Abstract
Background
Human papillomavirus (HPV) vaccination programs achieve substantial population-level impact, with effects extending beyond protection of vaccinated individuals. We assessed trends in HPV prevalence up to 8 years postvaccination among men and women in the Netherlands, where bivalent HPV vaccination, targeting HPV types 16/18, has been offered to (pre)adolescent girls since 2009 with moderate vaccination coverage.
Methods
We used data from the PASSYON study, a survey initiated in 2009 (prevaccination) and repeated biennially among 16- to 24-year-old visitors of sexual health centers. We studied genital HPV positivity from 2009 to 2017 among women, heterosexual men, and unvaccinated women using Poisson generalized estimating equation models, adjusted for individual- and population-level confounders. Trends were studied for 25 HPV types detected by the SPF10-LiPA25 platform.
Results
A total of 6354 women (64.7% self-reported unvaccinated) and 2414 heterosexual men were included. Percentual declines in vaccine types HPV-16/18 were observed for all women (12.6% per year [95% confidence interval {CI}, 10.6–14.5]), heterosexual men (13.0% per year [95% CI, 8.3–17.5]), and unvaccinated women (5.4% per year [95% CI, 2.9–7.8]). We observed significant declines in HPV-31 (all women and heterosexual men), HPV-45 (all women), and in all high-risk HPV types pooled (all women and heterosexual men). Significant increases were observed for HPV-56 (all women) and HPV-52 (unvaccinated women).
Conclusions
Our results provide evidence for first-order herd effects among heterosexual men against HPV-16/18 and cross-protective types. Additionally, we show second-order herd effects against vaccine types among unvaccinated women. These results are promising regarding population-level and clinical impact of girls-only bivalent HPV vaccination in a country with moderate vaccine uptake.
Keywords: human papillomavirus, HPV, vaccination, population effects, herd immunity, type replacement
This study presents trends in human papillomavirus (HPV) positivity since girls-only HPV vaccine introduction in the Netherlands, with moderate vaccine uptake. We show declining prevalences of vaccine types HPV-16/18 in heterosexual men and unvaccinated women, and of cross-protective types in heterosexual men.
Infections with human papillomavirus (HPV) are usually transient; however, persistent infections may induce illness in the anogenital or oropharyngeal regions among men and women. Most common are warts, but persistent infections with a high-risk (hr) HPV type can also lead to various cancers [1]. From 2006 onward, 3 prophylactic vaccines have been registered for prevention of HPV-related (pre)cancers, all targeting the most oncogenic hrHPV types 16 and 18. The Netherlands has included the bivalent HPV (2vHPV) vaccine, targeting HPV-16/18, in its national immunization program. HPV-16/18 vaccination is offered to 12-year-old girls in the routine program since 2010, after a one-off catch-up campaign in 2009 for 12- to 16-year-old girls (birth cohorts 1993–1996) [2]. So far, vaccine uptake among vaccine-eligible girls in the general population has fluctuated between 46% and 61% per year between 2009 and 2017 [3].
Vaccine effectiveness of HPV-16/18 vaccination against (persistent) infection with vaccine and cross-protective HPV types has been shown in vaccine recipients relative to controls [4, 5]. However, the population impact of HPV vaccination extends beyond direct protection of vaccinated individuals, as infection dynamics change through vaccination implementation. In countries achieving high coverage in girls-only quadrivalent HPV vaccination programs, also targeting low-risk (lr) HPV types 6 and 11, indirect benefits were evident early on through reduced prevalence of genital warts in unvaccinated young men [6]. Additionally, declining hrHPV prevalence in young vaccinated women was sufficient to provide herd protection to unvaccinated women within 6–7 years after initiation of girls-only HPV vaccination in settings with >80% coverage [7, 8]. Herd effects among unvaccinated women are mainly derived from unvaccinated, but indirectly protected, heterosexual men. However, men have been underrepresented in studies assessing population trends in HPV prevalence over time since vaccine introduction [9].
Previously, we demonstrated herd effects for vaccine types HPV-16/18 among heterosexual men 6 years after introduction of girls-only HPV-16/18 vaccination in the Netherlands [10]. Herd effects among unvaccinated women were not yet observed within 6 years postvaccination, presumably due to the moderate vaccine uptake in the Netherlands. In a girls-only vaccination program, herd protection of unvaccinated women constitutes a second-order effect and is strongly determined by vaccination coverage [11]. As we observed herd effects among heterosexual men within 6 years postvaccination, we hypothesized that herd effects among unvaccinated women in the Netherlands should also become measurable with prolonged monitoring. Continued monitoring of trends in type-specific HPV prevalence over time is also relevant for detection of type replacement, a still unresolved possibility in the wake of HPV vaccination [12].
To further assess the population impact of the girls-only HPV-16/18 vaccination program in the Netherlands on postvaccination trends in vaccine-targeted and nonvaccine HPV types, we updated and expanded our previous analyses. Here, we present trends in HPV positivity of 25 HPV types from prevaccination up to 8 years postvaccination among 16- to 24-year-old women and heterosexual men visiting sexual health centers (SHCs) throughout the Netherlands.
