Abstract
Background
Antibiotic use is the strongest modifiable risk factor for the development of Clostridioides difficile infection, but prescribers lack quantitative information on comparative risks of specific antibiotic courses. Our objective was to estimate risks of C. difficile infection associated with receipt of specific antibiotic courses.
Methods
We conducted a longitudinal case-cohort analysis representing over 90% of Ontario nursing home residents, between 2012 and 2017. Our primary exposure was days of antibiotic receipt in the prior 90 days. Adjustment covariates included: age, sex, prior emergency department or acute care stay, Charlson comorbidity index, prior C. difficile infection, acid suppressant use, device use, and functional status. We examined incident C. difficile infection, including cases identified within the nursing home, and those identified during subsequent hospital admissions. Adjusted and unadjusted regression models were used to measure risk associated with 5- to 14-day courses of 18 different antibiotics.
Results
We identified 1708 cases of C. difficile infection (1.27 per 100 000 resident-days). Longer antibiotic duration was associated with increased risk: 10- and 14-day courses incurred 12% (adjusted relative risk [ARR] = 1.12, 95% confidence interval [CI]: 1.09, 1.14) and 27% (ARR = 1.27, 95% CI: 1.21,1.30) more risk compared to 7-day courses. Among 7-day courses with similar indications: moxifloxacin resulted in 121% more risk than amoxicillin (ARR = 2.21, 95% CI: 1.67, 3.08), ciprofloxacin engendered 89% more risk than nitrofurantoin (ARR = 1.89, 95% CI: 1.45, 2.68), and clindamycin resulted in 112% (ARR = 2.12, 95% CI: 1.32, 3.78) more risk than cloxacillin.
Conclusions
C. difficile infection risk increases with antibiotic duration, and there are wide disparities in risks associated with antibiotic courses used for similar indications.
Keywords: Clostridioides difficile infection, CDI, cohort study, antibiotics, comparative effectiveness
We found large variations in C. difficile infection risk for antibiotic courses used for the same common indications. Risk could be substantially reduced if prescribers selected shorter durations of lower risk agents.
Antibiotic use is the most important modifiable risk factor for the development of Clostridioides difficile infection. Antibiotic-associated C. difficile infection risk is driven by the duration of antimicrobial exposure, as well as the class of antimicrobial agent received [1–4]. Several meta-analyses have found that 2nd and 3rd generation cephalosporins, fluoroquinolones, and clindamycin have particularly strong tendencies to precipitate C. difficile infection [5, 6], but few studies have examined differences between specific agents within each class [7, 8]. The time elapsed since receipt is also an important qualifier of antibiotic risk; risk is concentrated in the first 90 days after antibiotic exposure [4]. No studies have examined the incremental impact of duration on risk for specific antibiotics, or compared risks associated with alternative prescribing options for common indications.
Physician prescribing choices may strongly impact patient C. difficile infection risk. Variability in initiation, duration, and specific agent prescribed is driven by physician choice more than patient factors [9, 10]. Potential adverse effects do not figure strongly in clinician antibiotic prescribing decisions [11], and clinicians often overestimate benefits and underestimate harms of treatments [12]. In the case of antibiotics, this could be driven by a lack of readily available, comparative information on antibiotic harms.
Our objective was to compare the incremental risk of C. difficile infection that exposure to a given antibiotic, for a given amount of time, confers on a patient, using a representative cohort of long-term care residents, a group at high risk for antibiotic related harms. The resulting granular estimates of C. difficile infection risk could be used by clinicians to better weigh the harms and benefits of different antibiotic prescribing choices, minimize C. difficile infection risk, and maximize the health and well-being of patients.
METHODS
Data Sources
This study was made possible by comprehensive, individual-level and Ontario-wide, medico-administrative datasets, held at the ICES (formerly the Institute for Clinical Evaluative Sciences) in Toronto, Canada. These datasets were linked using unique encoded identifiers and analyzed at ICES. We included data sets corresponding to: mandated quarterly nursing home assessments from the Continuing Care Reporting System [13], physician billing claims to the Ontario Health Insurance Program, International Classification of Diseases version 10 coded discharge abstracts corresponding to ambulatory care, same day surgeries, and hospital admissions [14], and demographics from Ontario’s population registry. Information on antibiotic prescribing was ascertained by the Ontario Drug Benefit database, which covers nonhospital antibiotic dispensing only for Ontarians 65 years and older, and which has been shown to be over 99% accurate [15].
Study Design
This study employed a longitudinal case-cohort design. The study was longitudinal in that the unit of analysis was a resident-day, to ensure that the timing of C. difficile infection relative to antibiotic exposures could be captured accurately. Days on which residents developed C. difficile infection were case-days, and days on which residents did not develop C. difficile infection were control-days [2]. As such, a single resident could contribute both case- and control-days. Time-varying exposures, including antibiotics, were measured for each day [16]. The study used a case-cohort design in that controls were randomly sampled with a known probability of selection, and unlike a nested case-control study, controls were not matched to cases [17]. Because we anticipated over 150 million resident-day records and lacked the computing power for this size of data set, we selected 100% of C. difficile infection case-days and a 0.1% simple random sample of control-days. This design allowed us to analyze a data set that was almost 1000 times smaller than the full cohort, while obtaining results that case-cohort theory suggests should be indistinguishable from the full cohort analysis, because there were 80 controls per case (exceeding the 10:1 rule of thumb) [18].
