Abstract
Background:
Chronic hepatitis B infection is an important contributor to mortality in the United States, yet impact of available and effective oral antivirals on mortality among infected individuals is unknown.
Aim(s):
To compare risks and predictors of mortality in a recent time period between those with chronic, prior and no hepatitis B infection.
Methods:
This is a population-based cohort study of National Health and Nutrition Examination Surveys participants between 1999 and 2014 linked to National Death Index data. Adults aged 20 years or older with hepatitis B serologic testing were included. Outcomes of all-cause and liver-related mortality were evaluated using Cox regression.
Results:
Of 39,206 participants, 192 (0.5%) had chronic and 2694 (6.9%) had prior hepatitis B infection. The all-cause age/sex-standardized mortality rates for chronic, prior and uninfected were 21.4, 15.1 and 11.8 per 1000 person-years, respectively. Liver-related mortality occurred at respective rates of 4.1, 0.3 and 0.1 per 1000 person-years. In multivariable analyses, those with chronic infection had 1.9-fold (95% CI 1.1–3.3) increased hazard of all-cause mortality and 13.3-fold (95% CI 3.9–45.5) increased hazard of liver-related mortality compared to uninfected. Predictors of all-cause mortality among chronic infection included heavy alcohol use (HR 18.3, 95% CI 3.3–100.6) and higher alanine aminotransferase (HR 1.02, 95% CI 1.00–1.03).
Conclusions:
Mortality among adults living with chronic hepatitis B infection still exceeds that of uninfected despite availability of improved therapeutics. Identification of chronic infection, initiation of treatment among eligible, and modulation of co-factors for disease progression are needed to improve survival.
Keywords: chronic hepatitis B infection, mortality, viral hepatitis, hepatitis B virus, epidemiology
Introduction
Globally, viral hepatitis causes 1.3 million deaths per year, on par with other communicable diseases such as human immunodeficiency virus (HIV), malaria and tuberculosis1. Among those with chronic infection, the major driver of mortality is development of cirrhosis and hepatocellular carcinoma (HCC). Chronic hepatitis B virus (HBV) infection accounts for over 30% of cirrhosis and 50% of HCC cases worldwide2. While future estimates of HBV prevalence are expected to decline with broader uptake of vaccination, immigration from endemic regions has kept HBV prevalence in the US relatively constant over the past three decades3. Thus, an estimated 800,000 to 2.2 million individuals living in the US remain at risk for adverse liver-related outcomes and increased mortality3, 4.
Mortality due to chronic HBV infection in the US has not been well characterized, particularly since the introduction in the mid-2000s of oral HBV antiviral therapies with minimal to no resistance (i.e. entecavir and tenofovir) with long-term use 5, 6. Existing studies on HBV mortality relying on death certificate records reported no significant change in deaths attributable to HBV infection between 1999 and 2007, followed by a small temporal decline in deaths between 2007 and 20167, 8. Death certificate studies, however, may be subject to under-reporting of HBV status, as well as ascertainment and misclassification biases9, 10 and accurate capture of HBV-related mortality are crucial to inform policymakers on gaps in HBV care and prioritization of treatment.
The objective of this study was to evaluate mortality among a population-based cohort of adults with chronic HBV infection in the era of effective HBV therapies (1999–2014) by linking serologic evidence of HBV infection to death using the National Health and Nutrition Examination Surveys (NHANES)-Linked Mortality Dataset.
Materials and Methods
National Health and Nutrition Examination Survey
NHANES is a cross-sectional, stratified, multistage probability survey of a representative non-institutionalized civilian US population. Previously completed in 6-year cycles, the continuous NHANES has sampled every 2 years since 1999. NHANES samples in four-stages: 1) selection of primary sampling units (PSUs) typically individual counties; 2) selection of segments within each county; 3) selection of households within segments; and 4) selection of individuals within each household.
Cohort Selection
The study cohort included all NHANES participants for survey years 1999 to 2014 aged 20 years or older with available hepatitis B serologies. Laboratory testing included immunoassays for qualitative detection of hepatitis B core antibody (anti-HBc, total) and hepatitis B surface antigen (HBsAg) (Ortho CD VITROS Anti-HBc and HBsAg test; Ortho Clinical Diagnostics, Raritan, NJ). Only participants with a positive anti-HBc test had HBsAg testing performed11. Participants were classified into 3 groups: (1) no HBV infection if negative anti-HBc test, (2) prior HBV if positive anti-HBc test but negative HBsAg test, and (3) chronic HBV infection if positive tests for both anti-HBc and HBsAg.
Covariate Definitions
The following covariate definitions were used in this study:
> Cohort years: 1999–2006 and 2007–2014.
> Poverty index: calculated by dividing family income by the poverty guidelines. A poverty index below 1 was considered below the poverty line.
> Ever smoking: defined as reporting at least 100 cigarettes used over lifetime.
> Alcohol use: none, low (>0-<4 drinks/day in men and >0-<2 drinks/day in women) and heavy (≥4 drinks/day in men and ≥2 drinks/day in women). Due to 19% missing data, participants who responded “Yes” to “Have you had more than 12 drinks over your lifetime?” but failed to report amount or frequency of use were imputed as low alcohol use.
> Hypertension: average systolic or diastolic blood pressures ≥140 mm Hg or ≥90 mm Hg or self-reported history of oral antihypertensive medication use.
> Diabetes: hemoglobin A1c ≥6.5% or self-reported history of oral hypoglycemics or insulin use.