METHODS
Study Design
For this updated analysis, we used data from the PASSYON (Papillomavirus Surveillance Among STI Clinic Youngsters in the Netherlands) study: a biennial cross-sectional survey to assess HPV prevalence among young visitors to SHCs [10]. In the Netherlands, SHCs offer sexually transmitted infection (STI) testing to those with increased risk, including individuals ≤24 years of age. The study design has been described previously [13]. In brief, the PASSYON study was initiated in 2009 (prevaccination) and repeated in 2011, 2013, 2015, and 2017 in SHCs throughout the country (Supplementary Figure 1). Male and female SHC visitors 16–24 years of age were approached for participation and asked to collect a genital self-swab (Copan Diagnostics, Italy). Women were instructed to take a vaginal sample, while men took a penile sample. A questionnaire on sexual risk behavior, demographics, and vaccination status was collected, which was supplemented with information from regular SHC consults. The Medical Ethical Committee (University of Utrecht, the Netherlands), provided a waiver for full medical ethical review (protocol number 08/397). Data were obtained using a unique code per person and all participants gave informed consent.
Laboratory Analyses
HPV laboratory testing was conducted similarly across all study years [13]. In brief, DNA was extracted using the MagnaPure platform (Total Nucleic Acid Isolation Kit, Roche, the Netherlands) and HPV DNA was amplified using SPF10 primer sets and detected using DNA enzyme-linked immunoassays (HPV-DEIA, DDL Diagnostics Laboratory, the Netherlands). Positive samples were genotyped with line-probe assay (HPV-LiPA25, DDL Diagnostics Laboratory, the Netherlands), which is able to detect hrHPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and lrHPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, and 74. Also HPV-68, -73, and -97 can be detected, but these types cannot be distinguished from each other and are therefore classified as HPV-68.
Statistical Analysis
Trends in HPV positivity were studied for all women (irrespective of vaccination status) and self-reported heterosexual men. To study second-order herd effects, we additionally analyzed trends in self-reported unvaccinated women (only women reporting to be unvaccinated).
The crude prevalence of hrHPV and lrHPV types was calculated for each study year, stratified by sex and vaccination status. Trends in type-specific HPV prevalence over time were assessed using generalized estimating equation (GEE) Poisson models with a log link function and robust error variance. Incorporation of an exchangeable correlation structure accounted for dependency of HPV types within individuals and ensured efficient estimation of regression coefficients. PASSYON year was added as a continuous variable to the model, resulting in a linear trend of HPV prevalence over time on a log scale. Percentual changes in HPV prevalence per year were estimated by exponentiating the (adjusted) regression coefficient for each HPV type.
To select possible confounding variables in the estimation of HPV prevalence trends, we first examined the study population over time regarding participant characteristics. Using χ 2 tests, we checked whether characteristics were comparable between different study years. Next, we studied the association between HPV positivity (any type) and participant characteristics, again using χ 2 tests. Characteristics associated with study year and with HPV positivity were included as explanatory variables in a logistic regression stepwise selection model (with P < .05 as entry and stay criteria), using HPV positivity as outcome. Variables included in the final model were used for adjustment in the Poisson GEE models. This process was performed separately for all women, men, and unvaccinated women.
Next to individual-level confounders indicated by the selection models, trends in HPV prevalence were adjusted for age group (16–20 vs 21–24 years) and for changes in SHC access policy (population-level confounder). Due to funding restrictions from 2015 onward, SHCs became stricter in prioritizing individuals at high risk for STIs, which could have resulted in a study population at systematically increased risk for HPV infection starting from 2015 [14]. As we assumed we were unable to fully adjust for this by only including changes over time in the known variables [10], we adjusted for policy change by including a categorical variable indicating the policy change from 2015 onward.
Additionally, we estimated pooled (adjusted) trends in HPV positivity over time. Pooled estimates were obtained as a weighted average of type-specific trends in the GEE Poisson models. Pooled trends were estimated for vaccine types (16/18), hrHPV types of the nonavalent vaccine (16/18/31/33/45/52/58), all hrHPV types (16/18/31/33/35/39/45/51/52/56/58/59), and all types measured in the SPF10-DEIA-LiPA25 assay. As the impact of vaccination among 16- to 24-year-olds on overall prevalence was likely to be still very low in 2011 [15], we performed sensitivity analyses pooling data from PASSYON years 2009 and 2011 to create more stable baseline measurements.
All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina). We performed complete case analyses, as none of the variables had >5% missing.
RESULTS
We included a total of 6354 women (1574 vaccinated [≥1 dose], 4111 unvaccinated, and 669 unknown, all self-reported) and 2414 heterosexual men who all provided a genital swab; the study population was 1524 in 2009, 1775 in 2011, 1816 in 2013, 1782 in 2015, and 1871 in 2017. The percentage of women reporting to be vaccinated increased over the years; 2.3% in 2009, 6.4% in 2011, 19.2% in 2013, 36.7% in 2015, and 54.1% in 2017. In total, 13.2% of vaccinated women were vaccinated within the regular program. Characteristics of the total study population are presented in Table 1, with the association between characteristics and study year and the association between characteristics and HPV positivity given separately for all women, heterosexual men, and unvaccinated women in Supplementary Tables 1–3. In general, sexual risk behavior increased over time in all groups.
Table 1.
