Abstract
All living cells interact dynamically with a constantly changing world. Eukaryotes, in particular, evolved radically new ways to sense and react to their environment. These advances enabled new and more complex forms of cellular behaviour in eukaryotes, including directional movement, active feeding, mating, and responses to predation. But what are the key events and innovations during eukaryogenesis that made all of this possible? Here we describe the ancestral repertoire of eukaryotic excitability and discuss five major cellular innovations that enabled its evolutionary origin. The innovations include a vastly expanded repertoire of ion channels, the emergence of cilia and pseudopodia, endomembranes as intracellular capacitors, a flexible plasma membrane and the relocation of chemiosmotic ATP synthesis to mitochondria, which liberated the plasma membrane for more complex electrical signalling involved in sensing and reacting. We conjecture that together with an increase in cell size, these new forms of excitability greatly amplified the degrees of freedom associated with cellular responses, allowing eukaryotes to vastly outperform prokaryotes in terms of both speed and accuracy. This comprehensive new perspective on the evolution of excitability enriches our view of eukaryogenesis and emphasizes behaviour and sensing as major contributors to the success of eukaryotes.
This article is part of the theme issue ‘Basal cognition: conceptual tools and the view from the single cell’.
Keywords: eukaryogenesis, excitability, motility, cilia, membranes, protists
1. Introduction
Cellular excitability is the capacity to generate dynamic responses to stimuli, often over millisecond timescales. An initially weak reaction can be amplified nonlinearly to trigger a strong or impulsive response. Traditionally, the term ‘excitability’ has been associated with neurons and their electrical properties [1], often manifesting as rapid changes in membrane potential and electrical spiking activity known as action potentials. More generally, however, an excitable dynamical system is capable of supporting not only all-or-nothing responses that are independent of the stimulus amplitude, but also other signal types. These can also propagate spatially, with or without attenuation. An excitable system often undergoes a characteristic excursion through state space, before returning to its original state after a refractory period has elapsed. Here, we invoke this extended definition of excitability to explore cellular phenomena that specifically drive whole-organism behaviour across prokaryotic and eukaryotic cells.
During eukaryogenesis, cells evolved new mechanisms of excitability to sense and react rapidly to their environment. In all cells, excitability is underpinned by the thermodynamics of interfaces. Interfaces are formed by biomembranes that bind regions with different ionic compositions. Excitability emerges as a biophysical consequence of charge separation across biological membranes. This is regulated by the passage of ions between different cellular compartments through ion channels or biochemical signals initiated by metabotropic receptors. These ionic currents then regulate effector systems, including the cilium or contractility apparatus. The ionic homeostasis of the compartments is maintained by active pumping by ATPase pumps [2]. Biosensing is achieved whenever this homeostasis is disturbed from its equilibrium or steady state, by perturbative influences, which can then propagate rapidly and directionally across the membrane [3].
In general, eukaryotic cells display more complex behaviours than prokaryotes (archaea and bacteria). The differences often are not only quantitative but also qualitative. During eukaryogenesis, radically new forms of sensing and reacting to stimuli have evolved. This enabled deterministic navigation in eukaryotes, particularly direct tracking of gradients or movement along with vectorial cues in three dimensions in open water (3D taxis). Many eukaryotes also actively select particles during feeding, explore substrates, or undergo regulated cell–cell fusion during sex. Phagotrophy is another characteristic that distinguishes eukaryotes from other forms of life.
What are the evolutionary origins of these new forms of behaviour? Are there universal features that we can identify, which clearly set eukaryotes apart from prokaryotes? Here we attempt to trace the evolutionary origins of eukaryotic excitability by invoking a combination of cell biology and physical principles.
This paper is organized into two main sections. We begin with a comprehensive overview of eukaryote-signature behaviours and contrast these to those exhibited by prokaryotes. We proceed to argue that beyond changes in gene complements, several major cellular innovations were likely critical to the emergence of these new forms of behaviour. These all evolved during eukaryogenesis and were likely to have been present in the last eukaryotic common ancestor (LECA). Key structural innovations include changes in the complement of membrane channels, compartmentalization and new types of motility-generating appendages (eukaryotic cilia/flagella and pseudopodia). Furthermore, a general increase in cell size and compartmentalization could have fundamentally changed the biophysical regimes accessible to eukaryotic cells.
In this article, we present a new perspective of how these cellular innovations prompted the ‘new physics’ that may have unlocked the evolution of new sensory and response strategies that were previously unavailable to prokaryotes. We have organized the paper along clear conceptual lines with the aim of bringing high-level order to a large, sometimes disconnected body of literature. As Henri Poincaré once suggested ‘Le savant doit ordonner … ’ [4].
2. Forms of excitability in eukaryotes
In this section, we give an overview of the forms of excitability, sensing and response that are characteristic of eukaryotes, and contrast these strategies with those encountered in prokaryotes. We first focus on single-cell strategies for tactic navigation. Self-locomotion, or motility, is an important feat that not only increases the efficacy of environmental exploration, but also enables cells to move toward more favourable environments, and away from harm. Motility control in the presence of spatio-temporal gradients is an ideal testbed for evaluating a cell's sensory performance. Apparently very different microscopic sensorimotor rules can nonetheless lead to similar macroscopic or end outcomes, namely, some form of net migration towards or away from a stimulus.
Among motile organisms, strategies for navigation are often diverse and highly organism-specific. There are three major strategies for cells to track gradients of external cues (e.g. chemicals, light, temperature), which we shall refer to as stochastic navigation, spatial sensing and helical klinotaxis. This is inspired by the classification of Dusenbery [5], and also similar to the convention adopted by Alvarez et al. [6] in the context of chemotaxis. We shall seek to understand how the propensity for prokaryotes and eukaryotes to adopt different strategies may have arisen from adaptations to different physical regimes.
In addition, there are passive forms of orientation, which we will not discuss in detail here. These include magnetotaxis in some proteobacteria and a euglenid alga [7–9]. There are further idiosyncratic forms of environmental tracking that do not fall into any of the above navigation categories, such as active regulation of buoyancy in non-motile diatoms in order to move up and down in the water column [10,11].
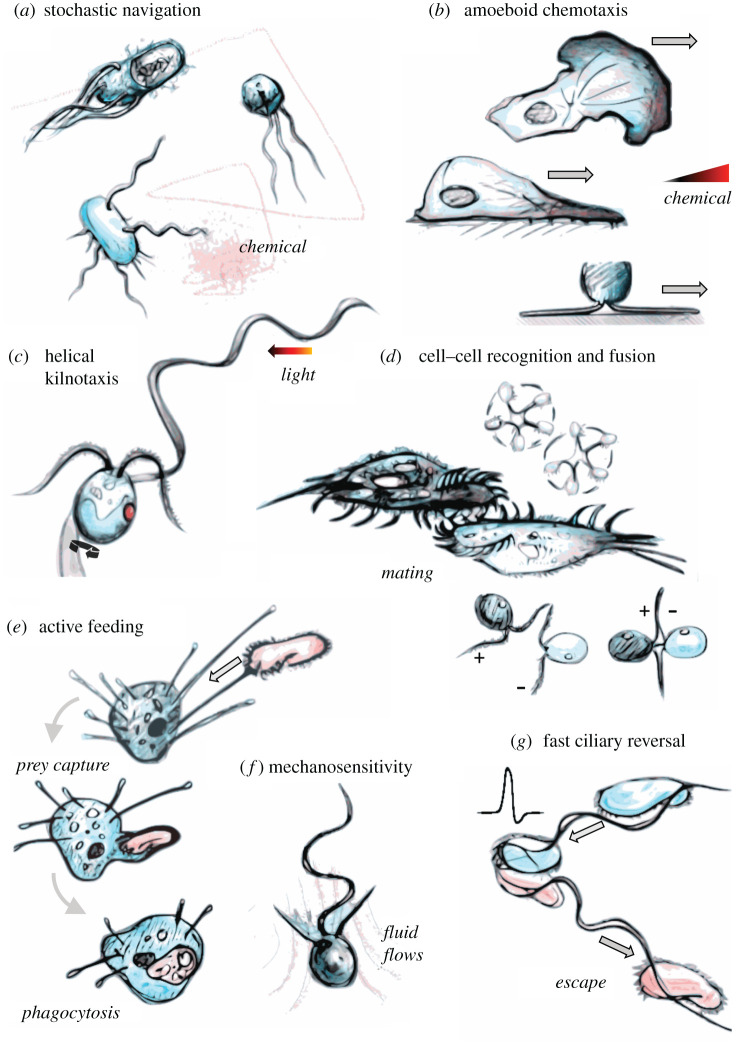
With the advent of enhanced sensory and navigational capabilities, eukaryotes became capable of more sophisticated behavioural sequences (summarized in figure 1 and table 1). These include nonlinear responses to mechanical stimulation and complex membrane dynamics, including cell engulfment and cell–cell fusion. Orderly sequences of excitable actions manifest themselves in the everyday processes in the life of a eukaryotic cell, for example, chemosensing or tracking of other cells by chemotaxis, followed by engulfment (phagocytosis) or cell fusion (sex).
Figure 1.
The many forms of cellular excitability. (a) Stochastic or non-oriented navigation strategies (e.g. prokaryotic chemotaxis), (b) sensing by spatial comparison (e.g. amoeboid chemotaxis), (c) sensing by helical klinotaxis (e.g. flagellate phototaxis), (d) cell–cell recognition as a prelude to fusion (e.g. ciliate conjugation and gametic fusion in Chlamydomonas), (e) active feeding by selective engulfment of prey organisms, (f) mechanosensitivity and flow interactions, and (g) ultrafast escape responses and reversal of ciliary beating by action potentials. (Online version in colour.)
Table 1.
Forms of cellular excitability in eukaryotes are contrasted with those in prokaryotes. See main text for references.
| forms of cellular excitability | prokaryotic examples | eukaryotic examples | made possible by |
|---|---|---|---|
| stochastic navigation | bacterial and archaeal chemotaxis, archaeal phototaxis | choanoflagellate aerotaxis, chemokinesis in some ciliates and flagellates, Micromonas phototaxis | any moving appendage or motility mechanism |
| spatial sensing | Synechocystis, Thioturbo danicus | amoebae, ciliates | spatially located sensor, large cell size |
| temporal taxes | Thiovulum | helical photo- and chemotaxis across eukaryotes | helical/chiral self-motion, fine motor control over cilia or flagella, temporal sensing, memory |
| cell fusion | some haloarchaea (incomplete), Borrelia (mostly OM, incomplete), Clostridium spp. (complete) | all gametic fusion events | cell–cell recognition, adhesion, in most eukaryotes mediated by cilia/flagella |
| active feeding by engulfment | some planctomycetes | many eukaryotic phagotrophs | deformable membrane, cell recognition, sometimes by specialized appendages, internal digestion |
| mechanosensitivity and flow interactions | osmosensation | many eukaryotes | mechanosensory channels (e.g. transient receptor potential), membrane fluidity |
| escape responses and action potentials | cable bacteria, some biofilms | many eukaryotes | voltage and calcium channels (e.g. Cav, Nav), often localized to cilia/flagella |
(a). Stochastic or non-oriented navigation
The response of an organism or cell to stimuli is not always directional. Instead stochastic navigation strategies—also termed kineses—exist (figure 1a). This form of navigation is most widespread among prokaryotes but also occurs in some eukaryotes. Stochastic navigation is a form of temporal sensing, during which changes in the concentration, e.g. of a chemical, are detected as the cell moves along a gradient. The most common strategy among prokaryotes consists of straight runs followed by brief turns [12] or tumbles to reorient the trajectory [13]. During positive chemotaxis, a sufficient rate of increase in the chemoattractant concentration will suppress tumbles, biasing the movement up the gradient [14]. Run-and-tumble chemotaxis does not involve directed turns and can therefore be referred to as statistical or indirect chemotaxis [5], or chemokinesis. Variations include run-and-reverse, run–reverse-and-flick [15] or run-and-stop [16]. It has been reported that some polarly flagellated bacteria exploit a buckling instability to steer, producing a peaked distribution around preferred turning angles [17]. These kinetic strategies depend on two-component signalling and generally apply to bacterial and archaeal chemotaxis and phototaxis [18,19]. Cells achieve biased migration according to generalized velocity-jump random walk processes [20].
Stochastic trial-and-error navigation by temporal sensing also exists among eukaryotes but is not as well-characterized. Recently, it was shown that aerotaxis (tracking of oxygen gradients) in choanoflagellates relies upon such a stochastic navigation strategy [21]. A similar mechanism operates during phototaxis in the pico-eukaryote Micromonas [22]. Many ciliates are capable of multiple gradient sensing strategies which may include a stochastic component, depending on the nature of the stimulus or irritant. Bacterial chemoattractants can also stimulate chemokinesis in Tetrahymena, leading to an increase in swimming speed [23]. The capacity for chemoattractants (e.g. glutamate, folate) and chemorepellents (toxins) to induce positive and negative kinetic effects in ciliates including Tetrahymena and Paramecium is well-established [24,25]. Different chemical signals induce a combination of more subtle movement responses, including klinokinesis (random reorientations) and orthokinesis (changes in speed). When exposed to extracellular GTP, some ciliates exhibit repetitive back-and-forth motion, a more complex form of excitability mediated by membrane potential [26].
(b). Deterministic steering by spatial comparison
In contrast with stochastic kineses, organisms can also tune their orientation with respect to a cue. Taxes are further subdivided [27] into tropotaxis, when gradients are measured directly at two different locations at the same time (spatial sensing), usually requiring at least two spatially separated receptors, and klinotaxis, when gradients are measured sequentially at two different times (temporal sensing) at the same receptor, while the organism is in motion (see §2c).
Sensing by spatial comparison (figure 1b) is a form of navigation that is a eukaryotic characteristic, with a few exceptional prokaryotic cases. Spatial chemosensing or light sensing relies on the differential excitation of one part of a cell coupled to directional movement along a gradient or vector. Among eukaryotes, spatial chemosensing is widespread across many supergroups and possibly traces back to the LECA. Most amoeboid eukaryotes are thought to perform chemotaxis through spatial sensing [28].
Amoeboid cells migrate by directional chemotaxis towards bacteria or other food sources. The mechanism is spatial sensing coupled to the directional extension of pseudopodia [29]. Such amoeboid chemotaxis has been demonstrated in members of many eukaryotic clades. Amoeboid cells of the excavate amoeboflagellate Naegleria fowleri (Discoba, Heterolobosea) show chemotaxis towards bacteria [30]. Similar directed chemotactic migration is present in Acanthamoeba [31], Entamoeba [32], Hartmannella [33], Dictyostelium and amoeboid cells in animals [29]. Directional chemotaxis also functions in syncytial forms like the plasmodia of the slime mould Physarum [34].
Chemotaxis in Dictyostelium is perhaps the best studied from a biophysical perspective [35]. In the absence of gradients, pseudopods extend randomly, but extensions become localized when gradients are detected. Cells can sense gradients of only 2% front-to-back and can migrate towards travelling waves of chemoattractant [36]. In very shallow gradients, cells extend pseudopodia stochastically and retain the ones oriented towards a source of chemoattractant [37]. Dictyostelium can also reconfigure its motility machinery to migrate against shear flows [38,39]. This active response relies on spatial sensing of differential hydrodynamic forces; just as during chemotaxis, directional pseudopodial motility occurs by an actin-polymerization-dependent mechanism.
There are other—likely more derived—forms of tropotaxes among eukaryotes. The euglenoid Peranema glides on surfaces and can efficiently move towards sources of food [40]. A long anterior flagellum and a short ventral flagellum are attached to an elongated cell body—which is itself capable of amoeboid-like movement in the absence of the flagella. Gliding by cilia is present in many species and relies on a surface mechanism mediated by interactions between transmembrane proteins and the substrate [41]. Red algae glide on surfaces and can move towards light by directional steering [42,43]. Since red algae generally lack cilia and helical swimming, their phototactic movement may rely on spatial sensing (shading or focusing by lipid droplets) and directional growth of cellular protrusions [44]. Filamentous eukaryotes with cell walls show directed orientation or growth along chemical gradients, including fungal hyphae [45], the pollen tube of plants [46] and plant roots undergoing hygrotropism [47].
Prokaryotes display spatial sensing only very sporadically or only as collectives. Multicellular communities of some bacteria display collective behaviour reminiscent of deterministic tracking by spatial comparison. Swarming Pseudomonas aeruginosa can track chemical trails by collective movement, even if the individual bacteria only move randomly [48]. Swarm colonies of the bacterium Rhodospirillum centenum show directional phototaxis through an unknown direction-sensing mechanism, possibly exhibiting colony-level shading [49,50]. Myxococcus xanthus swarming on agar plates turn towards plastic and glass beads by sensing deformations in surface texture (elasticotaxis) [51–53].
At the single-cell level, prokaryotes are too small for spatial sensing to be effective (§2f), with few exceptions. Some relatively large (2 × 6 µm) bipolar flagellated vibrioid bacteria (identified as Thioturbo danicus [54]) can track steep oxygen gradients and perform directional turns, likely by spatial sensing [55]. The cells are able to detect oxygen gradients across a distance of several micrometres along their long axis. Turning may rely on the faster rotation of the flagellar bundle at the cell pole exposed to higher oxygen levels. Another unusual form of spatial sensing is found in the cyanobacterium Synechocystis, which is able to follow directional light cues. These cells act as spherical microlenses to focus incoming light to the opposite side of the plasma membrane. This localized stimulus induces motility in the direction of light [56]. A further interesting example is presented by Pseudoalteromonas haloplanktis and Shewanella putrefaciens. These bacteria appear to steer towards and track individual free-swimming algae [57]. However, tracking may not be due to deterministic steering behaviour and can be explained by fluid dynamical effects due to the algal cell's vorticity field [58].
(c). Deterministic steering by temporal comparison
Klinotaxis relies on temporal comparisons and chiral self-motion (figure 1c). This is arguably the most sophisticated of cellular navigation strategies and occurs almost exclusively in eukaryotes. Helical self-propulsion at low Reynolds numbers arises naturally when cell-shape asymmetries are combined with periodic stroke patterns (e.g. of cilia and flagella) [59,60]. Direct taxes employing temporal sensing require the helical trajectories to have sufficiently large amplitude, as found in most freely swimming eukaryotes. During helical turns in a stimulus field, the cell tracks periodic changes in the stimulus, particularly in the direction perpendicular to the helix axis. By bending the helical trajectory in the stimulus direction, the cells can actively steer and migrate deterministically. Thus, helical klinotaxis is fundamentally different from stochastic navigation [5,6], and generally both more efficient and more robust to noise than other navigation types.
Diverse eukaryotes from distinct phyla use helical klinotaxis to track chemical gradients (such as diffusing from a food source) [61]. Helical klinotaxis has been described in various ciliates, the gametes of brown and green algae [62,63], the non-photosynthetic green alga Polytomella magna [64], animal sperm [65], fungal zoospores [66], the heterotrophic flagellates Cafeteria [61] and Euglena [67] and the dinoflagellate Peridiniopsis berolinensis [68]. The gametes of many organisms are also guided by helical chemotaxis for external fertilization [69].
A special form of chemotaxis is a prelude to gametic fusion. The helical klinotaxis of flagellated male gametes towards female gametes is common. Since cilia [70], meiosis [71] and thus gametogenesis trace back to LECA, it is possible that the gametes of LECA found each other by helical klinotaxis before sex. In order for gametes to interact, they are often attracted to each other by pheromones—likely some readily diffusible substance. This attraction has been extensively studied in species of brown algae. When the female gametes of brown algae settle on a surface, they secrete sexual pheromones (olefinic C11-hydrocarbons) [72,73], which attract the much more motile, biflagellated male gametes. The male gametes can either swim freely in three-dimensional helical trajectories, or swim close to the surface, moving in two-dimensional circular paths (referred to as thigmotaxis). The chemoattractant pheromones influence the beat pattern of the flagella, increasing the curvature of the helical swimming path [74]. Helical klinotaxis is also adopted by monoflagellated gametes, including fungal zoospores and animal sperm. The male gametes of the aquatic fungus Allomyces swim in a helical path interrupted by 'jerks’. The female gametes show little motility and secrete a pheromone (sirenin) that influences the swimming trajectories of male gametes, in order to guide their directional swimming [66].
In other examples of gametic attraction, it is not yet clear whether the mechanism involves helical klinotaxis or stochastic chemokinesis. When both partners are motile, chemoattraction need not be unidirectional. In the ciliate Blepharisma japonicum [75] single cells swim more slowly with increasingly circular trajectories around higher concentrations of gamone. Each partner can secrete a different pheromone, which has the potential to attract cells of the opposite mating type. In Spirostomum and Euplotes, characteristic pre-conjugation rituals (‘courtship’ or even mating dances) have been described [76,77].
