ABSTRACT
The HIV/AIDS prevalence in female sex workers (FSWs) and elderly male clients is increasing in Guangxi, China, but the transmission relationship between them remains unclear. This study aims to illuminate the transmission network between FSWs and elderly male clients using molecular epidemiological analyses. Phylogenetic analysis indicated that CRF01_AE was the dominant strain, followed by CRF07_BC and CRF08_BC in both groups. Multivariate logistic regression analysis indicated that viral loads of 50 to 1000 copies/mL, immunological treatment failure and CRF07_BC were risk factors for entering the transmission network. Transmission network analysis showed that CRF07_BC tended to form large clusters, whereas CRF01_AE tended to form multiple but small clusters. Two groups of 11 FSWs and 169 clients were intricately intertwined. Spatial analysis demonstrated the formation of hotspots and clusters of transmission sharing regional differences. In conclusion, our study provides direct genetic evidence of transmission linkages between FSWs and elderly male clients. Although the CRF01_AE subtype was still the predominant subtype in the region, the higher degree and larger clusters found in CRF07_BC illustrate a rapid and intensive uptrend, which is expected to increase its prevalence in the region in the future.
KEYWORDS: HIV/AIDS, female sex workers, elderly male clients, molecular epidemiology, transmission network
Introduction
China still faces the challenge of HIV/AIDS. In 2019, the annual HIV incidence reached 5.1/100,000, resulting in 963 thousand people living with HIV/AIDS. In addition, 21 thousand people died from AIDS, ranking first among Class B infectious diseases [1]. Guangxi is one of the provinces with the highest prevalence of HIV infection in China [2]. By the end of October, 2020, 97 thousand people were reported to be infected with HIV-1/AIDS, and the number of newly reported HIV cases reached 10 thousand people, which is higher than national averages.
In Guangxi Province, the main route of HIV transmission has shifted from initial intravenous drug use to heterosexual contact [3], which is associated with a faster spread of multiple genotypes that accelerate the progression of disease [4,5]. Female sex workers (FSWs) bear a tremendous burden of HIV infection. They also play a bridging role in the spread of the HIV epidemic from at-risk populations to the general public [6–8]. In Guangxi, commercial heterosexual transmission appears to be the primary mode of exposure for new HIV-1 infections among males. The cumulative number of reported HIV/AIDS cases in Guangxi aged 50 and older ranked first in China [3].
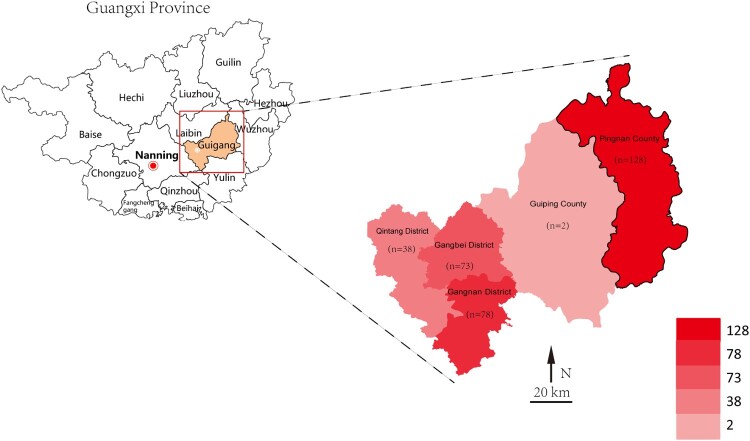
Recent studies have reported some generalities regarding HIV epidemics. The epidemic is generalized in terms of the late detection rate among men and the elderly in Guangxi, and prevalence tends to be significantly higher than the national average for the same period [9]. Furthermore, aging elderly adults continue to expand rapidly in Guangxi, and most of them are characterized by a low level of literacy, poor health awareness and absence of protection. All of the above has eventually led to the serious situation and the more strenuous task of prevention and treatment of HIV/AIDS in Guangxi Province. Guigang City, which is close to the provincial capital of Guangxi, is one of the cities with high HIV-1 prevalence in Guangxi Province. As a less developed area, Guigang City consists of 5 administrative regions (Figure 1) with a population of 5.56 million and an area of 10,600 km2. One of the epidemic characteristics is that the HIV-1 incidence among elderly men has increased dramatically in recent years, especially in the countryside [9–11]. Studies on the sources of infection and potential transmission routes among the elderly are of great significance for HIV/AIDS prevention and control.
Figure 1.
Geospatial distribution of the eligible patients in the study, 2009-2019.
Many studies concerning the role of commercial sex intercourse in the HIV-1 epidemic have been based on classic epidemiological methods [12,13]. However, most of them lack direct genetic evidence of transmission linkages between FSWs and their clients or the influence of transmission relationships on the HIV-1 epidemic. Targeted interventions on HIV prevalence between FSWs and elderly clients have a significant impact [14–16]. In recent years, utilization and advances in molecular epidemiology have corroborated critical aspects of the HIV epidemic [17–19]. Genetic analysis is helpful to develop interventions against HIV transmission [20–22]. A comprehensive analysis of HIV sequence, demographic and clinical data can clarify the subtype, distribution, and molecular transmission characteristics of HIV-1 infections and determine the influential spreader and hotspot regions involved. Therefore, in this study, we conducted a molecular epidemiological survey among FSWs and clients in Guigang City, a rural region of Guangxi. In this context, the findings of the present study are valuable for assessing the effect of commercial sex trade on local epidemics, and provide insights for other regions where commercial sex is prevalent.
Materials and methods
Participants
This study was conducted in Guigang City during January 2018 and March 2019. Inclusion criteria for older male clients included (1) local residents, (2) confirmed HIV/AIDS patients, (3) acquiring HIV-1 infection via heterosexual transmission, (4) history of transactional sex with FSWs, (5) no history of homosexual behaviour, and (6) no history of drug abuse. Inclusion criteria for FSWs included (1) at least 18 years old, (2) confirmed HIV-positive, (3) self-report of engaging in trading sex in change for money in the past 6 months, and (4) providing services to local residents. All participants in the study provided signed informed consent. Our recruitment was conducted at the local workplaces of transactional sex in Guigang City. Treatment eligibility referenced the Chinese National AIDS Free Antiretroviral Treatment Manual. Specifically, since 2008, the manual (2nd Edition) has recommended antiviral treatment for people with baseline CD4+ T cell counts less than 200 cells/μL. Then, in December 2012, the National Free ART Program (3rd Edition) implemented treatment initiation criteria for patients with baseline CD4+ T cell counts less than 350 cells/μL. After September 2016, according to the latest Chinese guideline (4th Edition), initiation of ART was performed among all people infected with HIV/AIDS. All patients included in the study were previously diagnosed with HIV infection between 2009 and 2019. Demographic data were collected through a verbally administered questionnaire, and the demographic information of all patients was obtained from the National HIV/AIDS Comprehensive Response Information Management System after obtaining informed consent. Venous blood (10 mL) was obtained from each subject. Plasma and blood cells were isolated by centrifugation and aliquots were stored at -80 °C. The study was approved by the Human Research Ethics Committee of Guangxi Medical University (Ethical Review No. 20170228-21).
