Abstract
How do people form their political beliefs? In an effort to address this question, we adopt a neuropsychological approach. In a natural experiment, we explored links between neuroanatomy and ideological preferences in two samples of brain lesion patients in New York City. Specifically, we compared the political orientations of patients with frontal lobe lesions, patients with amygdala lesions and healthy control subjects. Lesion type classification analyses revealed that people with frontal lesions held more conservative (or less liberal) beliefs than those with anterior temporal lobe lesions or no lesions. Additional analyses predicting ideology by extent of damage provided convergent evidence that greater damage in the dorsolateral prefrontal cortex—but not the amygdala—was associated with greater conservatism. These findings were robust to model specifications that adjusted for demographic, mood, and affect-related variables. Although measures of executive function failed to mediate the relationship between frontal lesions and ideology, our findings suggest that the prefrontal cortex may play a role in promoting the development of liberal ideology. Our approach suggests useful directions for future work to address the issue of whether biological developments precede political attitudes or vice versa—or both.
This article is part of the theme issue ‘The political brain: neurocognitive and computational mechanisms’.
Keywords: political ideology, brain lesions, neuroscience, neuropsychology, amygdala, dorsolateral prefrontal cortex
1. Introduction
‘I had always considered my thoughts as something abstract, but they weren't; they were as material as the heart beating in my chest . . . All of our systems, too—communism, capitalism, religion, science—they also originated in electrochemical currents flowing through this three-pound lump of flesh encased in the skull.'
Karl Ove Knausgaard [1]
The idea that even very complex political beliefs and social systems are the product of human minds is non-controversial. Still, the conclusion that these political outcomes can be linked to observable features of the organ that comprises mental states—the brain—is surprising to some. An emerging science of the neurobiology of politics has illustrated several ways in which researchers learn about the nature of political psychology by directly examining the brain [2–10]. In the current investigation, we take a neuropsychological approach to better understand the connection between specific brain regions and political attitudes, building on foundational work in political neuroscience.
Research in political neuroscience has identified links between brain functions and structures, on the one hand, and political ideology, on the other (for reviews, see [5,9,10]). This work builds on a rich literature that conceptualizes political ideology as a belief system that is motivated by basic social, cognitive and motivational needs (e.g. [11–13]). Theory and evidence indicate that liberals (or leftists) and conservatives (or rightists) are motivated by fairly different psychological preferences and tendencies. Whereas conservatism is associated with heightened preferences for cognitive consistency, closure and structure, as well as greater attention to threat and needs for security, liberalism is associated with increased tolerance for uncertainty, ambiguity and cognitive conflict [13].
Neuroscientific investigations have provided evidence that is highly congruent with the behavioural evidence [4], including some studies showing stronger evidence for ideological differences in patterns of neural activity than in observed behaviour (e.g. [14,15]). Liberals have been found to exhibit increased brain activity and more grey matter volume in the anterior cingulate cortex, a brain region important for detecting cognitive conflict [16,17]. Furthermore, conservatives have been found to possess more grey matter volume in the amygdala, a region that detects motivationally salient objects, including threatening stimuli [17]. These studies have helped to establish a growing body of research linking political beliefs and personality traits to specific neuropsychological and psychophysiological substrates [18,19].
The correlational nature of neuroimaging studies cannot help us to determine whether the brain influences political attitudes and behaviours or if it is the other way around (or both; see [5]). Lesion studies can provide a bridge between such correlational neuroimaging work and more direct tests of causality, such as approaches that directly manipulate regional brain function (e.g. [20]). Individuals who have experienced a significant biological alteration due to neuropathology and treatment through surgical interventions of focal areas of the brain provide a kind of quasi-experimental test group [21]. Lesion studies provide insight into whether a certain brain region may be necessary for a specific behavioural outcome, insofar as individuals with damage to that area exhibit behavioural responses that are different from those who do not have damage in that area.
Lesion studies can, therefore, help to illuminate the critical roles played by different brain regions. For instance, research on lesion patients has found that damage to the amygdala is associated with decreased fear [22]; damage to the dorsolateral prefrontal cortex (dlPFC) is associated with religious fundamentalism [23]; damage to the ventromedial prefrontal cortex (vmPFC) is associated with right-wing authoritarianism [24]; and damage to the hippocampus (i.e. in anterograde amnesia patients) is associated with impaired explicit—but not necessarily implicit—memory for political candidates [25]. Taken in conjunction, these studies suggest that these brain regions might be linked to the expression of political beliefs and behaviours.
In the present study, we examined a sample of clinically treated patients with focal lesions to explore the roles of the frontal cortex and amygdala in political ideology. In particular, we considered the possibility that certain brain regions would be integral to ideology by examining whether damage to one region was associated with differences in liberal–conservative orientation. The lesion method is used here to explore which neuroanatomical regions—and the cognitive functions associated with them—may be especially important when it comes to understanding ideological preferences.
(a). Frontal lobe lesions
Because previous research suggests a link between individual differences in cognitive flexibility and political orientation, we focused first on a neuroanatomical region that is responsible for many executive functions, namely the prefrontal cortex [26–31]. Functional neuroimaging studies have suggested that frontal lobe activity in the dlPFC is associated with processing political information that is inconsistent and associated with opposing candidates [32–35]. In addition, heightened dlPFC activity has been observed in those who change their candidate preferences in response to negative political advertising [36]. Transcranial random noise stimulation (tRNS) and functional magnetic resonance imaging (fMRI) studies suggest that enhanced activity in the dlPFC may be associated with political conservatism [35,37]. There is also evidence based on transcranial magnetic stimulation (TMS) that links decreased dlPFC activity to socio-cognitive processes such as stereotyping and punishment [38,39]. However, the existing evidence concerning the political significance of dlPFC activation (and deactivation) is ambiguous at best.
A few lesion studies, including those mentioned above, suggest that injuries to the dlPFC may have ideological consequences. The most pertinent study found that lesions in this area were associated with more religious fundamentalism, and the association was mediated by decreased openness and cognitive flexibility [23]. In other research, dlPFC damage was found to be associated with less cooperation in a public goods game, perhaps indicating increased individualistic selfishness [40]. Another study suggested that frontal lobe dysfunction due to frontotemporal dementia was associated with dramatic changes in personality, including political orientation [41]. However, to our knowledge, no lesion work has yet directly examined the link between dlPFC damage and political ideology.