While chemical or thermal gradients are diffuse, other classes of physical stimuli (such as light, gravity, flows) are inherently directional. Phototaxis is a particularly striking example of helical klinotaxis [78]. It likely evolved at least seven times independently during eukaryotic evolution and may not have been a property of the LECA. However, as a biophysical strategy, it is uniquely eukaryotic and was enabled by eukaryotic cellular organization, excitability and movement pattern.
For example, the biflagellate alga Chlamydomonas has a single eyespot which leads to an eightfold separation in perceived intensity from one side compared with the other [79]. A non-planar beat pattern leads to a helical trajectory which allows the organism to continuously scan three-dimensional space and modulate the helix axis towards or away from the light. A similar strategy of helical phototaxis can be found in other protists, including Euglena, brown algal swarmers and chytrid zoospores (reviewed in [78]).
In the colonial alga Volvox carteri, movement of thousands of flagella—which operate as individuals—achieves colony-level phototaxis by virtue of their common stimulus-response function and positional distribution on a spherical body [80]. In Euglena, the interplay between eyespot sensor placement and an intensity-dependent reorientation strategy leads to more complex responses to patterned light [81,82].
The most extreme examples of light sensing in a single eukaryotic cell are found in certain predatory dinoflagellates that have large and highly unusual ‘camera eyes’, called ocelloids—built from endosymbiotically acquired components. This unique organelle has superior light-gathering optics and can refract light to a retina-like structure [83,84]. In the warnowiid dinoflagellate Erythropsidinium, the design and sophistication of its eye suggests that it can do more than sense light gradients (as in most other protist photoreceptors) [85]. Ocelloid eyes are even capable of an active rolling or pivoting motion [86]. It is suggested that they can detect circularly polarized light—a tell-tale sign of prey dinoflagellates (polarotaxis). This extraordinary sensory capacity coincides with an arsenal of cellular weapons (e.g. harpoons, nematocysts) which this organism uses to impale its prey. The klinotaxis mechanism in this case must be highly refined, though the connection between signal and action on the flagellar beat pattern is unclear.
In contrast with its widespread use in eukaryotes, klinotaxis is largely absent from prokaryotes. Even though many prokaryotes move in a helical fashion [87,88], this alone is not generally sufficient for klinotaxis, as the radius of the helix must be large enough to allow sampling of gradients (see §2e). Perhaps the only example of true taxis in bacteria occurs in the large sulfide-oxidizing proteobacterium Thiovulum majus. Thiovulum cells are unusually large for a bacterium (5–25 µm) [89] and can swim at a speed of up to 615 µm s−1, one of the fastest swimming speeds ever recorded for a bacterium [90]. Thiovulum cells swim with multiple flagella in a left-handed helix and can directly track a gradient of oxygen by bending the helical trajectory as a function of changes in concentration [91]. Large groups of Thiovulum cells also form large-scale fronts owing to their chemotactic behaviour [92].
(d). Cell–cell recognition as a prelude to fusion
Sexual cycles consisting of meiosis and complete cell–cell fusion are unique to eukaryotes. From the perspective of excitability, the key properties of sex are the specific attraction and recognition by gametes, their complete fusion and the prevention of multiple rounds of fertilization. Eukaryotes undergo complete cell–cell fusion during sex and orchestrate this process with remarkable precision.
Mating starts with chemoattraction, often mediated by pheromones and helical klinotaxis (see §2c above). Adhesion and fusion are regulated by genetically determined mating types [93]. In the ciliate Euplotes patella, which expresses six mating types and at least three different pheromones (gamones), this attraction is thought to be combinatorial [94]. Gamete recognition can be accompanied by dramatic changes in behaviour. In Paramecium, there is a marked reduction in swimming speed following recognition [95]. In the biflagellate alga Chlamydomonas, cells of opposite mating type collide randomly, before fusing to form quadriflagellate zygotes. Adhesion occurs via different mating-type-specific glycoproteins expressed on the flagella (agglutinins) [96].
Cilia and flagella often participate in cell–cell fusion as mating organelles (figure 1d). In Paramecium aurelia, conjugation begins with the adhesion of specific cilia, and cells become adhered at the anterior-ventral side [97]. Membrane fusion then occurs, at surfaces where cilia degenerate, and pores form to provide cytoplasmic bridges between the partners. In Chlamydomonas, isogamous gametes of opposite mating types adhere head-to-head along the length of their flagella, with the eyespots on the same side of the cell [98]. The plus mating type then extends a tubular, actin-based mating structure which fuses with a smaller structure in the minus mating type. The temporary quadriflagellate dikaryons are also phototactic [99]. In Chlamydomonas, flagellar adhesion is also light-sensitive, in fact light switchable [100], which may explain the necessity of light for gametogenesis in this organism [101]. In oogamous brown algae, the male gametes attach to the female gamete with their anterior flagellum [102]. After one gamete fuses, the others are excluded. This is ensured by the formation of a fertilization barrier by the fusion of Golgi-derived vesicles underlying the plasma membrane [102]. It is unclear if initial chemotaxis and subsequent agglutinative contact are separate processes.
Among prokaryotes, cell–cell fusion is rare, and mostly only incomplete and reversible. In some haloarchaea, the exchange of genetic material can occur through incomplete cell–cell fusion, during which cells are connected by cytoplasmic bridges [103]. The selectivity of the mating process is regulated by surface glycosylation in Haloferax volcanii [104]. Among bacteria, transient outer membrane fusion has been observed in the myxobacterium Myxococcus xanthus [105]. Among cells of the spirochaete Borrelia, frequent outer membrane fusion and occasional inner membrane fusion were observed [106]. A recent study reported complete interspecies cell–cell fusion with large-scale exchange of cellular components between the bacteria Clostridium ljungdahlii and Clostridium acetobutylicum [107].
(e). Active feeding by selective engulfment
Another key trait of eukaryotes is predation by whole-cell engulfment, which is often proposed as one of the main innovations driving eukaryogenesis [108,109], an idea not without its critics [110]. Particles or even whole cells primed for engulfment may need to be first brought to a food vacuole by interception, by track-and-capture or by active filtration of self-generated currents [111].
Chemoattractants function to draw predators to prey, followed by specific and ordered activity sequences prior to prey engulfment, which may include cell attachment and the formation of specialized cellular protrusions. In the slime mould Dictyostelium, a G-protein-coupled receptor (GPCR), folic acid receptor 1, recognizes both diffusible chemoattractants and surface molecules of bacterial prey [112,113]. The dinoflagellate Oxyrrhis marina uses a mannose-binding lectin as a feeding receptor for recognizing prey [114].
In many cases, phagotrophic predators have highly specialized feeding strategies. The heliozoan Actinophrys sol can intercept and consume ciliate prey as large as itself by adhering to the ciliate with pseudopod extensions called axopodia, which then wrap around to completely enclose the prey [115]. Many protists possess specialized structures to facilitate prey immobilization and capture [116,117]. Phagotrophic euglenids actively shovel and manipulate prey organisms toward feeding grooves. The freshwater eukaryovorous euglenid Heteronema has been described to ensnare Chlamydomonas cells whole, by coordinating the action of multiple hook-bearing and mucus-covered flagella into the flagellar pocket [118]. The euglenid Peranema uses feeding rods to first pierce prey cells [119], before sucking out their contents directly into a feeding vacuole (myzocytosis). Photoautotrophic or osmotrophic species have much more reduced feeding apparatuses. Some dinoflagellates make use of a pseudopodial pallium to envelop and engulf large and awkwardly shaped prey (e.g. diatoms) [120].
Most ciliates, despite having an ordered and largely rigid structure (pellicle), restrict phagocytosis to a single expandable feeding cavity. This structure, known as the cytostome, is often decorated with a ring of specialized cilia. After first paralysing its prey with toxicysts, Didinium can engulf and pass a Paramecium cell as large as itself through its cytostome [121,122]. This process is associated with calcium-dependent electrical activity [123]. Hypostome ciliates make use of a cylindrical cytopharyngeal basket, which constricts to pass its filamentous prey (e.g. cyanobacteria) into a coil deep within the cytoplasm, at rates of up to 15 µm s−1 [124]. This is accompanied by rapid incorporation into the developing food vacuole of new membrane recycled from vesicular fusion. Generally, the feeding cavity must enlarge and acidify before the prey can be digested [125].
In filter feeders, ensembles of cilia and flagella coordinate to pump fluid at relatively high speeds (up to 1 mm s−1), often sweeping particles directionally into a feeding apparatus [126]. In collared choanoflagellates and the sessile ciliate Vorticella, food particles are sorted by size. In other species, mechanical filtration may be supplemented by adhesive surfaces for added particle selectivity.
Predatory behaviour also exists in prokaryotes [127], but owing to their inability to engulf, proceeds in a very different way. Predatory bacteria generally inflict chemical lysis upon prey cells, or attack collectively. Single-cell strategies are rare. Bdellovibrio hunts bacteria [128] but the tracking mechanism does not appear to be chemosensory but rather dependent on hydrodynamic entrapment [129]. A newly described planctomycete [130] can engulf other bacteria and pico-eukaryotes, via a mechanism that resembles but is not thought to be homologous to eukaryotic phagocytosis. However, this is a singular system which is not distributed among prokaryotes.
(f). Mechanosensitivity and flow interactions
Eukaryotes are particularly susceptible to mechanical stimuli and changes in membrane geometry. Many eukaryotes exhibit mechanosensitivity. This allows them to respond actively to hydromechanical signals transmitted remotely through the fluid, without need for direct contact with a potential predator or prey [131–133]. Marine ciliates can perform powerful jumps in response to predator-induced feeding currents at shear rates (magnitude of flow velocity gradients) of 1–10 s−1 [134,135].
More graded responses to mechanical cues are controlled by specialized ion channels and transmitted directly through the membrane [136]. These may be localized to cilia and flagella, which display an active load response [137,138]. Hair-like extensions of cilia (mastigonemes) are also implicated in mechanosensing in Chlamydomonas [139]. A combination of rheosensing and chemosensing guides sperm motility through the mammalian oviduct. Dictyostelium reorients actively to shear flows, i.e. gradients in flow velocity [38].
The mechanosense is also manifest in gravitaxis, as well as gyrotaxis—the interaction between motility, gravity and hydrodynamic torques in many marine protists. In the ciliate Loxodes, active control of membrane potential and statocyst-like organelles is thought to determine the sign of gravitaxis [140,141]. Some protists have also adapted to life in the ocean by exploiting sharp vertical gradients and undergoing ballistic diel migration [142]. The gravity-sensing mechanism remains unclear, but is likely to involve a passive shape-dependent mechanical component [143], in addition to active regulatory mechanisms.
Planktonic microorganisms show a great deal of resilience against turbulent ecosystems [144]. For this they must be able to integrate and respond to multisensory information: such as light, chemicals, flows and gravity. Such single-cell responses can lead to large-scale population structures such as algal blooms, even formation of photo-gyro-gravitactic bioconvection patterns and instabilities [145,146]. In general, behavioural transitions are mediated by stimuli-dependent ionic currents and an excitable membrane, coupled to some form of self-locomotion. Cross-responses are even possible (where one type of stimulus elicits a change in the cell's response to another stimulus type). The male gametes of brown algae switch the sign of phototaxis from positive to negative when exposed to chemoattractants [147]. Self-movement in a fluid will alter local gradients, providing an opportunity for reafferent feedback.
Prokaryotes may be too small to respond actively to shear flows. Bacterial rheotaxis (reorientation to shear flow) has been shown to be a passive phenomenon [148]. However bacteria are capable of osmoregulation and do possess ion channels which sense membrane tension [149], and mechanosensation in Escherichia coli has been demonstrated to rely on voltage-induced calcium flux [150]. The torque produced by the flagellar motor (therefore rotation speed) is also load-dependent [151,152]. Other appendages (e.g. pili) for surface movement are candidate mechanosensors and may reflect adaptation to substrate adherence for community living and biofilm formation [153].
(g). Escape responses and action potentials
Stimuli that have the potential to harm or kill demand more immediate detection. This is fundamentally distinct from navigation or exploration, in terms of the timescales available for response. Most motile species harbour a form of phobic or emergency response distinct from their steady state locomotion. Escape reactions are not strictly oriented—but commonly involve backward movement, sometimes with a negatively geotactic component [154]. Additional chemical self-defence strategies may be deployed, for example extrusion of trichocysts by Paramecium [155] or ejection of extrusomes in other ciliates [156].
In flagellate algae, abrupt changes in light intensity or intense photic stimuli induce rapid flagellar reversal and transient backward swimming [85,157]. In green algae, this action may be mediated by the contractile root fibre which alters the angle between basal bodies [158]. Cells can also react at speed to unexpected mechanical stimuli. All-or-none contractions in the stalked ciliate Vorticella can occur at rates of 8 cm s−1 [159]. In some species of heliozoa, axopods can completely retract within 20 ms in order to draw in trapped prey for phagocytosis [159,160].
These fast reactions are usually induced by action potentials—unidirectional electrical pulses involving fast, regenerative changes in membrane potential. While all cells display some electrical activity, phylogenetic evidence suggests that the capacity to propagate action potentials may have been an ancestral eukaryotic trait supported by the LECA. These may have emerged in response to accidental membrane damage and sudden calcium influx [161]. Bioelectrical signalling in the form of action potentials occurs orders of magnitude faster than any other signalling modalities, e.g. chemical diffusion, protein phosphorylation etc.
In order to initiate fast escape responses, these may have been coupled directly to the motility apparatus—particularly to flexible, membrane-continuous structures such as cilia and pseudopodia. Loss of voltage-gated sodium/calcium channels is further correlated with loss of cilia in many taxa. In protists, all-or-none action potentials occur almost exclusively in association with ciliary membranes [162–164], with the exception of some non-ciliated diatoms [165,166]. Graded potentials occur in amoebae, also for movement control [167].
In Chlamydomonas, action-potential-like flagellar currents induce photophobic responses and flagella reversal (via the voltage-gated calcium channel Cav2), while photoreceptor currents elicit much milder responses [168]. Here, a mechanosensory channel of the transient receptor potential (TRP) family (TRP11) is localized to the ciliary base, while Cav2 is localized only to the distal regions of cilia [169,170]. In Paramecium, hyperpolarizations increase ciliary beat frequency, while depolarizations have the opposite effect and eventually lead to a ciliary reversal. Depolarizations above a certain threshold result in action potentials, owing to opening of Cav channels located exclusively in the ciliary membrane [171,172]. Potassium channels—also residing in the membrane—help restore the resting membrane potential.
Eukaryotes manipulate their membrane potential to achieve transitions between different behaviours. Complex bioelectric sequences have been recorded in association with integrated feeding and predation behaviours in Favella [173]. Repetitive behaviours arise from rhythmic spiking. In ciliates, rhythmic depolarizations control fast and slow walking by tentacle-like compound cilia called cirri [174], enabling escape from dead ends [175] and courtship rituals in conjugating gametes [77,94]. In Stentor, action potentials produce whole-body contractions [176]. Finally, excitable systems operating close to bifurcations may admit limit cycles, which manifest as repetitive or rhythmic electrical spiking and repetitive behaviours. Ultimately, this may lead to habituation [177,178].
In prokaryotes, action-potential-like phenomena have been observed in biofilms [179] and also single cells. The archaeon Halobacterium salinarium shows a photophobic response characterized by a 180° reversal of its swimming direction induced by a reversal in the direction of flagellar rotation. At least some aspects of this response are likely mediated by changes in membrane potential by bacteriorhodopsin, a light-driven proton pump [180]. Action potential-like phenomena in prokaryotes are dissimilar from classical eukaryotic action potentials. The former are less reproducible, slower and exhibit a broader distribution in pulse amplitude and duration [150] (table 1).
3. Cellular and biophysical innovations underpinning eukaryotic excitability
In this section, we give an overview of the cellular innovations that contributed to the emergence of new forms of excitability during eukaryogenesis. These are (i) an extended repertoire of membrane receptors, channels and pumps, (ii) motility by cilia and pseudopodia, (iii) endomembranes and mitochondria as ionic compartments and intracellular capacitors, (iv) a flexible and reconfigurable membrane, (v) a larger size, (vi) new strategies for sensing. We identified these features as of major significance for the origin of eukaryotic excitability, but the list may not be exhaustive. We discuss how these factors contributed to the novel forms of excitability highlighted in the preceding sections and how they underpin new regimes of cellular biophysics that are only accessible by eukaryotic cells.
We shall embed our discussions within the most generally accepted framework for eukaryogenesis. This starts with an archaeal host related to the Asgard archaeal lineage [181]. This host acquired the mitochondrial symbiont—related to alphaproteobacteria—by internalization. There are several versions of this model, and from our perspective, some of the most intensely debated details are less relevant (e.g. how early or late and through which intermediate stages did mitochondria evolve) [182]. We focus here only on the key eukaryotic novelties of membrane topology, motility, ionic currents and other ingredients necessary for excitability, and how these may be contrasted with prokaryotic cell biology. Of note, there are also alternative—and in our view less plausible—cell evolution scenarios for eukaryogenesis, e.g. involving three symbiotic partners [183], which we will not consider here.
(a). An expanded repertoire of ion channels, pumps and membrane receptors
In eukaryotes, there is a vastly expanded repertoire of membrane channels, pumps and receptors, distributed across a highly compartmentalized cell. Comparative genomics indicates much of this diversity evolved during eukaryogenesis in stem eukaryotes and was present in the LECA (e.g. [184]).
The various fast and slow ionic currents generated by receptors and ion-selective channels underlie the responses of eukaryotic cells to diverse sensory stimuli and injury. The regulation of motility, contractility, mechanosensation, tactic and temperature responses all rely on membrane excitability. The complexification and diversification of ion channels and receptor pathways was one of the major innovations that underpinned the evolution of the new forms of excitability in eukaryotes. In parasites, this diversity can be dramatically reduced. In parasitic trypanosomes, voltage-gated channels are represented by one type, a reduction from ten types in their free-living relative Bodo saltans [185].
The regulation of calcium signalling illustrates the exuberance of systems eukaryotes evolved to control the flux of a single ion. Its influx and extrusion are regulated by various types of Ca2+ channels and pumps, including store-operated Ca2+ channels [186], Ca2+ ATPases, and voltage-gated, ligand-gated or mechanosensitive Ca2+ channels, including TRP channels.
The levels of free calcium are low in the cytoplasm and high in the endoplasmic reticulum (ER). Intracellular Ca2+ is kept low by the action of the plasma membrane calcium-transporting ATPase (PMCA), which counters the influx of Ca2+ at the plasma membrane. The influx of Ca2+ into the ER in turn is controlled by the sarcoplasmic/endoplasmic reticulum calcium ATPase Ca2+ pumps (SERCA). The ER and plasma membrane calcium systems are interlinked. The activation of plasma membrane receptors (e.g. some GPCRs) through second messengers can gate the inositol trisphosphate receptor (InsP3R) in the ER to induce Ca2+ release. The levels of Ca2+ in the ER then influence store-operated Ca2+ entry by plasma membrane Ca2+-release-activated Ca2+ (CRAC) channels (with the ORAI pore subunit [187]). Mitochondria are also involved in Ca2+ signalling. Many eukaryotes harbour a mitochondrial Ca2+ uniporter [188].
The core Ca2+ transport systems of ER and plasma membrane channels and pumps have homologues across diverse eukaryotes and were likely present in the LECA. These include SERCA, PMCA, InsP3R, trimeric intracellular cation-specific channels (TRIC) and ORAI [184,189,190]. However, some of the machinery of calcium signalling evolved in the common ancestor of Apusozoa, animals and fungi, representing a post-LECA diversification of ionic signalling (e.g. the sperm-specific CatSper Ca2+ channel complex) [191].
Calcium signalling mediates diverse excitable phenomena across eukaryotic taxa. For example, in the ciliate Tetrahymena, phagocytosis depends on calcium signalling [192]. In fungi, pulsatile calcium signalling events accompany cellular events, including cell–cell contact and polarized growth [193]. In the slime mould Dictyostelium, speed modulation in migrating cells in response to shear stress is dependent on G-protein signalling acting through plasma membrane calcium channels and IP3-mediated internal calcium-store release [29,112,194]. In ciliates, voltage-gated Ca2+ channels regulate motility [162] and temperature responses [195].
