Abstract
Every autumn, monarch butterflies migrate from North America to their overwintering sites in Central Mexico. To maintain their southward direction, these butterflies rely on celestial cues as orientation references. The position of the sun combined with additional skylight cues are integrated in the central complex, a region in the butterfly's brain that acts as an internal compass. However, the central complex does not solely guide the butterflies on their migration but also helps monarchs in their non-migratory form manoeuvre on foraging trips through their habitat. By comparing the activity of input neurons of the central complex between migratory and non-migratory butterflies, we investigated how a different lifestyle affects the coding of orientation information in the brain. During recording, we presented the animals with different simulated celestial cues and found that the encoding of the sun was narrower in migratory compared to non-migratory butterflies. This feature might reflect the need of the migratory monarchs to rely on a precise sun compass to keep their direction during their journey. Taken together, our study sheds light on the neural coding of celestial cues and provides insights into how a compass is adapted in migratory animals to successfully steer them to their destination.
Keywords: insect, central complex, navigation, orientation, Lepidoptera
1. Introduction
Migration requires the use of a sophisticated form of orientation exhibited widely across taxa, from birds and sea turtles to insects [1]. One of the most well-known insect migrations is accomplished by monarch butterflies (Danaus plexippus), which travel every autumn from North America to overwintering locations in Central Mexico [2,3]. To maintain their migratory direction, these butterflies rely on the sun as their main orientation reference [4]. In addition, they integrate time of day information from circadian clocks in the antennae [5,6] and perhaps also in the brain [7] into the compass network. This guarantees that the butterflies can maintain a constant southerly direction even though the azimuthal position of the sun changes over the course of a day [4,8]. When the sun is occluded on overcast days, the butterflies may rely on other orientation cues, such as the polarized skylight [9], and/or the Earth's magnetic-field [10] to maintain their course. In addition, the spectral gradient present in the sky [11] could also serve as an orientation signal, as demonstrated in other insects [12].
The neural network that processes sky compass information in monarch butterflies has previously been described in detail [13,14]. While photoreceptors of the butterfly's dorsal rim area (DRA) detect the polarized skylight, the remaining photoreceptors on the compound eye detect unpolarized light [7,15]. The polarized and unpolarized signals converge in the same compass network and are transferred to a region of the central brain, called the bulb [16]. There, tangential (TL) neurons have their synaptic input and transmit the information to the central body lower division of the central complex (figure 1a). Recent studies have shown that the central complex plays a key role in processing skylight information in insects [13,17–19] and is involved in a variety of orientation tasks [20,21]. While not explicitly demonstrated in monarchs, the central complex probably functions as the internal compass during the migration by matching the actual heading direction with the desired southward direction [22].
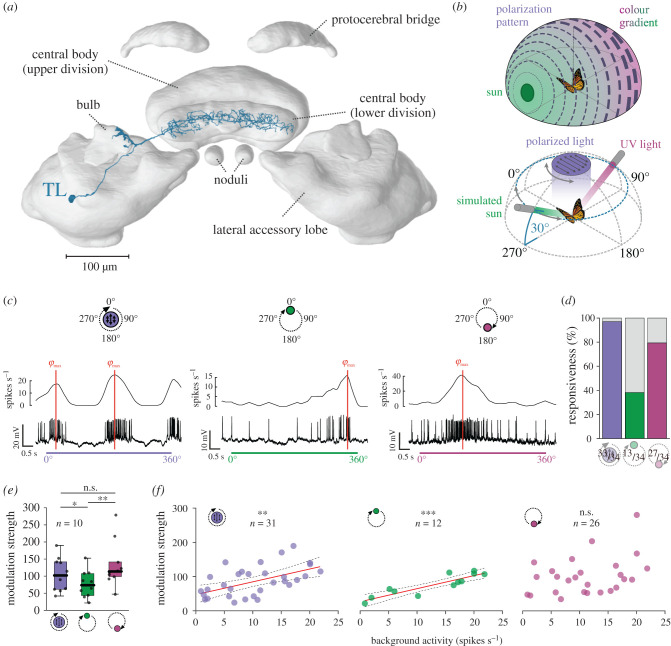
Figure 1.
Neural responses of TL neurons to simulated celestial cues. (a) TL neuron (blue) visualized in the monarch butterfly central complex. Modified from [16]. (b) Top: schematic drawing of the celestial cues in nature. Concentric circles illustrate the polarization pattern around the sun (green), with the line thickness denoting the degree of polarization. The colour gradient between the solar (green) and antisolar (magenta) hemisphere is shown. Bottom: schematic of the stimuli used during recordings. The polarization stimulus was presented in the animal's dorsal visual field (with the 0°-polarization angle aligned in parallel to the anterior-posterior body axis). The green/UV light spot (set 180° apart) were moved on a circular path (both at an elevation of 30°) around the animal. (c) Intracellular recordings from different TL neurons show the responses to polarized light (left), the green (middle) and the UV light spot (right). The schematic drawing (top) indicates the initial positions of each stimulus. The preferred directions (φmax) of the neuron are shown as red lines. (d) The responsiveness of the neurons to the different light cues (polarized light (left), green light (middle), UV light (right)). Coloured bars indicate the percentage of responsive neurons. (e) Modulation strengths of the TL neurons that responded significantly to all tested light stimuli (n = 10). These neurons showed a higher modulation to UV and polarized light compared to the green light (pPOL versus GREEN = 0.01, sign rank = 52; pUV versus GREEN = 0.002, sign rank = 0, n = 10; Wilcoxon-signed rank test). Individual data points are shown as black dots. Boxes indicate interquartile ranges. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines show the median. (f) The modulation strength of the TL neurons to polarized and the green light correlated linearly with the background activity (ppolarized light = 0.002, F = 12.32, ; pgreen light < 0.001, F = 41.97, ), which was not the case for the UV light (p = 0.072, ρSpearman = 0.35). The linear regressions are shown as solid red lines and the 95% confidence intervals are shown as dashed lines. n.s., not significant (e,f); *p < 0.05 (e), **p < 0.01 (e,f); ***p < 0.001 (e,f). (Online version in colour.)
