Abstract
Summary
Background
Satralizumab, a humanised monoclonal antibody targeting the interleukin-6 receptor, reduced the risk of relapse in patients with neuromyelitis optica spectrum disorder (NMOSD) when added to immunosuppressant therapy. This study assessed the safety and efficacy of satralizumab monotherapy in patients with the disorder.
Methods
In this phase 3, double-blind, placebo-controlled, parallel-group trial, we enrolled adults aged 18–74 years with aquaporin-4 antibody seropositive or seronegative NMOSD at 44 investigational sites in 13 countries. Eligible participants had experienced at least one documented NMOSD attack or relapse in the past 12 months and had a score of 6·5 or less on the Expanded Disability Status Scale. Exclusion criteria included clinical relapse 30 days or fewer before baseline. Participants were randomly assigned (2:1) to receive satralizumab 120 mg or visually matched placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Taking immunosuppressants concomitantly was prohibited. The primary endpoint was time to the first protocol-defined relapse, based on the intention-to-treat population and analysed with stratification for two randomisation factors (previous therapy for prevention of attacks and nature of the most recent attack). Safety was assessed in all participants who received at least one dose of satralizumab or placebo. The double-blind phase was due to last until 44 protocol-defined relapses occurred or 1 · 5 years after random assignment of the last patient enrolled, whichever occurred first; participants could enter an open-label phase after the occurrence of a protocol-defined relapse or at the end of the double-blind phase. The study is registered with ClinicalTrials.gov, NCT02073279.
Findings
95 (57%) of 168 screened participants were randomly assigned to treatment (63 to satralizumab; 32 to placebo) between Aug 5, 2014, and April 2, 2017. Protocol-defined relapses occurred in 19 (30%) patients receiving satralizumab and 16 (50%) receiving placebo (hazard ratio 0 ·45, 95% Cl 0·23–0 · 89; p=0–018). 473·9 adverse events per 100 patient-years occurred in the satralizumab group, as did 495·2 per 100 patient-years in the placebo group; the incidence of serious adverse events and adverse events leading to withdrawal was similar between groups.
Interpretation
Satralizumab monotherapy reduced the rate of NMOSD relapse compared with placebo in the overall trial population, with a favourable safety profile. The patient population included a ratio of aquaporin-4 antibody seropositive and seronegative patients that was reflective of clinical practice. Satralizumab has the potential to become a valuable treatment option for patients with NMOSD.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is an autoimmune neurological disease,1–3 with an estimated global pooled prevalence of 1·82 per 100000 people.4 The disorder is characterised by inflammatory lesions in the optic nerve, spinal cord, brainstem, and cerebrum,5 causing potentially severe motor and sensory impairment, bladder dysfunction, vision loss, pain, and other debilitating symptoms.3–5 Recovery is variable, and inflammatory attacks often result in permanent disability.1–3 Untreated, the risks of severe disability or death are substantial.1 Prevention of relapses and reduction of the impact of symptoms associated with the disorder are the foremost priorities for disease management.6
NMOSD is distinct from multiple sclerosis,3,7 the treatments for which are generally ineffective for NMOSD and, in some cases, might be harmful.6 However, patients with NMOSD can often be initially diagnosed with multiple sclerosis.8 Such misdiagnoses not only expose patients to inappropriate treatment, but also increase the risk of disability from untreated attacks,6 showing the importance of early and accurate diagnosis. Therapeutic strategies for the prevention of relapse have predominantly relied on off-label use of various immunosuppressants; however, the efficacy of these drugs in NMOSD has not been established in phase 3 trials.2,6,9
Autoantibodies against the water channel protein aquaporin-4 (AQP4) are implicated in the pathophysiology of NMOSD.10 AQP4 autoantibodies (AQP4-IgG) are a key diagnostic marker in the disorder and are not found in other demyelinating disorders.7 More than two-thirds of patients meeting dinical criteria for NMOSD are AQP4-IgG seropositive,7,11 but some are AQP4-IgG-seronegative.5,7,11 The seronegative population is heterogeneous, with several potential mechanisms involved in disease pathophysiology.5
Interleukin-6 (IL-6) is thought to have a key role in the disease activity of NMOSD.12 IL-6 promotes the differentiation of naive T cells into inflammatory T-helper-17 cells,13 which in the presence of IL-6 further stimulate the differentiation of B cells into plasmablasts that produce AQP4-IgG.14 IL-6 also increases the permeability of the blood-brain barrier, allowing penetration of AQP4-IgG and proinflammatory cells into the CNS.15 Within the CNS, IL-6 secreted by activated astrocytes16 might directly damage oligodendrocytes and axons, promoting demyelination and neurological deficits.17 IL-6 also regulates the expression of complement C3, a component required for complement pathway activation.16 Serum and CSF IL-6 levels are elevated during and after relapse, are associated with increased disability following relapse,12,18 and correlate with CSF AQP4-IgG titres.19
Satralizumab is a subcutaneously administered, humanised monoclonal antibody that binds to membrane-bound and soluble IL-6 receptors, preventing IL-6 from binding and inhibiting the IL-6 signalling pathways involved in inflammation.20–21 Satralizumab dissociates from the receptors at an acidic pH within endosomes and is returned to circulation,22 prolonging the plasma half-life of the drug.22
Satralizumab reduced the risk of relapse in NMOSD when added to baseline immunosuppressants in the SAkuraSky study.20 We did a phase 3 randomised trial (SAkuraStar) to assess the safety and efficacy of satralizumab monotherapy versus placebo in adult patients with NMOSD.
Methods
Study design
SAkuraStar was a phase 3, multicentre, randomised, double-blind, placebo-controlled parallel-group study of satralizumab monotherapy, followed by an open-label extension period. The study took place at 44 investigational sites, induding university hospitals and clinics, in 13 countries: Bulgaria, Canada, Croatia, Georgia, Italy, Malaysia, Poland, Romania, South Korea, Taiwan, Turkey, the USA, and Ukraine. Approval was obtained from the local ethics committee or institutional review board at each study centre. The trial was conducted in accordance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the Declaration of Helsinki.
Participants
Eligible participants were adults (aged 18–74 years) who had either AQP4-IgG seropositive or seronegative neuromyelitis optica using the 2006 Wingerchuk criteria,23 or AQP4-IgG seropositive NMOSD with either single or recurrent events of longitudinally extensive myelitis (≥3 vertebral segment spinal cord MRI lesions) or optic neuritis.7 The term NMOSD is used throughout this paper to refer to both groups of patients, in accordance with 2015 guidelines.5 The number of AQP4-IgG seronegative patients was limited to about 30% of the total population of the study (additional enrolment for AQP4-IgG seronegative patients was stopped after Dec 2,2016, except for patients under screening at that time, when 64 patients had already been randomly assigned). Patients were required to have clinical evidence of at least one documented attack in the 12 months before screening and a score of 6 · 5 or less on the Expanded Disability Status Scale (EDSS).
