Abstract
Background
Ventricular arrhythmia is a leading cause of cardiac death among patients with post‐infarction left ventricular aneurysm (PI‐LVA). The effect of coronary revascularization in PI‐LVA patients with ventricular tachyarrhythmia remains unknown. This study aims to investigate the impact of revascularization therapy on clinical outcomes in these patients.
Methods
A total of 238 PI‐LVA patients were enrolled, and 59 patients were presented with sustained ventricular tachycardia (VT) or ventricular fibrillation (VF). Patients were classified into 4 groups by treatment strategies (medical or revascularization) and the presence of VT/VF: group 1 (n = 57): VT/VF− and revascularization−; group 2 (n = 122): VT/VF− and revascularization+; group 3 (n = 34): VT/VF+ and revascularization+; and group 4 (n = 25): VT/VF+ and revascularization‐. The clinical outcomes were compared, and the primary endpoint was cardiac death or heart transplantation.
Results
Patients were followed up for 45 ± 16 months, and 41 patients (17.2%) reached the primary endpoint. Kaplan–Meier analysis showed that in VT/VF− patients, revascularization associated with higher cardiac survival compared with medical therapy (log‐rank p = .002), but in VT/VF+ patients, revascularization did not predict better cardiac outcome (log‐rank p = .901). Cox regression analysis revealed PET‐EF (HR 4.41, 95% CI: 1.72–11.36, p = .002) and moderate/severe mitral regurgitation (HR 2.32, 95% CI: 1.02–5.30, p = .046) as independent predictors of adverse cardiac outcome in patients with VT/VF.
Conclusion
PI‐LVA patients with VT/VF are at high risk of adverse cardiac outcome, and coronary revascularization does not mitigate this risk, although revascularization was associated with higher cardiac survival in PI‐LVA patients without VT/VF.
Keywords: post‐infarction left ventricular aneurysm, revascularization, ventricular tachyarrhythmia
1. INTRODUCTION
Post‐infarction left ventricular aneurysm (PI‐LVA) has been recognized as a consequence of acute transmural myocardial infarction. Revascularization and surgical treatment of PI‐LVA have been evolving for decades, with 5‐year survival rate varying between 58% and 80% and 10‐year overall survival being about 34%(Cosgrove et al., 1989; Ruzza et al., 2017). Ventricular arrhythmia is a common and serious complication of PI‐LVA (Ning et al., 2018). Previous randomized trials have demonstrated that ventricular arrhythmias in post‐infarct patients are significant contributors to mortality, especially in patients with reduced left ventricular ejection fraction (LVEF; Myerburg & Junttila, 2012). Even after appropriate coronary revascularization therapy, the risk of ventricular arrhythmia and sudden cardiac death (SCD) remains high, no matter the level of LVEF is relatively preserved or not, nor the revascularization is complete or not (Carson et al., 2013; Mondesert et al., 2016). In consideration of the above factors, the purpose of this study was to investigate the impact of revascularization on clinical outcomes in PI‐LVA patients with ventricular tachyarrhythmia.
2. MATERIALS AND METHODS
2.1. Study population
238 patients diagnosed as PI‐LVA for the first time between January 2012 and September 2016 were enrolled in this study. The definition of PI‐LVA contains two elements: (1) history of previous myocardial infarction confirmed by medical records, electrocardiography, and coronary angiography and (2) PI‐LVA is strictly defined as the presence of segment of the LV wall protruding from the expected outline of the ventricular chamber and displaying dyskinesis by ultrasonography examination or coronary angiography. The exclusion criteria were patients with cardiomyopathy, valvular disease, congenital heart disease, previous coronary artery bypass grafting (CABG), or aneurysm resection surgery. Acute myocardial infarction (AMI) within 8 weeks was also excluded. All decisions concerning the treatment strategy were made at the discretion of the referring physicians and were not based on a random assignment. All of the patients underwent SPECT and PET images test to evaluate the myocardial viability. Coronary revascularization was defined as percutaneous coronary intervention (PCI) or CABG therapy. This study conforms to the principles outlined in the Declaration of Helsinki.
