Abstract
Purpose
To study the demographic, clinical and topographic characteristics of keratoconus patients in Jordan.
Methods
A retrospective study which was conducted at King Abdullah University Hospital, Northern Jordan. The patients who visited our outpatient clinic from March 2015 to September 2020 and had a definite diagnosis of keratoconus were included in this study. Demographic and clinical data, including age, gender, family history, past ocular history, ophthalmic examination, and topographic parameters, were collected and analysed. Keratoconus severity was classified according to K mean readings.
Results
A total of 234 patients with keratoconus were evaluated in this study. The majority of patients (73, 31.2%) were between the ages of 20 and 24. Allergic conjunctivitis was the most frequent past ocular history. Fifty-five patients (23.5%) had a family history of keratoconus. Regarding severity, most of the eyes were mild (63.3%), followed by moderate (24.7%), and then severe (11.9%). The severity of keratoconus was significantly associated with gender (p<0.001). No correlation was found between family history and severity.
Conclusion
Most of the Keratoconus patients were young, with a mean age of 25.9 years. The majority were mild in severity, with more females presented in the severe stage. The study reported high rate of family history (23.5%) in comparison to similar studies. Therefore, screening of family members of Keratoconus patients is advisable.
Keywords: keratoconus, keratoconus characteristics, demographic profile, topographic parameters, Jordan
Introduction
Keratoconus (KC) is a progressive corneal ectasia, characterized by thinning and protrusion of the cornea, leading to irregular astigmatism and myopia development,1,2 As a consequence, KC patients complain of blurred and distorted vision, halos, and/or regular changes in their prescriptions of spectacles.3,4 It progresses gradually, and may lead to severe vision impairment. Therefore, detection and efficient treatment of this disease is of paramount importance.5 The average prevalence in general population is 1.38 per 1000 population.6 Prevalence of KC varies widely across different populations depending on many factors like geographic location, genetic factors, diagnostic criteria used in different countries; it can range from 9 per 100,000 in Japan to 2300 per 100,000 in India.7
The etiology of the disease remains unknown. However, there is evidence of genetic inheritance and possible association with systemic disease.8 An association between KC, atopy and excessive eye rubbing has been reported in some studies.2,9,10 The occurrence of KC is also suggested to be greater in countries with dusty, hot and dry climates.11 It is believed that the environmental factors in genetically susceptible patients may cause the disease.11
Awareness of the relationship between demographic factors, clinical characteristics and topographic indices will help to plan a regular management protocol that not only important for diagnosis but also to organize an effective early detection and screening program.12–15 Several studies have examined these parameters in various ethnic groups.16–19
In Jordan, Abu Ameerh et al studied the epidemiological aspects of a group of KC patients who were referred for keratoplasty.20 The present study aimed to comprehensively investigate and study the demographic, clinical, and topographic characteristics of KC patients who attended a tertiary hospital in the north of Jordan.
Materials and Methods
Study Design and Setting
This retrospective descriptive study was conducted at King Abdullah University Hospital (KAUH), Northern Jordan. The data of 263 patients who attended the ophthalmology clinic between March 2015 and September 2020 were retrieved from the electronic medical records database system of KAUH.
Sample Size and Data Collection
A total of 234 patients with KC were included in this study. Initially, a total of 263 patients were enrolled. However, 29 patients were excluded because of missing data. All available demographic and clinical data were collected, including age, gender, family history, systemic diseases, past ocular history. Data of ophthalmic examination were also collected, which included: visual acuity test using Snellen chart, manifest refraction, slit-lamp biomicroscopy findings and corneal topography parameters using Pentacam tomography (Oculus, Wetzlar, Germany). Diagnosis of KC was based on slit lamp exam (Central stromal thinning, Fleisher ring, Vogt’s stria, and apical scar), refraction, and findings on corneal topography. Impact on daily activity and Occupation were assessed through interviews, either in person or over the phone. A questionnaire specific to our patients was constructed by reference to 25-item National Eye Institute Visual Functioning Questionnaire (NEI-VFQ 25) and used to assess the quality of vision-related daily activity.21 It included questions evaluating the following: Ocular Pain, Near Activities, Distance Activities, Social Functioning, Dependency, Driving, and peripheral vision.
