Abstract
Background
Tuberculosis (TB) is a major cause of morbidity and mortality among incarcerated populations globally. We performed mass TB screening in 3 prisons and assessed yield, efficiency, and costs associated with various screening algorithms.
Methods
Between 2017 and 2018, inmates from 3 prisons in Brazil were screened for TB by symptom assessment, chest radiography, sputum testing by Xpert MTB/RIF fourth-generation assay, and culture. Chest radiographs were scored by an automated interpretation algorithm (Computer-Aided Detection for Tuberculosis [CAD4TB]) that was locally calibrated to establish a positivity threshold. Four diagnostic algorithms were evaluated. We assessed the yield (percentage of total cases found) and efficiency (prevalence among those screened) for each algorithm. We performed unit costing to estimate the costs of each screening or diagnostic test and calculated the cost per case detected for each algorithm.
Results
We screened 5387 prisoners, of whom 214 (3.9%) were diagnosed with TB. Compared to other screening strategies initiated with chest radiography or symptoms, the trial of all participants with a single Xpert MTB/RIF sputum test detected 74% of all TB cases at a cost of US$249 per case diagnosed. Performing Xpert MTB/RIF screening tests only on those with symptoms had a similar cost per case diagnosed (US$255) but missed 35% more cases (73 vs 54) as screening all inmates.
Conclusions
In this prospective study in 3 prisons in a high TB burden country, we found that testing all inmates with sputum Xpert MTB/RIF was a sensitive approach, while remaining cost-efficient. These results support use of Xpert MTB/RIF for mass screening in TB-endemic prisons.
Keywords: mass screening, tuberculosis, algorithms, prisons, cost-effectiveness
In this prospective study, we performed mass tuberculosis screening in Brazilian prisons and assessed yield, efficiency, and costs associated with various screening algorithms. We found that testing all participants with the sputum Xpert MTB/RIF assay was sensitive and cost-efficient.
(See the Editorial Commentary by Woodman and Grandjean on pages 778–9.)
Tuberculosis (TB) is the leading cause of death by an infectious disease worldwide [1] and as a response, the World Health Assembly set a goal to reduce the global TB incidence by 90% by 2035 [2]. Despite an elevated focus on TB and increased funding, the TB burden is declining by only 1%–2% per year globally. To reach global targets, complementary interventions are needed to supplement current TB control. Recently, there has been a push to target interventions to populations with a high TB burden to reduce disease incidence and transmission to the broader community [3, 4].
Prisons frequently have a very high burden of TB [5]. A meta-analysis of 19 studies found that the incidence of TB in prisons was 23 times greater than the surrounding population [5]. This high incidence leads to markedly elevated transmission rates. For example, 3 prisoner cohorts from Brazil, Colombia, and Iran have shown annual tuberculin conversion rates between 15% and 25% [6–8]. Effective case detection for TB in prisons is necessary to reduce ongoing transmission.
Despite the high rates of TB in prisoners and the potential importance of this population in the overall epidemic [3, 5, 9], few studies have assessed the efficiency and costs of different approaches for screening for TB among incarcerated populations [7]. Studies reporting the yield of TB detected in prisons rarely compare distinct screening modalities or report their costs. Additionally, the use of sensitive molecular diagnostic tests, such as the Xpert MTB/RIF assay, for TB screening among prisoners has not been widely explored. Studies using mathematical modeling suggest that annual mass screening can reduce the incidence of TB in prisons [3, 10]. However, there are no specific guidelines on how screening should be performed. Due to costs and a lack of evidence on effective screening approaches in this population, few prisons in low- and middle-income countries perform systematic screening for TB. Our objective was to identify effective and efficient approaches to TB screening in prisons that could be implemented in low- and middle-income countries.
METHODS
Study Population
This study was carried out in 3 prisons in Mato Grosso do Sul, Brazil. Brazil’s national prison population is > 700 000 individuals, the third largest globally [11]. Three prisons were included in this study: Penitenciária Estadual de Dourados (PED), Estabelecimento Penal Jair Ferreira de Carvalho (EPJFC), and Instituto Penal de Campo Grande (IPCG). These prisons exclusively incarcerate males ≥18 years old and were selected because they are the largest in the state and had the highest TB infection and disease rates in preliminary studies [7, 12].
