Abstract
Background
Eleven criteria correlating electrocardiogram (ECG) findings with reduced left ventricular ejection fraction (LVEF) have been previously published. These have not been compared head‐to‐head in a single study. We studied their value as a screening test to identify patients with reduced LVEF estimated by cardiac magnetic resonance (CMR) imaging.
Methods
ECGs and CMR from 548 patients (age 61 + 11 years, 79% male) with previous myocardial infarction (MI), from the DETERMINE and PRE‐DETERMINE studies, were analyzed. Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each criterion for identifying patients with LVEF ≤ 30% and ≤ 40% were studied. A useful screening test should have high sensitivity and NPV.
Results
Mean LVEF was 40% (SD = 11%); 264 patients (48.2%) had LVEF ≤ 40%, and 96 patients (17.5%) had LVEF ≤ 30%. Six of 11 criteria were associated with a significant lower LVEF, but had poor sensitivity to identify LVEF ≤ 30% (range 2.1%–55.2%) or LVEF ≤ 40% (1.1%–51.1%); NPVs were good for LVEF ≤ 30% (range 82.8%–85.9%) but not for LVEF ≤ 40% (range 52.1%–60.6%). Goldberger's third criterion (RV4/SV4 < 1) and combinations of maximal QRS duration > 124 ms + either Goldberger's third criterion or Goldberger's first criterion (SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV) had high specificity (95.4%–100%) for LVEF ≤ 40%, although seen in only 48 (8.8%) patients; predictive values were similar on subgroup analysis.
Conclusions
None of the ECG criteria qualified as a good screening test. Three criteria had high specificity for LVEF ≤ 40%, although seen in < 9% of patients. Whether other ECG criteria can better identify LV dysfunction remains to be determined.
Keywords: cardiac magnetic resonance imaging, coronary artery disease, electrocardiology, left ventricular ejection fraction, myocardial infarction
1. INTRODUCTION
Ischemic heart disease with myocardial infarction (MI) is a common cause of left ventricular (LV) failure. Loss of ventricular muscle due to MI results in LV systolic dysfunction, decrease in LV contractility, ventricular remodeling, and heart failure. In clinical practice, the most common measure of LV function is the left ventricular ejection fraction (LVEF) (Luemns et al., 2015). LVEF has limitations as a true measure of contractility as it is also influenced by ventricular afterload and preload. Nevertheless, it is commonly used in clinical practice because it is easy to conceptualize and can be measured non‐invasively by trans‐thoracic echocardiography (TTE) (Borlaug & Kass, 2011). Cardiac magnetic resonance (CMR) imaging, though not as widely available as TTE, has now become the gold standard for measurement of ventricular volumes and ejection fraction, because of its ability to deal with three‐dimensional structures without relying on geometric assumptions (de Haan et al., 2014).
In clinical cardiology, the electrocardiogram (ECG) remains the first‐line diagnostic test for evaluation of patients with suspected heart disease due to its ease of use, low cost, and near‐universal availability. A few previous studies have attempted to identify ECG findings that correlate with reduced EF (Bounous et al., 1988; Chinitz et al., 2008; Cincin et al., 2012; Goldberger, 1982; Momiyama et al., 1994; Palmeri et al., 1982). In one such study, Goldberger described a triad of ECG findings in patients with symptomatic dilated idiopathic cardiomyopathy and found that their presence correlated well with reduced EF on TTE (Goldberger, 1982). Other authors have described other ECG criteria that have correlated with a reduced LVEF in patients with prior MI; in most of these studies, EF was assessed using TTE (Bounous et al., 1988; Chinitz et al., 2008; Cincin et al., 2012; Momiyama et al., 1994; Palmeri et al., 1982). However, most of these criteria have not been externally validated head‐to‐head in a single study in patients with previous myocardial infarction.
ECG changes after myocardial infarction include loss of R‐wave amplitude and appearance of QS wave which correlate with the location and size of the infarct. The modified Selvester QRS score has been found to correlate reasonably well with infarct size on cardiac MR imaging (Geerse et al., 2009). As infarct size is an important determinant of decrease in LVEF following myocardial infarction, it would be reasonable to correlate ECG changes with reduction LVEF. Although echocardiography is most commonly used to estimate LVEF, the present study was performed to assess the diagnostic value of the previously described ECG criteria to identify reduced LV function in patients with previous MI from two large studies (the DETERMINE study and the PRE‐DETERMINE study) in which CMR imaging was used to diagnose previous MI and estimate LVEF.
2. METHODS
2.1. Study population
Patients with a clinical history of myocardial infarction were identified from the Defibrillators to Reduce Risk by Magnetic Resonance Imaging (DETERMINE) Trial and Registry and the PRE‐DETERMINE study. The DETERMINE Trial (ClinicalTrials.gov ID NCT00487279) was a multicenter randomized trial which sought to test the hypothesis that implantable cardioverter‐defibrillator (ICD) therapy would improve survival over optimal medical therapy in patients with coronary artery disease (CAD), with LVEF > 35% and infarct mass > 10% as estimated by CMR (Kadish et al., 2009). Patients screened for the DETERMINE study but who had LVEF ≤ 35%, infarct mass ≤ 10%, and/or an ICD already implanted were enrolled in the DETERMINE Registry. All patients were required to undergo CMR imaging to assess LVEF. Other patients screened and otherwise ineligible or unwilling to participate in the randomized trial were offered enrollment in either the DETERMINE Registry or the PRE‐DETERMINE Study.
The PRE‐DETERMINE Study (ClinicalTrials.gov ID NCT01114269) is a prospective, multicenter study of 5,763 patients with CAD, all with documentation of prior MI and/or mild to moderate LV dysfunction (LVEF 35%–50%), to determine the value of biomarkers of inflammation, membrane stabilization, fibrosis, and myocardial dysfunction in predicting risk of ventricular arrhythmic events. CMR imaging was not required as part of the study protocol (Clinicaltrials.gov., 2018).
Of 5,993 patients enrolled in the above studies, cine and late gadolinium enhanced CMR images were collected in 920 patients from 64 field sites across the United States. Patients with poor CMR or ECG image quality that precluded quantitative analysis or had an interval of > 1 year between CMR and ECG were excluded. Patients with left bundle branch block (LBBB) were also excluded as the changes in the QRS morphology due to bundle branch block would have confounded the occurrence of the previously described ECG criteria associated with reduced LVEF.