> Hyperlipidemia: cholesterol >200 mg/dL, low-density lipoprotein ≥130 mg/dL, HDL <40 mg/dL for men and <50 mg/dL for women, or self-reported history of oral cholesterol medication use12.
> Obesity: body mass index (BMI) ≥30 kg/m2. Since we were unable to determine Asian race, the lower recommended cut-off of ≥25 could not be applied.
> HBV treatment: yes, if HBV therapy identified by generic drug name as one of participant’s prescription medications.
Mortality Outcome Assessment
Primary outcome of this study was all-cause mortality and secondary outcome was liver-related mortality. We determined mortality status using the NHANES Linked Mortality File, in which NHANES 1999–2014 participants were matched through a probabilistic matching algorithm with multiple identifiers to the US National Death Index (NDI)13. Participant follow-up continued from enrollment into NHANES until death. Participants not matched with a death record were considered alive and censored at the end of follow-up (December 31, 2015). Participants with incomplete identifiers necessary for the probabilistic matching algorithm (N=48) were excluded from the mortality analysis13. To evaluate liver-related mortality, we obtained restricted access to cause-specific mortality data; data were accessed through the Research Data Center. We utilized the Underlying Causes of Death (113) Recode variable and defined liver-related mortality as death from viral hepatitis (B15-B19), malignant neoplasms of liver and intrahepatic bile ducts (C22), and chronic liver disease and cirrhosis (K70, K73-K74).
Statistical Analysis
All analyses accounted for weighting (due to differential probability of selection through oversampling, nonresponse and adjustment to independent population controls) and complex survey design of NHANES. Weights were given by NHANES for each 2-year cycle and combined to create 4-, 8- and 16-year weights to be applied at appropriate steps in analysis as recommended by NHANES statistical guidelines14. Variance estimation was performed using Taylor Series Linearization.
Population characteristics collected at NHANES enrollment examination were estimated as weighted means and percentages with standard errors (SE) and HBV status (no, prior, or chronic infection). Hypothesis testing was performed using t-test for continuous variables and chi-square test for proportions. All-cause and liver-related mortality rates (per 1000 person-years), standardized to age and sex distribution of US population, were calculated by HBV status and cohort years using the direct method of standardization. Both age- and sex- standardization was performed using the 2000 US census population as the standard. Linear trend over time by 4-year intervals was examined using linear contrasts.
We performed left-truncated Cox proportional hazards regression to estimate the hazard ratios (HRs) and 95% CIs for risk of all-cause and liver-related mortality in chronic and prior HBV infection compared to no infection. Left-truncation better controls for age, the principal confounder of a mortality outcome, than does time-on-study. Characteristics with a univariate pvalue <0.1, known associations with HBV-related mortality (race, foreign-born, and alanine aminotransferase [ALT]), or of interest for the study (HBV status and cohort years) were evaluated in multivariable modeling. Backward elimination (p<0.05) was used to select the adjusted multivariable models, retaining covariates related to HBV mortality or of interest to the study, regardless of statistical significance. Predictors of all-cause mortality among chronic HBV were also similarly examined. We tested for interaction between HBV status and cohort years (1999–2006 and 2007–2014) to examine change in hazard over time. Data were analyzed using SAS-SUDAAN and STATA v14.0. All tests were 2-sided and p<0.05 was considered statistically significant.
Results
Overall Characteristics of US Adults between 1999–2014 by HBV Status
A total of 39,206 NHANES participants met inclusion criteria, of which 2694 (6.9%) had prior HBV infection and 192 (0.5%) had chronic infection (Figure 1). In the prior HBV infection group (positive HBV core antibody test), 77% were also positive for HBV surface antibody. Demographic characteristics stratified by HBV status are reported in Table 1. Within the chronic HBV group, mean age was 47 years, 58% were male, and 44% identified “other” as race/ethnicity which included Asian race; 31% of those with prior infection and 10% with no infection identified “other” as race. The majority with chronic HBV (50%) were foreign-born; foreign-born comprised 41% with prior infection and 15% with no infection. Other than proportion with diabetes (13%), those with chronic infection had slightly lower prevalence of metabolic co-morbidities, such as hypertension (28%), hyperlipidemia (62%), and obesity (18%), compared to the other two groups. Mean ALT was highest among chronically infected (40 U/L) and lowest among uninfected (26 U/L). Eleven (5.7%) of 192 with chronic infection reported current antiviral therapy for HBV, including lamivudine, adefovir, emtricitabine-tenofovir, entecavir and tenofovir alone. Past antiviral therapy was not captured.
Figure 1.