Besides Ca2+, several other ions are involved in excitability phenomena and are regulated by dedicated pathways. For example, in the Paramecium membrane, one study reported six different ionic conductances [196], but there are probably many more, based on the high number of ion channels in the genome [197]. Diatoms and coccolithophores display voltage-activated cation and anion currents [166,198]
Sensory receptor pathways responsible for mediating responses to external cues also originated early in eukaryotes and some were likely present in LECA. These include members of the TRP and GPCR families. TRP channels at the plasma membrane and in the ciliary membrane mediate several excitable phenomena. For example, TRP channels are involved in mechanosensation-induced bioluminescence in the dinoflagellate Lingulodinium polyedra [199]. Rheotaxis in shear flow is dependent on a PKD2-like TRP channel in the amoeba Dictyostelium discoideum [200]. In the excavate Euglena gracilis, Ca2+ influx via a mechanosensitive TRP channel mediates negative gravitaxis [201]. In the green alga Chlamydomonas reinhardtii, there are several TRP channels involved in various sensory processes. The mechanosensory avoidance reaction of the cells requires the expression of TRP11 localized to the base of flagella [169]. Another flagellar TRP channel, CrPKD2, is important for flagella-dependent mating [202], whereas TRP1 is a thermo-sensitive channel that opens at increased temperatures [203].
Prokaryotes also have channels and transporters involved in various aspects of signalling and cell physiology such as chemotaxis [204–208]. These channels can be homologous to the eukaryotic channels, for example the TRIC channels are conserved across bacteria, archaea and eukaryotes [209]. However, the prokaryotic channels are often simpler and sporadically distributed. For example, most eukaryotic voltage-gated channels are tetrameric whereas prokaryotic channels are monomers [210,211] (but see [166]). The mitochondrial Ca2+ uniporter is widespread in eukaryotes but found only sporadically in bacteria [188]. Eukaryotes thus distinguish themselves by the diversity and complexity of channels, pumps and receptors.
(b). Motility by cilia and pseudopodia
There may be as many as 18 distinct motility types across all forms of life [212]. Among these, notable eukaryotic motilities include free-swimming by cilia and migration by pseudopodia. The earliest eukaryote may have been an amoeboflagellate alternating between cilia-driven and amoeboid locomotion. The amoeboid-to-flagellate transition is common among eukaryotes, occurring in Rhizaria [213], Amoebozoa, Naegleria [214], several opisthokonts including choanoflagellates [215], filastereans [216] and early-branching fungi [217,218].
These new forms of motility rely on eukaryotic-signature cell biology, including dynamic actin and microtubule cytoskeleton and membrane-bound cilia. Pseudopodia are involved in directional motility, feeding and sensing, as we discussed (in §2b) for the case of chemotaxis in Dictyostelium [37]. These actin-filled structures and their regulation by WASP and SCAR proteins trace back to LECA [218]. Here we focus on the uniquely eukaryotic biology of cilia (confusingly, also referred to as flagella in their longer forms) and discuss how they contribute to behaviour and sensing.
We briefly contrast the elaborate regulation, stroke patterns and gait control available for cilia-based motility, with the control of prokaryotic flagella and archaella [219–222]. All three appendage types share some physical similarities. They are all slender structures that create drag anisotropy when moving through a fluid. Net propulsion is achieved by breaking time-reversal symmetry with propagating waves or chiral rotation. Asymmetric interactions also mediate gliding and crawling [223–225].
Beyond these similarities, cilia stand out with a unique propulsion-generating machinery that is very different from that of bacterial flagella or archaella. Bacterial flagella and archaeal archaella are extracellular structures, composed only of a few proteins plus a rotary motor and membrane-embedded base structure, whereas membrane-bound cilia have over 500 proteins [226]. Unlike either of the prokaryotic structures, which are driven by rotary motors from one end, dynein motors populate the entire length of cilia. This is known as distributed force actuation [227], in stark contrast with boundary actuation (from only one end) in the prokaryotic appendages.
The continuity of ciliary membranes as an extension of the plasma membrane enables fast reactions. Active amplification at these locations reduces transduction times from sensor to motor actuators (locomotor appendages), thereby leaving more time for sensory integration or sampling (see §2f). At excitable interfaces, spontaneous fluctuations (due to noisy ion channels) of the resting membrane potential can amplify into action potentials. In flagellates and ciliates, this appears as spontaneous swimming reversals, which can occur in the absence of stimuli [228,229]. Artificial deciliation abolished the calcium-dependent excitability in Paramecium [230,231], further highlighting the possible ciliary origin of eukaryotic action potentials [232]. Leveraging mutants defective in ion-gating, several phospholipids were shown to be localized exclusively to the ciliary membrane of Paramecium [233].
While prokaryotes can manipulate the sense (and sometimes speed) of flagella rotation, the flexible and distributed architecture of cilia enables many more degrees of freedom. Dynein activity can be modulated intracellularly to produce a spectrum of beating modes within the same structure [234]. For instance, in green algal cilia, calcium induces a switch from a forward swimming mode with an asymmetric waveform (low-frequency) to a reverse mode with a symmetric waveform (high-frequency) in tens of milliseconds [228,235]. The beat pattern and asymmetry of sperm flagella are controlled by calcium and cAMP [236,237].
The capacity for subtle control of ciliary shape and movement underpins the exclusively eukaryotic trait of helical klinotaxis. Multiflagellate algae modulate the parameters of helical trajectories to realign with gradients in a graded, intensity-dependent manner—during Chlamydomonas phototaxis, photons incident on the eyespot induce a transient membrane depolarization [168] which alters the flagellar beat pattern [238]. Steering arises when the two flagella respond differently to this signal [239,240].
By contrast, during E. coli run-and-tumble, multiple flagella rotate in the same sense during swimming, but unbundling (when one or more motors rotate in the opposite sense) is stochastic. During stochastic chemotaxis, cells can only control the propensity for directional switching but not the new swimming direction. Some prokaryotes (e.g. Sinorhizobium meliloti, Rhodobacter sphaeroides) can also control motor speed [241–243].
Eukaryotes with multiple cilia can also have fine control over the movement sequence, or gait. This higher-level control requires the precise positioning of centrioles and cilia [244]. Multiciliary coordination can invoke hydrodynamic as well as intracellular means—including electrical signalling [136] or basal-body coupling [245]. Ciliary control dictates the walking gaits of Euplotes [246], as well as diverse swimming gaits in algal flagellates, including the breaststroke, trot and gallop. Distinguished cilia on the same cell may be found alternately in sequences of excitation or quiescence [247].
Owing to their larger size and active coordination, cilia can generate faster propulsion and flows. Collectively they can be organized across scales [239,248] for specific tasks. In ciliates and other filter feeders, cilia differentiate into oral cilia for feeding and somatic cilia for swimming. Bioelectrical signalling controls mouth ciliature for complex feeding behaviours, as well as the ciliary beat direction to direct feeding flows [163].
Cilia arose to support not only eukaryotic motility but also sensing [249,250]. The continuity of the membrane around the cilium (and other eukaryotic appendages) is integral to these dual functions. In addition to the various sensory receptors localized to the cilium (discussed in §3a above), cilia and other eukaryotic appendages are sustained out of equilibrium by ATP-dependent mechanochemical cycles, which produce unique dynamical signatures [251,252]. Macroscale flux cycles in the phase space of movement patterns (broken detailed balance) have been observed in cilia [251], in hair cell bundles [253], in the motor patterns of intact swimmers [228] and in reconstituted cytoskeletal networks [253,254]. These exhibit active fluctuations that are orders of magnitude higher than thermal noise and may further enhance sensory perception, particularly to mechanical stimuli and shear flows.
Besides cilia and pseudopodia, cytoskeletal polarization and patterning led to further, highly specialized forms of movement [255]. These involve dynamic mobilization of cellular protrusions for prey capture or manipulation (see §2e). Haptophyte algae have a unique organelle known as a haptonema—a slender microtubular structure distinct from cilia, which coils at high speed to detect prey and other mechanical cues [256]. In Vorticella and Zoothamnium, contractile proteins in the stalk organelle (spasmoneme) can undergo extremely rapid mechanochemical contraction–extension cycles, fuelled by calcium [257,258]. The axopods of Heliozoa, which are involved in feeding, and contain bundles of microtubules, undergo rapid shortening by microtubule catastrophe [259,260]. In many predatory ciliates, ciliary bundles or cirri form specialized feeding structures that undergo dramatic shape changes to internalize prey.
(c). Origins of mitochondria and endomembranes as intracellular capacitors
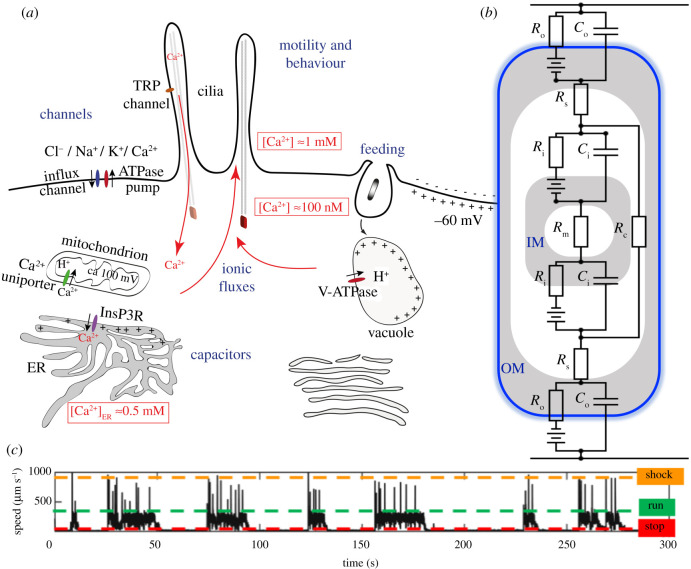
The eukaryotic cell is distinguished from prokaryotic cells by a complex endomembrane topology [261–263]. The endomembrane system contains several charged compartments (figure 2a)—multiple membranous structures, including the ER, the vacuole and mitochondria, often with closely stacked lamellae (e.g. ER, plastids). This sophisticated structural organization evolved during eukaryogenesis and is critical to eukaryotic excitability.
Figure 2.
Eukaryotic membranes control dynamic intracellular signalling. (a) Massive diversification of lipids and ion channels creates compartments and circuits within a flexible and excitable endomembrane system. (b) The mitochondrion has a distinctive two-layer topology with unique capacitative properties (IM, inner membrane; OM, outer membrane; Ci, Co are IM, OM capacitances; Ri, Ro and Rm are IM, OM and matrix resistances; Rs is the resistance of the inner membrane space; Rc is a ‘shunt’ resistance [264] arising from cristae extending across the intermembrane space). (c) Excitable signalling in cilia manifests as fast transitions or escape responses, as observed here in an octoflagellate protist [228] which switches between three distinct behavioural states (run, stop and shock).
One important structural innovation was the compartmentalization of chemiosmotic ATP production. In eukaryotes, ATP synthesis occurs in the mitochondria, commonly during aerobic respiration [265]. By contrast, prokaryotes use their plasma membrane for ATP synthesis, where ATP synthase complexes use the transmembrane electrochemical gradient of protons or Na+ ions [266]. During eukaryogenesis, the original archaeal plasma membrane A0A1 ATP synthase [266,267] became internalized, evolving into the vacuolar H+-ATPase (ATP-driven proton pump), which acidifies vacuoles [268]. Free from the burden of chemiosmotic ATP production, the eukaryotic plasma membrane can now assume novel signalling roles.
Another important aspect of eukaryotic endomembrane organization is the presence of several charged compartments with distinct ionic composition. These distinct compartments function as closed cellular capacitors [269,270]. The compartments are separated by membrane layers with low conductivity that form a physical barrier between the conductive internal and external fluid. These cellular capacitors actively release and replenish charges, gated by channels and pumps, which alter potential differences across membranes. In neurons, the speed of charge propagation from a synapse is inversely proportional to the specific capacitance (Cm, capacitance per unit area of the membrane) of the membrane. For the plasma membrane of animal cells, this is estimated to be approximately 1 µF cm−2 [271].
The ER reversibly stores and releases charge in the form of Ca2+ ions. The tunable capacitance of the ER depends on the flow of external ions into the cell by capacitative Ca2+ entry [272]. The acidified vacuole is positively charged relative to the cytosol, maintaining a vacuolar capacitance. Mitochondria and chloroplasts with their double membranes have unique capacitative properties (figure 2b) [264,273].
We propose that in eukaryotes, the presence of multiple circuits consisting of these capacitors and their gating machineries represents a novel form of information storage and parallel processing not seen in prokaryotes. These capacitors, and the control of their rapid charging and discharging by active currents, form new types of cellular logic gates. The organization of the eukaryotic endomembrane system has three important functional consequences for cellular capacitance. First, the network of thin membranes creates a large surface area for charge storage and high capacitance—bilayer membranes are typically only 5 nm thick. For parallel plates (membranes) of area A separated by thickness d and dielectric constant, ε0, the capacitance is C = ε0A/d. For a spherical capacitor of radius r, this is modified to C = 4πε0r2/d. The capacitance of compressible membranes is also sensitive to mechanical compression; for a 5 nm thick membrane, a 1 nm decrease in thickness can significantly increase capacitance [274].
Second, membrane topology, comprising nested or closely apposed membranes, greatly influences charge distribution. Where multiple membranes are stacked in parallel, resistances add reciprocally, while capacitances add linearly. The placement of different capacitors in a cell influences charge redistribution, particularly during dynamic phenomena such as motility and feeding. Membrane-bound organelles can be as close as 10 nm from the plasma membrane [275]. This physical proximity further ensures that coordinated signalling, or cross-talk, can occur near-synchronously across the different compartments. Changes in capacitance were measured in phagocytosing macrophages [275,276].
The third feature of the system is its ability to create and sustain nonlinear cycles of charging and discharging—a form of rapid bioelectric signalling. We illustrate this with a basic bilayer membrane (resistance R, capacitance C); here, the voltage–current relationship is given by [277,278] V = IR(1 − e−t/τ), where τ = RC is a time constant for capacitative charging and discharging. This is typically of the order of 100 ns, but can be up to microseconds for larger cells [279]. These currents propagate throughout the cell [280], introducing temporal delays and thereby controlling the timing of signalling events, as has been demonstrated in nerve cells [281,282]. The function of multi-organellar capacitative phenomena in excitability remains to be explored experimentally. If such phenomena are important, we would expect them to have a regulatory function, particularly in cells with tightly apposed ionic compartments—for example, in the flagellar pocket and associated membranes (ER, Golgi, mitochondrion, contractile vacuole) of trypanosomes [283], or the various extrusive organelle complexes such as the pellicular system in ciliates, with alveoli, cilia, mitochondria and trichocysts [277,278,284].
We conclude that during eukaryogenesis, the evolution of compartmentalized capacitors significantly increased the degrees of freedom available for intracellular electrical signalling, making critical contributions to eukaryotic excitability and behaviour. This critical function of the complex eukaryotic endomembrane system as a master regulator of behaviour and physiology extends beyond its bioenergetic or metabolic advantages. By analogy with electronics, eukaryotic cells constitute a complex and dynamic network of coupled resistors and interleaved capacitors as charge sources or sinks, which are associated with multiple time constants. Collectively, these circuits and motifs function as timers, frequency filters, tuners and logic gates, whence complex behaviours can ensue.
(d). Flexible and excitable membranes
Except when covered by a cell wall, as are some fungal and plant cells, eukaryotic cells are morphable and undergo shape changes not seen in prokaryotes. One of the key steps of eukaryogenesis was the loss of the rigid glycoprotein cell wall of the archaea-derived host cell [285]. In archaea, the ‘rigid structure and extremely tight adhesion or interdigitation of the glycoprotein cell walls […] represent an invincible obstacle' [266, p. 572].
The flexible plasma membrane in eukaryotes was a prerequisite for the evolution of total cell fusion, engulfment and membrane dynamics. The endomembrane system is suggested to have evolved rapidly and the LECA to have possessed an elaborate membrane-trafficking system including ER, endosomes and stacked Golgi [286]. This was supported by a complex machinery of membrane-sculpting proteins (e.g. SNAREs, small GTPases, ESCRT complex, septins, coatomer), some of it with an origin in the Asgard archaea [287,288]. For example, according to sequence reconstructions, the LECA may have had as many as 23 Rab GTPases [289]. In the absence of a protective cell wall, eukaryotes may have become more prone to localized injury and membrane rupture. This could have contributed to the evolution of membrane repair and recycling mechanisms and excitable signalling [161].
Besides its flexibility, the plasma membrane harbours the greatest diversity of channels and receptors in eukaryotes and—with the inclusion of the highly deformable ciliary membrane—became the most important surface for excitability. Eukaryotic membranes not only have diverse pumps and channels (see §3a above) but also have thousands of distinct lipid species [290] compared with only hundreds in prokaryotic membranes. The same tendency for amphipathic lipids to self-assemble into layers occurs sub-cellularly in eukaryotes to form multiple boundaries that delimit individual organelles. Lipid diversity also encodes organellar identity (different compartments made up of different lipid species), thus preventing them from coalescing. This diversity may have indirectly contributed to maintaining distinct capacitative identities for electrical signalling in organelles.
Enrichment by eukaryotic sterols (particularly cholesterol) and sphingolipids, which form lipid rafts, is a signature eukaryotic trait [291]. Small changes in lipid structure can have dramatic consequences on membrane thermodynamics [292]. Raft clustering and sorting is thought to precede pinching and budding of vesicles, and are critical for membrane and vesicular trafficking. The composition of plasma membranes resides at a critical point [293], which has been proposed to reduce the energetic costs of membrane compartmentalization and reconfiguration. Sterols are also major regulators of the activity of voltage-gated ion channels, which in turn function to alter the capacitance and conductance of eukaryotic membranes [261,294] (see §3c above). A diverse compositional repertoire further ensures that leakage currents can be matched exactly.
Membrane shape depends on a complex interplay of proteins and lipids, and is highly sensitive to the heterogeneous distribution of lipids [295], which promotes the formation of bends and curvatures [296]. Excitability also depends on membrane fluidity [297]. Eukaryotic sterols have a profound effect on fluidity, channel permeability and thickness [298]. Increased membrane fluidity was directly associated with changes in swimming speed in Tetrahymena [299]. In nerve membranes, anaesthetics are suggested to alter excitability by altering membrane fluidity [300].
Distortion of a fluid bilayer also provides a unique force-sensing mechanism as a form of membrane mechanosensitivity [301,302]. Shear forces and tension can activate transmembrane mechanosensory channels in a number of organisms, triggering action potentials [303]. Fluid shear has been shown to activate G-proteins [304], as well as increase membrane fluidity in endothelial cells [305] and the light-producing dinoflagellate Lingulodinium polyedrum (Alveolata) [306]. In another dinoflagellate, Pyrocystis lunula, the critical threshold for bioluminescence was estimated to be approximately 0.1 µN, about thrice the shear stress on the cell wall [307]. Meanwhile the force required to activate rapid behavioural switches (shock response) in a small prasinophyte alga can be under 10 pN [228]. Mechanical stimuli must do work to open transduction channels [308]. The amount of work required is a measure of sensitivity, which is much higher in eukaryotes (table 2). In bullfrog hair bundles, Ca2+ can shift the single-channel activation force by approximately 3 pN (equivalent to 1–2 kBT) [313].
Table 2.