2.2. Cardiac magnetic resonance
All patients included in this study underwent cine and late gadolinium enhanced (LGE) cardiac magnetic resonance (CMR) imaging, which were analyzed by a CMR core laboratory (Northwestern University Cardiovascular Imaging Core Laboratory). CMR studies were excluded if the short axis stack did not include the entire LV from the mitral valve plane to the apex, or if image artifact (such as wrap, poor respiratory or ECG gating, and/or improper inversion time selection) precluded quantitative analysis. Quantitative analysis was performed using Qmass software (Medis, Leiden, the Netherlands). Endocardial and epicardial borders were manually planimetered on cine short axis images at systole and diastole for calculation of LVEF.
2.3. 12‐lead electrocardiogram
All 12‐lead ECGs in this study were analyzed at a core ECG laboratory (IQVIA, Connected Devices; formerly known as Quintiles Cardiac Safety Services, Mumbai, India). Paper ECGs were scanned (Fujitsu Scanner model Fi‐5120C, Tokyo, Japan) to a PNG file format at a resolution of 300 dots per inch (dpi). The scanned ECGs were then analyzed on‐screen using a mouse‐driven ECG measurement software tool (Cardio Calipers version 3.3, Iconico Inc, New York, NY) by a team of trained readers (Panicker et al., 2009). The ECG analysis included amplitude and duration measurements of the individual components of the P wave, QRS complex, ST segment, and T wave in each of the 12 leads, as well as overall morphological interpretation of the ECG waveform. ECGs were excluded if measurements were not possible in two or more leads due to noise or artifact.
2.4. ECG criteria for detection of reduced left ventricular ejection fraction (LVEF)
A literature search was performed using PubMed, Google Scholar, and Scopus to identify prior studies, which used or defined ECG criteria for estimation of LVEF or for detection of a reduced LVEF. Based on the literature search, we found 11 ECG criteria that had been found to correlate with LVEF (Bounous et al., 1988; Chinitz et al., 2008; Cincin et al., 2012; Goldberger, 1982; Momiyama et al., 1994; Palmeri et al., 1982). Of these, there were 7 distinct ECG criteria, while the other 4 were combinations of these 7 distinct criteria (Table 1). We looked for the presence or absence of each of the 11 ECG criteria in each ECG and correlated these with LVEF estimated by CMR.
Table 1.
ECG Criteria, method of assessing left ventricular ejection fraction (LVEF), and patient characteristics from previous studies correlating ECG findings with LV function4‐9
| Number | ECG criteria | ECG descriptor | Author | Method for assessing LV function | Patients studied (MI or No MI) |
|---|---|---|---|---|---|
| 1 | SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV (Goldberger's first criterion) | Prominent precordial QRS amplitudes | Goldberger4 | Echocardiography (LVEF) | Both |
| 2 | Mean QRS amplitude in each of the limb leads ≤ 0.8mv (Goldberger's second criterion) | Low voltage in limb leads | |||
| 3 | RV4/SV4 < 1 (Goldberger's third criterion) | Poor R‐wave progression | |||
| 4 | Goldberger's triad | Combination of all 3 Goldberger's criteria | |||
| 5 | Maximal QRS duration ≥ 124 ms | QRS duration | Cincin et al.5 | Echocardiography (LVEF) | No MI |
| 6 | Maximal QRS duration + Goldberger's first criterion | QRS duration and prominent precordial QRS amplitudes | |||
| 7 | Maximal QRS duration + Goldberger's third criterion | QRS duration and low voltage in limb leads | |||
| 8 | Maximal QRS duration + Goldberger's first and third criteria | QRS duration, prominent precordial QRS amplitudes, and poor R‐wave progression | |||
| 9 | All voltage ratios of RV6/RI, RII, RIII ≥ 3 | Prominent QRS voltage in precordial leads and low QRS voltage in limb leads | Momiyama et al6 | Echocardiography | No MI (Dilated Cardiomyopathy) |
| 10 | Simplified Selvester QRS score ≥ 10 for LVEF ≤ 30 and Simplified Selvester QRS score ≥ 7 for LVEF ≤ 40 | Scores based on Q wave duration and R/Q ratio in limb leads I,II, aVL, and aVF and Q wave duration and R/Q or R/S ratio in precordial leads | Bounous EP et al7 and Palmeri et al8 | Multigated radionuclide angiography | Only MI |
| 11 | QRS voltage < 5 mm in all limb leads and > 10 mm in at least 2 contiguous precordial leads | Low voltage in limb leads without low voltage in precordial leads | Chinitz et al9 | Echocardiography (LVEF) | Both |
Abbreviation: MI, Myocardial infarction.
2.5. Statistical analysis
Baseline characteristics of patients such as age, gender, history of hypertension, diabetes, smoking, impaired renal function, and medications used were considered. Continuous numeric data were summarized as mean ± standard deviation (SD) and categorical data by numbers and percentages. The LVEF in patients meeting each of the 11 ECG criteria were compared with those not meeting the ECG criterion using unpaired t test. The diagnostic utility of each of the 11 ECG criteria to identify patients with reduced LVEF as defined by 2 cutoff values (LVEF ≤ 30% and LVEF ≤ 40%) was assessed. Sensitivity, specificity, and positive and negative predictive values were calculated to determine if any of these ECG criteria could act as a screening test in identifying patients with LVEF ≤ 30% or LVEF ≤ 40%. To study whether any of the ECG criteria performed better in specific subgroups of patients based on baseline characteristics, subgroup analysis was performed based on gender (females and males), age (≥60 years and < 60 years), diabetes (present, absent), hypertension (present, absent), and number of prior episodes of myocardial infarction (prior MI = 1 and > 1).
As each patient's ECG could meet more than one criterion, the total number of criteria that were met in each ECG was considered and its correlation with LVEF was assessed using Spearman's rank correlation. The proportion of patients with low LVEF (using cutoffs of 30% and 40%) with increasing number of ECG criteria was also evaluated using the chi‐square test for trend as a sensitivity analysis. All statistical analyses were performed using SAS software (version 9.4, SAS Institute Inc., Cary, NC, USA).