Population
We identified residents of nursing homes in Ontario, Canada, between 2012 and 2017. We included only residents aged 66 years of age or older in order to ensure 1 year of baseline prescribing information in the included cohort. We then selected 100% of case-days and 0.1% of control-days. After the case-cohort selection, we excluded resident-days with: (1) multiple antibiotics within a 90-day retrospective window to enable attribution of C. difficile risk to individual agents, (2) a hospitalization, rehabilitation, or continuing care stay in the prior 1–30 days, since antibiotic exposures in these centers are not captured in the Ontario Drug Benefit database (note that the day of admission itself was not excluded to enable detection of inpatient C. difficile infection), (3) a history of C. difficile infection in the prior 60 days, and (4) receipt of C. difficile treatment agents in the prior 90 days (oral vancomycin, metronidazole, and fidaxomicin) that did not meet the case definition, to ensure only incident C. difficile infections were included, and not the postinfection period, recurrent cases, or prolonged C. difficile infection treatment.
Case Definition
The primary outcome was incident C. difficile infection, as identified in nursing homes and hospitals. In nursing homes, C. difficile infection was identified by either a physician visit billing corresponding to diarrhea (ICD-9 code 009) or a resident assessment with C. difficile infection, combined with initiation of a C. difficile infection treatment agent. Treatment agents were defined as metronidazole, oral vancomycin, or fidaxomicin. Among residents transferred to a hospital, C. difficile infection was identified as an emergency unit or hospital admission with diagnosis code corresponding to C. difficile infection (ICD-10 code A04.7), which has been shown to have good concordance with laboratory identified infection (κ = 0.64) and neither over- nor underestimates incidence [19]. The date of incident infection was defined as the date of treatment agent initiation (nursing homes) or the day of emergency unit or hospital admission (hospitals).
Antibiotic Risk Factors
Antibiotics dispensed were assessed via the Ontario Drug Benefit database. Only antibiotics with a systemic route of administration (oral or parenteral) were included. For each resident-day, we measured the following, within a 90-day retrospective window: (1) the type of antibiotic received, if any, (2) days of antibiotic therapy, and (3) days elapsed since most recent antibiotic receipt.
Other Risk Factors
We captured 14 resident-level covariates that could potentially confound an association between antibiotic receipt and C. difficile infection, including: age (66–75, 76–85, ≥86) and sex from the population registry, days of emergency department or acute care stay in the prior 31–90 days (0, 1–7, ≥8 days), Charlson comorbidity index (0, 1, 2–3, ≥4) [20] and history of C. difficile infection in the prior 2 years, 2 variables related to acid suppressant use in the prior 90 days (proton pump or H2 antagonist receipt), 5 variables related to functional status (requiring assistance with transferring, dressing, eating, hygiene, and toileting), and 2 variables related to device use in the prior 30 days (dialysis, feeding tube) from quarterly nursing home assessments [13].
Statistical Analysis
Due to the case-cohort study design used in this study, we incorporated sampling weights to reflect the source population when calculating the cohort size, the prevalence of risk factors, and the incidence of C. difficile infection [18]. Sampling weights were 1 for case-days and 1000 for control-days, corresponding to the inverse of the selection probability.
We used logistic random-effects models to measure the probability of incident C. difficile infection on a given resident-day. In order to simultaneously account for both duration effects and waning effects after cessation, important qualifiers of antibiotic-associated risk [1, 4, 16], we developed 2 antibiotic exposure metrics. The “no waning” antibiotic metric was measured as the days having receipt of the antibiotic in the prior 90 days transformed using the log2(X + 1) transformation in order to account for the logarithmic relationship between antibiotic days and C. difficile infection risk. The “linear waning” antibiotic metric multiplied the “no waning” antibiotic metric by antibiotic recency which varied between 0 (no receipt in prior 90 days) and 1 (current receipt). Specifically, recency was measured as 1-X/90, where X was the number of days since a resident’s most recent antibiotic. Linear combinations of the no waning and linear waning metrics could simultaneously capture both cumulative exposure in terms of days of therapy and waning effects.
Unadjusted models included both antibiotic metrics, which were allowed to vary according to the type of antibiotic received, parameterized as a random-effect interaction [21]. Adjusted models included the same variables as the unadjusted model, with the addition of the 14 individual-level covariates.
We predicted resident C. difficile infection risk for each of the 18 antibiotics for durations between 5 and 14 days [22] using the unadjusted and adjusted models developed above. For unadjusted 90-day incidence, we extracted predicted probabilities over a 90-day period, with day 1 defined as the day of antibiotic initiation and then summed these probabilities to obtain the cumulative 90-day probability of C. difficile infection. In total, there were 180 risk estimates (18 × 10) for patients with a history of antibiotic use and 1 risk estimate for patients without antibiotic use, for a total of 181 risk estimates. Adjusted 90-day incidence was measured analogously but using the prediction at the means approach [23].
We compared different risk estimates using the risk ratio measure: (1) initiating a 7-day antibiotic course compared to no antibiotic exposure, (2) a longer duration of the same antibiotic versus a shorter duration, or (3) substituting an antibiotic (same 7-day duration but for different agents). We presented clinically important comparisons within urinary infective agents: ciprofloxacin or trimethoprim-sulfomethoxazole (co-trimoxazole) versus nitrofurantoin; and respiratory infective agents: moxifloxacin, levofloxacin or amoxicillin-clavulanate versus amoxicillin; clindamycin or cephalexin versus cloxacillin.
We estimated 95% confidence intervals (CIs) for risk estimates and risk ratios by iteratively bootstrapping the model and then recalculating the risk estimates and risk ratios 1000 times [24].