Characteristics of the Study Population for All Women, Heterosexual Men, and Unvaccinated Women
| Characteristic | All Women (n = 6354) |
Heterosexual Men (n = 2414) |
Unvaccinated Women (n = 4111) |
|||
|---|---|---|---|---|---|---|
| Age | ||||||
| 16–20 y | 2478 | (39.0) | 691 | (28.6) | 1336 | (32.5) |
| 21–24 y | 3876 | (61.0) | 1723 | (71.4) | 2775 | (67.5) |
| Self-defined ethnicity | ||||||
| Dutch | 5514 | (86.8) | 1933 | (80.1) | 3579 | (87.1) |
| Not Dutch | 837 | (13.2) | 479 | (19.9) | 530 | (12.9) |
| Educational levela | ||||||
| Low/middle | 1550 | (24.5) | 708 | (29.4) | 964 | (23.5) |
| High | 4773 | (75.5) | 1699 | (70.6) | 3133 | (76.5) |
| Sexual preference | ||||||
| Heterosexual | 6070 | (95.5) | 2414 | (100.0) | 3940 | (95.8) |
| Gay or bisexual | 284 | (4.5) | 0 | (0.0) | 171 | (4.2) |
| Age of sexual debut | ||||||
| ≤14 y | 813 | (13.0) | 405 | (16.9) | 504 | (12.4) |
| 15–16 y | 3036 | (48.3) | 961 | (40.2) | 1934 | (47.5) |
| ≥17 y | 2428 | (38.7) | 1024 | (42.9) | 1630 | (40.1) |
| No. of sex partners in past 6 mo | ||||||
| 0–1 | 1960 | (30.9) | 499 | (20.7) | 1342 | (32.7) |
| 2–3 | 3047 | (48.0) | 876 | (36.3) | 1956 | (47.6) |
| 4–5 | 947 | (14.9) | 510 | (21.1) | 591 | (14.4) |
| ≥6 | 394 | (6.2) | 529 | (21.9) | 219 | (5.3) |
| No. of lifetime sex partners | ||||||
| 0–2 | 682 | (10.9) | 130 | (5.6) | 438 | (10.8) |
| 3–4 | 1176 | (18.9) | 245 | (10.6) | 770 | (19.0) |
| 5–6 | 1220 | (19.6) | 284 | (12.3) | 789 | (19.5) |
| 7–14 | 2196 | (35.2) | 749 | (32.4) | 1417 | (35.0) |
| ≥15 | 962 | (15.4) | 903 | (39.1) | 635 | (15.7) |
| Anal sex past 6 mo | ||||||
| No | 5527 | (87.4) | 2021 | (84.8) | 3568 | (87.2) |
| Yes, insertive only | 0 | (0.0) | 351 | (14.7) | 0 | (0.0) |
| Yes, receptive only | 796 | (12.6) | 3 | (0.2) | 526 | (12.8) |
| Yes, both insertive and receptive | 0 | (0.0) | 7 | (0.3) | 0 | (0.0) |
| Notified for STIb | ||||||
| No | 5630 | (89.1) | 1992 | (82.9) | 3684 | (90.0) |
| Yes | 688 | (10.9) | 410 | (17.1) | 408 | (10.0) |
| STI-related symptomsb | ||||||
| No | 4818 | (76.4) | 1742 | (72.7) | 3100 | (75.9) |
| Yes | 1491 | (23.6) | 655 | (27.3) | 984 | (24.1) |
| Self-reported history of any STI | ||||||
| No | 3549 | (56.2) | 1298 | (54.0) | 2361 | (57.6) |
| Yes | 1683 | (26.6) | 508 | (21.1) | 1087 | (26.6) |
| Never tested | 1089 | (17.2) | 598 | (24.9) | 648 | (15.8) |
| Genital chlamydia infectionb | ||||||
| No | 5403 | (85.5) | 2010 | (83.8) | 3534 | (86.4) |
| Yes | 914 | (14.5) | 388 | (16.2) | 557 | (13.6) |
| Steady partner | ||||||
| No | 3883 | (62.6) | 1359 | (58.3) | 2470 | (61.5) |
| Yes, for 0–5 mo | 1366 | (22.0) | 584 | (25.1) | 908 | (22.6) |
| Yes, ≥6 mo | 959 | (15.4) | 386 | (16.6) | 641 | (15.9) |
| Condom use past 6 mo, casual partnerc | ||||||
| Inconsistent | 2624 | (41.5) | 1139 | (47.6) | 1601 | (39.1) |
| Consistent | 2243 | (35.5) | 810 | (33.8) | 1525 | (37.2) |
| No casual partners past 6 mo | 1455 | (23.0) | 445 | (18.6) | 972 | (23.7) |
Data are presented as No. (%). Numbers vary because of missing values.
Abbreviation: STI, sexually transmitted infection.
aHigh educational level included school of higher general secondary education, pre–university education, university of applied sciences, and university. Low/middle educational level included all other levels of education.
bBased on information of the sexual health center visit.
cInconsistent included reporting never, rarely, and “sometimes I do, sometimes I do not” condom use. Consistent included reporting often or always condom use.