3. RESULTS
3.1. Study population
A total of 843 patients were enrolled from 54 sites in the DETERMINE study and registry. After exclusion of 61 patients due to insufficient CMR image coverage/quality and 4 patients who withdrew from the study, 754 CMR studies from the DETERMINE study were eligible for inclusion in this analysis. Of the 5,764 patients enrolled in the PRE‐DETERMINE study, 77 patients from 19 sites had clinically ordered CMR studies. After exclusion of 15 MRI studies for insufficient image coverage/quality, 62 CMR studies from the PRE‐DETERMINE study were eligible for inclusion in this analysis. An additional 149 patients were excluded for the following reasons: insufficient ECG data for computation of criteria (n = 113), no prior history of MI (n = 62), >1 year between ECG and CMR (n = 46), presence of LBBB (n = 26), and missing LVEF values (n = 15). Thus, 548 patients (age 61 ± 11 years, 78% men, 22% women) that included 510 from the DETERMINE study and 38 from the PRE‐DETERMINE study were finally considered for this analysis. Patient demographics are shown in Table 2. The LVEF ranged from 9.5% to 69.1%, with a mean LVEF of 40.3% (SD 11%) (Figure 1). Of the 548 patients, 264 (48.2%) had LVEF ≤ 40% and 96 of these had LVEF ≤ 30% (36.4% of patients with LVEF ≤ 40% and 17.5% of all patients).
Table 2.
Descriptive baseline characteristics of patient population (n = 548)
| Baseline characteristics | Number of patients (%) (n = 548) |
|---|---|
| Age a | 61 ± 11 a |
| Gender | |
| Male | 430 (78.5%) |
| Female | 118 (21.5%) |
| Hypertension | 397 (72.5%) |
| Diabetes Mellitus | 177 (32.3%) |
| Smoking history | |
| Current | 75 (13.7%) |
| Former | 306 (55.8%) |
| Never | 167 (30.5%) |
| Impaired renal function | |
| Serum creatinine ≥ 2.0 mg/dl | 4 (0.7%) |
| Location of myocardial infarction on CMR | |
| Anterior | 263 (48%) |
| Lateral | 96 (17.5%) |
| Inferior | 170 (31.0%) |
| None | 19 (3.5%) |
| Medication | |
| ACE Inhibitors or Angiotensin II receptor blockers | 455 (83%) |
| ACE Inhibitors/Angiotensin II receptor blockers/Aldosterone inhibitors | 461 (84.1%) |
| Antiplatelets (clopidogrel, orasugrel, ticagrelor) | 38 (6.9%) |
| β‐blockers | 502 (91.6%) |
| Diuretics | 227 (41.4%) |
| Statins | 499 (91.1%) |
Values are mean ± SD.
Figure 1.
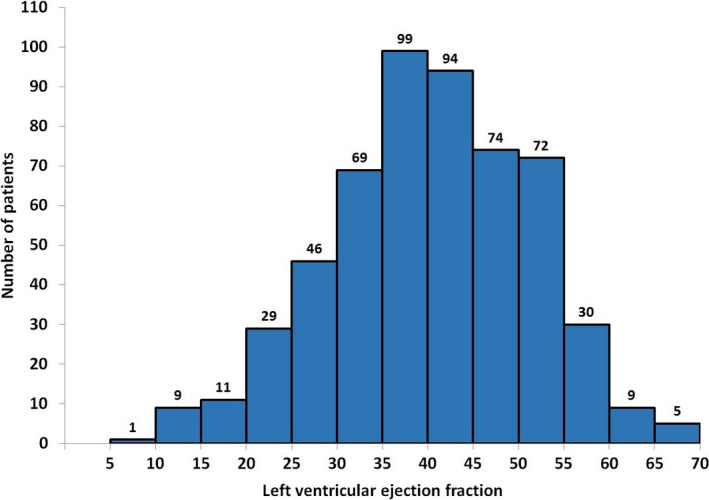
Distribution of left ventricular ejection fraction estimated from cardiac magnetic resonance (CMR) imaging in 548 patients included for the evaluation of ECG criteria
3.2. ECG criteria and left ventricular ejection fraction
The LVEF in patients meeting an ECG criterion was significantly lower than LVEF in those not meeting the ECG criterion for 6 of the 11 ECG criteria studied (Table 3). For 3 criteria, the difference in LVEF did not reach statistical significance and for two criteria there were ≤ 3 patients with ECGs meeting the criteria and hence p‐values could not be calculated. The mean LVEF ranged from 27% to 38.1% in patients meeting one of these 6 ECG criteria compared to a mean LVEF of 40.4% to 42.5% in patients not meeting the ECG criterion.
Table 3.
