Abstract
Nearly 17 years ago China launched its National HIV/AIDS Response Program, yet the epidemic still is not slowing. New cases and new deaths increase every year—in 2005, 40 711 people living with human immunodeficiency virus (HIV; PLWH) were diagnosed and 5729 died, whereas in 2019, 148 598 PLWH were diagnosed and 31 522 died. Moreover, the estimated PLWH population in China has risen to >1.25 million. However, epidemic data are worryingly complex and difficult to interpret, presenting challenges to the redirection and refocusing of efforts toward achievement of control. Here we present three “windows” into China’s epidemic data. From these viewpoints, it appears we still do not know how much infection exists, how much transmission is occurring, and in what contexts transmission happens. The enigma that is China’s HIV epidemic must be better understood. A new research agenda must be developed and executed if we are to change the future of HIV in China.
Keywords: HIV infection, transmission, diagnosis, case reporting, epidemic
Nearly 17 years into China’s National HIV/AIDS Response Program, the epidemic has not slowed, and the data are complex and difficult to interpret, presenting challenges to achieving control. A new research agenda is must be developed, prioritized, funded, and executed.
Expectations have been high that China’s massive, comprehensive, coordinated, and well-funded National HIV/AIDS Response Program would successfully bring about control of China’s human immunodeficiency virus (HIV) epidemic [1, 2]. Indeed, important gains have been made: testing, linkage to care, and treatment initiation have been dramatically improved [3, 4]; the National Free Antiretroviral Therapy Program has been rapidly scaled up, and treatment coverage has been expanded and regimens optimized [4]; and losses all along the care cascade have been reduced [5]. Prevention and harm reduction measures to protect key high-risk groups [6–9], advances to secure the blood supply [10], and training interventions to minimize occupational exposure risk [11] have been implemented and repeatedly enhanced. Infrastructure development projects aimed at bettering surveillance [12], case reporting, investigation, and management [13], and laboratory capacity and quality assurance [14] have been prioritized. Monitoring for virological failure [15, 16] and drug resistance [17] are improving, and availability of second-line therapy is expanding [4].
Nevertheless, China’s epidemic still shows no signs of slowing down [1, 2, 18]. National HIV/AIDS Response Program leaders thought they had a good overall understanding of the epidemic. Program components, each enormous, were developed alongside powerful data systems, which were integrated, upgraded, and relaunched in 2008 as China’s HIV/AIDS Comprehensive Response Information Management System (CRIMS) [19]. This has meant that an abundance of reasonably good quality data has been available for the generation of reports on a broad variety of metrics at local, county, district, prefecture, province, and national levels. Furthermore, extensive research has resulted in a substantial published literature of observational reports, interventional studies, and clinical trials to supplement official programmatic data. Although each of these program components is admittedly imperfect and in need of further development [4–17], the data are complex, in some cases contradictory, and generally difficult to interpret. Increasingly, China’s HIV/AIDS epidemic seems to be something of an enigma—a puzzle of complex and contradictory data that must be better understood in order to course-correct and begin to achieve control. The objective of this article is to illustrate this using three “windows” into China’s HIV epidemic data. From these viewpoints, it appears we still do not know how much infection exists, how much transmission is occurring, and in what contexts transmission is occurring.
How Much HIV Infection Is There?
Periodically since 2005, experts from the National Center for AIDS/STD Control and Prevention (NCAIDS) at the Chinese Center for Disease Control and Prevention (China CDC), the Joint United Nations Program on HIV/AIDS (UNAIDS), and the World Health Organization (WHO) have conducted a joint estimation process, using the “workbook” method, for the purpose of evaluating the status of China’s HIV epidemic [20]. Although the total estimated number of people living with HIV (PLWH) (ie, diagnosed plus undiagnosed) had steadily increased from 2005 to 2015, the estimated annual number of new infections had remained largely unchanged since 2007. Estimates of new infections were 50 000 (40 000–60 000) in 2007, 48 000 (41 000–55 000) in 2009, 48 000 (41 000–54 000) in 2011, 45 000 (38 000–55 000) in 2013, and 45 000 (41 000–50 000) in 2015 [18, 20–22]. These data strongly suggested that incidence had stabilized.
