for each broken tooth area, the area (in µm2) of failure and subsequently an average breaking area for each tooth type and tooth part could be determined (table 1). Then breaking stress was calculated from the breaking force and the mean broken area for the corresponding tooth type and tooth part. When failure involved the radular membrane (i.e. teeth broke as rows), the area of failure was rather difficult to measure which led to artefacts; thus no breaking stress was calculated but the force values needed for structural failure were analysed. Statistical analyses on breaking stress and forces were performed with JMP Pro, v. 13 (SAS Institute, Cary, NC, 1989–2007). Mean values and standard deviations were calculated. Shapiro–Wilk-W-test was used for testing of normality and one-way ANOVA followed by a Tukey–Kramer test for detecting homogeneous groups (for connecting letters, see figure 1e). Force and breaking stress values of the teeth were compared for dry and wet condition (figure 1e).
Table 1.
Force needed for failure of central, lateral and marginal teeth—single or row—in dry and wet condition; n = quantity of analysed experiments (each experiment = one structural failure with corresponding force measurement). The average breaking area of individually (single) failing teeth is listed. When a single central or a single lateral tooth failed, it always broke at its denticle; a single marginal tooth always broke at its stylus. Breaking stress of the individually failing teeth, calculated for each condition, is presented. χ2 < 0.5. s.d. = standard deviation.
| tooth type | force, mN, mean ± s.d. |
breaking area of single tooth, µm2, mean ± s.d. | breaking stress, Mpa, mean ± s.d. |
||||
|---|---|---|---|---|---|---|---|
| row = failed membrane |
single tooth |
single tooth |
|||||
| wet | dry | wet | dry | wet | dry | ||
| central tooth | 979.50 ± 381.14 (n = 14) |
teeth only failed individually (n = 0) |
302.43 ± 168.36 (n = 2) |
111.50 ± 0.71 (n = 7) |
403 ± 24 | 750.44 ± 417.76 | 276.67 ± 1.75 |
| lateral tooth | 799.83 ± 313.47 (n = 17) |
teeth only failed individually (n = 0) |
teeth only failed as row (n = 0) |
78.25 ± 47.46 (n = 4) |
294 ± 19 | teeth only failed as row thus BS was not calculated | 266.16 ± 161.45 |
| marginal teeth | teeth only failed individually (n = 0) |
teeth only failed individually (n = 0) |
171.57 ± 24.78 (n = 7) |
49.53 ± 18.11 (n = 20) |
456 ± 16 | 376.25 ± 54.35 | 108.61 ± 39.71 |
Data on Young's modulus and hardness measurements (ZMB 220.077, ZMB 220.143 and ZMH150008/999, n = 7 specimens) were taken from Krings et al. [29]). Overall, cusps are the hardest and stiffest parts, followed by the styli, and finally the bases as the softest and most flexible parts. Central teeth possess the stiffest and hardest cusps, followed by the lateral tooth cusps, and finally marginal tooth cusps. Hardness and Young's modulus in the teeth studied seem strongly related.
For SEM images of one unbroken radula (figure 1a), the buccal mass was extracted, treated with proteinase K according to the protocol of Holznagel [48], cleaned for a few seconds in an ultrasonic bath, mounted on an aluminium stub, coated with carbon and visualized with the SEM Zeiss LEO 1525 (One Zeiss Drive, Thornwood, NY).
3. Results
(a). Tooth failure and fractured area
Under dry condition the thin and slender marginal teeth broke at their styli close to their bases, whereas the broader and thicker central and lateral teeth broke at their denticles (area of failure is given in table 1). Under wet condition the marginals broke at their styli as well, but when applying force on wet lateral and central ones the teeth themselves were only occasionally damaged at level of the denticles, because they were elastic enough to undergo strong bending amplitude and to get support from the adjacent tooth rows. Here each tooth type failed together with teeth of the same type from adjacent rows due to the ripping of the chitinous radular membrane, embedding and holding the teeth, under shear force applied in the tested anterior–posterior direction (figure 1f).