While monarchs are best known for their autumn long-distance migration, completion of the migratory cycle is achieved by several generations. After overwintering in Central Mexico, migratory monarchs return north in the spring to southern states of the United States [23,24]. Recolonization of the breeding sites is not completed by the overwintered butterflies, but requires at least two spring generations that continue travelling north until they reach their summer habitat and produce non-migratory summer generations [14,25]. Thus, North American monarch butterflies occur in either a migratory or non-migratory form [26]. While migratory butterflies are physiologically adapted to effectively perform their long journey, non-migratory butterflies spend their time foraging in more restricted areas [27]. The transition from a generation of non-migratory to migratory butterflies significantly affects the volumes of compass neuropils within the central complex [16]. While these changes might be correlated with adaptations to the migration of these butterflies, their physiological consequence on the network remains unclear. To study this, we recorded the activity of the TL neurons intracellularly, while simultaneously presenting different simulated skylight cues to migratory and non-migratory butterflies. We found that the tuning width of the TL neurons to a green light spot representing the sun was narrower in migratory butterflies. In addition, the neurons in migratory butterflies exhibited a higher angular sensitivity to the sun in their frontal visual field, which matches the sun's position during their southward migration. Taken together, this supports the idea that the compass of migratory butterflies is adapted for their long-distance migration by allowing them to keep their flight direction with high precision over the course of the day.
2. Methods
(a). Animals
Recordings from the non-migratory monarch butterflies (Danaus plexippus) were performed from April to September 2018, 2019 in Würzburg (Germany). The butterflies were obtained as pupae from the Costa Rica Entomology Supply. After eclosion, the adult butterflies were kept in an incubator (I-30 VL, Percival Scientific) at 12 L : 12 D condition, 25°C and at 50% relative humidity. The animals' diet consisted of 15% sugar water solution ad libitum. Migratory animals were collected in College Station, TX, USA from October to November in 2018, 2019 and recordings were performed at this location. Wild-caught animals were kept in glassine envelopes and the incubator (I-30 VL, Percival Scientific) was adjusted to an 11 L : 13 D cycle with 23°C set during light and 12°C during dark phases to simulate the conditions during the autumn season. Animals were fed every other day with a 20% honey solution.
(b). Electrophysiology
Although the recordings were performed on two different electrophysiology set-ups (one at the University of Wuerzburg and one at Texas A&M University), both the equipment used and procedures for recordings from migratory and non-migratory animals were similar. To ensure stable recordings, the legs and wings were clipped off and the animals were fixed on a metal holder. After opening the head capsule, the neural sheath was removed using fine tweezers to expose the brain. At least one of the antennae remained intact to prevent the loss of time compensation [6]. During recording, the head capsule was filled with ringer solution (150 mM NaCl, 3 mM KCl, 10 mM TES, 25 mM sucrose, 3 mM CaCl2). Electrodes (50–200 MΩ) were drawn from borosilicate glass (inner/outer diameter: 0.75/1.5 mm, inner filament diameter: 0.2 mm; Hilgenberg) using a Flaming/Brown puller (P-97, Sutter Instruments) and were filled with 1 M KCl and loaded with 4% Neurobiotin (Vector Laboratories). In addition, a silver wire was immersed in the head capsule as a reference. A micromanipulator (Leica Microsystems) was used to position the electrode in the brain. Action potentials of single neurons were amplified using an intracellular amplifier (BA-03X, npi Elelctronic). The signals were digitized (Power1401, CED) with a resolution of 1–20 kHz and recorded using the Spike2.9 software (CED). After recording, the Neurobiotin was iontophoretically injected with 1–3.5 nA into the neuron for about 3–5 min and the brain was dissected out of the head. To identify the recorded neuron, we followed the immunohistochemical protocol described in [17] (for details, see the electronic supplementary methods, Histology and imaging). During cell injections, we often co-labelled several TL subtypes (electronic supplementary material, figure S1a). We were therefore not able to define from which TL subtype (TL2a/b or TL3, [16]) exactly our recordings were obtained. The similarity in the general tuning characteristic between migratory and non-migratory TL neurons suggests that, even if different TL subtypes may have different functional roles in monarch butterflies, this did not affect our comparisons between migratory and non-migratory TL neurons.
(c). Visual stimulus
To stimulate the butterflies with simulated skylight cues during recording, UV (365 nm, OSRAM) and green (520 nm, OSRAM) LEDs were mounted on a rotation stage (DT-50, PI miCos). The rotation stage was positioned dorsally to the animal. As the butterfly's DRA is sensitive to UV light [7,15], a UV-LED was mounted at the centre of the rotation stage. The light of the LED was passed through a diffuser (quarter white diffusion, LEE Filters) and a UV permeable polarizer (Bolder Vision Optik). Additionally, four arms were attached perpendicular to each other to the rotation stage. One arm was equipped with a green and the arm opposite, with a UV LED at the head of the arms. The stimulus was positioned in such a way that both LEDs were set at an elevation of 30° to the animal. All LEDs (unpolarized and polarized light) were adjusted to the same photon flux of about 1.4 × 1014 photons cm−2 s−1 using a spectrometer (Maya200 Pro, Ocean Optics) and measured in the position in which the animals would be facing the stimuli during the experiments. The angular extent of the polarization stimulus at the butterfly eye was 10.42° in non-migratory and 9.55° in migratory butterflies owing to slight differences in the set-ups. The angular size of the unpolarized light spots were 1.44° in non-migratory and 1.32° in migratory butterflies. The motions of the rotation stage were controlled via a custom-written Matlab script (v.R2019b, MathWorks). Similar to previous experiments [13], the polarizer orientation was changed with an angular velocity of 60° s−1. Likewise, the unpolarized light spots were moved on a circular path around the animal at a velocity of 60° s−1. The rotation stage was turned clockwise and counterclockwise while the tested cue (either the polarized or unpolarized light) was turned on.