Study Procedures
All prisoners were invited to participate, and those who accepted provided written informed consent. The study was approved by the Federal University of Grande Dourados, the National Committee on Research Ethics (number 2.195.047), and the Institutional Review Board of Stanford University (number 40285). Each participant was then interviewed using a standardized questionnaire to collect demographic and clinical information. We asked each participant about TB-related symptoms according to World Health Organization (WHO) guidelines [13, 14]. All participants were instructed to produce a sputum sample with a target volume of at least 2 mL. On this primary sample, the Xpert MTB/RIF assay (Cepheid, Sunnyvale; fourth genetation; hereinafter Xpert) was performed; the remainder of the sample was transported to the support laboratory for culture. A second sputum sample was collected on the following day for a second culture. Participants who were unable to produce sputum were coached by nursing staff; however, sputum induction was not performed, and many participants were unable to produce a sample. Participants without sufficient samples were included in the study.
All participants underwent posterior-anterior chest radiography (CXR). Chest radiographs were then evaluated with Computer-Aided Detection for Tuberculosis (CAD4TB) software version 5 [15]. CAD4TB assigns a quality assessment to a CXR, produces a heat map indicating areas with possible abnormalities, and designates a score between 1 and 100 related to the likelihood of radiological abnormalities suggestive of a TB diagnosis. CAD4TB was calibrated with training data from radiographic images of participants with (n = 80) and without (n = 200) microbiologically confirmed TB. Training data demonstrated high accuracy (area under the curve = 0.88), with a sensitivity and specificity > 80% using a CAD4TB score ≥ 60. Participants with a CAD4TB score ≥ 60 were clinically reevaluated; those who had been unable to produce sputum on the first occasion were given another opportunity and additional coaching to produce a sample for testing by Xpert assay. Those participants with negative results or who were still unable to produce sputum were assessed by a physician. All TB cases identified during screening were provided free treatment according to national guidelines [16].
Derivation of Mass Diagnostic Screening Algorithms
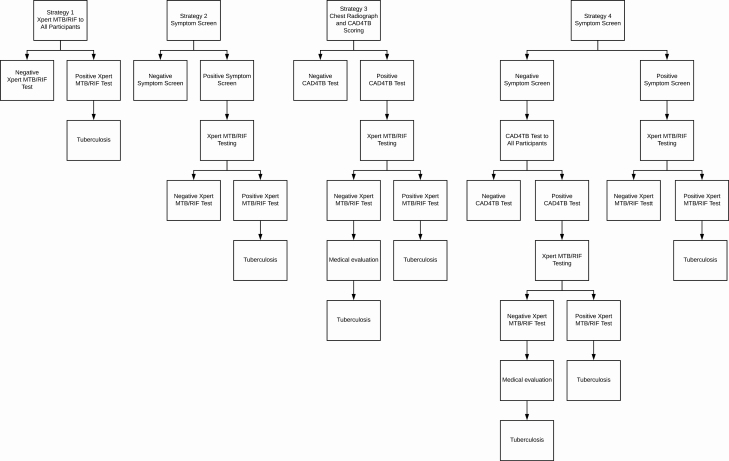
All participants were prospectively and systematically screened as outlined above; we then retrospectively evaluated 4 hypothetical, intensive screening algorithms (Figure 1) consisting of more limited sets of diagnostics:
Figure 1.
Outline of tuberculosis screening strategies assessed among prisoners in the study. Abbreviations: CAD4TB, Computer-Aided Detection for Tuberculosis; MTB, Mycobacterium tuberculosis; RIF, rifampicin.
• Strategy 1: Sputum testing by Xpert for all participants who could produce sputum at the moment of questionnaire, regardless of presence of symptoms.
• Strategy 2: Sputum testing by Xpert only for those who reported any TB-related symptom of any duration and who could produce sputum at the moment of questionnaire.
• Strategy 3: CXR with CAD4TB scoring for all participants. Those with CAD4TB score ≥ 60 underwent sputum testing by Xpert.
• Strategy 4: Symptom screening, followed by sputum Xpert testing for participants who reported a TB-related symptom. Those without any TB symptoms underwent CXR with CAD4TB scoring. Sputum collection and Xpert testing were then offered to participants with a CAD4TB score ≥ 60.
Outcome Definitions
We followed national Brazilian guidelines and WHO definitions for TB diagnosis. We defined a TB case as any individual with a positive sputum Xpert, sputum culture, or with a physician diagnosis based on clinical-epidemiological data and radiographic abnormalities. All participants with TB were administered a rapid human immunodeficiency virus (HIV) test and evaluated through a nursing and medical examination.
Analytical Approach and Cost Evaluation
We calculated the cost of each screening procedure: symptom screening interviews, Xpert, culture, and radiographic and clinical evaluations. These costs include equipment, maintenance, consumables, and personnel time.
In our calculations, we assumed the equipment used during mass screening would remain useful for a period of 10 years and amortized the cost over this period. Personnel time was calculated based on the salary of staff members involved in each screening component, time devoted to each component of screening, and the number of individuals who could be screened during that unit time. We calculated average unit cost by dividing total cost of each diagnostic procedure by the total number of procedures during the study period. The cost of each Xpert cartridge was US$9.90.