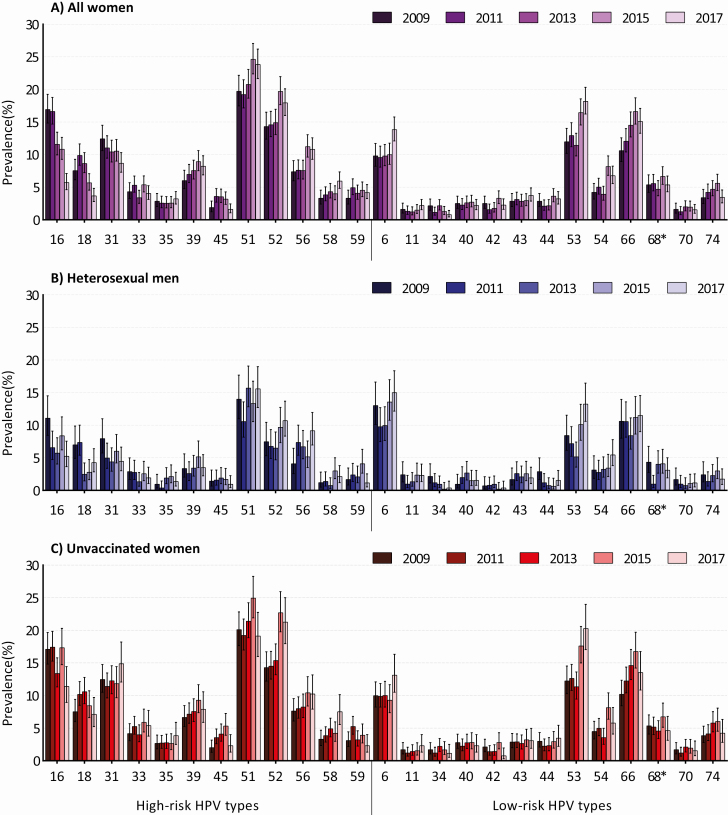
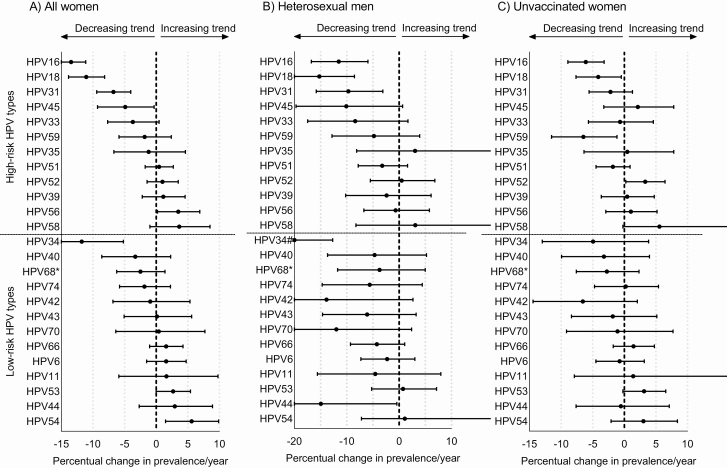
Figure 1 displays the crude HPV prevalence over time for all women, heterosexual men, and unvaccinated women. HPV prevalence was positively affected by the SHC policy change from 2015 onward. After adjustment for age and changes in individual-level characteristics over time, policy change was predicted to have elevated the HPV type-specific positivity by 9% among women and up to 30% among men in trend analyses. Overall, after adjustment for age and selected participant characteristics, and more so after adjustment for policy change, declining trends in HPV prevalence over time became stronger, while increasing trends became weaker (Supplementary Table 4). In the final adjusted GEE models, decreasing trends in both vaccine types HPV-16 and HPV-18 were estimated for women, heterosexual men, and unvaccinated women separately (Figure 2). The pooled percentual decline in HPV-16/18 prevalence per year was 12.6% (95% confidence interval [CI], 10.6%–14.5%) among all women, 13.0% (95% CI, 8.3%–17.5%) among heterosexual men, and 5.4% (95% CI, 2.9%–7.8%) among unvaccinated women (Table 2). Declining trends were also observed for cross-protective types. We estimated significantly declining trends in the prevalence of HPV-31, with a 6.8% annual decline (P < .001) among all women and a 9.7% annual decline (P = .005) among men, and in the prevalence of HPV-45, with a 4.9% annual decline (P = .036) among all women. The decline in HPV-45 prevalence of 10.4% annually among heterosexual men was borderline nonsignificant (P = .065). Other significantly declining trends in adjusted analyses were seen for HPV-59 among unvaccinated women and for low-risk types HPV-34 (all women and heterosexual men) and HPV-44 (heterosexual men).
Figure 1.
Prevalence of human papillomavirus (HPV) for the different years of the Papillomavirus Surveillance Among STI Clinic Youngsters in the Netherlands (PASSYON) study among all women (A), heterosexual men (B), and unvaccinated women (C). From 2015 onward, the access policy at the sexual health centers had changed, leading to prioritizing of individuals at high risk for sexually transmitted infections. *HPV-68 also includes HPV-73 and HPV-97.
Figure 2.
Percentual change in prevalence of high-risk and low-risk human papillomavirus (HPV) types per year, among all women (A), heterosexual men (B), and unvaccinated women (C). Percentual change in prevalence per year was calculated by exponentiating the adjusted regression coefficients of study year, which was added as a continuous variable in generalized estimating equation analyses. For the exact Percentual changes per year, see Supplementary Table 4. *HPV-68 also includes HPV-73 and HPV-97. #Point estimate for HPV-34 among heterosexual men was –26%. The x-axes differ between all women, heterosexual men, and unvaccinated women. Regression coefficients for all women were adjusted for age, policy change at the sexual health center, lifetime sex partners, history of any sexually transmitted infection (STI), steady partner, notified for STI, sex partners past 6 months, and condom use with casual partner. Regression coefficients for heterosexual men were adjusted for age, policy change at the sexual health center, lifetime sex partners, and history of any sexually transmitted infection. Regression coefficients for the unvaccinated women were adjusted for age, policy change at the sexual health center, lifetime sex partners, history of any sexually transmitted infection, notified for STI, sex partners past 6 months, and condom use with casual partner.