(b). Breaking force
For single teeth the highest forces (table 1 and figure 1e) were needed to break the central teeth, followed by the laterals and finally marginals. Significantly higher force was needed to break individual teeth in wet than in dry condition (p < 0.001; table 2).
Table 2.
Results from ANOVA for breaking force and breaking stress.
| source | df | sum of squares | mean square | F ratio | p-value |
|---|---|---|---|---|---|
| analysis of variance for breaking force | |||||
| species | 6 | 10689746.0 | 1 781 624 | 31.2611 | <0.0001* |
| error | 64 | 3647471.0 | 56 992 | ||
| total | 70 | 14337217.0 | |||
| analysis of variance for breaking stress | |||||
| species | 4 | 2196678.2 | 549 170 | 16.3859 | <0.0001* |
| error | 35 | 1173016.5 | 33 515 | ||
| total | 39 | 3369694.8 | |||
In all experiments, marginals broke as single teeth, but central and lateral teeth displayed a different mechanical behaviour; the quantity of experiments in which the row or the individual tooth was broken is presented in table 1. When comparing conditions (dry or wet) most of the experiments resulting in broken rows of central and lateral teeth were carried out under wet condition, because when force was applied to the dry central or lateral teeth, they failed individually at the level of their denticles and in most cases independently from the adjacent teeth. A breakage of lateral and central teeth under wet condition means that membrane failure occurred, because teeth were able to gain mechanical support by the adjacent tooth rows until the shear forces resulted in a ruptured membrane. Significant higher force was required for the breaking of tooth rows than for the individual tooth (p < 0.001; table 2).
(c). Breaking stress
The highest breaking stresses (table 1 and figure 1e) were needed for breaking the central teeth, followed by the laterals and finally marginals. Teeth in wet condition were able to resist significantly higher stresses than those in dry condition (p < 0.001; table 2).
4. Discussion
Water content has a high influence on the mechanical properties of various biological materials. In general, hardness and stiffness of wet materials is lower than these of same dry materials, whereas dry materials have lower fracture toughness than wet ones. This seems to be a general principle for biomaterials and was confirmed for invertebrates (e.g. insect cuticle [49–60]), but also vertebrates (e.g. mammal horn tissue [61,62]; bones, e.g. [63,64]). Only few studies on enamel and dentine revealed a higher hardness in tests under wet condition [65]. For Mollusca, the degree of water in the context of functional gradients had been studied in the chitinous squid beak [66,67]. For radular teeth, it had been reported that hydrated teeth display about 15% reduction in Young's modulus and hardness [16].
The influence of the water content on the here examined failure of radular structures is rather high; our breaking stress experiments revealed that significantly higher forces must be exerted on the tooth cusps to break teeth under wet condition. The reason for this is an increased flexibility of the chitinous teeth and of the embedding membrane in wet condition. The higher flexibility allows the marginal teeth to decrease the possibility of being broken by obstacles during feeding, because the teeth are able to deform and slip away. For central and lateral teeth, the higher flexibility allows bending at the stylus or tooth basis and using teeth of the same type from adjacent rows in anterior–posterior direction as a kind of support, distributing the stress from the hard and stiff tooth cusps not only to the stylus of the same tooth, but also to the styli of the adjacent teeth. This interaction and the subsequent stress redistribution were already proposed by Hickman [29,31,34–36] and is supported by experimental results of the present study. The bending of the teeth is additionally enabled by the elasticity of the embedding radular membrane; this chitinous structure anchors the teeth, but additionally reduces tooth failure by functioning as cushion.