Summary of key biophysical scaling relationships. See main text for references and for *rare prokaryotic examples that defy the general trend. (We have assumed throughout: kBT = 4.11 × 10−21 J = 4.11 pN nm, and μ/ρ = 10−2 cm2 s−1 is the kinematic viscosity of water at room temperature). Symbols are defined in the text.
| physical parameter | scaling | (typical) prokaryote | (typical) eukaryote | comments |
|---|---|---|---|---|
| Reynolds number | Re = ρUL/μ | Re = 10−5–10−3
(L = 1–10 μm, U = 10–100 μm s−1) |
Re = 10−3–1 (L = 10–1000 μm, U = 10–1000 μm s−1) |
unicellular eukaryotes and prokaryotes are viscosity dominated |
| Péclet number (fix D = 103 μm2 s−1 for small molecule) |
Pe = UL/D | Pe = 10−2–1 (L, U as above) |
Pe = 1–103
(L, U as above) |
prokaryotes are diffusion-limited and cannot reach* Pe > 1 |
| diffusion constant (passive) |
D0 = kBT/(6πμa) |
D0 = 0.22 μm2 s−1
(a = 1 μm) |
D0 = 0.02 μm2 s−1
(a = 10 μm) |
eukaryotes 10× less susceptible to linear diffusion |
| rotational diffusion (passive) | Drot = kBT/(8πμa3) |
Drot = 0.16 rad2 s−1
(a = 1 μm) |
Drot = 1.6 × 10−4 rad2 s−1
(a = 10 μm) |
eukaryotes 1000× less susceptible to rotational diffusion |
| effective diffusion (active, depends on motility strategy) |
Da = v2τ/3 (τ = free-flight time, v = speed of runs) |
Da ≈ 133 μm2 s−1
(v ≈ 20 μm s−1, τ ≈ 1 s) |
Da ≈ 3.3 × 10−2 cm2 s−1
(v ≈ 100 μm s−1, τ ≈ 10 s) |
empirical: E. coli: [309] D ≈ 10–100 μm2 s−1; C. reinhardtii: [310] D ≈ 10−3 cm2 s−1; P. caudatum: [311] D ≈ 10−2 cm2 s−1 |
| relevant for stochastic navigation | expected angular deviation in time τ θrms = √(2Drτ) |
θrms = 32° (for τ = 1 s) |
θrms = 1° (for τ = 1 s) |
prokaryotes are severely limited by rotational diffusion (cannot steer) |
| relevant for spatial sensing | too small* | useful strategy for large and slow-moving cells | common strategy for amoeboid eukaryotes | |
| relevant for helical klinotaxis | too small* | useful strategy to reorient toward vectorial cues | common strategy for free-swimming eukaryotes | |
| sensitivity to mechanical stimuli (membrane tension) | ratio of channel opening and closing probabilities ΔE = work done to open channel (approx. sensitivity) |
bacterial MscS and MscL channels (osmotic nanovalves): [301] 5 approximately 10 mN m−1 |
hair cells, single-channel gating stiffness: [308] approximately 1 mN m−1 Piezo1: [312] approximately 1.4 mN m−1 |
1 kBT is 4 nm2 change in area under tension of 1 mN m−1
lytic tension of pure lipid bilayer approximately 20 mN m−1 |
Eukaryotic membranes are capable of significant topological reorganization, notably during wound repair, phagocytosis and cell–cell fusion. Plasma membranes reversibly deform during cell migration or feeding to sustain non-energy-minimizing structures such as slender protrusions [314]. The constant spatio-temporal remodelling and turnover of membranes is a eukaryotic trait. Vigorous membrane turnover is observed in some species of Acanthamoeba, at an estimated complete turnover rate of several times per hour [315]. Membrane reorganization, even fusion, can occur between predatory suctorians and their prey [316].
(e). Increase in cell size
If one were allowed to speak of a stereotypical eukaryotic cell, it would be about an order of magnitude larger than a stereotypical prokaryote. Cell size in single-celled eukaryotes ranges from 1 µm [317] to several centimetres (some macroalgae, such as Acetabularia), while prokaryotes are typically 1–10 µm and can be diffraction-limited; the smallest mycoplasmas are approximately 0.2 µm [318]. Single-celled eukaryotes are found in most extant phyla. The largest ciliate has approximately 106 times the volume of the smallest eukaryote.
Cell size imposes fundamental limits on the ability to generate as well as control self-movement. We highlight two physical consequences of increasing size at the border between prokaryotes and eukaryotes. The first concerns the usefulness of self-movement for migration, while the second, the usefulness of self-generated flows for active feeding or nutrient sensing.
Virtually all single-celled organisms reside at low Reynolds numbers (Re; table 2), where viscosity dominates inertia [319]. At these scales there is no inertial coasting, so organisms stop instantaneously when they stop propelling themselves. Self-movement underlies navigation, active feeding, mating and many forms of excitability (§2, figure 1). However, owing to thermal noise, cells below a critical size can gain no absolute benefit from self-propulsion, regardless of the locomotion mechanism.
To see why, first consider an immotile cell of radius a immersed in a fluid of viscosity μ. It will be subject to Brownian motion, subject to both translational and rotational diffusion. The translational diffusion constant is given by the Stokes–Einstein law D0 = kBT/6πμa and has units of length2/time, where kBT (≈ 4.11 × 10−21 J at room temperature) is the thermal energy at temperature T. Meanwhile rotational diffusion Dr = kBT/8πμa3 (units of rad2/time), gives rise to a timescale τ = 1/2Dr over which the orientation of the cell will be ‘reset’.
An actively motile cell will have a higher effective translational diffusion Da, which depends on the detailed movement strategy, or repertoire [13,17,20]. For run-and-tumble (random reorientations after each tumble), Da ≈ v2τ/3. For prokaryotes, a typical run speed is v = 10 µm s−1, and the maximum free-flight time τ can be limited by Dr above. The ratio quantifies the benefit of active swimming to passive diffusion, which improves rapidly with increasing size and speed. Dusenbery used this expression to estimate that below a critical radius ac ≈ 0.64 µm, active swimming is no longer useful [320]. This may explain the paucity of motile bacteria with radius less than ac. This motility divide is also present within the green algal order Mamiellophyceae [321], which contains some of the smallest free-living eukaryotes (pico-eukaryotes): the non-motile Ostreococcus (approx. 1 µm), and the motile species Micromonas (approx. 2 µm), which swims with an average speed of approximately 20 µm s−1 [22].
There is thus a dramatic advantage to having even slightly larger size. Rotational diffusion Dr determines how much a swimmer can move in a certain direction before a noise-driven trajectory reorientation [322]. For a prokaryote with a = 1 µm, Dr ≈ 0.16 rad2 s−1, whereas for a eukaryote with a = 10 µm, Dr ≈ 10−4 rad2 s−1, rotational diffusion is negligible. Active swimming enables eukaryotes to achieve several-fold increase in sensory integration time and self-control over directional persistence (table 2). Eukaryotes can therefore afford to move at higher speeds in order to achieve the same level of signal discrimination (more in §3f).
The second physical consequence concerns how cell size influences solute transport. For a perfect spherical adsorber of radius a, the number of particles (present at ambient concentration C0) arriving at the surface per unit time is given by J = 4πDC0a. Meanwhile an organism's metabolic needs scale much faster, according to volume, or a3. The energy cost per unit volume of cell required to double the nutrient flux at the cell surface is estimated to be [323].
This means that while diffusion alone is sufficient to ensure prokaryotes are well-stirred, eukaryotes must have acquired alternative means of advecting flows. Cilia and flagella are often used to create large-scale flows (up to mm s−1) for filter feeding, or for replenishing the local nutrient concentration around cells [324,325]. For a cell with length L, moving at speed U, the importance of advection over diffusion is captured by the Péclet number Pe = UL/D, the ratio between advective (L/U) and diffusive timescales (L2/D). For a 1 µm prokaryote with a typical speed of 10 µm s−1 and D ≈ 10−5 cm2 s−1 (small molecules), Pe = 0.01, so no physiological amount of movement can improve diffusive uptake. Meanwhile eukaryotes readily achieve Pe numbers of order unity or above (figure 3), so that active stirring is beneficial for nutrient redistribution (mass transfer) and enables scanning through larger volumes of fluid [329].
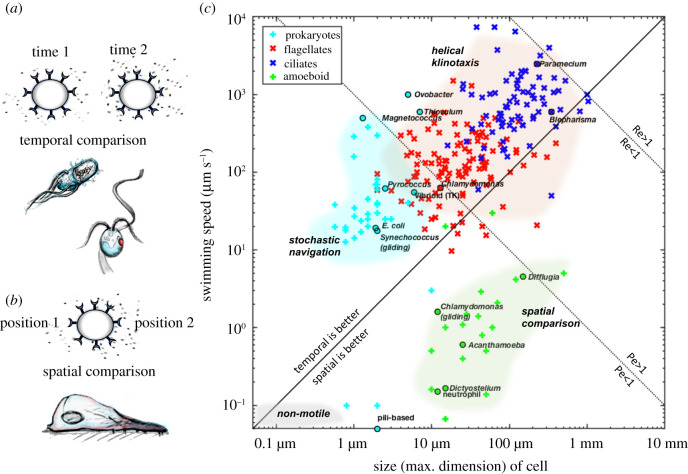
Figure 3.
Eukaryotes unlocked new biophysical regimes. (a) Temporal sensing strategies typically involve comparing the signal at two slightly different times—this can be stochastic and rotational diffusion limited (as in many prokaryotes), or involve self-steering (as in many ciliates and flagellates). (b) Spatial sensing strategies involve comparing the signal at two different positions at the same time (as in most amoeboid cells). (c) These distinctions create a prokaryote–eukaryote divide with respect to behaviour, as visualized here in a phase space of organism size versus speed (log–log scale). Three phase boundaries partition this space (see also table 2), namely, the Reynolds number (Re), Péclet number (Pe) and the relative advantage of temporal versus spatial sensing (plotted here for an integration time τ = 1 s). Data points are collated from the literature, where some species of particular interest are highlighted. (In particular see: amoeba [326], flagellates and ciliates [327] and marine bacteria [328]. The full dataset is available as supplementary material.)
While there are multiplicitous routes to increasing size and complexity, overcoming the diffusive bottleneck by active transport may have been a key step in eukaryogenesis, and multicellularity. Eukaryotic-signature motilities (dynamic cytoskeleton) also enabled nutrient redistribution inside large cells. Increasing size presents new opportunities for the creation of specialized organelles, such as mitochondria, feeding apparatuses and sensors.
Importantly, most prokaryotes cannot steer deterministically in bulk fluid, nor is it advantageous to do so due to excessive rotational diffusion. Exceptions include a vibrioid bacterium [55], and magnetotatic bacteria including the bilophotrichous Magnetococcus marinus [330], and the multicellular magnetotactic prokaryotes (MMPs; approx. 10 µm) [331]. The latter comprise multiple undifferentiated cells whose flagella exhibit some degree of coordination to achieve fast swimming and directional switching, aided by the Earth's magnetic field.
(f). New strategies for sensing and computation
All motile organisms larger than the critical size limit ac can access new strategies for sampling and sensing their environment beyond simple diffusion. Prokaryotes and eukaryotes use fundamentally distinct motility machinery and have evolved distinct sensing mechanisms (with rare exceptions). Cells are remarkable sensors, able to monitor weak signals with a precision approaching physical limits [332]. Examples include chemoreception in bacteria [333,334] and in sperm [65], photon counting in vision [335] and frequency discrimination in the human ear [336]. It is often physical rather than biological processes that set the limits of cellular sensing and response at the microscale.
Below, we discuss scaling laws that unequivocally constrain sensory fidelity at the microscale. These determine how the sensory signal-to-noise ratio (SNR) depends upon the navigation strategy and the nature of the stimulus, through parameters such as cell size, swimming speed and sensory integration time. Here, we focus on limits imposed by different strategies for chemosensing and photosensing, and discuss the implications of these limits for cell evolution and the origins of eukaryotic excitability.
Thermal noise is ever-present, arising from molecular encounters (between receptors and ligands), photons, noise in chemical reactions, gene expression etc. Signal detection reduces to stochastic counting of molecules of chemoattractant, or photons (figure 3a). For simplicity, we assume that gradients decay exponentially with a length scale ℓd. Typically, for chemicals, we can have ℓd ≈ 0.1 cm [320]. For light, the length scale will depend more strongly on the medium. We will use the same length scale of ℓd ≈ 0.1 cm for simplicity.
For chemosensing, molecules diffuse independently. So the number n of molecules bound by cellular receptors will be Poissonian, n = 4πDC0τa, where D is the diffusion constant, C0 the concentration, τ is the available integration or measurement time and a is the cell radius. During spatial sensing of a chemical, cells compare concentrations at two locations separated by Δx = 2a. Here the signal is S = (J+ − J−)τ = 6πDC0a2τ/ℓd, where J± are the diffusive currents to two halves of a stationary sphere [320]. For a single measurement, the noise is (Poissonian), with signal-to-noise ratio ℓd. If instead a motile cell moving at speed v performs temporal sensing, S = C0Δx/ℓd, this time Δx = vτ (in the regime Δx ≪ ℓd). Here, the measurement error is reduced by taking multiple measurements over a time τ, so that N = C0(2πDC0aτ)−1/2 [323], and ℓd. Similarly, during photosensing, the photon count n = IAfτ depends on I, the light intensity (photons per unit area per unit time), the area of the sensor A, the fractional absorbance f (≪1) and the integration time τ. For spatial comparisons, , so that ℓd). Meanwhile for temporal comparisons, ℓd.
In all cases, the SNR depends strongly on τ. For light or chemical signals, the relative SNR between the two strategies scales similarly (with different prefactors): . Similar expressions can be derived for other cues, such as temperature, or for variations upon this sensory strategy [337]. Thus large, slowly moving cells will sense more effectively by spatial comparison, as in large amoeboid eukaryotes which crawl on substrates [29].
By contrast, small fast-moving cells use temporal sensing [338]. Below a certain size (approx. 1 µm), cells become severely limited by rotational diffusion, so prokaryotes cannot maintain their orientation for long enough to steer deterministically toward gradients. Such cells must adopt stochastic random walks. We estimate that in 1 s the expected mean-squared angular displacement, given by , is about 30° (table 2). Only cells that are large enough can access the third strategy—helical klinotaxis. Here cells rotating around an axis can maintain stable helical swimming. Periodic stroke patterns produce superhelical trajectories over long times [339]. Cells detect gradients perpendicular to the helix axis and gradually steer toward gradients by adjusting the alignment between the swimming direction and gradient (error correction). Receptor organelles and sensors are localized to specific regions of the cell to further increase signal discrimination during active movement. For angular rotation speed ω, we require ω ≫ Dr which sets a minimum cell size for helical klinotaxis: e.g. a cell that rotates once per second about its axis must have a diameter of least 6 µm to achieve ω ≈ 10Dr. Thus klinotaxis is not physically accessible for most prokaryotes. Here, the SNR for chemotaxis depends strongly on the signal gradient and scales as R2τ where R and τ are the helix radius and period, respectively [340,341].
Eukaryotic cells are in general several times larger than the wavelength of visible light and can use the cell body to focus light. For example, an eye-less strain of Chlamydomonas [342] or multicellular Dictyostelium slugs [343,344] can focus and align with directional light. In prokaryotes, this mechanism has only been described in cyanobacteria, which operate at the physical limit of focusing [56,345].
In reality, even spatial sensing strategies have a temporal component [346]. Cells measure over a range of timescales, bound by a minimum reaction time and a maximum memory recall. This leads to band-pass signal processing, as has been shown in some bacteria [347]. Since eukaryotes can access a wider range of timescales, they can respond to a wider range of signals. To achieve the same SNR, a larger size can compensate for faster movement, or a shorter reaction time. The entropic and energetic costs of performing cellular computations may have eventually promoted multicellularity or colonial-living, rather than building bigger and more complex single cells. In both pro- and eukaryotes, cell–cell communication can indeed enhance population-level sensing [348,349].
In summary, eukaryotes unlocked novel strategies for improving the sensory SNR. This principally results from an increase in size, polarization of sensors and improved ability to control the swimming direction. The resulting behavioural stratification which partitions the eukaryotes from the prokaryotes can be visualized on a two-dimensional plot of cell size and speed (figure 3c).
4. Concluding remarks
With increasing size and structural complexity, eukaryotes developed novel strategies for environmental exploration. Different eukaryotic lineages are characterized by unique combinations of feeding modalities, control pathways and organization of motility appendages. Eukaryote-signature capabilities are most clearly manifest in emergent mechanisms of motility control, particularly of membrane-bound appendages such as cilia. Eukaryotes, thus endowed, became capable of programmed responses to complex stimuli in a more finely tuned manner. In this article, we have identified specific yet universal changes in cellular and membrane architecture that we propose were crucial for such organisms to overcome many of the challenges associated with life and survival.
Though our list may not be exhaustive, it offers a refreshing perspective for tracing the possible scenarios of eukaryogenesis, which emphasizes the need to consider signalling and behaviour at the whole-cell level. As prokaryotes evolved colonial-living (e.g. biofilms) to overcome the bottlenecks encountered at the microscale in terms of sensing and fluid-interactions, single-celled eukaryotes instead were able to attain a level of behavioural sophistication unmatched in single-celled prokaryotes. Given the strong scaling of size with rotational diffusion, we find that even a modest increase in size may have been sufficient to enable greater self-control and movement persistence (figure 3), and therefore a significant improvement in signal discrimination and decision-making. Such capabilities likely coevolved with cellular and structural innovations.
We suggest that interfacial excitability—the capacity for small potential differences to feed back and suddenly amplify—is critical for the novel functionality of eukaryotic membranes. Although the question of why prokaryotic membranes do not appear to be capable of propagating reproducible, millisecond action potentials remains open, the answer is likely to lie with the massive diversification of proteins and lipids in eukaryotic membranes. We propose that the compositional complexification of eukaryotic membranes conferred excitability to eukaryotes. This diversification enhanced the precision with which control can be exerted over the thermodynamic state of the membrane, its capacitance, fluidity, as well as the identity and magnitude of transmembrane ion fluxes. In this regime, even small changes in physical parameters can feed back and amplify nonlinearly.
In eukaryotes, the integration of fast sensory and excitable motility elements may thus have unlocked new physical regimes of memory and information processing in cells (for example, spatio-temporal navigation requires significant computational bit depth). The capacity to precisely control and generate action potentials, leading to fast escape responses or behavioural transitions, may be a strategy for self-protection from accidental damage. Single-cell neuronal computation [350] may thus have had ancient roots tracing back to ancestral eukaryotes that enacted distributed control over motile appendages [247]. These mechanisms of cellular computation may have been the basis for the origin of nervous systems and more advanced forms of cognition in Metazoa [351]. These concepts are addressed in other contributions in this and the subsequent theme issue on basal cognition.
The acute ‘environmental sensibility’ of excitable membrane interfaces was probably a key trait of ancestral eukaryotes. Excitability enabled eukaryotes to move, sense, react, feed and finally to fulfil the Biblical injunction, to multiply (paraphrasing Mills et al. [352]), in radically new ways. It would be hard to escape the conclusion that excitability had a major contribution to the success of eukaryotes and enabled them to conquer all but the most extreme habitats on the planet. What we hope we could also show is that the divide separating eukaryotes from prokaryotes is as wide for excitability and behaviour as for most other cellular features. Future models of eukaryogenesis ought to account for the origins of excitability to paint a fuller picture of this momentous transition in evolution.
Contributor Information
Kirsty Y. Wan, Email: k.y.wan2@exeter.ac.uk.
Gáspár Jékely, Email: g.jekely@exeter.ac.uk.
Data accessibility
Data collated from published sources were used to generate figure 3c. These data are uploaded as electronic supplementary material.
Authors' contributions
K.Y.W. and G.J. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
K.Y.W. gratefully acknowledges funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme under grant agreement no. 853560: ‘EvoMotion’—Moving around without a brain: Evolution of basal cognition in single-celled organisms. G.J. would like to thank the Leverhulme Trust for funding (RPG-2018-392).