However, there were other indicators that caused program leaders to suspect that incidence was actually continuing to rise—stabilized estimates of new infections did not make sense in light of the still high and rising estimates of PLWH (diagnosed and undiagnosed), surveillance data, and case reporting data. Therefore, when the “workbook” method was again employed in 2017, the resulting estimates were very controversial. So, a new method was used in 2018—the “Spectrum/EPP” (ie, the Spectrum and Estimation and Projection Package program) method [23, 24]. The result was a total infected population size (diagnosed and undiagnosed) of 1.25 million at the end of 2018, with an estimated 80 000 (60 000–105 000) new HIV infections having occurred during that year [18]. Both estimates were well above what would have been expected based on trends that had been relatively consistent since 2005 (Figure 1A).
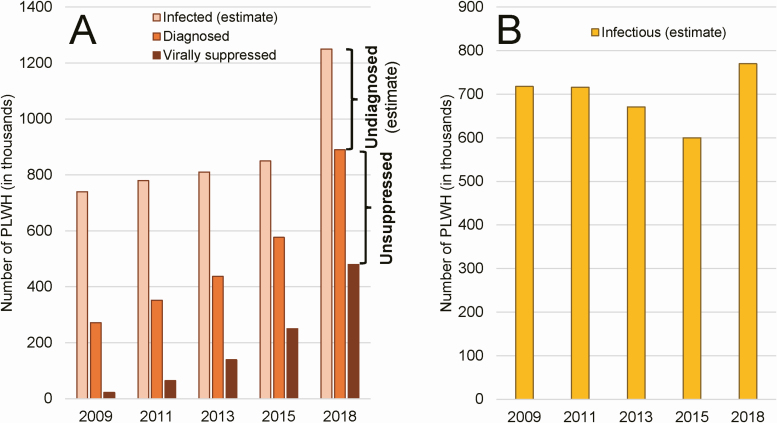
Figure 1.
Estimating the number of infected and infectious PLWH in China. A, Total number of people infected with HIV (ie, diagnosed and undiagnosed) is estimated periodically via a joint assessment process performed by NCAIDS/China CDC, UNAIDS, and WHO. Numbers of diagnosed PLWH and virally suppressed PLWH are obtained from case reporting data in CRIMS. Difference between the estimated number of people infected and the actual number of people diagnosed equals the estimated number of undiagnosed PLWH (ie, those who are infected but remain unaware of their status). Likewise, difference between the number of diagnosed PLWH and the number of virally suppressed PLWH equals the number of unsuppressed PLWH. B, Summed together, the undiagnosed and the unsuppressed equal an estimated number of PLWH who are infectious and capable of transmitting their HIV infection on to others. Abbreviations: CDC, Centers for Disease Control and Prevention; CRIMS, Comprehensive Response Information Management System; HIV, human immunodeficiency virus; NCAIDS, National Center for AIDS/STD Control and Prevention; PLWH, people living with HIV; UNAIDS, United Nations Program on HIV/AIDS; WHO, World Health Organization.
All things being equal, if the estimated numbers of annual new infections are correct, then the annual numbers of people who are newly at risk of transmitting infection (ie, those who are infectious) should have also stabilized. However, this ignores the already large population of PLWH who are not being treated or not being treated effectively. Recent modeling studies have found that >97% of new infections are caused by transmission from either PLWH who are undiagnosed, meaning they are infected but remain unaware of their infection, or by PLWH who are unsuppressed, meaning they have been diagnosed but have not achieved viral suppression [25, 26]. The sum of these 2 groups can serve as an estimate of the size of the infectious population.
Perhaps we can use the estimated size of the infectious population to answer the question, how much HIV infection is there in China? As an indicator of epidemic control, the decline observed from 2011 through 2015 would seem to suggest that China was gaining control of its epidemic. However, in 2018, the 1.25 million PLWH estimated using the new method (Figure 1A), less a total of 890 000 diagnosed PLWH in that year, results in an estimated 360 000 infected but undiagnosed people, all of whom were unknowingly capable of passing on their infection. Likewise, a total of 890 000 diagnosed PLWH, less a total of 480 000 virally suppressed PLWH in 2018, results in an estimated 410 000 people who were diagnosed but not virally suppressed, all of whom are also capable of passing on their infection. Summing the undiagnosed (ie, 360 000) and the unsuppressed (ie, 410 000) yields the estimated number of infectious PLWH in China in 2018, approximately 770 000 (Figure 1B). This value is alarming—almost 800 thousand people in China can create 1 or more new infections and what was previously thought to be a steadily declining trend has been interrupted by an abrupt increase of approximately 33% between 2015 and 2018 in the total estimated number of infectious PLWH in China.