Exclusion criteria included clinical relapse 30 days or fewer before baseline; any prior treatment with any agent targeting the IL-6 inhibition pathway, alemtuzumab treatment, total body irradiation, or bone marrow transplantation; treatment in the past 6 months with an anti-B-lymphocyte antigen CD20, eculizumab, anti-B lymphocyte stimulator, or any other multiple sclerosis disease-modifying treatment; treatment in the past 2 years with an anti-T-cell surface glycoprotein CD4, cladribine, cyclophosphamide, or mitoxantrone; or treatment with any other investigational drug within 3 months prior to baseline. Patients who were having a protocol-defined relapse, as adjudicated by the Clinical Endpoint Committee (CEC), or those who remained in the study when the double-blind period ended without having protocol-defined relapse, were eligible to enter the open-label extension period (appendix p 5). Patients who had a relapse that was adjudicated by the CEC to be not defined by the protocol remained in the double-blind period until a protocol-defined relapse or the end of the period. All patients provided written informed consent before randomisation.
Randomisation and masking
Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously during the double-blind period using Interactive Web Response and Voice Response Systems, which assigned patients to a treatment group using the pre-defined randomisation list. Study treatments (satralizumab and placebo) were supplied by Chugai Pharmaceutical (Tokyo, Japan) in identical vials and were identical in packaging, labelling, schedule of administration, and appearance. The 2:1 ratio was selected to decrease the probability of a patient being assigned placebo. Randomisation was stratified by previous therapy for relapse prevention (B-cell depleting therapy vs immunosuppressants or other) and the nature of the most recent attack in the year before screening (patient’s first clinical attack vs relapse). Administration of study treatment occurred on the same day as randomisation.
Patients and all study-site personnel were masked to treatment assignment until all patients completed the double-blind period or discontinued early from the study. Unmasking was permitted for treatment of a medical emergency in which the knowledge of the study treatment received would affect the treatment of the emergency, decided on a case-by-case basis by the investigator. Relapse was assessed separately from the treating investigator, who was responsible for patient care.
Laboratory data were also blinded before the primary analysis, including serum satralizumab concentration, high sensitivity C-reactive protein, IL-6, soluble IL-6 receptor, anti-satralizumab antibody, AQP4-IgG status (except screening), plasmablasts, and complement (C3, C4, and CH50).
Procedures
Once randomly assigned, patients received subcutaneous satralizumab or placebo at weeks 0, 2, and 4, and every 4 weeks thereafter in the double-blind period. Satralizumab was administered on the same dosing schedule during the open-label extension period. Based on sample size and power calculations, the double-blind period was initially planned to end after 44 protocol-defined relapses. However, to prevent prolonged exposure to a drug with an unknown risk–benefit balance, the end of the double-blind period was later modified to include a maximal duration of 1·5 years after random assignment of the last patient enrolled.
Immunosuppressants (eg, azathioprine, mycophenolate mofetil) were prohibited during the study. Corticosteroids and intravenous immunoglobulin were also prohibited except as rescue therapy; rescue therapy (eg, pulse intravenous corticosteroids) was permitted for treatment of relapse (protocol-defined or non-protocol defined). Analgesics were permitted for pain management during the study.
Outcomes
The primary outcome was the time to the first protocol-defined relapse in the double-blind period. Protocol-defined relapses were new or worsening objective neurological symptoms with at least one of the following: increase of 1·0 or more EDSS points from a baseline EDSS score of more than 0 (or increase of ≥2·0 EDSS points from a baseline EDSS score of 0); increase of 2 · 0 or more points on at least one appropriate symptom-specific functional system score for either pyramidal, cerebellar, brainstem, sensory, bowel or bladder, or a single eye; increase of 1·0 or more points on more than one symptom-specific functional system score with a baseline of at least 1·0; or increase of 1·0 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1 · 0. Symptoms were required to be attributable to NMOSD, persisting for more than 24 h, and not attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. EDSS and functional system scores were assessed within 7 days of a patient reporting their symptoms to the clinical trial coordinating centre. Relapses were adjudicated by a CEC masked to treatment assignment. The CEC adjudicated relapses as they occurred, following investigator assessment of relapse.
The following sensitivity analyses were performed to evaluate the robustness of the primary endpoint: time to first clinical relapse; time to first treated clinical relapse; time to first treated clinical relapse judged by the sponsor to be an optic neuritic event; and time to first protocol-defined relapse adjudicated by the CEC (regardless of 7-day EDSS assessment limit). Clinical relapses were defined as both protocol-defined relapses and investigator-assessed relapses that did not meet the criteria for a protocol-defined relapse.
Predefined key secondary outcomes were change in the Visual Analogue Scale (VAS) pain score and the Functional Assessment of Chronic Illness Therapy (FACIT) fatigue score24 from baseline to week 24. The VAS is a subjective measure of pain consisting of a 100 mm line with two endpoints representing “no pain” (left, 0) and “pain as bad as it could be” (right, 100). Patients were asked to rate their pain by placing a mark on the line corresponding to their current level of pain. The distance along the line from the “no pain” marker was then measured with a ruler. Scores were given out of 100. The FACIT fatigue scale includes 13 questions that measure fatigue and asthenia for patients with chronic or life-threatening illnesses. For each question, a patient rates his or her condition for the past week on a 5-point Likert scale ranging from 0 (“not at all”) to 4 (“very much”). The score was converted into a figure ranging from 0 to 52, with higher scores indicating less fatigue and better functioning.24
Additional predefined secondary outcomes were the proportion of relapse-free patients, annualised relapse rate, and the change in scores from baseline at 24-week intervals in the following measurements: 36-item Short Form Health Survey (consisting of eight sections, with each scale transformed into a scale of 0 to 100 and lower scores indicating increased disability); EuroQol-5 dimensions (scored on a scale of −0 · 109 to 1, with higher scores reflecting better health); timed 25-foot walk speed; modified Rankin Scale (scored from 0 to 6, with 0 reflecting no symptoms at all and 6 meaning the patient is dead); Zarit Burden Interview (scored from 0 to 88, with 0 indicating no burden and higher scores more burden on caregivers); EDSS (scored from 0 to 10, with 0 reflecting a normal neurological exam and 10 meaning death due to NMOSD); visual acuity (Snellen chart); and low-contrast visual acuity (Sloan chart).
Safety assessments consisted of monitoring and recording adverse events, including the severity of adverse events, serious adverse events, infections (including serious and opportunistic), injection-related reactions, and anaphylactic reactions. Relapses were not categorised as adverse events. Adverse events were coded using MedDRA version 16.1. Adverse events were analysed in the safety analysis population (all patients who received at least one dose of study treatment) in terms of percentage incidence, and as rates by exposure time (number of events per 100 patient years of exposure and the associated 95% Cl) to adjust for any differences in duration of exposure.