As mentioned in the introduction, the ability of a structure to transfer force can often be linked with its Young's modulus and its ability to bear load with its hardness and breaking stress. This effect was also revealed in the studied radular teeth: the failure of the individual tooth usually takes place in the softest and most flexible part of the tooth, the stylus, whereas the cusps as the hardest and stiffest parts are not as prone to failure. This result agrees with finite-element-analysis scenarios on Spekia zonata's teeth [36] displaying high values of stress and strain in the styli, whereas the harder and stiffer tooth cusps are almost not affected from stress and strain under load (figure 1g). Overall, lateral and central ones were able to resist to higher stress in comparison to the marginals, which is also congruent the results of Krings et al. [36]. The dry ones were however so brittle, that they broke at the region of their denticles, the part where the force was applied. Under native (wet) condition central and lateral teeth failed only due to ripping of the underlying radular membrane, because the wet ones were capable of bending to the adjacent row and relying on it, as mentioned above. The radular membrane, composed of parallel chitinous fibres, is the thinnest radular component (15–16 µm thickness in S. zonata, see [4]) and is the most flexible radular part under wet condition. However, the membrane seems to be the structure limiting the force that can be exerted by the teeth during foraging.
The mechanical behaviour of teeth seems to depend also on their attachment area with this membrane. Central and lateral teeth have a significantly larger (p < 0.001) anchorage area than the marginal teeth [4]. This in turn may lead to a better stress redistribution from the cusps over the stylus to the radular membrane in central and lateral teeth while interacting with the solid feeding substrate during scratching action. The smaller attachment area of the marginal teeth allows a large range of deflection possibly preventing tooth failure when hitting a larger and hard obstacle.
The analysed mechanical behaviour of the distinct tooth types tested here under wet (native) condition allows us to verify the past hypotheses on Spekia's tooth functionality [29,36]. Since central and lateral teeth can resist to higher stresses than the marginal ones, they are rather capable of loosening algae from rocks whereas marginal teeth gather the particles afterwards.
In the future, we will address the functionality of radular teeth from more species by analysing breaking stresses; by relating ingesta with radular performance we hope to gain insight into processes like trophic specialization.
Supplementary Material
Acknowledgements
We thank Heinz Büscher from Basel for collecting specimens at Lake Tanganyika, Thomas M. Kaiser from the CeNak, University of Hamburg, for discussing results and Peter Stutz from the Mineralogical-Petrographic Institute, University of Hamburg, for the great support in the sample preparation. We thank Oliver Hawlitschek from the CeNak, University of Hamburg, for his help in the name finding process of the manuscript. We are highly grateful for the supporting and constructive comments of the three anonymous reviewers and the editorial office.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.2jm63xsnj [68].
Authors' contribution
W.K. performed experiments, analysed the data, wrote the manuscript and drew the figures. A.K. set up the experiments and supported the data analysis. S.G. initiated the project, discussed the data, figures and contributed to the biomechanical aspects of the manuscript. All authors contributed to and approved the manuscript for publication.