2.2. Data collection
Arrhythmic findings including ventricular tachycardia/ventricular fibrillation (VT/VF), frequent premature ventricular contraction (PVC, >5 per min), and atrial fibrillation/atrial flutter (AF/AFL) were collected and analyzed. Data on the clinical management of arrhythmia, including pharmacologic treatment, catheter ablation, and implantation of an implantable cardioverter defibrillator (ICD) or cardiac resynchronized therapy cardioverter defibrillation (CRTD), were also collected. Two independent reviewers evaluated all arrhythmia data. Sustained VT was defined as tachycardia of ventricular origin lasting for more than 30 s, which was either self‐terminated or required electrical/pharmacological cardioversion due to hemodynamic collapse. VT/VF occurred within 48 hr of AMI symptom onset was not included in this study.
2.3. Analysis of SPECT and PET images
All of the patients enrolled in this study were assessed with 99mTcm‐ MIBI SPECT rest perfusion imaging and 18F‐FDG‐PET metabolism imaging, and MIBI and FDG myocardial activity were graded with 5‐point score and 17‐segment model (Holly et al., 2010; Wei et al., 2014). The segment with the highest perfusion activity was defined as normal, and the relative perfusion and metabolic activity in the residual segments were graded (0 = normal radiotracer activity, 1 = mildly reduced activity, 2 = moderately reduced activity, 3 = severely reduced activity, and 4 = absence of activity). The mismatch score (MMS) of the LV was calculated as the summed rest perfusion score minus the summed FDG score. LV functional parameters, including EDV, ESV, and LVEF, were automatically calculated by QGS software with manual correction (version 3.1, Cedars‐Sinai Medical Center, Los Angeles, CA, USA).
2.4. Grouping and follow‐up
Patients were divided into 4 groups according to the presence of VT/VF and treatment strategies: group 1 (n = 57): VT/VF− and revascularization−; group 2 (n = 122): VT/VF− and revascularization+; group 3 (n = 34): VT/VF+ and revascularization+; and group 4 (n = 25): VT/VF+ and revascularization‐. Long‐term follow‐up was performed by review of patients’ clinical records and by phone contact with patients or their relatives. The average follow‐up time was 45 ± 16 months. The primary endpoint consisted of cardiac death or heart transplantation.
2.5. Statistical analysis
Continuous variables were presented as mean value ± SD and compared using Student's t test. Categorical variables were presented as frequencies and percentages and compared by chi‐squared or fisher test. Cardiac survival curves were generated by the Kaplan–Meier method and compared by the log‐rank test. Logistic regression analysis was used to evaluate variables associated with VT/VF development. To determine independent predictors of primary endpoint, Cox proportional hazards models with stepwise backward elimination methods were used. Hazard ratios (HRs) are presented with the 95% confidence intervals (CIs). Statistical significance was defined as a two‐sided p value < .05. All statistical analyses were performed using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA).
3. RESULTS
3.1. Population characteristics: VT/VF+ vs VT/VF−group
The baseline clinical characteristics of patients were showed in Table 1. There were 205 males (86%) and 33 women (14%), with an average age of 58 ± 10 years old. All of the patients had a history of ST‐elevated MI (STEMI), and about 87% of the location of infarction was anterior (mid‐anterior, apical‐anterior, extensive anterior), 5% of the location was lateral and inferior, and 8% of the patients had concurrent anterior and lateral/inferior MI. The baseline ECG for all the 238 patients showed pathological Q waves. Among the 238 patients, 59 presented with VT/VF. A total of 156 patients had coronary revascularization therapy, 57 patients received PCI therapy, and 99 received CABG treatment (plus aneurysmectomy = 60). Patients in VT/VF+ group did not differ from VT/VF− group with respect to gender, age, and management therapy, and the majority of the patients underwent revascularization therapy (59% in VT/VF− group, 68% in VT/VF+ group, p = .208). Risk factors such as hypertension, diabetes mellitus, and hyperlipidemia were not different between the two groups. Patients in VT/VF+ group were more likely to have enlarged LVEDD assessed in ultrasound and EDV and ESV analyzed in PET imaging study. Furthermore, LVEF and PET‐EF were both substantially lower in VT/VF+ group. Patients with VT/VF+ had a higher prevalence of AF/AFL and PVC. A significantly elevated incidence of multivessel disease was observed in the VT/VF− group. MMS was significantly higher in VT/VF+ group (11 vs 6, p = .000). In VT/VF+ group, 56 presented with sustained VT and 3 presented with VF. There were 18 patients who complained syncope and 14 received external cardioversion therapy. Thirteen patients underwent implantation of ICD, and radiofrequency catheter ablation (RFCA) was performed in 3 patients.