Severity Assessment and Grouping
Severity assessment was done by using K mean readings. The severity was classified into three groups, including mild: < 48 Diopter (D), moderate: 48–53 D, and severe: > 53 D. Patients were grouped according to age into four bands: <20 years, 20–24 years, 25–29 years, and ≥30 years.
Ethics
This study was approved ethically by the Institutional Review Board (IRB) of Jordan University of Science and Technology (Irbid, Jordan). Patients’ consent to review their medical records was not required by the IRB because the research involved minimal risk, as the review of subjects’ medical records was for limited information. The information was not sensitive in nature. A precaution was taken to limit the record review to specified data. Contacting subjects to obtain their consent could be considered an invasion of privacy and cause subjects undue anxiety. All study procedures were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments.
Statistical Analysis
SPSS 22.0 version was used to perform the statistical analysis. All variables were compared according to the age bands, gender and severity of the disease. Descriptive statistics were used to summarize the data. Variables were expressed as the mean ± standard deviation (mean ± SD). Chi-square analysis was used to find the association between categorical variables. Statistical significance between the mean of three or more independent groups was assessed by using a one-way analysis of variance (ANOVA). Spearman correlation coefficient was used to assess the correlation between age and K mean. A P value of less than 0.05 was considered statistically significant.
Results
Patients’ Characteristics
The socio-demographic and relevant characteristics of 234 patients with KC, 119 (50.9%) males and 115 (49.1%) females, are shown in Table 1. Their mean age was 25.9 (SD = 6.9) years. Almost one-third of patients were between the age of 20 to 24 years (n=73, 31.2%), and 30 (12.8%) patients were in the pediatric age group (≤ 18 years old). The majority of patients were unemployed.
Table 1.
The Socio-Demographic and Relevant Characteristics of 234 Patients with KC
| n (%) | |
|---|---|
| Gender | |
| Male | 119 (50.9) |
| Female | 115 (49.1) |
| Age in years | |
| < 20 | 40 (17.1) |
| 20–24 | 73 (31.2) |
| 25–29 | 60 (25.6) |
| ≥ 30 | 61 (26.1) |
| Occupation | |
| Employed office based | 33 (14.1) |
| Employed outside worker | 27 (11.5) |
| Unemployeda | 109 (46.6) |
| Unknown | 65 (27.7) |
| Affecting his/her daily activity | 58 (24.8) |
| Presence of systemic disease | 19 (8.1) |
| Past ocular history (POH) | 60(25.6) |
| Family history of KC | 55 (23.5) |
| Previous Cross linking (CXL) | 28 (12.0) |
| Consanguinity degree in presence of family history of KC | |
| 1st degree | 7 (2.9) |
| 2nd degree | 34 (14.5) |
| 3rd degree | 6 (2.6) |
| 4th degree | 8 (3.4) |
Note: aStudents are included.
About one-fourth (24.8%) of the patients reported that their vision-related daily activity was affected by their KC disease. About 8.1% of the patients had a systemic disease like Asthma, DM, hypothyroidism, or Down syndrome. About 23.5% of patients had a family history of KC.
Sixty (25.6%) patients had a previous history of eye disease or surgery. Allergic conjunctivitis (n=32, 13.7%) was the most frequent POH of KC patients, followed by Vernal keratoconjunctivitis (n=14, 6%). Seven (2.9%) patients had Penetrating keratoplasty (PKP) in the contralateral eye, two (0.9%) patients had strabismus surgery, three (1.3%) patients underwent phacoemulsification surgery, and 2 (0.9%) had intrastromal ring insertion.
Clinical Presentation of KC
The clinical presentation of KC patients is shown in Table 2. Decreased vision (70.9%) was the most common symptom associated with KC patients followed by frequent changes of glasses (9%) and seeking for refractive surgery (7.3%).