CD4+ T cell counts and viral loads
CD4+ T cell counts were determined in fresh whole blood on a FACS Calibur (Becton Dickinson, San Jose, CA, USA) using TRITEST three-color CD4/CD8/CD3 reagent and TRUCOUNT tubes (Becton Dickinson). The HIV-1 viral loads were determined by real-time reverse transcription and polymerase chain reaction (RT-PCR) using a Cobas TaqMan 48 HIV-1 test with the high purity system (COBAS TaqMan 48, Amp link version 3.2; Roche Molecular Systems, Branchburg, NJ, USA).
Viral RNA/DNA extraction, cDNA synthesis, and amplification
For samples with HIV RNA >1000 copies/mL, HIV-1 RNA was extracted from 200 μL of plasma using the Tianlong RNA Extraction Kit (Tian Long, Beijing, China). Then, cDNA synthesis was performed using the reverse transcription kit (Takara, Dalian, China). For samples with HIV RNA ≤1000 copies/mL, proviral HIV-1 DNA was extracted from PBMCs using a whole-blood genomic DNA extraction kit according to the manufacturer’s instructions (Aidlab Inc, Beijing, China).
Reverse transcription and nested PCR amplification of the HIV-1 partial polymerase (pol) gene (nt 2253-3446 HXB2) were performed as previously described [23]. Upon successful amplification, the amplified products were then identified by electrophoresis using agarose gels (1%) and finally subjected to direct sequencing.
Sequence alignment and analysis
The obtained sequences were visually inspected, manually edited and then assembled with Sequencer 5.1. Next, quality control procedures were implemented to remove potential contaminant sequences in the online HIV Sequence Database. Then, sequences were aligned, edited, and analyzed by BioEdit software. In total, 320 sequences were obtained for subsequent analysis.
Phylogenetic analyses
Initially subtype analysis of HIV-1 pol region sequences was performed by NCBI Genotyping. A group of HIV-1 pol region reference sequences were downloaded from the Los Alamos HIV-1 sequence database. FastTree 2.2.10 and FigTree v1.4.1 software were utilized to further estimate an approximately-maximum likelihood (ML) phylogenetic tree based on GTR + CAT (general time reversible & category) nucleotide substitution models. Phylogenetic trees were plotted with labels for branch bootstrap ≥90. Subtypes in the HIV-1 pol region were finally identified using the online NCBI genotyping tool and ML phylogenetic trees.
Characteristics of transmission clusters
The transmission network was inferred based on the nucleotide genetic distances (GDs) between HIV-1 pol sequences from each participant. Hyphy2.2.4 software was used for GD computation based on the TN93 model. Then, calculated GDs were filtered using SAS 9.3 for GDs less than 1.5%, and the genetic transmission networks were generated at various GDs using Cytoscape v3.6.1. Degree indicates connectivity and represents the number of links or edges connecting to the other individuals in a molecular transmission network. Clusters are defined as those connected components of the network comprising two or more nodes. The optimum GD was defined as the GD when the maximum number of clusters was identified in the transmission network. To further uncover the role that patients played in the formation of networks, we took the region as a factor influencing the direction of propagation into consideration. First, edges between individuals in molecular transmission networks were translated into links between the townships and counties they represented to investigate possible transmission models between regions. Edges between individuals in molecular transmission networks were translated into links between the townships and counties they represented, and then the spatial distribution of HIV/AIDS combining epidemiological and genetic data was analyzed using the R package Circlize.
Definition
According to the National AIDS Free Antiviral Treatment Manual Version 4 (China), immunological failures were defined as CD4+ T cell counts reduced to baseline levels or below or remaining below 100 cells/mm3 after the initiation of the treatment regardless of viral loads.
Statistical analysis
The results were analyzed by standard statistical tests using SPSS (SPSS version 25.0, IBM Corp., Armonk, NY, USA, 2017). Median, standard deviation (SD) and interquartile ranges (for continuous variables), or frequencies (for categorical variables) were employed to describe participants’ demographic and clinical data. Categorical data were compared using chi-square tests, Fisher’s exact tests or nonparametric (Mann-Whitney, Kruskal-Wallis) tests. Logistical regression analysis was applicable to investigate the difference and association. All statistical tests were two sides, with significance determined at p<0.05.
Results
Demographic characteristics of the study population
Of the 349 participants, 47 female sex workers and 302 elderly male clients were involved. After excluding low-quality samples, 91.7% (320/349) of samples were successfully sequenced from 43 FSWs and 277 male clients. The sequences were then subjected to subsequent analysis. Among the eligible samples, 40.3% (129/320) lived in Pingnan County, 22.8% (73/320) were from Gangbei District, 24.4% (78/320) were from Gangnan District, 11.9% (38/320) were in Qintang District, and two were from Guiping City (Figure 1).
We provided baseline characteristics for eligible patients grouped by gender (Table 1). Of the 320 screened HIV-1-infected individuals, the median age of FSWs was 49 years old (IQR 41-54), and the youngest was 33 years old. The median age of clients was 64 years old (IQR 57-69), and the oldest was 79 years old. Among these patients, FSWs were mainly divorced or widowed (53.5%), and the majority (77.9%) of male clients were married. In addition, the vast majority (94.7%) had less than 12 years of schooling. Farmers accounted for 88.8% (246/277) of the clients. The median baseline CD4+ T count was 346.5 (IQR 241-417) cells/μL in the FSW group, and 167 (IQR 58-301) cells/μL in the elderly male client group. A total of 58.1% (25/43) of FSWs and 88.1% (244/277) of clients received antiviral treatment.
Table 1.
Characteristics of studied female sex workers and their older male clients in Guigang, Guangxi, 2009–2019.