Competing interests
We declare we have no competing interests.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Chapman AD. 2009. Numbers of living species in Australia and the world, 2nd edn. Toowoomba, Australia: Australian Biodiversity Information Services. [Google Scholar]
- 2.Wanninger A, Wollesen T. 2019. The evolution of molluscs. Biol. Rev. 94, 102-115. ( 10.1111/brv.12439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guralnick R, Smith K. 1999. Historical and biomechanical analysis of integration and dissociation in molluscan feeding, with special emphasis on the true limpets (Patellogastropoda: Gastropoda). J. Morphol. 241, 175-195. () [DOI] [PubMed] [Google Scholar]
- 4.Krings W, Brütt J-O, Gorb S, Glaubrecht M. 2020. Tightening it up: diversity of the chitin anchorage of radular teeth in paludomid freshwater gastropods. Malacologia 63, 77-94. ( 10.4002/040.063.0108) [DOI] [Google Scholar]
- 5.Vortsepneva EV. 2020. Radula morphology of Clione limacina (Phipps, 1774) (Gastropoda: Heterobranchia: Gymnosomata). Inverteb. Zool. 17, 291-309. ( 10.15298/invertzool.17.3.06) [DOI] [Google Scholar]
- 6.Runham NW, Thornton PR. 1967. Mechanical wear of the gastropod radula: a scanning electron microscope study. J. Zool. 153, 445-452. ( 10.1111/j.1469-7998.1967.tb04976.x) [DOI] [Google Scholar]
- 7.van der Wal P, Giesen H, Videler J. 1999. Radular teeth as models for the improvement of industrial cutting devices. Mater. Sci. Eng. C 7, 129-142. ( 10.1016/S0928-4931(99)00129-0) [DOI] [Google Scholar]
- 8.Shaw JA, Macey DJ, Brooker LR, Clode PL. 2010. Tooth use and wear in three iron-biomineralizing mollusc species. Biol. Bull. 218, 132-144. ( 10.1086/BBLv218n2p132) [DOI] [PubMed] [Google Scholar]
- 9.Ukmar-Godec T, Kapun G, Zaslansky P, Faivre D. 2015. The giant keyhole limpet radular teeth: a naturally-grown harvest machine. J. Struct. Biol. 192, 392-402. ( 10.1016/j.jsb.2015.09.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mikovari A, Williams J, Oakes F, Lincicum RB, Zellmer AJ, Martin GG. 2015. Radula development in the giant key-hole limpet Megathura crenulata. J. Shellfish Res. 34, 893-902. ( 10.2983/035.034.0319) [DOI] [Google Scholar]
- 11.Runham NW. 1962. Rate of replacement of the molluscan radula. Nature 194, 992-993. ( 10.1038/194992b0) [DOI] [Google Scholar]
- 12.Runham NW, Isarankura K. 1966. Studies on radula replacement. Malacologia 5, 73. [Google Scholar]
- 13.Mackenstedt U, Märkel K. 1987. Experimental and comparative morphology of radula renewal in pulmonates (Mollusca, Gastropoda). Zoomorphology 107, 209-239. ( 10.1007/BF00312262) [DOI] [Google Scholar]
- 14.Padilla DK, Dittman DE, Franz J, Sladek R. 1996. Radular production rates in two species of Lacuna Turton (Gastropoda: Littorinidae). J. Molluscan Stud. 62, 275-280. ( 10.1093/mollus/62.3.275) [DOI] [Google Scholar]
- 15.Shaw JA, Macey DJ, Brooker LR. 2008. Radula synthesis by three species of iron mineralizing molluscs: production rate and elemental demand. J. Mar. Biol. Assoc. UK 88, 597-601. ( 10.1017/S0025315408000969) [DOI] [Google Scholar]
- 16.Weaver JC, et al. 2010. Analysis of an ultra hard magnetic biomineral in chiton radular teeth. Mater. Today 13, 42-52. ( 10.1016/S1369-7021(10)70016-X) [DOI] [Google Scholar]