Ejection fraction in patients meeting an ECG criterion versus patients not meeting the ECG criterion (N = 548)
| ECG Criteria | Patients with ECG meeting criteria | Patients with ECG not meeting criteria | p value for difference in EF | |||
|---|---|---|---|---|---|---|
| N (%) | Ejection fraction (mean ± SD) | N (%) | Ejection fraction (mean ± SD) | |||
| 1 | Goldberger's first criterion—SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV | 14 (2.6%) | 30.5 ± 11.7 | 531 (97.4%) | 40.5 ± 10.9 | 0.0007 |
| 2 | Goldberger's second criterion—Total QRS amplitude in each of the limb leads ≤ 0.8 mv | 154(28.1%) | 40.6 ± 10.6 | 394(71.9%) | 40.2 ± 11.2 | 0.7440 |
| 3 | Goldberger's third criterion—RV4/SV4 < 1 | 231 (43.1%) | 38.1 ± 11.0 | 305 (56.9%) | 41.9 ± 10.8 | <0.0001 |
| 4 | Goldberger's triad (all 3 criteria present) | 0 | – | 548 (100.0%) | – | – |
| 5 | Maximal QRS duration ≥ 124 ms5 | 115 (21.0%) | 36.4 ± 10.8 | 433 (79.0%) | 41.3 ± 10.9 | <0.0001 |
| 6 | Maximal QRS duration + Goldberger's first criterion5 | 3 (0.6%) | 27.0 ± 4.3 | 545 (99.5%) | 40.4 ± 11.0 | 0.0365 |
| 7 | Maximal QRS duration + Goldberger's third criterion5 | 48 (8.8%) | 34.4 ± 11.1 | 500 (91.2%) | 40.9 ± 10.9 | 0.0001 |
| 8 | Maximal QRS duration + Goldberger's first and third criteria5 | 1 (0.2%) | 27.0 | 547 (99.8%) | 40.3 ± 11.0 | – |
| 9 | All voltage ratios of RV6/RI, RII, RIII ≥ 36 | 4 (0.8%) | 30.9 ± 20.3 | 526 (99.3%) | 40.5 ± 10.9 | .4170 |
| 10 | Simplified Selvester QRS Score of ≥ 78 | 221 (40.3%) | 37.0 ± 10.2 | 327 (59.7%) | 42.5 ± 11.0 | <.0001 |
| Simplified Selvester QRS Score of ≥ 108 | 84 (15.3) | 35.5 ± 10.0 | 464(84.7%) | 41.2 ± 11.0 | <.0001 | |
| 11 | QRS voltage less than 5 mm in all limb leads and greater than 10 mm in at least 2 contiguous precordial Leads9 | 5 (0.9%) | 38.7 ± 10.8 | 543 (99.1%) | 40.3 ± 11.0 | .7474 |
On comparison of proportion of patients with ejection fraction ≤ 30% (n = 96) and patients with ejection fraction > 30% (n = 452) for each of these criteria, the difference was statistically significant for 5 of these 6 ECG criteria (Table 4a). The proportion of patients with LVEF ≤ 40% was also significantly higher when one of the same 5 criteria was present in the ECG. (Table 4b).
Table 4.
Comparison of number of patients with EF cutoff (a) ≤ 30% (b) ≤ 40% in patients meeting an ECG criterion and patients not meeting the ECG criterion (N = 548)
| ECG criteria | Patients with ECG meeting criteria | Patients with ECG meeting criteria | Patients with ECG not meeting criteria | Patients with ECG not meeting criteria | p value | |||
|---|---|---|---|---|---|---|---|---|
| N (%) | Ejection fraction ≤ 30% | Ejection fraction > 30% | N (%) | Ejection fraction ≤ 30% | Ejection fraction > 30% | |||
| (a) | ||||||||
| 1 | Goldberger's first criterion—SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV | 14 (2.6%) | 8 (57.1% of 14) | 6 (42.9% of 14) | 531 (97.4%) | 88 (16.6% of 531) | 443 (83.4% of 531) | .0001 |
| 2 | Goldberger's second criterion—Total QRS amplitude in each of the limb leads ≤ 0.8mv | 154 (28.1%) | 23 (14.9% of 154) | 131 (85.1% of 154) | 394 (71.9%) | 73 (18.5% of 394) | 321 (81.5% of 394) | .3199 |
| 3 | Goldberger's third criterion—RV4/SV4 < 1 | 231 (43.1%) | 53 (22.9% of 231) | 178 (77.1% of 231) | 305 (56.9%) | 43 (14.1% of 305) | 262 (85.9% of 305) | .0082 |
| 4 | Goldberger's triad (all 3 criteria present) | 0 | ‐ | ‐ | 548 (100.0%) | 96 (17.5% of 548) | 452 (82.5% of 548) | – |
| 5 | Maximal QRS duration ≥ 124 ms5 | 115 (21.0%) | 29 (25.2% of 115) | 86 (74.8% of 115) | 433 (79.0%) | 67 (15.5% of 433) | 366 (84.5% of 433) | .0145 |
| 6 | Maximal QRS duration + Goldberger's first criterion5 | 3 (0.6%) | 2 (66.7% of 3) | 1 (33.3% of 3) | 545 (99.5%) | 94 (17.3% of 545) | 451 (82.8% of 545) | .1378 |
| 7 | Maximal QRS duration + Goldberger's third criterion5 | 48 (8.8%) | 15 (31.3% of 48) | 33 (68.8% of 48) | 500 (91.2%) | 81 (16.2% of 500) | 419 (83.8% of 500) | .0088 |
| 8 | Maximal QRS duration + Goldberger's first and third criteria5 | 1 (0.2%) | 1 (100.0% of 1) | 0 | 547 (99.8%) | 95 (17.4% of 547) | 452 (82.6% of 547) | .3924 |
| 9 | All voltage ratios of RV6/RI, RII, RIII ≥ 36 | 4 (0.8%) | 2 (50.0% of 4) | 2 (50.0% of 4) | 526 (99.3%) | 89 (16.9% of 526) | 437 (83.1% of 526) | .2791 |
| 10 | Simplified Selvester QRS Score of ≥ 108 | 84 (15.3%) | 24 (28.6% of 84) | 60 (71.4% of 84) | 464 (84.7%) | 72 (15.5% of 464) | 392 (84.5% of 464) | .0038 |
| 11 | QRS voltage less than 5 mm in all limb leads and greater than 10 mm in at least 2 contiguous precordial Leads9 | 5 (0.9%) | 1 (20.0% of 5) | 4 (80.0% of 5) | 543 (99.1%) | 95 (17.5% of 543) | 448 (82.5% of 543) | 1.0000 |