What do these data mean? Do these estimated numbers of infectious PLWH help us predict numbers of new infections in subsequent years? Both methods used to estimate total and new infections are imperfect, but is one more accurate than the other? Hundreds of technical experts from across China have been involved in these exercises, as have international experts including top technical specialists from WHO and UNAIDS. The outcomes of each of these exercises were checked to ensure they made logical sense and biological sense, and it is widely believed that these estimates are the best approximation possible with currently available knowledge and technology.
Unfortunately, the only thing that can be concluded is that, no matter the estimation method used, China missed the 2020 deadline for the “First 90” UNAIDS target (90% of PLWH are diagnosed) [27]; considerable work remains if it is to achieve the 95–95–95 targets by 2030 [27], yet we still do not know how much HIV infection there is today in China.
How Much Transmission Is Occurring?
Knowing that the number of newly diagnosed PLWH is not the same as the number of newly infected PLWH, the idea behind scaling up HIV testing has been that, over time, more and more undiagnosed PLWH will be diagnosed, and the number of PLWH who remain undiagnosed in society will become smaller and smaller. Many leaders of China’s National HIV/AIDS Response Program expected that after more than a decade of aggressive HIV testing scale up, the annual numbers of newly diagnosed PLWH would gradually decline. However, rather than decline, case reporting data show that the absolute number of new HIV diagnoses in the country continues to rise year after year (Figure 2A) [2, 4]. But what does this mean? The number of newly diagnosed cases in a year is determined by 2 factors—the number of undiagnosed PLWH in society and the coverage of the society’s HIV testing programs. On the one hand, if there are very few undiagnosed PLWH, then even a near-universal HIV screening effort will not generate large numbers of new diagnoses. On the other hand, if there are many undiagnosed PLWH but a very limited HIV testing program, then there would be an extremely small number of PLWH being diagnosed during the year.
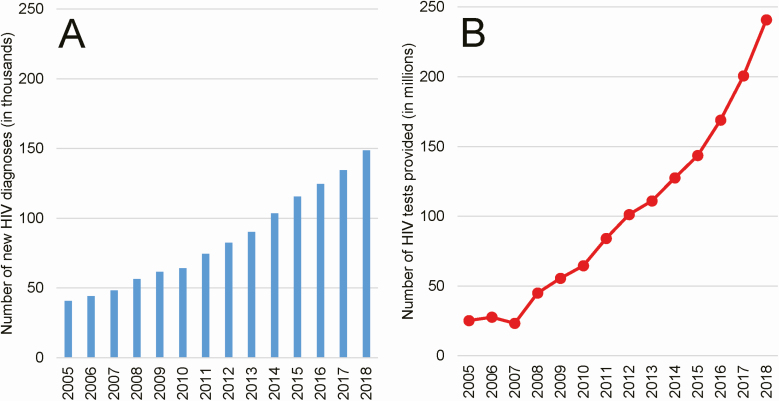
Figure 2.
Number of new HIV diagnoses made and number of HIV tests provided. A, Annual numbers of new diagnoses (in thousands; not new infections) are from case reporting data available in CRIMS. This is not a measure of incidence because many in China are only diagnosed after they have had HIV infection for many years. B, Annual numbers of HIV screening tests provided (in millions; tests actually taken, not just offered) is also from CRIMS. Because HIV tests may not be provided outside of government-run public health and medical settings and authorized community-based organizations, these data are highly accurate with one exception: HIV self-testing is increasing yet not counted in CRIMS. Thus, data from more recent years (ie, 2016–18) are likely an understatement of true testing coverage. Abbreviations: CRIMS, Comprehensive Response Information Management System; HIV, human immunodeficiency virus.
In China, HIV testing has been massively scaled up (Figure 2B) [28]. In 2005, <3700 testing sites provided 23.3 million tests, whereas by the end of 2018, nearly 25 000 sites provided 240.9 million tests to China’s citizens [18]. Although the increase in newly diagnosed HIV cases year after year is certainly partially driven by this scale-up in HIV testing, it also indicates that the population of PLWH in China who remain undiagnosed must still be very large.