References
- 1.Izhikevich EM. 2010. Dynamical systems in neuroscience: the geometry of excitability and bursting. Cambridge, MA: MIT Press. [Google Scholar]
- 2.Gadsby DC. 2009. Ion channels versus ion pumps: the principal difference, in principle. Nat. Rev. Mol. Cell Biol. 10, 344-352. ( 10.1038/nrm2668) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodgkin AL, Huxley AF. 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500-544. ( 10.1113/jphysiol.1952.sp004764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poincaré H. 1902. La science et l'hypothese [Science and hypothesis], ch. IX. [In French.] Paris, France: Flammarion. [Google Scholar]
- 5.Dusenbery DB. 1992. Sensory ecology: how organisms acquire and respond to information. New York, NY: W. H. Freeman & Company. [Google Scholar]
- 6.Alvarez L, Friedrich BM, Gompper G, Benjamin Kaupp U. 2014. The computational sperm cell. Trends Cell Biol. 24, 198-207. ( 10.1016/j.tcb.2013.10.004) [DOI] [PubMed] [Google Scholar]
- 7.Chen Y-R, Zhang R, Du H-J, Pan H-M, Zhang W-Y, Zhou K, Li J-H, Xiao T, Wu L-F.. 2015. A novel species of ellipsoidal multicellular magnetotactic prokaryotes from Lake Yuehu in China. Environ. Microbiol. 17, 637-647. ( 10.1111/1462-2920.12480) [DOI] [PubMed] [Google Scholar]
- 8.Frankel RB, Blakemore RP. 1989. Magnetite and magnetotaxis in microorganisms. Bioelectromagnetics 10, 223-237. ( 10.1002/bem.2250100303) [DOI] [PubMed] [Google Scholar]
- 9.de Araujo FF, Pires MA, Frankel RB, Bicudo CE.. 1986. Magnetite and magnetotaxis in algae. Biophys. J. 50, 375-378. ( 10.1016/S0006-3495(86)83471-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrieta J, Barreira A, Tuval I. 2015. Microscale patches of nonmotile phytoplankton. Phys. Rev. Lett. 114, 128102. ( 10.1103/PhysRevLett.114.128102) [DOI] [PubMed] [Google Scholar]
- 11.Gemmell BJ, Oh G, Buskey EJ, Villareal TA. 2016. Dynamic sinking behaviour in marine phytoplankton: rapid changes in buoyancy may aid in nutrient uptake. Proc. R. Soc. B 283, 20161126. ( 10.1098/rspb.2016.1126) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Götz R, Schmitt R. 1987. Rhizobium meliloti swims by unidirectional, intermittent rotation of right-handed flagellar helices. J. Bacteriol. 169, 3146-3150. ( 10.1128/JB.169.7.3146-3150.1987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg HC. 1984. Random walks in biology, pp. 149–153. Princeton, NJ: Princeton University Press. ( 10.1515/9781400820023) [DOI]
- 14.Adler J. 1965. Chemotaxis in Escherichia coli. Cold Spring Harb. Symp. Quant. Biol. 30, 289-292. ( 10.1101/SQB.1965.030.01.030) [DOI] [PubMed] [Google Scholar]
- 15.Xie L, Altindal T, Chattopadhyay S, Wu X-L.. 2011. Bacterial flagellum as a propeller and as a rudder for efficient chemotaxis. Proc. Natl Acad. Sci. USA 108, 2246-2251. ( 10.1073/pnas.1011953108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosser G, Baker RE, Armitage JP, Fletcher AG. 2014. Modelling and analysis of bacterial tracks suggest an active reorientation mechanism in Rhodobacter sphaeroides. J. R. Soc. Interface 11, 20140320. ( 10.1098/rsif.2014.0320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Son K, Guasto JS, Stocker R. 2013. Bacteria can exploit a flagellar buckling instability to change direction. Nat. Phys. 9, 494-498. ( 10.1038/nphys2676) [DOI] [Google Scholar]
- 18.Rudolph J, Oesterhelt D. 1995. Chemotaxis and phototaxis require a CheA histidine kinase in the archaeon Halobacterium salinarium. EMBO J. 14, 667-673. ( 10.1002/j.1460-2075.1995.tb07045.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szurmant H, Ordal GW. 2004. Diversity in chemotaxis mechanisms among the bacteria and archaea. Microbiol. Mol. Biol. Rev. 68, 301-319. ( 10.1128/MMBR.68.2.301-319.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taktikos J, Stark H, Zaburdaev V. 2013. How the motility pattern of bacteria affects their dispersal and chemotaxis. PLoS ONE 8, e81936. ( 10.1371/journal.pone.0081936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkegaard JB, Bouillant A, Marron AO, Leptos KC, Goldstein RE. 2016. Aerotaxis in the closest relatives of animals. eLife 5, e18109. ( 10.7554/eLife.18109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henshaw R, Jeanneret R, Polin M. 2019. Phototaxis of the dominant marine pico-eukaryote Micromonas sp.: from population to single cell. bioRxiv, 740571. ( 10.1101/740571) [DOI]
- 23.Leick V. 1992. Chemotactic properties, cellular binding and uptake of peptides and peptide derivatives: studies with Tetrahymena thermophila. J. Cell Sci. 103, 565-570. [DOI] [PubMed] [Google Scholar]
- 24.Van Houten J. 1978. Two mechanisms of chemotaxis in Paramecium. J. Comp. Physiol. 127, 167-174. ( 10.1007/bf01352301) [DOI] [Google Scholar]
- 25.Leick V, Koppelhus U, Rosenberg J. 1994. Cilia-mediated oriented chemokinesis in Tetrahymena thermophila. J. Eukaryot. Microbiol. 41, 546-553. ( 10.1111/j.1550-7408.1994.tb01515.x) [DOI] [PubMed] [Google Scholar]
- 26.Sehring IM, Plattner H. 2004. Ca2+ oscillations mediated by exogenous GTP in Paramecium cells: assessment of possible Ca2+ sources. Cell Calcium 36, 409-420. ( 10.1016/j.ceca.2004.04.001) [DOI] [PubMed] [Google Scholar]
- 27.Fraenkel GS, Gunn DL. 1940. The orientation of animals, kineses, taxes and compass reactions. Oxford, UK: Clarendon Press. [Google Scholar]
- 28.Shi C, Iglesias PA. 2013. Excitable behavior in amoeboid chemotaxis. Wiley Interdiscip. Rev. Syst. Biol. Med. 5, 631-642. ( 10.1002/wsbm.1230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaney KF, Huang C-H, Devreotes PN. 2010. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39, 265-289. ( 10.1146/annurev.biophys.093008.131228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marciano-Cabral F, Cline M. 1987. Chemotaxis by Naegleria fowleri for bacteria. J. Protozool. 34, 127-131. ( 10.1111/j.1550-7408.1987.tb03147.x) [DOI] [PubMed] [Google Scholar]
- 31.Schuster FL, Rahman M, Griffith S. 1993. Chemotactic responses of Acanthamoeba castellanii to bacteria, bacterial components, and chemotactic peptides. Trans. Am. Microsc. Soc. 112, 43-61. ( 10.2307/3226781) [DOI] [Google Scholar]
- 32.Urban T, Jarstrand C, Aust-Kettis A. 1983. Migration of Entamoeba histolytica under agarose. Am. J. Trop. Med. Hyg. 32, 733-737. ( 10.4269/ajtmh.1983.32.733) [DOI] [PubMed] [Google Scholar]
- 33.McIntyre J, Jenkin CR. 1969. Chemotaxis in the free living amoeba (Hartmannella rhysodes). Aust. J. Exp. Biol. Med. Sci. 47, 625-632. ( 10.1038/icb.1969.156) [DOI] [PubMed] [Google Scholar]
- 34.Knowles DJ, Carlile MJ. 1978. The chemotactic response of plasmodia of the myxomycete Physarum polycephalum to sugars and related compounds. J. Gen. Microbiol. 108, 17-25. ( 10.1099/00221287-108-1-17) [DOI] [PubMed] [Google Scholar]
- 35.King JS, Insall RH. 2009. Chemotaxis: finding the way forward with Dictyostelium. Trends Cell Biol. 19, 523-530. ( 10.1016/j.tcb.2009.07.004) [DOI] [PubMed] [Google Scholar]
- 36.Skoge M, Yue H, Erickstad M, Bae A, Levine H, Groisman A, Loomis WF, Rappel W-J.. 2014. Cellular memory in eukaryotic chemotaxis. Proc. Natl Acad. Sci. USA 111, 14 448-14 453. ( 10.1073/pnas.1412197111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrew N, Insall RH. 2007. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat. Cell Biol. 9, 193-200. ( 10.1038/ncb1536) [DOI] [PubMed] [Google Scholar]
- 38.Fache S. 2005. Calcium mobilization stimulates Dictyostelium discoideum shear-flow-induced cell motility. J. Cell Sci. 118, 3445-3458. ( 10.1242/jcs.02461) [DOI] [PubMed] [Google Scholar]
- 39.Décavé E, Garrivier D, Bréchet Y, Fourcade B, Bruckert F. 2002. Shear flow-induced detachment kinetics of Dictyostelium discoideum cells from solid substrate. Biophys. J. 82, 2383-2395. ( 10.1016/s0006-3495(02)75583-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YT. 1950. Investigations of the biology of Peranema trichophorum (Euglenineae). Q. J. Microsc. Sci. 91, 279-308. [PubMed] [Google Scholar]
- 41.Bloodgood RA. 1988. Gliding motility and the dynamics of flagellar membrane glycoproteins in Chlamydomonas reinhardtii. J. Protozool. 35, 552-558. ( 10.1111/j.1550-7408.1988.tb04151.x) [DOI] [PubMed] [Google Scholar]
- 42.Pringsheim EG. 1968. Kleine Mitteilungen über Flagellaten und Algen [Short contributions on flagellates and algae]. Arch. Mikrobiol. 63, 1-6. [In German.] ( 10.1007/bf00407057) [DOI] [PubMed] [Google Scholar]
- 43.Ohnuma M, Misumi O, Kuroiwa T. 2011. Phototaxis in the unicellular red algae Cyanidioschyzon merolae and Cyanidium caldarium. Cytologia 76, 295-300. ( 10.1508/cytologia.76.295) [DOI] [Google Scholar]
- 44.Maschmann S, Ruban K, Wientapper J, Walter WJ. 2020. Phototaxis of the unicellular red alga Cyanidioschyzon merolae is mediated by novel actin-driven tentacles. Int. J. Mol. Sci. 21, 6209. ( 10.3390/ijms21176209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sbrana C, Giovannetti M. 2005. Chemotropism in the arbuscular mycorrhizal fungus Glomus mosseae. Mycorrhiza 15, 539-545. ( 10.1007/s00572-005-0362-5) [DOI] [PubMed] [Google Scholar]
- 46.Shimizu KK, Okada K. 2000. Attractive and repulsive interactions between female and male gametophytes in Arabidopsis pollen tube guidance. Development 127, 4511-4518. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi H. 1997. Hydrotropism: the current state of our knowledge. J. Plant Res. 110, 163-169. ( 10.1007/BF02509304) [DOI] [PubMed] [Google Scholar]
- 48.Netotea S, Bertani I, Steindler L, Kerényi A, Venturi V, Pongor S. 2009. A simple model for the early events of quorum sensing in Pseudomonas aeruginosa: modeling bacterial swarming as the movement of an ‘activation zone’. Biol. Direct 4, 6. ( 10.1186/1745-6150-4-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilde A, Mullineaux CW. 2017. Light-controlled motility in prokaryotes and the problem of directional light perception. FEMS Microbiol. Rev. 41, 900-922. ( 10.1093/femsre/fux045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ragatz L, Jiang ZY, Bauer CE, Gest H. 1995. Macroscopic phototactic behavior of the purple photosynthetic bacterium Rhodospirillum centenum. Arch. Microbiol. 163, 1-6. ( 10.1007/BF00262196) [DOI] [PubMed] [Google Scholar]
- 51.Dworkin M. 1983. Tactic behavior of Myxococcus xanthus. J. Bacteriol. 154, 452-459. ( 10.1128/JB.154.1.452-459.1983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stanier RY. 1942. A note on elasticotaxis in myxobacteria. J. Bacteriol. 44, 405-412. ( 10.1128/jb.44.4.405-412.1942) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fontes M, Kaiser D.. 1999. Myxococcus cells respond to elastic forces in their substrate. Proc. Natl Acad. Sci. USA 96, 8052-8057. ( 10.1073/pnas.96.14.8052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muyzer G, Yildirim E, van Dongen U, Kühl M, Thar R.. 2005. Identification of “Candidatus Thioturbo danicus,” a microaerophilic bacterium that builds conspicuous veils on sulfidic sediments. Appl. Environ. Microbiol. 71, 8929-8933. ( 10.1128/aem.71.12.8929-8933.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thar R, Kuhl M.. 2003. Bacteria are not too small for spatial sensing of chemical gradients: an experimental evidence. Proc. Natl Acad. Sci. USA 100, 5748-5753. ( 10.1073/pnas.1030795100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schuergers N, et al. 2016. Cyanobacteria use micro-optics to sense light direction. eLife 5, e12620. ( 10.7554/eLife.12620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barbara GM, Mitchell JG. 2003. Bacterial tracking of motile algae. FEMS Microbiol. Ecol. 44, 79-87. ( 10.1111/j.1574-6941.2003.tb01092.x) [DOI] [PubMed] [Google Scholar]
- 58.Locsei JT, Pedley TJ. 2009. Bacterial tracking of motile algae assisted by algal cell's vorticity field. Microb. Ecol. 58, 63-74. ( 10.1007/s00248-008-9468-6) [DOI] [PubMed] [Google Scholar]
- 59.Shapere A, Wilczek F. 1989. Geometry of self-propulsion at low Reynolds number. J. Fluid Mech. 198, 557. ( 10.1017/s002211208900025x) [DOI] [PubMed] [Google Scholar]
- 60.Rossi M, Cicconofri G, Beran A, Noselli G, DeSimone A. 2017. Kinematics of flagellar swimming in Euglena gracilis: helical trajectories and flagellar shapes. Proc. Natl Acad. Sci. USA 114, 13 085-13 090. ( 10.1073/pnas.1708064114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fenchel T, Blackburn N. 1999. Motile chemosensory behaviour of phagotrophic protists: mechanisms for and efficiency in congregating at food patches. Protist 150, 325-336. ( 10.1016/S1434-4610(99)70033-7) [DOI] [PubMed] [Google Scholar]
- 62.Tsubo Y. 1961. Chemotaxis and sexual behavior in Chlamydomonas. J. Protozool. 8, 114-121. ( 10.1111/j.1550-7408.1961.tb01191.x) [DOI] [Google Scholar]
- 63.Kinoshita N, Nagasato C, Motomura T. 2017. Phototaxis and chemotaxis of brown algal swarmers. J. Plant Res. 130, 443-453. ( 10.1007/s10265-017-0914-8) [DOI] [PubMed] [Google Scholar]
- 64.Nultsch W, Häder M.. 1984. Light-induced chemotactic responses of the colorless flagellate,Polytomella magna, in the presence of photodynamic dyes. Arch. Microbiol. 139, 21-27. ( 10.1007/bf00692706) [DOI] [Google Scholar]
- 65.Jikeli JF, et al. 2015. Sperm navigation along helical paths in 3D chemoattractant landscapes. Nat. Commun. 6, 7985. ( 10.1038/ncomms8985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pommerville J. 1978. Analysis of gamete and zygote motility in Allomyces. Exp. Cell Res. 113, 161-172. ( 10.1016/0014-4827(78)90096-4) [DOI] [PubMed] [Google Scholar]
- 67.Bowne SW Jr, Bowne GD. 1967. Taxis in Euglena. Exp. Cell Res. 47, 545-553. ( 10.1016/0014-4827(67)90010-9) [DOI] [PubMed] [Google Scholar]
- 68.Häder M. 1984. Light-induced chemotactic reactions in the dinoflagellates, Peridiniopsis berolinensis. Photochem. Photobiol. 40, 533-537. ( 10.1111/j.1751-1097.1984.tb04629.x) [DOI] [Google Scholar]
- 69.Kholodnyy V, Gadêlha H, Cosson J, Boryshpolets S. 2020. How do freshwater fish sperm find the egg? The physicochemical factors guiding the gamete encounters of externally fertilizing freshwater fish. Rev. Aquac. 12, 1165-1192. ( 10.1111/raq.12378) [DOI] [Google Scholar]
- 70.Satir P, Mitchell DR, Jékely G. 2008. How did the cilium evolve? Curr. Top. Dev. Biol. 85, 63-82. ( 10.1016/S0070-2153(08)00803-X) [DOI] [PubMed] [Google Scholar]
- 71.Speijer D, Lukeš J, Eliáš M. 2015. Sex is a ubiquitous, ancient, and inherent attribute of eukaryotic life. Proc. Natl Acad. Sci. USA 112, 8827-8834. ( 10.1073/pnas.1501725112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller DG, Jaenicke L, Donike M, Akintobi T. 1971. Sex attractant in a brown alga: chemical structure. Science 171, 815-817. ( 10.1126/science.171.3973.815) [DOI] [PubMed] [Google Scholar]
- 73.Müller DG, Kawai H, Stache B, Fölster E, Boland W. 1990. Sexual pheromones and gamete chemotaxis in Analipus japonicus (Phaeophyceae). Experientia 46, 534-536. ( 10.1007/bf01954258) [DOI] [Google Scholar]
- 74.Kinoshita N, Shiba K, Inaba K, Fu G, Nagasato C, Motomura T. 2016. Flagellar waveforms of gametes in the brown alga Ectocarpus siliculosus. Eur. J. Phycol. 51, 139-148. ( 10.1080/09670262.2015.1109144) [DOI] [Google Scholar]
- 75.Sugiura M, Shiotani H, Suzaki T, Harumoto T. 2010. Behavioural changes induced by the conjugation-inducing pheromones, gamone 1 and 2, in the ciliate Blepharisma japonicum. Eur. J. Protistol. 46, 143-149. ( 10.1016/j.ejop.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 76.Seshachar BR, Padmavathi PB. 1959. Conjugation in Spirostomum. Nature 184, 1510-1511. ( 10.1038/1841510b0)13825422 [DOI] [Google Scholar]
- 77.Stock C, Krüppel T, Key G, Lueken W. 1999. Sexual behaviour in Euplotes raikovi is accompanied by pheromone-induced modifications of ionic currents. J. Exp. Biol. 202, 475-483. [DOI] [PubMed] [Google Scholar]
- 78.Jékely G. 2009. Evolution of phototaxis. Phil. Trans. R. Soc. B 364, 2795-2808. ( 10.1098/rstb.2009.0072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schaller K, David R, Uhl R. 1997. How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys. J. 73, 1562-1572. ( 10.1016/S0006-3495(97)78188-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drescher K, Goldstein RE, Tuval I. 2010. Fidelity of adaptive phototaxis. Proc. Natl Acad. Sci. USA 107, 11 171-11 176. ( 10.1073/pnas.1000901107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Häder D-P. 1993. Simulation of phototaxis in the flagellate Euglena gracilis. J. Biol. Phys. 19, 95-108. ( 10.1007/BF00700253) [DOI] [Google Scholar]
- 82.Tsang ACH, Lam AT, Riedel-Kruse IH. 2018. Polygonal motion and adaptable phototaxis via flagellar beat switching in the microswimmer Euglena gracilis. Nat. Phys. 14, 1216-1222. ( 10.1038/s41567-018-0277-7) [DOI] [Google Scholar]
- 83.Nilsson D-E, Marshall J. 2020. Lens eyes in protists. Curr. Biol. 30, R458-R459. ( 10.1016/j.cub.2020.01.077) [DOI] [PubMed] [Google Scholar]
- 84.Gavelis GS, Hayakawa S, White RA III, Gojobori T, Suttle CA, Keeling PJ, Leander BS. 2015. Eye-like ocelloids are built from different endosymbiotically acquired components. Nature 523, 204-207. ( 10.1038/nature14593) [DOI] [PubMed] [Google Scholar]
- 85.Foster KW, Smyth RD. 1980. Light antennas in phototactic algae. Microbiol. Rev. 44, 572-630. ( 10.1128/MR.44.4.572-630.1980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gómez F. 2017. The function of the ocelloid and piston in the dinoflagellate Erythropsidinium (Gymnodiniales, Dinophyceae). J. Phycol. 53, 629-641. ( 10.1111/jpy.12525) [DOI] [PubMed] [Google Scholar]
- 87.Darnton NC, Turner L, Rojevsky S, Berg HC. 2007. On torque and tumbling in swimming Escherichia coli. J. Bacteriol. 189, 1756-1764. ( 10.1128/JB.01501-06) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nan B. 2017. Bacterial gliding motility: rolling out a consensus model. Curr. Biol. 27, R154-R156. ( 10.1016/j.cub.2016.12.035) [DOI] [PubMed] [Google Scholar]
- 89.de Boer W, La Riviere JW, Houwink AL. 1961. Observations on the morphology of Thiovulum majus Hinze. Antonie Van Leeuwenhoek 27, 447-456. ( 10.1007/BF02538470) [DOI] [PubMed] [Google Scholar]
- 90.Garcia-Pichel F. 1989. Rapid bacterial swimming measured in swarming cells of Thiovulum majus. J. Bacteriol. 171, 3560-3563. ( 10.1128/JB.171.6.3560-3563.1989) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thar R, Fenchel T. 2001. True chemotaxis in oxygen gradients of the sulfur-oxidizing bacterium Thiovulum majus. Appl. Environ. Microbiol. 67, 3299-3303. ( 10.1128/AEM.67.7.3299-3303.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petroff A, Libchaber A. 2014. Hydrodynamics and collective behavior of the tethered bacterium Thiovulum majus. Proc. Natl Acad. Sci. USA 111, E537-E545. ( 10.1073/pnas.1322092111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Goodenough U, Heitman J. 2014. Origins of eukaryotic sexual reproduction. Cold Spring Harb. Perspect. Biol. 6, a016154. ( 10.1101/cshperspect.a016154) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kimball RF. 1942. The nature and inheritance of mating types in Euplotes patella. Genetics 27, 269-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kitamura A, Hiwatashi K. 1984. Inactivation of cell movement following sexual cell recognition in Paramecium caudatum. Biol. Bull. 166, 156-166. ( 10.2307/1541438) [DOI] [Google Scholar]