(d). Data analysis
Neurons were included in the analysis if they fulfilled the following criteria: (i) stable baseline throughout the whole recording, (ii) consistency in shape of action potentials, (iii) spiking amplitude above the noise ratio, and (iv) neuron identifiable based on the tracer injection. The data were exported from Spike2 for further evaluation in Matlab using a custom-written script that included the CircStat toolbox [28] and the CircHist function [29].
Action potentials were detected using a manually set threshold. Background activity was evaluated based on a 3–6 s section of the recording prior to light stimulation. As the recording quality can affect the observed background activity (a lower electrode resistance can lead to more spikes s−1), we ensured that the background activity did not correlate with the electrode resistance (electronic supplementary material, figure S1b). The same segment as for the background activity was used to determine the background variability [30]. Sliding averages of the responses were obtained by applying a low-pass filter to the inter-spike-intervals. The maximum spike rate during stimulation was calculated by defining the highest action potential rate for each rotation and averaging it across all rotations. To calculate the neuron's preferred directions (φmax) for each light stimulus, each action potential was assigned to a degree according to the stimulus position. The preferred direction was then determined based on a circular unimodal (unpolarized light) or bimodal (polarized light) distribution. The modulation strength of each neuron, which contains information about the response strength to a light cue (a higher modulation value indicates a stronger response), was calculated in 20° bins according to [17]. To obtain the tuning curves, spike frequencies were calculated in 5° bins and the preferred direction (φmax) of each rotation was shifted to 0°. The firing rates from all rotations were averaged to obtain mean tuning curves for each neuron. The resulting curves were then normalized by the area below the curve. The tuning width for each cell was determined by calculating the full width at half maximum. The angular sensitivity (change of firing rate) of the neurons was calculated by determining the difference in the spike rate between adjacent bins. To analyse the distribution and compare the data statistically, we used linear and circular statistics (for details, see the electronic supplementary material, Statistics). All data are reported as mean ± standard deviation (s.d.).
3. Results
(a). General tuning characteristics of tangential neurons
We recorded from 34 TL neurons: 17 from migratory and 17 from non-migratory monarch butterflies. In many insects, a green light spot is interpreted as the sun's direction [17,31,32]. During recordings, a green light spot was therefore moved on a circular path around the butterfly to simulate a rotation of the animal under the sun (figure 1b). To test if the neurons encode the celestial spectral gradient, we observed the neural response to a moving UV light spot. A rotation of the butterfly under the polarization pattern was simulated through a full rotation of a zenithal polarizer. TL neurons typically exhibited a bimodal response to the polarizer (figure 1c, left) and a unimodal response to the unpolarized light spots (figure 1c; middle, right). However, not every neuron showed a significant modulation to the tested stimuli; 33 of the 34 recorded neurons showed a significant response to the polarization stimulus (figure 1d; p < 0.05; Rayleigh test). Out of the 34 tested neurons, 13 (38.23%) TL cells responded to the green light spot (figure 1d; p < 0.05; Rayleigh test). Interestingly, the neurons' responsiveness to the UV light was much higher (79.41%). Ten of the tested TL neurons responded to all three stimuli with a significant modulation of the firing rate (figure 1e). In these cells, the modulation strength was higher to the polarization (105.22 ± 49.65, mean ± s.d.) and the UV stimulus (133.37 ± 67.08) than to the green light (76.26 ± 39.53). Hereby, the modulation strength increased with the background activity when the neurons were stimulated with polarized light or the green light (figure 1f; electronic supplementary material, figure S2). A similar trend towards higher modulation strengths at higher background activities was also observed for the UV light spot. Taken together, our results show that TL neurons code for different skylight cues in monarch butterflies.
(b). Comparison of the responses in migratory and non-migratory butterflies
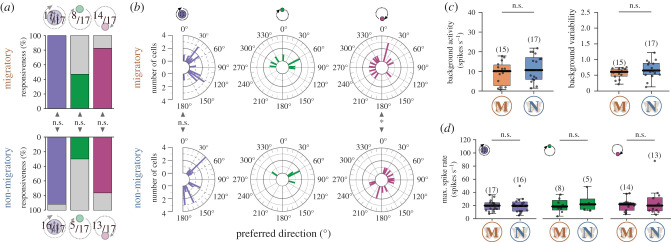
Next, we compared the TL-neuron responses of migratory and non-migratory butterflies. The responsiveness of the TL neurons to the presented stimuli did not differ between migratory and non-migratory monarchs (figure 2a), suggesting that skylight cues are equally relevant for the functioning of TL neurons in both forms. The stimulus position that evokes the strongest average response of a neuron is referred to as the preferred direction (φmax; figure 1c). In general, the preferred directions of the TL neurons to polarized light and the UV light covered all possible directions, while the preferred directions to the green light were clustered around 0° (electronic supplementary material, figure S3a). The preferred directions of the TL neurons to polarized light did not differ significantly between the forms (figure 2b, left plots). We could also not detect any obvious difference in the preferred directions to the green light between the migratory and non-migratory TL neurons (figure 2b, middle plots). However, the distribution of the UV preferred directions differed significantly between the two forms (figure 2b, right plots) because there were more migratory ones tuned to the left and more non-migratory ones tuned to the right visual field of the animals. We also calculated the angular differences between the preferred directions (Δφmax; electronic supplementary material, figure S3b). In TL neurons of both forms, the spatial relationship between the responses to polarized light and the green/UV light spot deviated from the natural 90°-relationship. In most of the TL cells, the relationship between the green and UV light was clustered between 0–90°, irrespective of the butterflies’ form (electronic supplementary material, figure S3b). Taken together, the preferred directions did not show any obvious differences between migratory and non-migratory butterflies to polarized light and the green light, suggesting that the recorded neurons were representing a wide range of the classical TL neuron population.