However, it is difficult to separate the relative contributions of HIV testing scale-up and incidence of new infections to the effect we can see, which is an increase in new diagnoses over time. This is especially true when one considers the persistent problem of late diagnosis in China. A recent study estimated that newly diagnosed PLWH in China had already had HIV infection for a mean of 6.3 years at the time of their diagnosis [29]. Thus, in the China setting, after many years scaling up HIV testing, new diagnoses are still not the same thing as new infections.
So what can these data tell us about China’s HIV epidemic? How much transmission is occurring in China today? Is China losing or gaining control? If we subscribe to a definition of epidemic control that is based on estimated HIV incidence [30, 31], then the answer is we do not know. These data cannot tell us because of the disparity between date of infection and date of diagnosis.
Fortunately, there are tools to help separate recent from long-term infection. BED capture-enzyme immunoassays and limiting antigen-avidity assays have been used for many years in China in studies in high prevalence areas and in key populations, and more recently for surveillance at sentinel sites [14]. However, these methods have limitations that are exacerbated by high and increasing genetic diversity in human immunodeficiency virus type 1 strains in China as well as variability in viral loads, immune function, and antiretroviral therapy use among Chinese PLWH [32]. Newer and faster recency assays have been developed, but they have yet to be employed on a broad scale in China [32, 33].
In What Contexts Is Transmission Occurring?
The HIV epidemic in China began with outbreaks among people who inject drugs in the remote southwest region in the late 1980s and among former plasma donors in rural central provinces in the mid-1990s [2]. For more than 2 decades, the epidemic remained concentrated both geographically and within key high-risk groups. However, it has since shifted, and the dominant transmission route has been sexual contact for several years now [2]. Overall, the proportion of all newly diagnosed infections attributed to the sexual contact transmission route has increased from 11% in 2005 to 96% in 2017. In this 12-year period, the heterosexual contact transmission route increased from 11% of all newly diagnosed HIV infections to 70%, and male-male sexual contact increased from near zero to 26% (Figure 3A) [18].
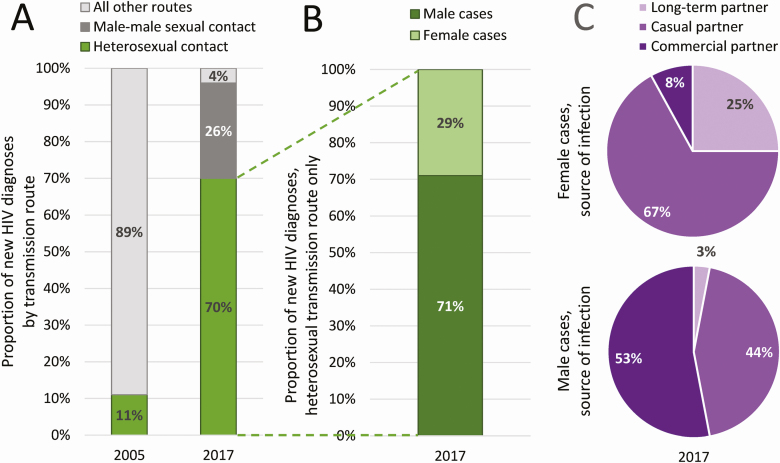
Figure 3.
Contexts of HIV sexual transmission. A, Among newly diagnosed cases, only 11% were attributed to sexual contact in 2005, whereas in 2017, 96% were attributed to sexual contact: 70% heterosexual contact and 26% male-male sexual contact (ie, among MSM). B, Among heterosexual cases in 2017, 71% were males and 29% were females. C, Taking a closer look at the sources of these infections, 53% of male heterosexual cases report acquiring HIV from commercial partners compared to only 8% of females, and only 3% of males report acquiring HIV from their spouse or long-term partner compared to 25% of females. Although these data are from case reports in CRIMS, they are based on risk behavior self-reported by newly diagnosed PLWH during routine epidemiological investigation. Abbreviations: CRIMS, Comprehensive Response Information Management System; HIV, human immunodeficiency virus; MSM, men who have sex with men; PLWH, people living with HIV.
Although the shifting of transmission routes over time and the expansion of the HIV epidemic beyond high-risk groups and into the general population was not unexpected [34], the level of complexity in the data on heterosexual transmission has been surprising, making it difficult to interpret and presenting challenges to the development of interventions meant to curb transmission.