Table 2.
The Clinical Presentation of KC Patients
| Clinical Presentation | n | % |
|---|---|---|
| Decreased vision | 166 | 70.9 |
| Eye deviation | 1 | 0.4 |
| Frequent change of glasses | 21 | 9.0 |
| Headache | 5 | 2.1 |
| Incidental | 11 | 4.7 |
| Itching | 2 | 0.9 |
| Multiple | 6 | 2.6 |
| Screening family history of KC | 3 | 1.3 |
| Seeking refractive surgery | 17 | 7.3 |
| Uncomfortable with glasses | 1 | 0.4 |
| White lesion on the cornea | 1 | 0.4 |
| Total | 234 | 100.0 |
Keratoconus Severity
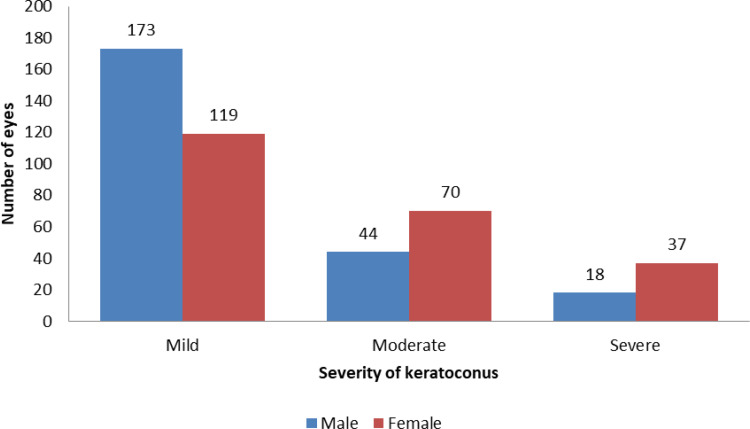
The number of eyes, according to KC severity, is shown in Figure 1. Seven eyes of 7 patients were excluded because they had Penetrating keratoplasty in the other eye due to the advanced stage of KC. The number of eyes in the mild stage of KC was 292 eyes (63.3%), followed by moderate (114, 24.7%) and severe stage (55, 11.9%). Among men and women, 73.5% and 25.8% of eyes had mild KC, respectively.
Figure 1.
Number of eyes according to KC severity.
Spearman correlation coefficient showed no significant correlation between age and K mean (r = −.001; p-value = 0.983).However, women were significantly more likely than men to have severe KC (17.25 vs.7.2%, p-value < 0.001). The distribution of the severity in individual eyes did not vary significantly according to family history of KC (p = 0.264).
Clinical and Topographic Parameters
The mean of quantitative parameters, including BCVA, sphere, cylinder, K mean, K max, and cornea thinnest location according to age and gender, are shown in Table 3, Table 4, respectively. There was a significant association between gender and the following parameters: BCVA, sphere, K max, and k mean. However, the results showed that only sphere was significantly associated with age.
Table 3.
Clinical and Topographic Parameters According to Gender
| Female | Male | Total | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | P-value | |
| BCVAa | 0.6 | 0.3 | 0.710 | 0.2797 | 0.673 | 0.2825 | 0.004 |
| Sphere | −1.7 | 2.5 | −0.8 | 2.3 | −1.2 | 2.5 | 0.000 |
| Cylinder | −2.9 | 1.7 | −2.8 | 1.6 | −2.8 | 1.6 | 0.499 |
| Cornea thinnest location | 455.5 | 55.5 | 461.9 | 50.0 | 458.8 | 52.8 | 0.192 |
| K meanb | 48.9 | 5.8 | 46.5 | 4.2 | 47.7 | 5.2 | 0.000 |
| K maxc | 55.6 | 9.5 | 53.2 | 7.7 | 54.4 | 8.7 | 0.003 |
Notes: aBest-corrected visual acuity; bkeratometry mean; ckeratometry maximum.
Table 4.