Sensitivity Analyses
We reran our prediction models on the following subsets: (1) residents without any hospital exposure in the prior 90 days, (2) residents without a prior history of C. difficile, and (3) using just a single, randomly selected, observation per resident.
RESULTS
Population
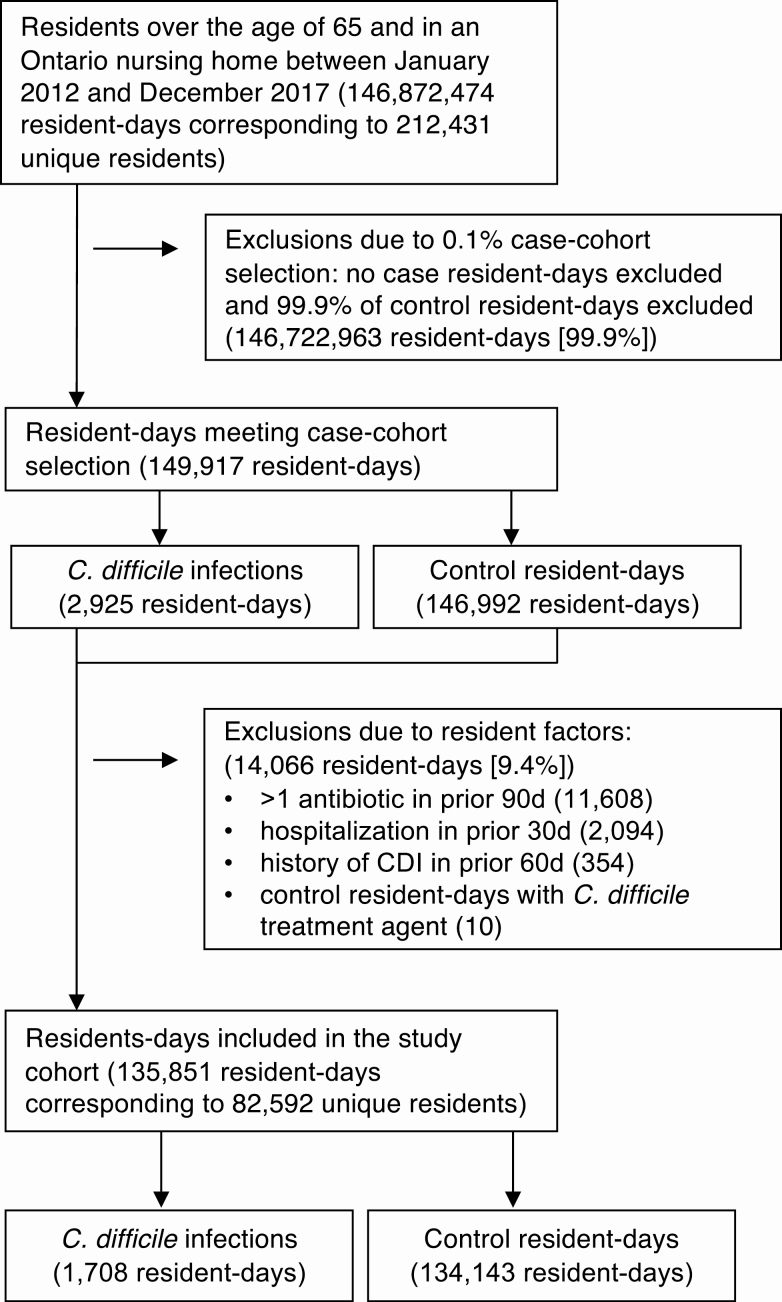
The source longitudinal cohort included 146.9 million days of follow-up, which was reduced to 149 917 days after the initial 0.1% selection of control resident-days (Figure 1). In sum, 9.4% of the remaining resident-days were excluded based on resident-level exclusion criteria, of which receipt of > 1 antibiotic in the prior 90 days was the most significant, leaving 135 851 resident-days among 82 592 unique residents in the final cohort (mean of 1.6 days of observation per resident).
Figure 1.
Study flow diagram. Abbreviation: CDI, Clostridium difficile infection.
C. difficile Infection Incidence
Of these 135 851 resident-days, we identified 1708 cases of C. difficile infection and 134 143 control resident-days for a case-control ratio of 80:1. Accounting for sampling weights [18], the incidence of C. difficile infection was 1.27 per 100 000 resident-days. Of the 1708 cases, 680 were identified as outpatients, whereas 1028 were identified in-hospital.
Cohort Characteristics
The majority (54.7%) of residents were 86 or over, and 71.4% of residents were female (Table 1). A high proportion of residents had received a proton pump inhibitor in the prior 90 days (39.4%).
Table 1.