To calculate the cost per case detected, we assumed the definition of fixed and variable costs. Fixed costs are costs that apply to the entire cohort, regardless of how many people are screened, such as purchasing and maintaining equipment and software. Variable costs are costs related to use, such as human resources, inputs, and evaluation of each radiographic image in CAD4TB. The cost per case detected was calculated for each strategy by multiplying the average unit cost by the number of procedures performed in each strategy and then dividing by the cases detected in the strategy. The values in Brazilian reais were converted to US dollars using the quotation of 28 November 2018 (R$3.87 = US$1.00).
RESULTS
Study Population
Between November 2017 and July 2018, we screened 5387 of 6054 eligible study participants (88.9%). Reasons for not participating included lack of interest, lack of clothing to leave the cell, and fear of meeting members of rival groups. Participating inmates had a median age of 30.5 years (Table 1). More than half of participants were smokers (58.3%) and used some type of illicit drug in the past year (58.8%), and 70.3% were previously incarcerated. More than 71.4% of participants reported knowing a person diagnosed with TB, and 8.2% reported having prior TB. During the study period, a total of 214 participants were diagnosed with pulmonary TB, equating to a prevalence of 3973 per 100 000 participants (95% confidence interval, 3483–4528). Disaggregating by prison, we identified TB prevalence of 5567/100 000 (101/1814) in EPJFC, 3607/100 000 (82/2273) in PED, and 2384/100 000 (31/1300) in IPCG.
Table 1.
Sociodemographic Characteristics and Risk Factors for Tuberculosis Among Screened Inmates
| Variables | Total (N = 5387) | TB Cases (n = 214) | No TB (n = 5173) | P Value |
|---|---|---|---|---|
| Prison unit | ||||
| PED | 2272 (42.2) | 82 (38.3) | 2191 (42.4) | .24 |
| EPJFC | 1814 (33.7) | 101 (47.2) | 1713 (33.1) | < .01 |
| IPCG | 1300 (24.1) | 31 (14.5) | 1269 (24.5) | < .01 |
| Median age, y (IQR) | 30.5 (25–37) | 30 (25–37) | 31 (25–37) | < .01 |
| Ethnicity | ||||
| Mixed | 3312 (61.5) | 136 (63.6) | 3176 (61.3) | .52 |
| White | 1306 (24.2) | 49 (22.9) | 1257 (24.3) | .64 |
| Black | 617 (11.5) | 23 (10.7) | 593 (11.4) | .76 |
| Indigenous | 144 (2.6) | 6 (2.8) | 138 (2.6) | > .99 |
| Asian | 8 (0.1) | 0 (0.0) | 8 (0.2) | > .99 |
| < 8 y of schooling | 3540 (65.7) | 154 (72.0) | 3386 (65.4) | .04 |
| Current smoker | 3139 (58.3) | 161 (75.2) | 2978 (57.5) | < .01 |
| Illicit drug use over the last year | 3172 (58.8) | 169 (78.9) | 3003 (58.0) | < .01 |
| BCG vaccinated | 4736 (87.9) | 185 (86.4) | 4551 (87.9) | .49 |
| Previous TB | 482 (8.2) | 56 (26.2) | 426 (8.2) | < .01 |
| Know someone with TB | 3849 (71.4) | 181 (84.6) | 3668 (70.9) | < .01 |
| Report any WHO TB symptoms | 2127 (39.4) | 174 (81.3) | 1953 (37.7) | < .01 |
| Report cough | 1527 (28.3) | 151 (70.6) | 1376 (26.6) | < .01 |
| Previously incarcerated | 3786 (70.3) | 167 (78.0) | 3619 (70.0) | < .01 |
Data are presented as no. (%) unless otherwise indicated.
Abbreviations: BCG, Bacillus Calmette-Guérin; EPJFC, Estabelecimento Penal Jair Ferreira de Carvalho; IPCG, Instituto Penal de Campo Grande; IQR, interquartile range; PED, Penitenciária Estadual de Dourados; TB, tuberculosis; WHO, World Health Organization.