Clinical and Topographic Parameters According to Age
| <20 | 20–24 | 25–29 | ≥ 30 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | P-value | |
| BCVAa | 0.7 | 0.3 | 0.7 | 0.3 | 0.7 | 0.3 | 0.6 | 0.3 | 0.7 | 0.3 | 0.249 |
| Sphere | −0.7 | 2.0 | −0.7 | 1.6 | −1.6 | 2.4 | −1.8 | 3.3 | −1.2 | 2.5 | 0.000 |
| Cylinder | −2.6 | 1.5 | −2.7 | 1.7 | −3.0 | 1.7 | −3.0 | 1.6 | −2.8 | 1.6 | 0.223 |
| Cornea thinnest location | 460.0 | 48.7 | 460.9 | 50.7 | 454.9 | 63.1 | 459.3 | 46.8 | 458.8 | 52.8 | 0.828 |
| K meanb | 47.5 | 5.0 | 47.6 | 5.2 | 47.6 | 4.6 | 47.9 | 5.9 | 47.7 | 5.2 | 0.955 |
| K maxc | 53.6 | 7.6 | 54.9 | 8.8 | 54.3 | 8.2 | 54.5 | 9.7 | 54.4 | 8.7 | 0.757 |
Notes: aBest-corrected visual acuity; bkeratometry mean; ckeratometry maximum.
Discussion
Keratoconus (KC) is a bilateral corneal, non-inflammatory condition characterized by gradual thinning and apical protrusion.22 It may result in a significant decrease in vision, which can contribute to the need for corneal transplantation at a young age.23 In different countries, KC prevalence varies, suggesting the possible role of genetics in its etiology.8,11 Several studies evaluated the demographic, clinical characteristics of patients with KC in different countries.24–27 In the present study, we assessed the characteristics of 234 KC patients from a tertiary hospital in the northern region of Jordan.
The male patients were slightly higher than the female patients in our study. Several studies have reported that the prevalence of KC was high in males.19,27,28 While other studies did not find differences between males and females,7,29,30 it is still unclear whether gender differences are significant or not.
In this study, the majority of KC patients were found in the age band of 20 to 24 years. The mean age was 25.9 years. Several studies conducted in various countries demonstrated that the mean age of KC patients was between 18 and 24 years in Asian countries,31–33 in the USA was 20 to 39 years,22,30 and 20 to 35 years in European countries.16,34
KC significantly influences the vision-related quality of life.35,36 Our findings showed that about one-fourth of the patients reported that their vision-related daily activity was affected by their KC disease. About 23.5% of the patients had a family history of KC. The family history rate was reported as 15%37 and 13.5%22 in KC patients in several large population studies. According to our results; the presence of family history does not affect severity. Szczotka-Flynn et al reported the same in their study.38
Allergic conjunctivitis was the most frequent childhood complaint of the patients in this study. It has been hypothesized that during forceful eye rubbing seen in patients with allergic conjunctivitis, protease activity increases and contributes to the development and progression of KC.39 Similarly, a recent study by Bencharki et al40 reported that allergic conjunctivitis plays a significant role in the progression and severity of KC. Many different theories have been suggested to explain the correlation between eye rubbing and development of KC, like decrease in keratocytes density and changes in intraocular pressure (IOP) as a result of forced eye rubbing may lead to KC development and progression.41
According to the findings of our study, most of the patients had a mild level of KC (63.6%) followed by moderate (24.7%) and severe (11.9%), in a recent study; Rana et al had similar results where mild cases were the most.42 Many Factors could explain the results. Using newer techniques and protocols for diagnosis and increasing awareness of KC disease may play a role in detecting the disease in the early stage.