Building on prior research, we investigated whether frontal lobe lesions would be associated with increased conservatism (or decreased liberalism). We also considered the possibility that changes to executive function due to frontal lesions might exert ideological effects. Our approach was inherently exploratory because the existing evidence based on multiple methodological approaches is inconclusive concerning the relationship between the prefrontal cortex and political orientation. Nevertheless, we compared patients with frontal lobe lesions with patients with amygdala lesions as well as healthy control participants (with no lesions). This allowed us to assess whether the prefrontal cortex is a necessary region for promoting liberalism.
(b). Amygdala lesions
We also examined the possibility that the amygdala—a neural structure involved in the detection of emotionally salient stimuli, including objects connoting uncertainty and threat (e.g. [42,43])—would play a role in the formation of political beliefs. Neuroimaging studies reveal that the amygdala is especially active when people ponder issue positions and political candidates with which they agree [33,44,45]. Furthermore, people who show decreased amygdala activation in response to counter-attitudinal evidence are more likely to change their minds [46]. Larger amygdala volume has also been found to predict political conservatism [17], in addition to being (a) positively associated with system justification (that is, the tendency to accept existing social, economic, and political arrangements as fair and legitimate), and (b) negatively associated with participation in protests aimed at changing the societal status quo [47]—attitudinal and behavioural characteristics linked to political conservatism.
Although we know of no amygdala lesion studies that have focused on human political behaviour, there is some previous work suggesting that the amygdala plays a key role in threat detection and various forms of social behaviour [48–50]. In rhesus monkeys, for instance, amygdala damage impairs the ability to accurately perceive external risks as well as the social status of conspecifics [51–54]. A famous human patient with rare focal bilateral amygdala lesions due to Urbach–Wiethe disease failed to express normal fear and distress reactions in response to a range of potentially frightening objects and situations, including snakes, spiders, haunted houses and horror movies [22]. Studies of other human patients with amygdala lesions found that damage to this region was associated with diminished loss aversion [55].
In light of these varied findings, we explored the possible role of the amygdala in the development of political ideology. Because the amygdala is clearly linked to threat detection [22,51], and behavioural evidence suggests that conservatives are more sensitive to potential threats in their environment [56–58], we explored the possibility that people with amygdala lesions would be more likely to hold liberal attitudes than people with frontal lobe lesions and healthy control participants.
2. Materials and methods
(a). Participants
Sixty-two participants (mean age = 38 years; 31 female; 40 White/European-American, 10 Black/African-American, 5 Asian/Asian-American, 7 Latino/Hispanic) were recruited from the Patient Registry for the Study of Perception, Emotion & Cognition (PROSPEC) at New York University (NYU). This registry includes patients who have damaged brain tissue and/or surgical resections of brain tissue to treat brain pathology, as well as matched healthy control participants with normal brains. Most of the lesion patients in our study had damaged brain tissue because of surgical interventions to treat tumours or focal epilepsy. Therefore, the cause of their brain damage was almost surely unrelated to political orientation. The study was approved by the University Committee on Activities Involving Human Subjects (UCAIHS) at NYU, and all participants provided written informed consent.
Using neuroanatomical masks created by a board-certified neuropsychologist, the lesion patients were classified by their primary tissue damage. This procedure resulted in the classification of 18 patients with primary damage in the frontal lobe and 26 patients with primary lesions in the anterior temporal lobe (ATL), which includes the amygdala. Eighteen healthy control subjects with no brain damage were also recruited from the registry (see table 1 for demographic details of each group). These three classifications served as ‘natural' experimental groups, which were equivalent with respect to age (F2,58 = 0 .47, p = 0 .63), gender (χ2(2) = 1.78, p = 0 .41), years of education (F2,59 = 0.35, p = 0 .71), and intelligence (F2,59 = 0.03, p = 0 .97) as measured by the WAIS-IV (Wechsler Adult Intelligence Scale IV, Full-Scale IQ; [59]). Racial identification was unevenly distributed between the lesion groups to a marginally significant degree (χ2(6) = 10.63, p = 0 .10), so we conducted additional models with demographic covariates.
Table 1.
Demographics and neuropsychological status of lesion classification groups. **p < 0.01, *p < 0.05, +p < 0.1. Standard deviations in parentheses. WAIS-IV FSIQ = Wechsler Adult Intelligence Scale IV, Full Scale IQ; BDI = Beck Depression Inventory 2nd edn; BAI = Beck Anxiety Inventory; STAI = State–Trait Anxiety Inventory; PANAS = Positive and Negative Affect Schedule; WCST = Wisconsin Card Sorting Task; Stroop = Stroop Colour-Word Test; TMT-A = Trail Making Test Part A; TMT-B = Trail Making Test Part B.
| group | frontal lesion (N = 18) | amygdala (ATL) lesion (N = 26) | healthy control (N = 18) | F or χ2 |
|---|---|---|---|---|
| demographics | ||||
| age | 39.72 (12.95) | 36.20 (9.62) | 38.67 (14.72) | F = 0.47 |
| gender |
M = 11 F = 7 |
M = 13 F = 13 |
M = 7 F = 11 |
χ2 = 1.78 |
| education | 15.94 (1.89) | 15.54 (2.23) | 16.00 (1.85) | F = 0.35 |
| racial identification | White = 14 Black = 2 Asian = 1 Hispanic = 1 |
White = 19 Black = 2 Asian = 1 Hispanic = 4 |
White = 7 Black = 6 Asian = 3 Hispanic = 2 |
χ2 = 10.63 |
| cognitive and affective tests | ||||
| WAIS-IV FSIQ | 105.28 (13.43) | 105.46 (12.37) | 104.17 (28.53) | F = 0.03 |
| BDI | 9.00 (8.37) | 9.54 (8.67) | 2.83 (3.55) | F = 4.86* |
| BAI | 7.28 (6.63) | 7.00 (6.98) | 2.28 (2.56) | F = 4.25* |
| STAI trait | 41.00 (12.44) | 40.81 (10.48) | 38.17 (8.21) | F = 0.43 |
| STAI state | 32.11 (9.54) | 32.42 (10.26) | 32.33 (10.03) | F = 0.01 |
| PANAS positive | 34.39 (8.49) | 34.81 (7.63) | 33.56 (7.01) | F = 0.14 |
| PANAS negative | 20.11 (9.18) | 21.46 (6.80) | 15.94 (6.15) | F = 3.05† |
| WCST total categories completed | 3.06 (1.76) | 3.58 (1.50) | 3.67 (1.61) | F = 0.78 |
| WCST perseverative errors | 40.72 (7.50) | 44.96 (8.70) | 45.72 (10.88) | F = 1.64 |
| WCST accuracy rate | 0.67 (0.20) | 0.75 (0.14) | 0.72 (0.21) | F = 1.11 |
| Stroop | 50.76 (10.96) | 54.35 (8.27) | 53.39 (10.16) | F = 0.72 |
| TMT-A (time in seconds) | 29.94 (11.96) | 22.15 (6.70) | 25.11 (10.49) | F = 5.51** |
| TMT-B (time in seconds) | 64.12 (27.64) | 57.96 (17.50) | 60.94 (23.38) | F = 0.51 |