| Variable | Total | FSWs, n (%) | Clients, n (%) |
|---|---|---|---|
| Total | 320 (1) | 43 (13.4) | 277 (86.6) |
| Age (median, IQR) | 63 (56, 68) | 49 (41, 54) | 64 (57, 69) |
| Marital status | |||
| Single | 9 (2.8) | 4 (9.3) | 5 (1.8) |
| Married | 231 (72.2) | 16 (37.2) | 215 (77.9) |
| Divorced/widowed | 79 (24.7) | 23 (53.5) | 56 (20.3) |
| Ethnicity | |||
| Han | 269 (84.1) | 32 (74.4) | 237 (85.9) |
| Zhuang | 45 (14.1) | 8 (18.6) | 37 (13.4) |
| Other | 5 (1.6) | 3 (7.0) | 2 (0.7) |
| Education (years) | |||
| < 6 | 222 (69.4) | 39 (90.7) | 183 (66.3) |
| 6-9 | 81 (25.3) | 3 (7.0) | 78 (28.3) |
| > 9 | 16 (5.0) | 1 (2.3) | 15 (5.4) |
| Baseline CD4+ T cell counts (median, IQR) | 191 (77, 333) | 346.5 (241, 417) | 167 (58, 301) |
| Latest CD4+ T cell counts (median, IQR) | 294 (153, 432) | 394 (269, 582) | 258 (139, 411) |
| Viral loads (copies/mL) | |||
| < 50 | 104 (32.5) | 3 (7.0) | 101 (36.6) |
| 50-1000 | 33 (10.3) | 0 (0) | 33 (12.0) |
| > 1000 | 17 (5.3) | 9 (20.9) | 8 (2.9) |
| Unknown | 166 (51.9) | 31 (72.1) | 134 (48.6) |
| ART | |||
| Yes | 269 (84.3) | 25 (58.1) | 244 (88.1) |
| No | 50 (15.7) | 18 (41.9) | 32 (11.9) |
Phylogenetic analysis of the screened participants
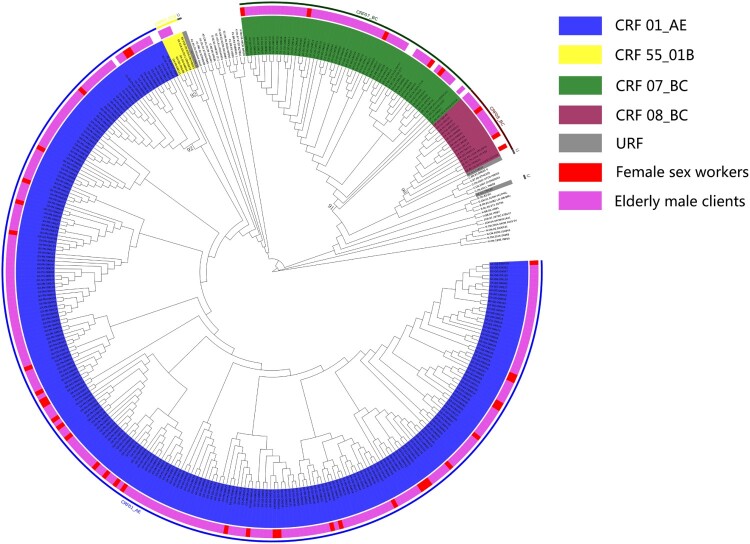
We performed phylogenetic analyses to understand the subtype distribution of 320 available sequences. Phylogenetic analyses revealed that the main prevalent subtype of this population was CRF01_AE (78.1%, 250/320) followed by CRF07_BC (15.6%, 50/320), CRF08_BC (4.1%, 13/320), CRF55_01B (0.9%, 3/320) and unique recombinant forms (URFs) (0.9%, 3/320) (Figure 2). In FSWs, 81.4% of sequences were classified as CRF01_AE, 11.6% as CRF07_BC, and 7.0% as CRF08_BC (Table 2). CRF01_AE (77.9%, 215/276) was the dominant subtype among elderly male clients followed by CRF07_BC (16.3%, 45/276), CRF08_BC (3.6%, 10/276), CRF55_01B (1.1%, 3/276) and URFs (1.1%, 3/276) (Table 3). For local female respondents, the variables of marital status (χ2=27.012, p<0.001) and immunological failure (χ2=7.023, p=0.031) were both significantly different among the HIV-1 subtypes. More FSWs infected with CRF01_AE were married, divorced or widowed, whereas relatively more FSWs infected with CRF07_BC were single. For their elderly male clients, ethnicity (χ2=20.152 p=0.013), baseline CD4+ T cell count (χ2=15.495, p=0.004) and latest CD4+ T cell count (χ2=13.530, p=0.009) were factors significantly associated with different subtypes. CRF01_AE was predominantly identified in Han infections, whereas CRF07_BC was relatively more prevalent among Zhuang minority infections. Moreover, patients with CRF01_AE infection had a lower baseline CD4+ T cell count and latest CD4+ T cell count than patients with CRF07_BC infection.
Figure 2.
ML phylogenetic tree of sequences obtained from female sex workers and elderly male clients. The various colors represent different subtypes and different attributes. URF, unique recombinant forms.
Table 2.
Demographic characteristics of female sex workers according to the HIV-1 subtype.
| Variable | CRF01_AE | CRF07_BC | CRF08_BC | χ2/Z | P |
|---|---|---|---|---|---|
| Total | 35 (81.4) | 5 (11.6) | 3 (7.0) | ||
| Age (years) | 1.424 | 0.591 | |||
| < 45 | 10 (28.6) | 3 (60.0) | 1 (33.3) | ||
| 45-54 | 17 (48.6) | 1 (20.0) | 2 (66.7) | ||
| 55-64 | 8 (22.9) | 1 (20.0) | 0 (0) | ||
| ≥ 65 | 0 (0) | 0 (0) | 0 (0) | ||
| Marital status | 27.012 | <0.001 | |||
| Single | 2 (5.7) | 2 (40.0) | 0 (0) | ||
| Married | 14 (40.0) | 1 (20.0) | 1 (33.3) | ||
| Divorced/widowed | 19 (54.3) | 2 (40.0) | 2 (66.7) | ||
| Ethnicity | 3.353 | 0.405 | |||
| Han | 26 (74.3) | 4 (80.0) | 2 (66.7) | ||
| Zhuang | 7 (20.0) | 1 (20.0) | 0 (0) | ||
| Other/Unknown | 2 (5.7) | 0 (0) | 1 (33.3) | ||
| Education (years) | 0.669 | 1.000 | |||
| < 6 | 32 (91.4) | 4 (80.0) | 3 (1) | ||
| 6-9 | 2 (5.7) | 1 (20.0) | 0 (0) | ||
| > 9 | 1 (2.9) | 0 (0) | 0 (0) | ||
| Baseline CD4+ T counts (cells/μL) | 1.474 | 0.478 | |||
| < 100 | 0 (0) | 0 (0) | 0 (0) | ||
| 100-199 | 4 (13.3) | 0 (0) | 0 (0) | ||
| 200-349 | 12 (40.0) | 1 (20.0) | 2 (66.7) | ||
| 350-499 | 9 (30.0) | 4 (80.0) | 0 (0) | ||
| ≥ 500 | 5 (16.7) | 0 (0) | 1 (33.3) | ||
| Latest CD4+ T counts (cells/μL) | 4.476 | 0.107 | |||
| < 100 | 0 (0) | 0 (0) | 0 (0) | ||
| 100-199 | 2 (7.1) | 0 (0) | 0 (0) | ||
| 200-349 | 4 (14.3) | 2 (40.0) | 2 (66.7) | ||
| 350-499 | 10 (35.7) | 3 (60.0) | 1 (33.3) | ||
| ≥ 500 | 12 (42.9) | 0 (0) | 0 (0) | ||
| Immunological failure | 7.023 | 0.031 | |||
| Yes | 1 (5.3) | 2 (66.7) | 1 (33.3) | ||
| No | 18 (94.7) | 1 (33.3) | 2 (66.7) | ||
| Viral loads (copies/mL) | 2.945 | 0.517 | |||
| < 50 | 2 (5.7) | 1 (20.0) | 0 (0) | ||
| 50-1000 | 0 (0) | 0 (0) | 0 (0) | ||
| > 1000 | 7 (20.0) | 1 (20.0) | 1 (33.3) | ||
| Unknown | 26 (74.3) | 3 (60.0) | 2 (66.7) |
Table 3.