Table 2.
Pooled Trends in Percentual Change of Human Papillomavirus Prevalence per Year Among All Women, Heterosexual Men, and Unvaccinated Women
| Vaccine Type | All Women, % (95% CI) |
Heterosexual Men, % (95% CI) |
Unvaccinated Women, % (95% CI) |
|---|---|---|---|
| Bivalent vaccine typesa | –12.58 (–14.53 to –10.59) | –13.04 (–17.54 to –8.30) | –5.38 (–7.84 to –2.87) |
| Nonavalent vaccine hrHPV typesb | –5.73 (–7.42 to –4.02) | –7.82 (–11.81 to –3.64) | –1.28 (–3.40 to .87) |
| All hrHPV typesc | –3.02 (–4.61 to –1.42) | –5.29 (–8.96 to –1.48) | –1.29 (–3.22 to .67) |
| All hrHPV and lrHPV typesd | –1.59 (–3.13 to –.03) | –4.10 (–7.69 to –.38) | –0.67 (–2.53 to 1.22) |
Pooled trends were obtained as a weighted average of the type-specific trends in the generalized estimating equation Poisson models. Percentual change in prevalence per year was calculated by exponentiating the adjusted regression coefficients of study year.
Abbreviations: CI, confidence interval; hrHPV, high-risk human papillomavirus; lrHPV, low-risk human papillomavirus.
aIncluding HPV types 16 and 18.
bIncluding HPV types 16, 18, 31, 33, 45, 52, and 58.
cIncluding HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59.
dIncluding HPV types 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 70, 74, and 68/73/97.
Also increasing trends in HPV prevalence were observed. In adjusted GEE models, the prevalence of HPV-56/54 increased among all women, and the prevalence of HPV-52 increased among unvaccinated women. A complete overview of the trends for all 25 HPV types is provided in Figure 2.
Pooling trends of hrHPV types of the nonavalent vaccine and all hrHPV types (as a weighted average) resulted in a 5.7% and 3.0% annual decline among women and in a 7.8% and 5.3% annual decline among heterosexual men (Table 2). The pooled trend of all measured HPV types including hrHPV and lrHPV types was declining among women and heterosexual men (1.6% and 4.1% annual decline, respectively). Among unvaccinated women, none of the pooled trends were statistically significant.
Sensitivity analyses in which the first two PASSYON years were pooled and considered as one baseline measurement yielded comparable results regarding adjusted trends for HPV vaccine types. Some type specific estimates became more pronounced, for example, the decline of HPV-45/33 among all women, whereas others were attenuated, such as the decrease in HPV-31 among heterosexual men. Increasing trends in HPV-56 among all women and in HPV-52 among unvaccinated women were no longer statistically significant (Supplementary Table 5).
DISCUSSION
We assessed trends in type-specific HPV prevalence for 25 HPV types up to 8 years after HPV-16/18 vaccination implementation in the Netherlands. We demonstrated significant population impact of girls-only vaccination on vaccine-type HPV infection, with HPV-16/18 prevalence declining each year by 13% among women and heterosexual men, and by 5.4% among unvaccinated women. We also demonstrated significant declines in HPV-31 and HPV-45 among women and heterosexual men, providing strong evidence that cross-protection of the 2vHPV vaccine extends to unvaccinated individuals. Our results show that HPV-16/18 vaccination induces herd effects against vaccine and cross-protective HPV types in a setting with moderate vaccination uptake.
Decreasing trends in HPV-16/18 prevalence were observed among all groups. For women, this is partly explained by an increased proportion of vaccinated women over time who benefit from direct protection of HPV-16/18 vaccination. In a previous analysis with data up to 6 years postvaccination, we reported that heterosexual men already benefited indirectly from this through herd protection [10]. This finding is reiterated in the current analyses with data up to 8 years postvaccination. Additionally, we were now able to measure reductions in HPV-16/18 prevalence among unvaccinated women, which constitutes a second-order effect that takes more time to develop. Meanwhile, no effects of vaccination implementation were observed among men who have sex with men in the same period [16]. Our results are in line with observations from the United States, where vaccine coverage has been suboptimal as well (around 50%) and herd effects among unvaccinated women were not yet present 3–6 years after vaccination, but became measurable 5–8 years after vaccination [17].
Cross-protection of HPV-16/18 vaccination has been most clearly established for HPV types 31/33/45 [18]. In line with this cross-protection, the current analyses showed significantly decreasing trends in HPV-31/45 among all women, and in HPV-31 among heterosexual men. Declining trends in HPV-33 among all women and in HPV-45 among heterosexual men were also pronounced, albeit nonsignificant. Declines in cross-protective HPV types were not yet observed in previous analyses [10]. Although natural fluctuation could occur over time, consistency of these results suggests that cross-protection of the 2vHPV vaccine also leads to herd effects for these types, although second-order herd effects against cross-protective types remain to be demonstrated. Second-order effects against the pooled outcome HPV-31/33/45 were seen in Scotland 7 years after 2vHPV vaccine introduction, but this was in a setting with a much higher vaccination uptake of around 90% [7]. Likewise, in a community-randomized trial with moderate vaccination uptake, significant second-order herd effects could only be demonstrated in the sex-neutral vaccination arm, and not in the girls-only vaccination arm [19]. Presumably, the speed at which herd effects become apparent is a composite of vaccine effectiveness, which is lower against HPV-31/33/45 as compared to HPV-16/18, and vaccination coverage. Therefore, we suspect herd protection against cross-protective HPV types will also become apparent in unvaccinated women in the Netherlands with prolonged follow-up.