**p < 0.01, *p < 0.05, †p < 0.1.
(b). Procedure
As part of a larger battery of neuropsychological tests and questionnaires administered to all patients and control participants in the registry during initial PROSPEC inclusion, participants in our study answered surveys administered by a board-certified neuropsychologist assessing psychological characteristics and political ideology (see electronic supplementary material, table S1 for correlations among behavioural measures). All de-identified data and analysis code are publicly available at https://osf.io/xcwgy/.
(i). Political ideology
Our primary dependent variable was political ideology. Participants reported their political ideology using a self-placement item ranging from 1 (extremely liberal) to 6 (neither) to 11 (extremely conservative) on general political orientation (‘Where on the following scale of political orientation would you place yourself (overall, in general)?’). Prior research has established that a single-item self-report measure of general political orientation is strongly predictive of voting behaviour in nationally representative American samples, among many other things [12]. To explore more specific dimensions of ideology [60], participants also reported their political orientation in terms of social (‘In terms of social and cultural issues, how liberal or conservative are you?’) and economic (‘In terms of economic issues, how liberal or conservative are you?’) issues. On average, participants were slightly liberal overall (M = 4.73, s.d. = 2.24) and in terms of both social (M = 4.39, s.d. = 2.54) and economic issues (M = 5.31, s.d. = 2.71).
(ii). Affective and cognitive tests
A battery of standard affective and cognitive tests for brain lesion patients was administered to all participants. Tests of mood and affect were administered to rule out the possibility that differences in general emotional experiences would account for differences in ideology between the lesion classification groups.1 These tests included measures of depression, state and trait anxiety, and general positive and negative affect. Clinical depression was measured using the Beck Depression Inventory (BDI-II; [63]). The mean level of endorsed depressive symptoms was 7.44 (s.d. = 7.92), which is considered to be in the range of normal ‘ups and downs' in mood. Ratings of recently experienced anxiety symptoms were evaluated using Beck Anxiety Inventory (BAI; [64]). The average level of anxiety symptoms was 5.71 (s.d. = 6.24), which is considered to be low (with possible scores ranging from 0 to 21). We used the State–Trait Anxiety Inventory to obtain self-ratings of chronic anxiety as well as acute symptoms (STAI; [65]). The average state anxiety score was 32.31 (s.d. = 9.83), and the average trait anxiety score was 40.10 (s.d. = 10.41); both scores are considered to be below the level of clinical significance. Finally, general positive and negative affect was assessed with the Positive and Negative Affect Schedule (PANAS; [66]). The average positive affect score was 34.32 (s.d. = 7.61) and the average negative affect score was 19.47 (s.d. = 7.65). Both of these scores are close to the averages for non-clinical populations. As expected, measures of affect and mood disorders were highly intercorrelated (see electronic supplementary material, table S1 for bivariate correlations).
Previous research suggests that (a) lesions of the frontal lobe affect executive functions such as response inhibition and flexible set switching [26–31], and (b) measures of cognitive flexibility are associated with liberal ideology [13,67,68]. We therefore selected a set of cognitive tests from the neuropsychological battery to determine whether differences in cognitive functioning, especially executive functioning, in patients with various brain lesions might account for differences in political ideology.
Specifically, we analysed data from three tests of executive function that have been extensively used and standardized for clinical populations: the Wisconsin Card Sorting Test (WCST), focusing on the number of total categories completed, the perseverative errors score, and the overall accuracy rate as measures of flexible pattern response switching [69,70]; the colour–word interference score from the Stroop Test (STROOP), a measure of response inhibition [71]; and the Trail Making Test (TMT) Parts A and B, a measure of executive response switching [72]. As expected, the cognitive tests were moderately correlated with one another (see electronic supplementary material, table S1 for bivariate correlations).
3. Results
(a). Predicting political orientation by primary lesion classification
We began with a focal test using the full sample to examine possible differences in ideology between primary lesion classification groups as identified by a board-certified neuropsychologist. Although the frontal and anterior temporal lobes are both regions that serve multiple functions, the purpose of starting with a comparison between lesion classification groups was to explore whether broad functional dissociation for executive function (in the frontal lobe) and emotional and memory functions (in the ATL) might be related to political orientation. We modelled general political orientation as a linear function of dummy coded primary lesion classification. The lesion classification regression model suggested there were marginally significant differences among the groups, F2,59 = 2.76, p = 0 .07 (figure 1 and table 2, Model 1). Specifically, frontal lesion patients (M = 5.75, s.d. = 2.38) were more politically conservative than patients with ATL lesions (M = 4.31, s.d. = 1.98; p = 0 .03), and they were marginally more conservative than healthy control subjects (M = 4.33, s.d. = 2.25; p = 0 .06). There was no difference between the ATL lesion patients and healthy controls in terms of political orientation (p = 0 .97).2
Figure 1.
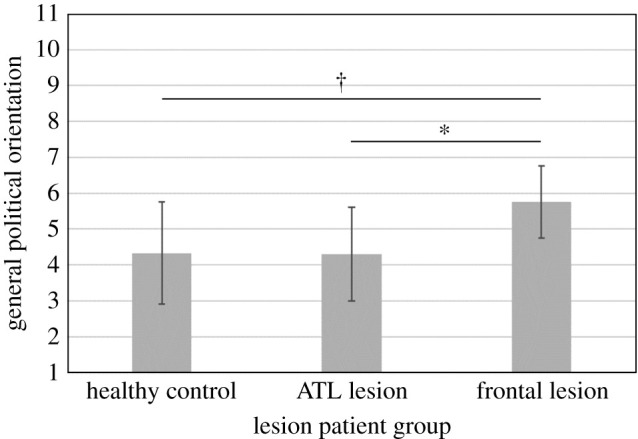
General political orientation as a function of primary lesion group. Note: Higher values on general political orientation reflect greater conservatism, and lower scores reflect greater liberalism. Error bars represent 95% confidence intervals (*p < 0.05, †p < 0.10). ATL=anterior temporal lobe (includes amygdala damage). Patients with damage to their frontal cortex reported more conservative political orientation than ATL patients and matched healthy control participants.