- 96.Goodenough UW, Weiss RL. 1975. Gametic differentiation in Chlamydomonas reinhardtii. III. Cell wall lysis and microfilament-associated mating structure activation in wild-type and mutant strains. J. Cell Biol. 67, 623-637. ( 10.1083/jcb.67.3.623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Watanabe T. 1982. Correlation between ventral surface structures and local degeneration of cilia during conjugation in Paramecium. J. Embryol. Exp. Morphol. 70, 19-28. [PubMed] [Google Scholar]
- 98.Holmes JA, Dutcher SK. 1989. Cellular asymmetry in Chlamydomonas reinhardtii. J. Cell Sci. 94, 273-285. [DOI] [PubMed] [Google Scholar]
- 99.Hegemann P, Musgrave A. 1991. Phototaxis of Chlamydomonas eugametos pairs is controlled by the mt+ partner. J. Plant Physiol. 138, 285-291. ( 10.1016/s0176-1617(11)80289-0) [DOI] [Google Scholar]
- 100.Kreis CT, Le Blay M, Linne C, Makowski MM, Bäumchen O.. 2018. Adhesion of Chlamydomonas microalgae to surfaces is switchable by light. Nat. Phys. 14, 45-49. ( 10.1038/nphys4258) [DOI] [Google Scholar]
- 101.Treier U, Fuchs S, Weber M, Wakarchuk WW, Beck CF. 1989. Gametic differentiation in Chlamydomonas reinhardtii: light dependence and gene expression patterns. Arch. Microbiol. 152, 572-577. ( 10.1007/bf00425489) [DOI] [Google Scholar]
- 102.Forbes MA, Hallam ND. 1978. Gamete structure and fertilization in the brown alga Hormosira banksii (Turner) Decaisne. Br. Phycol. J. 13, 299-310. ( 10.1080/00071617800650361) [DOI] [Google Scholar]
- 103.Rosenshine I, Tchelet R, Mevarech M. 1989. The mechanism of DNA transfer in the mating system of an archaebacterium. Science 245, 1387-1389. ( 10.1126/science.2818746) [DOI] [PubMed] [Google Scholar]
- 104.Shalev Y, Turgeman-Grott I, Tamir A, Eichler J, Gophna U. 2017. Cell surface glycosylation is required for efficient mating of Haloferax volcanii. Front. Microbiol. 8, 1253. ( 10.3389/fmicb.2017.01253) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ducret A, Fleuchot B, Bergam P, Mignot T. 2013. Direct live imaging of cell–cell protein transfer by transient outer membrane fusion in Myxococcus xanthus. eLife 2, e00868. ( 10.7554/elife.00868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kudryashev M, Cyrklaff M, Alex B, Lemgruber L, Baumeister W, Wallich R, Frischknecht F. 2011. Evidence of direct cell–cell fusion in Borrelia by cryogenic electron tomography. Cell Microbiol. 13, 731-741. ( 10.1111/j.1462-5822.2011.01571.x) [DOI] [PubMed] [Google Scholar]
- 107.Charubin K, Modla S, Caplan JL, Papoutsakis ET. 2020. Interspecies microbial fusion and large-scale exchange of cytoplasmic proteins and RNA in a syntrophic Clostridium coculture. mBio 11, e02030-20. ( 10.1128/mbio.02030-20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cavalier-Smith T. 2009. Predation and eukaryote cell origins: a coevolutionary perspective. Int. J. Biochem. Cell Biol. 41, 307-322. ( 10.1016/j.biocel.2008.10.002) [DOI] [PubMed] [Google Scholar]
- 109.Martijn J, Ettema TJG. 2013. From archaeon to eukaryote: the evolutionary dark ages of the eukaryotic cell. Biochem. Soc. Trans. 41, 451-457. ( 10.1042/BST20120292) [DOI] [PubMed] [Google Scholar]
- 110.Martin WF, Tielens AGM, Mentel M, Garg SG, Gould SB. 2017. The physiology of phagocytosis in the context of mitochondrial origin. Microbiol. Mol. Biol. Rev. 81, e00008-17. ( 10.1128/MMBR.00008-17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hausmann K, Bradbury PC (eds). 1996. Ciliates: cells as organisms. Stuttgart, Germany: Vch Pub. [Google Scholar]
- 112.Pan M, Neilson MP, Grunfeld AM, Cruz P, Wen X, Insall RH, Jin T. 2018. A G-protein-coupled chemoattractant receptor recognizes lipopolysaccharide for bacterial phagocytosis. PLoS Biol. 16, e2005754. ( 10.1371/journal.pbio.2005754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan M, Xu X, Chen Y, Jin T. 2016. Identification of a chemoattractant G-protein-coupled receptor for folic acid that controls both chemotaxis and phagocytosis. Dev. Cell. 36, 428-439. ( 10.1016/j.devcel.2016.01.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wootton EC, Zubkov MV, Jones DH, Jones RH, Martel CM, Thornton CA, Roberts EC. 2007. Biochemical prey recognition by planktonic protozoa. Environ. Microbiol. 9, 216-222. ( 10.1111/j.1462-2920.2006.01130.x) [DOI] [PubMed] [Google Scholar]
- 115.Hausmann K, Patterson DJ. 1982. Pseudopod formation and membrane production during prey capture by a heliozoon (feeding by Actinophrys, II). Cell Motility 2, 9-24. ( 10.1002/cm.970020103) [DOI] [Google Scholar]
- 116.Leander BS. 2020. Predatory protists. Curr. Biol. 30, R510-R516. ( 10.1016/j.cub.2020.03.052) [DOI] [PubMed] [Google Scholar]
- 117.Coyle SM, Flaum EM, Li H, Krishnamurthy D, Prakash M. 2019. Coupled active systems encode an emergent hunting behavior in the unicellular predator Lacrymaria olor. Curr. Biol. 29, 3838-3850.e3. ( 10.1016/j.cub.2019.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Breglia SA, Yubuki N, Leander BS. 2013. Ultrastructure and molecular phylogenetic position of Heteronema scaphurum: a eukaryovorous euglenid with a cytoproct. J. Eukaryot. Microbiol. 60, 107-120. ( 10.1111/jeu.12014) [DOI] [PubMed] [Google Scholar]
- 119.Triemer RE. 1997. Feeding in Peranema trichophorum revisited (Euglenophyta). J. Phycol. 33, 649-654. ( 10.1111/j.0022-3646.1997.00649.x) [DOI] [Google Scholar]
- 120.Jacobson DM, Anderson DM. 1986. Thecate heterophic dinoflagellates: feeding behavior and mechanisms. J. Phycol. 22, 249-258. ( 10.1111/j.1529-8817.1986.tb00021.x) [DOI] [Google Scholar]
- 121.Mast SO. 1909. The reactions of Didinium nasutum (Stein) with special reference to the feeding habits and the function of trichocysts. Biol. Bull. 16, 91-118. ( 10.2307/1536126) [DOI] [Google Scholar]
- 122.Small EB, Marszalek DS. 1969. Scanning electron microscopy of fixed, frozen, and dried protozoa. Science 163, 1064-1065. ( 10.1126/science.163.3871.1064) [DOI] [PubMed] [Google Scholar]
- 123.Hara R, Asai H, Naitoh Y. 1985. Electrical responses of the carnivorous ciliate Didinium nasutum in relation to discharge of the extrusive organelles. J. Exp. Biol. 119, 211-224. [Google Scholar]
- 124.Hausmann K, Peck RK. 1979. The mode of function of the cytopharyngeal basket of the ciliate Pseudomicrothorax dubius. Differentiation 14, 147-158. ( 10.1111/j.1432-0436.1979.tb01023.x) [DOI] [PubMed] [Google Scholar]
- 125.Plattner H. 2017. Signalling in ciliates: long- and short-range signals and molecular determinants for cellular dynamics. Biol. Rev. Camb. Phil. Soc. 92, 60-107. ( 10.1111/brv.12218) [DOI] [PubMed] [Google Scholar]
- 126.Fenchel T. 1980. Suspension feeding in ciliated Protozoa: structure and function of feeding organelles. Arch. Protist. 123, 239-260. ( 10.1016/s0003-9365(80)80009-1) [DOI] [Google Scholar]
- 127.Pérez J, Moraleda-Muñoz A, Marcos-Torres FJ, Muñoz-Dorado J. 2016. Bacterial predation: 75 years and counting! Environ. Microbiol. 18, 766-779. ( 10.1111/1462-2920.13171) [DOI] [PubMed] [Google Scholar]
- 128.Rendulic S. 2004. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303, 689-692. ( 10.1126/science.1093027) [DOI] [PubMed] [Google Scholar]
- 129.Jashnsaz H, et al. 2017. Hydrodynamic hunters. Biophys. J. 112, 1282-1289. ( 10.1016/j.bpj.2017.02.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shiratori T, Suzuki S, Kakizawa Y, Ishida K-I. 2019. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat. Commun. 10, 5529. ( 10.1038/s41467-019-13499-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mathijssen AJTM, Culver J, Bhamla MS, Prakash M. 2019. Collective intercellular communication through ultra-fast hydrodynamic trigger waves. Nature 571, 560-564. ( 10.1038/s41586-019-1387-9) [DOI] [PubMed] [Google Scholar]
- 132.Kiørboe T, Jiang H. 2013. To eat and not be eaten: optimal foraging behaviour in suspension feeding copepods. J. R. Soc. Interface 10, 20120693. ( 10.1098/rsif.2012.0693) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Visser AW. 2001. Hydromechanical signals in the plankton. Mar. Ecol. Prog. Ser. 222, 1-24. ( 10.3354/meps222001) [DOI] [Google Scholar]
- 134.Jakobsen HH. 2002. Escape of protists in predator-generated feeding currents. Aquat. Microb. Ecol. 26, 271-281. ( 10.3354/ame026271) [DOI] [Google Scholar]
- 135.Jakobsen HH, Everett LM, Strom SL. 2006. Hydromechanical signaling between the ciliate Mesodinium pulex and motile protist prey. Aquat. Microb. Ecol. 44, 197-206. ( 10.3354/ame044197) [DOI] [Google Scholar]
- 136.Naitoh Y, Eckert R. 1969. Ionic mechanisms controlling behavioral responses of Paramecium to mechanical stimulation. Science 164, 963-965. ( 10.1126/science.164.3882.963) [DOI] [PubMed] [Google Scholar]
- 137.Wan KY, Goldstein RE. 2014. Rhythmicity, recurrence, and recovery of flagellar beating. Phys. Rev. Lett. 113, 238103. ( 10.1103/PhysRevLett.113.238103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Friedrich BM. 2018. Load response of shape-changing microswimmers scales with their swimming efficiency. Phys. Rev. E 97, 042416. ( 10.1103/PhysRevE.97.042416) [DOI] [PubMed] [Google Scholar]
- 139.Liu P, Lou X, Wingfield JL, Lin J, Nicastro D, Lechtreck K. 2020. Chlamydomonas PKD2 organizes mastigonemes, hair-like glycoprotein polymers on cilia. J. Cell Biol. 219, e202001122. ( 10.1083/jcb.202001122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Häder D-P, Hemmersbach R, Lebert M. 2005. Gravity and the behavior of unicellular organisms. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 141.Bean B. 1984. Microbial geotaxis. In Membranes and sensory transduction (eds Colombetti G, Lenci F), pp. 163-198. Boston, MA: Springer. ( 10.1007/978-1-4613-2675-5_5) [DOI] [Google Scholar]
- 142.Schuech R, Menden-Deuer S. 2014. Going ballistic in the plankton: anisotropic swimming behavior of marine protists. Limnol. Oceanogr. Fluids Environ. 4, 10.1215/21573689-2647998. ( 10.1215/21573689-2647998) [DOI] [Google Scholar]
- 143.Kage A, Omori T, Kikuchi K, Ishikawa T. 2020. The shape effect of flagella is more important than bottom-heaviness on passive gravitactic orientation in Chlamydomonas reinhardtii. J. Exp. Biol. 223, jeb205989. ( 10.1242/jeb.205989) [DOI] [PubMed] [Google Scholar]
- 144.Guasto JS, Rusconi R, Stocker R. 2012. Fluid mechanics of planktonic microorganisms. Annu. Rev. Fluid Mech. 44, 373-400. ( 10.1146/annurev-fluid-120710-101156) [DOI] [Google Scholar]
- 145.Bees MA. 2020. Advances in bioconvection. Annu. Rev. Fluid Mech. 52, 449-476. ( 10.1146/annurev-fluid-010518-040558) [DOI] [Google Scholar]
- 146.Wheeler JD, Secchi E, Rusconi R, Stocker R. 2019. Not just going with the flow: the effects of fluid flow on bacteria and plankton. Annu. Rev. Cell Dev. Biol. 35, 213-237. ( 10.1146/annurev-cellbio-100818-125119) [DOI] [PubMed] [Google Scholar]
- 147.Kinoshita-Terauchi N, Shiba K, Umezawa T, Matsuda F, Motomura T, Inaba K. 2019. A brown algal sex pheromone reverses the sign of phototaxis by cAMP/Ca2+-dependent signaling in the male gametes of Mutimo cylindricus (Cutleriaceae). J. Photochem. Photobiol. B 192, 113-123. ( 10.1016/j.jphotobiol.2019.01.010) [DOI] [PubMed] [Google Scholar]
- 148.Marcos, Fu HC, Powers TR, Stocker R.. 2012. Bacterial rheotaxis. Proc. Natl Acad. Sci. USA 109, 4780-4785. ( 10.1073/pnas.1120955109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cox CD, Bavi N, Martinac B. 2018. Bacterial mechanosensors. Annu. Rev. Physiol. 80, 71-93. ( 10.1146/annurev-physiol-021317-121351) [DOI] [PubMed] [Google Scholar]
- 150.Bruni GN, Weekley RA, Dodd BJT, Kralj JM.. 2017. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. Proc. Natl Acad. Sci. USA 114, 9445-9450. ( 10.1073/pnas.1703084114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wadhwa N, Phillips R, Berg HC. 2019. Torque-dependent remodeling of the bacterial flagellar motor. Proc. Natl Acad. Sci. USA 116, 11 764-11 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nirody JA, Nord AL, Berry RM. 2019. Load-dependent adaptation near zero load in the bacterial flagellar motor. J. R. Soc. Interface 16, 20190300. ( 10.1098/rsif.2019.0300) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dufrêne YF, Persat A. 2020. Mechanomicrobiology: how bacteria sense and respond to forces. Nat. Rev. Microbiol. 18, 227-240. ( 10.1038/s41579-019-0314-2) [DOI] [PubMed] [Google Scholar]
- 154.Jakobsen HH. 2001. Escape response of planktonic protists to fluid mechanical signals. Mar. Ecol. Prog. Ser. 214, 67-78. ( 10.3354/meps214067) [DOI] [Google Scholar]
- 155.Harumoto T, Miyake A. 1991. Defensive function of trichocysts in Paramecium. J. Exp. Zool. 260, 84-92. ( 10.1002/jez.1402600111) [DOI] [Google Scholar]
- 156.Rosati G, Modeo L. 2003. Extrusomes in ciliates: diversification, distribution, and phylogenetic implications. J. Eukaryot. Microbiol. 50, 383-402. ( 10.1111/j.1550-7408.2003.tb00260.x) [DOI] [PubMed] [Google Scholar]
- 157.Holland EM, Harz H, Uhl R, Hegemann P. 1997. Control of phobic behavioral responses by rhodopsin-induced photocurrents in Chlamydomonas. Biophys. J. 73, 1395-1401. ( 10.1016/S0006-3495(97)78171-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hayashi M, Yagi T, Yoshimura K, Kamiya R. 1998. Real-time observation of Ca2+-induced basal body reorientation in Chlamydomonas. Cell Motil. Cytoskeleton 41, 49-56. ( 10.1002/(sici)1097-0169(1998)41:1) [DOI] [PubMed] [Google Scholar]
- 159.Moriyama Y, Hiyama S, Asai H. 1998. High-speed video cinematographic demonstration of stalk and zooid contraction of Vorticella convallaria. Biophys. J. 74, 487-491. ( 10.1016/s0006-3495(98)77806-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ando M, Shigenaka Y. 1989. Structure and function of the cytoskeleton in heliozoa: I. Mechanism of rapid axopodial contraction in Echinosphaerium. Cell Motil. Cytoskeleton 14, 288-301. ( 10.1002/cm.970140214) [DOI] [PubMed] [Google Scholar]
- 161.Brunet T, Arendt D. 2016. From damage response to action potentials: early evolution of neural and contractile modules in stem eukaryotes. Phil. Trans. R. Soc. B 371, 20150043. ( 10.1098/rstb.2015.0043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eckert R, Brehm P. 1979. Ionic mechanisms of excitation in Paramecium. Annu. Rev. Biophys. Bioeng. 8, 353-383. ( 10.1146/annurev.bb.08.060179.002033) [DOI] [PubMed] [Google Scholar]
- 163.Eckert R, Naito Y. 1972. Bioelectric control of locomotion in the ciliates. J. Protozool. 19, 237-243. ( 10.1111/j.1550-7408.1972.tb03444.x) [DOI] [PubMed] [Google Scholar]
- 164.Wood DC. 1982. Membrane permeabilities determining resting, action and mechanoreceptor potentials in Stentor coeruleus. J. Comp. Physiol. A 146, 537-550. ( 10.1007/bf00609450) [DOI] [Google Scholar]
- 165.Taylor AR. 2009. A fast Na+/Ca2+-based action potential in a marine diatom. PLoS ONE 4, e4966. ( 10.1371/journal.pone.0004966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Helliwell KE, Chrachri A, Koester JA, Wharam S, Verret F, Taylor AR, Wheeler GL, Brownlee C. 2019. Alternative mechanisms for fast Na+/Ca2+ signaling in eukaryotes via a novel class of single-domain voltage-gated channels. Curr. Biol. 29, 1503-1511.e6. ( 10.1016/j.cub.2019.03.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bingley MS, Thompson CM. 1962. Bioelectric potentials in relation to movement in amoebae. J. Theor. Biol. 2, 16-32. ( 10.1016/s0022-5193(62)80024-1) [DOI] [Google Scholar]
- 168.Harz H, Hegemann P. 1991. Rhodopsin-regulated calcium currents in Chlamydomonas. Nature 351, 489-491. ( 10.1038/351489a0) [DOI] [Google Scholar]
- 169.Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. 2011. Mechanoreception in motile flagella of Chlamydomonas. Nat. Cell Biol. 13, 630-632. ( 10.1038/ncb2214) [DOI] [PubMed] [Google Scholar]
- 170.Fujiu K, Nakayama Y, Yanagisawa A, Sokabe M, Yoshimura K. 2009. Chlamydomonas CAV2 encodes a voltage-dependent calcium channel required for the flagellar waveform conversion. Curr. Biol. 19, 133-139. ( 10.1016/j.cub.2008.11.068) [DOI] [PubMed] [Google Scholar]
- 171.Umbach JA. 1981. pH and membrane excitability in Paramecium caudatum. Los Angeles, CA: University of California. [Google Scholar]
- 172.Dunlap K. 1977. Localization of calcium channels in Paramecium caudatum. J. Physiol. 271, 119-133. ( 10.1113/jphysiol.1977.sp011993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Echevarria ML, Wolfe GV, Taylor AR. 2016. Feast or flee: bioelectrical regulation of feeding and predator evasion behaviors in the planktonic alveolate Favella sp. (Spirotrichia). J. Exp. Biol. 219, 445-456. ( 10.1242/jeb.121871) [DOI] [PubMed] [Google Scholar]
- 174.Lueken W, Ricci N, Krüppel T. 1996. Rhythmic spontaneous depolarizations determine a slow-and-fast rhythm in walking of the marine hypotrich Euplotes vannus. Eur. J. Protist. 32, 47-54. ( 10.1016/s0932-4739(96)80038-1) [DOI] [Google Scholar]
- 175.Kunita I, Kuroda S, Ohki K, Nakagaki T. 2014. Attempts to retreat from a dead-ended long capillary by backward swimming in Paramecium. Front. Microbiol. 5, 270. ( 10.3389/fmicb.2014.00270) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wood DC. 1988. Habituation in Stentor: produced by mechanoreceptor channel modification. J. Neurosci. 8, 2254-2258. ( 10.1523/JNEUROSCI.08-07-02254.1988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Jennings HS. 1899. Studies on reactions to stimuli in unicellular organisms. III Reactions to localized stimuli in Spirostomum and Stentor. Am. Nat. 33, 373-389. ( 10.1086/277256) [DOI] [Google Scholar]
- 178.Dexter JP, Prabakaran S, Gunawardena J. 2019. A complex hierarchy of avoidance behaviors in a single-cell eukaryote. Curr. Biol. 29, 4323-4329.e2. ( 10.1016/j.cub.2019.10.059) [DOI] [PubMed] [Google Scholar]