Another declining trend is observed for HPV-34, showing a decrease in both women and heterosexual men. Of the lrHPV types, HPV-34 is phylogenetically most closely related to HPV-16 [20]. Hence, the observed decrease could be related to vaccine introduction, although cross-protection against HPV-34 has not been noticed before. Furthermore, we also observed increasing trends for a few HPV types; for HPV-54/56 among all women, and for HPV-52 among unvaccinated women. Increasing trends in HPV-58/53 were borderline nonsignificant among (unvaccinated) women. Interestingly, HPV-53/54/56 are phylogenetically very distant from the vaccine types and are located on different clades (α6 and α13, respectively), and are therefore the least likely to benefit from cross-reactivity of vaccine-induced immune responses [20]. However, HPV-52 is relatively closely related to HPV-16 (both on clade α9) while also showing an increase [21]. Together, these findings could signify early effects of type replacement, but an increasing HPV prevalence over time could also be due to unmasking and secular trends irrespective of vaccination, for example, due to behavioral changes over time [22]. No significantly increasing trends in HPV prevalence were observed among men, and all increases disappeared in sensitivity analyses. However, detection of type replacement in our study was complicated by the SHC policy change during our study period, and adjusting for this policy change could have resulted in an overcorrection. Other studies assessing trends in type-specific HPV prevalence also showed increases in HPV types following vaccination implementation. In meta-analyses, increases were observed in HPV-39/52/53/73 [23], and in a community-randomized trial a tendency for increasing prevalence of HPV-39/51 was observed among unvaccinated participants [24]. In both studies, results were inconsistent when analyzed by age or birth cohort, and other studies reported no increases in HPV types [25, 26]. Because type replacement following HPV vaccination probably takes many years to develop if present [12], continued surveillance is needed on a type-specific level. Additionally, eventual replacement in disease burden also depends on the oncogenic potential of HPV types becoming more common, emphasizing the need for type-specific surveillance in (pre)cancer screening following vaccination implementation.
The current study has some limitations. First, the prioritization of high-risk individuals eligible for testing at SHCs has changed due to policy changes over the last years. While we corrected for this by including both individual- and population-level confounders in our model, we cannot rule out residual bias. Still, declines in prevalence were already observed without adjustment, both for vaccine-targeted and cross-protective types. Second, vaccination status was self-reported and could be subject to recall bias. If part of the unvaccinated women were truly vaccinated, this would result in an overestimation of the decreasing trends for vaccine types among unvaccinated women. However, previous confirmation analyses based on serology showed good correlations between self-reported vaccination status and observed antibody levels [4]. Therefore, the possible bias by using self-reported vaccination status in this setting can be considered minimal. Third, only 1 year of official prevaccination data was available, affecting our ability to consider possible background trends or natural fluctuations in HPV prevalence. In sensitivity analyses, we repeated all trend analyses in which study years 2009 and 2011 were pooled and considered to represent the prevaccination situation, and these yielded comparable results.
Our current findings indicate that transmission of vaccine-targeted and cross-protective HPV types is decreasing throughout the population. Our study included a high-risk population with a higher HPV prevalence compared to the general Dutch population, hampering generalizability [27]. As the population-level impact of HPV vaccination is generally attenuated in a high-prevalence setting, the estimates of herd protection as provided in this study are probably conservative, and population effects of girls-only HPV-16/18 vaccination in the Netherlands are likely to be stronger in the general population [28]. Our findings also emphasize the importance of monitoring nonvaccine HPV types. Prevalence changes of hrHPV types other than HPV-16/18 are relevant to assess the residual risk of (pre-)cancerous lesions and screening need in vaccinated cohorts. Our results showed declining pooled trends in all hrHPV types and all hrHPV and lrHPV types together, among both women and heterosexual men. This is reassuring for the overall benefit of HPV-16/18 vaccination and demonstrates that the 2vHPV vaccine generates broad-spectrum protection against HPV infections.
In conclusion, the current study showed substantial population-level impact of girls-only HPV-16/18 vaccination in a high-risk study population in the Netherlands, a country with moderate vaccination coverage. Apart from significant declines in vaccine-type HPV infections, we also demonstrated that cross-protection of HPV-16/18 vaccination extended to unvaccinated individuals. Our study provides unique documentation of the unfolding of first- and second-order herd effects, and suggests a significant eventual clinical impact of a girls-only HPV vaccination program.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A. B. S. developed the PASSYON (Papillomavirus Surveillance Among STI Clinic Youngsters in the Netherlands) study design. The Public Health Services (PHS) collected the data. P. J. W. coordinated the data collection. A. J. K. coordinated the laboratory analyses. P. J. W. and J. H. led the statistical analyses and data interpretation (with oversight from J. A. B., H. E. M., J. B., C. J. P. A. H., and B. H.). B. B. assisted with data interpretation. P. J. W. and J. H. drafted the manuscript. All authors contributed to drafting and revision of the paper and all authors read and approved the final manuscript. The corresponding author had full access to all of the data and final responsibility to submit for publication.