In the current study, the severity of KC was significantly associated with gender (P <0.001), where more females presented with severe disease; several studies reported the effect of hormonal levels in the biomechanics of cornea and hence the progression of KC.43,44 Moreover, it is believed that an increase in estrogen level during pregnancy, menstrual cycle, or during hormonal replacement therapy (HRT) may influence the stiffness of the cornea and therefore play a role in the progression of KC.44,45
On the contrary, our findings did not show a significant association between KC severity and age, which agrees with Rafati et al.25 Unfortunately, there is no worldwide consensus on the classification of KC severity; similar studies used different methods of severity characterization; this may influence the results and hence the comparability between studies.
The study is limited by its retrospective nature; some data were missing or not documented. The study setting was also one of the drawbacks of this study; we studied KC patients who visited our hospital, reducing the results’ generalizability and increasing the selection bias. In this regard, more population-based studies are therefore warranted.
Conclusion
In this study, the majority of the KC patients were between 20 and 24 years old. Most of the patients with KC had a mild level of severity. Allergic conjunctivitis was the most past ocular history. There was a significant association between gender and severity, whereas severity did not vary according to a family history of KC. However, the rate of family history (23.5%) was high in comparison to other countries. We advise to screen the family members of patients with KC.
The results of this study can be used during KC screening in Jordan, so early detection and intervention can be provided, and thus, better vision-related quality of life would be maintained for KC patients.
Disclosure
The authors report no funding or conflicts of interest for this work.
References
- 1.Kanski JJ, Bowling B. Clinical Ophthalmology: A Systematic Approach. Elsevier Health Sciences; 2011. [Google Scholar]
- 2.Romero-Jimenez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Contact Lens Anterior Eye. 2010;33(4):157–166. doi: 10.1016/j.clae.2010.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Lim N, Vogt U. Characteristics and functional outcomes of 130 patients with keratoconus attending a specialist contact lens clinic. Eye. 2002;16(1):54–59. doi: 10.1038/sj.eye.6700061 [DOI] [PubMed] [Google Scholar]
- 4.Weed KH, MacEwen CJ, Giles T, Low J, McGhee CNJ. The Dundee University Scottish Keratoconus study: demographics, corneal signs, associated diseases, and eye rubbing. Eye. 2008;22(4):534–541. doi: 10.1038/sj.eye.6702692 [DOI] [PubMed] [Google Scholar]
- 5.Kymes SM, Walline JJ, Zadnik K, Sterling J, Gordon MO; Group CLE of KS. Changes in the quality-of-life of people with keratoconus. Am J Ophthalmol. 2008;145(4):611–617. doi: 10.1016/j.ajo.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemi H, Heydarian S, Hooshmand E, et al. The prevalence and risk factors for keratoconus: a systematic review and meta-analysis. Cornea. 2020;39(2):263–270. doi: 10.1097/ICO.0000000000002150 [DOI] [PubMed] [Google Scholar]
- 7.Omer K. Epidemiology of keratoconus worldwide. Open Ophthalmol J. 2018;12(1):289. doi: 10.2174/1874364101812010289 [DOI] [Google Scholar]
- 8.Bykhovskaya Y, Margines B, Rabinowitz YS. Genetics in keratoconus: where are we? Eye Vis. 2016;3(1):1–10. doi: 10.1186/s40662-016-0047-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khor W-B, Wei RH, Lim L, Chan CM, Tan DT. Keratoconus in Asians: demographics, clinical characteristics and visual function in a hospital-based population. Clin Exp Ophthalmol. 2011;39(4):299–307. doi: 10.1111/j.1442-9071.2010.02458.x [DOI] [PubMed] [Google Scholar]