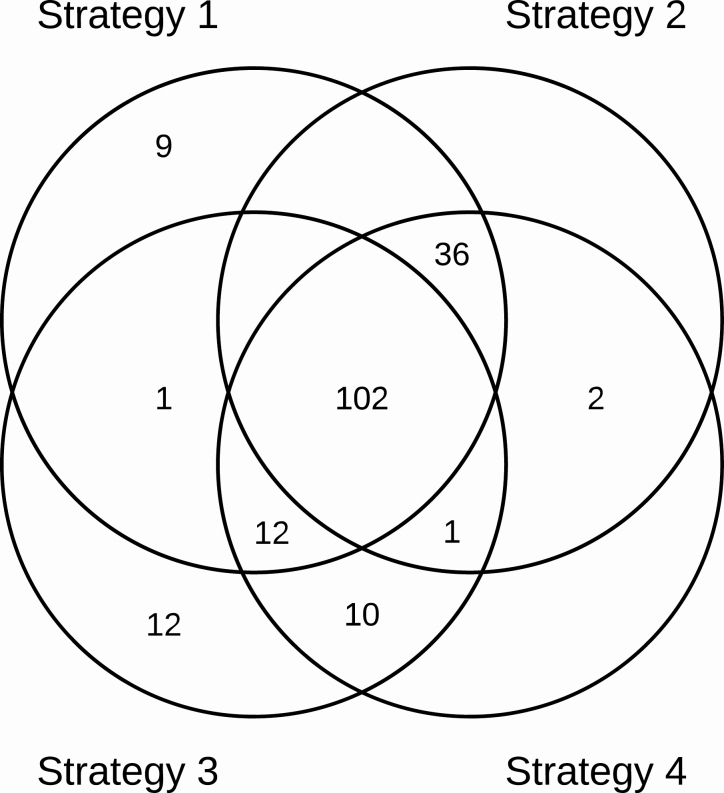
Of the diagnosed cases on the initial visit, 172 (80.3%) were diagnosed by the Xpert assay and sputum culture. At the initial visit, sputum was obtained from 1467 inmates. Among these, Xpert was performed on almost all participants (1452 [98.9%]) and detected 160 TB cases (Figure 2). Culture was performed on 1385 participants and 12 additional cases were identified by culture. Sputum smear tests were performed on 1386 participants; among the 214 TB cases who had smear microscopy performed, 49 (22.8%) had a positive smear. All TB cases that tested smear-positive were positive by Xpert. Among 1295 participants who were culture and/or Xpert negative, 261 had a CAD4TB score ≥ 60. According to the study protocol, these participants were reevaluated for TB, 114 did Xpert, and 22 were diagnosed (11 by Xpert and 11 by clinical evaluation). Among participants who did not produce a sputum sample at the initial visit (n = 3920), 523 (13.3%) had a CAD4TB score ≥ 60. A second attempt was made to collect sputum among these participants, of which 155 (29.6%) were successful and 9 were Xpert positive. A further 11 cases were clinically diagnosed after physician reevaluation. Among cases of active TB, 4 (1.9%) were HIV positive.
Figure 2.
Tuberculosis (TB) cases overlap of screening strategies. Strategy 1: Xpert MTB/RIF assay for all prisoners. Strategy 2: Xpert test only for those who reported any TB symptom. Strategy 3: Chest radiography, those with Computer-Aided Detection for Tuberculosis (CAD4TB) score ≥ 60 undergo Xpert test. Strategy 4: Symptom screening, followed by Xpert test, those without any TB symptoms undergo chest radiography with CAD4TB scoring followed by Xpert test. Abbreviations: MTB, Mycobacterium tuberculosis; RIF, rifampicin.
Accuracy and Yield of Symptom- and Radiograph-based Screening
In the initial screening interview, 2127/5387 patients (39.5%) reported at least 1 WHO-defined TB symptom; the most common of the symptoms was cough (71.8%). Symptom screening alone had a sensitivity and specificity of 81.3% and 61.6%, respectively. If screening was initiated based on cough alone rather than a comprehensive symptom screen, 151 (70.6%) cases would be detected (Table 2). The sensitivity of CXR with a CAD4TB score ≥60 was 77.1% and specificity was 85.6%.
Table 2.
Predictive Value of World Health Organization Tuberculosis Symptom Screen, Cough, and Computer-Aided Detection for Tuberculosis Score in 5387 Screened Inmates
| Symptoms | Cough | CAD4TB Score | No. of Individuals (% of Total Cohort) | No. of Cases (TB Prevalence) | % of all TB Cases Detected |
|---|---|---|---|---|---|
| Absent | No | < 60 | 2793 (51.8) | 7 (0.2) | 3.3 |
| ≥ 60 | 467 (8.7) | 33 (7.0) | 15.4 | ||
| Total | 3260 (60.5) | 40 (1.2) | 18.7 | ||
| Present | No | < 60 | 498 (9.2) | 6 (1.2) | 2.8 |
| ≥ 60 | 102 (2.0) | 17 (16.6) | 7.9 | ||
| Yes | < 60 | 1189 (22.0) | 36 (3.0) | 16.8 | |
| ≥ 60 | 338 (6.3) | 115 (34.0) | 53.8 | ||
| Total | 2127 (39.5) | 174 (8.2) | 81.3 |
Abbreviations: CAD4TB, Computer-Aided Detection for Tuberculosis; TB, tuberculosis.