Table 2.
Hierarchical linear regression models predicting general political orientation from lesion classification groups (Model 1) and with demographic covariates (Model 2). ***p < 0.001, **p < 0.01, *p < 0.05, †p < 0.1. Standard errors in parentheses. Reference group is healthy control.
| general political orientation |
||
|---|---|---|
| variable | Model 1 | Model 2 |
| frontal lesion | 1.42† (0.73) | 1.91* (0.75) |
| ATL lesion | −0.03 (0.67) | 0.34 (0.69) |
| age | 0.02 (0.28) | |
| female | −0.45 (0.57) | |
| non-White | 1.61* (0.61) | |
| education | −0.34 (0.29) | |
| WAIS IQ | 0.48 (0.29) | |
| constant | 4.33*** (0.51) | 3.68*** (0.72) |
| F | 2.76† | 2.41* |
| d.f. | 2, 59 | 7, 53 |
| adjusted R² | 0.05 | 0.14 |
We conducted two additional types of analyses to assess the robustness of the association between frontal lobe lesions and political orientation. First, we adjusted the model for the demographic variables of age, gender, race, education and IQ (table 2, Model 2) and observed that frontal lesion patients were indeed significantly more conservative than ATL lesion patients (p = 0 .02) and healthy controls (p = 0 .01). Moreover, hierarchical regression modelling revealed that the inclusion of lesion classification explained a significant amount of variance in political orientation above and beyond the effects of demographic variables (Δ(adjusted R2) = 0 .09, p =0 .02). Second, because we observed some differences in depression, anxiety, and negative affect between the lesion groups and the healthy control group (table 1), we assessed three additional lesion classification models adjusting for BDI, BAI and the PANAS negative scale, respectively. Results revealed that lesion classification continued to be a significant predictor of political orientation above and beyond the effects of the affect variables.3
We also conducted a more exploratory analysis assessing differences between lesion classification groups in terms of ideological extremity, in light of prior work suggesting a link between cognitive inflexibility and extremity [73]. Specifically, we examined ideological extremity on general, social, and economic dimensions both as the absolute distance from the ideology scale midpoint and separately for those on the left versus right on the ideology scale. However, we failed to observe any statistically significant associations between lesion type and any of the indicators of ideological extremity, whether we adjusted for demographic variables or not (electronic supplementary material, tables S4–S6).
(b). Predicting political orientation from percentage of damage in dlPFC and amygdala
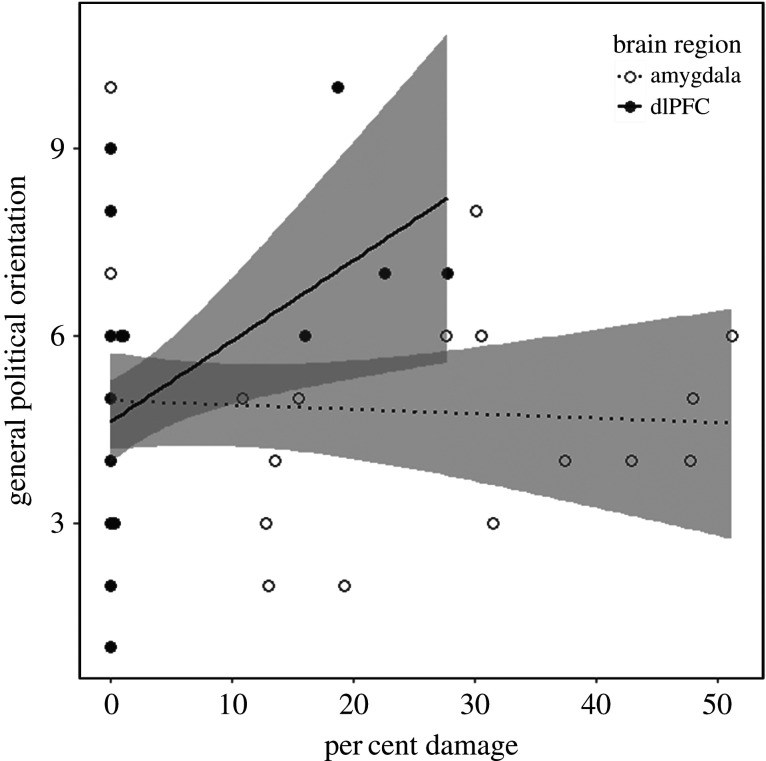
We conducted a complementary analysis on a subset of participants for whom we had information on the degree of damage to focal brain regions (n = 43), using percentage of damage in the regions of interest as a continuous predictor of general political orientation (see [40]). This enabled us to compare per cent damage in the amygdala and dlPFC to assess the relationship between neural structure and political ideology in a more targeted manner. Although these analyses were conducted on a smaller subsample, they provide a finer grained analysis that complements our lesion classification analyses. Consistent with the lesion classification analyses, linear regression models predicting general political orientation by per cent damage in the dlPFC and in the amygdala revealed that greater damage in the dlPFC was associated with conservatism, even after adjusting for demographic variables (figure 2 and table 3). Degree of amygdala damage was unrelated to political orientation.4
Figure 2.
General political orientation as a function of percentage of damage in the dlPFC and amygdala regions. Note: Error bands represent 95% confidence intervals.
Table 3.