Figure 2.
Comparison of the general tuning characteristics of TL neurons in migratory and non-migratory butterflies. (a) Responsiveness to the different light stimuli (polarized light (left); green light (middle); UV light (right)) did not differ between the migratory and non-migratory TL neurons (ppolarized light = 1.00, χ2 = 0.00; pgreen light = 0.48, χ2 = 0.50; puv light = 1.00, χ2 = 0.00; χ2 test). (b) The preferred directions to polarized light (left), the green light (middle) and the UV light (right) in migratory (top; n = 17) and non-migratory (bottom; n = 17) TL neurons. The preferred directions did not differ between the forms for the polarized light (p = 0.57, W = 1.13, Mardia–Watson–Wheeler test), but for the UV light (p = 0.01, W = 8.62, Mardia–Watson–Wheeler test). Owing to the low sample size, a statistical analysis of the distributions was not performed for the preferred directions to the green light. (c) Background activity (left, p = 0.35, Z = −0.94, Wilcoxon rank-sum test) and background variability (right, p = 0.21, Z = −1.25, Wilcoxon rank-sum test) did not differ between migratory (M) and non-migratory (N) neurons. (d) Maximum spiking rates did not differ between the forms (ppolarized light = 0.73, Z = 0.34; pgreen light = 0.83, rank sum = 54.00; pUV light = 0.87, Z = 0.17; Wilcoxon rank-sum test). Sample size is shown in brackets. Individual data points are shown as black dots. Boxes indicate interquartile ranges. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines show the median. n.s.: not significant (a–d), *p < 0.05 (b). (Online version in colour.)
(c). Comparison of general physiological properties
We next compared the basic physiological properties of the TL neurons between the forms. On average, the background activity of the TL neurons in migratory butterflies was 9.25 ± 5.8 spikes s−1; n = 15), which was not different from the background activity observed in non-migratory TL neurons (11.61 ± 6.75 spikes s−1; n = 17; figure 2c, left plot). Similarly, the background variability did not differ either between groups (figure 2c, right plot). In addition, we investigated if the butterflies' form could influence the neurons' response to the tested skylight cues by comparing their maximum spike rate during stimulation (figure 2d). The maximum spike rate of the migrants TL neurons to polarized light was on average 19.67 ± 8.08 spikes s−1 (n = 17) and was comparable to the TL neurons of non-migratory butterflies (19.61 ± 11.29 spikes s−1; n = 16). Similarly, the responses to the green (max. spike ratemigratory = 20.00 ± 10.78 spikes s−1; n = 8; max. spike ratenon-migratory = 24.26 ± 14.4 spikes s−1; n = 5) or UV light (max. spike ratemigratory = 20.92 ± 9.53 spikes s−1; n = 14; max. spike ratenon-migratory = 24.77 ± 21.57 spikes s−1; n = 13) did not differ between the two monarch forms (figure 2d). Together with our findings that TL neurons display similar background activity/variability in migratory and non-migratory monarchs, these data suggest that the butterfly's form does not influence the general physiological properties of TL neurons.
(d). Comparison of functional tuning characteristics
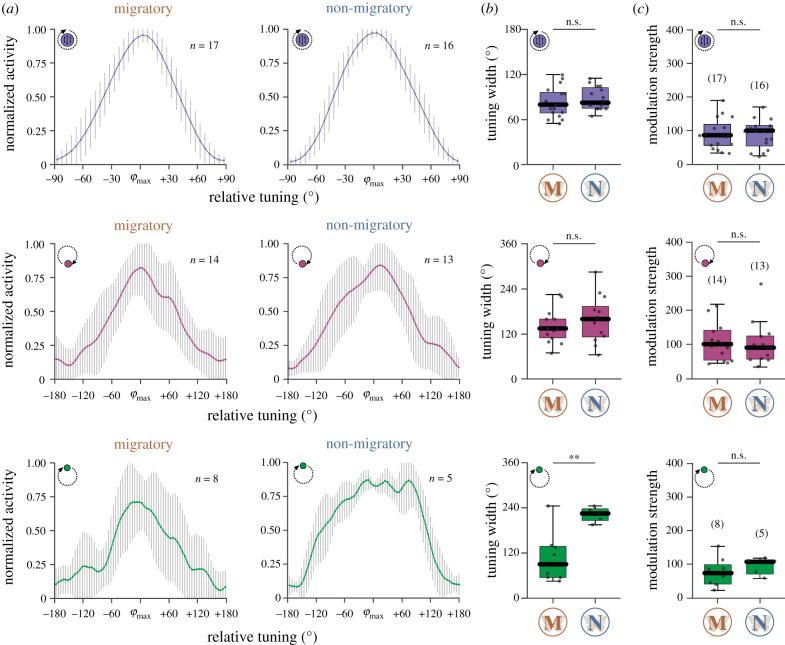
We next compared the tuning curves of the responses to the presented stimuli between the migratory and non-migratory TL neurons (figure 3a). For each TL cell, we generated individual tuning curves and shifted the preferred directions to 0° (electronic supplementary material, figure S4). The average tuning width of migratory TL neurons to polarized light (82.94 ± 19.93°) was similar to the tuning width observed in non-migratory butterflies (88.13 ± 15.04°; figure 3a,b). Similarly, the tuning width of the TL neurons to the UV light spot did not differ between migratory (140.71 ± 44.41°) and non-migratory (159.62 ± 61.56°) butterflies (figure 3a,b). By contrast, while the tuning curve in migratory butterflies was relatively narrow (106.88 ± 67.61°), the tuning curve in response to the green light in non-migratory butterflies was consistently broader (222.00 ± 19.87°; figure 3a,b; electronic supplementary material, figure S4). To exclude the possibility that the narrower tuning curves could result from stronger responses to the green light in TL neurons of migratory monarchs, we also compared the modulation strength of the responses. Neither the modulation strength to polarized light (Mmigratory = 89.68 ± 45.72, n = 17; Mnon-migratory = 88.64 ± 42.72, n = 16), to the UV light (Mmigratory = 107.94 ± 55.01, n = 14; Mnon-migratory = 103.31 ± 64.49, n = 13), or to the green light (Mmigratory = 72.12 ± 42.94, n = 8; Mnon-migratory = 93.20 ± 25.81, n = 5) differed between migratory and non-migratory butterflies (figure 3c) suggesting that the narrower tuning to the green light in migrants does not arise from differences in response strength.