Demographic characteristics of elderly male clients according to the HIV-1 subtype.
| Variable | CRF01_AE | CRF07_BC | CRF08_BC | CRF55_01B | URF | χ2/Z | P |
|---|---|---|---|---|---|---|---|
| Total | 215 (77.9) | 45 (16.3) | 10 (3.6) | 3 (1.1) | 3 (1.1) | ||
| Age (years) | 0.877 | 0.926 | |||||
| < 45 | 2 (0.9) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 45-54 | 25 (11.6) | 5 (11.1) | 0 (0) | 1 (33.3) | 1 (33.3) | ||
| 55-64 | 88 (40.9) | 20 (44.4) | 2 (20.0) | 1 (33.3) | 0 (0) | ||
| ≥ 65 | 100 (46.5) | 20 (44.4) | 8 (80.0) | 1 (33.3) | 2 (66.7) | ||
| Marital status | 6.378 | 0.681 | |||||
| Single | 3 (1.4) | 2 (4.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Married | 167 (77.7) | 34 (75.6) | 9 (90.0) | 2 (66.7) | 3 (1) | ||
| Divorced/widowed | 45 (20.9) | 9 (20.0) | 1 (10.0) | 1 (33.3) | 0 (0) | ||
| Ethnicity | 20.152 | 0.013 | |||||
| Han | 193 (89.8) | 31 (68.9) | 8 (80.0) | 3 (1) | 2 (66.7) | ||
| Zhuang | 21 (9.8) | 13 (28.9) | 2 (20.0) | 0 (0) | 1 (33.3) | ||
| Other/Unknown | 1 (0.5) | 1 (2.2) | 0 (0) | 0 (0) | 0 (0) | ||
| Education (years) | 1.304 | 0.523 | |||||
| < 6 | 144 (67.0) | 28 (62.2) | 8 (80.0) | 1 (33.3) | 2 (66.7) | ||
| 6-9 | 58 (27.0) | 16 (35.6) | 2 (20.0) | 1 (33.3) | 1 (33.3) | ||
| > 9 | 13 (6.0) | 1 (2.2) | 0 (0) | 1 (33.3) | 0 (0) | ||
| Baseline CD4+ T cell counts (cells/μL) | 15.495 | 0.004 | |||||
| < 100 | 83 (38.8) | 8 (17.8) | 1 (10.0) | 2 (66.7) | 1 (33.3) | ||
| 100-199 | 43 (20.1) | 12 (26.7) | 4 (40.0) | 1 (33.3) | 2 (66.7) | ||
| 200-349 | 53 (24.8) | 9 (20.0) | 4 (40.0) | 0 (0) | 0 (0) | ||
| 350-499 | 25 (11.7) | 9 (20.0) | 0 (0) | 0 (0) | 0 (0) | ||
| ≥ 500 | 10 (4.7) | 7 (15.6) | 1 (10.0) | 0 (0) | 0 (0) | ||
| Latest CD4+ T cell counts (cells/μL) | 13.530 | 0.009 | |||||
| < 100 | 40 (18.6) | 6 (13.3) | 0 (0) | 1 (33.3) | 1 (33.3) | ||
| 100-199 | 43 (20.0) | 6 (13.3) | 4 (40.0) | 1 (33.3) | 2 (66.7) | ||
| 200-349 | 66 (30.7) | 7 (15.6) | 3 (30.0) | 1 (33.3) | 0 (0) | ||
| 350-499 | 37 (17.2) | 14 (31.1) | 3 (30.0) | 0 (0) | 0 (0) | ||
| ≥ 500 | 29 (13.5) | 12 (26.7) | 0 (0) | 0 (0) | 0 (0) | ||
| Immunological failure | 2.839 | 0.537 | |||||
| Yes | 28 (14.6) | 7 (17.5) | 1 (14.3) | 0 (0) | 1 (50.0) | ||
| No | 164 (85.4) | 33 (82.5) | 6 (85.7) | 2 (1) | 1 (50.0) | ||
| Viral loads (copies/mL) | 11.500 | 0.446 | |||||
| < 50 | 73 (34.0) | 22 (48.9) | 4 (40.0) | 0 (0) | 2 (66.7) | ||
| 50-1000 | 27 (12.6) | 6 (13.3) | 0 (0) | 0 (0) | 0 (0) | ||
| > 1000 | 6 (2.8) | 2 (4.4) | 0 (0) | 0 (0) | 0 (0) | ||
| Unknown | 109 (50.7) | 15 (33.3) | 6 (60.0) | 3 (1) | 1 (33.3) |
Transmission network analysis
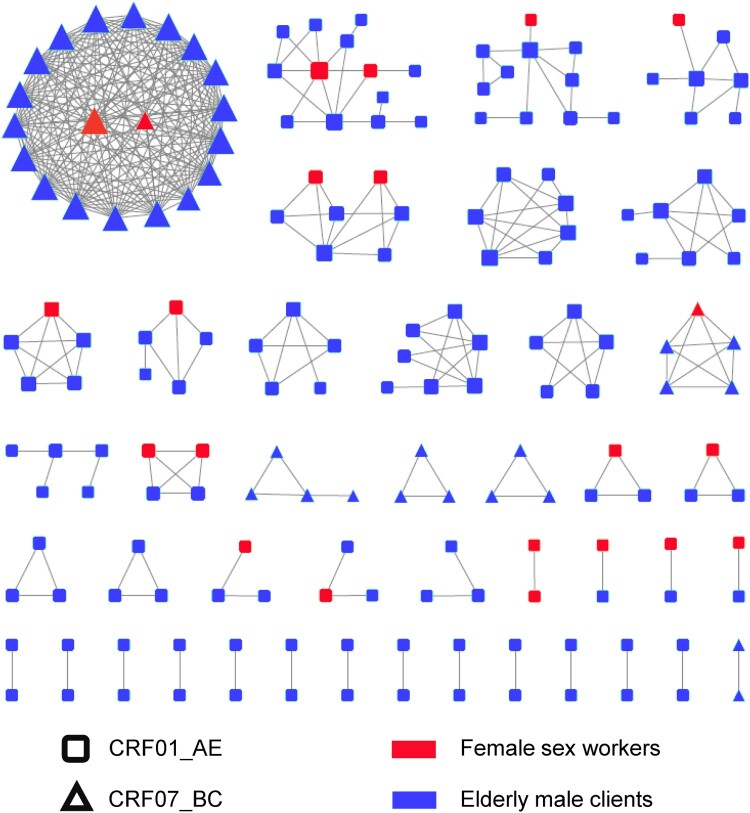
Then relationships for HIV transmission among different subtypes were observed by transmission network analysis. The genetic distance between pairs of sequences was calculated using the Tamura-Nei method. Transmission networks were constructed under a series of genetic distance thresholds ranging from 1–2%. Finally, we chose a threshold of a genetic distance confined to 1.2% to identify the maximum number of clusters in this study (Figure S1). Overall, 180 (56.3%) sequences were segregated into 43 networks, comprising 180 nodes and 361 edges (Figure 3). Out of the 180 patients, 11 were FSWs, and 169 were clients. Of these, 33.9% (61/180) of patients had 1 link, and 66.1% (119/180) had ≥2 links. Within the identified networks, 57.0% (143/251) of CRF01_AE individuals entered the networks and formed 37 clusters ranging in size from 2 to 13, whereas 74% (37/50) of CRF07_BC formed 6 clusters from 2 to 20. Moreover, 95.5% (172/180) of the individuals in the network received a diagnosis of HIV-1 infection from 2016 to 2019 (Figure S2). Network topology parameters were calculated using the Network Analyzer tool. The networks had a clustering coefficient (namely, how nodes shape clusters) of 0.530, characteristic path length (the desired distance between two connected nodes) of 1.484, network diameter (the longest shortest path among all pairs of nodes) of 4 and an average number of neighbors (the mean number of connections per node) of 4.011.