Statistical analysis
Efficacy analyses were based on the intention-to-treat population. Primary analysis was planned for the earlier of either 44 protocol-defined relapses, or 1·5 years after the random assignment of the last patient. Sample size considerations were based on the following seven assumptions: (1) a two-sided log-rank test; (2) at least 80% power at the 5% significance level; (3) the hazard ratio (HR) of satralizumab versus placebo for an initial 2 months from randomisation is 1·0; (4) the HR after an initial 2 months from randomisation is 0 · 25; (5) the time to the first protocol-defined relapse in the placebo arm following an exponential distribution, with hazard rate for 1 year h(t)=l·1295; (6) a 2-year dropout rate of 10%; and (7) 2:1 allocation with satralizumab and placebo. Based on these assumptions, 44 protocol-defined relapse events that were confirmed by the CEC were needed for the primary analysis. Additionally, based on the Monte Carlo simulation under the assumed setting, the number of required patients was 90. The target effect of 0·25 HR was estimated using case reports in patients with NMOSD, in which anti-IL-6 therapy reduced relapse counts from a total of four to one event per 6 months.25
The Kaplan-Meier method was used to estimate the distribution of time to the first protocol-defined relapse for each group. Relapse-free rates (with 95% CIs) and HRs were used every 24 weeks to describe the distribution of time to the first protocol-defined relapse. A two-sided log-rank test was used, stratified by previous therapy for the prevention of NMOSD attack (B-cell-depleting therapy vs immunosuppressants or other) and by the nature of the most recent attack in the year before screening (patient’s first clinical attack vs relapse). The treatment effect was expressed as HR and 95% Cl using a Cox proportional hazards model, again stratified by previous therapy (B-cell-depleting therapy vs immunosuppressants or other) and nature of the most recent attack in the year before screening (first clinical attack vs relapse). The following were treated as censored: discontinuation from double-blind period; ongoing at cutoff date; or entering the open-label extension period after having a clinical relapse before implementation of protocol version 5 (Nov 5, 2015), which required that patients could enter the open-label extension only after a protocol-defined relapse that was confirmed by the CEC.
For the two key secondary analyses specified in the protocol, we used ANCOVA including the treatment group as a fixed effect with baseline measurements and stratification factors (previous treatment [B-cell depleting therapy vs immunosuppressants or other] and nature of last attack [patient’s first clinical attack vs relapse]) as covariates. Missing data were imputed using the baseline-observation-carried-forward method; if a patient’s week-24 value was missing, then the baseline value was imputed. Results were presented as means (95% CIs). Additionally, a sensitivity analysis of the key secondary endpoints was done using ANCOVA (change from baseline to week 24) using multiple imputation methods with random hot deck multiple imputation, the treatment group as fixed effect, and the baseline measurements and stratification factors as covariates.
With the exception of annualised relapse rate, all additional secondary analyses were analysed using the mixed-effect model repeated measures method, including treatment group, protocol-specified visit, and treatment-by-visit interaction as fixed effects, with the baseline measurements and stratification factors as covariates, and visit as a repeated measure; the unstructured covariance matrix was assumed in the model. Annualised relapse rate was calculated as the total number of relapses experienced by the patients in each treatment arm divided by the patient-years at risk for each year of the study period; the 95% Cl was based on the Poisson distribution. Annualised relapse rate based on the patient-years at risk in the total study period was also calculated. To account for different study treatment exposure durations among patients, log-transformed exposure time was included in the model as an offset variable.
The serial gatekeeping method was used to control the rate of false positives for the primary and two key secondary outcomes. These outcomes were analysed in hierarchical order, beginning with the primary outcome, followed by VAS pain, and ending with FACIT fatigue. A fixed-sequence approach was applied to control the overall significance level at 5%. p values were not presented if statistical significance was not met for an outcome that was higher in the testing hierarchy.
A prespecified subgroup analysis was done for the time to first protocol-defined relapse by AQP4-IgG serological status at screening (positive vs negative by central lab test result using ELISA M23 assay).
All statistical outputs were produced in a secure and validated environment, using SAS version 9.2 or later. The database system used was DataLabs version 5.2.1 or later.
Role of the fundi ng source
The study funder, Chugai Pharmaceutical, a member of the Roche group, designed the trial, provided the trial drug and placebo, paid for professional medical writing, and analysed the data. The study was designed in collaboration with the SAkuraStar steering committee and in consultation with regulatory agencies. Data were collected by investigators (appendix p 2) and were analysed by the sponsor after database lock. Chugai, Roche, and the steering committee reviewed the results, and contributed to the writing of the manuscript. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. All authors vouch for the fidelity of trial conduct to the protocol, accuracy of results reporting, and accuracy of adverse event reporting.
Results
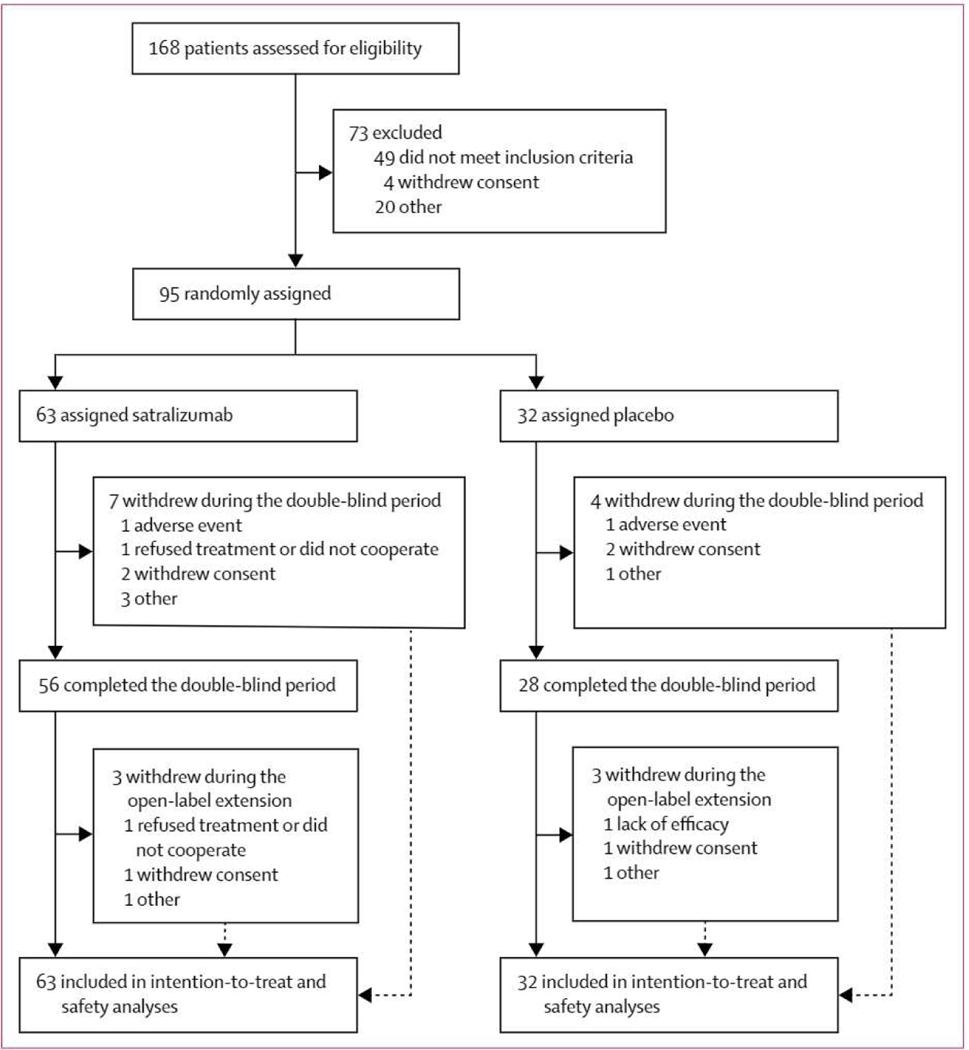
95 (57%) of 168 patients screened were randomly assigned to treatment with either satralizumab (n=63) or placebo (n=32; figure 1, table 1, appendix p 12) between Aug 5,2014, and April 2, 2017. Numbers of patients enrolled by country is available (appendix p 13). The double-blind treatment period ended as planned, 1·5 years after the random assignment of the last enrolled patient. Disease history and baseline characteristics were well balanced overall, apart from sex; 77 (81%) of the 95 participants were women (46 [73%] of 63 in the satralizumab group vs 31 [97%] in the placebo group). The mean scores for pain and fatigue at baseline were low in both groups (table 1). The median treatment duration in the double-blind period was 92·3 weeks (range 0–202 weeks; IQR 40·3–120·4) in the satralizumab group and 54·6 weeks (2–216 weeks; IQR 15·2–94·9) in the placebo group. The median treatment duration for all patients receiving satralizumab in the double-blind and extension periods was 95 · 9 weeks (5–206 weeks; IQR 71 · 9–164 · 6).