- 179.Yang C-Y, Bialecka-Fornal M, Weatherwax C, Larkin JW, Prindle A, Liu J, Garcia-Ojalvo J, Süel GM. 2020. Encoding membrane-potential-based memory within a microbial community. Cell Syst. 10, 417-423.e3. ( 10.1016/j.cels.2020.04.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grishanin RN, Bibikov SI, Altschuler IM, Kaulen AD, Kazimirchuk SB, Armitage JP, Skulachev VP. 1996. delta psi-mediated signalling in the bacteriorhodopsin-dependent photoresponse. J. Bacteriol. 178, 3008-3014. ( 10.1128/JB.178.11.3008-3014.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Williams TA, Cox CJ, Foster PG, Szöllősi GJ, Embley TM. 2020. Phylogenomics provides robust support for a two-domains tree of life. Nat. Ecol. Evol. 4, 138-147. ( 10.1038/s41559-019-1040-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zachar I, Szathmáry E. 2017. Breath-giving cooperation: critical review of origin of mitochondria hypotheses. Biol. Direct 12, 19. ( 10.1186/s13062-017-0190-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.López-García P, Moreira D. 2020. The syntrophy hypothesis for the origin of eukaryotes revisited. Nat. Microbiol. 5, 655-667. ( 10.1038/s41564-020-0710-4) [DOI] [PubMed] [Google Scholar]
- 184.Schäffer DE, Iyer LM, Burroughs AM, Aravind L. 2020. Functional innovation in the evolution of the calcium-dependent system of the eukaryotic endoplasmic reticulum. Front. Genet. 11, 34. ( 10.3389/fgene.2020.00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jackson AP, et al. 2016. Kinetoplastid phylogenomics reveals the evolutionary innovations associated with the origins of parasitism. Curr. Biol. 26, 161-172. ( 10.1016/j.cub.2015.11.055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stathopulos PB, Ikura M. 2017. Store operated calcium entry: from concept to structural mechanisms. Cell Calcium 63, 3-7. ( 10.1016/j.ceca.2016.11.005) [DOI] [PubMed] [Google Scholar]
- 187.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. 2006. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230-233. ( 10.1038/nature05122) [DOI] [PubMed] [Google Scholar]
- 188.Bick AG, Calvo SE, Mootha VK. 2012. Evolutionary diversity of the mitochondrial calcium uniporter. Science 336, 886. ( 10.1126/science.1214977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Alzayady KJ, Sebé-Pedrós A, Chandrasekhar R, Wang L, Ruiz-Trillo I, Yule DI. 2015. Tracing the evolutionary history of inositol, 1, 4, 5-trisphosphate receptor: insights from analyses of Capsaspora owczarzaki Ca2+ release channel orthologs. Mol. Biol. Evol. 32, 2236-2253. ( 10.1093/molbev/msv098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Plattner H, Verkhratsky A. 2015. The ancient roots of calcium signalling evolutionary tree. Cell Calcium 57, 123-132. ( 10.1016/j.ceca.2014.12.004) [DOI] [PubMed] [Google Scholar]
- 191.Cai X, Clapham DE. 2012. Ancestral Ca2+ signaling machinery in early animal and fungal evolution. Mol. Biol. Evol. 29, 91-100. ( 10.1093/molbev/msr149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gonda K, Komatsu M, Numata O. 2000. Calmodulin and Ca2+/calmodulin-binding proteins are involved in Tetrahymena thermophila phagocytosis. Cell Struct. Funct. 25, 243-251. ( 10.1247/csf.25.243) [DOI] [PubMed] [Google Scholar]
- 193.Kim H-S, Czymmek KJ, Patel A, Modla S, Nohe A, Duncan R, Gilroy S, Kang S. 2012. Expression of the Cameleon calcium biosensor in fungi reveals distinct Ca2+ signatures associated with polarized growth, development, and pathogenesis. Fungal Genet. Biol. 49, 589-601. ( 10.1016/j.fgb.2012.05.011) [DOI] [PubMed] [Google Scholar]
- 194.Chen M-Y, Insall RH, Devreotes PN. 1996. Signaling through chemoattractant receptors in Dictyostelium. Trends Genet. 12, 52-57. ( 10.1016/0168-9525(96)81400-4) [DOI] [PubMed] [Google Scholar]
- 195.Kuriu T, Saitow F, Nakaoka Y, Oosawa Y. 1998. Calcium current activated by cooling in Paramecium. J. Comp. Physiol. A 183, 135-141. ( 10.1007/s003590050241) [DOI] [Google Scholar]
- 196.Kung C, Saimi Y. 1982. The physiological basis of taxes in Paramecium. Annu. Rev. Physiol. 44, 519-534. ( 10.1146/annurev.ph.44.030182.002511) [DOI] [PubMed] [Google Scholar]
- 197.Martinac B, Saimi Y, Kung C. 2008. Ion channels in microbes. Physiol. Rev. 88, 1449-1490. ( 10.1152/physrev.00005.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Taylor AR, Brownlee C. 2003. A novel Cl− inward-rectifying current in the plasma membrane of the calcifying marine phytoplankton Coccolithus pelagicus. Plant Physiol. 131, 1391-1400. ( 10.1104/pp.011791) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lindström JB, Pierce NT, Latz MI. 2017. Role of TRP channels in dinoflagellate mechanotransduction. Biol. Bull. 233, 151-167. ( 10.1086/695421) [DOI] [PubMed] [Google Scholar]
- 200.Lima WC, Vinet A, Pieters J, Cosson P. 2014. Role of PKD2 in rheotaxis in Dictyostelium. PLoS ONE 9, e88682. ( 10.1371/journal.pone.0088682) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Häder D-P, Hemmersbach R.. 2017. Gravitaxis in Euglena. Adv. Exp. Med. Biol. 979, 237-266. ( 10.1007/978-3-319-54910-1_12) [DOI] [PubMed] [Google Scholar]
- 202.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. 2007. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J. Cell Biol. 179, 501-514. ( 10.1083/jcb.200704069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McGoldrick LL, Singh AK, Demirkhanyan L, Lin T-Y, Casner RG, Zakharian E, Sobolevsky AI. 2019. Structure of the thermo-sensitive TRP channel TRP1 from the alga Chlamydomonas reinhardtii. Nat. Commun. 10, 4180. ( 10.1038/s41467-019-12121-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Domínguez DC, Guragain M, Patrauchan M. 2015. Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 57, 151-165. ( 10.1016/j.ceca.2014.12.006) [DOI] [PubMed] [Google Scholar]
- 205.Domínguez DC. 2018. Calcium signaling in prokaryotes. Calcium Signal Transduct. ( 10.5772/intechopen.78546) [DOI] [Google Scholar]
- 206.Tisa LS, Adler J.. 1995. Cytoplasmic free-Ca2+ level rises with repellents and falls with attractants in Escherichia coli chemotaxis. Proc. Natl Acad. Sci. USA 92, 10 777-10 781. ( 10.1073/pnas.92.23.10777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ordal GW. 1977. Calcium ion regulates chemotactic behaviour in bacteria. Nature 270, 66-67. ( 10.1038/270066a0) [DOI] [PubMed] [Google Scholar]
- 208.Ordaq GW, Fields RB. 1977. A biochemical mechanism for bacterial chemotaxis. J. Theor. Biol. 68, 491-500. ( 10.1016/0022-5193(77)90100-x) [DOI] [PubMed] [Google Scholar]
- 209.Silverio ALF, Saier MH Jr. 2011. Bioinformatic characterization of the trimeric intracellular cation-specific channel protein family. J. Membr. Biol. 241, 77-101. ( 10.1007/s00232-011-9364-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Fux JE, Mehta A, Moffat J, Spafford JD. 2018. Eukaryotic voltage-gated sodium channels: on their origins, asymmetries, losses, diversification and adaptations. Front. Physiol. 9, 1406. ( 10.3389/fphys.2018.01406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Durell SR, Guy HR. 2001. A putative prokaryote voltage-gated Ca2+ channel with only one 6TM motif per subunit. Biochem. Biophys. Res. Commun. 281, 741-746. ( 10.1006/bbrc.2001.4408) [DOI] [PubMed] [Google Scholar]
- 212.Miyata M, et al. 2020. Tree of motility – a proposed history of motility systems in the tree of life. Genes Cells 25, 6-21. ( 10.1111/gtc.12737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cavalier-Smith T, Chao EE, Lewis R. 2018. Multigene phylogeny and cell evolution of chromist infrakingdom Rhizaria: contrasting cell organisation of sister phyla Cercozoa and Retaria. Protoplasma 255, 1517-1574. ( 10.1007/s00709-018-1241-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fritz-Laylin LK, et al. 2010. The genome of Naegleria gruberi illuminates early eukaryotic versatility. Cell 140, 631-642. ( 10.1016/j.cell.2010.01.032) [DOI] [PubMed] [Google Scholar]
- 215.Brunet T, Albert M, Roman W, Spitzer DC, King N. 2020 A flagellate-to-amoeboid switch in the closest living relatives of animals. bioRxiv, 2020.06.26.171736. ( 10.1101/2020.06.26.171736) [DOI] [PMC free article] [PubMed]
- 216.Tikhonenkov DV, Hehenberger E, Esaulov AS, Belyakova OI, Mazei YA, Mylnikov AP, Keeling PJ. 2020. Insights into the origin of metazoan multicellularity from predatory unicellular relatives of animals. BMC Biol. 18, 39. ( 10.1186/s12915-020-0762-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Karpov SA, Vishnyakov AE, Moreira D, López-García P. 2019. The ultrastructure of Sanchytrium tribonematis (Sanchytriaceae, Fungi incertae sedis) confirms its close relationship to Amoeboradix. J. Eukaryot. Microbiol. 66, 892-898. ( 10.1111/jeu.12740) [DOI] [PubMed] [Google Scholar]
- 218.Fritz-Laylin LK, Lord SJ, Dyche Mullins R. 2017. WASP and SCAR are evolutionarily conserved in actin-filled pseudopod-based motility. J. Cell Biol. 216, 1673-1688. ( 10.1083/jcb.201701074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Alam M, Oesterhelt D. 1984. Morphology, function and isolation of halobacterial flagella. J. Mol. Biol. 176, 459-475. ( 10.1016/0022-2836(84)90172-4) [DOI] [PubMed] [Google Scholar]
- 220.Lowe G, Meister M, Berg HC. 1987. Rapid rotation of flagellar bundles in swimming bacteria. Nature 325, 637-640. ( 10.1038/325637a0) [DOI] [Google Scholar]
- 221.Albers S-V, Jarrell KF. 2018. The archaellum: an update on the unique archaeal motility structure. Trends Microbiol. 26, 351-362. ( 10.1016/j.tim.2018.01.004) [DOI] [PubMed] [Google Scholar]
- 222.Albers S-V, Jarrell KF. 2015. The archaellum: how Archaea swim. Front. Microbiol. 6, 23. ( 10.3389/fmicb.2015.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mullins RD. 2010. Cytoskeletal mechanisms for breaking cellular symmetry. Cold Spring Harb. Perspect. Biol. 2, a003392. ( 10.1101/cshperspect.a003392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Poulsen NC, Spector I, Spurck TP, Schultz TF, Wetherbee R. 1999. Diatom gliding is the result of an actin-myosin motility system. Cell Motil. Cytoskeleton 44, 23-33. () [DOI] [PubMed] [Google Scholar]
- 225.Lewis OL, Zhang S, Guy RD, del Álamo JC. 2015. Coordination of contractility, adhesion and flow in migrating Physarum amoebae. J. R. Soc. Interface 12, 20141359. ( 10.1098/rsif.2014.1359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Beeby M, Ferreira JL, Tripp P, Albers S-V, Mitchell DR. 2020. Propulsive nanomachines: the convergent evolution of archaella, flagella, and cilia. FEMS Microbiol. Rev. 44, 253-304. ( 10.1093/femsre/fuaa006) [DOI] [PubMed] [Google Scholar]
- 227.Machin KE. 1958. Wave propagation along flagella. J. Exp. Biol. 35, 796-806. [Google Scholar]
- 228.Wan KY, Goldstein RE. 2018. Time irreversibility and criticality in the motility of a flagellate microorganism. Phys. Rev. Lett. 121, 058103. ( 10.1103/PhysRevLett.121.058103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Moolenaar WH, De Goede J, Verveen AA.. 1976. Membrane noise in Paramecium. Nature 260, 344-346. ( 10.1038/260344a0) [DOI] [PubMed] [Google Scholar]
- 230.Nakaoka Y, Imaji T, Hara M, Hashimoto N. 2009. Spontaneous fluctuation of the resting membrane potential in Paramecium: amplification caused by intracellular Ca2+. J. Exp. Biol. 212, 270-276. ( 10.1242/jeb.023283) [DOI] [PubMed] [Google Scholar]
- 231.Machemer H, Ogura A. 1979. Ionic conductances of membranes in ciliated and deciliated Paramecium. J. Physiol. 296, 49-60. ( 10.1113/jphysiol.1979.sp012990) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ogura A, Takahashi K. 1976. Artificial deciliation causes loss of calcium-dependent responses in Paramecium. Nature 264, 170-172. ( 10.1038/264170a0) [DOI] [PubMed] [Google Scholar]
- 233.Eisenbach L, Ramanathan R, Nelson DL. 1983. Biochemical studies of the excitable membrane of Paramecium tetraurelia. IX. Antibodies against ciliary membrane proteins. J. Cell Biol. 97, 1412-1420. ( 10.1083/jcb.97.5.1412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sleigh MA, Barlow DI. 1982. How are different ciliary beat patterns produced? Symp. Soc. Exp. Biol. 35, 139-157. [PubMed] [Google Scholar]
- 235.Schmidt JA, Eckert R. 1976. Calcium couples flagellar reversal to photostimulation in Chlamydomonas reinhardtii. Nature 262, 713-715. ( 10.1038/262713a0) [DOI] [PubMed] [Google Scholar]
- 236.Lindemann CB, Kanous KS. 1989. Regulation of mammalian sperm motility. Arch. Androl. 23, 1-22. ( 10.3109/01485018908986783) [DOI] [PubMed] [Google Scholar]
- 237.Jansen V, Alvarez L, Balbach M, Strünker T, Hegemann P, Kaupp UB, Wachten D. 2015. Controlling fertilization and cAMP signaling in sperm by optogenetics. eLife 4, e05161. ( 10.7554/eLife.05161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kamiya R, Witman GB. 1984. Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J. Cell Biol. 98, 97-107. ( 10.1083/jcb.98.1.97) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Wan KY. 2018. Coordination of eukaryotic cilia and flagella. Essays Biochem. 62, 829-838. ( 10.1042/EBC20180029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Isogai N, Kamiya R, Yoshimura K. 2000. Dominance between the two flagella during phototactic turning in Chlamydomonas. Zool. Sci. 17, 1261-1266. ( 10.2108/zsj.17.1261) [DOI] [Google Scholar]
- 241.Schmitt R. 2002. Sinorhizobial chemotaxis: a departure from the enterobacterial paradigm. Microbiology 148, 627-631. ( 10.1099/00221287-148-3-627) [DOI] [PubMed] [Google Scholar]
- 242.Platzer J, Sterr W, Hausmann M, Schmitt R. 1997. Three genes of a motility operon and their role in flagellar rotary speed variation in Rhizobium meliloti. J. Bacteriol. 179, 6391-6399. ( 10.1128/JB.179.20.6391-6399.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Packer HL, Lawther H, Armitage JP. 1997. The Rhodobacter sphaeroides flagellar motor is a variable-speed rotor. FEBS Lett. 409, 37-40. ( 10.1016/S0014-5793(97)00473-0) [DOI] [PubMed] [Google Scholar]
- 244.Bornens M. 2018. Cell polarity: having and making sense of direction—on the evolutionary significance of the primary cilium/centrosome organ in Metazoa. Open Biol. 8, 180052. ( 10.1098/rsob.180052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wan KY, Goldstein RE. 2016. Coordinated beating of algal flagella is mediated by basal coupling. Proc. Natl Acad. Sci. USA 113, E2784-E2793. ( 10.1073/pnas.1518527113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Erra F, Iervasi A, Ricci N, Banchetti R. 2001. Movement of the cirri during the creeping of Euplotes crassus (Ciliata, Hypotrichida). Can. J. Zool. 79, 1353-1362. ( 10.1139/z01-030) [DOI] [Google Scholar]
- 247.Wan KY. 2020. Synchrony and symmetry-breaking in active flagellar coordination. Phil. Trans. R. Soc. B 375, 20190393. ( 10.1098/rstb.2019.0393) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gilpin W, Bull MS, Prakash M. 2020. The multiscale physics of cilia and flagella. Nat. Rev. Phys. 2, 74-88. ( 10.1038/s42254-019-0129-0) [DOI] [Google Scholar]
- 249.Jékely G. 2011. Origin and early evolution of neural circuits for the control of ciliary locomotion. Proc. R. Soc. B 278, 914-922. ( 10.1098/rspb.2010.2027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. 2011. Evolution: tracing the origins of centrioles, cilia, and flagella. J. Cell Biol. 194, 165-175. ( 10.1083/jcb.201011152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Battle C, Broedersz CP, Fakhri N, Geyer VF, Howard J, Schmidt CF, MacKintosh FC. 2016. Broken detailed balance at mesoscopic scales in active biological systems. Science 352, 604-607. ( 10.1126/science.aac8167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gnesotto FS, Mura F, Gladrow J, Broedersz CP. 2018. Broken detailed balance and non-equilibrium dynamics in living systems: a review. Rep. Prog. Phys. 81, 066601. ( 10.1088/1361-6633/aab3ed) [DOI] [PubMed] [Google Scholar]
- 253.Martin P, Hudspeth AJ, Julicher F. 2001. Comparison of a hair bundle's spontaneous oscillations with its response to mechanical stimulation reveals the underlying active process. Proc. Natl Acad. Sci. USA 98, 14 380-14 385. ( 10.1073/pnas.251530598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Brangwynne CP, Koenderink GH, MacKintosh FC, Weitz DA. 2008. Nonequilibrium microtubule fluctuations in a model cytoskeleton. Phys. Rev. Lett. 100, 118104. ( 10.1103/physrevlett.100.118104) [DOI] [PubMed] [Google Scholar]
- 255.Marshall WF. 2020. Pattern formation and complexity in single cells. Curr. Biol. 30, R544-R552. ( 10.1016/j.cub.2020.04.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Nomura M, Atsuji K, Hirose K, Shiba K, Yanase R, Nakayama T, Ishida K-I, Inaba K. 2019. Microtubule stabilizer reveals requirement of Ca-dependent conformational changes of microtubules for rapid coiling of haptonema in haptophyte algae. Biol. Open 8, bio036590. ( 10.1242/bio.036590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Katoh K, Kikuyama M. 1997. An all-or-nothing rise in cytosolic. J. Exp. Biol. 200, 35-40. [DOI] [PubMed] [Google Scholar]
- 258.Amos WB. 1971. Reversible mechanochemical cycle in the contraction of Vorticella. Nature 229, 127-128. ( 10.1038/229127a0) [DOI] [PubMed] [Google Scholar]
- 259.Tilney LG, Porter KR. 1965. Studies on microtubules in Heliozoa. I. The fine structure of Actinosphaerium nucleofilum (Barrett), with particular reference to the axial rod structure. Protoplasma 60, 317-344. ( 10.1007/BF01247886) [DOI] [PubMed] [Google Scholar]
- 260.Febvre-Chevalier C, Febvre J. 1992. Microtubule dissassembly in vivo: intercalary destabilization and breakdown of microtubules in the heliozoan Actinocoryne contractilis. J. Cell Biol. 118, 585-594. ( 10.1083/jcb.118.3.585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Balajthy A, Hajdu P, Panyi G, Varga Z. 2017. Sterol regulation of voltage-gated K channels. Curr. Top. Membr. 80, 255-292. ( 10.1016/bs.ctm.2017.05.006) [DOI] [PubMed] [Google Scholar]
- 262.Gould SB. 2018. Membranes and evolution. Curr. Biol. 28, R381-R385. ( 10.1016/j.cub.2018.01.086) [DOI] [PubMed] [Google Scholar]
- 263.Terasaki M, et al. 2013. Stacked endoplasmic reticulum sheets are connected by helicoidal membrane motifs. Cell 154, 285-296. ( 10.1016/j.cell.2013.06.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Padmaraj D, Pande R, Miller JH, Wosik J, Zagozdzon-Wosik W. 2014. Mitochondrial membrane studies using impedance spectroscopy with parallel pH monitoring. PLoS ONE 9, e101793. ( 10.1371/journal.pone.0101793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Roger AJ, Muñoz-Gómez SA, Kamikawa R. 2017. The origin and diversification of mitochondria. Curr. Biol. 27, R1177-R1192. ( 10.1016/j.cub.2017.09.015) [DOI] [PubMed] [Google Scholar]
- 266.Schäfer G, Engelhard M, Müller V. 1999. Bioenergetics of the Archaea. Microbiol. Mol. Biol. Rev. 63, 570-620. ( 10.1128/MMBR.63.3.570-620.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lewalter K, Müller V. 2006. Bioenergetics of archaea: ancient energy conserving mechanisms developed in the early history of life. Biochim. Biophys. Acta 1757, 437-445. ( 10.1016/j.bbabio.2006.04.027) [DOI] [PubMed] [Google Scholar]