TABLE 1.
Clinical characteristics of PI‐LVA patients
| VT/VF− | VT/VF+ | |||
|---|---|---|---|---|
| Revascularization− group 1(n = 57) | Revascularization+ group 2(n = 122) | Revascularization+ group 3(n = 34) | Revascularization − group 4(n = 25) | |
| Male gender, n | 42(74) | 111(91)* | 30(88)* | 22(88)* |
| Age (year) | 56 ± 10 | 58 ± 10 | 57 ± 9 | 61 ± 9 |
| Diabetes, n | 17(30) | 29(24) | 11(32) | 6(24) |
| Hypertension, n | 28(49) | 55(45) | 15(44) | 10(40) |
| Hyperlipidemia, n | 40(70) | 70(57) | 21(62) | 17(68) |
| NYHAⅢ‐Ⅳ, n | 21(37) | 21(17)* | 21(62)*, ** | 12(48)**, *** |
| LVEDD (mm) | 62 ± 8 | 58 ± 6* | 63 ± 7** | 63 ± 9** |
| LVEF (%) | 37 ± 9 | 41 ± 8* | 35 ± 7** | 38 ± 10** |
| PET‐EDV (ml) | 193 ± 67 | 161 ± 46* | 217 ± 48*, ** | 210 ± 15*, ** |
| PET‐ESV (ml) | 138 ± 63 | 106 ± 43* | 161 ± 43*, ** | 156 ± 70*, ** |
| PET‐EF (%) | 31 ± 11 | 37 ± 11 | 26 ± 6*, ** | 27 ± 9*, ** |
| LVEF ≤ 35%, n | 38(67) | 55(45)* | 28(82)*, ** | 17(68)**, *** |
| Moderate/severe MR, n | 27(47) | 41(34)* | 20(59)*, ** | 8(32)*, *** |
| Multivessel disease, n | 28(49) | 63(52) | 10(29) | 8(32) |
| AF/AFL, n | 6(11) | 10(8) | 16(47)*, ** | 8(32)*, ** |
| PVC, n | 11(19) | 20(16) | 10(29)*, ** | 9(36)*, ** |
| ICD, n | 0 | 0 | 9(26) | 4(16) |
| Syncope, n | 0 | 0 | 10(29) | 8(32) |
| MMS | 5 ± 5 | 6 ± 6 | 9 ± 10*, ** | 12 ± 9*, ** |
| Aneurysm location, n | ||||
| Apex | 56(98) | 119(98) | 32(94) | 24(96) |
| Anterior | 1(2) | 3(2) | 1(3) | 1(4) |
| Posterior | 0 | 0 | 0 | 0 |
| Inferior | 0 | 0 | 1(3) | 0 |
Data are number (with percentage in parentheses) or mean ± SD.
Abbreviations: AF/AFL, atrial fibrillation/atrial flutter; EDV, end diastolic volume; ESV, end systolic volume; ICD, implantable cardioverter defibrillator; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; MMS, mismatch scoreMR, mitral regurgitation; NYHA, New York heart association classification; PI‐LVA, post‐infarction left ventricular aneurysm; PVC, premature ventricular contraction; VT/VF, ventricular tachycardia/ventricular fibrillation.
p < .05 compared to group 1.
p < .05 compared to group 2.
p < .05 compared to group 3.
3.2. Factors associated with VT/VF development in PI‐LVA patients
In this study, univariate logistic regression analysis showed that the potential predictors associated with VT/VF development were multivessel disease, MMS, moderate/severe MR, LVEDD, LVEF, PET‐EF ≤ 35%, PET‐EDV, PET‐ESV, and PET‐EF (seen in Table 2). The results of multivariate logistic regression analysis using the above 9 factors are presented in Table 2. MMS was predictive of VT/VF development (p = .000). In addition, patients with enlarged PET‐EDV and lower PET‐EF had higher odds of VT/VF. Multivessel disease was a negative factor of VT/VF, which means prevalence of VT/VF was significantly higher in patients with non‐multivessel disease.