Figure 1: Trial profile.
Data cutoff on Oct 12,2018.
Table 1:
Baseline characteristics
| Patients receiving satralizumab (n=63) | Patients receiving placebo (n=32) | |
|---|---|---|
| Age, years | 45·3 (12·0) | 40·5 (10·5) |
| Age at clinical presentation, years | 36·4(107) | 39·3 (13·3) |
| Sex | ||
| Male | 17(27%) | 1 (3%) |
| Female | 46 (73%) | 31(97%) |
| Diagnosis* | ||
| Neuromyelitis optica | 47(75%) | 24(75%) |
| Neuromyelitis optica spectrum disorder | 16 (25%) | 8 (25%) |
| AQP4-lgG seropositive | 41(65%) | 23 (72%) |
| Annualised relapse rate | 1·4 (0·6) | 1·5(07) |
| Expanded Disability Status Scale score | 3·9 (1·5) | 3·7(1·6) |
| Visual Analogue Scale pain score | ||
| Mean (SD) | 31·7(28·9) | 27·6(30·8) |
| Median (range) | 25 (0–94) | 9 (0–90) |
| Functional Assessment of Chronic Illness Therapy fatigue score | ||
| Mean (SD) | 30·6(11·7) | 29·7(12·9) |
| Median (range) | 30(6–52) | 31(5–48) |
| Race or ethnicity | ||
| American Indian or Alaska Native | 2(3%) | 0 |
| Asian (non-Japanese) | 8 (13%) | 6 (19%) |
| Black or African American | 13 (21%) | 3 (9%) |
| White | 37(59%) | 22 (69%) |
| Other | 3(5%) | 1(3%) |
| Previous treatment | ||
| B-cell-depleting therapy | 8 (13%) | 4(13%) |
| Immunosuppressants or other | 55(87%) | 28 (88%) |
| Disease duration, weeks | 317·8 (340·9) | 214·7(201·3) |
| Type of most recent attack | ||
| First attack | 7(11%) | 4(13%) |
| Relapse | 56(89%) | 28 (88%) |
Data are mean (SD) or n (%).
Patients had neuromyelitis optica by Wingerchuk and colleagues’ 2006 criteria23 (aquaporin-4 autoantibodies [AQP4-lgG] seropositive or seronegative); or neuromyelitis optica spectrum disorder by Wingerchuk and colleagues’ 2007 criteria7 (AQP4-lgG seropositive only), with idiopathic single or recurrent events of longitudinally extensive myelitis (≥3 vertebral segment spinal cord MRI lesion) or single, recurrent, or simultaneous bilateral optic neuritis.
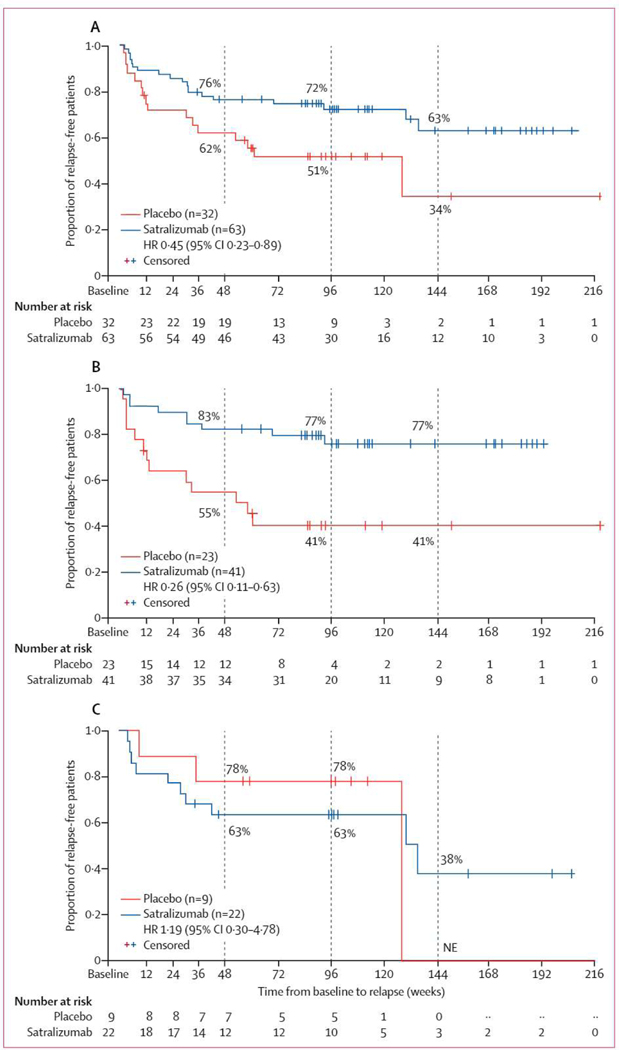
Overall, 35 protocol-defined relapses were observed in the double-blind period. 19 (30%) of the 63 patients receiving satralizumab had a protocol-defined relapse, compared with 16 (50%) of the 32 patients receiving placebo (HR 0·45, 95% Cl 0·23–0·89; p=0·018; table 2, figure 2A). Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab. The Kaplan-Meier method suggested that 76% (95% Cl 64–85) of 63 patients on satralizumab had not relapsed at 48 weeks (and 72%, 59–82, after 96 weeks), compared with 62% (43–76) of 32 patients on placebo at 48 weeks (and 51% [32–67] at 96 weeks; figure 2A, appendix p 13).