- 10.Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M. Characteristics and associations of keratoconus patients. Contact Lens Anterior Eye. 2015;38(3):199–205. doi: 10.1016/j.clae.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 11.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:1–19. doi: 10.1155/2015/795738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fink BA, Wagner H, Steger-May K, et al. Differences in keratoconus as a function of gender. Am J Ophthalmol. 2005;140(3):459–e1. doi: 10.1016/j.ajo.2005.03.078 [DOI] [PubMed] [Google Scholar]
- 13.Mihaltz K, Kovacs I, Takacs G, Nagy ZZ. Evaluation of keratometric, pachymetric, and elevation parameters of keratoconic corneas with Pentacam. Cornea. 2009;28(9):976–980. doi: 10.1097/ICO.0b013e31819e34de [DOI] [PubMed] [Google Scholar]
- 14.Hashemi H, Beiranvand A, Yekta A, Maleki A, Yazdani N, Khabazkhoob M. Pentacam top indices for diagnosing subclinical and definite keratoconus. J Curr Ophthalmol. 2016;28(1):21–26. doi: 10.1016/j.joco.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wygledowska-Promie Ska D, Zawojska I. Procedure for keratoconus detection according to the Rabinowitz-Rasheed method–personal experience. Klin Oczna. 2000;102(4):241–244. [PubMed] [Google Scholar]
- 16.Barrientos YF, Moreno SG, Soto ML. Estimated prevalence and clinical characteristics of keratoconus in the healthcare setting of the Hospital Costa del Sol, Spain. J Emmetropia J Cataract Refract Corneal Surg. 2014;5(1):15–21. [Google Scholar]
- 17.Rashid ZA, Millodot M, Evans KSE. Characteristics of keratoconic patients attending a specialist contact lens clinic in Kenya. Middle East Afr J Ophthalmol. 2016;23(4):283. doi: 10.4103/0974-9233.194074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gokhale NS. Epidemiology of keratoconus. Indian J Ophthalmol. 2013;61(8):382. doi: 10.4103/0301-4738.116054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohd-Ali B, Abdu M, Yaw CY, Mohidin N. Clinical characteristics of keratoconus patients in Malaysia: a review from a cornea specialist centre. J Optom. 2012;5(1):38–42. doi: 10.1016/j.optom.2012.01.002 [DOI] [Google Scholar]
- 20.Abu Ameerh MA, Al Refai RM, Al Bdour MD. Keratoconus patients at Jordan University Hospital: a descriptive study. Clin Ophthalmol. 2012;6:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visual function questionnaire 25 | National eye institute [WWW document]. n.d.. Available from: https://www.nei.nih.gov/learn-about-eye-health/resources-for-health-educators/outreach-materials/visual-function-questionnaire-25. Accessed February2, 2021.
- 22.Zadnik K, Barr JT, Gordon MO, Edrington TB. Biomicroscopic signs and disease severity in keratoconus. Cornea. 1996;15(2):139–146. doi: 10.1097/00003226-199603000-00006 [DOI] [PubMed] [Google Scholar]
- 23.Brierly SC, Izquierdo JL, Mannis MJ. Penetrating keratoplasty for keratoconus. Cornea. 2000;19(3):329–332. doi: 10.1097/00003226-200005000-00014 [DOI] [PubMed] [Google Scholar]
- 24.Assiri AA, Yousuf BI, Quantock AJ, Murphy PJ. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br J Ophthalmol. 2005;89(11):1403–1406. doi: 10.1136/bjo.2005.074955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafati S, Hashemi H, Nabovati P, et al. Demographic profile, clinical, and topographic characteristics of keratoconus patients attending at a tertiary eye center. J Curr Ophthalmol. 2019;31(3):268–274. doi: 10.1016/j.joco.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farrag AA. Keratoconus in the Omani Population; 2015.