TABLE 2.
Logistic regression analysis for variables associated with VT/VF development
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95%CI) | P value | |
| Multivessel disease | 0.397 (0.21–0.74) | .004 | 0.256 (0.09–0.67) | .006 |
| MMS | 1.096 (1.04–1.15) | .000 | 1.120 (1.06–1.19) | .000 |
| Moderate/severe MR | 2.005 (1.14–3.52) | .016 | ||
| LVEDD | 1.079 (1.03–1.12) | .000 | ||
| LVEF | 0.951 (0.91–0.98) | .008 | ||
| PET‐EF ≤ 35% | 3.167 (1.60–6.27) | .001 | ||
| PET‐EDV | 1.012 (1.01–1.02) | .000 | 1.013(1.01–1.02) | .000 |
| PET‐ESV | 1.013 (1.01–1.02) | .000 | ||
| PET‐EF | 0.921 (0.89–0.95) | .000 | 0.922 (0.88–0.97) | .001 |
3.3. Prognostic factors for PI‐LVA patients: VT/VF+ vs VT/VF−group
During a follow‐up period of 45 ± 16 months, 41 patients experienced cardiac death (n = 40) or heart transplantation (n = 1), and there were 3 patients died from non‐cardiac reasons: 1 from lung cancer, 1 from cerebral infarction, and 1 from cerebral hemorrhage. Kaplan–Meier survival analysis showed that the survival in group 2 was significantly higher than that in other 3 groups (p = .001, seen in Figure 1). In VT/VF− patients (group 1 and 2), revascularization therapy was associated with a significantly higher survival compared with medical therapy, whereas in VT/VF+ patients (groups 3 and 4), revascularization therapy was not associated with a better survival (Figure 2).
FIGURE 1.

The Kaplan–Meier survival analysis for 4 groups. The survival in group 2 (VT/VF− and revascularization+) was significantly higher than that in other 3 groups
FIGURE 2.
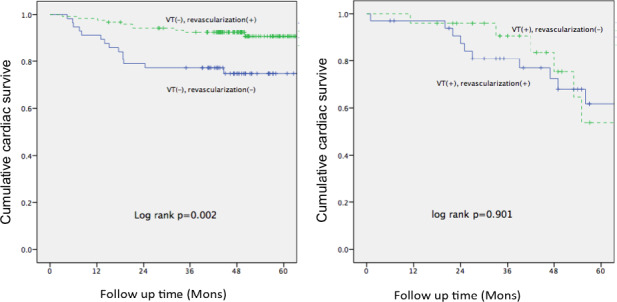
The Kaplan–Meier survival analysis in PI‐LVA patients according to the presence of VT/VF and treatment strategies. p values were calculated with the log‐rank test. Cumulative cardiac survive are shown for VT/VF− (left panel) and VT/VF+ (right panel) subgroup
In PI‐LVA patients with VT/VF, univariate Cox regression analysis revealed PET‐EDV (p = .004), PET‐ESV (p = .001), PET‐EF (p = .001), PET‐EF < 35% (p = .003), VT/VF (p = .017), AF/AFL (p = .024), moderate/severe MR (p = .003), and revascularization therapy (p = .011) were predictors for cardiac survival. Multivariate Cox analysis identified PET‐EF (p < .001) and moderate/severe MR (p < .001) as independent predictors of adverse cardiac outcome (seen in Table 3).
TABLE 3.