Nursing Home Resident Cohort Characteristics and Exposures, 2012–2017
| Variable | Cohort resident-days (000s)a | C. difficile infection (N) | Incidence Rate per 100 000 resident-days (95% CI) |
|---|---|---|---|
| Total | 134 145 (100%) | 1708 (100%) | 1.27 (1.21, 1.34) |
| Age | |||
| 66 to 75 | 16 144 (12.0%) | 223 (13.1%) | 1.38 (1.21, 1.58) |
| 76 to 85 | 44 666 (33.3%) | 617 (36.1%) | 1.38 (1.27, 1.49) |
| ≥86 | 73 335 (54.7%) | 868 (50.8%) | 1.18 (1.11, 1.27) |
| Female | 95 782 (71.4%) | 1119 (65.5%) | 1.17 (1.10, 1.24) |
| Hospital visit in prior 31–90 db | |||
| None | 120 950 (90.2%) | 972 (56.9%) | .80 (.75, .86) |
| Any | 13 195 (9.8%) | 736 (43.1%) | 5.58 (5.18, 6.00) |
| 1 to 7 d | 10 006 (7.5%) | 489 (28.6%) | 4.89 (4.46, 5.34) |
| ≥8 d | 3140 (2.3%) | 247 (14.5%) | 7.87 (6.92, 8.91) |
| Charlson comorbidities | |||
| 0 | 90 962 (67.8%) | 662 (38.8%) | .73 (.67, .79) |
| 1 | 18 381 (13.7%) | 291 (17%) | 1.58 (1.41, 1.78) |
| 2 to 3 | 17 721 (13.2%) | 478 (28%) | 2.70 (2.46, 2.95) |
| ≥4 | 7080 (5.3%) | 277 (16.2%) | 3.91 (3.47, 4.40) |
| History of C. difficile infection | 1989 (1.5%) | 312 (18.3%) | 15.7 (14.0, 17.5) |
| Acid suppressants in prior 90 d | |||
| Proton pump inhibitor | 52 915 (39.4%) | 933 (54.6%) | 1.76 (1.65, 1.88) |
| H2 antagonist | 4732 (3.5%) | 91 (5.3%) | 1.92 (1.55, 2.36) |
| Device use in prior 30 d | |||
| Feeding tube | 958 (0.7%) | 80 (4.7%) | 8.35 (6.62, 10.4) |
| Dialysis | 654 (0.5%) | 58 (3.4%) | 8.87 (6.73, 11.5) |
| Functional status, requires assistance … | |||
| Transferring | 99 413 (74.1%) | 1402 (82.1%) | 1.41 (1.34, 1.49) |
| Dressing | 118 575 (88.4%) | 1576 (92.3%) | 1.33 (1.26, 1.40) |
| Eating | 48 959 (36.5%) | 628 (36.8%) | 1.28 (1.18, 1.39) |
| With hygiene | 112 334 (83.7%) | 1519 (88.9%) | 1.35 (1.29, 1.42) |
| Toileting | 118 800 (88.6%) | 1548 (90.6%) | 1.30 (1.24, 1.37) |
Abbreviation: CI, confidence interval.
aWeighted to reflect the source population (case weight = 1, control weight = 1000).
bNote that residents with a hospital visit in the prior 30 d were excluded from the cohort.
Cohort Antibiotic Exposures
One fifth (20.4%) of residents in the cohort had exposure to antibiotics in the prior 90 days (Table 2). The 6 most common antibiotics were cephalexin, ciprofloxacin, amoxicillin, cotrimoxazole, nitrofurantoin, and moxifloxacin, which together accounted for 67.2% of antibiotic exposures in the cohort. The next 6 most common antibiotics included levofloxacin, amoxicillin-clavulanate, azithromycin, cefuroxime, norfloxacin, and clarithromycin, which together accounted for an additional 22.1% of antibiotic exposures. The next 6 most common antibiotics included clindamycin, cloxacillin, cefprozil, tetracycline, cefixime, and trimethoprim, accounting for just 8.7% of antibiotic exposures. Seven antibiotics, of which penicillin V and ceftriaxone were the most common, were rarely dispensed and accounted for the remaining 2% of antibiotic exposures.
Table 2.
Prevalence of Antibiotic Exposures in the Nursing Home Resident Cohort
| Variable | Cohort resident-days (000s)a | Mean Days of Antibiotic in Prior 90 da | C. difficile Infection (N, %) | Incidence Rate per 100 000 resident-days (95% CI) |
|---|---|---|---|---|
| Antibiotic exposure in prior 90 d | ||||
| None | 106 766 (79.6%) | 0 | 883 (51.7%) | 0.83 (.77, .88) |
| Any | 27 379 (20.4%) | 11.6 | 825 (48.3%) | 3.01 (2.81, 3.23) |
| Type of antibiotic | ||||
| Cephalexin | 3641 (2.7%) | 11.0 | 123 (7.2%) | 3.38 (2.81, 4.03) |
| Ciprofloxacin | 3405 (2.5%) | 9.6 | 123 (7.2%) | 3.61 (3.00, 4.31) |
| Amoxicillin | 3409 (2.5%) | 9.6 | 57 (3.3%) | 1.67 (1.27, 2.17) |
| Cotrimoxazole | 2932 (2.2%) | 15.7 | 65 (3.8%) | 2.22 (1.71, 2.83) |
| Nitrofurantoin | 2667 (2.0%) | 19.7 | 35 (2.0%) | 1.31 (.91, 1.83) |
| Moxifloxacin | 2343 (1.7%) | 8.4 | 140 (8.2%) | 5.98 (5.03, 7.05) |
| Levofloxacin | 1814 (1.4%) | 8.2 | 42 (2.5%) | 2.32 (1.67, 3.13) |
| Amoxicillin-clavulanate | 1508 (1.1%) | 9.3 | 67 (3.9%) | 4.44 (3.44, 5.64) |
| Azithromycin | 1140 (0.8%) | 8.8 | 34 (2.0%) | 2.98 (2.07, 4.17) |
| Cefuroxime | 848 (0.6%) | 8.6 | 36 (2.1%) | 4.25 (2.97, 5.88) |
| Norfloxacin | 742 (0.6%) | 13.0 | 8 (0.5%) | 1.08 (.47, 2.12) |
| Clarithromycin | 602 (0.4%) | 8.6 | 8 (0.5%) | 1.33 (.57, 2.62) |
| Clindamycin | 526 (0.4%) | 8.6 | 32 (1.9%) | 6.08 (4.16, 8.59) |
| Cloxacillin | 440 (0.3%) | 10.8 | 13 (0.8%) | 2.95 (1.57, 5.05) |
| Cefprozil | 352 (0.3%) | 9.3 | 11 (0.6%) | 3.13 (1.56, 5.59) |
| Tetracycline | 174 (0.1%) | 45.6 | 0 (0%) | 0.00 (.00, 2.12) |
| Cefixime | 144 (0.1%) | 8.6 | 14 (0.8%) | 9.72 (5.32, 16.3) |
| Trimethoprim | 152 (0.1%) | 72.0 | 1–5 (0.0–0.3%)b | NA |
| Other c | 539 (0.4%) | 14.6 | 15 (0.9%) | 2.78 (1.56, 4.59) |
Abbreviation: CI, confidence interval; NA, not applicable.