| ECG criteria | Patients with ECG meeting criteria | Patients with ECG meeting criteria | Patients with ECG not meeting criteria | Patients with ECG not meeting criteria | p value | |||
|---|---|---|---|---|---|---|---|---|
| N (%) | Ejection fraction ≤ 40% | Ejection fraction > 40% | N (%) | Ejection fraction ≤ 40% | Ejection fraction > 40% | |||
| (b) | ||||||||
| 1 | Goldberger's first criterion—SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV | 14 (2.6%) | 13 (92.9% of 14) | 1 (7.1% of 14) | 531 (97.4%) | 250 (47.1% of 531) | 281 (52.9% of 531) | .0019 |
| 2 | Goldberger's second criterion—Total QRS amplitude in each of the limb leads ≤ 0.8mv | 154 (28.1%) | 74 (48.1% of 154) | 80 (51.9% of 154) | 394 (71.9%) | 190 (48.2% of 394) | 204 (51.8% of 394) | .9712 |
| 3 | Goldberger's third criterion—RV4/SV4 < 1 | 231 (43.1%) | 132 (57.1% of 231) | 99 (42.9% of 231) | 305 (56.9%) | 127 (41.6% of 305) | 178 (58.4% of 305) | .0004 |
| 4 | Goldberger's triad (all 3 criteria present) | 0 | – | – | 548 (100.0%) | 264 (48.2% of 548) | 284 (51.8% of 548) | – |
| 5 | Maximal QRS duration ≥ 124 ms5 | 115 (21.0%) | 77 (67.0% of 115) | 38 (33.0% of 115) | 433 (79.0%) | 187 (43.2% of 433) | 246 (56.8% of 433) | <.0001 |
| 6 | Maximal QRS duration + Goldberger's first criterion5 | 3 (0.55%) | 3 (100.0% of 3) | 0 | 545 (99.5%) | 261 (47.9% of 545) | 284 (52.1% of 545) | .2217 |
| 7 | Maximal QRS duration + Goldberger's third criterion5 | 48 (8.8%) | 35 (72.9% of 48) | 13 (27.1% of 48) | 500 (91.2%) | 229 (45.8% of 500) | 271 (54.2% of 500) | .0003 |
| 8 | Maximal QRS duration + Goldberger's first and third criteria5 | 1 (0.2%) | 1 (100.0% of 1) | 0 | 547 (99.8%) | 263 (48.1% of 547) | 284 (51.9% of 547) | .9708 |
| 9 | All voltage ratios of RV6/RI, RII, RIII ≥ 36 | 4 (0.8%) | 2 (50.0% of 4) | 2 (50.0% of 4) | 526 (99.2%) | 251 (47.7% of 526) | 275 (52.3% of 526) | 1.0000 |
| 10 | Simplified Selvester QRS Score of ≥ 78 | 221 (40.33%) | 135 (61.09% of 221) | 86 (38.91% of 221) | 327 (59.67%) | 129 (39.45% of 327) | 198 (60.55% of 327) | <.0001 |
| 11 | QRS voltage less than 5 mm in all limb leads and greater than 10 mm in at least 2 contiguous precordial Leads9 | 5 (0.9%) | 3 (60.0% of 5) | 2 (40.0% of 5) | 543 (99.1%) | 261 (48.1% of 543) | 282 (51.9% of 543) | .9346 |
3.3. Diagnostic value of ECG criteria
Computation of the predictive characteristics (Sensitivity and specificity, positive predictive value and negative predictive value) of the ECG criteria showed that none of the 11 criteria had a combination of high sensitivity and negative predictive values that would make them qualify as a good screening test (Figure 2). All 11 criteria had poor sensitivity ranging from 1% to 55.2% in detecting patients with LVEF ≤ 30% and from 1.1% to 51.1%, in detecting patients with LVEF ≤ 40%. The negative predictive values for these criteria were reasonably good in patients with LVEF ≤ 30% and ranged from 81.6% to 85.9%. Goldberger's third criterion (RV4/SV4 < 1) had the highest negative predictive value (85.9%) for identifying patients with LVEF ≤ 30% followed by maximal QRS duration ≥ 124 ms (84.5%). In contrast, the negative predictive values for patients with LVEF ≤ 40% were poor and range between 51.6% and 60.6%.
Figure 2.
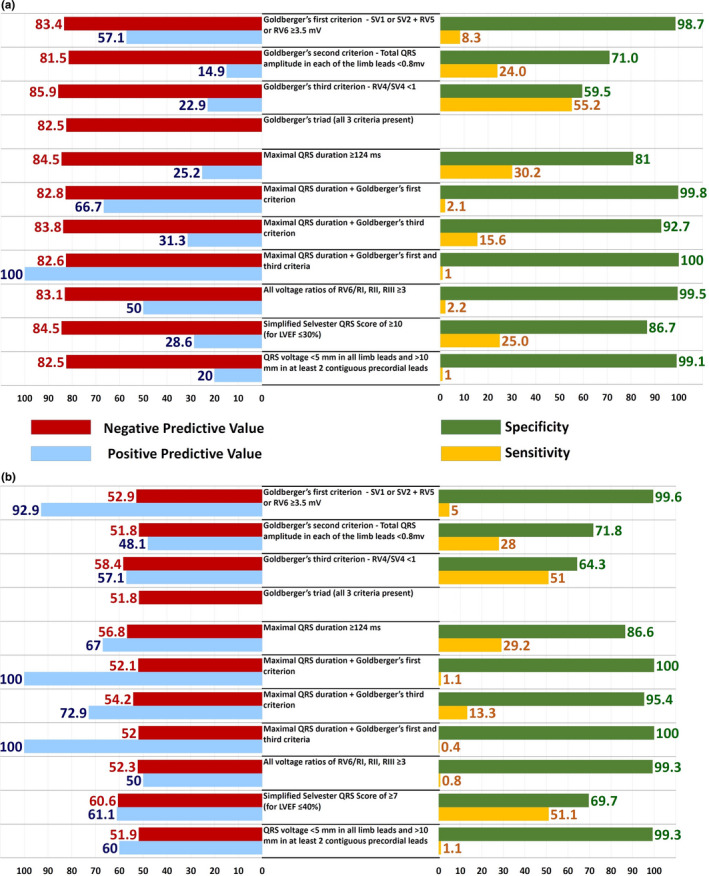
Negative predictive value (NPV), positive predictive value (PPV), specificity, and sensitivity and for each of the ECG criteria with LVEF cutoff of 30% (Panel a) and LVEF cutoff of 40% (Panel b). (N = 548)
For identification of patients with LVEF ≤ 30%, the combination of “Maximal QRS duration + Goldberger's first + third criteria” had a positive predictive value of 100%, but was seen in only one of our 548 patients. For all other criteria, the positive predictive value ranged from 14.9% to 66.7% (Figure 2). For identification of patients with LVEF ≤ 40%, Goldberger's first criterion (SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV) had a positive predictive value of 92.9%. Of the 14 patients with ECGs meeting this criterion, 13 had LVEF ≤ 40%. The positive predictive value of this criterion increased to 100% when it was combined with the criterion of “Maximal QRS duration > 124 ms,” but this was seen in only 3 of the 548 patients in the study. The combination criteria of “Maximal QRS duration + Goldberger's first + third criteria” also had a positive predictive value of 100%, but was seen in only one of the 548 patients. For all other criteria, the positive predictive values ranged from 47.6% to 72.9% (Figure 2).