Hierarchical linear regression models predicting general political orientation from ROI damage as continuous variables (Model 1) and with demographic covariates (Model 2). ***p < 0.001, **p < 0.01, *p < 0.05, +p < 0.1. Standard errors in parentheses.
| general political orientation |
||
|---|---|---|
| variable | Model 1 | Model 2 |
| dlPFC % damage | 0.13* (0.05) | 0.15* (0.06) |
| amygdala % damage | −0.003 (0.02) | −0.002 (0.02) |
| age | 0.01 (0.33) | |
| female | −0.10 (0.71) | |
| non-White | 1.26† (0.67) | |
| education | 0.24 (0.41) | |
| WAIS IQ | 0.21 (0.33) | |
| constant | 4.65*** (0.39) | 4.17*** (0.64) |
| F | 3.36* | 1.52 |
| d.f. | 2, 40 | 7, 35 |
| adjusted R² | 0.10 | 0.08 |
(c). Exploratory analyses: cognitive tests
Finally, we explored the possibility that the nature of brain lesions could impact political ideology via changes to executive functioning as assessed by a range of cognitive tests, following previous work in neuropsychology (e.g. [26–31]). Specifically, we aimed to determine (a) whether there were differences between the different lesion classification groups on tests of flexible response-switching and response inhibition, and, if so, (b) whether such differences might help to explain the observed differences in political ideology. Thus, we first compared the primary lesion classification groups on each of the cognitive tests and found that although mean score differences trended in the direction consistent with expectations, none was statistically significant except for TMT-A. Frontal patients took more time than ATL patients and healthy controls on the Trail Making Tests. Although frontal lesion patients tended to perform worse than ATL patients and healthy controls on the Stroop task and the WCST measures with respect to total categories completed, number of perseverative errors, and accuracy rate, these group differences were not statistically significant (table 1).
Because only one significant association between lesion classification type and cognitive test score was observed, we conducted a single mediation model [74]. On an exploratory basis, we assessed a simple mediation model using the psych package's mediate function in R, requesting 95% confidence intervals using 10 000 resamples, to examine whether time spent on TMT-A helped to explain the relationship between frontal lesions and political orientation (following the bootstrapping technique outlined by [75] and [76], for small sample sizes). The 95% confidence interval for the indirect effect of frontal lesion classification through TMT-A on political orientation was {−0.59, 0.54}, which was considered to be non-significant because the interval included 0.
4. Discussion
We found that frontal lobe lesion patients reported more conservative (or less liberal) political orientation than patients with damage to their anterior temporal lobe and healthy control participants with no history of brain damage. ATL lesion patients were as liberal as healthy control participants. These findings were robust to various model specifications, including those that adjusted for demographic, mood and affect-related variables. Moreover, the extent of damage in the dlPFC was positively associated with self-reported conservatism, whereas the extent of damage in the amygdala was not associated with ideology. These results were not attributable to ideological extremity, which was unrelated to brain damage.
The results of our study suggest that the prefrontal cortex may be a region that is integral to the expression of liberal political attitudes, insofar as damage to this brain region was associated with a more conservative orientation. The results are also suggestive of the possibility that the amygdala is an important structure for the development of conservative attitudes, given that ATL lesion patients were less conservative than frontal lobe lesion patients. This would be consistent with prior work linking greater amygdala volume to conservatism [17] and system justification [47]. At the same time, ATL lesion patients were no more liberal than healthy control participants, and the degree of amygdala damage was not associated with ideology, so, at least in this context, it appears that the amygdala per se is not a necessary structure for conservatism. We hope that future work will be able to assess causal pathways more directly, perhaps by obtaining information about political attitudes before and after a planned surgical resection of brain tissue.
Based on research linking (a) political liberalism to cognitive flexibility and control and (b) executive functioning to frontal lobe activity, we explored the possibility that patients with frontal lobe lesions were more conservative owing in part to diminished executive functioning. However, this possibility was not borne out in this study. We examined performance on three established tests of executive function; the differences we observed were in the expected direction, but they were not statistically significant, possibly because our sample was too small to provide sufficient statistical power. It is possible that specific cognition type could be useful to consider. For instance, it may be that social or identity-related cognitive functions are especially pertinent when it comes to linking frontal cortex function to political ideology (see [10]). Future research would do well to investigate these possibilities in larger samples, possibly by combining several small samples of patients with different types of lesions.
Some have argued that the study of political ideology requires the measurement of multiple dimensions, such as social and economic attitudes ([60]; but see [77]). We had no specific predictions regarding social versus economic dimensions of ideology (as opposed to overall liberalism–conservatism), but we did administer individual items to measure them separately. We found no evidence that brain lesions were differentially linked to social versus economic attitudes, nor to ideological extremity on any of these dimensions. Nevertheless, future research based on larger (and more diverse) samples would do well to explore these possibilities.
In conclusion, we have undertaken a neuropsychological investigation of political orientation by focusing on patients with different brain lesions. Our findings speak to the question of whether certain brain regions are necessary for the development of specific political beliefs, opinions and values. It may be worth noting that by exploring brain lesions we are not in any way suggesting that holding liberal or conservative attitudes is reflective of neural deficits or damage. Rather, the lesion method illuminates which neuroanatomical regions—and the cognitive functions related to them—may be necessary for understanding the development of political ideology. It is also important to keep in mind that studies of brain structure, including lesion studies, do not rule out effects of neural reorganization and malleability [78]. Accordingly, we strongly caution against deterministic or essentialized interpretations of our research (see [79]). Moreover, we theorize that the relationship between neurobiology and ideology is dynamic and reciprocal (see [5]), bearing in mind Nudo's [78, p. 1] observation that ‘behavioural experience is the most potent modulator of brain plasticity'. Along these lines, we look forward to future research that specifies the ways in which ideological experiences may shape the structures and functions of the human brain.
Acknowledgements
We are grateful to Shawn Kim, Jacob Martin and Sonia Yanovsky for research assistance, as well as Leor Zmigrod and three anonymous reviewers for astute and helpful comments on the manuscript.
Endnotes
Previous studies suggest that patients with amygdala lesions do not exhibit markedly different generalized mood states compared with healthy controls [48,61,62].
We also examined political orientation on social and economic dimensions as a function of lesion type. However, lesion classification models predicting social conservatism (electronic supplementary material, table S2) and economic conservatism (electronic supplementary material, table S3) did not explain a significant amount of variance in either dimension of ideology, whether we adjusted for demographic variables or not. Pairwise comparisons between lesion classification groups also failed to produce consistent results. Therefore, we decided not to explore potential differences between social and economic dimensions of ideology any further in this study.
Adjusting for depression (BDI), frontal lesion patients were more conservative than ATL/amygdala lesion patients (p = 0.04) and healthy controls (p = 0.03). Adjusting for anxiety (BAI), frontal lesion patients were more conservative than ATL/amygdala lesion patients (p = 0.03) and healthy controls (p = 0.04). And finally, adjusting for negative affect (PANAS negative), frontal lesion patients were more conservative than ATL/amygdala lesion patients (p = 0.04) and healthy controls (p = 0.04).