a Weighted to reflect the source population (case weight = 1, control weight = 1000).
b Exact value suppressed.
c Includes penicillin V, ceftriaxone, and other less frequently prescribed antibiotics.
Comparative C. difficile Infection Risks of Antibiotic Courses
The 90-day risk of C. difficile infection among residents without antibiotics was 0.81 per 1000 residents (Table 3), compared to 1.90 among those with a 7-day course of antibiotics. A 7-day course of antibiotics was associated with a 1.80-fold increase in C. difficile infection risk (ARR = 1.80 for 7-day course versus no antibiotics, 95% CI: 1.55, 1.97).
Table 3.
90-day Incidence of C. difficile Infection for Antibiotic Courses
| Unadjusted 90-day Incidence (per 1000) | Unadjusted Relative Risk (95% CI) | Adjusted a 90-day Incidence (per 1000) | Adjusted a Relative Risk (95% CI) | |
|---|---|---|---|---|
| Initiation (7 d) | ||||
| No antibiotic | 0.81 | Reference | 0.57 | Reference |
| Any antibiotic | 1.90 | 2.34 (2.01, 2.53) | 1.02 | 1.80 (1.55, 1.97) |
| Clindamycin | 4.12 | 5.08 (3.55, 6.97) | 2.29 | 4.04 (2.74, 5.72) |
| Fluoroquinolones | ||||
| Moxifloxacin | 3.66 | 4.50 (3.77, 5.24) | 1.92 | 3.39 (2.83, 4.03) |
| Ciprofloxacin | 2.38 | 2.93 (2.49, 3.45) | 1.07 | 1.90 (1.57, 2.25) |
| Levofloxacin | 1.78 | 2.20 (1.66, 2.74) | 0.87 | 1.55 (1.11, 1.98) |
| Norfloxacin | 0.92 | 1.14 (.62, 1.57) | 0.61 | 1.09 (.65, 1.50) |
| Cephalosporins | ||||
| Cefixime | 4.74 | 5.83 (3.51, 9.66) | 2.41 | 4.26 (2.41, 7.42) |
| Cefuroxime | 2.66 | 3.28 (2.42, 4.29) | 1.39 | 2.46 (1.61, 3.45) |
| Cefprozil | 2.12 | 2.61 (1.49, 3.89) | 1.07 | 1.89 (1.13, 3.02) |
| Cephalexin | 1.96 | 2.41 (2.01, 2.80) | 1.05 | 1.85 (1.54, 2.15) |
| Macrolides | ||||
| Azithromycin | 2.23 | 2.75 (1.91, 3.69) | 1.22 | 2.15 (1.53, 2.95) |
| clarithromycin | 1.20 | 1.48 (.82, 2.06) | 0.74 | 1.32 (.73, 1.85) |
| Penicillins | ||||
| Amoxicillin-clavulanate | 2.89 | 3.55 (2.82, 4.34) | 1.37 | 2.43 (1.89, 3.08) |
| Cloxacillin | 1.90 | 2.35 (1.53, 3.28) | 1.08 | 1.90 (1.21, 2.79) |
| Amoxicillin | 1.39 | 1.71 (1.28, 2.11) | 0.87 | 1.53 (1.15, 1.92) |
| Sulfonamides and Trimethoprim | ||||
| Cotrimoxazole | 1.56 | 1.92 (1.52, 2.34) | 0.85 | 1.50 (1.19, 1.83) |
| Trimethoprim | 1.28 | 1.58 (.73, 3.48) | 0.73 | 1.29 (.73, 2.95) |
| Nitrofurantoin | 1.08 | 1.33 (.95, 1.70) | 0.57 | 1.00 (.72, 1.25) |
| Tetracycline | 0.86 | 1.06 (.65, 1.23) | 0.53 | 0.94 (.65, 1.11) |
| Duration | ||||
| 7 d | 1.90 | Reference | 1.02 | Reference |
| vs 5 d | 1.68 | 0.88 (.87, .90) | 0.93 | 0.91 (.90, .93) |
| vs 10 d | 2.19 | 1.15 (1.13, 1.17) | 1.14 | 1.12 (1.09, 1.14) |
| vs 14 d | 2.53 | 1.33 (1.27, 1.36) | 1.29 | 1.27 (1.21, 1.30) |
| Selection (7 d) | ||||
| Nitrofurantoin | 1.08 | Reference | 0.57 | Reference |
| vs cotrimoxazole | 1.56 | 1.45 (1.05, 2.10) | 0.85 | 1.50 (1.10, 2.21) |
| vs ciprofloxacin | 2.38 | 2.21 (1.65, 3.24) | 1.07 | 1.89 (1.45, 2.68) |
| Amoxicillin | 1.39 | Reference | 0.87 | Reference |
| vs levofloxacin | 1.78 | 1.28 (.91, 1.86) | 0.87 | 1.01 (.70, 1.45) |
| vs amoxicillin-clavulanate | 2.89 | 2.08 (1.53, 2.96) | 1.37 | 1.58 (1.15, 2.28) |
| vs moxifloxacin | 3.66 | 2.63 (2.03, 3.55) | 1.92 | 2.21 (1.67, 3.08) |
| Cloxacillin | 1.90 | Reference | 1.08 | Reference |
| vs cephalexin | 1.96 | 1.03 (.71, 1.59) | 1.05 | .97 (.65, 1.56) |
| vs clindamycin | 4.12 | 2.17 (1.35, 3.85) | 2.29 | 2.12 (1.32, 3.78) |
Abbreviation: CI, confidence interval.