For instance, with most newly diagnosed HIV cases each year attributed to heterosexual transmission (ie, currently 70%; Figure 3A), why has HIV prevalence among female sex workers (FSW) and their male clients remained consistently very low? Indeed, HIV prevalence at the national level among these 2 vulnerable groups has never climbed above 1% [7, 18, 35]. Granted, national-level figures such as this can hide locally high concentrations of HIV among risk groups, but we would still expect national-level prevalence in these risk groups to already be well above its historical value. Furthermore, epidemiological investigations (which includes contact tracing) of newly diagnosed cases seem to contradict these data. In 2017, 71% of all heterosexually acquired cases were male, and 29% were female (Figure 3B). Among male cases, 53% reported the source of their infection as commercial sex partners (ie, FSW) and 44% reported the source of their infection as casual partners (ie, nonmarital, noncommercial partners). By contrast, only 8% of female cases reported commercial sex partners and 67% reported casual partners as the source of their HIV infection (Figure 3C) [18]. How can these seemingly conflicting observations be reconciled? Where does the HIV infection come from? Perhaps the sentinel surveillance program failed to survey representative samples of FSW and their male clients or perhaps exposures were not accurately reported.
The complexity of heterosexual transmission in China is illustrated also by the older adult male demographic (ie, men >60 years of age). There has been a significant increase in HIV among older adult men who report heterosexual contact as their infection route. The absolute number of newly diagnosed cases among this group has increased each year and is up from 4800 in 2010 to 19 800 in 2017. Additionally, the older adult heterosexual male population represents an increasingly large proportion of all newly diagnosed cases each year, up from 7.4% in 2010 to 14.7% in 2017 [18]. HIV sexual transmission has been traditionally assumed to be a risk for younger, more sexually active people. However, in China at least, this assumption is clearly incorrect. Although these figures likely reflect the troubling problem of late diagnosis in China [29], they may also suggest a rise in the use of erectile dysfunction medication (EDM) or their less expensive alternatives, which in China are typically unregulated, illegally manufactured aphrodisiacs of unknown chemical composition. Studies among older Chinese men have suggested that EDM or aphrodisiac use, especially among those engaged in low-cost commercial sex and/or condomless sex, have greater risk of HIV and other sexually transmitted infections [36, 37]. Clearly these data indicate an urgent need for prevention and testing interventions in this vulnerable group.
It is likely that the HIV transmission route is misclassified as a result of social desirability bias. Stigma and discrimination toward MSM are still prevalent in China. An unpublished epidemiology data quality assessment study in China found that about 8–10% of MSM reported their HIV infections to be heterosexually acquired. But a correction for this bias based on this study is insufficient to account for the the overall trends observed. Guangxi Province has the highest proportion of elderly male HIV cases nationwide, and local epidemiologists there have found that most of these elderly men are widowed, divorced, or separated and that they visit low-fee FSW. Thus, it seems that data quality issues alone cannot simply explain the phenomenon observed.
However, if reducing incidence is the key to gaining control of the HIV epidemic in China [30, 31], then the heterosexual transmission route must be addressed. But how do we effectively and efficiently find people at risk of acquiring HIV infection via heterosexual sex to intervene with prevention measures if they are not members of traditionally recognized high-risk groups? Molecular epidemiology studies and phylogenetic analyses can sort out these types of transmission network challenges. However, in China, these techniques have thus far been used primarily to describe the genetic diversity and prevalence of HIV variants: little work has been done to leverage these methods to explore HIV transmission networks in China [38].
Moving Forward
We have presented 3 “windows” into China’s epidemic data. From these viewpoints, it appears that we still do not know how much infection there is, how much transmission is occurring, and in what contexts transmission happens. The enigma that is China’s HIV epidemic must be better understood. Perhaps China’s new “mega-cohort” studies (ie, a cohort of more than 10 million people followed since 2011) will provide some insight. Perhaps renewed emphasis on more detailed contact tracing will help if it is accompanied by concerted efforts to eliminate stigma and discrimination. Surely some answers must lie in existing programmatic information, which could be disaggregated in more precise and different ways to look for new patterns. Clearly, a comprehensive, integrated review of all National HIV/AIDS Response Program data needs to be conducted and then supplemented with other sources of nongovernmental data. However, the best way forward is with a new research agenda. A new, comprehensive HIV research agenda must be thoughtfully developed, highly prioritized, appropriately funded, and urgently executed, and new evidence generated must be quickly used to drive broad-sweeping programmatic changes. This is the only way that China can begin to curb incidence and gain control of its HIV epidemic. This is the only way to change the future of HIV—and change the future for PLWH—in China.