Figure 3.
Comparison of the tuning shapes between migratory (M) and non-migratory (N) animals to polarized light (top), UV light (middle) and the green light (bottom). (a) Averaged tuning curves (bin size 5°) of the TL cells in migratory (left) and non-migratory animals (right). The n-size refers to the number of analysed cells. The error bars indicate the standard deviations. (b) While the tuning widths (full width at half maximum) of the responses to polarized light (p = 0.41, t-distribution = −0.84) and the UV light (p = 0.37, t-distribution = −0.92, unpaired t-test) were similar between the forms, a difference was found for the green light (p = 0.004, t-distribution = −3.66, unpaired t-test). (c) The modulation strengths to polarized light (p = 0.99, Z = 0.02), the UV light (p = 0.79, Z = 0.27) and the green light (p = 0.44, rank sum = 50.00) did not differ between migratory and non-migratory butterflies. Individual data points are represented by black dots. Boxes of the box plots display the interquartile ranges. Whiskers extend to the 2.5th and 97.5th percentiles. Black horizontal lines indicate the median. Sample size is shown in brackets (c). n.s., not significant (b,c)., **p < 0.01 (b). (Online version in colour.)
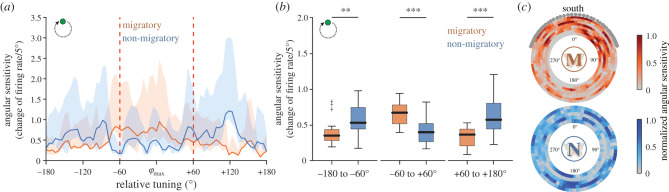
The precision of a compass increases by a high alteration of the neurons' spiking rate as the animal rotates around its body axis [12]. To test if the different tuning widths affect the rotational sensitivity of the TL network, we also compared the angular sensitivity, i.e. the change of firing rate, of the neurons between migratory and non-migratory butterflies (figure 4a). Interestingly, the angle relative to the preferred direction at which the neurons exhibited the highest sensitivity differed between the forms: while the angular sensitivity to the green light was significantly higher in the sector around the preferred direction in the migratory TL cells, the angular sensitivity of the non-migratory cells was higher in the sectors that were about 120° away from the preferred direction (figure 4b). As expected, these differences in the angular sensitivity were not observed when stimulating with polarized light or the UV light (electronic supplementary material, figure S5). Thus, although the tuning curves in response to the green light were broader in non-migratory butterflies, the TL neurons in both forms have the angular sensitivity to code for the sun. To investigate how the spatial difference in the angular sensitivity affects the precision of the compass, we analysed the angular sensitivity of each TL cell with respect to the position of the green light stimulus (figure 4c). In contrast to the TL neurons in non-migratory butterflies (p = 0.91; V-test, n = 5), in migratory TL neurons the highest angular sensitivities were significantly centred in the animals' anterior visual fields (p < 0.001; V-test, n = 8). Interestingly, this increased sensitivity in the front of the animals correlated with the sun azimuth during their southward migration (figure 4c). In summary, the TL neurons in migrants are well suited to maintain the sun in front of the animal and might help the butterflies to keep their southerly direction with a high precision during their long-distance migration.
Figure 4.
Comparison of the angular sensitivity to a moving green light spot. (a) The change of firing rate (bin size: 5°) to the green light stimulus with respect to the preferred direction of the cells (φmax) is shown for migratory (orange) and non-migratory (blue) TL neurons. Shaded areas indicate the 25–75% quantiles. Red vertical dashed lines separate the sectors that are statistically analysed in (b). (b) The mean angular sensitivity of the neurons to the green light. The TL neurons in migratory butterflies were more sensitive around the φmax (–60° to 60° (middle); p < 0.001, Z = 4.46, Wilcoxon-rank sum test), while the non-migratory neurons were more sensitive at an angular distance of about 120° away from the φmax (−180° to −60° (left); p < 0.022, Z = −3.06; +180° to +60° (right); p < 0.001, Z = −4.42, Wilcoxon rank sum test). (c) The circular plots show the normalized angular sensitivity in relation to the position of the green light for migratory (M, n = 8) and non-migratory (N, n = 5) TL neurons. Each row shows the angular sensitivity of one TL neuron. Grey dots at the perimeter of the upper plot indicate the sun azimuth over the course of a day (with respect to south) during the migration (1 November, College Station, TX). **p < 0.01 (b); ***p < 0.001 (b). (Online version in colour.)
4. Discussion
(a). General characteristics of tangential neurons
We showed that monarch TL neurons encode different skylight cues, which is in line with previous findings [13]. Each TL neuron is tuned to a specific polarization angle as observed in TL neurons in locusts, crickets, beetles [17,19,33], and butterflies [13], and may additionally encode the sun. Interestingly, the TL neurons, preferred directions to the green light were directed towards the animal's frontal visual field. Currently, we do not know what causes this bias or if it has any functional implication. The observed differences in the cells' responsiveness to polarized light/UV light versus green light is most likely a bias in the way of detecting the neurons during experiments. The polarization response helped in identifying a compass neuron and this was then tested using the green and UV light spot. Interestingly, the neurons showed higher responsiveness and modulation strength to the UV light than to the green light. This indicates that the UV photoreceptors in the butterflies' eye are either more sensitive than the green photoreceptors or that the UV light information is integrated over a larger array of ommatidia than the green light information.