Cox regression analysis in PI‐LVA patients with VT/VF
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |
| Male gender | 1.916 (0.91–4.02) | .059 | ||
| PET‐ESV | 1.008 (1.00–1.01) | .001 | ||
| PET‐EDV | 1.007 (1.00–1.01) | .004 | ||
| PET‐EF | 0.945 (0.91–0.98) | .001 | 4.41 (1.72–11.36) | .002 |
| PET‐EF < 35% | 3.240 (1.49–7.02) | .003 | ||
| VT/VF | 2.015 (1.07–3.79) | .017 | ||
| AF/AFL | 2.411 (1.06–5.45) | .024 | ||
| Moderate/severe MR | 2.661 (1.41–5.03) | .003 | 2.32 (1.02–5.30) | .046 |
| Revascularization therapy | 0.451 (0.24–0.83) | .011 | ||
4. DISCUSSION
4.1. Effects of revascularization therapy in patients with VT/VF
Despite the decline in cardiovascular deaths over the past several decades, the incidence of SCD as a proportion of overall cardiovascular deaths has increased (Niemeijer et al., 2015; Srinivasan & Schilling, 2018). VT/VF remains a leading cause of cardiac death among patients with previous myocardium infarction (Colquitt et al., 2014; Greenberg et al., 2004). Electroanatomic mapping and direct palpation indicated that LVA had clear borders (Liu et al., 2018). Viable but hypoperfused myocardium is often localized in those areas, which is ideal for VT/VF to develop (Canty et al., 2004; Partington et al., 2011).
Subgroup analysis in VT/VF+ patients in this study indicated that revascularization therapy did not predict long‐term cardiac prognosis. A previous study showed no reduction in appropriate ICD therapies in patients after complete revascularization. Another study reported a comparable incidence of recurrent arrhythmias in patients with VAs before vs after surgical revascularization (Nageh et al., 2014). This may be explained that in post‐infarction patients, the presence of irreversible substrate of ventricular arrhythmias cannot be reliably excluded even with complete revascularization. In a study of 64 patients with spontaneous VT and prior MI, VT remained inducible in more than 81% of the patients despite complete revascularization, and over 50% of them experienced arrhythmia recurrence (Brockes et al., 2002). Although revascularization therapy could be a positive predictor of cardiac survival in PI‐LVA patients without VT/VF, revascularization did not predict a survival benefit compared with medical therapy in PI‐LVA patients with VT/VF.
4.2. MMS assessment and its association with VT/VF
In this study, we assessed the myocardial viability by SPECT rest perfusion imaging and FDG‐PET metabolism imaging. MMS was significantly higher in VT/VF+ group than that in VT/VF− group (10.6 ± 10 vs 5.5 ± 6, p = .000). Patients underwent revascularization therapy also had relatively elevated MMS compared with those with drug therapy (6.5 ± 7 vs 6 ± 7, p = .05). Logistic regression analysis showed that MMS was associated with VT/VF development. Preclinical studies suggest myocardial hibernation may cause cellular hypertrophy, altered calcium handling, and prolongation of action potentials leading to triggered activity and heterogeneous repolarization (Canty & Fallavollita, 2005; Fallavollita et al., 2014).
It has been proven that the non‐uniform anisotropy can form zones of slow conduction and result in regions of conduction block. This facilitates reentrant excitation, which is the major underlying mechanism of post‐infarction VT (Tschabrunn et al., 2016). MI also causes injury of cardiac sympathetic nerves. Previous basic and clinical studies have suggested the importance of sympathetic activation in the development of lethal ventricular arrhythmias, and inhomogeneity in myocardial sympathetic innervation is vulnerable to arrhythmic death (Tomaselli & Zipes, 2004). The PAREPET study showed that sympathetic denervation predicts SCD independently of LVEF and infarct volume (Fallavollita et al., 2014). In this study, Cox regression analysis did not illustrated MMS as a predictor of clinic outcome in PI‐LVA patients. Substudy of Surgical Treatment of Ischemic Heart Failure (STICH) trial (Bonow et al., 2011) and the Canadian PPAR study (Beanlands et al., 2007) reported a lack of association between the presence of myocardial viability and mortality when the treatment was allocated to revascularization or medical therapy. Because therapy decision in this study was not based on the MMS, and medical or revascularization therapy were not standardized, further trials are needed to explore the prognostic significance of myocardium viability in PI‐LVA patients. Although revascularization is considered the gold standard treatment for improving left ventricular function and reducing ischemic‐driven VT/VF after MI, the arrhythmia substrate is not modified in a significant proportion of PI‐LVA patients. Patients might benefit from a more aggressive modification of neurohormonal and electrical activation.