Notes
Author contributions. Z. W. wrote the initial draft of the manuscript. All authors contributed to many revisions and approved the final version for submission.
Acknowledgments. The authors thank Richard A. Stone, Ronald L. Moolenaar, and R. J. Simonds for their valuable insights, feedback, and encouragement.
Disclaimer. The views and opinions expressed here belong to the authors alone, and do not necessarily reflect the official views or endorsement of their affiliated institutions.
Financial support. This work was funded by China’s National Health Commission (grant number 2018ZX10721102). The study sponsor had no role in the writing of the report and in the decision to submit the paper for publication. Z. W. had final responsibility for the decision to submit for publication.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Wu Z, Detels R, McGoogan JM. Challenges and future directions. In: Wu Z, Wang Y, Detels R, Bulterys M, McGoogan JM, eds. HIV/AIDS in China—epidemiology, prevention, and treatment. Singapore: Springer, 2020:675–85. [Google Scholar]
- 2. Wu Z, Chen J, Scott SR, McGoogan JM. History of the HIV epidemic in China. Curr HIV/AIDS Rep 2019; 16:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu Z, Tang Z, Mao Y, et al. Testing and linkage to HIV care in China: a cluster-randomised trial. Lancet HIV 2017; 4:e555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao W, Hsieh E, Li T. Optimizing treatment for adults with HIV/AIDS in China: successes over two decades and remaining challenges. Curr HIV/AIDS Rep 2020; 17:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ma Y, Dou Z, Guo W, et al. The human immunodeficiency virus care continuum in China: 1985–2015. Clin Infect Dis 2018; 66:833–9. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Zou X, Xu Y, et al. The decade-long Chinese methadone maintenance therapy yields large population and economic benefits for drug users in reducing harm, HIV and HCV disease Burden. Front Public Health 2019; 7:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang H, Hsieh E, Wang L, Liao S. HIV/AIDS among female sex workers in China: epidemiology and recent prevention strategies. Curr HIV/AIDS Rep 2020; 17:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchwood TD, He J, Smith MK, et al. “Getting to Zero” among men who have sex with men in China: a review of the HIV care continuum. Curr HIV/AIDS Rep 2019; 16:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen H, Yang X, Zhu Q, et al. Treatment for HIV prevention study in southwestern areas of China. Infect Dis Model 2018; 3:249–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin YH, Li CQ, Liu Z. Blood donation in China: sustaining efforts and challenges in achieving safety and availability. Transfusion 2015; 55:2523–30. [DOI] [PubMed] [Google Scholar]
- 11. Wu Q, Xue XF, Shah D, Zhao J, Hwang LY, Zhuang G. Knowledge, attitude, and practices regarding occupational HIV exposure and protection among health care workers in China: census survey in a rural area. J Int Assoc Provid AIDS Care 2016; 15:363–9. [DOI] [PubMed] [Google Scholar]
- 12. Cui Y, Guo W, Li D, et al. Estimating HIV incidence among key affected populations in China from serial cross-sectional surveys in 2010–2014. J Int AIDS Soc 2016; 19:20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Z, Mao Y, McGoogan JM. National HIV/AIDS case management. In: Wu Z, Wang Y, Detels R, Bulterys M, McGoogan JM, eds. HIV/AIDS in China—epidemiology, prevention and treatment. Singapore: Springer, 2020:491–502. [Google Scholar]
- 14. Jiang Y. HIV laboratory network and quality assurance system. In: Wu Z, Wang Y, Detels R, Bulterys M, McGoogan JM, eds. HIV/AIDS in China—epidemiology, prevention and treatment. Singapore: Springer, 2020:75–102. [Google Scholar]
- 15. Ma Y, Dou Z, McGoogan JM, Wu Z. Reply to Zhang et al. Clin Infect Dis 2018; 67:809–10. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Wu Z, McGoogan JM, et al. Nationwide cohort study of antiretroviral therapy timing: treatment dropout and virological failure in China, 2011–2015. Clin Infect Dis 2019; 68:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuo L, Liu K, Liu H, et al. Trend of HIV-1 drug resistance in China: a systematic review and meta-analysis of data accumulated over 17 years (2001–2017). EClinicalMedicine 2020; 18:100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Center for AIDS/STD Control and Prevention. 2018 China national HIV/STD/HCV program implementation report. Beijing: Chinese Center for Disease Control and Prevention, 2019. [Google Scholar]