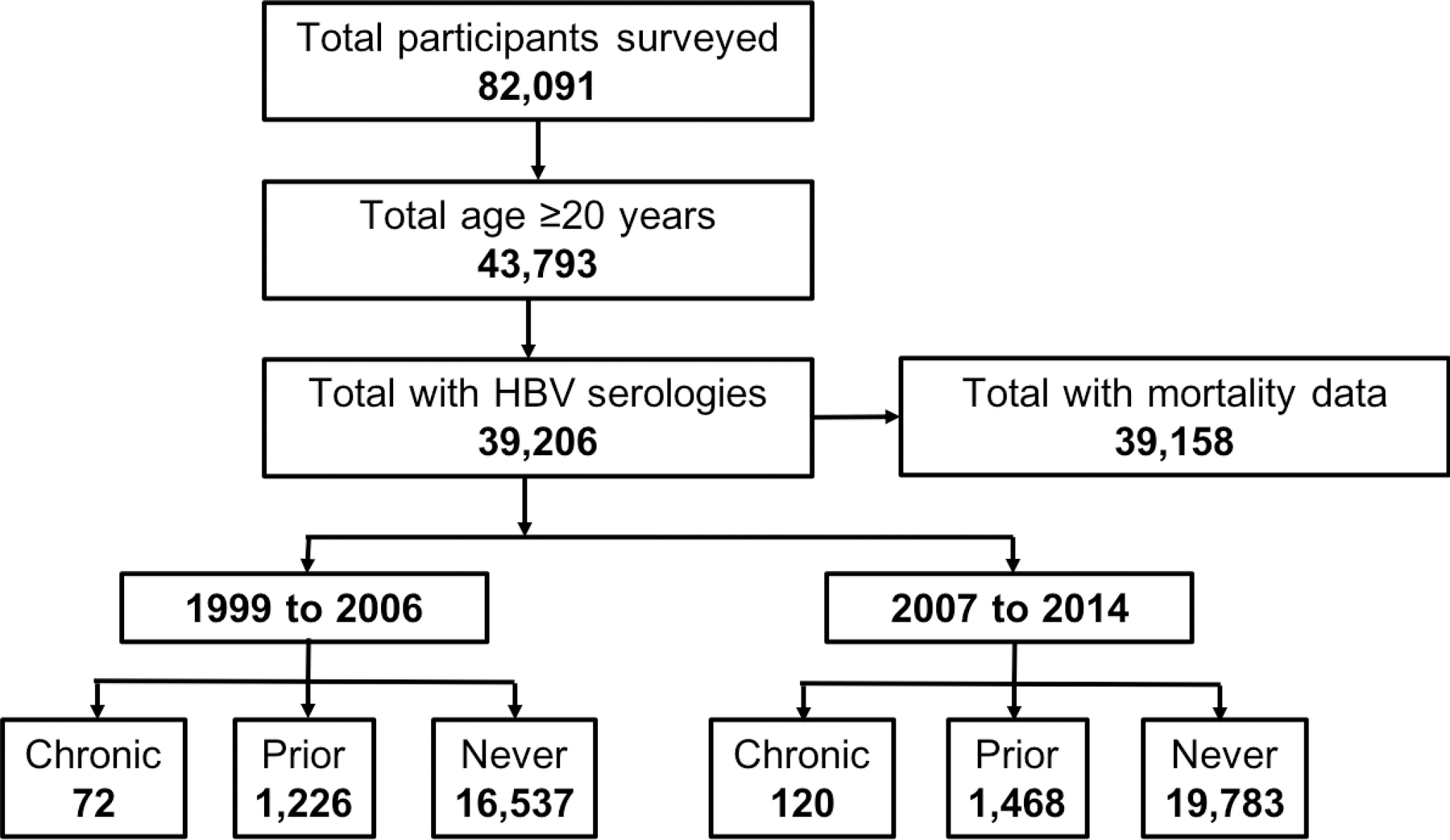
NHANES Cohort Selection
Abbr: HBV=hepatitis B virus; anti-HBc=hepatitis B core antibody; HBsAg=hepatitis B surface antigen
“Chronic” infection defined as anti-HBc+/HBsAg+; “Prior” infection defined as anti-HBc+/HBsAg-; “Never” infection defined as anti-HBc-/HBsAg-
Table 1.
Baseline Characteristics Among Adults Aged 20–85 Years by Hepatitis B Status
| No infection | Prior infection | Chronic infection | |
|---|---|---|---|
| Characteristics | N=36,320 | N=2694 | N=192 |
| Age, years | 46.2 ± 0.1 | 47.4 ± 0.2 | 47.2 ± 0.5 |
| Male, % | 47.9 ± 0.2 | 53.4 ± 1.6 | 58.2 ± 3.9 |
| Race, % | |||
| Mexican | 8.0 ± 0.6 | 4.0 ± 0.6 | 2.5 ± 1.3 |
| White | 72.3 ± 1.1 | 38.5 ± 1.8 | 27.0 ± 4.0 |
| Black | 9.7 ± 0.6 | 26.5 ± 1.5 | 26.7 ± 3.9 |
| Otherb | 10.0 ± 0.6 | 31.0 ± 1.9 | 43.8 ± 4.3 |
| Foreign-born, % | 14.5 ± 0.7 | 40.6 ± 2.0 | 50.2 ± 5.3 |
| Married, % | 64.5 ± 0.5 | 56.8 ± 1.6 | 55.8 ± 5.6 |
| Poverty Index >1, % | 13.9 ± 0.5 | 21.6 ± 1.2 | 19.8 ± 3.9 |
| Education, % | |||
| <HS | 17.5 ± 0.5 | 27.1 ± 1.3 | 24.1 ± 3.7 |
| HS or eq | 23.8 ± 0.5 | 24.0 ± 1.4 | 24.2 ± 3.8 |
| >HS (college) | 58.7 ± 0.8 | 48.9 ± 1.7 | 51.7 ± 4.6 |
| Ever smoker, % | 53.2 ± 0.6 | 48.9 ± 1.6 | 54.4 ± 4.2 |
| Alcohol use, % | |||
| None | 22.6 ± 0.7 | 25.4 ± 1.3 | 26.2 ± 4.1 |
| Low | 75.3 ± 0.7 | 72.0 ± 1.3 | 72.3 ± 4.4 |
| Heavyc | 2.1 ± 0.1 | 2.6 ± 0.4 | 1.5 ± 1.5a |
| BMI, kg/m2 | 28.5 ± 0.1 | 27.3 ± 0.2 | 26.8 ± 0.9 |
| Waist circumference, cm | 97.7 ± 0.2 | 94.8 ± 0.5 | 93.0 ± 1.6 |
| Diabetes, % | 9.0 ± 0.2 | 11.0 ± 0.6 | 13.4 ± 3.2 |
| Hypertension, % | 29.7 ± 0.3 | 29.6 ± 1.0 | 27.6 ± 4.1 |
| Hyperlipidemiad, % | 68.9 ± 0.4 | 68.4 ±1.4 | 61.7 ± 4.0 |
| Obesity, % | 34.0 ± 0.4 | 27.2 ± 1.4 | 17.8 ± 3.9 |
| ALT, U/L | 25.6 ± 0.1 | 28.2 ± 0.5 | 40.2 ± 6.0 |
| AST, U/L | 25.2 ± 0.1 | 28.2 ± 0.6 | 36.1 ± 4.6 |
| ALP, U/L | 68.6 ± 0.3 | 69.9 ± 0.7 | 70.3 ± 2.4 |
| Total bilirubin, mg/dL | 0.7 ± 0.004 | 0.7 ± 0.01 | 0.8 ± 0.03 |
| Platelet count, cells/uL | 257.8 ± 0.7 | 251.0 ± 2.0 | 230.3 ± 5.9 |
| Albumin, g/dL | 4.3 ± 0.004 | 4.3 ± 0.01 | 4.3 ± 0.04 |
| Creatinine, mg/dL | 0.88 ± 0.002 | 0.88 ± 0.01 | 0.89 ± 0.03 |
| HCV-infected, % | 0.7 ± 0.06 | 7.4 ± 0.7 | 2.9 ± 1.4a |
Data are presented as mean or % ± SE
BMI = body mass index; ALT = alanine aminotransferase; AST = aspartate aminotransferase, ALP = alkaline phosphatase; GGT = gamma glutamyl-transferase; HCV = hepatitis C virus
Does not meet the standard of statistical reliability and precision (relative SE≥30%)
Includes non-Mexican Hispanics, Aleut, Eskimo, American Indian, and Asian or Pacific islander.
Defined as >4 drinks/day in men and >2 drinks/day in women.