3.4. LVEF and number of ECG criteria
Using a cutoff of LVEF ≤ 30%, the proportion of patients with reduced LVEF steadily increased with an increasing number of ECG criteria met from 9.15% for 0 criteria to 18.6% for 1, 21.1% for 2, 29.4% for 3, and 50% for 4 criteria (p = .0019 by chi‐squared for trend; Table 5). This was also observed on using a cutoff of LVEF ≤ 40%. The proportion of patients with reduced LVEF increased from 29.1% for 0 criteria to 46.8% for 1, 55.3% for 2, 67.4% for 3, and 83.3% for 4 criteria (p < .0001) (Table 5).
Table 5.
Left ventricular ejection fraction (LVEF) versus number of ECG criteria met in 548 subjects with previous MI (N = 548)
| Number of ECG criteria met a | Left ventricular ejection fraction cutoff of 30% | Left ventricular ejection fraction cutoff of 40% | ||||
|---|---|---|---|---|---|---|
| Total number of patients b (N) | LVEF ≤ 30% | Left ventricular ejection fraction > 30% | Total number of patients b (N) | LVEF ≤ 40% | Left ventricular ejection fraction > 40% | |
| 0 | 164 | 15 (9.15%) | 149 (90.9%) | 141 | 41 (29.1%) | 100 (70.9%) |
| 1 | 220 | 41 (18.6%) | 179 (81.4%) | 171 | 80 (46.8%) | 91 (53.2%) |
| 2 | 109 | 23 (21.1%) | 86 (78.9%) | 141 | 78 (55.3%) | 63 (44.7%) |
| 3 | 51 | 15 (29.4%) | 36 (70.6%) | 89 | 60 (67.4%) | 29 (32.6%) |
| 4 | 4 | 2 (50%) | 2 (50%) | 6 | 5 (83.3%) | 1 (16.7%) |
| >4 | 0 | 0 | 0 | 0 | 0 | 0 |
| χ2 (Chi‐square for Trend) P Value | 0.0019 | <0.0001 | ||||
Simplified Selvester QRS Score of ≥ 10 was used as cutoff for LVEF ≤ 30% and Simplified Selvester QRS Score of ≥ 7 was used as cutoff for LVEF ≤ 40%.
Number of patients with ECGs meeting the specified number of criteria. The numbers in these columns differ for LVEF ≤ 30% and LVEF ≤ 40% because different cutoff values of the Simplified Selvester QRS Score were used in the two groups.
3.5. Subgroup analysis
We also studied the diagnostic value of the 6 potentially useful ECG criteria in specific subgroups based on baseline characteristics including gender (females and males), age (≥60 years and age < 60 years), diabetes (present, absent), hypertension (present, absent), and number of prior episodes of myocardial infarction (prior MI = 1 and > 1).
All 6 criteria had poor sensitivity ranging from 0% to 62.1% in detecting patients with LVEF ≤ 30% and from 0% to 57.3%, in detecting patients with LVEF ≤ 40% (see Supplementary data file). The negative predictive values for these criteria were reasonably good in patients with LVEF ≤ 30% and ranged from 76.6% to 88.3%. In contrast, the negative predictive values for patients with LVEF ≤ 40% were poor and range between 45.2% and 64.4% (see Supplementary data file). The sensitivity and negative predictive values in the subgroups were not significantly different from that in the overall group of patients (see Supplementary data file).
4. DISCUSSION
We identified 11 previously defined ECG criteria that had been found to correlate with reduced LVEF; these included 7 independent criteria, and 4 criteria that were combinations of the 7 independent criteria. We studied the diagnostic value of these 11 criteria in a set of 548 patients, who were part of the DETERMINE Trial and Registry and the PRE‐DETERMINE study, all of whom had prior history of myocardial infarction. Of the 548 patients, 264 patients (48.2%) had a LVEF ≤ 40% and 96 patients (17.5%) had a LVEF ≤ 30%.
We found a statistically significant difference in the LVEF in patients with and without the presence of a particular ECG criterion for only 4 of the 7 independent criteria and 2 of the 4 combination criteria (Table 3). We therefore further studied the predictive characteristics of these 6 ECG criteria to identify patients with LVEF ≤ 30% of LVEF ≤ 40%.
Electrocardiography is typically used as a screening test for heart disease, which could subsequently be confirmed by other more specific tests. We started with the assumption that to serve as a useful screening test, these ECG criteria should be highly sensitive and should identify most patients with depressed LVEF, who could then be subjected to a more specific test like echocardiography or cardiac MRI (Leong et al., 2010). Moreover, a useful screening test, if negative, should also give reasonable assurance that the LVEF is likely to be normal (high negative predictive value) (Won et al., 2015).
In 1982, Goldberger prospectively studied 2000 consecutive ambulatory and in‐hospital patients, after excluding patients who had undergone cardiac surgery within the preceding six months and patients with idiopathic congestive cardiomyopathy. He described a triad of ECG findings, all of which were present in only 32 patients (1.6%) of his patients, 29 of whom showed evidence of LV dysfunction (LVEF ≤ 40%); 20 of these had ischemic heart disease (Goldberger, 1982). These criteria were (a) SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV, (b) total QRS amplitude in each of the limb leads ≤ 0.8 mV, and (c) RV4/SV4 < 1. Goldberger attributed these ECG findings to a spatial shift in the QRS vector perpendicular to the frontal plane and toward the transverse plane as a consequence of ventricular dilation. Interestingly, none of our patients had an ECG meeting all criteria of the triad. Two other studies have evaluated these criteria in patients with LV dysfunction. Cincin et al studied 143 patients with heart failure that included 106 with LV dysfunction defined as LVEF < 50 and 92 with coronary artery disease. Only 10 patients of the 106 fulfilled all three criteria (sensitivity 9.4%, specificity 100%, positive predictive value 100%, and negative predictive value 27.8%) (Cincin et al., 2012). In another study, Lopez et al studied 51 patients with severe LV dysfunction defined by LVEF ≤ 20%; 7 of these had coronary artery disease (Lopez et al., 2012; Madias, 2012). The ECG triad was present in only in 1 out of their 51 patients. Thus, the triad seems to be a very insensitive criterion to identify patients with reduced ejection fraction.