- 17.Lu D, Barber AH. 2012. Optimized nanoscale composite behaviour in limpet teeth. J. R. Soc. Interface 9, 1318-1324. ( 10.1098/rsif.2011.0688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grunenfelder LK, de Obaldia EE, Wang Q, Li D, Weden B, Salinas C, Wuhrer R, Zavattieri P, Kisailus D. 2014. Biomineralization: Stress and damage mitigation from oriented nanostructures within the radular teeth of Cryptochiton stelleri. Adv. Funct. Mater. 24/39, 6085. ( 10.1002/adfm.201401091) [DOI] [Google Scholar]
- 19.Barber AH, Lu D, Pugno NM. 2015. Extreme strength observed in limpet teeth. J. R. Soc. Interface 12, 20141326. ( 10.1098/rsif.2014.1326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ukmar-Godec T, et al. 2017. Materials nanoarchitecturing via cation-mediated protein assembly: making limpet teeth without mineral. Adv. Mater. 29, 1701171. ( 10.1002/adma.201701171) [DOI] [PubMed] [Google Scholar]
- 21.Breure ASH, Gittenberger E. 1981. The rock-scraping radula, a striking case of convergence (Mollusca). Neth. J. Zool. 32, 307-312. ( 10.1163/002829681X00347) [DOI] [Google Scholar]
- 22.Steneck RS, Watling L. 1982. Feeding capabilities and limitation of herbivorous molluscs: a functional group approach. Mar. Biol. 68, 299-319. ( 10.1007/BF00409596) [DOI] [Google Scholar]
- 23.Kesler DH, Jokinen EH, Munns WR Jr. 1986. Trophic preferences and feeding morphology of two pulmonate snail species from a small New England pond, USA. Can. J. Zool. 64, 2570-2575. ( 10.1139/z86-377) [DOI] [Google Scholar]
- 24.Black R, Lymbery A, Hill A. 1988. Form and function: size of radular teeth and inorganic content of faeces in a guild of grazing molluscs at Rottnest Island, Western Australia. J. Exp. Mar. Biol. Ecol. 121, 23-35. ( 10.1016/0022-0981(88)90021-4) [DOI] [Google Scholar]
- 25.Blinn W, Truitt RE, Pickart A. 1989. Feeding ecology and radular morphology of the freshwater limpet Ferrissia fragilis. J. N. Am. Benthol. Soc. 8, 237-242. ( 10.2307/1467327) [DOI] [Google Scholar]
- 26.Ilken K. 1999. Feeding ecology of the Antarctic herbivorous gastropod Laevilacunaria antarctica Martens. J. Exp. Mar. Biol. Ecol. 236, 133-148. ( 10.1016/S0022-0981(98)00199-3) [DOI] [Google Scholar]
- 27.Nishi M, Kohn AJ. 1999. Radular teeth of Indo-Pacific molluscivorous species of Conus: a comparative analysis. J. Molluscan Stud 65, 483-497. ( 10.1093/mollus/65.4.483) [DOI] [Google Scholar]
- 28.Duda TF, Kohn AJ, Palumbi SR. 2001. Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol. J. Linn. Soc. 73, 391-409. ( 10.1006/bijl.2001.0544) [DOI] [Google Scholar]
- 29.Krings W, Kovalev A, Glaubrecht M, Gorb SN. 2019. Differences in the Young modulus and hardness reflect different functions of teeth within the taenioglossan radula of gastropods. Zoology 137, 125713. ( 10.1016/j.zool.2019.125713) [DOI] [PubMed] [Google Scholar]
- 30.Hickman CS. 1980. Gastropod radulae and the assessment of form in evolutionary paleontology. Paleobiology 6, 276-294. ( 10.1017/S0094837300006801) [DOI] [Google Scholar]
- 31.Padilla DK. 2004. Form and function of radular teeth of herbivorous molluscs: focus on the future. Am. Malacolog. Bull. 18, 163-168. [Google Scholar]
- 32.Solem A. 1972. Malacological applications of scanning electron microscopy. II. Radular structure and functioning. Veliger 14, 327-336. [Google Scholar]
- 33.Morris TE, Hickman CS. 1981. A method for artificially protruding gastropod radulae and a new model of radula function. Veliger 24, 85-89. [Google Scholar]
- 34.Hickman CS. 1984. Implications of radular tooth-row functional-integration for archaeogastropod systematics. Malacologia 25, 143-160. [Google Scholar]
- 35.Herrera SA, et al. 2015. Stylus support structure and function of radular teeth in Cryptochiton stelleri. Paper presented at 20th Int. Conf. on Composite Materials Copenhagen, Denmark, 19–24 July. [Google Scholar]