Figure 3.
Molecular transmission networks of HIV-1 among female commercial sex workers and their elderly male clients in Guigang City, Guangxi. This diagram was arranged by cluster sizes. Different shapes represent various subtypes: rectangle: CRF01_AE; triangle: CRF07_BC. Orange: female sex workers; blue: elderly male clients of FSWs. The sizes of those shapes were dictated by degree.
To explore features among the clustering and nonclustering individuals in the networks, we investigated differences between the two groups by using a logistic regression model (Table 4). For the univariate level, baseline CD4+ T cell counts (p=0.083), immunological failure (p=0.073), viral loads of 50 to 1000 copies/mL (p=0.033) and CRF07_BC (p=0.027) were significantly associated with clustering. Multivariate logistic regression analyses showed that patients with viral loads of 50 to 1000 copies/mL (OR: 2.958, 95% CI: 1.110-7.881) and suffering from immunological treatment failure (OR: 2.171, 95% CI: 1.114-4.230) were significantly associated with clustering. Additionally, compared with CRF01_AE, CRF07_BC was significantly related to clustering (OR: 2.320, 95% CI: 1.065-5.051).
Table 4.
Comparative analysis of sociodemographic characteristics of Clustering and Nonclustering individuals in Guigang City, Guangxi, 2009-2019.
| Demographic | Clustering, n (%) | Nonclustering, n (%) | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |||
| Total | 180 (56.3) | 140 (43.8) | ||||
| Gender | ||||||
| Female | 22 (12.2) | 21(15.1) | 1 | - | 1 | - |
| Male | 158 (87.8) | 118 (84.9) | 1.166 (0.626-2.170) | 0.628 | 0.807 (0.144-4.526) | 0.807 |
| Age (years) | ||||||
| < 45 | 7 (3.9) | 9 (6.5) | 1 | - | 1 | - |
| 45-54 | 33 (18.3) | 20 (14.4) | 2.121 (0.683-6.588) | 0.193 | 2.097 (0.420-10.479) | 0.367 |
| 55-64 | 68 (37.8) | 51 (36.7) | 1.714 (0.598-4.910) | 0.315 | 1.133 (0.211-6.085) | 0.884 |
| ≥ 65 | 72 (40.0) | 59 (42.4) | 1.569 (0.551-4.466) | 0.399 | 1.204 (0.220-6.594) | 0.831 |
| Occupation | ||||||
| Commercial services | 18 (10.0) | 16 (11.4) | 1 | - | 1 | - |
| Farmer | 141 (78.3) | 109 (77.9) | 1.150 (0.561-2.358) | 0.703 | 2.106 (0.358-12.374) | 0.410 |
| Other/Unknown | 21 (11.7) | 15 (10.7) | 1.244 (0.484-3.201) | 0.650 | 3.340 (0.522-21.369) | 0.203 |
| Marital status | ||||||
| Single | 4 (2.2) | 5 (3.6) | 1 | - | 1 | - |
| Married | 131 (72.8) | 100 (71.9) | 1.637 (0.429-6.256) | 0.471 | 3.943 (0.758-20.504) | 0.103 |
| Divorced/widowed | 45 (25.0) | 34 (24.5) | 1.654 (0.413-6.630) | 0.477 | 3.666 (0.676-19.887) | 0.132 |
| Ethnicity | ||||||
| Han | 151 (83.9) | 118 (84.3) | 1 | - | 1 | - |
| Zhuang | 27 (15.0) | 18 (12.9) | 1.172 (0.616-2.230) | 0.628 | 0.749 (0.343-1.634) | 0.467 |
| Other/Unknown | 2 (1.1) | 4 (2.9) | 0.391 (0.070-2.170) | 0.283 | 0.208 (0.014-3.148) | 0.257 |
| Education (years) | ||||||
| < 6 | 130 (72.2) | 92 (66.2) | 1 | - | 1 | - |
| 6-9 | 43 (23.9) | 38 (27.3) | 0.801 (0.480-1.336) | 0.395 | 0.604 (0.328-1.111) | 0.105 |
| > 9 | 7 (3.9) | 9 (6.5) | 0.550 (0.198-1.531) | 0.550 | 0.386 (0.111-1.346) | 0.135 |
| Baseline CD4+ T cell counts (cells/μL) | ||||||
| < 100 | 48 (27.3) | 47 (34.3) | 1 | - | 1 | - |
| 100-199 | 34 (19.3) | 32 (23.4) | 1.040 (0.555-1.950) | 0.902 | 1.157 (0.561-2.384) | 0.693 |
| 200-349 | 49 (27.8) | 32 (23.4) | 1.499 (0.823-2.732) | 0.186 | 1.969 (0.955-4.059) | 0.066 |
| 350-499 | 31 (17.6) | 16 (11.7) | 1.897 (0.919-3.917) | 0.083 | 1.758 (0.756-4.090) | 0.190 |
| ≥ 500 | 14 (8.0) | 10 (7.3) | 1.371 (0.554-3.391) | 0.495 | 1.279 (0.431-3.794) | 0.657 |
| Immunological failure | ||||||
| Yes | 51 (33.3) | 27 (23.3) | 1 | - | 1 | - |
| No | 102 (66.3) | 89 (76.7) | 1.648 (0.954-2.846) | 0.073 | 2.171 (1.114-4.230) | 0.023 |
| Viral loads (copies/mL) | ||||||
| < 50 | 60 (33.3) | 44 (31.4) | 1 | - | 1 | - |
| 50-1000 | 26 (14.4) | 7 (5.0) | 2.724 (1.085-6.840) | 0.033 | 2.958 (1.110-7.881) | 0.030 |
| > 1000 | 7 (3.9) | 10 (7.1) | 0.513 (0.181-1.454) | 0.209 | 0.443 (0.125-1.571) | 0.208 |
| Unknown | 87 (48.3) | 79 (56.4) | 0.808 (0.493-1.324) | 0.397 | 0.780 (0.423-1.439) | 0.427 |
| Subtype | ||||||
| CRF01_AE | 143 (79.4) | 108 (77.1) | 1 | - | 1 | - |
| CRF07_BC | 37 (20.6) | 13 (9.3) | 2.150 (1.090-4.241) | 0.027 | 2.320 (1.065-5.051) | 0.034 |
| Other | 0 (0) | 19 (13.6) | 0 | 0.998 | 0 | 0.998 |
CI, confidence interval.