4.3. ICD therapy in PI‐LVA patients
In this study, all of the utilization of ICD was for the purpose of secondary prevention of SCD, and only 22% (13/59) of the sustained VT/VF patients underwent ICD implantation. ICD Implantation rates in real‐world practice in our country remained suboptimal. The barriers to ICD therapy maybe the physician's inability to address the necessary for an ICD or the expensive cost of the device. Patients’ refusal of ICD therapy could be the fear of being shocked, unawareness of the importance of ICD therapy, and so on. The logistic regression analysis indicated that MMS measured by PET/SPECT was significantly associated with VT/VF development in PI‐LVA patients. MMS zones in the myocardium showing reduced or abolished perfusion and preserved metabolism, and MMS is a measure of the extent and severity of viability. Zhang et al (Zhang et al., 2008) observed that aneurysmal MMS was a negative independent predictor for long‐term cardiac survival. They explanted that the possible mechanism may be the high arrhythmogenic potential of viable myocardium. Assessment of MMS could be complementary to measurement of LVEF for risk stratification of VT/VF. The MMS could merit as an important marker for risk stratification and may bring refinements for prescription of ICD therapy.
4.4. Limitations
There were potential limitations that should be taken into account in this study. Firstly, this is a retrospective study in a single institution, and selection bias may present. Even though multivariable Cox regression analysis is used to minimize residual confounding, we cannot ensure that unmeasured confounding take place in our analyses. Secondly, the treatment decisions were made at the discretion of the referring physicians, and there is a lack of insight into reasons whether or not to revascularize the diagnosed coronary artery lesions.
5. CONCLUSIONS
In PI‐LVA patients combined with VT/VF, revascularization therapy did not predict a survival benefit over medical therapy. It is expected that there is plenty of room for outcome improvement after revascularization in PI‐LVA patients with ventricular tachyarrhythmia. MMS, PET‐EDV, PET‐EF, and multivessel disease associated with development of VT/VF. PET‐EF and moderate/severe MR were independent negative predictors of cardiac survival. These findings may give insight into potential risk stratification and treatment strategies.
CONFLICTS OF INTEREST
None declared.
Ning X, Yang Z, Ye X, et al. Impact of revascularization in patients with post‐infarction left ventricular aneurysm and ventricular tachyarrhythmia. Ann Noninvasive Electrocardiol.2021;26:e12814. 10.1111/anec.12814
Funding informationNational Natural Science Foundation of China (81700307).
DATA AVAILABILITY STATEMENT
Author elects to not share data.
REFERENCES
- Beanlands, R. S. B. , Nichol, G. , Huszti, E. , Humen, D. , Racine, N. , Freeman, M. , Gulenchyn, K. Y. , Garrard, L. , deKemp, R. , Guo, A. , Ruddy, T. D. , Benard, F. , Lamy, A. , & Iwanochko, R. M. (2007). F‐18‐fluorodeoxyglucose positron emission tomography imaging‐assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: A randomized, controlled trial (PARR‐2). Journal of the American College of Cardiology, 50(20), 2002–2012. 10.1016/j.jacc.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Bonow, R. O. , Maurer, G. , Lee, K. L. , Holly, T. A. , Binkley, P. F. , Desvigne‐Nickens, P. , Drozdz, J. , Farsky, P. S. , Feldman, A. M. , Doenst, T. , Michler, R. E. , Berman, D. S. , Nicolau, J. C. , Pellikka, P. A. , Wrobel, K. , Alotti, N. , Asch, F. M. , Favaloro, L. E. , She, L. , … Panza, J. A. (2011). Myocardial viability and survival in ischemic left ventricular dysfunction. New England Journal of Medicine, 364(17), 1617–1625. 10.1056/NEJMoa1100358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockes, C. , Rahn‐Schonbeck, M. , Duru, F. , Candinas, R. , Seifert, B. , & Turina, M. (2002). ICD implantation with and without combined myocardial revascularisation–incidence of ICD therapy and late survival. The Thoracic and Cardiovascular Surgeon, 50(6), 333–336. 10.1055/s-2002-35741 [DOI] [PubMed] [Google Scholar]