Acknowledgments. The authors thank Hein Boot (deceased), Elske van Logchem, Naomi van Marm, Suzan Leussink, Tim Severs, Rutger Schepp, and Rianne Vriend for their valuable contributions to the design or execution of the study. Furthermore, the sexual health centers, including all nurses and physicians, within the PHS and the hospitals are acknowledged for their effort. The authors acknowledge the medical microbiologic laboratories and the analysts for storage and testing of the samples. Medical Microbiological Laboratories: Certe: D. Adema, R. Buist-Arkema, D. Luijt, S. Meijer, J. Schirm. ETZ Hospital Tilburg: A. Buiting, H. Verbakel, P. van Esch, J. Verweij. Erasmus Medical Center: A. van der Eijk. University Medical Center Utrecht: F. Verduyn Lunel, S. Lakbiach, R. Schuurman. Public Health Laboratory Amsterdam: D. Abma, K. Adams, S. Bruisten, I. Linde, P. Oostvogel, C. Touwen, W. Vermeulen. Maastricht University Medical Center: J. Nelissen, P. Wolffs. Jeroen Bosch Hospital: N. van Duijvendijk, P. Schneeberger. Radboud University Medical Center: M. Dinnissen–van Poppel, W. Melchers. Izore: M. Hooghiemstra, H. Huisman, J. Weel. LabMicTA: F. Bosma, F. Geeraedts, I. Polman. Isala: P. van Goor, M. Wolfhagen. Rijnstate: E. van Koolwijk, M. Peters, C. Swanink, R. Tiemessen. 356 Medical Laboratory Dr Stein & Collegae: J. Janssen, M. Pelsers. Canisius Wilhelmina Hospital: W. de Waal. Public Health Services: PHS Drenthe: G. Aalfs. PHS IJsselland: H. van Buel. PHS Gelderland-Zuid: C. van Bokhoven-Rombouts, P. Cornelissen, M. Kersten, C. van Ruitenbeek, I. Molenaar. University Medical Center Utrecht: E. Doorn. PHS Rotterdam-Rijnmond: H. Götz, M. Illidge, J. Stam, E. Swaders. PHS Groningen: F. Postma. PHS Zuid Limburg: A M. Niekamp, M. Smit. PHS Fryslân: D. Bukasa, M. Chirandjilal, T. Taconis. PHS Twente: M. de Graas, I. Hondelink, C. Kampman. PHS Hart voor Brabant: M. van de Pas. PHS Amsterdam: T. Heijman, A. Hogewoning, M. van Rooijen. PHS Gelderland-Midden: F. Neienhuijsen, M. Pelgrim.
Disclaimer. The funders had no role in study design, data collection and analysis, interpretation of data, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the Netherlands Ministry of Health, Welfare and Sport.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Medical Microbiological Laboratories and Public Health Services:
D Adema, R Buist-Arkema, D Luijt, S Meijer, J Schirm, A Buiting, H Verbakel, P van Esch, J Verweij, A van der Eijk, F Verduyn Lunel, S Lakbiach, R Schuurman, D Abma, K Adams, S Bruisten, I Linde, P Oostvogel, C Touwen, W Vermeulen, J Nelissen, P Wolffs, N van Duijvendijk, P Schneeberger, M Dinnissen–van Poppel, W Melchers, M Hooghiemstra, H Huisman, J Weel, F Bosma, F Geeraedts, I Polman, P van Goor, M Wolfhagen, E van Koolwijk, M Peters, C Swanink, R Tiemessen, J Janssen, M Pelsers, W de Waal, G Aalfs, H van Buel, C van Bokhoven-Rombouts, P Cornelissen, M Kersten, C van Ruitenbeek, I Molenaar, E Doorn, H Götz, M Illidge, J Stam, E Swaders, F Postma, A M Niekamp, M Smit, D Bukasa, M Chirandjilal, T Taconis, M de Graas, I Hondelink, C Kampman, M van de Pas, T Heijman, A Hogewoning, M van Rooijen, F Neienhuijsen, and M Pelgrim
References
- 1. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017; 141:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qendri V, Schurink-Van ‘t Klooster TM, Bogaards JA, Berkhof J. Ten years of HPV vaccination in the Netherlands: current evidence and future challenges in HPV-related disease prevention. Expert Rev Vaccines 2018; 17:1093–104. [DOI] [PubMed] [Google Scholar]
- 3. van Lier E, Oomen P, Giesbers H, et al. Vaccinatiegraad en jaarverslag Rijksvaccinatieprogramma Nederland 2018. Report No.: 2019-0015. Bilthoven, the Netherlands: National Institute for Public Health and the Environment, 2019. [Google Scholar]
- 4. Woestenberg PJ, King AJ, van Benthem BHB, et al. ; Medical Microbiological Laboratories and the Public Health Services . Bivalent vaccine effectiveness against type-specific HPV positivity: evidence for cross-protection against oncogenic types among Dutch STI clinic visitors. J Infect Dis 2018; 217:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Donken R, King AJ, Bogaards JA, Woestenberg PJ, Meijer CJLM, de Melker HE. High effectiveness of the bivalent human papillomavirus (HPV) vaccine against incident and persistent HPV infections up to 6 years after vaccination in young Dutch women. J Infect Dis 2018; 217:1579–89. [DOI] [PubMed] [Google Scholar]
- 6. Korostil IA, Ali H, Guy RJ, Donovan B, Law MG, Regan DG. Near elimination of genital warts in Australia predicted with extension of human papillomavirus vaccination to males. Sex Transm Dis 2013; 40:833–5. [DOI] [PubMed] [Google Scholar]
- 7. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 8. Machalek DA, Garland SM, Brotherton JML, et al. Very low prevalence of vaccine human papillomavirus types among 18- to 35-year old Australian women 9 years following implementation of vaccination. J Infect Dis 2018; 217:1590–600. [DOI] [PubMed] [Google Scholar]