- 268.Gogarten JP, et al. 1989. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc. Natl Acad. Sci. USA 86, 6661-6665. ( 10.1073/pnas.86.17.6661) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ray S, Kassan A, Busija AR, Rangamani P, Patel HH. 2016. The plasma membrane as a capacitor for energy and metabolism. Am. J. Physiol. Cell Physiol. 310, C181-C192. ( 10.1152/ajpcell.00087.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Everitt CT, Haydon DA. 1968. Electrical capacitance of a lipid membrane separating two aqueous phases. J. Theor. Biol. 18, 371-379. ( 10.1016/0022-5193(68)90084-2) [DOI] [PubMed] [Google Scholar]
- 271.Gentet LJ, Stuart GJ, Clements JD. 2000. Direct measurement of specific membrane capacitance in neurons. Biophys. J. 79, 314-320. ( 10.1016/S0006-3495(00)76293-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Locke EG, Bonilla M, Liang L, Takita Y, Cunningham KW. 2000. A homolog of voltage-gated Ca2+ channels stimulated by depletion of secretory Ca2+ in yeast. Mol. Cell. Biol. 20, 6686-6694. ( 10.1128/MCB.20.18.6686-6694.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Carraretto L, Teardo E, Checchetto V, Finazzi G, Uozumi N, Szabo I. 2016. Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: from molecular identification to function. Mol. Plant 9, 371-395. ( 10.1016/j.molp.2015.12.004) [DOI] [PubMed] [Google Scholar]
- 274.Heimburg T. 2012. The capacitance and electromechanical coupling of lipid membranes close to transitions: the effect of electrostriction. Biophys. J. 103, 918-929. ( 10.1016/j.bpj.2012.07.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pichler H, Gaigg B, Hrastnik C, Achleitner G, Kohlwein SD, Zellnig G, Perktold A, Daum G. 2001. A subfraction of the yeast endoplasmic reticulum associates with the plasma membrane and has a high capacity to synthesize lipids. Eur. J. Biochem. 268, 2351-2361. ( 10.1046/j.1432-1327.2001.02116.x) [DOI] [PubMed] [Google Scholar]
- 276.Holevinsky KO, Nelson DJ. 1998. Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys. J. 75, 2577-2586. ( 10.1016/S0006-3495(98)77703-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Hausmann K. 1978. Extrusive organelles in protists. Int. Rev. Cytol. 52, 197-276. ( 10.1016/S0074-7696(08)60757-3) [DOI] [PubMed] [Google Scholar]
- 278.Stelly N, Halpern S, Nicolas G, Fragu P, Adoutte A. 1995. Direct visualization of a vast cortical calcium compartment in Paramecium by secondary ion mass spectrometry (SIMS) microscopy: possible involvement in exocytosis. J. Cell Sci. 108, 1895-1909. [DOI] [PubMed] [Google Scholar]
- 279.Frey W, White JA, Price RO, Blackmore PF, Joshi RP, Nuccitelli R, Beebe SJ, Schoenbach KH, Kolb JF. 2006. Plasma membrane voltage changes during nanosecond pulsed electric field exposure. Biophys. J. 90, 3608-3615. ( 10.1529/biophysj.105.072777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Schoenbachk KH, Joshi RP, Kolb JF, Chen N, Stacey M, Buescher ES, Beebe SJ, Blackmon P. 2005. Ultrashort electrical pulses open a new gateway into biological cells. In Conf. Rec. 26th Int. Power Modulator Symp. 2004 and 2004 High-Voltage Workshop, San Francisco, CA, 2004, pp. 205-209. Piscataway, NJ: IEEE. ( 10.1109/modsym.2004.1433545) [DOI] [Google Scholar]
- 281.Rall W. 1959. Branching dendritic trees and motoneuron membrane resistivity. Exp. Neurol. 1, 491-527. ( 10.1016/0014-4886(59)90046-9) [DOI] [PubMed] [Google Scholar]
- 282.Jaeger D, De Schutter E, Bower JM.. 1997. The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study. J. Neurosci. 17, 91-106. ( 10.1523/JNEUROSCI.17-01-00091.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lacomble S, Vaughan S, Gadelha C, Morphew MK, Shaw MK, McIntosh JR, Gull K. 2009. Three-dimensional cellular architecture of the flagellar pocket and associated cytoskeleton in trypanosomes revealed by electron microscope tomography. J. Cell Sci. 122, 1081-1090. ( 10.1242/jcs.045740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kugrens P, Lee RE, Corliss JO. 1994. Ultrastructure, biogenesis, and functions of extrusive organelles in selected non-ciliate protists. Protoplasma 181, 164-190. ( 10.1007/bf01666394) [DOI] [Google Scholar]
- 285.Cavalier-Smith T. 2014. The neomuran revolution and phagotrophic origin of eukaryotes and cilia in the light of intracellular coevolution and a revised tree of life. Cold Spring Harb. Perspect. Biol. 6, a016006. ( 10.1101/cshperspect.a016006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Dacks JB, Field MC. 2007. Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J. Cell Sci. 120, 2977-2985. ( 10.1242/jcs.013250) [DOI] [PubMed] [Google Scholar]
- 287.Zaremba-Niedzwiedzka K, et al. 2017. Asgard archaea illuminate the origin of eukaryotic cellular complexity. Nature 541, 353-358. ( 10.1038/nature21031) [DOI] [PubMed] [Google Scholar]
- 288.Neveu E, Khalifeh D, Salamin N, Fasshauer D. 2020. Prototypic SNARE proteins are encoded in the genomes of Heimdallarchaeota, potentially bridging the gap between the prokaryotes and eukaryotes. Curr. Biol. 30, 2468-2480.e5. ( 10.1016/j.cub.2020.04.060) [DOI] [PubMed] [Google Scholar]
- 289.Elias M, Brighouse A, Gabernet-Castello C, Field MC, Dacks JB. 2012. Sculpting the endomembrane system in deep time: high resolution phylogenetics of Rab GTPases. J. Cell Sci. 125, 2500-2508. ( 10.1242/jcs.101378) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Simons K, Sampaio JL. 2011. Membrane organization and lipid rafts. Cold Spring Harb. Perspect. Biol. 3, a004697. ( 10.1101/cshperspect.a004697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rasmussen B, Fletcher IR, Brocks JJ, Kilburn MR. 2008. Reassessing the first appearance of eukaryotes and cyanobacteria. Nature 455, 1101-1104. ( 10.1038/nature07381) [DOI] [PubMed] [Google Scholar]
- 292.Klose C, Ejsing CS, García-Sáez AJ, Kaiser H-J, Sampaio JL, Surma MA, Shevchenko A, Schwille P, Simons K. 2010. Yeast lipids can phase-separate into micrometer-scale membrane domains. J. Biol. Chem. 285, 30 224-30 232. ( 10.1074/jbc.M110.123554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Honerkamp-Smith AR, Veatch SL, Keller SL. 2009. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta Biomembranes 1788, 53-63. ( 10.1016/j.bbamem.2008.09.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. 2010. Cholesterol and ion channels. Subcell. Biochem. 51, 509-549. ( 10.1007/978-90-481-8622-8_19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Heimburg T, Jackson AD.. 2005. On soliton propagation in biomembranes and nerves. Proc. Natl Acad. Sci. USA 102, 9790-9795. ( 10.1073/pnas.0503823102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Prinz WA, Hinshaw JE. 2009. Membrane-bending proteins. Crit. Rev. Biochem. Mol. Biol. 44, 278-291. ( 10.1080/10409230903183472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Corda D, Pasternak C, Shinitzky M. 1982. Increase in lipid microviscosity of unilamellar vesicles upon the creation of transmembrane potential. J. Membr. Biol. 65, 235-242. ( 10.1007/BF01869967) [DOI] [PubMed] [Google Scholar]
- 298.Bolotina V, Omelyanenko V, Heyes B, Ryan U, Bregestovski P. 1989. Variations of membrane cholesterol alter the kinetics of Ca2+-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 415, 262-268. ( 10.1007/BF00370875) [DOI] [PubMed] [Google Scholar]
- 299.Goto M, Ohki K, Nozawa Y. 1982. Evidence for a correlation between swimming velocity and membrane fluidity of Tetrahymena cells. Biochim. Biophys. Acta 693, 335-340. ( 10.1016/0005-2736(82)90440-0) [DOI] [PubMed] [Google Scholar]
- 300.Lee AG. 1976. Model for action of local anaesthetics. Nature 262, 545-548. ( 10.1038/262545a0) [DOI] [PubMed] [Google Scholar]
- 301.Kung C, Martinac B, Sukharev S. 2010. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313-329. ( 10.1146/annurev.micro.112408.134106) [DOI] [PubMed] [Google Scholar]
- 302.Keren K. 2011. Cell motility: the integrating role of the plasma membrane. Eur. Biophys. J. 40, 1013-1027. ( 10.1007/s00249-011-0741-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zimmermann U, Beckers F. 1978. Generation of action potentials in Chara corallina by turgor pressure changes. Planta 138, 173-179. ( 10.1007/BF00391175) [DOI] [PubMed] [Google Scholar]
- 304.Gudi S, Nolan JP, Frangos JA. 1998. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc. Natl Acad. Sci. USA 95, 2515-2519. ( 10.1073/pnas.95.5.2515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Haidekker MA, L'Heureux N, Frangos JA. 2000. Fluid shear stress increases membrane fluidity in endothelial cells: a study with DCVJ fluorescence. Am. J. Physiol. Heart Circ. Physiol. 278, H1401-H1406. ( 10.1152/ajpheart.2000.278.4.H1401) [DOI] [PubMed] [Google Scholar]
- 306.Mallipattu SK, Haidekker MA, Von Dassow P, Latz MI, Frangos JA.. 2002. Evidence for shear-induced increase in membrane fluidity in the dinoflagellate Lingulodinium polyedrum. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 409-416. ( 10.1007/s00359-002-0315-9) [DOI] [PubMed] [Google Scholar]
- 307.Jalaal M, Schramma N, Dode A, de Maleprade H, Raufaste C, Goldstein RE.. 2020. Stress-induced dinoflagellate bioluminescence at the single cell level. Phys. Rev. Lett. 125, 028102. ( 10.1103/PhysRevLett.125.028102) [DOI] [PubMed] [Google Scholar]
- 308.Hudspeth AJ, Choe Y, Mehta AD, Martin P. 2000. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc. Natl Acad. Sci. USA 97, 11 765-11 772. ( 10.1073/pnas.97.22.11765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Patteson AE, Gopinath A, Goulian M, Arratia PE. 2015. Running and tumbling with E. coli in polymeric solutions. Scient. Rep. 5, 15761. ( 10.1038/srep15761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Polin M, Tuval I, Drescher K, Gollub JP, Goldstein RE.. 2009. Chlamydomonas swims with two ‘gears’ in a eukaryotic version of run-and-tumble locomotion. Science 325, 487-490. ( 10.1126/science.1172667) [DOI] [PubMed] [Google Scholar]
- 311.Kawakubo T, Tsuchiya Y. 1981. Diffusion coefficient of Paramecium as a function of temperature1. J. Protozool. 28, 342-344. ( 10.1111/j.1550-7408.1981.tb02862.x) [DOI] [Google Scholar]
- 312.Lewis AH, Grandl J. 2015. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4, e12088. ( 10.7554/eLife.12088) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Cheung ELM, Corey DP. 2006. Ca2+ changes the force sensitivity of the hair-cell transduction channel. In Auditory mechanisms: processes and models (eds Nuttall AL, Ren T, Gillespie P, Grosh K, de Boer E), pp. 277-285. Singapore: World Scientific. ( 10.1142/9789812773456_0047) [DOI] [Google Scholar]
- 314.Jékely G, Arendt D. 2006. Evolution of intraflagellar transport from coated vesicles and autogenous origin of the eukaryotic cilium. Bioessays 28, 191-198. ( 10.1002/bies.20369) [DOI] [PubMed] [Google Scholar]
- 315.Bowers B, Olszewski TE. 1972. Pinocytosis in Acanthamoeba castellanii. Kinetics and morphology. J. Cell Biol. 53, 681-694. ( 10.1083/jcb.53.3.681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Benwitz G. 1984. Die Entladung der Haptocysten von Ephelota gemmipara (Suctoria, Ciliata) [Mode of discharge of haptocysts in Ephelota gemmipara (Suctoria, Ciliata)] . Z. Naturforsch. C 39, 812-817. [In German with English abstract.] ( 10.1515/znc-1984-7-821) [DOI] [Google Scholar]
- 317.Derelle E, et al. 2006. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc. Natl Acad. Sci. USA 103, 11 647-11 652. ( 10.1073/pnas.0604795103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Razin S. 1992. Peculiar properties of mycoplasmas: the smallest self-replicating prokaryotes. FEMS Microbiol. Lett. 100, 423-431. ( 10.1111/j.1574-6968.1992.tb05735.x) [DOI] [PubMed] [Google Scholar]
- 319.Purcell EM. 1976. Life at low Reynolds number. AIP Conf. Proc. 28, 49-64. ( 10.1063/1.30370) [DOI] [Google Scholar]
- 320.Dusenbery DB. 1997. Minimum size limit for useful locomotion by free-swimming microbes. Proc. Natl Acad. Sci. USA 94, 10 949-10 954. ( 10.1073/pnas.94.20.10949) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Marin B, Melkonian M. 2010. Molecular phylogeny and classification of the Mamiellophyceae class. nov. (Chlorophyta) based on sequence comparisons of the nuclear- and plastid-encoded rRNA operons. Protist 161, 304-336. ( 10.1016/j.protis.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 322.Taylor GI. 1922. Diffusion by continuous movements. Proc. Lond. Math. Soc. s2-20, 196-212. ( 10.1112/plms/s2-20.1.196) [DOI] [Google Scholar]
- 323.Berg HC, Purcell EM. 1977. Physics of chemoreception. Biophys. J. 20, 193-219. ( 10.1016/s0006-3495(77)85544-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Higdon JJL. 1979. The generation of feeding currents by flagellar motions. J. Fluid Mech. 94, 305-330. ( 10.1017/s002211207900104x) [DOI] [Google Scholar]
- 325.Wan KY, Hürlimann SK, Fenix AM, McGillivary RM, Makushok T, Burns E, Sheung JY, Marshall WF. 2020. Reorganization of complex ciliary flows around regenerating Stentor coeruleus. Phil. Trans. R. Soc. B 375, 20190167. ( 10.1098/rstb.2019.0167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Claussen M, Schmidt S. 2017. Locomotion pattern and pace of free-living amoebae - a microscopic study. In Microscopy and imaging science: practical approaches to applied research and education (ed. Mendez-Vilas A), pp. 223-230. Badajoz, Spain: Formatex Research Center. [Google Scholar]
- 327.Lisicki M, Velho Rodrigues MF, Goldstein RE, Lauga E. 2019. Swimming eukaryotic microorganisms exhibit a universal speed distribution. eLife 8, e44907. ( 10.7554/eLife.44907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Johansen JE, Pinhassi J, Blackburn N, Zweifel UL, Hagström Å. 2002. Variability in motility characteristics among marine bacteria. Aquat. Microbiol. Ecol. 28, 229-237. ( 10.3354/ame028229) [DOI] [Google Scholar]
- 329.Childress S, Koehl MAR, Miksis M. 1987. Scanning currents in Stokes flow and the efficient feeding of small organisms. J. Fluid Mech. 177, 407-436. ( 10.1017/s0022112087001022) [DOI] [Google Scholar]
- 330.Bente K, Mohammadinejad S, Charsooghi MA, Bachmann F, Codutti A, Lefèvre CT, Klumpp S, Faivre D. 2020. High-speed motility originates from cooperatively pushing and pulling flagella bundles in bilophotrichous bacteria. eLife 9, e47551. ( 10.7554/eLife.47551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Winklhofer M, Abraçado LG, Davila AF, Keim CN, de Barros HGPL. 2007. Magnetic optimization in a multicellular magnetotactic organism. Biophys. J. 92, 661-670. ( 10.1529/biophysj.106.093823) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Bialek W. 1987. Physical limits to sensation and perception. Annu. Rev. Biophys. Biophys. Chem. 16, 455-478. ( 10.1146/annurev.bb.16.060187.002323) [DOI] [PubMed] [Google Scholar]
- 333.Bicknell BA, Dayan P, Goodhill GJ. 2015. The limits of chemosensation vary across dimensions. Nat. Commun. 6, 7468. ( 10.1038/ncomms8468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Endres RG, Wingreen NS. 2008. Accuracy of direct gradient sensing by single cells. Proc. Natl Acad. Sci. USA 105, 15 749-15 754. ( 10.1073/pnas.0804688105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Rieke F, Baylor DA. 1998. Single-photon detection by rod cells of the retina. Rev. Mod. Phys. 70, 1027-1036. ( 10.1103/revmodphys.70.1027) [DOI] [Google Scholar]
- 336.Hudspeth AJ. 1983. The hair cells of the inner ear. Scient. Am. 248, 54-64. ( 10.1038/scientificamerican0183-54) [DOI] [PubMed] [Google Scholar]
- 337.Tan RZ, Chiam K-H. 2018. A computational model for how cells choose temporal or spatial sensing during chemotaxis. PLoS Comput. Biol. 14, e1005966. ( 10.1371/journal.pcbi.1005966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Dusenbery DB. 1998. Spatial sensing of stimulus gradients can be superior to temporal sensing for free-swimming bacteria. Biophys. J. 74, 2272-2277. ( 10.1016/S0006-3495(98)77936-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Cortese D, Wan KY. 2020. Control of helical navigation by three-dimensional flagellar beating. BioRxiv, 2020.09.27.315606. ( 10.1101/2020.09.27.315606) [DOI]
- 340.Kromer JA, Märcker S, Lange S, Baier C, Friedrich BM. 2018. Decision making improves sperm chemotaxis in the presence of noise. PLoS Comput. Biol. 14, e1006109. ( 10.1371/journal.pcbi.1006109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Friedrich BM, Jülicher F. 2009. Steering chiral swimmers along noisy helical paths. Phys. Rev. Lett. 103, 068102. ( 10.1103/PhysRevLett.103.068102) [DOI] [PubMed] [Google Scholar]
- 342.Ueki N, et al. 2016. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA 113, 5299-5304. ( 10.1073/pnas.1525538113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Miura K, Siegert F.. 2000. Light affects cAMP signaling and cell movement activity in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 97, 2111-2116. ( 10.1073/pnas.040554497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Francis DW. 1964. Some studies on phototaxis of Dictyostelium. J. Cell. Comp. Physiol. 64, 131-138. ( 10.1002/jcp.1030640113) [DOI] [PubMed] [Google Scholar]
- 345.Nilsson D-E, Colley NJ. 2016. Comparative vision: can bacteria really see? Curr. Biol. 26, R369-R371. ( 10.1016/j.cub.2016.03.025) [DOI] [PubMed] [Google Scholar]
- 346.Tweedy L, Thomason PA, Paschke PI, Martin K, Machesky LM, Zagnoni M, Insall RH. 2020. Seeing around corners: cells solve mazes and respond at a distance using attractant breakdown. Science 369, eaay9792. ( 10.1126/science.aay9792) [DOI] [PubMed] [Google Scholar]
- 347.Segall JE, Block SM, Berg HC.. 1986. Temporal comparisons in bacterial chemotaxis. Proc. Natl Acad. Sci. USA 83, 8987-8991. ( 10.1073/pnas.83.23.8987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Fancher S, Mugler A. 2017. Fundamental limits to collective concentration sensing in cell populations. Phys. Rev. Lett. 118, 078101. ( 10.1103/physrevlett.118.078101) [DOI] [PubMed] [Google Scholar]
- 349.Mehta P, Goyal S, Long T, Bassler BL, Wingreen NS. 2009. Information processing and signal integration in bacterial quorum sensing. Mol. Syst. Biol. 5, 325. ( 10.1038/msb.2009.79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.London M, Häusser M. 2005. Dendritic computation. Annu. Rev. Neurosci. 28, 503-532. ( 10.1146/annurev.neuro.28.061604.135703) [DOI] [PubMed] [Google Scholar]
- 351.Cook ND, Carvalho GB, Damasio A. 2014. From membrane excitability to metazoan psychology. Trends Neurosci. 37, 698-705. ( 10.1016/j.tins.2014.07.011) [DOI] [PubMed] [Google Scholar]
- 352.Mills DR, Peterson RL, Spiegelman S. 1967. An extracellular Darwinian experiment with a self-duplicating nucleic acid molecule. Proc. Natl Acad. Sci. USA 58, 217-224. ( 10.1073/pnas.58.1.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collated from published sources were used to generate figure 3c. These data are uploaded as electronic supplementary material.