Table 2:
Trial endpoints for intention-to-treat population
| Patients receiving satralizumab |
Patients receiving placebo |
Difference (95% Cl) | |||
|---|---|---|---|---|---|
| Patients (n=63) | Change from baseline (95% Cl) | Patients (n=32) | Change from baseline (95% Cl) | ||
| Primary endpoint | |||||
| Protocol-defined relapse | 19(30%) | .. | 16 (50%) | .. | HR 0·45 (0·23 to 0·89); p=0·018 |
| Secondary endpoints | |||||
| VAS pain score at week 24, mean*† | 62 | −2·74 (−11·20 to 5·73) | 32 | −5·95 (−15·55 to 3·65) | 3·21 (−5·09 to 11·52); p=0·44 |
| FACIT fatigue score at week 24, mean*‡ | 62 | 5·71 (2·51 to 8·91) | 32 | 3·60 (−0·01 to 7·22) | 2·11 (−1·01 to 5·22) |
| Annualised relapse rate§ | 63 | 0·17 (0·10 to 0·26)§ | 32 | 0·41 (0·24 to 0·67)§ | 0·41 (0·21 to 0·79) |
| SF-36 score at week 24, mean | |||||
| Physical component summary score | 54 | 2·54 (0·26 to 4·82) | 20 | 3·59 (0·63 to 6·55) | .. |
| Mental component summary score | 54 | 4·84 (1·54to 8·13) | 20 | 1·39 (−2·86 to 5·65) | .. |
| Physical functioning domain | 54 | 3·59 (0·86 to 6·31) | 20 | 4·24 (1·02 to 7·46) | .. |
| Role-physical domain | 54 | 4·31 (0·97 to 7·64) | 20 | 4·89 (0·64 to 9·14) | .. |
| Bodily pain domain | 54 | 0·90 (−2·40 to 4·20) | 20 | 0·43 (−3·66 to 4·51) | .. |
| General health domain | 54 | 3·24 (−0 07 to 6·54) | 20 | 2·69 (−1·23 to 6·61) | .. |
| Vitality domain | 54 | 5·81 (2·33 to 9·28) | 20 | 3·35 (−1·05 to 7·75) | .. |
| Social role functioning domain | 54 | 4·89 (1·05 to 8 73) | 20 | 4·60 (−0·32 to 9·52) | .. |
| Role-emotional domain | 54 | 5·08 (0·98 to 9·18) | 20 | 0·98 (−4·44 to 6·39) | .. |
| Mental health domain | 54 | 3·56 (0·82 to 6·30) | 20 | 2·07 (−1·51 to 5·64) | .. |
| EQ-5D score at week 24, mean | 53 | 0 03 (−0·03 to 0·10) | 20 | 0·04 (−0·05 to 0·12) | .. |
| Timed 25-foot walk speed at week 24, mean¶ | 53 | 0·0004 (−0·0118 to 0·0127) |
19 | 0·0006 (−0·0137 to 0·0150) |
.. |
| Mean Modified Rankin Scale at week 24 | 54 | −0 03 (−0·29 to 0·23) | 19 | −0·19 (−0·52 to 0·13) | .. |
| Mean Zarit Burden Interview score at week 24 | 4 | −6·24 (−31·78 to 19·29) | 6 | −3·61 (−19·47 to 12·25) | .. |
| Mean EDSS score at week 24 | 53 | −0·34 (−0·62 to−0·05) | 20 | −0·17 (−0·52 to 0·19) | .. |
| Visual acuity (Snellen chart) at week 24‖ | |||||
| Right eye | 53 | 0·01 (−0·15 to 0·17) | 20 | −0·10 (−0·32 to 0·12) | .. |
| Left eye | 53 | 0·04 (−019 to 0·27) | 20 | 0·08 (−0·19 to 0·34) | .. |
| Low-contrast visual acuity (low-contrast Sloan letter chart) at week 24|| | |||||
| 100% chart | 49 | 0·6 (−3·8 to 5·1) | 19 | −0·4 (−4·8 to 4·0) | .. |
| 2·5% chart | 49 | 4·2 (−1·7 to 10·1) | 19 | 0·7 (−5·4 to 6·7) | .. |
| 1·25% chart | 49 | −1·4 (−6·4 to 3·7) | 19 | −4·0 (−9·7 to 1·7) | .. |
Data are n (%) or n (95% Cl) unless otherwise specified. HR=hazard ratio. VAS=Visual Analogue Scale. FACIT=Functional Assessment of Chronic Illness Therapy. EQ-5D=EuroQol-five dimensions. SF-36=36-item Short Form Generic Health Survey. EDSS=Expanded Disability Status Scale.
Baseline VAS and FACIT data were unavailable for one patient in the satralizumab group.
Week-24 VAS scores were imputed forten patients in the satralizumab arm and 12 patients in the placebo arm.
Week-24 FACIT fatigue scores were imputed for nine patients in the satralizumab arm and 12 patients in the placebo arm.
Annualised rate is not change from baseline.
Speed is calculated as 1 divided by the time of a 25-foot walk (s).
Figures are adjusted mean based on mixed-effect model repeated measure analysis; positive numbers indicate improvements in vision. Snellen chart scores were converted to logMAR figures presented as adjusted mean change in test distance divided by letter size, expressed as a decimal score. Low contrast Sloan letter chart figures are presented as the mean number of letters read correctly from a distance of 2 m at high (100%), 2·5%, and 1·25% contrast levels.
Figure 2: Time to first protocol-defined relapse during the double-blind period in the overall patient cohort (A), the subgroup seropositive for aquaporin-4 antibodies (B), and the subgroup seronegative for aquaporin-4 antibodies (C).
Patients who did not experience a protocol-defined relapse were censored at the end date of the double-blind period. The dashed lines represent 48 weeks, 96 weeks, and 144 weeks from baseline. The numbers within the figures represent the proportion of relapse-free patients. HR=hazard ratio. NE=not evaluable.
In the AQP4-IgG seropositive subgroup, nine (22%) of 41 patients receiving satralizumab versus 13 (57%) of 23 receiving placebo experienced a protocol-defined relapse (HR 0·26, 95% Cl 0·11–0·63; figure 2B). In the seropositive subgroup, 83% (95% Cl 67–91) of patients receiving satralizumab had not relapsed by 48 weeks and 77% (59–87) had not by 96 weeks, whereas in those receiving placebo, 55% (33–73) had not relapsed by 48 weeks and 41% (21–60) had not by 96 weeks (figure 2B). In the AQP4-IgG seronegative subgroup, ten (46%) of 22 patients receiving satralizumab experienced a protocol-defined relapse versus three (33%) of nine patients receiving placebo (HR 1·19, 0·30–4·78; figure 2C). In the seronegative subgroup, 63% (40–80) of patients receiving satralizumab and 78% (36–94) receiving placebo had not relapsed at both 48 weeks and 96 weeks (figure 2C).
For the prespecified key secondary outcomes, the adjusted mean of the VAS pain score change from baseline did not differ significantly between the two groups (between-group difference in mean score change 3·21, −5·09 to 11·52; p=0·44; table 2). The between-group difference in mean score change for FACIT fatigue from baseline to week 24 was 2·11 (−1·01 to 5·22; table 2). Week-24 VAS scores were imputed by the baseline-observation-carried-forward method for ten patients in the satralizumab arm and 12 patients in the placebo arm, and for FACIT fatigue, week-24 scores were imputed by the same method for nine patients in the satralizumab arm and 12 patients in the placebo arm. The findings of the exploratory sensitivity analyses into the key secondary endpoints were consistent with these results (appendix p 14). Results of the additional secondary endpoints are shown in table 2.
The sensitivity analysis of time to first clinical relapse, including both protocol-defined and non-protocol-defined relapses, showed no evidence of risk reduction (HR 0·74, 95% Cl 0·41–1·35; appendix p 10). The evidence was also weak for time to first treated clinical relapse of an optic neuritis event (HR 0·43, 0·15–1·20). Other sensitivity analyses were consistent with the results of the primary analysis, with HR estimates favouring satralizumab (for time to first treated clinical relapse [HR 0 · 46, 0 · 24–0 · 88]; and time to first protocol-defined relapse adjudicated by the CEC, regardless of 7-day EDSS assessment limit [HR 0·49, 0·25–0·95]; appendix pp 11–13).