Heinze et al. [16] divided the monarch TL neurons anatomically into three subtypes that innervate different layers in the central body lower division. We were not able to find any physiological differences between TL subtypes and, as we often co-labelled several TL subtypes (electronic supplementary material, figure S1a), were not always able to define from which subtypes our recordings were obtained. All three subtypes of monarch TL neuron are GABA-ergic [34] and inhibit the downstream heading-direction network, termed CL1 (E-PG in fruit flies) neurons, in the central body lower division (ellipsoid body in fruit flies [35]). Our results here show that the background activity of the TL neurons correlates with the modulation strength to polarized light and the green light spot. Thus, the sensitivity could be altered by changing the background activity of individual TL cells and with it, the inhibitory input on the CL1 network. Interestingly, TL neurons also receive circadian [36] and self-motion signals [37] in fruit flies. These inputs could modify the background activity of individual TL neurons in monarch butterflies and change the sensitivity for coding heading-direction information in a time-of-day or context-dependent (walking versus flying) manner.
(b). Integration of different celestial cues
The skylight polarization angles vibrate at 90° to the sun's position in nature. This spatial relationship, however, is not reflected in the TL cells in our study, in line with previous results from TL neurons in monarch butterflies [13]. This deviation originates in the DRA, which is not centred in the zenith in monarchs [15]. Compass neurons in the brain need to match the relationship between polarized light and the sun in a time-of-day dependent manner owing to the change of the sun's elevation over the course of the day [38]. Although such a time-dependent ‘elevation-compensation' has previously been reported in the monarch TL neurons [13], our TL recordings were too limited in terms of number of cells tuned to the simulated sun, to fully re-evaluate this.
Most of the TL cells' preferred direction was not affected by the spectral content of the light spot which is in line with previous reports in monarchs [13]. We found one migratory TL neuron that exhibited a 180° relationship between the green and UV light (electronic supplementary material, figure S2b). Such a neuron would help monarchs distinguish between solar and antisolar hemisphere based on spectral information. However, responses of compass neurons to spectral cues strongly depend on the light intensity [39]. Thus, more TL neurons might be detected that encode the celestial spectral information if tested at the correct light intensities.
(c). Behavioural context and neural plasticity
The volume of brain regions differs dramatically between the non-migratory and migratory forms in monarch butterflies and desert locusts [16,40]. In both species, this does not affect the general tuning properties of compass neurons [41, this study]. We here observed that the tuning width of the TL neurons in response to the simulated sun was narrower in migratory monarchs than in non-migrants, which led to a higher angular sensitivity in the frontal visual field. While the detailed neural mechanisms are unclear, this higher sensitivity may allow the migratory butterflies to precisely maintain the sun in their frontal visual field during their southward migration. This is essential during their long journeys in which an accumulation of even small deviations from the optimal course could lead to energetically costly detours. Despite being able to maintain a constant direction using a sun stimulus indoors [42], non-migratory butterflies do not show a group orientation to the south [26]. In line with this, we did not find any obvious bias in the spatial distribution of the TL neurons' angular sensitivity in non-migratory butterflies.
The TL neurons' input regions, the bulb, is highly conserved among insects and consists of large synapses [37,43–45]. This region has been shown to be highly plastic in Cataglyphis ants with the number of large synapses increasing in ants that change from workers to foragers [44]. Thus, the bulb represents an attractive candidate site where the differences in the tuning width might be established in the butterfly's brain. Future anatomical experiments comparing the large synapses of migratory and non-migratory butterflies may reveal differences depending on the behavioural context of the butterflies. Combined with a behavioural assay in which the butterflies maintain constant headings to a simulated sun [42], we will next be able to quantify the narrower tuning in migratory TL neurons on the precision of the butterflies compass.
Supplementary Material
Acknowledgements
We thank Samantha Iiams, Aldrin Lugena, Guijun Wan and Ying Zhang for helping capture migratory monarchs in College Station TX and for checking them for Ophryocystis elektroscirrha. We thank Anna Stoeckl, Emily Baird and James Foster for their helpful comments on the manuscript and Johannes Spaethe for providing us with a spectrometer. In addition, we thank Sergio Siles (butterflyfarm.co.cr) and Marie Gerlinde Blaese for providing us with monarch butterfly pupae in Würzburg.
Data accessibility
Raw data and analyses scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dv41ns1wr [46].
Authors' contributions
Study design: T.A.T.N., C.M., B.e.J. Conducted experiments: T.A.T.N. Analysis of data: T.A.T.N., M.J.B., B.e.J. Interpretation of data: T.A.T.N., M.J.B., C.M., B.e.J. Drafting of the manuscript: T.A.T.N., B.e.J. Critical review of the manuscript: M.J.B., C.M. Acquired funding: B.e.J. All authors approved of the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by the Emmy Noether program of the Deutsche Forschungsgemeinschaft granted to B.e.J. (GZ: EL784/1-1).