We also conducted parallel analyses (n = 25) excluding healthy control participants, which yielded results consistent with the larger sample. Among lesion patients, the degree of damage in the dlPFC (M = 3.5%, s.d. = 8.13) was significantly associated with political orientation (p = 0.04), whereas degree of damage in the amygdala (M = 16.62%, s.d. = 18) was not (p = 0.44). Adjusting for demographic covariates, dlPFC damage was marginally associated with political orientation (p < 0.10), whereas amygdala damage was not (p = 0.37).
Data accessibility
All de-identified data and analysis code are available at https://osf.io/xcwgy/.
Authors' contributions
H.H.N., J.T.J. and J.J.V.B. designed the study; M.R.M. collected the data; H.H.N. analysed the data with input from J.T.J., M.R.M. and J.J.V.B.; H.H.N. drafted the manuscript with revisions from J.T.J., M.R.M. and J.J.V.B.
Competing interests
We declare we have no competing interests.
Funding
This study was supported in part by the National Science Foundation (grant no. 1349089 to J.V.B.). We received no other funding for this study.
References
- 1.Knausgaard KO. 2015. The terrible beauty of brain surgery. The New York Times Magazine, 30 December 2015. See https://www.nytimes.com/2016/01/03/magazine/karl-ove-knausgaard-on-the-terrible-beauty-of-brain-surgery.html. [Google Scholar]
- 2.Cacioppo JT, Visser PS. 2003. Political psychology and social neuroscience: strange bedfellows or comrades in arms? Polit. Psychol. 24, 647-656. ( 10.1046/j.1467-9221.2003.00345.x) [DOI] [Google Scholar]
- 3.Haas IJ. 2016. Political neuroscience. In Neuroimaging personality, social cognition, and character (eds Absher JR, Cloutier J), pp. 355-370. New York, NY: Academic Press. [Google Scholar]
- 4.Jost JT, Amodio DM. 2012. Political ideology as motivated social cognition: behavioural and neuroscientific evidence. Motiv. Emot. 36, 55-64. ( 10.1007/s11031-011-9260-7) [DOI] [Google Scholar]
- 5.Jost JT, Nam HH, Amodio DM, Van Bavel JJ. 2014. Political neuroscience: the beginning of a beautiful friendship. Polit. Psychol. 35, 3-42. ( 10.1111/pops.12162) [DOI] [Google Scholar]
- 6.Landau-Wells M, Saxe R. 2020. Political preferences and threat perception: opportunities for neuroimaging and developmental research. Curr. Opin. Behav. Sci. 34, 58-63. ( 10.1016/j.cobeha.2019.12.002) [DOI] [Google Scholar]
- 7.Lieberman MD, Schreiber D, Ochsner KN. 2003. Is political cognition like riding a bicycle? How cognitive neuroscience can inform research on political thinking. Polit. Psychol. 24, 681-704. ( 10.1046/j.1467-9221.2003.00347.x) [DOI] [Google Scholar]
- 8.Nam HH, Jost JT, Feldman S. 2017. The neurobiology of fairness and social justice: an introduction. Social Justice Res. 30, 289-299. ( 10.1007/s11211-017-0296-z) [DOI] [Google Scholar]
- 9.Nam HH. 2020. Neuroscientific approaches to the study of system justification. Curr. Opin. Behav. Sci. 34, 205-210. ( 10.1016/j.cobeha.2020.04.003) [DOI] [Google Scholar]
- 10.Van Bavel JJ, Pereira A. 2018. The partisan brain: an identity-based model of political belief. Trends Cogn. Sci. 22, 213-224. ( 10.1016/j.tics.2018.01.004) [DOI] [PubMed] [Google Scholar]
- 11.Hatemi PK, McDermott R. 2016. Give me attitudes. Annu. Rev. Polit. Sci. 19, 331-350. ( 10.1146/annurev-polisci-103113-034929) [DOI] [Google Scholar]
- 12.Jost JT. 2006. The end of the end of ideology. Am. Psychol. 61, 651-670. ( 10.1037/0003-066X.61.7.651) [DOI] [PubMed] [Google Scholar]
- 13.Jost JT. 2017. Ideological asymmetries and the essence of political psychology. Polit. Psychol. 38, 167-208. ( 10.1111/pops.12407) [DOI] [Google Scholar]
- 14.Ahn WY. et al. 2014. Nonpolitical images evoke neural predictors of political ideology. Curr. Biol. 24, 2693-2699. ( 10.1016/j.cub.2014.09.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber D, Fonzo G, Simmons AN, Dawes CT, Flagan T, Fowler JH, Paulus MP. 2013. Red brain, blue brain: evaluative processes differ in Democrats and Republicans. PLoS ONE 8, e52970. ( 10.1371/journal.pone.0052970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amodio DM, Jost JT, Master SL, Yee CM. 2007. Neurocognitive correlates of liberalism and conservatism. Nat. Neurosci. 10, 1246-1247. ( 10.1038/nn1979) [DOI] [PubMed] [Google Scholar]
- 17.Kanai R, Feilden T, Firth C, Rees G. 2011. Political orientations are correlated with brain structure in young adults. Curr. Biol. 21, 677-680. ( 10.1016/j.cub.2011.03.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hibbing JR, Smith KB, Alford JR. 2014. Differences in negativity bias underlie variations in political ideology. Behav. Brain Sci. 37, 297-307. ( 10.1017/S0140525X13001192) [DOI] [PubMed] [Google Scholar]
- 19.Hibbing JR, Smith KB, Alford JR. 2014. Predisposed: liberals, conservatives, and the biology of political differences. New York, NY: Routledge. [Google Scholar]
- 20.Ruff CC, Ugazio G, Fehr E. 2013. Changing social norm compliance with noninvasive brain stimulation. Science 342, 482-484. ( 10.1126/science.1241399) [DOI] [PubMed] [Google Scholar]
- 21.Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. 2005. Method matters: an empirical study of impact in cognitive neuroscience. J. Cogn. Neurosci. 17, 850-858. ( 10.1162/0898929054021139) [DOI] [PubMed] [Google Scholar]
- 22.Feinstein JS, Adolphs R, Damasio A, Tranel D. 2011. The human amygdala and the induction and experience of fear. Curr. Biol. 21, 34-38. ( 10.1016/j.cub.2010.11.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong W, Cristofori I, Bulbulia J, Krueger F, Grafman J. 2017. Biological and cognitive underpinnings of religious fundamentalism. Neuropsychologia 100, 18-25. ( 10.1016/j.neuropsychologia.2017.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asp E, Ramchandran K, Tranel D. 2012. Authoritarianism, religious fundamentalism, and the human prefrontal cortex. Neuropsychology 26, 414-421. ( 10.1037/a0028526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coronel JC, Duff MC, Warren DE, Federmeier KD, Gonsalves BD, Tranel D, Cohen NJ. 2012. Remembering and voting: theory and evidence from amnesic patients. Am. J. Polit. Sci. 56, 837-848. ( 10.1111/j.1540-5907.2012.00608.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson SW, Damasio H, Jones RD, Tranel D. 1991. Wisconsin Card Sorting Test performance as a measure of frontal lobe damage. J. Clin. Exp. Neuropsychol. 13, 909-922. ( 10.1080/01688639108405107) [DOI] [PubMed] [Google Scholar]