We further analyzed the features of large transmission networks. Transmission clusters consisting of more than 10 nodes were defined as large transmission networks. In our study, the sizes of networks ranged between 2 and 20. Three large transmission clusters in our study consisted of 20, 13 and 11 persons. The largest cluster contained 2 FSWs and 18 elderly male clients, with an average degree of 18, and three-quarters of these individuals lived in Gangbei District. Within the second largest cluster, 2 FSWs and 11 clients living in Pingnan County were found. The third largest cluster included 1 FSW and 10 male clients who were all from Gangnan District. All patients in the largest cluster were infected with CRF07_BC, whereas the second and third largest clusters were all CRF01_AE strains (Figure 3). Furthermore, the sizes of clusters had a significant correlation with subtypes (χ2=22.108, p<0.001) (Figure S3).
Geographic analysis of genetic transmission networks
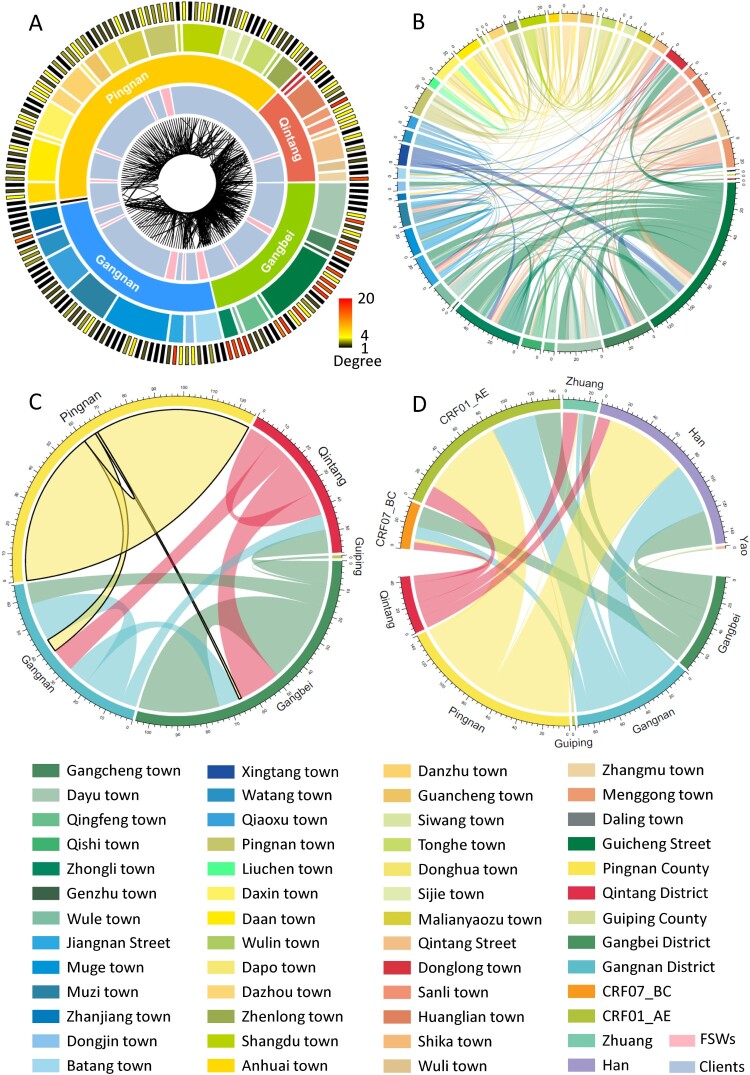
We further analyzed the geographical location of patients in the transmission networks. A majority of individuals with high degrees were located in Gangbei District followed by Qintang District and Gangnan District (Figure 4A). Similarly, the linkages connecting two individuals at the molecular genetic level appeared to be much stronger and more disordered around Gangbei and Qintang District than Pingnan County, demonstrating that much more complicated propagative relationships existed. In addition, transmission occurred not only inside or outside townships, but also cross districts. Moreover, the interactions between townships were clearer, and the complexity of the transmission was completely highlighted (Figure 4B). Most patients in Pingnan County tended to be connected with patients in the same county, but patients from Gangbei, Gangnan and Qintang Districts were cross-district connected (Figure 4C). The differences in subtypes (χ2=27.967, p<0.001) and ethnicity (χ2=60.748, p<0.001) of patients falling into the transmission networks among different regions were significant (Figure 4D, Table S1). The results indicated that the distribution of subtypes in Pingnan County differed significantly from that in the other counties and regions.
Figure 4.
Distribution, interconnections and demographic characteristics of the individuals involved in the molecular genetic transmission network. A. Each bar in the outermost circle represented an individual in the network. The colors depended on degrees: black: 1 degree; yellow: 4 degrees; red: 20 degrees. The second circle was the region. The innermost circle was the interconnection between individuals, and this connection originated from the edges of the molecular transmission network. B. To further visualize town-to-town connection. C. The interrelationships between regions were manifested. D. The diagram graphically depicted the relationship between the location and ethnicities and subtypes, respectively.
Discussion
In this population-based study, we found that elderly male clients with HIV-1 infection tended to be married, and most of the clients were farmers who resided in rural areas, which was consistent with other studies [24,25]. Additionally, all of them reported no homosexual behaviour, 31.8% (96/302) of them had no spouse or regular partner, and 67.9% (205/302) had a spouse or partner. Among these spouses and partners (not enrolled in this study), 69.8% (143/205) were HIV-seronegative, 25.9% (53/205) were positive, and the remaining were undetected. Thus, rural elderly individuals living with HIV/AIDS should be prioritized in HIV prevention. In addition, a previous study found that the proportion of FSWs in the higher age groups has increased over the years in Guangxi [14]. Similarly, FSWs in our study appeared to be old and 90.7% (39/43) of them received primary school or lower. Their clients were all elderly men in rural areas due to their poor socioeconomic status. These characteristics of low-fee FSWs were consistent with findings in similar studies and indicated a positive association with HIV prevalence [26–28]. Furthermore, mobility and privacy resulted in a low percentage of FSWs choosing antiretroviral treatment, which made it easier to spread the virus to their clients. In addition, other barriers, such as discrimination and stigma, were also responsible for weak consciousness of active testing, prevention and treatment [29]. Therefore, we could take full advantage of diverse modern technologies, such as the internet and social apps or health service centres of communities and villages, to conduct regular and interesting educational activities among FSWs and their clients.