References
- 1.Mouritsen H. 2018. Long-distance navigation and magnetoreception in migratory animals. Nature 558, 50-59. ( 10.1038/s41586-018-0176-1) [DOI] [PubMed] [Google Scholar]
- 2.Reppert SM, de Roode JC. 2018. Demystifying monarch butterfly migration. Curr. Biol. 28, R1009-R1022. ( 10.1016/j.cub.2018.02.067) [DOI] [PubMed] [Google Scholar]
- 3.Merlin C, Iiams SE, Lugena AB. 2020. Monarch butterfly migration moving into the genetic era. Trends Genet. 36, 689-701. ( 10.1016/j.tig.2020.06.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouritsen H, Frost BJ. 2002. Virtual migration in tethered flying monarch butterflies reveals their orientation mechanisms. Proc. Natl Acad. Sci. USA 99, 10 162-10 166. ( 10.1073/pnas.152137299) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlin C, Gegear RJ, Reppert SM. 2009. Antennal circadian clocks coordinate sun compass orientation in migratory monarch butterflies. Science 325, 1700-1704. ( 10.1126/science.1176221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guerra PA, Merlin C, Gegear RJ, Reppert SM. 2012. Discordant timing between antennae disrupts sun compass orientation in migratory monarch butterflies. Nat. Commun. 3, 958. ( 10.1038/ncomms1965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sauman I, Briscoe AD, Zhu H, Shi D, Froy O, Stalleicken J, Yuan Q, Casselman A, Reppert SM. 2005. Connecting the navigational clock to sun compass input in monarch butterfly brain. Neuron 46, 457-467. ( 10.1016/j.neuron.2005.03.014) [DOI] [PubMed] [Google Scholar]
- 8.Froy O, Gotter AL, Casselman AL, Reppert SM. 2003. Illuminating the circadian clock in monarch butterfly migration. Science 300, 1303-1305. ( 10.1126/science.1084874) [DOI] [PubMed] [Google Scholar]
- 9.Reppert SM, Zhu H, White RH. 2004. Polarized light helps monarch butterflies navigate. Curr. Biol. 14, 155-158. ( 10.1016/j.cub.2003.12.034) [DOI] [PubMed] [Google Scholar]
- 10.Guerra PA, Gegear RJ, Reppert SM. 2014. A magnetic compass aids monarch butterfly migration. Nat. Commun. 5, 4164. ( 10.1038/ncomms5164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coemans MA, Vos Hzn JJ, Nuboer JF. 1994. The relation between celestial colour gradients and the position of the sun, with regard to the sun compass. Vision Res. 34, 1461-1470. ( 10.1016/0042-6989(94)90148-1) [DOI] [PubMed] [Google Scholar]
- 12.el Jundi B, Pfeiffer K, Heinze S, Homberg U. 2014. Integration of polarization and chromatic cues in the insect sky compass. J. Comp. Physiol. A 200, 575-589. ( 10.1007/s00359-014-0890-6) [DOI] [PubMed] [Google Scholar]
- 13.Heinze S, Reppert SM. 2011. Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69, 345-358. ( 10.1016/j.neuron.2010.12.025) [DOI] [PubMed] [Google Scholar]
- 14.Reppert SM, Guerra PA, Merlin C. 2016. Neurobiology of monarch butterfly migration. Annu. Rev. Entomol. 61, 25-42. ( 10.1146/annurev-ento-010814-020855) [DOI] [PubMed] [Google Scholar]
- 15.Stalleicken J, Labhart T, Mouritsen H. 2006. Physiological characterization of the compound eye in monarch butterflies with focus on the dorsal rim area. J. Comp. Physiol. A 192, 321-331. ( 10.1007/s00359-005-0073-6) [DOI] [PubMed] [Google Scholar]
- 16.Heinze S, Florman J, Asokaraj S, el Jundi B, Reppert SM. 2013. Anatomical basis of sun compass navigation II: the neuronal composition of the central complex of the monarch butterfly. J. Comp. Neurol. 521, 267-298. ( 10.1002/cne.23214) [DOI] [PubMed] [Google Scholar]
- 17.el Jundi B, Warrant EJ, Byrne MJ, Khaldy L, Baird E, Smolka J, Dacke M. 2015. Neural coding underlying the cue preference for celestial orientation. Proc. Natl Acad. Sci. USA 112, 11 395-11 400. ( 10.1073/pnas.1501272112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giraldo YM, Leitch KJ, Ros IG, Warren TL, Weir PT, Dickinson MH. 2018. Sun navigation requires compass neurons in Drosophila. Curr. Biol. 28, 2845-2852. ( 10.1016/j.cub.2018.07.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pegel U, Pfeiffer K, Homberg U. 2018. Integration of celestial compass cues in the central complex of the locust brain. J. Exp. Biol. 221, 1-15. ( 10.1242/jeb.171207) [DOI] [PubMed] [Google Scholar]
- 20.Neuser K, Triphan T, Mronz M, Poeck B, Strauss R. 2008. Analysis of a spatial orientation memory in Drosophila. Nature 453, 1244-1247. ( 10.1038/nature07003) [DOI] [PubMed] [Google Scholar]
- 21.Ofstad TA, Zuker CS, Reiser MB. 2011. Visual place learning in Drosophila melanogaster. Nature 474, 204-207. ( 10.1038/nature10131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinze S. 2017. Unraveling the neural basis of insect navigation. Curr. Opin. Insect Sci. 24, 58-67. ( 10.1016/j.cois.2017.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Urquhart FA. 1964. Monarch butterfly (Danaus plexippus) migration studies: autumnal movement. Proc. Entomol. Soc. Ont. 95, 23-33. [Google Scholar]
- 24.Urquhart FA, Urquhart NR. 1979. Vernal migration of the monarch butterfly (Danaus P. plexippus, Lepidoptera: Danaidae) in North America from the overwintering site in the neo-volcanic plateau of Mexico. Can. Entomol. 111, 15-18. ( 10.4039/Ent11115-1) [DOI] [Google Scholar]
- 25.Brower LP. 1995. Understanding and misunderstanding the migration of the monarch butterfly (Nymphalidae) in North America: 1857–1995. J. Lepid Soc. 49, 304-385. [Google Scholar]