Based on non-fasting values
Complete mortality follow-up data were available for 39,158 out of 39,206 participants (99.9%) and nearly all participants with chronic HBV (191/192, 99.5%). The mean duration of follow-up for all participants was 7.9 ± 4.5 years. Overall, there were 5042 deaths over 309,348 person-years of follow-up.
Changes in Characteristics of Adults with Chronic HBV Over Time
Of a total 192 chronically infected participants, 72 (38%) entered NHANES between 1999–2006 and 120 (62%) between 2007–2014 (Supplemental Table 1). A greater proportion were “Other race” in the latter time period (40% vs 47%). Proportion foreign-born also increased from 41% to 57%. Reporting of ever smoking (at least 100 cigarettes) increased from 49% to 60% while no alcohol use increased from 22% to 29%. Mean BMI and waist circumference increased slightly, but the proportion categorized as obese remained similar (under 20%). Mean ALT was higher between 1999–2006 compared to 2007–2014 with values of 47 vs 36 U/L, respectively. Only mean platelet count was significantly different between cohort years with decrease from 248 to 215 cells/uL (p=0.004).
Of 29 total deaths among chronic HBV between 1999–2014, the majority of deaths were attributed to other causes (n=16; 57%), followed by heart disease (n=6; 21%) and malignant neoplasms (n=5; 18%) (results for two categories were suppressed due to low number of persons). Among decedents, 55% were men and the median age at death was 66 years (IQR 57–81).
Age- and Sex-Standardized Mortality Rates
Age- and sex-adjusted all-cause mortality rate for the entire cohort over the 16-year period was 12.1 per 1000 person-years (Supplemental Table 2). The overall mortality rate was 11.8 per 1000 person-years among uninfected, 15.1 per 1000 person-years among prior infected, and 21.4 per 1000 person-years among chronically infected. There was a significant trend towards decreasing all-cause mortality rates among all groups when mortality rates were examined in 4-year intervals (p-value for linear trend=0.01 for chronic infection, p=0.02 for prior infection and p<0.001 for no infection) (Figure 2). Overall age- and sex-adjusted liver-related mortality rates were highest among those with chronic infection, estimated at 4.1 per 1000 person-years (95% CI 1.9–10.0). Estimated liver-related mortality in those with prior infection was lower at 0.3 per 1000 person-years (95% CI 0.1–0.6) and lowest in those without infection at 0.1 per 1000 person-years (95% CI 0.1–0.2). Notably no liver-related deaths occurred in participants with chronic infection recruited between 2007–2014.
Figure 2.