- 9. Drolet M, Bénard É, Pérez N, Brisson M; HPV Vaccination Impact Study Group . Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019; 394:497–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woestenberg PJ, Bogaards JA, King AJ, et al. ; Medical Microbiological Laboratories and the Public Health Services . Assessment of herd effects among women and heterosexual men after girls-only HPV16/18 vaccination in the Netherlands: a repeated cross-sectional study. Int J Cancer 2019; 144:2718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malagón T, Laurie C, Franco EL. Human papillomavirus vaccination and the role of herd effects in future cancer control planning: a review. Expert Rev Vaccines 2018; 17:395–409. [DOI] [PubMed] [Google Scholar]
- 12. Man I, Vänskä S, Lehtinen M, Bogaards JA. Human papillomavirus genotype replacement: still too early to tell? [manuscript published online ahead of print 27 January 2020]. J Infect Dis 2020; doi:1093/infdis/jiaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vriend HJ, Boot HJ, van der Sande MA; Medical Microbiological Laboratories; Municipal Health Services . Type-specific human papillomavirus infections among young heterosexual male and female STI clinic attendees. Sex Transm Dis 2012; 39:72–8. [DOI] [PubMed] [Google Scholar]
- 14. Visser M, van Aar F, van Oeffelen A, et al. Sexually transmitted infections in the Netherlands in 2016. Bilthoven, the Netherlands: National Institute for Public Health and the Environment, 2017. [Google Scholar]
- 15. Vriend HJ, Bogaards JA, van der Klis FR, et al. ; Medical Microbiological Laboratories, Municipal Health Services . Patterns of human papillomavirus DNA and antibody positivity in young males and females, suggesting a site-specific natural course of infection. PLoS One 2013; 8:e60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woestenberg PJ, van Benthem BH, Bogaards JA, et al. HPV infections among young MSM visiting sexual health centers in the Netherlands: opportunities for targeted HPV vaccination. Vaccine 2020; 38:3321–9. [DOI] [PubMed] [Google Scholar]
- 17. Oliver SE, Unger ER, Lewis R, et al. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J Infect Dis 2017; 216:594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malagón T, Drolet M, Boily MC, et al. Cross-protective efficacy of two human papillomavirus vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:781–9. [DOI] [PubMed] [Google Scholar]
- 19. Lehtinen M, Luostarinen T, Vänskä S, et al. Gender-neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomized trial (III). Int J Cancer 2018; 143:2299–310. [DOI] [PubMed] [Google Scholar]
- 20. Bogaards JA, van der Weele P, Woestenberg PJ, van Benthem BHB, King AJ. Bivalent human papillomavirus (HPV) vaccine effectiveness correlates with phylogenetic distance from HPV vaccine types 16 and 18. J Infect Dis 2019; 220:1141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology 2013; 445:2–10. [DOI] [PubMed] [Google Scholar]
- 22. Tota JE, Ramanakumar AV, Jiang M, et al. Epidemiologic approaches to evaluating the potential for human papillomavirus type replacement postvaccination. Am J Epidemiol 2013; 178:625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesher D, Soldan K, Lehtinen M, et al. Population-level effects of human papillomavirus vaccination programs on infections with nonvaccine genotypes. Emerg Infect Dis 2016; 22:1732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gray P, Palmroth J, Luostarinen T, et al. Evaluation of HPV type-replacement in unvaccinated and vaccinated adolescent females-post-hoc analysis of a community-randomized clinical trial (II). Int J Cancer 2018; 142:2491–500. [DOI] [PubMed] [Google Scholar]
- 25. Mesher D, Panwar K, Thomas SL, et al. The impact of the national HPV vaccination program in England using the bivalent HPV vaccine: surveillance of type-specific HPV in young females, 2010–2016. J Infect Dis 2018; 218:911–21. [DOI] [PubMed] [Google Scholar]
- 26. Feiring B, Laake I, Christiansen IK, et al. Substantial decline in prevalence of vaccine-type and nonvaccine-type human papillomavirus (HPV) in vaccinated and unvaccinated girls 5 years after implementing HPV vaccine in Norway. J Infect Dis 2018; 218:1900–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mollers M, Vriend HJ, van der Sande MA, et al. Population- and type-specific clustering of multiple HPV types across diverse risk populations in the Netherlands. Am J Epidemiol 2014; 179:1236–46. [DOI] [PubMed] [Google Scholar]
- 28. Baussano I, Lazzarato F, Ronco G, Franceschi S. Impacts of human papillomavirus vaccination for different populations: a modeling study. Int J Cancer 2018; 143:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.