aAdjusted for 14 resident-level covariates: age, sex, days of emergency department or acute care stay in the prior 31–90 days, Charlson comorbidity index, and history of C. difficile infection in the prior 2 years, acid suppressant use in the prior 90 days, functional status, and device use in the prior 30 days.
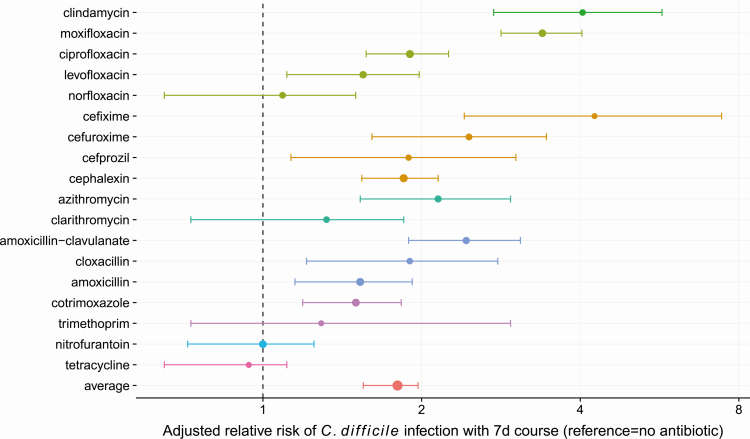
Antibiotic risks were heterogeneous. When examining 7-day courses of antibiotics, agents conferring the highest risks (Figure 2) were cefixime (ARR = 4.26, 95% CI: 2.41, 7.42), clindamycin (ARR = 4.04, 95% CI: 2.74, 5.72), moxifloxacin (ARR = 3.39, 95% CI: 2.83, 4.03), and amoxicillin-clavulanate (ARR = 2.43, 95% CI: 1.89, 3.08).
Figure 2.
Forest plot of adjusted relative risks and 95% confidence intervals for 90-day C. difficile infection incidence associated with different 7-day antibiotic courses. The reference is the incidence in residents with no antibiotic receipt in the prior 90 days.
We compared 7-day courses of antibiotics with similar indications: ciprofloxacin engendered 89% more risk than nitrofurantoin (ARR = 1.89, 95% CI: 1.45, 2.68), moxifloxacin resulted in 121% more risk than amoxicillin (ARR = 2.21, 95% CI: 1.67, 3.08), and clindamycin resulted in 112% (ARR = 2.12, 95% CI: 1.32, 3.78) more risk than cloxacillin. Among fluoroquinolones, a 7-day course of moxifloxacin conferred significantly more C. difficile infection risk compared to the same duration of either ciprofloxacin (ARR = 1.79, 95% CI: 1.40, 2.30) or levofloxacin (ARR = 2.20, 95% CI: 1.62, 3.11).
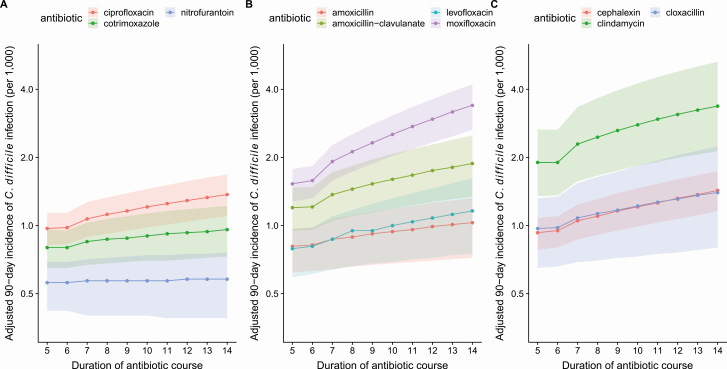
Longer antibiotic durations were associated with greater C. difficile infection risk. On average, compared to a 7-day course, a 14-day course was associated with 27% more risk (ARR = 1.27, 95% CI: 1.21, 1.30), while a 5-day course was associated with 9% less risk (ARR = 0.91, 95% CI: .90, .93). However, the strength of the duration-risk association was heterogeneous; longer durations of low-risk agents (nitrofurantoin) did not increase risk substantially, while longer durations of high-risk agents (moxifloxacin) were particularly harmful (Figure 3).
Figure 3.
Adjusted 90-day incidence of C. difficile infection (per 1000 residents) for (A) ciprofloxacin, cotrimoxazole, nitrofurantoin, (B) amoxicillin, amoxicillin-clavulanate, levofloxacin, moxifloxacin, and (C) cephalexin, clindamycin, cloxacillin, for durations of 5 to 14 days. Shaded regions represent 95% confidence intervals.