The prevalence of TB was very low (0.2%) among participants with no symptoms and a CAD4TB score < 60, and this group comprised just over half (51.6%) of all participants. Prevalence among participants with no TB symptoms but CAD4TB score ≥ 60, comprising 8.7% of the cohort, was 7.0%. Among the 22.0% of participants with cough and CAD4TB score < 60, prevalence was 3.0%. The highest-risk group consisted of participants with both cough and CAD4TB score ≥ 60, in whom prevalence was 34.0%; although only 6.3% of participants met both criteria, this accounted for 53.8% of all TB cases detected.
Costs and Efficiency of Screening Strategies
Among diagnostic modalities used during mass screening, the highest cost per participant was for Xpert (US$19.20), followed by CXR with CAD4TB scoring (US$6.28), clinical evaluation (US$2.60), and symptom screening interviews (US$1.90) (Table 3). The costliest component of Xpert was consumables (54.8%). For CAD4TB score, CXR, human resources, equipment, and CAD4TB score analysis contributed almost equally to main costs (29.6%, 29.0%, and 29.8%, respectively).
Table 3.
Total and Unit Cost for Each Screening or Diagnostic Procedure
| Category | Total Cost of the Item, 2018 US$ | Unit Cost, 2018 US$ | |
|---|---|---|---|
| Interview (n = 5387) | |||
| Fixed costs | Equipmenta | 127.63 | 0.02 |
| Variable costs | Human resources | 10 027.80 | 1.86 |
| Inputs | 105.21 | 0.02 | |
| Total | 10 260.64 | 1.9 | |
| Clinical evaluation (n = 764) | |||
| Variable costs | Human resources | 1986.40 | 2.60 |
| Total | 1986.40 | 2.60 | |
| Radiograph (n = 5387) | |||
| Fixed costs | Equipmenta | 9840.73 | 1.83 |
| CAD4TB software | 667.00 | 0.12 | |
| Transport mobile diagnostic unit | 3875.96 | 0.72 | |
| Variable costs | Human resources | 10 027.80 | 1.86 |
| CAD4TB score | 9427.25 | 1.75 | |
| Total | 33 838.74 | 6.28 | |
| Xpert MTB/RIF assay (n = 1743) | |||
| Fixed costs | Equipmenta | 4954.98 | 2.84 |
| Maintenance | 5167.95 | 2.97 | |
| Variable costs | Human resources | 5013.90 | 2.87 |
| Inputs | 18 339.23 | 10.52 | |
| Total | 33 476.06 | 19.20 |
Abbreviations: CAD4TB, Computer-Aided Detection for Tuberculosis; MTB, Mycobacterium tuberculosis; RIF, rifampicin; US, United States.
aProjected cost for a useful life of 10 years, based on the examinations made for 1 year.
The cost per case detected for all strategies ranged from US$249 to US$395 (Table 4). Strategy 1 (Xpert assay for all participants) resulted in the second highest yield, detecting 74% of all cases, at lowest cost (US$249) per case detected. Strategy 2 (Xpert for individuals with any symptom) had a low cost per case detected (US$255) but resulted in lower yield (65%) compared with strategies 1 and 4. Strategies 2 and 3 had lower yield (65% and 64%, respectively) than strategies 1 and 4. And strategies 3 and 4 had a higher cost per case diagnosed (US$370 and US$395, respectively).
Table 4.
Yield and Cost per Case Diagnosed for 4 Tuberculosis Screening Strategies
| Strategies | Cases Diagnosed | Missed Cases | % Yield (95% CI) | Participants Screened With Xpert, No. | Mean Cost per Case Detected, US$ |
|---|---|---|---|---|---|
| All cases | 214 | … | … | … | 485 |
| Comparator groups | |||||
| Strategy 1: Sputum Xpert for all participants | 160 | 54 | 74 (68–80) | 1452 | 249 |
| Strategy 2: Symptom screening If positive: Xpert | 141 | 73 | 65 (59–71) | 1163 | 255 |
| Strategy 3: Chest radiography (CAD4TB) If score ≥ 60: Xpert | 138 | 76 | 64 (57–70) | 383 | 370 |
| Strategy 4: Symptom screening If positive, Xpert If negative, CXR (CAD4TB) followed by Xpert if score ≥60 | 163 | 51 | 76 (70–81) | 1248 | 395 |
Abbreviations: CAD4TB, Computer-Aided Detection for Tuberculosis; CI, confidence interval; CXR, chest radiograph; MTB, Mycobacterium tuberculosis; RIF, rifampicin; US, United States; Xpert, MTB/RIF assay.