- 27.Millodot M, Shneor E, Albou S, Atlani E, Gordon-Shaag A. Prevalence and associated factors of keratoconus in Jerusalem: a cross-sectional study. Ophthalmic Epidemiol. 2011;18(2):91–97. doi: 10.3109/09286586.2011.560747 [DOI] [PubMed] [Google Scholar]
- 28.Gorskova EN, Sevost’ianov EN. Epidemiology of keratoconus in the Urals. Vestn Oftalmol. 1998;114(4):38–40. [PubMed] [Google Scholar]
- 29.Pearson AR, Soneji B, Sarvananthan N, Sandford-Smith JH. Does ethnic origin influence the incidence or severity of keratoconus? Eye. 2000;14(4):625–628. doi: 10.1038/eye.2000.154 [DOI] [PubMed] [Google Scholar]
- 30.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101(3):267–273. doi: 10.1016/0002-9394(86)90817-2 [DOI] [PubMed] [Google Scholar]
- 31.Li SW, Li ZX, Shi WY, Zeng QY, Jin XM. Clinical features of 233 cases of keratoconus. Zhonghua Yan Ke Za Zhi. 2005;41(7):610. [PubMed] [Google Scholar]
- 32.Tanabe U, Fujiki K, Ogawa A, Ueda S, Kanai A. Prevalence of keratoconus patients in Japan. Nihon Ganka Gakkai Zasshi. 1985;89(3):407. [PubMed] [Google Scholar]
- 33.Sharma R, Titiyal JS, Prakash G, Sharma N, Tandon R, Vajpayee RB. Clinical profile and risk factors for keratoplasty and development of hydrops in north Indian patients with keratoconus. Cornea. 2009;28(4):367–370. doi: 10.1097/ICO.0b013e31818cd077 [DOI] [PubMed] [Google Scholar]
- 34.Nielsen K, Hjortdal J, Ehlers N, Ehlers N. Incidence and prevalence of keratoconus in Denmark. Acta Ophthalmol Scand. 2007;85(8):890–892. doi: 10.1111/j.1600-0420.2007.00981.x [DOI] [PubMed] [Google Scholar]
- 35.Aydin Kurna S, Altun A, Gencaga T, Akkaya S, Sengor T. Vision related quality of life in patients with keratoconus. J Ophthalmol. 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner H, Barr JT, Zadnik K; Group CLE of K (CLEK) S. Collaborative longitudinal evaluation of keratoconus (CLEK) study: methods and findings to date. Contact Lens Anterior Eye. 2007;30(4):223–232. doi: 10.1016/j.clae.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ihalainen A. Clinical and epidemiological features of keratoconus genetic and external factors in the pathogenesis of the disease. Acta Ophthalmol Suppl. 1986;178:1–64. [PubMed] [Google Scholar]
- 38.Szczotka-Flynn L, Slaughter M, McMahon T, et al. Disease severity and family history in keratoconus. Br J Ophthalmol. 2008;92(8):1108–1111. doi: 10.1136/bjo.2007.130294 [DOI] [PubMed] [Google Scholar]
- 39.Sharma N, Vajpayee R, Maharana P, Rao K. Ocular allergy and keratoconus. Indian J Ophthalmol. 2013;61(8):407. doi: 10.4103/0301-4738.116063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bencharki B. Comparative and Prospective Study of Kerato-Allergic Conjunctivitis Associated with Keratoconus. 2019:2.
- 41.Najmi H, Mobarki Y, Mania K, et al. The correlation between keratoconus and eye rubbing: a review. Int J Ophthalmol. 2019;12(11):1775–1781. doi: 10.18240/ijo.2019.11.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rana RS, Bajracharya L, Gurung R. Clinical profile on keratoconus presenting at a tertiary eye care centre-Tilganga institute of ophthalmology. Nepal J Ophthalmol. 2019;11(2):138–144. doi: 10.3126/nepjoph.v11i2.27818 [DOI] [PubMed] [Google Scholar]
- 43.McKay TB, Hjortdal J, Sejersen H, Asara JM, Wu J, Karamichos D. Endocrine and metabolic pathways linked to keratoconus: implications for the role of hormones in the stromal microenvironment. Sci Rep. 2016;6(1):25534. doi: 10.1038/srep25534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spoerl E, Zubaty V, Raiskup‐Wolf F, Pillunat LE. Oestrogen‐induced changes in biomechanics in the cornea as a possible reason for keratectasia. Br J Ophthalmol. 2007;91(11):1547–1550. doi: 10.1136/bjo.2007.124388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coco G, Kheirkhah A, Foulsham W, Dana R, Ciolino JB. Keratoconus progression associated with hormone replacement therapy. Am J Ophthalmol Case Rep. 2019;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]