To enable prescribers to compare the relative risks of alternative specific antibiotic courses, we developed a searchable table (https://rebrand.ly/cdiffrisk) to allow the comparison of 90-day C. difficile infection risk between 2 antibiotic courses defined by antibiotic type (among the 18 antibiotics included in this study) and duration (5–14 days), for a total of over 32 000 potential comparisons. For example, the searchable table can be used to show that a 5-day ciprofloxacin course is associated with substantially higher risk than a 7-day nitrofurantoin course (ARR = 1.72, 95% CI: 1.29, 2.49).
Sensitivity Analyses
We used linear regression (Supplementary Data—Figure) to compare the ARRs for the 18 antibiotics in the main analysis versus the same analysis: (1) excluding patients with a recent hospitalization in the prior 90 days (R2 = 0.93), (2) excluding patients with first-time C. difficile infection (R2 = 0.98), and (3) including only a single observation per resident (R2 = 0.99).
CONCLUSIONS
Our study has shown that antibiotic prescribing choices lead to differences in C. difficile infection risk, with risk being a function of the decision to initiate, the duration dispensed, and the type of antibiotic selected. We have quantified these differential risks using a real-world population-based cohort of nursing home residents with comprehensive antibiotic exposure information.
Antibiotics are frequently prescribed for longer than necessary, driven by prescriber training and habit [9, 10]. We found that each additional day of antibiotic exposure was associated with increased C. difficile infection risk. Relative to a 7-day course, a 14-day course was associated with 27% more risk, whereas a shorter 5-day course was associated with 9% less risk. Shorter courses of 5–7 days have similar clinical efficacy compared to longer courses for uncomplicated urinary tract infections, pneumonia, and cellulitis [25]. When antibiotics are necessary, prescribers can use this information to select the shortest effective duration and minimize patient risk.
Consistent with previous meta-analyses [5, 6, 26], we showed that clindamycin, fluoroquinolones, and cephalosporins were the highest risk classes and that tetracyclines were a low risk class [27, 28]. We also identified important within-class differences in risk which prior studies were not powered to investigate. First, we showed that amoxicillin-clavulanate [6] conferred high risk, providing further evidence that penicillin-beta-lactamase combinations should be considered apart from other penicillins and prescribed with caution [29]. Second, we showed that moxifloxacin, a respiratory fluoroquinolone with extended anaerobic coverage, caused greater risk than ciprofloxacin and levofloxacin. A prior study, using Ontario outpatient data from the early 2000s, found that moxifloxacin had 37% higher risk than levofloxacin and ciprofloxacin, but this finding was not statistically significant [7]. This study, using a longitudinal model capturing detailed antibiotic exposures, has enabled better measurement of antibiotic risks.
Our study quantified the comparative risks of real-world prescribing choices. For example, prescribing a 7-day course of nitrofurantoin (an agent with good clinical efficacy for treatment of cystitis) [30], rather than ciprofloxacin, would lead to 47% less C. difficile risk. Similarly, for suspected or proven pneumococcal community-acquired pneumonia, amoxicillin is equally effective as the broader spectrum moxifloxacin [31]; however, this study found that a 7-day course of amoxicillin would lead to 55% less C. difficile infection risk compared to the same duration of moxifloxacin. These quantitative comparisons provide information for prescribers and patients to make informed choices [32] and minimize C. difficile risk.
Our study had certain limitations. Our dispensing data, although highly comprehensive for all Ontarians over 65 years of age, did not capture antibiotics administered during hospitalizations. We addressed this by excluding patients with a recent hospitalization in the prior 30 days from the main analysis. Furthermore, a sensitivity analysis excluding all residents with a hospitalization in the prior 90 days was also conducted and showed similar antibiotic risks (R2 = 0.93). Some have highlighted confounding due to multiple antibiotic exposures as a common weakness of C. difficile risk studies [7]. We have eliminated this weakness by examining only residents receiving a single antibiotic type in the prior 90 days. However, this meant that we were unable to consider the impacts of antibiotic combinations. Although a strength of this study was the comprehensive exposure information in the nursing home cohort, we cannot be certain how well these findings generalize to younger patients and community dwelling elderly. Finally, our study only delineates the comparative risks of C. difficile and ignores other differences in antibiotic harms (allergy, organ toxicity, drug-drug interactions, and selection for antimicrobial resistance) and benefits (spectrum of coverage, potency, tissue penetration). Despite these limitations, this study provides much needed information on antibiotic risks that in the past could not be easily quantified.
C. difficile infection is an important antibiotic associated harm, and harms related to antibiotic prescribing may not be sufficiently considered when clinicians make prescribing decisions [11, 12]. We measured the relative risks across a wide range of antibiotic prescribing choices and found wide disparities in risks. The results of this study can be used by clinicians to weigh the potential harms of antibiotic prescribing choices to prevent C. difficile infection and improve patient outcomes.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgements. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The authors also thank Kwaku Adomako and Arezou Saedi for administrative support.
Disclaimer. The funder had no role in the conduct of the research.
Financial support. This study was funded by an unrestricted grant from the Canadian Institutes of Health Research (funding reference number 141798).