- Canty, J. M. Jr , & Fallavollita, J. A. (2005). Hibernating myocardium. Journal of Nuclear Cardiology, 12(1), 104–119. 10.1016/j.nuclcard.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Canty, J. M. Jr , Suzuki, G. , Banas, M. D. , Verheyen, F. , Borgers, M. , & Fallavollita, J. A. (2004). Hibernating myocardium: Chronically adapted to ischemia but vulnerable to sudden death. Circulation Research, 94(8), 1142–1149. 10.1161/01.RES.0000125628.57672.CF [DOI] [PubMed] [Google Scholar]
- Carson, P. , Wertheimer, J. , Miller, A. , O'Connor, C. M. , Pina, I. L. , Selzman, C. , Sueta, C. , She, L. , Greene, D. , Lee, K. L. , Jones, R. H. , & Velazquez, E. J. (2013). The STICH trial (Surgical Treatment for Ischemic Heart Failure): Mode‐of‐death results. JACC: Heart Fail, 1(5), 400–408. 10.1016/j.jchf.2013.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquitt, J. L. , Mendes, D. , Clegg, A. J. , Harris, P. , Cooper, K. , Picot, J. , & Bryant, J. (2014). Implantable cardioverter defibrillators for the treatment of arrhythmias and cardiac resynchronisation therapy for the treatment of heart failure: Systematic review and economic evaluation. Health Technology Assessment, 18(56), 1–560. 10.3310/hta18560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove, D. M. , Lytle, B. W. , Taylor, P. C. , Stewart, R. W. , Golding, L. A. , Mahfood, S. , & Loop, F. D. (1989). Ventricular aneurysm resection. Trends in Surgical Risk. Circulation, 79(6 Pt 2), I97–101. [PubMed] [Google Scholar]
- Fallavollita, J. A. , Heavey, B. M. , Luisi, A. J. , Michalek, S. M. , Baldwa, S. , Mashtare, T. L. , Hutson, A. D. , deKemp, R. A. , Haka, M. S. , Sajjad, M. , Cimato, T. R. , Curtis, A. B. , Cain, M. E. , & Canty, J. M. (2014). Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. Journal of the American College of Cardiology, 63(2), 141–149. 10.1016/j.jacc.2013.07.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, H. , Case, R. B. , Moss, A. J. , Brown, M. W. , Carroll, E. R. , Andrews, M. L. , & Investigators, M.‐I. (2004). Analysis of mortality events in the Multicenter Automatic Defibrillator Implantation Trial (MADIT‐II). Journal of the American College of Cardiology, 43(8), 1459–1465. 10.1016/j.jacc.2003.11.038 [DOI] [PubMed] [Google Scholar]
- Holly, Thomas A. , Abbott, Brian G. , Al‐Mallah, Mouaz , Calnon, Dennis A. , Cohen, Mylan C. , DiFilippo, Frank P. , Ficaro, Edward P. , Freeman, Michael R. , Hendel, Robert C. , Jain, Diwakar , Leonard, Scott M. , Nichols, Kenneth J. , Polk, Donna M. , & Soman, Prem (2010). Single photon‐emission computed tomography. Journal of Nuclear Cardiology, 17(5), 941–973. 10.1007/s12350-010-9246-y [DOI] [PubMed] [Google Scholar]
- Liu, C. , Wang, L. , Li, B. O. , Wang, J. , Hu, Y. , Li, S. , Yu, Y. , & Gu, C. (2018). Surgical linear ablation for ventricular tachycardia with postinfarction ventricular aneurysm. Journal of Surgical Research, 228, 211–220. 10.1016/j.jss.2018.02.031 [DOI] [PubMed] [Google Scholar]
- Mondésert, B. , Khairy, P. , Schram, G. , Shohoudi, A. , Talajic, M. , Andrade, J. G. , Dubuc, M. , Guerra, P. G. , Macle, L. , Roy, D. , Dyrda, K. , Thibault, B. , Barrero, M. , Diaz, A. , Kouz, S. , McNicoll, S. , Nowakowska, D. , & Rivard, L. (2016). Impact of revascularization in patients with sustained ventricular arrhythmias, prior myocardial infarction, and preserved left ventricular ejection fraction. Heart Rhythm, 13(6), 1221–1227. 10.1016/j.hrthm.2016.01.019 [DOI] [PubMed] [Google Scholar]