- 36.Krings W, Marcé-Nogué N, Karabacak H, Glaubrecht M, Gorb SN. 2020. Finite element analysis of individual taenioglossan radular teeth (Mollusca). Acta Biomater. 115, 317-332. ( 10.1016/j.actbio.2020.08.034) [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Meyers MA, Zhang Z, Ritchie RO. 2017. Functional gradients and heterogeneities in biological materials: design principles, functions, and bioinspired applications. Prog. Mater. Sci. 88, 467-498. ( 10.1016/j.pmatsci.2017.04.013) [DOI] [Google Scholar]
- 38.Bendsøe MP. 1989. Optimal shape design as a material distribution problem. Struct. Optim. 1, 193-202. ( 10.1007/BF01650949) [DOI] [Google Scholar]
- 39.Bendsøe MP. 1995. Optimization of structural topology, shape and material. Berlin, Germany: Springer. [Google Scholar]
- 40.Bendsøe MP, Kikuchi N. 1988. Generating optimal topologies in structural design using a homogenization method. Comput. Methods Appl. Mech. Eng. 71, 197-224. ( 10.1016/0045-7825(88)90086-2) [DOI] [Google Scholar]
- 41.Dumont ER, Grosse IR, Slater GJ. 2009. Requirements for comparing the performance of finite element models of biological structures. J. Theor. Biol. 256, 96-103. ( 10.1016/j.jtbi.2008.08.017) [DOI] [PubMed] [Google Scholar]
- 42.Freeman PW, Lemen CA. 2007. The trade-off between tooth strength and tooth penetration: predicting optimal shape of canine teeth. J. Zool. 273, 273-280. ( 10.1111/j.1469-7998.2007.00325.x) [DOI] [Google Scholar]
- 43.Woodward SP. 1859. On some new freshwater shells from Central Africa. Proc. Zool. Soc. Lond. 27, 348-351. [Google Scholar]
- 44.Brown D. 1994. Freshwater snails of Africa and their medical importance. London, UK: Taylor and Francis. [Google Scholar]
- 45.Glaubrecht M. 2008. Adaptive radiation of thalassoid gastropods in Lake Tanganyika, East Africa: morphology and systematization of a paludomid species flock in an ancient lake. Zoosystematics Evol. 84, 71-122. ( 10.1002/zoos.200700016) [DOI] [Google Scholar]
- 46.Krings W, Faust T, Kovalev A, Neiber MT, Glaubrecht M, Gorb SN. 2019. In slow motion: radula motion pattern and forces exerted to the substrate in the land snail Cornu aspersum (Mollusca, Gastropoda) during feeding. R. Soc. Open Sci. 6, 2054-5703. ( 10.1098/rsos.190222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosoda N, Gorb SN. 2012. Underwater locomotion in a terrestrial beetle: combination of surface de-wetting and capillary forces. Proc. R. Soc. B 279, 4236-4242. ( 10.1098/rspb.2012.1297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holznagel W. 1998. A nondestructive method for cleaning gastropod radulae from frozen, alcohol-fixed, or dried material. Am. Malacol. Bull. 14, 181-183. [Google Scholar]
- 49.Cusack S, Miller A. 1979. Determination of the elastic constants of collagen by Brillouin light scattering. J. Mol. Biol. 135, 39-51. ( 10.1016/0022-2836(79)90339-5) [DOI] [PubMed] [Google Scholar]
- 50.Thompson JI, Czernuszka JT. 1995. The effect of two types of cross-linking on some mechanical properties of collagen. Biomed. Mater. Eng. 5, 37-48. ( 10.3233/BME-1995-5105) [DOI] [PubMed] [Google Scholar]
- 51.Andersen SO, Peter MG, Roepstorff P. 1996. Cuticular sclerotization in insects. Comp. Biochem. Physiol 113B, 689-705. ( 10.1016/0305-0491(95)02089-6) [DOI] [Google Scholar]
- 52.Vincent JF, Wegst UG. 2004. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 33, 187-199. ( 10.1016/j.asd.2004.05.006) [DOI] [PubMed] [Google Scholar]
- 53.Schöberl T, Jäger IL. 2006. Wet or dry hardness, stiffness and wear resistance of biological materials on the micron scale. Adv. Eng. Mater. 8, 1164– 1169. ( 10.1002/adem.200600143) [DOI] [Google Scholar]