The rate of adverse events was 473 · 9 events per 100 patient-years in the satralizumab group and 495 · 2 events per 100 patient-years in the placebo group, and the rate of serious adverse events was similar between the two groups (table 3). One patient in each group withdrew from the study treatment (on day 271 in the placebo group and day 202 in the satralizumab group) because of an adverse event. The most commonly reported adverse events were urinary tract infection and upper respiratory tract infection (appendix p 18). Most adverse events were mild to moderate in intensity, and a higher rate of severe adverse events was reported in the satralizumab group (32·1 events per 100 patient-years) than in the placebo group (9·9 events per 100 patient-years). The severe adverse events in the satralizumab group were distributed in different system organ classes with small numbers in each category; most (27 [73%] of 37 events) were considered to be unrelated to the study treatment by the investigators. None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group. The overall rate of infections and serious infections was similar between the satralizumab and placebo groups, with no opportunistic infections reported in patients treated with satralizumab. Similar rates of injection-related reactions occurred in the satralizumab and placebo groups, including systemic injection-related reactions in four patients in the satralizumab group and one in the placebo group. Most injection-related reactions were resolved without interruption of taking the study drug. To date, there have been no deaths or anaphylactic reactions throughout the study, including the open-label extension period.
Table 3:
Adverse events in the double-blind period in the safety analysis population
| Patients receiving satralizumab (n=63; 115·2 patient-years) |
Patients receiving placebo (n=32; 40·6 patient-years) |
|||
|---|---|---|---|---|
| Number of patients | Events per 100 patient-years (95% Cl) | Number of patients | Events per 100 patient-years (95% Cl) | |
| Adverse events | 58(92%) | 473·9 (435 0–515·4) | 24(75%) | 495·2 (429·1–568·6) |
| Serious adverse events | 12 (19%) | 17·4(10·6–26·8) | 5 (16%) | 14·8 (5·4–32·2) |
| Severe adverse events | 17(27%) | 32·1 (22·6–44·3) | 2 (6%) | 9·9 (2·7–25·2) |
| Deaths | 0 | 0 (NE–3·2) | 0 | 0 (NE–9·1) |
| Infections* | 34(54%) | 99·8 (82·4–119·8) | 14(44%) | 162·6 (125·8–206·9) |
| Serious infections* | 6 (10%) | 5·2 (1·9–11·3) | 3(9%) | 9·9(2·7–25·2) |
| Injection-related reactions | 8 (13%) | 13·9 (7·9–22·6) | 5(16%) | 17·3 (6·9–35·5) |
| Anaphylactic reactions† | 0 | 0 (NE–3·2) | 0 | 0 (NE–9·1) |
Data are n (%) unless otherwise specified. NE=not evaluable.
MedDRA system organ class; infections and infestations.
Standardised MedDRA Queries anaphylaxis narrow term.
Discussion
Previous preclinical and clinical studies suggest a role for IL-6 in the pathophysiology of NMOSD.12,17 In the phase 3 SAkuraSky study20 in patients with the disorder, satralizumab as an add-on treatment to baseline immunosuppressants was shown to reduce the risk of relapse (HR 0·38; 95% Cl 0·16–0·88; p=0·018; risk reduction 62%). In this phase 3 trial, we showed that patients with NMOSD receiving satralizumab monotherapy also had significantly reduced rate of relapse compared with those receiving placebo.
Until recently, no phase 3 clinical trials had been completed in NMOSD, with evidence for off-label immunosuppressant treatments relying on retrospective analyses, observational studies, and case reports.2,6,9 Three disease-modifying clinical agents including satralizumab have now completed phase 3 clinical trials.20,26,27 Eculizumab, a terminal complement inhibitor, has been approved by the US Food and Drug Administration, the European Commission, and Japan’s Ministry of Health, Labour, and Welfare for the treatment of AQP4-IgG seropositive NMOSD,28 following positive trial results in patients receiving baseline immunosuppressants.26 Inebilizumab, which targets CD19, has shown positive results as monotherapy in a phase 2/3 trial of primarily AQP4-IgG seropositive patients (seronegative patients were permitted in the trial, but few patients enrolled).27 These treatments have been developed against a background of new diagnostic criteria published in 2015,5 which widen the spectrum of NMOSD and enable its clinical diagnosis, including in patients with AQP4-IgG seronegative disease.
SAkuraStar (along with the add-on study of satralizumab, SAkuraSky)20 enrolled both AQP4-IgG seropositive and seronegative patients, with the proportion of seronegative patients capped to reflect the approximate proportion of these patients in the overall population.7,11 Subgroup analysis by AQP4-IgG serostatus suggests that satralizumab reduced the risk of relapse in patients who were AQP4-IgG seropositive; however, there is insufficient evidence to indicate a risk reduction for the AQP4-IgG seronegative subgroup. The absence of observed efficacy in seronegative patients might be partly attributable to the greater degree of disease heterogeneity within the general AQP4-IgG seronegative subpopulation, as well as the small sample size.5 The study was not designed or powered to detect differences in efficacy within these subgroups.
No significant change in VAS pain score was observed, despite previous studies showing that IL-6 plays a part in neuropathic and inflammatory pain.29 The reasons behind this insignificant difference are complex. Although patients reported a wide range of baseline pain scores (from no pain to severe pain), the mean and median baseline pain scores were low. In this respect, the study patients differed from those in previous research, which found substantial levels of severe pain in patients with NMOSD, particularly in those with spinal cord lesions.30 Given that the starting point for pain in this study was low, it is possible that satralizumab had little effect on the average VAS pain score because there was limited scope for improvement in this outcome. A targeted study restricted to patients with high levels of baseline pain or those with myelitis, or both, might help to provide further clarity on this point. Additional confounding factors include the heterogeneity of pain syndromes and the ability of patients to use concomitant medications for pain during the study, including adjusting the dose as needed for pain control. Future analyses should consider heterogeneity of the patient population and concomitant medication use.
IL-6 levels, which have been shown to correlate with pain, are generally low in NMOSD during periods of low disease activity and become raised during relapse.18 In this study, patients who relapsed and subsequently entered the open-label extension period prior to the week 24 cutoff point were not included in the key secondary outcome assessment. Therefore, the VAS pain endpoint was unable to capture the effect of satralizumab on changes in pain due to relapse.
No improvement or worsening in fatigue was seen in either group. This finding is consistent with the results of a questionnaire study that found no correlation between IL-6 levels and fatigue, suggesting that the potential effect of satralizumab on fatigue might be small.31 Fatigue in NMOSD is multifaceted and is strongly affected by other factors commonly experienced by patients, such as depression and sleep disturbance.31
Satralizumab was well tolerated by patients with NMOSD; the majority of adverse events were mild to moderate in severity, and adverse events leading to treatment withdrawal were uncommon. A lower rate of infections (in terms of events per 100 patient-years, and including serious infections) was observed in patients treated with satralizumab than in the placebo group. Injection-related reactions were generally mild to moderate, and manageable without interrupting treatment. There was no anaphylaxis throughout the study. The safety profile of satralizumab monotherapy was similar to that observed with satralizumab as an add-on treatment to baseline immunosuppressants.20
The time to the first relapse was used for the primary outcome instead of annualised relapse rate in a fixed observation period. Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug. The trial design used unequal randomisation to minimise exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.