As is reported, viral genetics have implications for HIV acquisition, transmission, possible differential rates of disease progression, responses to antiretroviral therapy, patient survival rates and vaccine development [30–34]. In contrast to some previous reports in Guangxi [35,36], 6 individuals infected with CRF55_01B or URF strains presented among male clients, indicating increasing complexity of HIV circulation in Guangxi. CRF55_01B, which was initially found among men-having-sex-with-men (MSM), has expanded to heterosexuals, according to our results and earlier reports [37,38]. Subtype CRF01_AE was still the predominant subtype in recent years, and the percentage of CRF07_BC exceeded that of CRF08_BC. This finding revealed that timely urgent measures must be taken to retard the spread of CRF01_AE due to its high virulence, mortality and faster disease progression, particularly among elderly male clients. In contrast, CRF07_BC was reported to be the primary driver of HIV-1 infection among people who injected abused drugs [39]. Thus, there are reasons to be skeptical of the concurrency of transactional sex and drug addiction, which was evidenced to facilitate the recombination of HIV strains [40].
Network clustering analysis showed that 56.3% of the sequences formed a network of 43 clusters, a relatively high percentage compared to other transmission groups [41]. Moreover, we found three large clusters in which one FSW linked to multiple clients. These results provide proof of a close transmission relationship within the commercial sex group and suggest that FSWs might be drivers of local HIV-1 spread. Sociodemographic characteristics associated with clustering were also analyzed. Individuals with elevated CD4+ T cell counts, viral loads of 50 to 1000 copies/mL or CRF07_BC were more likely to enter the transmission network, implying the importance of screening out these high-risk patients. Moreover, compared with separated CRF01_AE, CRF07_BC had a relatively higher degree and was prone to form a larger cluster. These results might suggest a rapid and intensive increase in CRF07_BC, whereas CRF01_AE had a longer period of transmission [42], scattered distribution of infection sources or a higher evolutionary rate [43]. Transmission analysis suggested that comprehensive intervention should be designed for FSWs in rural areas, including improving the HIV testing rate, condom use education and immediate antiviral treatment, especially for those with multiple links. For HIV-1-positive clients, HIV detection of sex partners is also essential.
Our study further combined a genetic transmission network with spatial distribution, which could provide valuable information for the assessment, surveillance, prevention and control of HIV-1 infection. First, spatial clusters of CRF07_BC mainly emerged in Gangbei District followed by Gangnan District. In addition, 70% of patients within the largest cluster exhibited a complicated transmission pattern and a concentrated uncontrolled HIV-1 epidemic, which highlighted the severe situation of HIV-1 infection and difficulty of intervention in Gangbei District. These findings also indicated that HIV-1 circulation in Gangbei District was influenced by the migrant population; thus, public health officials should reinforce the management of these patients to guarantee access to health services. Second, CRF01_AE was the most extensively scattered genotype in Pingnan County. People in Pingnan County were evidenced to conduct local transactions. This finding emphasized the necessity of comprehensive surveillance for diverse genetic HIV-1 circulation among local residents. Therefore, there is an urgent need to strengthen controls and increase awareness of potential risk behaviours for local HIV-1 transmission.
There are a few inherent limitations in this study. First, the sample composition may not be representative of the entire group of FSWs and elderly male clients in Guigang City. Second, the molecular network could provide an inferred transmission relationship but cannot be directly related to epidemiological information due to a lack of details of the sex trade. Third, blood samples of the clients’ sexual partners were not collected in this study. We could not explore the possibility of transmitting HIV-1 from FSWs to clients and then to clients’ partners.
Taken together, this study focused on HIV transmission among elderly male clients and FSWs. Our study provides genetic evidence of transmission linkages between FSWs and elderly male clients, indicating that FSWs are the drivers of HIV-1 spreading to the elderly male population. In addition, the higher degree and larger clusters found in the CRF07_BC subtype illustrate a rapid and intensive uptrend of CRF07_BC, suggesting that there will be an increase in its prevalence in the region in the future.
Supplementary Material
Acknowledgments
We would like to express our gratitude to all participants involved in this study and the staff of Guigang Center for Disease Control and Prevention for their support.
Funding Statement
This study was supported by the National Natural Science and Technology Foundation of China (81960602 and 81971935), Guangxi Science Fund for Distinguished Young Scholars (2018GXNSFFA281001), Guangxi Key Research and Development Plan (GuikeAB18050022), Self-financing Project of Health Commission of Guangxi (Z20180857), Guangxi Bagui Scholar (to Junjun Jiang), Guangxi Medical University Training Program for Distinguished Young Scholars (to Junjun Jiang), and Youth Science Foundation of Guangxi Medical University (GXMUYSF201906).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- 1.China Center for Disease Control and Prevention 2020 . ([updated Apr 20; cited 2020 May 1]). Available from: http://www.nhc.gov.cn/jkj/s3578/202004/b1519e1bc1a944fc8ec176db600f68d1.shtml.
- 2.Wang Y, Yang Y, Shi X, et al. The spatial distribution pattern of human immunodeficiency virus/acquired immune deficiency syndrome in China. Geospat Health. 2016 May 31;11(2):414. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Luo L, Pan SW, et al. HIV epidemiology and prevention in Southwestern China: trends from 1996-2017. Curr HIV Res. 2019;17(2):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu M, Zhang W, Zhang X, et al. HIV-1 CRF01_AE strain is associated with faster HIV/AIDS progression in Jiangsu Province, China. Sci Rep. 2017 May 8;7(1):1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiguoya MW, Mann JK, Chopera D, et al. Subtype-specific differences in Gag-protease-driven replication capacity are consistent with intersubtype differences in HIV-1 disease progression. J Virol. 2017 Jul 1;91:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nsanzimana S, Mills EJ, Harari O, et al. Prevalence and incidence of HIV among female sex workers and their clients: modelling the potential effects of intervention in Rwanda. BMJ Glob Health. 2020 Aug;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen B, Chen J, Shao Y, et al. Need for intervention services for promotion of condom use by female Sex workers to consider size of entertainment venues: a cross-sectional study. Med Sci Monit Basic Res. 2019 Jan 1;25:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truong HM, Fatch R, Grant RM, et al. Characterization of HIV recent infection Among high-risk men at public STI clinics in mumbai. AIDS Behav. 2018 Jul;22(Suppl 1):70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge XM, Yang WM, Zhu QY, et al. Epidemiological characteristics of HIV/AIDS in Guangxi Zhuang Autonomous Region, 2010-2017. Zhonghua Liu Xing Bing Xue Za Zhi. 2019 Mar 10;40(3):315–321. [DOI] [PubMed] [Google Scholar]
- 10.Guo HS, Feng XX, Zhang Q, et al. Survival status and influencing factors of HIV/AIDS cases in liuzhou, 2008-2018. Zhonghua Liu Xing Bing Xue Za Zhi. 2020 Dec 10;41(12):2098–2103. [DOI] [PubMed] [Google Scholar]