- 27.Demakis GJ. 2010. Frontal lobe damage and tests of executive processing: a meta-analysis of the Category Test, Stroop Test, and Trail-Making Test. J. Clin. Exp. Neuropsychol. 26, 441-450. ( 10.1080/13803390490510149) [DOI] [PubMed] [Google Scholar]
- 28.Gläscher J, Adolphs R, Damasio H, Bechara A, Rudrauf D, Calamia M, Paul LK, Tranel D. 2012. Lesion mapping of cognitive control and value-based decision making in the prefrontal cortex. Proc. Natl Acad. Sci. USA 109, 14 681-14 686. ( 10.1073/pnas.1206608109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuss DT, Levine B, Alexander MP, Hong J, Palumbo C, Hamer L, Murphy KJ, Izukawa D. 2000. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia 38, 388-402. ( 10.1016/S0028-3932(99)00093-7) [DOI] [PubMed] [Google Scholar]
- 30.Stuss DT, Floden D, Alexander MP, Levine B, Katz D. 2001. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia 39, 771-786. ( 10.1016/S0028-3932(01)00013-6) [DOI] [PubMed] [Google Scholar]
- 31.Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. 1993. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 46, 175-199. ( 10.1016/0165-1781(93)90019-D) [DOI] [PubMed] [Google Scholar]
- 32.Kaplan JT, Freedman J, Iacoboni M. 2007. Us versus them: political attitudes and party affiliation influence neural response to faces of presidential candidates. Neuropsychologia 45, 55-64. ( 10.1016/j.neuropsychologia.2006.04.024) [DOI] [PubMed] [Google Scholar]
- 33.Knutson KM, Wood JN, Spampinato MV, Grafman J. 2006. Politics on the brain: an fMRI investigation. Social Neurosci. 1, 25-40. ( 10.1080/17470910600670603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westen D, Blagov PS, Harenski K, Kilts C, Hamann S. 2006. Neural bases of motivated reasoning: an fMRI study of emotional constraints on partisan political judgment in the 2004 US presidential election. J. Cogn. Neurosci. 18, 1947-1958. ( 10.1162/jocn.2006.18.11.1947) [DOI] [PubMed] [Google Scholar]
- 35.Zamboni G, Gozzi M, Krueger F, Duhamel JR, Sirigu A, Grafman J. 2009. Individualism, conservatism, and radicalism as criteria for processing political beliefs: a parametric fMRI study. Social Neurosci. 4, 367-383. ( 10.1080/17470910902860308) [DOI] [PubMed] [Google Scholar]
- 36.Kato J, Ide H, Kabashima I, Kadota H, Takano K, Kansaku K. 2009. Neural correlates of attitude change following positive and negative advertisements. Front. Behav. Neurosci. 3, 6. ( 10.3389/neuro.08.006.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chawke C, Kanai R. 2016. Alteration of political belief by non-invasive brain stimulation. Front. Hum. Neurosci. 9, 621. ( 10.3389/fnhum.2015.00621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brüne M, Scheele D, Heinisch C, Tas C, Wischniewski J, Güntürkün O. 2012. Empathy moderates the effect of repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex on costly punishment. PLoS ONE 7, e44747. ( 10.1371/journal.pone.0044747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cattaneo Z, Mattavelli G, Platania E, Papagno C. 2011. The role of the prefrontal cortex in controlling gender-stereotypical associations: a TMS investigation. Neuroimage 56, 1839-1846. ( 10.1016/j.neuroimage.2011.02.037) [DOI] [PubMed] [Google Scholar]
- 40.Wills J, FeldmanHall O, Meager MR, Van Bavel JJ, NYU PROSPEC Collaboration. 2018. Dissociable contributions of the prefrontal cortex in group-based cooperation. Soc. Cogn. Affect. Neurosci. 13, 349-356. ( 10.1093/scan/nsy023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K. 2001. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 57, 817-821. ( 10.1212/WNL.57.5.817) [DOI] [PubMed] [Google Scholar]
- 42.Cunningham WA, Brosch T. 2012. Motivational salience: amygdala tuning from traits, needs, values, and goals. Curr. Dir. Psychol. Sci. 21, 54-59. ( 10.1177/0963721411430832) [DOI] [Google Scholar]
- 43.Sander D, Grafman J, Zalla T. 2003. The human amygdala: an evolved system for relevance detection. Rev. Neurosci. 14, 303-316. ( 10.1515/revneuro.2003.14.4.303) [DOI] [PubMed] [Google Scholar]
- 44.Gozzi M, Zamboni G, Krueger F, Grafman J. 2010. Interest in politics modulates neural activity in the amygdala and ventral striatum. Hum. Brain Mapp. 31, 1763-1771. ( 10.1002/hbm.20976) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rule NO, Freeman JB, Moran JM, Gabrieli JD, Adams RB Jr, Ambady N. 2010. Voting behaviour is reflected in amygdala response across cultures. Soc. Cogn. Affect. Neurosci. 5, 349-355. ( 10.1093/scan/nsp046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaplan JT, Gimbel SI, Harris S. 2016. Neural correlates of maintaining one's political beliefs in the face of counterevidence. Scient. Rep. 6, 39589. ( 10.1038/srep39589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam HH, Jost JT, Kaggen L, Campbell-Meiklejohn D, Van Bavel JJ. 2018. Amygdala structure and the tendency to regard the social system as legitimate and desirable. Nat. Hum. Behav. 2, 133-138. ( 10.1038/s41562-017-0248-5) [DOI] [Google Scholar]
- 48.Adolphs R, Tranel D, Damasio H, Damasio A. 1994. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669-672. ( 10.1038/372669a0) [DOI] [PubMed] [Google Scholar]
- 49.Anderson AK, Phelps EA. 2001. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature 411, 305-309. ( 10.1038/35077083) [DOI] [PubMed] [Google Scholar]