Various additional steps were taken to minimise the risk of harm to patients in the study. A proactive screening procedure aimed to ensure that potential relapses were not missed, including weekly phone calls, patient training during the screening period to identify the signs and symptoms of a potential relapse, and instructions to contact the study site should patients experience any of these symptoms. All potential relapses were reported to and adjudicated by the CEC. The Independent Data Monitoring Committee reviewed unblinded safety data to recommend whether the trial should be stopped early, as needed. The study exclusion criteria prevented patients from participating in the study if they were considered to have a particularly high risk.
Using protocol-defined relapse as an outcome rather than clinical relapse reduced the risk of overestimating the number of relapses. The sensitivity analyses of clinical relapse and treated clinical relapse of an optic neuritis event did not provide evidence for a risk reduction as was shown for protocol-defined relapse for satralizumab versus placebo. However, the risk reduction for treated clinical relapse was very similar to the risk reduction observed for protocol-defined relapse, and most treated clinical relapses in both groups were adjudicated as protocol-defined relapses. This similarity suggests that protocol-defined relapse is a robust and clinically relevant outcome, reflecting relapses that are severe enough to trigger rescue therapy.
SAkuraStar enrolled a diverse and global patient population. Patients from a wide age group (20–70 years) were recruited, and the trial permitted individuals previously treated with B-cell depletion (at least 6 months prior to entry) or immunosuppressant therapy, as well as patients naive to NMOSD treatment. Both AQP4-IgG seropositive and seronegative patients were enrolled in a ratio consistent with that observed in clinical practice.7,11 Although some patients with specific comorbidities or laboratory abnormalities were excluded, the trial participants were overall representative of the real-world population of patients with NMOSD.
The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants.
In conclusion, treatment with satralizumab monotherapy in patients with NMOSD delayed and reduced the rate of relapse, particularly in patients who were AQP4-IgG seropositive. Based on these positive results, satralizumab has the potential to become a valuable treatment option for patients with NMOSD. Further analyses of data from this trial and the ongoing extension studies of satralizumab will provide additional insight on the effectiveness and long-term safety of satralizumab in NMOSD.
Supplementary Material
Research in context.
Evidence before this study
A PubMed search up to Aug 5,2014, for “[neuromyelitis optica], [NMO], [NMOSD], or [Devic’s disease]” identified 23 publications relating to clinical trials or clinical studies in neuromyelitis optica spectrum disorder (NMOSD). Potential treatment options discussed in these publications included azathioprine, interferon-beta, anti-tuberculosis treatment, mitoxantrone, and rituximab. These trials were either retrospective and observational, or small and prospective in nature. With interferon-beta the effects of treatment on the disorder were negative, whereas for azathioprine, anti-tuberculosis treatment, mitoxantrone, and rituximab the effects were modest, associated with poor tolerability, or warranted further investigation in phase 3 clinical trials.
Two additional PubMed searches were done up to Aug 5,2014, focusing on the role of interleukin-6 (IL-6) receptor blockade in NMOSD. These searches used the search terms ″([neuromyelitis optica] OR [NMO] OR [NMOSD] OR [Devic′s disease]) AND [IL-6 receptor blockade]″, and ″([neuromyelitis optica] OR [NMO] OR [NMOSD] OR [Devic′s disease]) AND [IL-6 receptor antagonist]″. Of the four studies identified, one was preclinical and suggestive of the potential benefit of IL-6 receptor-targeted therapy. The remaining three publications consisted of small, open label-studies (n=3–7) and a case report, all of which used an anti-IL-6 receptor blockade and showed the viability of pursuing treatments targeting this receptor to reduce the risk of relapse in patients with NMOSD.
Within the past 2 years, research in NMOSD has accelerated and yielded groundbreaking developments in potential therapeutic options, with positive results from trials of three distinct treatments: PREVENT (eculizumab, NCTOI892345), N-MOmentum (inebilizumab, NCT02200770), and SAkuraSky (satralizumab in combination with patients’ baseline immunosuppressants, NCT02028884).Todate, eculizumab, a terminal complement inhibitor, has been approved by the US Food and Drug Administration, the European Commission, and Japan’s Ministry of Health, Labour, and Welfare for the treatment of aquaporin-4 autoantibody seropositive NMOSD.
Added value of this study
SAkuraStarwas a double-blind, randomised, placebo-controlled phase 3 study assessing the efficacy and safety of satralizumab monotherapy. Satralizumab is a monoclonal antibody targeting the IL-6 receptor, which was designed with recycling antibody technology that increases the half-life of the drug in plasma. SAkuraStar enrolled a diverse and global patient population, reflective of real-world NMOSD cohorts at the time of study initiation. The trial population included aquaporin-4 antibody seropositive and seronegative patients, and patients naive to NMOSD treatment.
Implications of all the available evidence
Satralizumab monotherapy was found to be effective in reducing the rate of NMOSD attacks and showed a favourable safety profile. These results were consistent with the reported efficacy and safety of satralizumab in combination with baseline immunosuppressants (SAkuraSky), suggesting that satralizumab could be a valuable treatment option for patients with NMOSD. The ongoing extension studies will offer further insight into long-term safety and effectiveness of satralizumab in this disorder.
Acknowledgments
We thank the patients for participating in the trial and the study investigators (appendix p 2). We also thank the following project team members at Chugai Pharmaceutical: Naofumi Sugaya, Masayuki Haramura, and Takahito Yamada for their expert contribution to analysis planning and data interpretation; Angela Melia and Helen Thomas for study management; Junnosuke Matsushima for statistical analysis; and Nobuhiko Ishizuka for project management. Additionally, we thank the members of the Clinical Endpoint Committee (Zsolt Illés, David Brassat, and Mark Freedman) and the Independent Data Monitoring Committee (Fredrik Piehl, Robin Roberts, Ruth Whitham, and Takeo Sato). We also thank the following project team members at Roche for their critical review: Cristina Costantino, Xiujing Kou, Gaëlle Klingelschmitt, and H-Christian von Büdingen. Eloise Aston (ApotheCom, UK) was involved in generating and framing the first draft based on input from the authors and provided medical writing assistance together with David Mayes (ApotheCom, UK). Marta Lozano-Wilhelmi (ApotheCom, UK) copyedited and styled the manuscript according to journal requirements.
Funding Chugai Pharmaceutical (Roche).
Declaration of interests
AT reports grants, personal fees, and non-financial support from Chugai Pharmaceutical during the conduct of the study, grants and personal fees from Biogen, Roche, and Sanofi Genzyme, grants from Novartis, and personal fees from Teva Neuroscience outside the submitted work.