- Myerburg, R. J. , & Junttila, M. J. (2012). Sudden cardiac death caused by coronary heart disease. Circulation, 125(8), 1043–1052. 10.1161/CIRCULATIONAHA.111.023846 [DOI] [PubMed] [Google Scholar]
- Nageh, M. F. , Kim, J. J. , Chen, L. H. , & Yao, J. F. (2014). Implantable defibrillators for secondary prevention of sudden cardiac death in cardiac surgery patients with perioperative ventricular arrhythmias. Journal of the American Heart Association, 3(4), e000686. 10.1161/JAHA.113.000686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeijer, M. N. , van den Berg, M. E. , Leening, M. J. G. , Hofman, A. , Franco, O. H. , Deckers, J. W. , Heeringa, J. , Rijnbeek, P. R. , Stricker, B. H. , & Eijgelsheim, M. (2015). Declining incidence of sudden cardiac death from 1990–2010 in a general middle‐aged and elderly population: The Rotterdam Study. Heart Rhythm, 12(1), 123–129. 10.1016/j.hrthm.2014.09.054 [DOI] [PubMed] [Google Scholar]
- Ning, X. , Ye, X. , Si, Y. , Yang, Z. , Zhao, Y. , Sun, Q. I. , Chen, R. , Tang, M. , Chen, K. , Zhang, X. , & Zhang, S. (2018). Prevalence and prognosis of ventricular tachycardia/ventricular fibrillation in patients with post‐infarction left ventricular aneurysm: Analysis of 575 cases. Journal of Electrocardiology, 51(4), 742–746. 10.1016/j.jelectrocard.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Partington, S. L. , Kwong, R. Y. , & Dorbala, S. (2011). Multimodality imaging in the assessment of myocardial viability. Heart Failure Reviews, 16(4), 381–395. 10.1007/s10741-010-9201-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzza, Andrea , Czer, Lawrence S. C. , Arabia, Francisco , Vespignani, Roberta , Esmailian, Fardad , Cheng, Wen , De Robertis, Michele A. , & Trento, Alfredo (2017). Left Ventricular Reconstruction for Postinfarction Left Ventricular Aneurysm: Review of Surgical Techniques. Texas Heart Institute Journal, 44(5), 326–335. 10.14503/THIJ-16-6068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, N. T. , & Schilling, R. J. (2018). Sudden cardiac death and arrhythmias. Arrhythmia & Electrophysiology Review, 7(2), 111–117. 10.15420/aer.2018:15:2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaselli, G. F. , & Zipes, D. P. (2004). What causes sudden death in heart failure? Circulation Research, 95(8), 754–763. 10.1161/01.RES.0000145047.14691.db [DOI] [PubMed] [Google Scholar]
- Tschabrunn, C. M. , Roujol, S. , Nezafat, R. , Faulkner‐Jones, B. , Buxton, A. E. , Josephson, M. E. , & Anter, E. (2016). A swine model of infarct‐related reentrant ventricular tachycardia: Electroanatomic, magnetic resonance, and histopathological characterization. Heart Rhythm, 13(1), 262–273. 10.1016/j.hrthm.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, H. , Tian, C. , Schindler, T. H. , Qiu, M. , Lu, M. , Shen, R. , Tian, Y. , Zhao, S.‐H. , & Zhang, X. (2014). The impacts of severe perfusion defects, akinetic/dyskinetic segments, and viable myocardium on the accuracy of volumes and LVEF measured by gated (9)(9)mTc‐MIBI SPECT and gated (1)(8)F‐FDG PET in patients with left ventricular aneurysm: Cardiac magnetic resonance imaging as the reference. Journal of Nuclear Cardiology, 21(6), 1230–1244. 10.1007/s12350-014-9978-1 [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Liu, X.‐J. , Hu, S. , Schindler, T. H. , Tian, Y. , He, Z.‐X. , Gao, R. , Wu, Q. , Wei, H. , Sayre, J. W. , & Schelbert, H. R. (2008). Long‐term survival of patients with viable and nonviable aneurysms assessed by 99mTc‐MIBI SPECT and 18F‐FDG PET: A comparative study of medical and surgical treatment. Journal of Nuclear Medicine, 49(8), 1288–1298. 10.2967/jnumed.107.046730 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Author elects to not share data.