Of the 3 components of the Goldberger triad, the first criterion (SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV) was one of the 6 criteria that we identified as potentially useful to identify patients with LVEF above or below a cutoff of 40% or 30%. The sensitivity of this criterion to identify patients with LVEF ≤ 40% was only 5%, and the negative predictive value was 53%. The other 5 potentially useful criteria too had a low sensitivity: Goldberger's third criterion (RV4/SV4 < 1) and the Simplified Selvester QRS score had the best sensitivity of 51% and 51.1% each, and the sensitivity of the remaining 3 criteria ranged from 1.1% to 29.2%. The negative predictive values ranged from 52.1% to 60.6% for these 6 criteria. All 6 criteria performed better when used to identify patients with LVEF ≤ 30%: The negative predictive value improved and ranged from 82.8% to 85.9%, though sensitivity remained low and ranged from 2.1% to 55.2%. Here too, Goldberger's third criterion (RV4/SV4 < 1) performed the best with a negative predictive value of 85.9% and sensitivity of 55.2%. Thus, none of these criteria met the characteristics of a useful screening test (high sensitivity and high negative predictive value) (Schwartz, 2005) to identify patients with a LVEF ≤ 40%, although they were all more sensitive in identifying patients with LVEF ≤ 30%.
Another potentially useful criterion was the simplified Selvester QRS score, which was primarily developed as a method to estimate infarct size based on ECG findings (Wagner et al., 1982). It was subsequently found to correlate well with survival, and its prognostic value was explained largely by its correlation to left ventricular function (Palmeri et al., 1982). Palmeri et al studied the value of this scoring system for assessing left ventricular function after acute myocardial infarction and found a significant inverse linear relationship between the simplified Selvester score and LVEF (Palmeri et al., 1982). Their findings suggested that a simplified Selvester QRS score of ≥ 10 would identify patients with LVEF ≤ 30% and a score of ≥ 7 would identify patients with LVEF ≤ 40% (Palmeri et al., 1982). Although we found a statistically significant difference in the LVEF in patients with ECGs meeting this criterion and those without, the sensitivity (51%) and negative predictive value (60.6%) of this criterion were poor (mean ± SD of 37 ± 10.2% versus 42.5 ± 11%; p < .0001 for Selvester QRS score of ≥ 7 for identifying patients with LVEF ≤ 40%). Similarly, the sensitivity (25%) for Selvester QRS score of ≥ 10 for identifying patients with LVEF ≤ 30% was poor, though the negative predictive value was high (84.5%).
Although none of the previously defined criteria had sufficiently high sensitivity or negative predictive values to serve as a screening test, three criteria were highly specific in identifying patients with LVEF ≤ 40%. These were Goldberger's first criterion (SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV) with a specificity of 99.6%, Goldberger's first criterion plus maximal QRS duration ≥ 124 ms (specificity 100%) and Goldberger's third criterion plus maximal QRS duration ≥ 124 ms (specificity 95.4%). However, the number of patients with ECGs meeting these criteria was extremely small: Goldberger's first criterion was present in ECGs of only 14 (2.6%) patients of which 13 (92.9%) had LVEF ≤ 40%, Goldberger's first criterion plus maximal QRS duration ≥ 124 ms was seen in 3 patients (all had LVEF ≤ 40%), and Goldberger's third criterion (RV4/SV4 <1) plus maximal QRS duration ≥ 124 ms was seen in 48 patients (35 had LVEF ≤ 40%).
Subgroup analysis showed that diagnostic value of the 6 potentially useful ECG criteria did not differ in subgroups based on gender, age, diabetes, hypertension, and number of prior episodes of myocardial infarction and were not much better in any subgroup as compared to the overall study population.
5. CONCLUSION
Although echocardiography is most commonly used to quantify LVEF, we attempted to validate the usefulness of the 12 lead ECG to identify patients with previous MI who had reduced LVEF using existing ECG criteria. Our study differed from previous studies in that this was a large cohort of patients with previous MI where the presence of the infarct as well as the LVEF were confirmed by cardiac MRI. Moreover, ECGs as well as cardiac MRI scans were analyzed in a central laboratory, thereby limiting observer variability. Of the eleven previously defined ECG criteria studied for their value as a screening test to identify patients with reduced LVEF, LVEF was statistically significantly lower in patients with ECGs meeting 6 of these criteria. However, none of these criteria had a sufficiently high sensitivity or negative predictive value to serve as screening tests to identify patients with reduced LVEF. However, three criteria (presence of SV1 or SV2 + RV5 or RV6 ≥ 3.5 mV with or without a maximal QRS duration ≥ 124 ms and RV4/SV4 < 1 plus maximal QRS duration ≥ 124 ms) had high specificity to identify patients with reduced LVEF, even though these were seen in ≤ 9% of all patients studied. Our study suggests the need to develop better ECG criteria as the present ECG criteria do not permit the use of the 12‐lead ECG as a screening tool to identify patients with previous MI who have reduced LVEF. Whether other ECG criteria can better identify LV dysfunction remains to be determined.
Conflicts of Interest
None declared.
Ethical Approval
PRE‐DETERMINE Biologic Markers and MRI SCD Registry Study has been reviewed and approved by the Partners Human Research Committee (PHS) institutional review board (IRB).
Supporting information
Appendix S1
Acknowledgements
DETERMINE was supported by St Jude Medical, Inc; and PRE‐DETERMINE was supported by a research grant from the National Heart, Lung and Blood Institute (R01HL91069), by St. Jude Medical, Inc and a grant from the St. Jude Medical Foundation.