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011; 53:42–8. [DOI] [PubMed] [Google Scholar]
- 2. Brown KA, Jones M, Daneman N, et al. Importation, antibiotics, and Clostridium difficile infection in veteran long-term care: a multilevel case-control study. Ann Intern Med 2016; 164:787–94. [DOI] [PubMed] [Google Scholar]
- 3. Brown KA, Fisman DN, Moineddin R, Daneman N. The magnitude and duration of Clostridium difficile infection risk associated with antibiotic therapy: a hospital cohort study. PLoS One 2014; 9:e105454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kavanagh K, Pan J, Marwick C, et al. Cumulative and temporal associations between antimicrobial prescribing and community-associated Clostridium difficile infection: population-based case-control study using administrative data. J Antimicrob Chemother 2017; 72:1193–201. [DOI] [PubMed] [Google Scholar]
- 5. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother 2013; 57:2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slimings C, Riley TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother 2014; 69:881–91. [DOI] [PubMed] [Google Scholar]
- 7. Dhalla IA, Mamdani MM, Simor AE, Kopp A, Rochon PA, Juurlink DN. Are broad-spectrum fluoroquinolones more likely to cause Clostridium difficile-associated disease? Antimicrob Agents Chemother 2006; 50:3216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marwick CA, Yu N, Lockhart MC, et al. Community-associated Clostridium difficile infection among older people in Tayside, Scotland, is associated with antibiotic exposure and care home residence: cohort study with nested case-control. J Antimicrob Chemother 2013; 68:2927–33. [DOI] [PubMed] [Google Scholar]
- 9. Daneman N, Campitelli MA, Giannakeas V, et al. Influences on the start, selection and duration of treatment with antibiotics in long-term care facilities. CMAJ 2017; 189:E851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fernandez-Lazaro CI, Brown KA, Langford BJ, Daneman N, Garber G, Schwartz KL. Late-career physicians prescribe longer courses of antibiotics. Clin Infect Dis 2019; 69:1467–75. [DOI] [PubMed] [Google Scholar]
- 11. Livorsi D, Comer A, Matthias MS, Perencevich EN, Bair MJ. Factors influencing antibiotic-prescribing decisions among inpatient physicians: a qualitative investigation. Infect Control Hosp Epidemiol 2015; 36:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann TC, Del Mar C. Clinicians’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med 2017; 177:407–19. [DOI] [PubMed] [Google Scholar]
- 13. Mor V, Intrator O, Unruh MA, Cai S. Temporal and geographic variation in the validity and internal consistency of the Nursing Home Resident Assessment Minimum Data Set 2.0. BMC Health Services Research 2011; 11. Available at: https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-11-78. Accessed 13 August 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Quan H, Li B, Saunders LD, et al. ; IMECCHI Investigators . Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res 2008; 43:1424–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levy AR, O’Brien BJ, Sellors C, Grootendorst P, Willison D. Coding accuracy of administrative drug claims in the Ontario Drug Benefit database. Can J Clin Pharmacol 2003; 10:67–71. [PubMed] [Google Scholar]
- 16. Brown KA, Daneman N, Stevens VW, et al. Integrating time-varying and ecological exposures into multivariate analyses of hospital-acquired infection risk factors: a review and demonstration. Infect Control Hosp Epidemiol 2016; 37:411–9. [DOI] [PubMed] [Google Scholar]
- 17. Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986; 73:1–11. [Google Scholar]
- 18. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999; 52:1165–72. [DOI] [PubMed] [Google Scholar]
- 19. Dubberke ER, Butler AM, Nyazee HA, et al. ; Centers for Disease Control and Prevention Epicenters Program . The impact of ICD-9-CM code rank order on the estimated prevalence of Clostridium difficile infections. Clin Infect Dis 2011; 53:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 21. Gelman A, Hill J. Data analysis using regression and multilevel/hierarchical models. Cambridge: Cambridge University Press, 2007. [Google Scholar]
- 22. Manuel DG, Rosella LC, Hennessy D, Sanmartin C, Wilson K. Predictive risk algorithms in a population setting: an overview. J Epidemiol Community Health 2012; 66:859–65. [DOI] [PubMed] [Google Scholar]
- 23. Muller CJ, MacLehose RF. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol 2014; 43:962–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hesterberg T. Bootstrap. Wiley Interdiscip Rev Comput Stat 2011; 3:497–526. [Google Scholar]
- 25. Wald-Dickler N, Spellberg B. Short-course antibiotic therapy—replacing constantine units with “shorter is better.” Clin Infect Dis 2019; 69:1476–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother 2013; 68:1951–61. [DOI] [PubMed] [Google Scholar]
- 27. Tariq R, Cho J, Kapoor S, et al. Low risk of primary Clostridium difficile infection with tetracyclines: a systematic review and metaanalysis. Clin Infect Dis 2018; 66:514–22. [DOI] [PubMed] [Google Scholar]
- 28. Brown KA, Khanafer N, Daneman N, Fisman DN. Reply to “Are there reasons to prefer tetracyclines to macrolides in older patients with community-acquired pneumonia?”. Antimicrob Agents Chemother 2013; 57:4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kundrapu S, Sunkesula VCK, Jury LA, et al. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis 2016; 16:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huttner A, Verhaegh EM, Harbarth S, Muller AE, Theuretzbacher U, Mouton JW. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015; 70:2456–64. [DOI] [PubMed] [Google Scholar]
- 31. Petitpretz P, Arvis P, Marel M, Moita J, Urueta J; CAP5 Moxifloxacin Study Group . Oral moxifloxacin vs high-dosage amoxicillin in the treatment of mild-to-moderate, community-acquired, suspected pneumococcal pneumonia in adults. Chest 2001; 119:185–95. [DOI] [PubMed] [Google Scholar]
- 32. Coxeter P, Del Mar CB, McGregor L, Beller EM, Hoffmann TC. Interventions to facilitate shared decision making to address antibiotic use for acute respiratory infections in primary care. Cochrane Database Syst Rev 2015; Available at: http://doi.wiley.com/10.1002/14651858.CD010907.pub2. Accessed 13 August 2019. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.