DISCUSSION
Tuberculosis is a major infectious disease problem within prisons worldwide. However, there is a dearth of evidence concerning how to effectively detect TB while controlling costs in these environments. As a result, screening modalities in prisons globally remain variable, with few high-TB-burden countries enacting systematic screening policies in correctional facilities. In 3 prisons in Brazil, we found a very high prevalence (3973 per 100 000) of TB through systematic screening of inmates. This prevalence is higher to the identified in other studies in prisons in Brazil and other countries [17–21].
Strategies 1 and 4 had similar yields. We found that systematic Xpert testing among all individuals able to produce sputum was effective at a modest cost per case diagnosed of US$249 dollars, and detected only 3 fewer cases compared to strategy 4, which detected the most due to radiographically diagnosed cases. Implementing a symptom screen to identify individuals for sputum testing had a similar efficiency at US$255 per case detected. Strategies involving CXR were most costly and did not increase the overall yield compared with sputum Xpert testing alone. Together, these results suggest that testing all inmates able to produce sputum using Xpert may be an effective and affordable strategy in high-burden prisons.
There has been debate over the reliability of symptom screening to triage the use of diagnostics in high-risk populations, due to limitations in both sensitivity and specificity [22, 23]. We found that approximately 39.5% of all inmates reported at least 1 WHO-defined TB symptom [13], of which cough was the most common. Several studies show that mass screening using a cough-based strategy has moderate sensitivity [22, 24]. In this study, 81.3% of individuals with TB had at least 1 TB-related symptom, such that an algorithm beginning with symptom-screening would detect the majority of cases, while reducing the number of individuals who require testing. An additional 19 patients (8.8% of all cases) were detected by screening all individuals, irrespective of symptoms (Strategy 1); this required screening an additional 289 participants by Xpert, as most of the 3260 participants without symptoms also didn’t produce sputum. While symptom-based screening was the most efficient algorithm, screening everyone who could produce sputum identified more cases at comparable costs per case detected.
The high number of symptomatic participants may be due to the high frequency of smoking and illicit drug use in our population. Studies of the general population [25–27] show a lower prevalence of symptoms than studies with prison inmates [7, 22, 24]. Our study used cough of any duration as a symptom, rather than cough > 2 weeks as is commonly done in other studies, increasing sensitivity at the expense of specificity. Symptom-based screening in the context of mass screening may perform better in populations with a lower prevalence of smoking, in whom the specificity of cough is higher.
At the beginning of the study, we defined a CAD4TB threshold of ≥ 60 based on preliminary data indicating a sensitivity and specificity of approximately 80%. In this study, we found overall sensitivity to be 77.1% and specificity to be 82.8%. This sensitivity was slightly lower than that of symptom screening (81.3%), but specificity was much higher (60.5% for symptom screening). The cost of radiographic screening or symptom screening followed Xpert assay and CXR were considerably higher than that of symptom screening and Xpert for all individuals. The cost per case diagnosed for screening with Xpert assay for all individuals was lower. Alternative thresholds could be used to increase sensitivity of CXR with CAD4TB, at the expense of specificity, and further work is needed to identify optimal thresholds to maximize cost-effectiveness.
The strengths of our study include a representative sample of prisoners in Mato Grosso do Sul. The 3 prisons we screened house 32% of the state’s prison population [19, 21]. Our participation rate of 88.9% of the study’s target population is similar to previous recruitments performed by our group [7] and other mass screening initiatives [19]. We undertook a rigorous microcosting analysis to derive “real-world” costs of implementing various components of triage and diagnosis in prisons, which are critical to decisions of scaling up systematic screening in these settings.
There are several limitations to this study. A major challenge was that only 27.2% of participants were able to produce a sputum sample in initial visit, and sputum induction was not possible in this setting. As a result, we likely underestimated the true prevalence of TB. However, our estimates for the yield and cost per case diagnosed when screening all participants reflect this limitation in prisons, which is not just a study challenge but a real-world obstacle to screening. While sputum induction would likely improve yield, it is possible that the efficiency (prevalence among tested individuals) would be lower, and the cost per case diagnosed would likely be higher. Our findings do, however, underscore the need for non-sputum-based diagnostics to reach patients earlier in the TB disease spectrum [28–31].