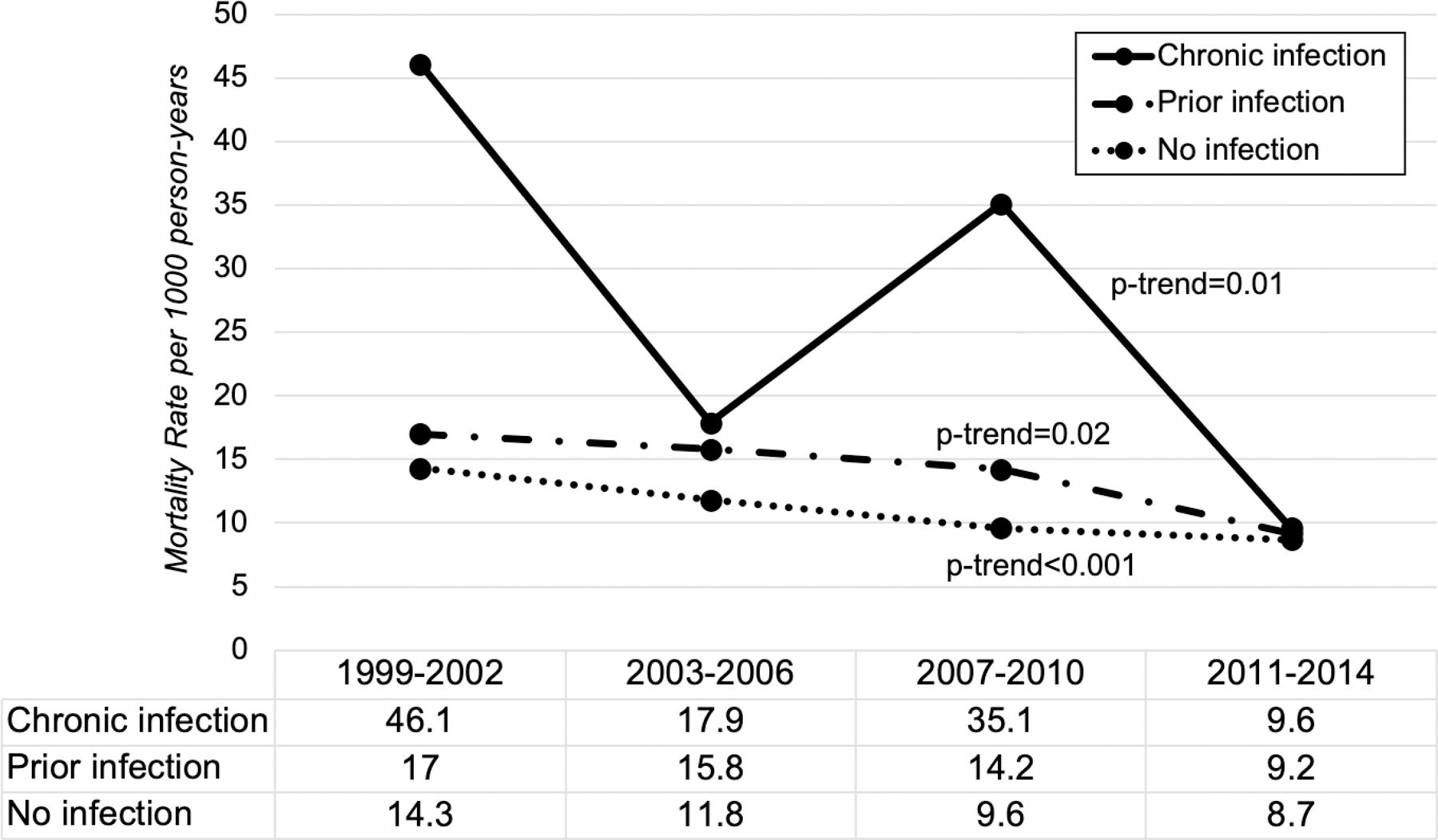
Trend in All-Cause Mortality Rates by Chronic Hepatitis B Status in 4-year Intervals (1999–2014)
All-cause age- and sex-standardized mortality rates for chronic infection (solid), prior infection (solid/dot) and no infection (dotted) presented per 1000 person-years by 4-year intervals between 1999 and 2014. P-value given for linear trend.
Risk of All-Cause and Liver-Related Mortality by HBV Status
In Cox regression analysis using no infection as the comparator group, the crude HR for all-cause mortality was 1.3 (95% CI 1.1–1.4; p=0.001) in the prior infection group and 1.9 (95% CI 1.1–3.3; p=0.02) in the chronic infection group (Table 2). In multivariate analysis, the HR was 1.1 (95% CI 1.0–1.3; p=0.2) in the prior infection group and 1.9 (95% CI 1.1–3.3; p=0.03) in the chronic infection group. There was no interaction between chronic infection and cohort years (p=0.62) suggesting the association between mortality and chronic infection did not differ statistically over the two time periods (1999–2006 vs 2007–2014).
Table 2.
Crude and Adjusted Risk of All-Cause and Liver-Related Mortality by Hepatitis B Status
| Hazard Ratio for All-Cause Mortality |
Hazard Ratio for Liver-Related Mortality |
|||||||
|---|---|---|---|---|---|---|---|---|
| HBV status | Crude | 95% CI | Adjusted† | 95% CI | Crude | 95% CI | Adjusted‡ | 95% CI |
| No infection | Ref | Ref | Ref | Ref | ||||
| Prior infection | 1.3 | 1.1–1.4 | 1.1 | 0.95–1.3 | 2.5 | 1.4–4.5 | 1.7 | 0.9–3.3 |
| Chronic infection | 1.9 | 1.1–3.3 | 1.9 | 1.1–3.3 | 17.0 | 6.1–47.6 | 13.3 | 3.9–45.5 |
Adjusted for age, sex, race/ethnicity, cohort years, foreign-born, marital status, poverty, education, smoking, alcohol use, diabetes, hypertension, hyperlipidemia, waist circumference, and alanine aminotransferase
Adjusted for age, race/ethnicity, cohort years, foreign-born, smoking, alcohol use, hyperlipidemia, waist circumference, and alanine aminotransferase
While liver-related mortality was 2.5-fold higher in prior infection compared to no infection in crude analysis (95% CI 1.4–4.5), the adjusted HR of 1.7 was not statistically significant in multivariable analysis (95% CI 0.9–3.3). Among participants with chronic infection, the adjusted analysis showed a 13.3-fold higher likelihood of dying from a liver-related cause than those with no infection (95% CI 3.9–45.5).