- 26.Zhu H, Gegear RJ, Casselman A, Kanginakudru S, Reppert SM. 2009. Defining behavioral and molecular differences between summer and migratory monarch butterflies. BMC Biol. 7, 1-14. ( 10.1186/1741-7007-7-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calvert WH. 2001. Monarch butterfly (Danaus plexippus L., nymphalidae) fall migration: flight behavior and direction in relation to celestial and physiographic cues. J. Lepid Soc. 55, 162-168. [Google Scholar]
- 28.Berens P. 2009. CircStat: A MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1-21. ( 10.18637/jss.v031.i10) [DOI] [Google Scholar]
- 29.Zittrell F, Pfeiffer K, Homberg U. 2020. Matched-filter coding of sky polarization results in an internal sun compass in the brain of the desert locust. Proc. Natl Acad. Sci. USA 117, 25 810-25 817. ( 10.1073/pnas.2005192117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuebler E, Thivierge J-P. 2014. Spiking variability: theory, measures and implementation in MATLAB. Quant. Meth. Psych. 10, 131-142. ( 10.20982/tqmp.10.2.p131) [DOI] [Google Scholar]
- 31.Edrich W, Neumeyer C, von Heiversen O. 1979. ‘Anti-sun orientation’ of bees with regard to a field of ultraviolet light. J. Comp. Physiol. A 134, 151-157. ( 10.1007/BF00610473) [DOI] [Google Scholar]
- 32.Rossel S, Wehner R. 1984. Celestial orientation in bees: the use of spectral cues. J. Comp. Physiol. A 155, 605-613. ( 10.1007/BF00610846) [DOI] [Google Scholar]
- 33.Sakura M, Lambrinos D, Labhart T. 2008. Polarized skylight navigation in insects: model and electrophysiology of e-vector coding by neurons in the central complex. J. Neurophysiol. 99, 667-682. ( 10.1152/jn.00784.2007) [DOI] [PubMed] [Google Scholar]
- 34.Homberg U, Humberg TH, Seyfarth J, Bode K, Perez MQ. 2018. GABA immunostaining in the central complex of dicondylian insects. J. Comp. Neurol. 526, 2301-2318. ( 10.1002/cne.24497) [DOI] [PubMed] [Google Scholar]
- 35.Fisher YE, Lu J, D'Alessandro I, Wilson RI. 2019. Sensorimotor experience remaps visual input to a heading-direction network. Nature 576, 121-125. ( 10.1038/s41586-019-1772-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamaze A, Kratschmer P, Chen KF, Lowe S, Jepson JEC. 2018. A Wake-promoting circadian output circuit in Drosophila. Curr. Biol. 28, 3098-3105. ( 10.1016/j.cub.2018.07.024) [DOI] [PubMed] [Google Scholar]
- 37.Seelig JD, Jayaraman V. 2013. Feature detection and orientation tuning in the Drosophila central complex. Nature 503, 262-266. ( 10.1038/nature12601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeiffer K, Homberg U. 2007. Coding of azimuthal directions via time-compensated combination of celestial compass cues. Curr. Biol. 17, 960-965. ( 10.1016/j.cub.2007.04.059) [DOI] [PubMed] [Google Scholar]
- 39.Kinoshita M, Pfeiffer K, Homberg U. 2007. Spectral properties of identified polarized-light sensitive interneurons in the brain of the desert locust Schistocerca gregaria. J. Exp. Biol. 210, 1350-1361. ( 10.1242/jeb.02744) [DOI] [PubMed] [Google Scholar]
- 40.Ott SR, Rogers SM. 2010. Gregarious desert locusts have substantially larger brains with altered proportions compared with the solitarious phase. Proc. R. Soc. B 277, 3087-3096. ( 10.1098/rspb.2010.0694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.el Jundi B, Homberg U. 2012. Receptive field properties and intensity-response functions of polarization-sensitive neurons of the optic tubercle in gregarious and solitarious locusts. J. Neurophysiol. 108, 1695-1710. ( 10.1152/jn.01023.2011) [DOI] [PubMed] [Google Scholar]
- 42.Franzke M, Kraus C, Dreyer D, Pfeiffer K, Beetz MJ, Stoeckl AL, Foster JJ, Warrant EJ, el Jundi B. 2020. Spatial orientation based on multiple visual cues in non-migratory monarch butterflies. J. Exp. Biol. 223, 1-12. ( 10.1242/jeb.223800) [DOI] [PubMed] [Google Scholar]
- 43.Held M, Berz A, Hensgen R, Muenz TS, Scholl C, Roessler W, Homberg U, Pfeiffer K. 2016. Microglomerular synaptic complexes in the sky-compass network of the honeybee connect parallel pathways from the anterior optic tubercle to the central complex. Front. Behav. Neurosci. 10, 91-105. ( 10.3389/fnbeh.2016.00186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt F, Stieb SM, Wehner R, Roessler W. 2016. Experience-related reorganization of giant synapses in the lateral complex: potential role in plasticity of the sky-compass pathway in the desert ant Cataglyphis fortis. Dev. Neurobiol. 76, 390-404. ( 10.1002/dneu.22322) [DOI] [PubMed] [Google Scholar]
- 45.Träger U, Wagner R, Bausenwein B, Homberg U. 2008. A novel type of microglomerular synaptic complex in the polarization vision pathway of the locust brain. J. Comp. Neurol. 506, 288-300. ( 10.1002/cne.21512) [DOI] [PubMed] [Google Scholar]
- 46.Nguyen TAT, Beetz MJ, Merlin C, el Jundi B. 2021. Data from: Sun compass neurons are tuned to migratory orientation in monarch butterflies. Dryad Digital Repository. ( 10.5061/dryad.dv41ns1wr) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Nguyen TAT, Beetz MJ, Merlin C, el Jundi B. 2021. Data from: Sun compass neurons are tuned to migratory orientation in monarch butterflies. Dryad Digital Repository. ( 10.5061/dryad.dv41ns1wr) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Raw data and analyses scripts are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.dv41ns1wr [46].