BMG reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study, grants from Chugai Pharmaceutical, Medlmmune, Genentech, MedDay, Patient-Centered Outcomes Research Institute, the National Institutes of Health, the National Multiple Sclerosis Society, and the Guthy Jackson Charitable Foundation, and personal fees from Novartis, Alexion, Celgene, Roche, and EMD Serono outside the submitted work. BMG is also an unpaid board member of the Transverse Myelitis Association. JLB reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study, personal fees from Viela Bio, Equillium, Frequency Therapeutics, Clene Neuroscience, Alexion, Genentech, and Roche, grants from Mallinckrodt, the National Institutes of Health, and the Guthy Jackson Charitable Foundation, and personal fees from Chugai Pharmaceutical outside the submitted work. Additionally, JLB has a patent issued for Aquaporumab. LS reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study. EF reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study, and grants and personal fees from AbbVie, Biogen, Celene, EMD Serono, MedDay, Novartis, Sanofi Genzyme, and TG Therapeutics outside the submitted work. SS reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study. TY reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study, grants and personal fees from Biogen, Novartis, Chiome Bioscience, Miraca Holdings, and Chugai Pharmaceutical, grants from Teva, and personal fees from Takeda, Sumitomo Dainippon, Mitsubishi Tanabe, Bayer, Japan Blood Products Organization, Otsuka, Kissei, and Daiichi Sankyo outside the submitted work. YT, YK, and PW are employees of Chugai Pharmaceutical, and AG-B was an employee of Chugai Pharmaceutical at the time of study. HG is an employee of Genentech. BGW reports personal fees and non-financial support from Chugai Pharmaceutical during the conduct of the study, and personal fees from Medlmmune, Alexion, Caladrius Biosciences, Roivant, Brainstorm Therapeutics, Novartis, Mitsubishi Tanabe, and Reistone Biopharma outside the submitted work. In addition, BGW receives royalties from RSR, Oxford University, Hospices Civil de Lyon, and MVZ Labor PD Dr Volkmann und Kollegen for a patent of NMO-IgG as a diagnostic test for neuromyelitis optica and related disorders.
Footnotes
Data sharing
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (http://www.clinicalstudydatarequest.com). For further details on Chugai Pharmaceutical’s data sharing policy and how to request access to related clinical study documents see www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html.
References
- 1.Jarius S, Wildemann B, Paul F. Neuromyelitis optica: clinical features, immunopathogenesis and treatment. Clin Exp Immunol 2014; 176:149–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinshenker BG, Wingerchuk DM. Neuromyelitis spectrum disorders. Mayo Clin Proc 2017; 92: 663–79. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RA, Mealy MA, Levy M. Treatment of neuromyelitis optica spectrum disorder: acute, preventive, and symptomatic. Curr Treat Options Neurol 2016; 18: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Etemadifar M, Nasr Z, Khalili B, Taherioun M, Vosoughi R. Epidemiology of neuromyelitis optica in the world: a systematic review and meta-analysis. Mult Scler Int 2015; 2015:174720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015; 85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kowarik MC, Soltys J, Bennett JL. The treatment of neuromyelitis optica. J Neuroophthalmol 2014; 34: 70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–15. [DOI] [PubMed] [Google Scholar]
- 8.Williamson M, McEnany M, Maxion-Bergemann S, Ong R. Epidemiologic temporal trends of neuromyelitis optic spectrum disorder in the United States from 2000 to 2015. 34th International Conference on Pharmacoepidemiology and Therapeutic Risk Management. Prague, Czech Republic; Aug 22–26, 2018. [Google Scholar]
- 9.Kleiter I, Gold R. Present and future therapies in neuromyelitis optica spectrum disorders. Neurotherapeutics 2016; 13:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev 2013; 93:1543–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sepúlveda M, Armangué T, Sola-Valls N, et al. Neuromyelitis optica spectrum disorders: comparison according to the phenotype and serostatus. Neurol Neuroimmunol Neuroinflamm 2016; 3: e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uzawa A, Mori M, Arai K, et al. Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler 2010; 16:1443–52. [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Li X, Xia J. Thl7 cells in neuromyelitis optica spectrum disorder: a review. Int J Neurosci 2016; 126:1051–60. [DOI] [PubMed] [Google Scholar]
- 14.Chihara N, Aranami T, Sato W, et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci USA 2011; 108: 3701–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeshita Y, Obermeier B, Cotleur AC, et al. Effects of neuromyelitis optica-IgG at the blood-brain barrier in vitro. Neurol Neuroimmunol Neuroinflamm 2016; 4: e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci 2012; 8:1254–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papadopoulos MC, Bennett JL, Verkman AS. Treatment of neuromyelitis optica: state-of-the-art and emerging therapies. Nat Rev Neurol 2014; 10: 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita T, Tateishi T, Isobe N, et al. Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS One 2013; 8: e61835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Içöz S, Tüzün E, Kürtüncü M, et al. Enhanced IL-6 production in aquaporin-4 antibody positive neuromyelitis optica patients. Int J Neurosci 2010; 120: 71–75. [DOI] [PubMed] [Google Scholar]
- 20.Yamamura T, Kleiter I, Fujihara K, et al. Trial of satralizumab in neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 2114–24. [DOI] [PubMed] [Google Scholar]
- 21.Schett G Physiological effects of modulating the interleukin-6 axis. Rheumatology (Oxford) 2018; 57(suppl 2): ii43–ii50. [DOI] [PubMed] [Google Scholar]
- 22.Igawa T, Ishii S, Tachibana T, et al. Antibody recycling by engineered pH-dependent antigen binding improves the duration of antigen neutralization. Nat Biotechnol 2010; 28:1203–07 [DOI] [PubMed] [Google Scholar]
- 23.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006; 66:1485–89. [DOI] [PubMed] [Google Scholar]
- 24.Webster K, Celia D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 2003; 1:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Araki M, Aranami T, Matsuoka T, et al. Clinical efficacy of anti-IL-6 receptor monoclonal antibody tocilizumab in three patients with neuromyelitis optica. European Committee for Treatment and Research in Multiple Sclerosis Congree. Lyon, France; Oct 10–13, 2012: poster 918. [Google Scholar]
- 26.Pittock SJ, Berthele A, Fujihara K, et al. Eculizumab in aquaporin-4-positive neuromyelitis optica spectrum disorder. N Engl J Med 2019; 381: 614–25. [DOI] [PubMed] [Google Scholar]
- 27.Cree BAC, Bennett JL, Kim HJ, et al. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394:1352–63. [DOI] [PubMed] [Google Scholar]
- 28.Alexion. SOLIRIS® (eculizumab) receives approval in Japan for the prevention of relapse in patients with neuromyelitis optica spectrum disorder (NMOSD). Nov 22, 2019. https://ir.alexion.com/news-releases/news-release-details/solirisr-eculizumab-receives-approval-japan-prevention-relapse (accessed Feb 20, 2020).
- 29.Zhou YQ, Liu Z, Liu ZH, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 2016; 13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tackley G, Vecchio D, Hamid S, et al. Chronic neuropathic pain severity is determined by lesion level in aquaporin 4-antibody-positive myelitis. J Neurol Neurosurg Psychiatry 2017; 88:165–69. [DOI] [PubMed] [Google Scholar]
- 31.Masuda H, Mori M, Uzawa A, et al. Validation of the Modified Fatigue Impact Scale and the relationships among fatigue, pain and serum interleukin-6 levels in patients with neuromyelitis optica spectrum disorder. J Neurol Sci 2018; 385: 64–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.