Predictors of Mortality among Adults with Chronic HBV
Factors associated with all-cause mortality among chronically infected adults in univariate analysis included foreign-born, smoking, alcohol use, hypertension, and ALT level (p<0.1). HBV treatment was not associated with mortality (p=0.36). The only independent predictors of all-cause mortality among chronically infected adults were heavy alcohol use (HR 18.3, 95% CI 3.3–100.6) and elevated ALT (HR 1.02 per 10-unit increase, 95% CI 1.00–1.03) (see Table 3).
Table 3.
Multivariable Predictors of All-Cause Mortality among Adults with Chronic Hepatitis B (n=191)
| Variables | Hazard Ratio | 95% CI | p-value |
|---|---|---|---|
| Sex | |||
| Male | 1.0 | ||
| Female | 0.8 | 0.2–2.9 | 0.75 |
| Cohort 8-yr | |||
| 1999–2006 | 1.0 | ||
| 2007–2014 | 1.4 | 0.5–3.9 | 0.56 |
| Race / Ethnicity | |||
| NH White | 1.0 | ||
| NH Black | 1.8 | 0.6–5.8 | 0.33 |
| Mexican | 2.8 | 0.6–14.2 | 0.21 |
| Other | 0.9 | 0.2–5.6 | 0.95 |
| Foreign Born | |||
| US | 1.0 | ||
| Foreign | 0.6 | 0.3–1.4 | 0.22 |
| Alcohol use | |||
| No | 1.0 | ||
| Low† | 1.1 | 0.4–3.3 | 0.86 |
| Heavy | 18.3 | 3.3–100.6 | <0.001 |
| ALT (U/L)‡ | |||
| Per 10 unit increase | 1.02 | 1.00–1.03 | 0.01 |
Missing alcohol use data imputed as low (n=24)
Missing for ALT value (n=2)
Discussion
Using population-based survey data, we demonstrate that mortality in the United States among chronically infected adults with HBV exceeded that of uninfected by two-fold over the past two decades. Deaths related to cirrhosis and HCC are postulated drivers of this difference, with chronically infected persons incurring a 13-fold increased risk of liver-related mortality over the same time period. While there was a numeric trend towards decreased overall mortality among chronic HBV-infected for the 1999–2014 time period, we did not detect a statistical difference, which may be attributed to lack of power from low HBV prevalence in the NHANES cohort or reflect insufficient penetrance of antiviral therapy among those with chronic HBV infection.
To date, population-based studies using national death certificate data have provided estimates of US death rates attributable to HBV, reported at 0.85 per 100,000 persons in 2007 and 0.67 per 100,000 in 2016 (annual percent change −2.1%)8, 15. However, population-based data on mortality risk among individuals with chronic HBV are lacking. The Chronic Hepatitis Cohort Study (CHeCS), recruited at four healthcare centers between 2006–2013, corroborates our study findings as they observed a similar 1.9-fold increased risk of all-cause mortality and 16-fold increased risk of liver-related mortality compared to the general US population10. A striking 81% did not have hepatitis B infection reported on their death certificates, emphasizing the limitations of death certificate analyses10.
There are many potential explanations for the persistent excess mortality over a time period in which improved HBV therapeutics have become increasingly available, with lamuvidine approved in 1998, entecavir in 2005 and tenofovir in 2008. Barriers to effective delivery of care along each step of the HBV care cascade, from insufficient screening and awareness to low provision or uptake of treatment in eligible patients, may be contributing. Across various clinical settings in the US, up to 40–60% of treatment-eligible patients are not on treatment16, 17. The low percentage currently on therapy in our cohort (6%) suggests uptake as an issue, although based on ALT alone only an additional 8% would be definitively eligible without additional clinical information. Diminished access to care, including drug availability and cost concerns, among at-risk populations such as immigrants, incarcerated and injection drug users is one possible obstacle18. The complexity of confirming treatment eligibility for chronic HBV and need for lifelong monitoring and therapy, in comparison to the more straightforward regimens for hepatitis C virus, may also contribute to inadequate uptake19. Furthermore, while HBV treatment improves outcomes, it does not completely ameliorate risk of HCC, particularly among those with a long duration of infection or pre-existing cirrhosis20. In one US study of which liver disease etiology was not considered, a decline in HCC (2.7% decrease per year) as cause of death among Asians in the same time period of our study was observed21, potentially reflecting improved screening practices or HBV treatment uptake/response. Thus, the impact of antiviral therapy on all-cause mortality among HBV-infected may be anticipated in future NHANES cohorts.
We found heavy alcohol use and higher ALT to be independent predictors of all-cause mortality among those with chronic HBV infection. While we acknowledge that use of simple imputation for missing alcohol use responses in our study may have biased alcohol risk estimates toward the null hypothesis, the mortality HR for the high alcohol use group remained statistically significant despite this. Alcohol use as an independent predictor of death among chronic HBV patients has been demonstrated in studies from Europe and Asia22–24. The combination of HBV and alcohol not unexpectedly leads to more rapid progression to cirrhosis than HBV alone and increased carcinogenesis, manifested by an up to 8-fold higher risk of HCC24–26. Furthermore, the overall rising rates of and mortality from alcohol-related liver disease and non-alcoholic fatty liver disease (NAFLD) in US, including among HBV-infected, may negate some of the mortality benefits from HBV therapy21, 27. Screening and counseling on the dangers of concurrent alcohol use need to be prioritized among those with existing liver injury due to HBV, including those without active disease or on treatment.