- 19. Mao Y, Wu Z, Poundstone K, et al. Development of a unified web-based national HIV/AIDS information system in China. Int J Epidemiol 2010; 39:ii79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu F, Wang N, Wu Z, et al. Estimating the number of people at risk for and living with HIV in China in 2005: methods and results. Sex Transm Infect 2006; 82:iii87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang L, Wang N, Wang L, et al. The 2007 estimates for people at risk for and living with HIV in China: progress and challenges. J Acquir Immune Defic Syndr 2009; 50:414–8. [DOI] [PubMed] [Google Scholar]
- 22. Wang N, Wang L, Wu Z, et al. ; National Expert Group on HIV/AIDS Estimation . Estimating the number of people living with HIV/AIDS in China: 2003–09. Int J Epidemiol 2010; 39:ii21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stover J, Brown T, Marston M. Updates to the spectrum/estimation and projection package (EPP) model to estimate HIV trends for adults and children. Sex Transm Infect 2012; 88 Suppl 2:i11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. UNAIDS. Methods for deriving UNAIDS estimates 2018. Geneva, Switzerland: UNAIDS, 2018. [Google Scholar]
- 25. Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175:588–96. [DOI] [PubMed] [Google Scholar]
- 26. Escudero DJ, Lurie MN, Mayer KH, et al. The risk of HIV transmission at each step of the HIV care continuum among people who inject drugs: a modeling study. BMC Public Health 2017; 17:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. UNAIDS. Fast-track: ending the AIDS epidemic by 2030. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 28. McGoogan JM, Wu Z. The revolution of HIV testing. In: Wu Z, Wang Y, Detels R, Bulterys M, McGoogan JM, eds. HIV/AIDS in China—epidemiology, prevention and treatment. Singapore: Springer, 2020:251–68. [Google Scholar]
- 29. Li AH, Wu ZY, Jiang Z, McGoogan JM, Zhao Y, Duan S. Duration of human immunodeficiency virus infection at diagnosis among new human immunodeficiency virus cases in Dehong, Yunnan, China, 2008–2015. Chin Med J (Engl) 2018; 131:1936–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. UNAIDS. Making the end of AIDS real: consensus building around what we mean by “epidemic control”—a meeting convened by the UNAIDS Science Panel. Geneva, Switzerland: UNAIDS, 2017. [Google Scholar]
- 31. Galvani AP, Pandey A, Fitzpatrick MC, Medlock J, Gray GE. Defining control of HIV epidemics. Lancet HIV 2018; 5:e667–70. [DOI] [PubMed] [Google Scholar]
- 32. Cai Q, Wang H, Huang L, Yan H, Zhu W, Tang S. Characterization of HIV-1 genotype specific antigens for the detection of recent and long-term HIV-1 infection in China. Virus Res 2019; 264:16–21. [DOI] [PubMed] [Google Scholar]
- 33. Kim AA, Behel S, Northbrook S, Parekh BS. Tracking with recency assays to control the epidemic: real-time HIV surveillance and public health response. AIDS 2019; 33:1527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu L, Jia M, Ma Y, et al. The changing face of HIV in China. Nature 2008; 455:609–11. [DOI] [PubMed] [Google Scholar]
- 35. Zhang L, Chow EP, Jing J, et al. HIV prevalence in China: integration of surveillance data and a systematic review. Lancet Infect Dis 2013; 13:955–63. [DOI] [PubMed] [Google Scholar]
- 36. Tang Z, Wu X, Li G, et al. Aphrodisiac use associated with HIV infection in elderly male clients of low-cost commercial sex venues in Guangxi, China: a matched case-control study. PLoS One 2014; 9:e109452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan HH, Wong ML, Chan RK. An epidemiological and knowledge, attitudes, beliefs and practices study of sexually transmitted infections in older men. Singapore Med J 2006; 47:886–91. [PubMed] [Google Scholar]
- 38. Ding Y, Ma Z, He J, et al. Evolving HIV epidemiology in Mainland China: 2009–2018. Curr HIV/AIDS Rep 2019; 16:423–30. [DOI] [PubMed] [Google Scholar]