We do not use testing for TB infection, either through a interferon gamma release assays (IGRA) or tuberculin skin test, in our diagnostic algorithms. Previous tuberculin skin test conversion studies in Brazilian prisons have demonstrated hyperendemic rates of transmission with an annual conversion above 25% [7, 12]. In a setting with such a high force of infection, it is unclear how tuberculin skin (or IGRA) testing would accurately discriminate TB disease.
We estimated costs assuming that diagnostic infrastructure (Xpert machines, radiography equipment) was not present; for prisons in which such investments have been made for routine diagnostic purposes, incremental costs per case diagnosed via mass screening may be lower. Finally, we evaluated a limited combination of commonly used diagnostics (symptom screening, Xpert, radiography); while many more combinations or algorithms are possible using, for example, different criteria for interpretation of these screening tools, we selected these to be simple and scalable for use in resource-constrained settings.
In summary, our results suggest that mass TB screening in high-burden prisons, conducted by sputum Xpert testing of all inmates or those with symptoms, is an effective approach to case detection at a modest cost per case detected. Chest radiography, while it has higher overall accuracy than symptom screening, was more costly and did not substantially improve yield compared with sputum-based screening of all participants. Active case finding by sputum testing with Xpert MTB/RIF assay should be scaled up in Brazilian prisons and other high-burden countries to address TB in incarcerated populations.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online.
Consisting of data provided by the authors to benefit the reader, the posted
materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. S. S., E. F. L., C. R., A. K., J. R. A., and J. C. were involved in the study conception and design. A. S. S., E. F. L., and F. L. were involved in the data collection. A. S. S., R. D. O., E. F. L., F. L., O. C., and L. M. were involved in the data analysis and manuscript drafting. C. G., T. C., A. K., J. R. A., and J. C. were involved in the study design and manuscript review. All authors read and approved the final manuscript.
Acknowledgments. The authors thank the State Health Department of Mato Grosso do Sul and State Agency of Administration Prisons for their full support during the study period; the study participants for their kind cooperation during the data collection process; and the Central Laboratory of the state of Mato Grosso do Sul for the support in the accomplishment of the laboratory tests. All data generated or analyzed during this study are included in this published article and its supplementary information files.
Disclaimer. All participants provided written informed consent prior to study participation. The study was approved by Federal University of Grande Dourados, the National Committee on Research Ethics (#2.195.047), and the Institutional Review Board and Stanford University (#40285).
Financial support. This study was supported by the US National Institutes of Health (grant number R01 AI130058).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Kyu HH, Maddison ER, Henry NJ, et al. The global burden of tuberculosis: results from the Global Burden of Disease Study 2015. Lancet Infect Dis 2018; 18:261–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zumla A, George A, Sharma V, Herbert RHN, Oxley A, Oliver M. The WHO 2014 global tuberculosis report—further to go. Lancet Glob Health 2015; 3:e10–2. [DOI] [PubMed] [Google Scholar]
- 3. Mabud TS, de Lourdes Delgado Alves M, Ko AI, et al. Evaluating strategies for control of tuberculosis in prisons and prevention of spillover into communities: An observational and modeling study from Brazil. PLoS Med 2019; 16:e1002737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cudahy PGT, Andrews JR, Bilinski A, et al. Spatially targeted screening to reduce tuberculosis transmission in high-incidence settings. Lancet Infect Dis 2019; 19:e89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baussano I, Williams BG, Nunn P, Beggiato M, Fedeli U, Scano F. Tuberculosis incidence in prisons: a systematic review. PLoS Med 2010; 7:e1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mamani M, Mahmudian H, Majzoobi MM, Poorolajal J. Prevalence and incidence rates of latent tuberculous infection in a large prison in Iran. Int J Tuberc Lung Dis 2016; 20:1072–7. [DOI] [PubMed] [Google Scholar]