Serum ALT is an important marker of hepatic inflammation and a key parameter in clinical decision making on HBV treatment initiation28. Encouragingly, the mean ALT among HBV-infected was lower in the later time period (36 U/L in 2007–2014) compared to the earlier time period (47 U/L in 1999–2006) and may indirectly suggest increased uptake of treatment. However, the ALT level may also reflect a secondary cause of liver disease such as NAFLD. Assessment and counseling for NAFLD risk factors should be incorporated into routine management of chronic HBV patients. Furthermore, the positive association between ALT level and mortality in this study is consistent with other observational studies, the largest of which (REVEAL-B study) demonstrated that ALT independently predicted development of cirrhosis and HCC29. We described in a previous study that less than half of NHANES participants with an elevated ALT and chronic HBV infection were aware of their disease30. For improvement in HBV-related mortality to be realized, evidence-based strategies to promote screening for HBV among individuals with elevated liver tests in the primary care setting should be implemented, followed by linkage to specialty care for treatment initiation31.
While the major strengths of this study are decreased selection bias from a population-based sample and robust linkage between serologic data and vital status, several limitations are present. First, mortality among chronic HBV and prior HBV-infected are likely underestimated by the exclusion of incarcerated and homeless populations in NHANES sampling. The low prevalence of chronic infection in the US resulted in a small number of infected patients despite the large number of total patients sampled, thus limiting the precision of our results. Absence of available viral and host characteristics, including HBV DNA, hepatitis B e-antigen status and cirrhosis, did not allow further risk stratification among those with chronic infection. While we had limited data on receipt of HBV therapy, we were unable to demonstrate or make direct conclusions on the impact of these therapies given small percentage (6%) on therapy. The addition of an HBV-specific questionnaire to NHANES (including self-reported treatment) starting in the 2013–2014 cycle will facilitate assessment of treatment influence in future studies. Due to long latency between disease onset and adverse outcomes, an even longer period of follow-up may be required to appreciate the full impact of current HBV treatments on a population level32. Notably these results may not be generalizable to other countries or regions outside of the US, however, despite small sample size with chronic infection, we do believe our results to be nationally representative of our racially diverse population with HBV.
In summary, the presence of HBV infection in chronically infected adults living in the US continues to contribute to excess mortality, both all-cause and liver-related, despite availability of highly effective antiviral therapy that can reduce these risks. With elimination of viral hepatitis a growing global reality, concurrent with developing a HBV functional cure, greater efforts are needed by policy makers and key stakeholders to expand treatment access and delivery across the broader HBV-infected US population.
Supplementary Material
Acknowledgements
We would like to thank Peter Bacchetti, PhD, Department of Epidemiology and Biostatistics, University of California, San Francisco and Meghan Morris, PhD, MPH, Department of Epidemiology and Biostatistics, University of California, San Francisco for their support and mentorship on this manuscript.
Declaration of funding interests:
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [T32 5T32DK060414-14] to KZ and the UCSF Liver Center (P30 DK026743). The study sponsor had no role in the study design in the collection, analysis, and interpretation of data.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the views of the Research Data Center, the National Center for Health Statistics, or the Centers for Disease Control and Prevention.
Statement of Interests
Authors’ declaration of personal interests
Dr. Terrault reports institutional grant support from Gilead Sciences. The other authors disclose no conflicts.
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, Abu-Raddad LJ, Assadi R, Bhala N, Cowie B, Forouzanfour MH, Groeger J, Hanafiah KM, Jacobsen KH, James SL, MacLachlan J, Malekzadeh R, Martin NK, Mokdad AA, Mokdad AH, Murray CJL, Plass D, Rana S, Rein DB, Richardus JH, Sanabria J, Saylan M, Shahraz S, So S, Vlassov VV, Weiderpass E, Wiersma ST, Younis M, Yu C, El Sayed Zaki M and Cooke GS. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016;388:1081–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perz JF, Armstrong GL, Farrington LA, Hutin YJ and Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529–38. [DOI] [PubMed] [Google Scholar]
- 3.Roberts H, Kruszon-Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB and Holmberg SD. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63:388–97. [DOI] [PubMed] [Google Scholar]
- 4.Kowdley KV, Wang CC, Welch S, Roberts H and Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–33. [DOI] [PubMed] [Google Scholar]
- 5.Wong GL, Tse YK, Chan HL, Yip TC, Tsoi KK and Wong VW. Oral nucleos(t)ide analogues reduce recurrence and death in chronic hepatitis B-related hepatocellular carcinoma. Aliment Pharmacol Ther 2016;43:802–13. [DOI] [PubMed] [Google Scholar]
- 6.Lim YS, Han S, Heo NY, Shim JH, Lee HC and Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014;147:152–61. [DOI] [PubMed] [Google Scholar]
- 7.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW and Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156:271–8. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Li AA, Perumpail BJ, Gadiparthi C, Kim W, Cholankeril G, Glenn JS, Harrison SA, Younossi ZM and Ahmed A. Changing Trends in Etiology-Based and Ethnicity-Based Annual Mortality Rates of Cirrhosis and Hepatocellular Carcinoma in the United States. Hepatology. 2019;69:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manos MM, Leyden WA, Murphy RC, Terrault NA and Bell BP. Limitations of conventionally derived chronic liver disease mortality rates: Results of a comprehensive assessment. Hepatology. 2008;47:1150–7. [DOI] [PubMed] [Google Scholar]
- 10.Bixler D, Zhong Y, Ly KN, Moorman AC, Spradling PR, Teshale EH, Rupp LB, Gordon SC, Boscarino JA, Schmidt MA, Daida YG, Holmberg SD and Investigators CH. Mortality Among Patients With Chronic Hepatitis B Infection: The Chronic Hepatitis Cohort Study (CHeCS). Clin Infect Dis 2019;68:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Health and Nutrition Examination Survey. 2013–2014 Data Documentation, Codebook, and Frequencies “Hepatitis B: core antibody, surface antigen, and Hepatitis D antibody (HEPBD_H)”. 2015;2020. [Google Scholar]
- 12.National Cholesterol Education Program Expert Panel on Detection E and Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Office of Analysis and Epidemiology. The Linkage of National Center for Health Statistics Survey Data to the National Death Index – 2015 Linked Mortality File (LMF): Methodology Overview and Analytic Considerations. March 2019.