- 11.Hu X, Liang B, Zhou C, et al. HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther. 2019 Mar 16;16(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalla LC, Herce ME, Edwards JK, et al. The burden of HIV among female sex workers, men who have sex with men and transgender women in Haiti: results from the 2016 priorities for local AIDS control efforts (PLACE) study. J Int AIDS Soc. 2019 Jul;22(7):e25281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon E, Chabata ST, Magutshwa S, et al. Estimating the population size of female Sex workers in Zimbabwe: comparison of estimates obtained using different methods in twenty sites and development of a national-level estimate. J Acquir Immune Defic Syndr. 2020 Sep 1;85(1):30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Abraham Bussell S, Shen Z, et al. Declining inconsistent condom use but increasing HIV and syphilis prevalence among older male clients of female sex workers: analysis from sentinel surveillance sites (2010-2015), guangxi, China. Medicine (Baltimore. 2016 May;95(22):e3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Hsieh E, Wang L, et al. HIV/AIDS among female sex workers in China: epidemiology and recent prevention strategies. Curr HIV/AIDS Rep. 2020 Apr;17(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mbita G, Mwanamsangu A, Plotkin M, et al. Consistent condom use and dual protection among female sex workers: surveillance findings from a large-scale, community-based combination HIV prevention program in Tanzania. AIDS Behav. 2020 Mar;24(3):802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su L, Feng Y, Liang S, et al. The origin and spread of CRF85_BC, driven by heterosexual transmission among older people in sichuan, China. BMC Infect Dis. 2020 Oct 19;20(1):772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M, Ma Y, Chen H, et al. Spatial clusters of HIV-1 genotypes in a recently infected population in yunnan, China. BMC Infect Dis. 2019 Jul 29;19(1):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Y, Zhou Y, Lu J, et al. Molecular epidemiology of HIV-1 in Jiangsu Province, southeast China: genotypes and HIV-1 transmission networks Among newly diagnosed Men having Sex with Men in 2017. AIDS Res Hum Retroviruses. 2021 Jan;37(1):62–69. [DOI] [PubMed] [Google Scholar]
- 20.Paraskevis D, Beloukas A, Stasinos K, et al. HIV-1 molecular transmission clusters in nine european countries and Canada: association with demographic and clinical factors. BMC Med. 2019;17(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stecher M, Hoenigl M, Eis-Hübinger AM, et al. Hotspots of transmission driving the local Human immunodeficiency virus epidemic in the cologne-bonn region, Germany. Clinical Infectious Diseases: an Official Publication of the infectious diseases society of America. 2019;68(9):1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wertheim JO, Kosakovsky Pond SL, Forgione LA, et al. Social and genetic networks of HIV-1 transmission in New York city. PLoS Pathog.. 2017;13(1):e1006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen R, Liang B, Wen B, et al. No difference in prevalence of transmitted drug resistance between injection drug users and non-injection drug users: a cross-sectional study among antiretroviral treatment-naive HIV patients. Intervirology. 2018;61(6):281–291. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, His JH, Wu X, et al. Disparities in HIV and syphilis prevalence and risk factors between older male clients with and without steady sex partners in southwestern rural China. BMC Infect Dis. 2017 Apr 12;17(1):269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadol P, Hoang TV, Le LV, et al. High HIV prevalence and risk among male clients of female sex workers in Hanoi and Ho Chi Minh City, Vietnam. AIDS Behav. 2017 Aug;21(8):2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou C, Rou K, Dong WM, et al. High prevalence of HIV and syphilis and associated factors among low-fee female sex workers in mainland China: a cross-sectional study. BMC Infect Dis. 2014 Apr 26;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan YG, Liu JJ, Zhang YJ, et al. HIV, other sexually transmitted infections, and risk behaviors among female sex workers in Liuzhou, China. Int J Gynaecol Obstet. 2015 Jan;128(1):18–22. [DOI] [PubMed] [Google Scholar]
- 28.Ngui EM, Kako PM, Dressel A, et al. The association of HIV status with rural-urban differences in wealth in Malawi: 2004-2015/16. AIDS Care. 2020 Aug 24: 1–7. doi: 10.1080/09540121.2020.1808157 [Epub ahead of print. PMID: 32835495]. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Z, Li X, Qiao S, et al. Using communication privacy management theory to examine HIV disclosure to sexual partners/spouses among PLHIV in Guangxi. AIDS Care. 2015;27(Suppl 1):73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gartner MJ, Roche M, Churchill MJ, et al. Understanding the mechanisms driving the spread of subtype C HIV-1. EBioMedicine. 2020 Mar;53:102682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemelaar J, Loganathan S, Elangovan R, et al. Country level diversity of the HIV-1 pandemic between 1990 and 2015. J Virol. 2020 Dec 22;95(2):e01580–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venner CM, Nankya I, Kyeyune F, et al. Infecting HIV-1 subtype predicts disease progression in women of Sub-saharan Africa. EBioMedicine. 2016 Nov;13:305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hemelaar J, Elangovan R, Yun J, et al. Global and regional molecular epidemiology of HIV-1, 1990-2015: a systematic review, global survey, and trend analysis. Lancet Infect Dis. 2019 Feb;19(2):143–155. [DOI] [PubMed] [Google Scholar]
- 34.Lin PH, Lai CC, Yang JL, et al. Slow immunological progression in HIV-1 CRF07_BC-infected injecting drug users. Emerg Microbes Infect. 2013 Dec;2(12):e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang H, Liang B, Zhou B, et al. Distribution of subtypes of pol gene in HIV-1 epidemic strains in Guangxi Zhuang Autonomous Region, 2010-2012. Zhonghua Yu Fang Yi Xue Za Zhi. 2016 Jan;50(1):79–84. [DOI] [PubMed] [Google Scholar]
- 36.Deng YQ, Li JJ, Fang NY, et al. Study on HIV-1 subtype among elderly male clients and female sex workers of low-cost venues in Guangxi Zhuang Autonomous Region, China. Zhonghua Liu Xing Bing Xue Za Zhi. 2017 Mar 10;38(3):326–330. [DOI] [PubMed] [Google Scholar]
- 37.Han X, Takebe Y, Zhang W, et al. A large-scale survey of CRF55_01B from men-who-have-sex-with-men in China: implying the evolutionary history and public health impact. Sci Rep. 2015 Dec 15;5:18147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Feng Y, Shen Z, et al. HIV-1 Transmissions among recently infected individuals in Southwest China are predominantly derived from circulating local strains. Sci Rep. 2018 Aug 27;8(1):12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolopoulos GK, Kostaki EG, Paraskevis D.. Overview of HIV molecular epidemiology among people who inject drugs in Europe and Asia. Infect Genet Evol. 2016 Dec;46:256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei H, Xing H, Hsi JH, et al. The sexually driven epidemic in youths in China's southwestern border region was caused by dynamic emerging multiple recombinant HIV-1 strains. Sci Rep. 2015 Jul 2;5:11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M, Ma Y, Chen H, et al. HIV-1 genetic transmission networks among men who have sex with men in Kunming, China. PLoS One. 2018;13(4):e0196548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng Y, He X, Hsi JH, et al. The rapidly expanding CRF01_AE epidemic in China is driven by multiple lineages of HIV-1 viruses introduced in the 1990s. AIDS. 2013 Jul 17;27(11):1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan H, Liu Z, Wu X, et al. Evolutionary characteristics and genetic transmission patterns of predominant HIV-1 subtypes among men who have sex with men in China. Int J Infect Dis. 2020 Jan;90:125–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.