- 54.Müller M, Olek M, Giesrig M, Schmitz H. 2008. Micromechanical properties of consecutive layers in specialized insect cuticle: the gula of Pachnoda marginata (Coleoptera, Scarabaeidae) and the infrared sensilla of Melanophila acuminate (Coleoptera, Buprestidae). J. Exp. Biol. 211, 2576.–. ( 10.1242/jeb.020164) [DOI] [PubMed] [Google Scholar]
- 55.Vincent JFV. 2009. If it's tanned it must be dry: a critique. J. Adhesion 85, 755-769. ( 10.1080/00218460903291296) [DOI] [Google Scholar]
- 56.Dirks JH, Dürr V. 2011. Biomechanics of the stick insect antenna: damping properties and structural correlates of the cuticle. J. Mech. Behav. Biomed. Mater. 4, 2031-2042. ( 10.1016/j.jmbbm.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 57.Klocke D, Schmitz H. 2011. Water as a major modulator of the mechanical properties of insect cuticle. Acta Biom. 7, 2935-2942. ( 10.1016/j.actbio.2011.04.004) [DOI] [PubMed] [Google Scholar]
- 58.Dirks JH, Taylor D. 2012. Fracture toughness of locust cuticle. J. Exp. Biol. 215, 1502-1508. ( 10.1242/jeb.068221) [DOI] [PubMed] [Google Scholar]
- 59.Aberle B, Jemmali R, Dirks J-H. 2017. Effect of sample treatment on biomechanical properties of insect cuticle. Arthropod. Struct. Dev. 46, 138-146. ( 10.1016/j.asd.2016.08.001) [DOI] [PubMed] [Google Scholar]
- 60.Parle E, Dirks JH, Taylor D. 2017. Damage, repair and regeneration in insect cuticle: the story so far, and possibilities for the future. Arthropod. Struct. Dev. 46, 49-55. ( 10.1016/j.asd.2016.11.008) [DOI] [PubMed] [Google Scholar]
- 61.Werth AJ, Harriss RW, Rosario MV, George JC, Sformo TL. 2016. Hydration affects the physical and mechanical properties of baleen tissue. R. Soc. Open Sci. 3, 160591. ( 10.1098/rsos.160591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang W, Zaheri A, Yang W, Kisailus D, Ritchie RO, Espinosa H, McKittrick J. 2019. How water can affect keratin: hydration-driven recovery of bighorn sheep (Ovis canadensis). Horns. Adv. Funct. Mater. 29, 1901077. ( 10.1002/adfm.201901077) [DOI] [Google Scholar]
- 63.Nyman JS, Roy A, Shen X, Acuna RL, Tyler JH, Wang X. 2006. The influence of water removal on the strength and toughness of cortical bone. J. Biomech. 39, 931-938. ( 10.1016/j.jbiomech.2005.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Granke M, Does MD, Nyman JS. 2015. The role of water compartments in the material properties of cortical bone. Calcif. Tissue Int. 97, 292-307. ( 10.1007/s00223-015-9977-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaiser TM, Braune C, Kalinka G, Schulz-Kornas E. 2018. Nano-indentation of native phytoliths and dental tissues: implications for herbivore-plant combat and dental wear proxies. Evol. Syst. 2, 55-63. ( 10.3897/evolsyst.2.22678) [DOI] [Google Scholar]
- 66.Miserez A, Li Y, Waite JH, Zok F. 2007. Jumbo squid beaks: Inspiration for design of robust organic composites. Acta Biomater. 3, 139-149. ( 10.1016/j.actbio.2006.09.004) [DOI] [PubMed] [Google Scholar]
- 67.Miserez A, Schnerberk T, Sun C, Zok FW, Waite JJ. 2008. The transition from stiff to compliant materials in squid beaks. Science 319, 1816-1819. ( 10.1126/science.1154117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krings W, Kovalev A, Gorb SN. 2021. Breaking force Spekia. Dryad Digital Repository. ( 10.5061/dryad.2jm63xsnj) [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Krings W, Kovalev A, Gorb SN. 2021. Breaking force Spekia. Dryad Digital Repository. ( 10.5061/dryad.2jm63xsnj) [DOI]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.2jm63xsnj [68].