- 50.Phelps EA, Cannistraci CJ, Cunningham WA. 2003. Intact performance on an indirect measure of race bias following amygdala damage. Neuropsychologia 41, 203-208. ( 10.1016/S0028-3932(02)00150-1) [DOI] [PubMed] [Google Scholar]
- 51.Amaral DG. 2006. The amygdala, social behavior, and danger detection. Ann. N.Y. Acad. Sci. 1000, 337-347. ( 10.1196/annals.1280.015) [DOI] [PubMed] [Google Scholar]
- 52.Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. 2006. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 120, 749-760. ( 10.1037/0735-7044.120.4.749) [DOI] [PubMed] [Google Scholar]
- 53.Rosvold HE, Mirsky AF, Pribram KH. 1954. Influence of amygdalectomy on social behaviour in monkeys. J. Comp. Physiol. Psychol. 47, 173-178. ( 10.1037/h0058870) [DOI] [PubMed] [Google Scholar]
- 54.Taubert J, Flessert M, Wardle SG, Basile BM, Murphy AP, Murray EA, Ungerleider LG. 2018. Amygdala lesions eliminate viewing preferences for faces in rhesus monkeys. Proc. Natl Acad. Sci. USA 115, 8043-8048. ( 10.1073/pnas.1807245115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Martino B, Camerer CF, Adolphs R. 2010. Amygdala damage eliminates monetary loss aversion. Proc. Natl Acad. Sci. USA 107, 3788-3792. ( 10.1073/pnas.0910230107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carraro L, Castelli L, Macchiella C. 2011. The automatic conservative: ideology-based attentional asymmetries in the processing of valenced information. PLoS ONE 6, e26456. ( 10.1371/journal.pone.0026456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jost JT, Stern C, Rule NO, Sterling J. 2017. The politics of fear: is there an ideological asymmetry in existential motivation? Social Cogn. 35, 324-353. ( 10.1521/soco.2017.35.4.324) [DOI] [Google Scholar]
- 58.Shook NJ, Clay R. 2011. Valence asymmetry in attitude formation: a correlate of political ideology. Social Psychol. Pers. Sci. 2, 650-655. ( 10.1177/1948550611405219) [DOI] [Google Scholar]
- 59.Wechsler D. 2008. Wechsler adult intelligence scale, 4th edn. San Antonio, TX: NCS Pearson. [Google Scholar]
- 60.Feldman S, Johnston C. 2014. Understanding the determinants of political ideology: implications of structural complexity. Polit. Psychol. 35, 337-358. ( 10.1111/pops.12055) [DOI] [Google Scholar]
- 61.Anderson AK, Phelps EA. 2002. Is the human amygdala critical for the subjective experience of emotion? Evidence of intact dispositional affect in patients with amygdala lesions. J. Cogn. Neurosci. 14, 709-720. ( 10.1162/08989290260138618) [DOI] [PubMed] [Google Scholar]
- 62.Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA. 2013. Fear and panic in humans with bilateral amygdala damage. Nat. Neurosci. 16, 270-272. ( 10.1038/nn.3323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beck AT, Steer RA, Brown GK. 1996. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 64.Beck AT, Epstein N, Brown G, Steer RA. 1988. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 56, 893-897. ( 10.1037/0022-006X.56.6.893) [DOI] [PubMed] [Google Scholar]
- 65.Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. 1983. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- 66.Watson D, Clark LA, Tellegen A. 1988. Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Social Psychol. 54, 1063. [DOI] [PubMed] [Google Scholar]
- 67.Eidelman S, Crandall CS, Goodman JA, Blanchar JC. 2012. Low-effort thought promotes political conservatism. Pers. Social Psychol. Bull. 38, 808-820. ( 10.1177/0146167212439213) [DOI] [PubMed] [Google Scholar]
- 68.Zmigrod L, Rentfrow PJ, Robbins TW. 2018. Cognitive underpinnings of nationalistic ideology in the context of Brexit. Proc. Natl Acad. Sci. USA 115, E4532-E4540. ( 10.1073/pnas.1708960115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant DA, Berg EA. 1948. A behavioural analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 38, 404-411. ( 10.1037/h0059831) [DOI] [PubMed] [Google Scholar]
- 70.Heaton RK. 1981. A manual for the Wisconsin Card Sorting Test. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- 71.Golden CJ. 1978. Stroop Color and Word Test: a manual for clinical and experimental uses. Chicago, IL: Stoelting Co. [Google Scholar]
- 72.Bowie CR, Harvey PD. 2006. Administration and interpretation of the Trail Making Test. Nat. Protoc. 1, 2277-2281. ( 10.1038/nprot.2006.390) [DOI] [PubMed] [Google Scholar]
- 73.Zmigrod L, Rentfrow PJ, Robbins TW. 2020. The partisan mind: is extreme political partisanship related to cognitive inflexibility? J. Exp. Psychol. Gen. 149, 407-418. ( 10.1037/xge0000661) [DOI] [PubMed] [Google Scholar]
- 74.Baron RM, Kenny DA. 1986. The moderator–mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J. Pers. Social Psychol. 51, 1173-1182. ( 10.1037/0022-3514.51.6.1173) [DOI] [PubMed] [Google Scholar]
- 75.Preacher KJ, Hayes AF. 2008. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods 40, 879-891. ( 10.3758/BRM.40.3.879) [DOI] [PubMed] [Google Scholar]
- 76.Shrout PE, Bolger N. 2002. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol. Methods 7, 422-445. ( 10.1037/1082-989X.7.4.422) [DOI] [PubMed] [Google Scholar]
- 77.Azevedo F, Jost JT, Rothmund T, Sterling J. 2019. Neoliberal ideology and the justification of inequality in capitalist societies: why social and economic dimensions of ideology are intertwined. J. Social Issues 75, 49-88. ( 10.1111/josi.12310) [DOI] [Google Scholar]
- 78.Nudo RJ. 2013. Recovery after brain injury: mechanisms and principles. Front. Hum. Neurosci. 7, 887. ( 10.3389/fnhum.2013.00887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dar-Nimrod I, Heine SJ. 2011. Genetic essentialism: on the deceptive determinism of DNA. Psychol. Bull. 137, 800-818. ( 10.1037/a0021860) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All de-identified data and analysis code are available at https://osf.io/xcwgy/.