Panicker GK, Narula DD, Albert CM, et al. Validation of electrocardiographic criteria for identifying left ventricular dysfunction in patients with previous myocardial infarction. Ann Noninvasive Electrocardiol.2021;26:e12812. 10.1111/anec.12812
REFERENCES
- Borlaug, B. A. , & Kass, D. A. (2011). Invasive hemodynamic assessment in heart failure. Cardiology Clinics, 29, 269–280. 10.1016/j.ccl.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Bounous, E. P. Jr , Califf, R. M. , Harrell, F. E. Jr , Hinohara, T. , Mark, D. B. , Ideker, R. E. , Selvester, R. H. , & Wagner, G. S. (1988). Prognostic value of the simplified Selvester QRS score in patients with coronary artery disease. Journal of the American College of Cardiology, 11, 35–41. 10.1016/0735-1097(88)90163-5 [DOI] [PubMed] [Google Scholar]
- Chinitz, J. S. , Cooper, J. M. , & Verdino, R. J. (2008). Electrocardiogram voltage discordance: Interpretation of low QRS voltage only in the limb leads. Journal of Electrocardiology, 41, 281–286. 10.1016/j.jelectrocard.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Cincin, A. , Ozben, B. , & Erdogan, O. (2012). Diagnostic utility of specific electrocardiographical parameters in predicting left ventricular function. Experimental & Clinical Cardiology, 17, 210–214. [PMC free article] [PubMed] [Google Scholar]
- Clinicaltrials.gov (2018). PRE‐DETERMINE Cohort Study – Clinicaltrials.gov [online]. Available at: https://clinicaltrials.gov/ct2/show/NCT01114269 [Accessed 01 Oct 2018].
- de Haan, S. , de Boer, K. , Commandeur, J. , Beek, A. M. , van Rossum, A. C. , & Allaart, C. P. (2014). Assessment of left ventricular ejection fraction in patients eligible for ICD therapy: Discrepancy between cardiac magnetic resonance imaging and 2D echocardiography. Netherlands Heart Journal, 22, 449–455. 10.1007/s12471-014-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerse, D. A. , Wu, K. C. , Gorgels, A. P. , Zimmet, J. , Wagner, G. S. , & Miller, J. M. (2009). Comparison between contrast‐enhanced magnetic resonance imaging and Selvester QRS scoring system in estimating changes in infarct size between the acute and chronic phases of myocardial infarction. Annals of Noninvasive Electrocardiology, 14, 360–365. 10.1111/j.1542-474X.2009.00327.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger, A. L. (1982). A specific ECG triad associated with congestive heart failure. Pacing and Clinical Electrophysiology, 5, 593–599. 10.1111/j.1540-8159.1982.tb02285.x [DOI] [PubMed] [Google Scholar]
- Kadish, A. H. , Bello, D. , Finn, J. P. , Bonow, R. O. , Schaechter, A. , Subacius, H. , Albert, C. , Daubert, J. P. , Fonseca, C. G. , & Goldberger, J. J. (2009). Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. Journal of Cardiovascular Electrophysiology, 20, 982–987. 10.1111/j.1540-8167.2009.01503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, D. P. , De Pasquale, C. G. , & Selvanayagam, J. B. (2010). Heart failure with normal ejection fraction: The complementary roles of echocardiography and CMR imaging. JACC: Cardiovascular Imaging, 3(4), 409–420. 10.1016/j.jcmg.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Lopez, C. , Ilie, C. C. , Glancy, D. L. , & Quintal, R. E. (2012). Goldberger’s electrocardiographic triad in patients with echocardiographic severe left ventricular dysfunction. American Journal of Cardiology, 109, 914–918. 10.1016/j.amjcard.2011.10.053 [DOI] [PubMed] [Google Scholar]
- Lumens, J. , Prinzen, F. W. , & Delhaas, T. (2015). Longitudinal strain: "Think Globally, Track Locally". JACC: Cardiovascular Imaging, 8, 1360–1363. 10.1016/j.jcmg.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Madias, J. E. (2012). Goldberger's electrocardiographic triad revisited. American Journal of Cardiology, 110, 160–161. 10.1016/j.amjcard.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Momiyama, Y. , Mitamura, H. , & Kimura, M. (1994). ECG characteristics of dilated cardiomyopathy. Journal of Electrocardiology, 27, 323–328. 10.1016/S0022-0736(05)80270-5 [DOI] [PubMed] [Google Scholar]
- Palmeri, S. T. , Harrison, D. G. , Cobb, F. R. , Morris, K. G. , Harrell, F. E. , Ideker, R. E. , Selvester, R. H. , & Wagner, G. S. (1982). A QRS scoring system for assessing left ventricular function after myocardial infarction. New England Journal of Medicine, 306(1), 4–9. 10.1056/NEJM198201073060102 [DOI] [PubMed] [Google Scholar]
- Panicker, G. K. , Karnad, D. R. , Joshi, R. , Shetty, S. , Vyas, N. , Kothari, S. , & Narula, D. (2009). Z‐score for benchmarking reader competence in a central ECG laboratory. Annals of Noninvasive Electrocardiology, 14, 19–25. 10.1111/j.1542-474X.2008.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, J. S. (2005). Clinical decision‐making in cardiology. In Zipes D., Libby P., Bonow R. O., & Braunwald E. (Eds.), Braunwald’s Heart Disease. A Textbook of Cardiovascular Medicine, 7th ed. (pp. 27–34). Elsevier Saunders. [Google Scholar]
- Wagner, G. S. , Freye, C. J. , Palmeri, S. T. , Roark, S. F. , Stack, N. C. , Ideker, R. E. , Harrell, F. E. Jr , & Selvester, R. H. (1982). Evaluation of a QRS scoring system for estimating myocardial infarct size. I. Specificity and observer agreement. Circulation, 65, 342–347. 10.1161/01.CIR.65.2.342 [DOI] [PubMed] [Google Scholar]
- Won, E. , Donnino, R. , Srichai, M. B. , Sedlis, S. P. , Feit, F. , Rolnitzky, L. , Miller, L. H. , Iqbal, S. N. , Axel, L. , Nguyen, B. , Slater, J. , & Shah, B. (2015). Diagnostic accuracy of cardiac magnetic resonance imaging in the evaluation of newly diagnosed heart failure with reduced left ventricular ejection fraction. American Journal of Cardiology, 116, 1082–1087. 10.1016/j.amjcard.2015.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