- 7. Paião DS, Lemos EF, Carbone AD, et al. Impact of mass-screening on tuberculosis incidence in a prospective cohort of Brazilian prisoners. BMC Infect Dis 2016; 16:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arroyave L, Keynan Y, López L, Marin D, Arbeláez MP, Rueda ZV. Negative latent tuberculosis at time of incarceration: identifying a very high-risk group for infection. Epidemiol Infect 2017; 145:2491–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bourdillon PM, Gonçalves CCM, Pelissari DM, et al. Increase in tuberculosis cases among prisoners, Brazil, 2009–2014. Emerg Infect Dis 2017; 23:496–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Legrand J, Sanchez A, Le Pont F, Camacho L, Larouze B. Modeling the impact of tuberculosis control strategies in highly endemic overcrowded prisons. PLoS One 2008; 3:e2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brazil Ministry of Justice and Public Security. National survey of prison information: INFOPEN update June/2016. Brasília: Ministry of Justice and Public Security, 2017. Available at: http://depen.gov.br/DEPEN/depen/sisdepen/infopen/relatorio_2016_22-11.pdf. Accessed 22 September 2018. [Google Scholar]
- 12. Carbone A da SS, Paião DSG, Sgarbi RVE, et al. Active and latent tuberculosis in Brazilian correctional facilities: a cross-sectional study. BMC Infect Dis 2015; 15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bone A, Aerts A, Grzemska M, et al. Tuberculosis control in prisons: a manual for programme managers. WHO/CDS/TB/2000.281.191. 2000. Available at: https://apps.who.int/iris/handle/10665/66823. Accessed 27 June 2019. [Google Scholar]
- 14. World Health Organization. Implementing the End TB strategy: the essentials. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 15. Delft Imaging Systems. CAD4TB: Computer-Aided Detection for Tuberculosis. Available at: https://www.delft.care/cad4tb. Accessed 26 June 2019. [Google Scholar]
- 16. Brazil Ministry of Health. Guidelines for tuberculosis control in Brazil. Brasília: Ministry of Health: Secretariat of Health Surveillance: Department of Epidemiological Surveillance, 2018. [Google Scholar]
- 17. Brazil Ministry of Health. Epidemiological bulletin 11. Brasília: Ministry of Health, 2018. Available at: http://portalarquivos2.saude.gov.br/images/pdf/2018/marco/26/2018-009.pdf. Accessed 6 February 2019. [Google Scholar]
- 18. Valença MS, Possuelo LG, Cezar-Vaz MR, et al. Tuberculosis in Brazilian prisons: an integrative literature review. Ciênc Amp Saúde Coletiva 2016; 21:2147–60. [DOI] [PubMed] [Google Scholar]
- 19. Pelissari DM, Kuhleis DC, Bartholomay P, et al. Prevalence and screening of active tuberculosis in a prison in the south of Brazil. Int J Tuberc Lung Dis 2018; 22:1166–71. [DOI] [PubMed] [Google Scholar]
- 20. Vinkeles Melchers NV, van Elsland SL, Lange JM, Borgdorff MW, van den Hombergh J. State of affairs of tuberculosis in prison facilities: a systematic review of screening practices and recommendations for best TB control. PLoS One 2013; 8:e53644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lemos AC, Matos ED, Bittencourt CN. Prevalence of active and latent TB among inmates in a prison hospital in Bahia, Brazil. J Bras Pneumol 2009; 35:63–8. [DOI] [PubMed] [Google Scholar]
- 22. Sanchez A, Gerhardt G, Natal S, et al. Prevalence of pulmonary tuberculosis and comparative evaluation of screening strategies in a Brazilian prison. Int J Tuberc Lung Dis 2005; 9:633–9. [PubMed] [Google Scholar]
- 23. Fournet N, Sanchez A, Massari V, et al. Development and evaluation of tuberculosis screening scores in Brazilian prisons. Public Health 2006; 120:976–83. [DOI] [PubMed] [Google Scholar]
- 24. Sanchez A, Larouzé B, Espinola AB, et al. Screening for tuberculosis on admission to highly endemic prisons? The case of Rio de Janeiro State prisons. Int J Tuberc Lung Dis 2009; 13:1247–52. [PubMed] [Google Scholar]
- 25. Hoa NB, Sy DN, Nhung NV, Tiemersma EW, Borgdorff MW, Cobelens FG. National survey of tuberculosis prevalence in Viet Nam. Bull World Health Organ 2010; 88:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Federal Republic of Nigeria. Report first national TB prevalence survey 2012, Nigeria. Abuja, Nigeria: Ministry of Health, 2012. Available at: https://www.who.int/tb/publications/NigeriaReport_WEB_NEW.pdf. Accessed 28 June 2019. [Google Scholar]
- 27. Soemantri S, Senewe FP, Tjandrarini DH, et al. Three-fold reduction in the prevalence of tuberculosis over 25 years in Indonesia. Int J Tuberc Lung Dis 2007; 11:398–404. [PubMed] [Google Scholar]
- 28. Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis 2018; 18:e199–210. [DOI] [PubMed] [Google Scholar]
- 29. Denkinger CM, Kik SV, Cirillo DM, et al. Defining the needs for next generation assays for tuberculosis. J Infect Dis 2015; 211(Suppl 2):S29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Keeler E, Perkins MD, Small P, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature 2006; 444(Suppl 1):49–57. [DOI] [PubMed] [Google Scholar]
- 31. Calligaro GL, Zijenah LS, Peter JG, et al. Effect of new tuberculosis diagnostic technologies on community-based intensified case finding: a multicentre randomised controlled trial. Lancet Infect Dis 2017; 17:441–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.