- 14.Division of the National Health and Nutrition Examination Surveys. The National Health and Nutrition Examination Survey (NHANES) Analytic and Reporting Guidelines. 2018.
- 15.Kim D, Li AA, Gadiparthi C, Khan MA, Cholankeril G, Glenn JS and Ahmed A. Changing Trends in Etiology-Based Annual Mortality From Chronic Liver Disease, From 2007 Through 2016. Gastroenterology. 2018;155:1154–1163 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu VD, Do A, Nguyen NH, Kim LH, Trinh HN, Nguyen HA, Nguyen KK, Nguyen M, Huynh A and Nguyen MH. Long-term follow-up and suboptimal treatment rates of treatment-eligible chronic hepatitis B patients in diverse practice settings: a gap in linkage to care. BMJ Open Gastroenterol 2015;2:e000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen VH, Le AK, Trinh HN, Chung M, Johnson T, Wong C, Wong C, Zhang J, Li J, Levitt BS, Nguyen HA, Nguyen KK, Henry L, Cheung R and Nguyen MH. Poor Adherence to Guidelines for Treatment of Chronic Hepatitis B Virus Infection at Primary Care and Referral Practices. Clin Gastroenterol Hepatol 2019;17:957–967 e7. [DOI] [PubMed] [Google Scholar]
- 18.Subic M and Zoulim F. How to improve access to therapy in hepatitis B patients. Liver Int 2018;38 Suppl 1:115–121. [DOI] [PubMed] [Google Scholar]
- 19.Mukhtar NA, Kathpalia P, Hilton JF, Lau G, Yu A, Grumbach K, Nguyen TT, Chan D and Khalili M. Provider, Patient, and Practice Factors Shape Hepatitis B Prevention and Management by Primary Care Providers. J Clin Gastroenterol 2017;51:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Han S, Kim N and Lim YS. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis B virus-endemic population. Hepatology. 2017;66:1454–1463. [DOI] [PubMed] [Google Scholar]
- 21.Tapper EB and Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montuclard C, Hamza S, Rollot F, Evrard P, Faivre J, Hillon P, Di Martino V and Minello A. Causes of death in people with chronic HBV infection: A population-based cohort study. J Hepatol 2015;62:1265–71. [DOI] [PubMed] [Google Scholar]
- 23.Ribes J, Cleries R, Rubio A, Hernandez JM, Mazzara R, Madoz P, Casanovas T, Casanova A, Gallen M, Rodriguez C, Moreno V and Bosch FX. Cofactors associated with liver disease mortality in an HBsAg-positive Mediterranean cohort: 20 years of follow-up. Int J Cancer. 2006;119:687–94. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N and Kumada H. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol 1998;28:930–8. [DOI] [PubMed] [Google Scholar]
- 25.Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH and Group R-HS. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 26.Lin CW, Lin CC, Mo LR, Chang CY, Perng DS, Hsu CC, Lo GH, Chen YS, Yen YC, Hu JT, Yu ML, Lee PH, Lin JT and Yang SS. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J Hepatol 2013;58:730–5. [DOI] [PubMed] [Google Scholar]
- 27.Liu A, Le A, Zhang J, Wong C, Wong C, Henry L and Nguyen MH. Increasing co-morbidities in chronic hepatitis B patients: experience in primary care and referral practices during 2000–2015. Clin Transl Gastroenterol 2018;9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr., Bzowej NH and Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iloeje UH, Yang HI, Jen CL, Su J, Wang LY, You SL, Chen CJ, Risk Evaluation of Viral Load E and Associated Liver Disease/Cancer-Hepatitis BVSG. Risk and predictors of mortality associated with chronic hepatitis B infection. Clin Gastroenterol Hepatol 2007;5:921–31. [DOI] [PubMed] [Google Scholar]
- 30.Zhou KT N Lack of Awareness of Hepatitis B and C Diagnoses in a Population-Based Survey of Adults Living in the United States. Paper presented at: Global Hepatitis Summit; 2018; Toronto, Canada. [Google Scholar]
- 31.Zhou K, Fitzpatrick T, Walsh N, Kim JY, Chou R, Lackey M, Scott J, Lo YR and Tucker JD. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect Dis 2016;16:1409–1422. [DOI] [PubMed] [Google Scholar]
- 32.Ganem D and Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med 2004;350:1118–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


